Enhanced Anionic Redox Reaction of Na-Layered Li-Containing Mn-Based Cathodes by Cu-Mediated Reductive Coupling Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Samples
2.3. Characterization
2.4. Electrochemical Measurement
3. Results and Discussion
3.1. Structure and Morphology
3.2. Electrochemical Performance
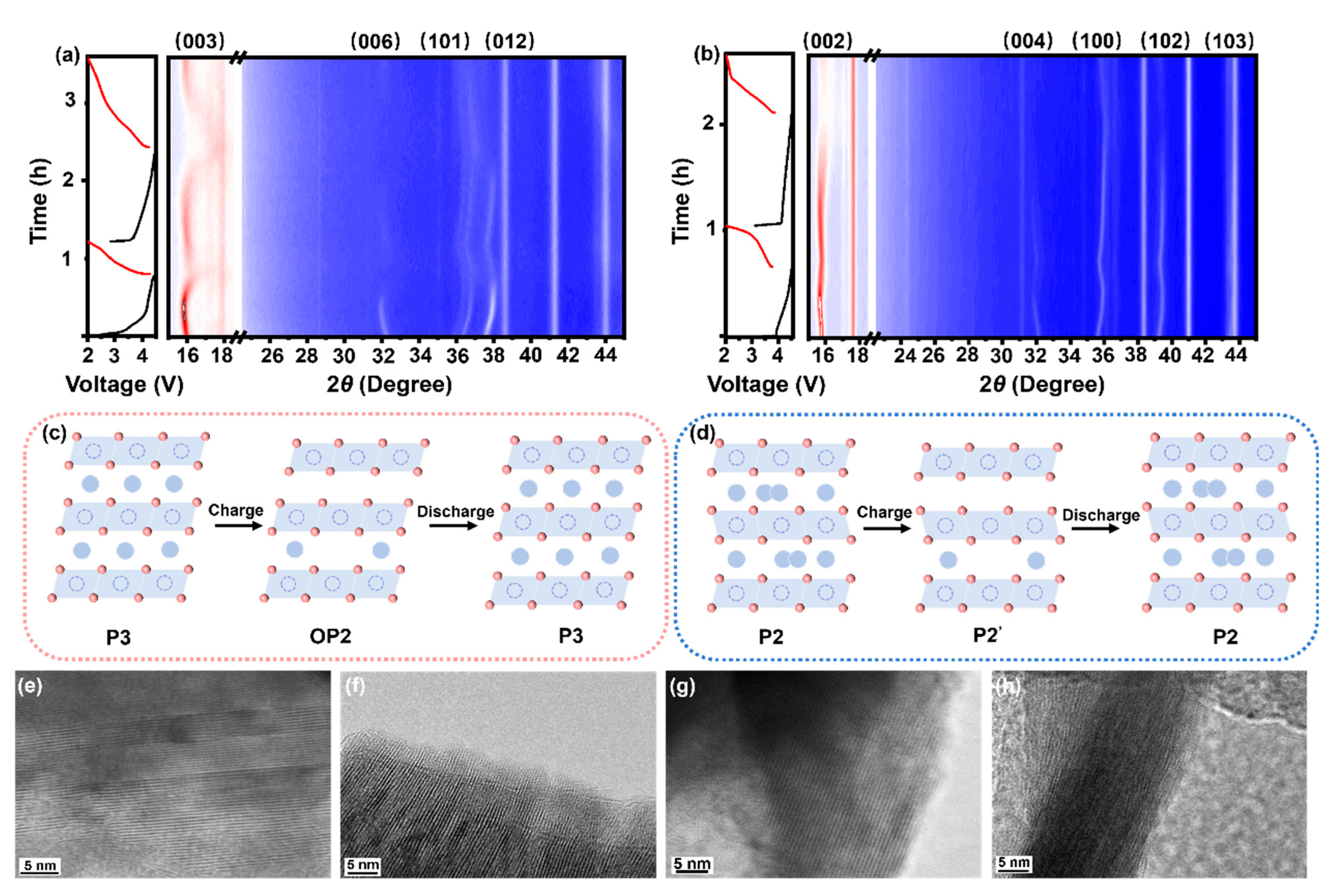
3.3. Structural Evolution During Cycling
3.4. Charge Compensation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.-J.; Jin, R.-X.; Fan, M.; Wang, W.-P.; Xin, S.; Wan, L.-J.; Guo, Y.-G. Sodium layered oxide cathodes: Properties, practicality and prospects. Chem. Soc. Rev. 2024, 53, 7828–7874. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhuo, H.; Wang, J.; Poon, F.; Sun, X.; Xiao, B. Recent advances in Mn-Rich layered materials for sodium-ion batteries. Adv. Funct. Mater. 2023, 33, 2212607. [Google Scholar] [CrossRef]
- Assat, G.; Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 2018, 3, 373–386. [Google Scholar] [CrossRef]
- Li, C.; Geng, F.; Hu, B. Anionic redox in Na-based layered oxide cathodes: A review with focus on mechanism studies. Mater. Today Energy 2020, 17, 100474. [Google Scholar] [CrossRef]
- Maitra, U.; House, R.A.; Somerville, J.; Tapia-Ruiz, N.; Lozano, J.G.; Guerrini, N.; Hao, R.; Luo, K.; Jin, L.; Perez-Osorio, M.A.; et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem. 2018, 10, 288–295. [Google Scholar] [CrossRef]
- Bai, X.; Sathiya, M.; Mendoza-Sánchez, B.; Iadecola, A.; Vergnet, J.; Dedryvère, R.; Saubanère, M.; Abakumov, A.M.; Rozier, P.; Tarascon, J.-M. Anionic redox activity in a newly Zn-doped sodium layered oxide P2-Na2/3Mn1−yZnyO2 (0 < y< 0.23). Adv. Energy Mater. 2018, 8, 1802379. [Google Scholar]
- de Boisse, B.M.; Nishimura, S.-i.; Watanabe, E.; Lander, L.; Tsuchimoto, A.; Kikkawa, J.; Kobayashi, E.; Asakura, D.; Okubo, M.; Yamada, A. Highly reversible oxygen-redox chemistry at 4.1 V in Na4/7−x[□1/7Mn6/7]O2 (□: Mn vacancy). Adv. Energy Mater. 2018, 8, 1800409. [Google Scholar] [CrossRef]
- Rong, X.; Liu, J.; Hu, E.; Liu, Y.; Wang, Y.; Wu, J.; Yu, X.; Page, K.; Hu, Y.-S.; Yang, W.; et al. Structure-Induced Reversible Anionic Redox Activity in Na Layered Oxide Cathode. Joule 2018, 2, 125–140. [Google Scholar] [CrossRef]
- Wu, J.; Zhuo, Z.; Rong, X.; Dai, K.; Lebens-Higgins, Z.; Sallis, S.; Pan, F.; Piper, L.F.J.; Liu, G.; Chuang, Y.-D.; et al. Dissociate lattice oxygen redox reactions from capacity and voltage drops of battery electrodes. Sci. Adv. 2020, 6, eaaw3871. [Google Scholar] [CrossRef]
