Functional Properties and Safety Considerations of Zinc Oxide Nanoparticles Under Varying Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Zinc Oxide Nanoparticles
2.2. Characterization of ZnO NPs
2.2.1. Transmission Electron Microscopy
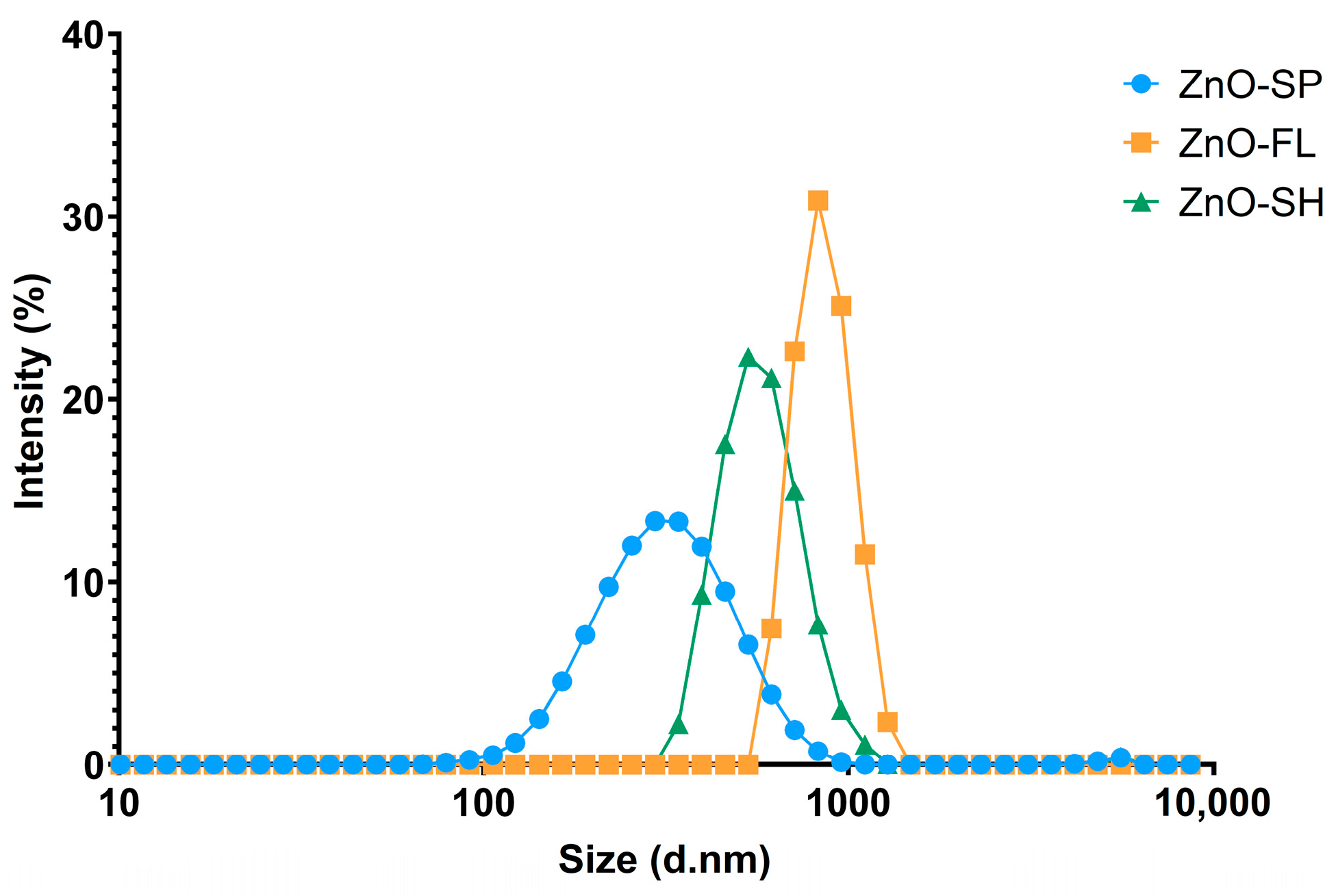
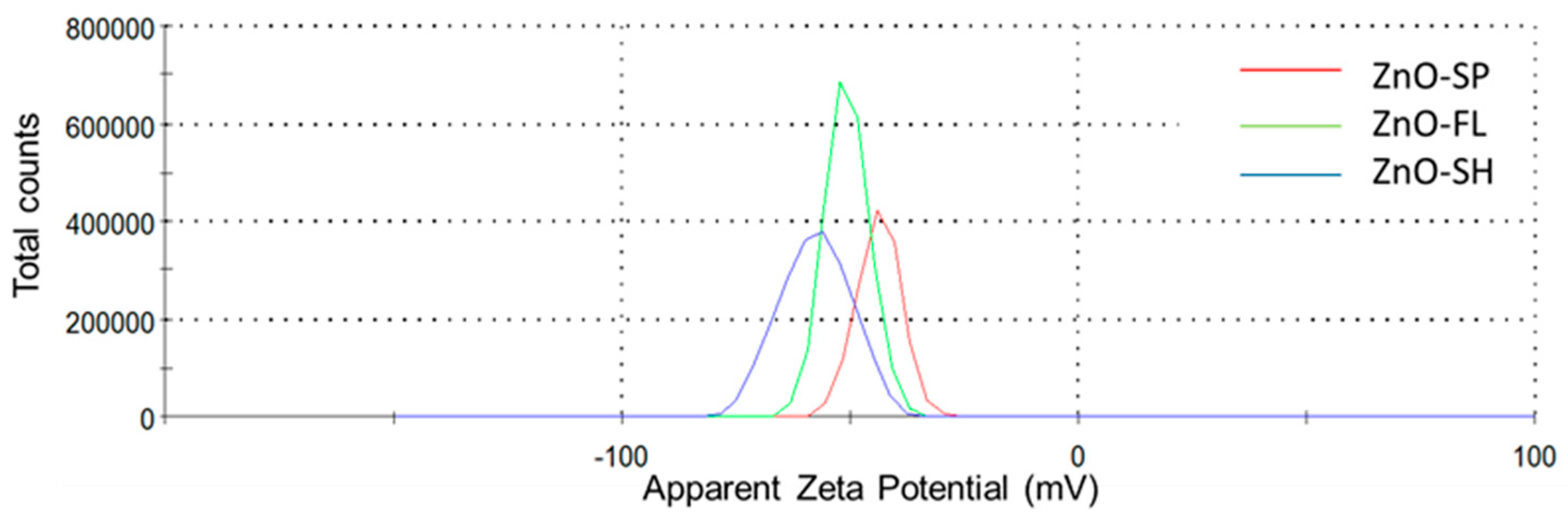
2.2.2. Dynamic Light Scattering
2.3. Functional Properties of ZnO NPs
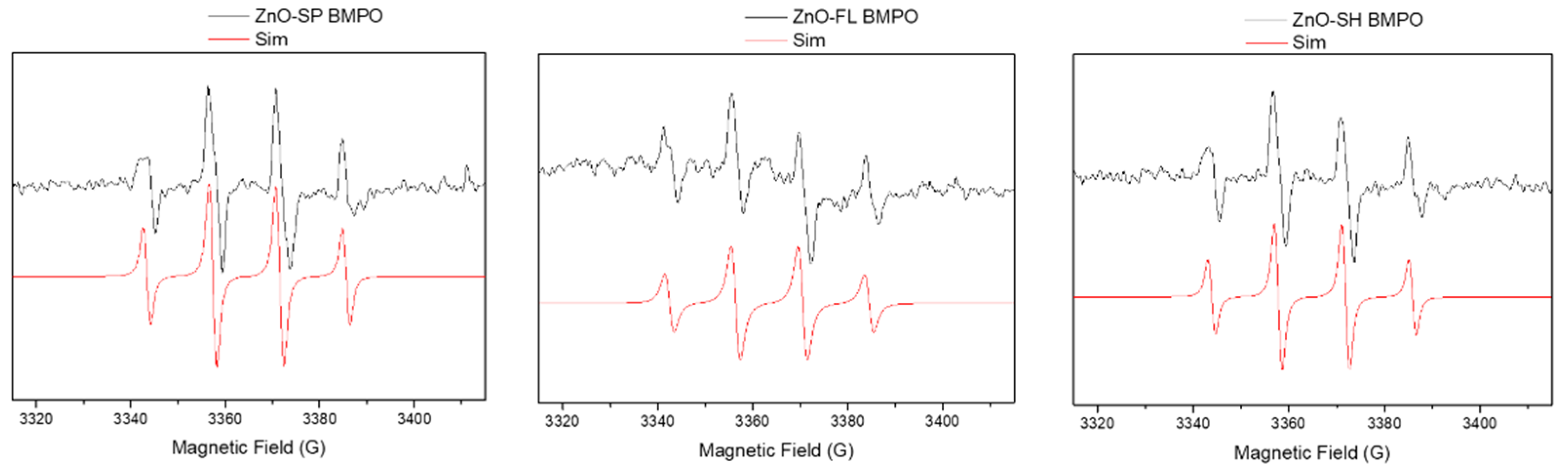
2.3.1. Generation of Reactive Oxygen Species
2.3.2. Antioxidant Activity
ABTS
DPPH
2.3.3. Antibacterial Activity
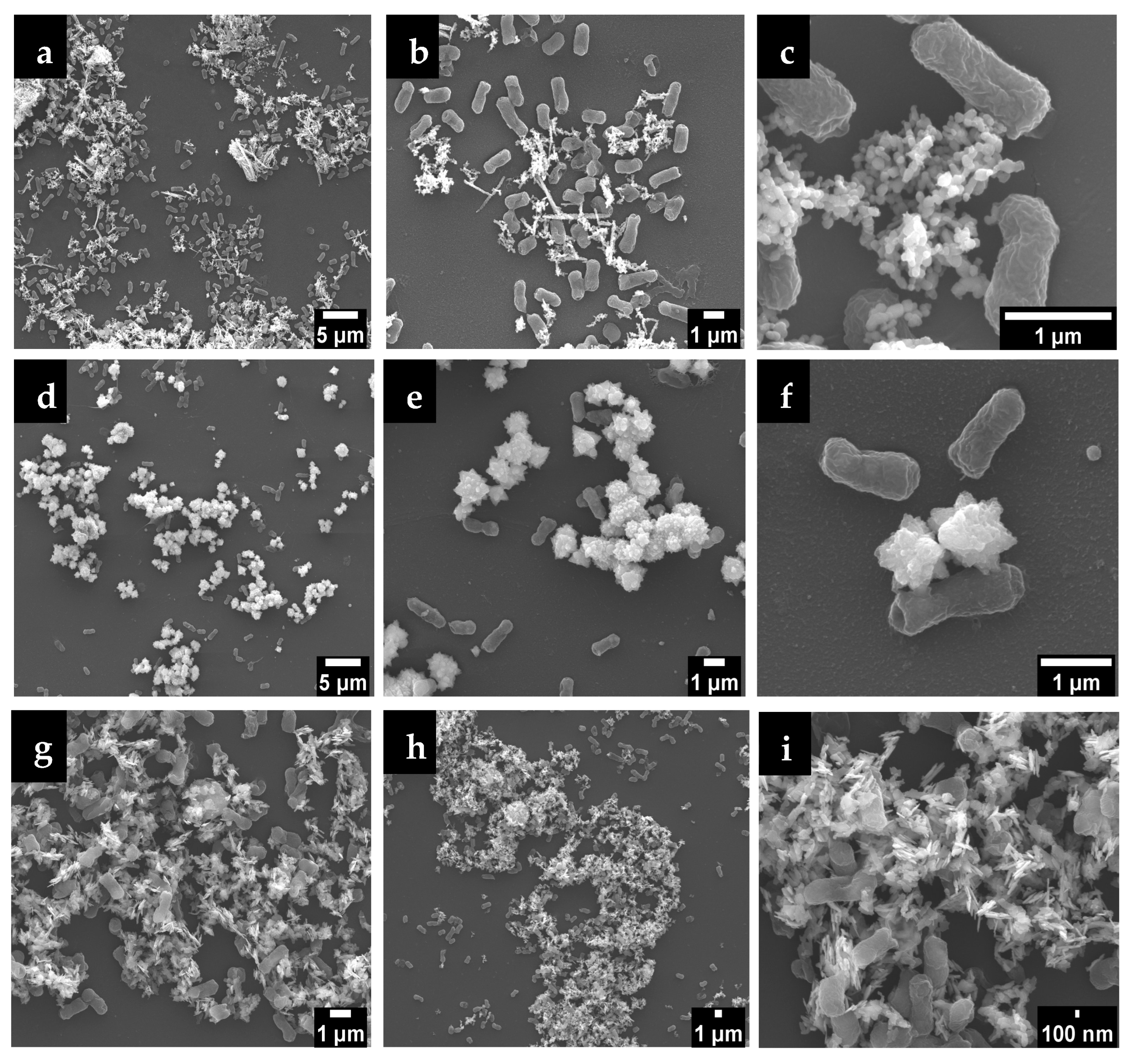
2.3.4. Interface E. coli/ZnO NPs and Stability of Particles
2.4. Data Handling and Statistical Analysis
3. Results and Discussion
3.1. Size and Morphology of ZnO NPs
3.2. Functional Properties
3.2.1. Generation of Reactive Oxygen Species
3.2.2. Antioxidant Activity
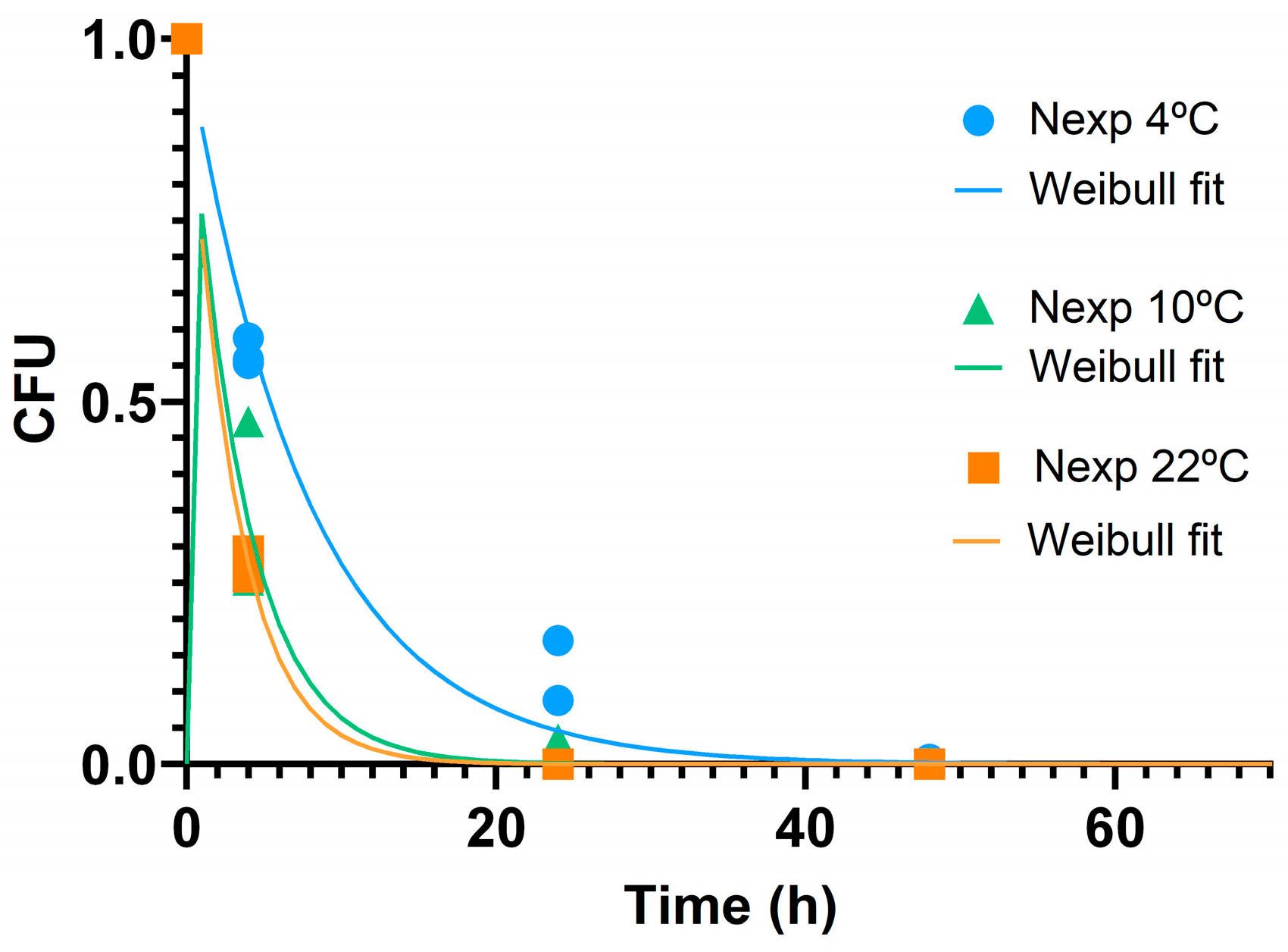
3.2.3. Antibacterial Activity and Effect of Temperature
3.2.4. Interface E. coli/ZnO NPs and Particles Stability
4. Safety Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Commission Recommendation of 10 June 2022 on the Definition of Nanomaterial (Text with EEA Relevance) 2022/C 229/01. Off. J. Eur. Union 2022, 229, 1–5. [Google Scholar]
- Egwu, C.N.; Babalola, R.; Udoh, T.H.; Esio, O.O. Nanotechnology: Applications, Challenges, and Prospects. In Advanced Manufacturing in Biological, Petroleum, and Nanotechnology Processing; Ayeni, O., Oladokun, O., Orodu, O.D., Eds.; Springer: Cham, Switzerland, 2022; pp. 3–15. [Google Scholar]
- Mekuye, B.; Abera, B. Nanomaterials: An Overview of Synthesis, Classification, Characterization, and Applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Vasiljevic, Z.Z.; Auger, S.; Vidic, J. Metal Oxide Nanoparticles for Safe Active and Intelligent Food Packaging. Trends Food Sci. Technol. 2021, 116, 655–668. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Lamsaf, H.; Sebastian, C.V.; Cerqueira, M.A.; Pastrana, L.; Teixeira, J.A. Active Packaging Systems Based on Metal and Metal Oxide Nanoparticles. In Nanotechnology—Enhanced Food Packaging; Rodriguez, R., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 143–181. [Google Scholar]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Poças, F.; Franz, R. Overview on European Regulatory Issues, Legislation, and EFSA Evaluations of Nanomaterials. In Nanomaterials for Food Packaging; López-Rubio, M.Á., Fabra, M.J., Lagarón, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 277–300. [Google Scholar]
- Singh, A.; Banerjee, S.L. Polymer Composites as Packaging Materials. In Industrial Applications of Polymer Composites; Nayak, S.K., Mohanty, S., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023; pp. 20–57. [Google Scholar]
- Mishra, B.; Panda, J.; Mishra, A.K.; Nath, P.C.; Nayak, P.K.; Mahapatra, U.; Sharma, M.; Chopra, H.; Mohanta, Y.K.; Sridhar, K. Recent Advances in Sustainable Biopolymer—Based Nanocomposites for Smart Food Packaging: A Review. Int. J. Biol. Macromol. 2024, 279, 135583. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal Oxide Nanoparticles as Antimicrobial Agents: A Promise for the Future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Anvar, A.A.; Ahari, H.; Ataee, M. Antimicrobial Properties of Food Nanopackaging: A New Focus on Foodborne Pathogens. Front. Microbiol. 2021, 12, 703412. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Soares Silva, F.A.G.; Carvalho, M.; Bento de Carvalho, T.; Gama, M.; Poças, F.; Teixeira, P. Antimicrobial Activity of In-Situ Bacterial Nanocellulose—Zinc Oxide Composites for Food Packaging. Food Packag. Shelf Life 2023, 40, 101201. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Mendes, A.R.; Granadeiro, C.M.; Leite, A.; Pereira, E.; Teixeira, P.; Poças, F. Optimizing Antimicrobial Efficacy: Investigating the Impact of Zinc Oxide Nanoparticle Shape and Size. Nanomaterials 2024, 14, 638. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified ABTS Method to Measure Antioxidant Capacity of Selected Small Fruits and Comparison to FRAP and DPPH Methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Huertas, J.P.; Ros-Chumillas, M.; Garre, A.; Fernández, P.S.; Aznar, A.; Iguaz, A.; Esnoz, A.; Palop, A. Impact of Heating Rates on Alicyclobacillus acidoterrestris Heat Resistance Under Non-Isothermal Treatments and Use of Mathematical Modelling to Optimize Orange Juice Processing. Foods 2021, 10, 1496. [Google Scholar] [CrossRef]