- Peng, B.; Zhou, Z.; Shi, J.; Huang, X.; Li, Y.; Ma, L. Earth-abundant Fe-Mn-based compound cathodes for sodium-ion batteries: Challenges and progress. Adv. Funct. Mater. 2024, 34, 2311816. [Google Scholar] [CrossRef]
- Li, N.; Zhao, E.; Zhang, Z.; Yin, W.; He, L.; Wang, B.; Wang, F.; Xiao, X.; Zhao, J. Gradient and de-clustered anionic redox enabled undetectable O2 formation in 4.5 V sodium manganese oxide cathodes. Adv. Mater. 2024, 36, 2408984. [Google Scholar] [CrossRef] [PubMed]
- House, R.A.; Maitra, U.; Perez-Osorio, M.A.; Lozano, J.G.; Jin, L.; Somerville, J.W.; Duda, L.C.; Nag, A.; Walters, A.; Zhou, K.-J.; et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 2020, 577, 502–508. [Google Scholar] [CrossRef]
- House, R.A.; Rees, G.J.; McColl, K.; Marie, J.-J.; Garcia-Fernandez, M.; Nag, A.; Zhou, K.-J.; Cassidy, S.; Morgan, B.J.; Saiful Islam, M.; et al. Delocalized electron holes on oxygen in a battery cathode. Nat. Energy 2023, 8, 351–360. [Google Scholar] [CrossRef]
- Lu, Z.H.; Dahn, J.R. In situ X-ray diffraction study of P2 Na2/3[Ni1/3Mn2/3]O2. J. Electrochem. Soc. 2001, 148, A1225–A1229. [Google Scholar] [CrossRef]
- Li, F.; Liu, R.; Liu, J.; Li, H. Voltage hysteresis in transition metal oxide cathodes for Li/Na-ion batteries. Adv. Funct. Mater. 2023, 33, 2300602. [Google Scholar] [CrossRef]
- Assat, G.; Foix, D.; Delacourt, C.; Iadecola, A.; Dedryvère, R.; Tarascon, J.-M. Fundamental interplay between anionic/cationic redox governing the kinetics and thermodynamics of lithium-rich cathodes. Nat. Commun. 2017, 8, 2219. [Google Scholar] [CrossRef]
- Jiang, N.; Yu, J.; Wu, Z.; Zhao, J.; Zeng, Y.; Li, H.; Meng, M.; He, Y.; Jiao, P.; Pan, H.; et al. Surface gradient desodiation chemistry in layered oxide cathode materials. Angew. Chem. -Int. Ed. 2024, 63, e202410080. [Google Scholar]
- Ren, H.; Zheng, L.; Li, Y.; Ni, Q.; Qian, J.; Li, Y.; Li, Q.; Liu, M.; Bai, Y.; Weng, S.; et al. Impurity-vibrational entropy enables quasi-zero-strain layered oxide cathodes for high-voltage sodium-ion batteries. Nano Energy 2022, 103, 107765. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, T.; Li, C.; Zhang, T.; Zhang, W.; Cui, S.; Shen, C.; Jiao, L.; Huang, H.; Xie, K. Lattice strengthening enables reversible anionic redox chemistry in sodium-ion batteries. Energy Storage Mater. 2025, 74, 103935. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Zhao, S.; Liu, Y.; Yang, Y.; Yu, H.; Lee, S.; Lee, G.-H.; Kang, Y.-M.; Liu, R.; et al. Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery. Nat. Commun. 2021, 12, 2256. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Z.; Hou, M.; Ni, Y.; Sun, H.; Jiao, P.; Li, H.; Zhang, W.; Zhang, L.; Zhang, K.; et al. Unraveling and suppressing the voltage decay of high-capacity cathode materials for sodium-ion batteries. Energy Environ. Sci. 2024, 17, 210–218. [Google Scholar] [CrossRef]
- Zou, P.; Yao, L.; Wang, C.; Lee, S.J.; Li, T.; Xin, H.L. Regulating cation interactions for zero-strain and high-voltage P2-type Na2/3Li1/6Co1/6Mn2/3O2 layered oxide cathodes of sodium ion batteries. Angew. Chem. -Int. Ed. 2023, 62, e202304628. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhuo, Z.; Xia, X.; Liu, T.; Shen, Y.; Yuan, C.; Zeng, P.; Cao, D.; Zou, Y.; Guo, J.; et al. Stabilized Oxygen Vacancy Chemistry toward High-Performance Layered Oxide Cathodes for Sodium-Ion Batteries. ACS Nano 2024, 18, 35052–35065. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, M.; Chen, G.; Tang, G.; Huang, Z.; Chu, M.; Qi, R.; Li, S.; Wang, R.; Wang, C.; et al. Triggering anionic redox activity in Fe/Mn-based layered oxide for high-performance sodium-ion batteries. Nano Energy 2022, 94, 106958. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Jin, J.; Shen, Q.; Hu, Y.; Song, X.; Li, H.; Qu, X.; Jiao, L.; Liu, Y. Boosting the reversibility and kinetics of anionic redox chemistry in sodium-ion oxide cathodes via reductive coupling mechanism. J. Am. Chem. Soc. 2023, 145, 22708–22719. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Ma, J.; Wei, G.; Ling, Y.; Zhang, R.; Huang, Y. Revisiting the Na2/3Ni1/3Mn2/3O2 cathode: Oxygen redox chemistry and oxygen release suppression. ACS Cent. Sci. 2020, 6, 232–240. [Google Scholar] [CrossRef]
- Cui, T.; Liu, L.; Xiang, Y.; Sheng, C.; Li, X.; Fu, Y. Facilitating an ultrastable O3-type cathode for 4.5 V sodium-ion batteries via a dual-reductive coupling mechanism. J. Am. Chem. Soc. 2024, 146, 13924–13933. [Google Scholar] [CrossRef]
- Li, B.; Kumar, K.; Roy, I.; Morozov, A.V.; Emelyanova, O.V.; Zhang, L.; Koc, T.; Belin, S.; Cabana, J.; Dedryvere, R.; et al. Capturing dynamic ligand-to-metal charge transfer with a long-lived cationic intermediate for anionic redox. Nat. Mater. 2022, 21, 1165–1174. [Google Scholar] [CrossRef]
- Li, B.; Zhuo, Z.; Zhang, L.; Iadecola, A.; Gao, X.; Guo, J.; Yang, W.; Morozov, A.V.; Abakumov, A.M.; Tarascon, J.-M. Decoupling the roles of Ni and Co in anionic redox activity of Li-rich NMC cathodes. Nat. Mater. 2023, 22, 1370–1379. [Google Scholar] [CrossRef]
- Seo, D.-H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef]