- Alvarenga, V.O.; Brito, L.M.; Lacerda, I.C.A. Application of Mathematical Models to Validate Emerging Processing Technologies in Food. Curr. Opin. Food Sci. 2022, 48, 100928. [Google Scholar] [CrossRef]
- Buzrul, S. The Weibull Model for Microbial Inactivation. Food Eng. Rev. 2022, 14, 45–61. [Google Scholar] [CrossRef]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; de Almeida, M.P.; Pereira, E.; Araújo, J.P.; Baptista, P.V.; Soares, L. A Direct Comparison of Experimental Methods to Measure Dimensions of Synthetic Nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef]
- Wilson, B.K.; Prud’homme, R.K. Nanoparticle Size Distribution Quantification from Transmission Electron Microscopy (TEM) of Ruthenium Tetroxide Stained Polymeric Nanoparticles. J. Colloid Interface Sci. 2021, 604, 208–220. [Google Scholar] [CrossRef]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Chapter 11—Characterization of Nanomaterials: Tools and Challenges. In Nanomaterials for Food Applications; Diez-Pascual, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar]
- Evans, A.; Song, P.; Peddie, C.; Evans, A. Particle Size Reduction to the Nanometer Range: A Promising Approach to Improve Buccal Absorption of Poorly Water-Soluble Drugs. Int. J. Nanomed. 2011, 6, 1245–1252. [Google Scholar] [CrossRef]
- Vinardell, M.; Llanas, H.; Marics, L.; Mitjans, M. In Vitro Comparative Skin Irritation Induced by Nano and Non-Nano Zinc Oxide. Nanomaterials 2017, 7, 56. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection and Characterisation of Radicals in Biological Materials Using EPR Methodology. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Joseph, J.; Zhang, H.; Karoui, H.; Kalyanaraman, B. Synthesis and Biochemical Applications of a Solid Cyclic Nitrone Spin Trap: A Relatively Superior Trap for Detecting Superoxide Anions and Glutathiyl Radicals. Free Radic. Biol. Med. 2001, 31, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Lakshmeesha, T.R.; Srikantaswamy, S.; Byrappa, K. The Correlation Among Morphology, Oxygen Vacancies and Properties of ZnO Nanoflowers. J. Mater. Sci. Mater. Electron. 2018, 29, 13551–13560. [Google Scholar] [CrossRef]