- Ben Yahia, M.; Vergnet, J.; Saubanere, M.; Doublet, M.-L. Unified picture of anionic redox in Li/Na-ion batteries. Nat. Mater. 2019, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.T.; Doublet, M.L.; Foix, D.; Gonbeau, D.; Walker, W.; et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 2013, 12, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zhu, J.; Hu, G.; Gao, H.; Li, Y.; Goodenough, J.B. Exploring reversible oxidation of oxygen in a manganese oxide. Energy Environ. Sci. 2016, 9, 2575–2577. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural classification and properties of the layered oxides. Phys. B+C 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, C.; Wu, X.; Hu, C.; Geng, F.; Shen, M.; Hu, B.; Hu, B.; Li, C. Inconsistency between superstructure stability and long-term cyclability of oxygen redox in Na layered oxides. Energy Environ. Sci. 2024, 17, 668–679. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, S.; Xu, X.; An, Z.; Han, X.; Cao, Y.; He, D.; Zhang, F.; Guo, H.; Liu, Y.; et al. Negative enthalpy doping stabilizes P2-type oxides cathode for high-performance sodium-ion batteries. Adv. Mater. 2025, 37, 2408012. [Google Scholar] [CrossRef]
- Wu, Z.; Ni, Y.; Tan, S.; Hu, E.; He, L.; Liu, J.; Hou, M.; Jiao, P.; Zhang, K.; Cheng, F.; et al. Realizing high capacity and zero strain in layered oxide cathodes via Lithium dual-site substitution for sodium-ion batteries. J. Am. Chem. Soc. 2023, 145, 9596–9606. [Google Scholar] [CrossRef]
- Kubota, K.; Asari, T.; Komaba, S. Impact of Ti and Zn dual-substitution in P2 type Na2/3Ni1/3Mn2/3O2 on Ni-Mn and Na-vacancy ordering and electrochemical properties. Adv. Mater. 2023, 35, 2300714. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Yao, Z.; Wang, J.; Sánchez-Lengeling, B.; Ding, F.; Qi, X.; Lu, Y.; Bai, X.; Li, B.; et al. Rational design of layered oxide materials for sodium-ion batteries. Science 2020, 370, 708–711. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, Z.; Wang, Q.; Li, H.; Wang, J.; Liu, M.; Ganapathy, S.; Lu, Y.; Cabana, J.; Li, B.; et al. Revealing high Na-content P2-type layered oxides as advanced sodium-ion cathodes. J. Am. Chem. Soc. 2020, 142, 5742–5750. [Google Scholar] [CrossRef]
- Li, K.; Xue, D. Estimation of electronegativity values of elements in different valence states. J. Phys. Chem. A 2006, 110, 11332–11337. [Google Scholar] [CrossRef] [PubMed]
- Vergnet, J.; Saubanère, M.; Doublet, M.-L.; Tarascon, J.-M. The structural stability of P2-layered Na-based electrodes during anionic redox. Joule 2020, 4, 420–434. [Google Scholar] [CrossRef]
- Dai, K.; Mao, J.; Zhuo, Z.; Feng, Y.; Mao, W.; Ai, G.; Pan, F.; Chuang, Y.-d.; Liu, G.; Yang, W. Negligible voltage hysteresis with strong anionic redox in conventional battery electrode. Nano Energy 2020, 74, 104831. [Google Scholar] [CrossRef]
- Cheng, C.; Chen, C.; Chu, S.; Hu, H.; Yan, T.; Xia, X.; Feng, X.; Guo, J.; Sun, D.; Wu, J.; et al. Enhancing the reversibility of lattice oxygen redox through modulated transition metal-oxygen covalency for layered battery electrodes. Adv. Mater. 2022, 34, 2201152. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Pei, Z.; Luan, D.; Lou, X.W. Foldable anode-free sodium batteries enabled by N,P-codoped carbon macroporous fibers incorporated with CoP nanoparticles. Sci. Adv. 2025, 11, eadv2007. [Google Scholar] [CrossRef]
- Luo, M.; Zheng, S.; Wu, J.; Zhou, K.; Zuo, W.; Feng, M.; He, H.; Liu, R.; Zhu, J.; Zhao, G.; et al. Identifying the anionic redox activity in cation-disordered Li1.25Nb0.25Fe0.50O2/C oxide cathodes for Li-ion batteries. J. Mater. Chem. A 2020, 8, 5115–5127. [Google Scholar] [CrossRef]
- Pandey, A.K.; Campéon, B.D.L.; Zafar, S.; Ishigaki, T.; Koyama, T.; Nakayama, M.; Yabuuchi, N. Designing metastable P3-type layered negative electrodes with high Na vacancy concentration for high-power sodium-ion batteries. Adv. Funct. Mater. 2025, 35, 2417830. [Google Scholar] [CrossRef]
- Rong, X.; Hu, E.; Lu, Y.; Meng, F.; Zhao, C.; Wang, X.; Zhang, Q.; Yu, X.; Gu, L.; Hu, Y.-S.; et al. Anionic redox reaction-induced high-capacity and low-strain cathode with suppressed phase transition. Joule 2019, 3, 503–517. [Google Scholar] [CrossRef]
- Liu, R.; Huang, W.; Liu, J.; Li, Y.; Wang, J.; Liu, Q.; Ma, L.; Kwon, G.; Ehrlich, S.N.; Wu, Y.; et al. Revealing the nature of binary-phase on structural stability of sodium layered oxide cathodes. Adv. Mater. 2024, 36, 2401048. [Google Scholar] [CrossRef]
- Matsui, M.; Mizukoshi, F.; Hasegawa, H.; Imanishi, N. Ca-substituted P3-type NaxNi1/3Mn1/3Co1/3O2 as a potential high voltage cathode active material for sodium-ion batteries. J. Power Sources 2021, 485, 229346. [Google Scholar] [CrossRef]
- Voronina, N.; Shin, M.-Y.; Kim, H.-J.; Yaqoob, N.; Guillon, O.; Song, S.H.; Kim, H.; Lim, H.-D.; Jung, H.-G.; Kim, Y.; et al. Hysteresis-suppressed reversible oxygen-redox cathodes for sodium-ion batteries. Adv. Energy Mater. 2022, 12, 2103939. [Google Scholar] [CrossRef]