- Mini, R.; Prabhu, V.; Poonkodi, K.; Vimaladevi, K.; Anusuya, M.; Vasuki, M. Bio-Synthesis, Characterization of ZnO Nanoparticles from Scoparia dulcis L. Plant Extract and Its In Vitro Antioxidant, Acetylcholinesterase Activity. Plant Sci. Today 2023, 10, 90–97. [Google Scholar] [CrossRef]
- Ramesh, A.M.; Pal, K.; Kodandaram, A.; Manjula, B.L.; Ravishankar, D.K.; Gowtham, H.G.; Basavaraju, P.; Raj, D.S.; Krishna, V.; Prashith Kekuda, T.R. Antioxidant and Photocatalytic Properties of Zinc Oxide Nanoparticles Phyto-Fabricated Using the Aqueous Leaf Extract of Sida acuta. Green Process. Synth. 2022, 11, 857–867. [Google Scholar] [CrossRef]
- Safawo, T.; Sandeep, B.; Pola, S.; Tadesse, A. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Tuber Extract of Anchote (Coccinia abyssinica (Lam.) Cong.) for Antimicrobial and Antioxidant Activity Assessment. OpenNano 2018, 3, 56–63. [Google Scholar] [CrossRef]
- Kokabi, M.; Nejad Ebrahimi, S. Polyphenol Enriched Extract of Pomegranate Peel; A Novel Precursor for the Biosynthesis of Zinc Oxide Nanoparticles and Application in Sunscreens. Pharm. Sci. 2020, 27, 102–110. [Google Scholar] [CrossRef]
- Ramana, V.; Rajeshkumar, S.; Jagadeesh, K. Review of the Environmentally Friendly Production of Zinc Oxide Nanoparticles and Its Antioxidant, Anti-Hyperlipidemic, and Anti-Diabetic Properties. J. Surv. Fish. Sci. 2023, 10, 117–127. [Google Scholar]
- Lee, J.; Choi, K.-H.; Min, J.; Kim, H.-J.; Jee, J.-P.; Park, B.J. Functionalized ZnO Nanoparticles with Gallic Acid for Antioxidant and Antibacterial Activity against Methicillin-Resistant S. aureus. Nanomaterials 2017, 7, 365. [Google Scholar] [CrossRef]
- Elbrolesy, A.; Abdou, Y.; Elhussiny, F.A.; Morsy, R. Novel Green Synthesis of UV-Sunscreen ZnO Nanoparticles Using Solanum lycopersicum Fruit Extract and Evaluation of Their Antibacterial and Anticancer Activity. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3750–3759. [Google Scholar] [CrossRef]