- Asl, H.Y.; Manthiram, A. Reining in dissolved transition-metal ions. Science 2020, 369, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yin, X.; Ning, D.; Chai, Y.; Du, R.; Hao, D.; Wang, C.; Liu, X.; Gao, R.; Wang, J.; et al. Crystal modulation of Mn-based layered oxide toward long-enduring anionic redox with fast kinetics for sodium-ion batteries. Angew. Chem. Int. Ed. 2025, 64, e202415450. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yin, L.; Ronne, A.; Zhang, Y.; Hu, Z.; Tan, S.; Wang, Q.; Song, B.; Li, M.; Rong, X.; et al. Stabilizing lattice oxygen redox in layered sodium transition metal oxide through spin singlet state. Nat. Commun. 2023, 14, 7665. [Google Scholar] [CrossRef]
- Zhao, C.; Yao, Z.; Wang, J.; Lu, Y.; Bai, X.; Aspuru-Guzik, A.; Chen, L.; Hu, Y.-S. Ti substitution facilitating oxygen oxidation in Na2/3Mg1/3Ti1/6Mn1/2O2 cathode. Chem 2019, 5, 2913–2925. [Google Scholar] [CrossRef]
- Cai, C.; Li, X.; Li, J.; Yu, R.; Hu, P.; Zhu, T.; Li, T.; Lee, S.; Xu, N.; Fan, H.; et al. Transition metal vacancy and position engineering enables reversible anionic redox reaction for sodium storage. Nat. Commun. 2025, 16, 100. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Jin, J.; Shen, Q.; Zhang, N.; Qu, X.; Liu, Y.; Jiao, L. Low-cost layered oxide cathode involving cationic and anionic redox with a complete solid-solution sodium-storage behavior. Energy Storage Mater. 2022, 47, 44–50. [Google Scholar] [CrossRef]
- Saubanere, M.; McCalla, E.; Tarascon, J.M.; Doublet, M.L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 2016, 9, 984–991. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Liu, C.; Zhao, S.; Li, F.; Li, H.; Wang, C.; Zhao, X.S. Enhanced Anionic Redox Reaction of Na-Layered Li-Containing Mn-Based Cathodes by Cu-Mediated Reductive Coupling Mechanism. Nanomaterials 2025, 15, 893. https://doi.org/10.3390/nano15120893
Li D, Liu C, Zhao S, Li F, Li H, Wang C, Zhao XS. Enhanced Anionic Redox Reaction of Na-Layered Li-Containing Mn-Based Cathodes by Cu-Mediated Reductive Coupling Mechanism. Nanomaterials. 2025; 15(12):893. https://doi.org/10.3390/nano15120893
Chicago/Turabian StyleLi, Danyang, Can Liu, Shu Zhao, Fujie Li, Hao Li, Chao Wang, and Xiu Song Zhao. 2025. "Enhanced Anionic Redox Reaction of Na-Layered Li-Containing Mn-Based Cathodes by Cu-Mediated Reductive Coupling Mechanism" Nanomaterials 15, no. 12: 893. https://doi.org/10.3390/nano15120893
APA StyleLi, D., Liu, C., Zhao, S., Li, F., Li, H., Wang, C., & Zhao, X. S. (2025). Enhanced Anionic Redox Reaction of Na-Layered Li-Containing Mn-Based Cathodes by Cu-Mediated Reductive Coupling Mechanism. Nanomaterials, 15(12), 893. https://doi.org/10.3390/nano15120893