- Irede, E.L.; Awoyemi, R.F.; Owolabi, B.; Aworinde, O.R.; Kajola, R.O.; Hazeez, A.; Adekanmbi, O. Cutting-Edge Developments in Zinc Oxide Nanoparticles: Synthesis and Applications for Enhanced Antimicrobial and UV Protection in Healthcare Solutions. RSC Adv. 2024, 14, 20992–21034. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–58. [Google Scholar]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO Size and Shape Effect on Antibacterial Activity and Cytotoxicity Profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of Size Scale and Morphology on Antibacterial Properties of ZnO Powders Hydrothermally Synthesized Using Different Surface Stabilizing Agents. Colloids Surf. B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef]
- Cai, Q.; Gao, Y.; Gao, T.; Lan, S.; Simalou, O.; Zhou, X.; Feng, Y.; Liu, C. Insight into Biological Effects of Zinc Oxide Nanoflowers on Bacteria: Why Morphology Matters. ACS Appl. Mater. Interfaces 2016, 8, 10109–10120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The Effect of Shape and Size of ZnO Nanoparticles on Their Antimicrobial and Photocatalytic Activities: A Green Approach. Bull. Mater. Sci. 2020, 43, 20. [Google Scholar] [CrossRef]
- Ashwini, J.; Aswathy, T.R.; Rahul, A.B.; Thara, G.M.; Nair, A.S. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Acacia caesia Bark Extract and Its Photocatalytic and Antimicrobial Activities. Catalysts 2021, 11, 1507. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable Synthesis of ZnO Nanoparticles and Their Morphology-Dependent Antibacterial and Optical Properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73. [Google Scholar] [CrossRef]
- Saliani, M.; Jalal, R.; Kafshadre Goharshadi, E. Effects of pH and Temperature on Antibacterial Activity of Zinc Oxide Nanofluid Against E. coli O157:H7 and Staphylococcus aureus. Jundishapur J. Microbiol. 2015, 8, e17115. [Google Scholar] [CrossRef]
- Sinegani, A.; Noroozi, O. Effect of Dosage and Particle Size of Natural Zeolite on the Survival of Escherichia coli in Soil. J. Water Environ. Nanotechnol. 2019, 4, 296–307. [Google Scholar]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic Investigation into Antibacterial Behaviour of Suspensions of ZnO Nanoparticles Against E. coli. J. Nanopartic. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO Nanomaterials: Current Advancements in Antibacterial Mechanisms and Applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Ding, Z.; Jiang, Y.; Liu, X. Nanoemulsions-Based Drug Delivery for Brain Tumors. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–358. [Google Scholar]
- Marsalek, R. Particle Size and Zeta Potential of ZnO. APCBEE Procedia 2014, 9, 13–17. [Google Scholar] [CrossRef]
- Melk, M.M.; El-Hawary, S.S.; Melek, F.R.; Saleh, D.O.; Ali, O.M.; El Raey, M.A.; Shalaby, E.A.; Eid, A.M.; Eldahshan, O.A. Antiviral Activity of Zinc Oxide Nanoparticles Mediated by Plumbago indica L. Extract Against Herpes Simplex Virus Type 1 (HSV-1). Int. J. Nanomed. 2021, 16, 8221–8233. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile Green Synthesis of Zinc Oxide Nanoparticles with Potential Synergistic Activity with Common Antifungal Agents Against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 774. [Google Scholar] [CrossRef]
- Rezaei, A.; Katoueizadeh, E.; Zebarjad, S.M. Investigation of the Parameters Affecting the Morphology of Zinc Oxide (ZnO) Nanoparticles Synthesized by Precipitation Method. Mater. Today Chem. 2022, 26, 101239. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The Effects of Interfacial Potential on Antimicrobial Propensity of ZnO Nanoparticles. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef]
- Pandey, R.K.; Ao, C.K.; Lim, W.; Sun, Y.; Di, X.; Nakanishi, H.; Soh, S. The Relationship Between Static Charge and Shape. ACS Cent. Sci. 2020, 6, 704–714. [Google Scholar] [CrossRef]
- Ramani, M.; Ponnusamy, S.; Muthamizhchelvan, C. From Zinc Oxide Nanoparticles to Microflowers: A Study of Growth Kinetics and Biocidal Activity. Mater. Sci. Eng. C 2012, 32, 2381–2389. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Investigating the Toxicological Effects of Nanomaterials in Food Packaging Associated with Human Health and the Environment. J. Hazard. Mater. Lett. 2024, 5, 100125. [Google Scholar] [CrossRef]
- Mazur, P.; Skiba-Kurek, I.; Mrowiec, P.; Karczewska, E.; Drożdż, R. Synergistic ROS-Associated Antimicrobial Activity of Silver Nanoparticles and Gentamicin Against Staphylococcus epidermidis. Int. J. Nanomed. 2020, 15, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Hasanabadi, P.; Malekshah, O.M.; Mohammadi, H. The Exposure and Hazards of Zinc Oxide Nanoparticles: In Vitro and In Vivo Studies. Pharm. Biomed. Res. 2023, 9, 77–84. [Google Scholar] [CrossRef]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific Opinion on the Safety Assessment of the Substance Zinc Oxide, Nanoparticles, for Use in Food Contact Materials. EFSA J. 2016, 14, 4408. [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific Opinion on the Safety Evaluation of the Substance Zinc Oxide, Nanoparticles, Uncoated and Coated with [3-(Methacryloxy)propyl] Trimethoxysilane, for Use in Food Contact Materials. EFSA J. 2015, 13, 4063. [Google Scholar]
- European Commission. Commission Regulation (EU) 2016/1416 of 24 August 2016 Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2016, 22–42. [Google Scholar]
- Motelica, L.; Ficai, D.; Oprea, O.; Ficai, A.; Trusca, R.-D.; Andronescu, E.; Holban, A.M. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef]

| R(t) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | ||||||||
| T (°C) | Time (h) | ZnO-SP | ZnO-FL | ZnO-SH | Control Bacteria | ZnO-SP | ZnO-FL | ZnO-SH | Control Bacteria |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | −0.31 ± 0.17 | −0.33 ± 0.03 | −0.24 ± 0.09 | −0.14 ± 0.07 | −0.06 ± 0.39 | −0.06 ± 0.22 | −0.05 ± 0.11 | −0.07 ± 0.04 | |
| 24 | −0.63 ± 0.60 | −0.51 ± 0.11 | −0.95 ± 0.22 | −0.32 ± 0.07 | −0.33 ± 0.16 | −0.07 ± 0.30 | −0.35 ± 0.11 | 0.10 ± 0.24 | |
| 48 | −0.46 ± 0.25 | −0.66 ± 0.04 | −2.30 ± 0.14 | −0.55 ± 0.13 | −0.61 ± 0.11 | −0.36 ± 0.77 | −0.72 ± 0.13 | −0.14 ± 0.03 | |
| 168 | −1.34 ± 0.85 | −0.84 ± 0.21 | −7.53 ± 0.08 | −0.80 ± 0.07 | −1.37 ± 1.06 | −0.53 ± 0.19 | −1.85 ± 0.22 | −0.58 ± 0.05 | |
| 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | −0.63 ± 0.15 | 0.08 ± 0.22 | −0.55 ± 0.09 | −0.06 ± 0.05 | −0.23 ± 0.31 | −0.18 ± 0.22 | −0.38 ± 0.48 | 0.05 ± 0.34 | |
| 24 | −1.97 ± 0.26 | −0.17 ± 0.18 | −7.53 ± 0.08 | −0.04 ± 0.15 | −1.85 ± 0.06 | −0.15 ± 0.16 | −0.80 ± 0.15 | 0.06 ± 0.31 | |
| 48 | −3.35 ± 0.33 | −0.58 ± 0.81 | −7.53 ± 0.08 | −0.04 ± 0.09 | −3.50 ± 0.36 | −0.42 ± 0.19 | −2.53 ± 0.36 | −0.05 ± 0.20 | |
| 168 | −7.53 ± 0.08 | −4.63 ± 2.71 | −7.53 ± 0.08 | −0.06 ± 0.10 | −6.83 ± 0.05 | −3.71 ± 1.32 | −6.83 ± 0.05 | −1.35 ± 1.05 | |
| Estimated Parameters | ||||
|---|---|---|---|---|
| Temperature (°C) | δ (h) | p | RSS | R2 |
| 4 | 1.769 | 0.222–0.227 | 0.023 | 0.982 |
| 10 | 1.567 | 0.430 | 0.031 | 0.970 |
| 22 | 1.509 | 0.484–0.503 | 0.001 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, A.R.; Granadeiro, C.M.; Leite, A.; Geiss, O.; Bianchi, I.; Ponti, J.; Mehn, D.; Pereira, E.; Teixeira, P.; Poças, F. Functional Properties and Safety Considerations of Zinc Oxide Nanoparticles Under Varying Conditions. Nanomaterials 2025, 15, 892. https://doi.org/10.3390/nano15120892
Mendes AR, Granadeiro CM, Leite A, Geiss O, Bianchi I, Ponti J, Mehn D, Pereira E, Teixeira P, Poças F. Functional Properties and Safety Considerations of Zinc Oxide Nanoparticles Under Varying Conditions. Nanomaterials. 2025; 15(12):892. https://doi.org/10.3390/nano15120892
Chicago/Turabian StyleMendes, Ana Rita, Carlos M. Granadeiro, Andreia Leite, Otmar Geiss, Ivana Bianchi, Jessica Ponti, Dora Mehn, Eulália Pereira, Paula Teixeira, and Fátima Poças. 2025. "Functional Properties and Safety Considerations of Zinc Oxide Nanoparticles Under Varying Conditions" Nanomaterials 15, no. 12: 892. https://doi.org/10.3390/nano15120892
APA StyleMendes, A. R., Granadeiro, C. M., Leite, A., Geiss, O., Bianchi, I., Ponti, J., Mehn, D., Pereira, E., Teixeira, P., & Poças, F. (2025). Functional Properties and Safety Considerations of Zinc Oxide Nanoparticles Under Varying Conditions. Nanomaterials, 15(12), 892. https://doi.org/10.3390/nano15120892








