Dispersion Stability and Tribological Properties of Cold Plasma-Modified h-BN Nanofluid
Abstract
1. Introduction
2. Experimental Details
2.1. Experimental Setup
2.2. Material
2.3. Experimental Steps
2.3.1. Cold Plasma-Modified h-BN Nanoparticles
2.3.2. Preparation of h-BN Nanofluid
2.3.3. Friction and Wear Test
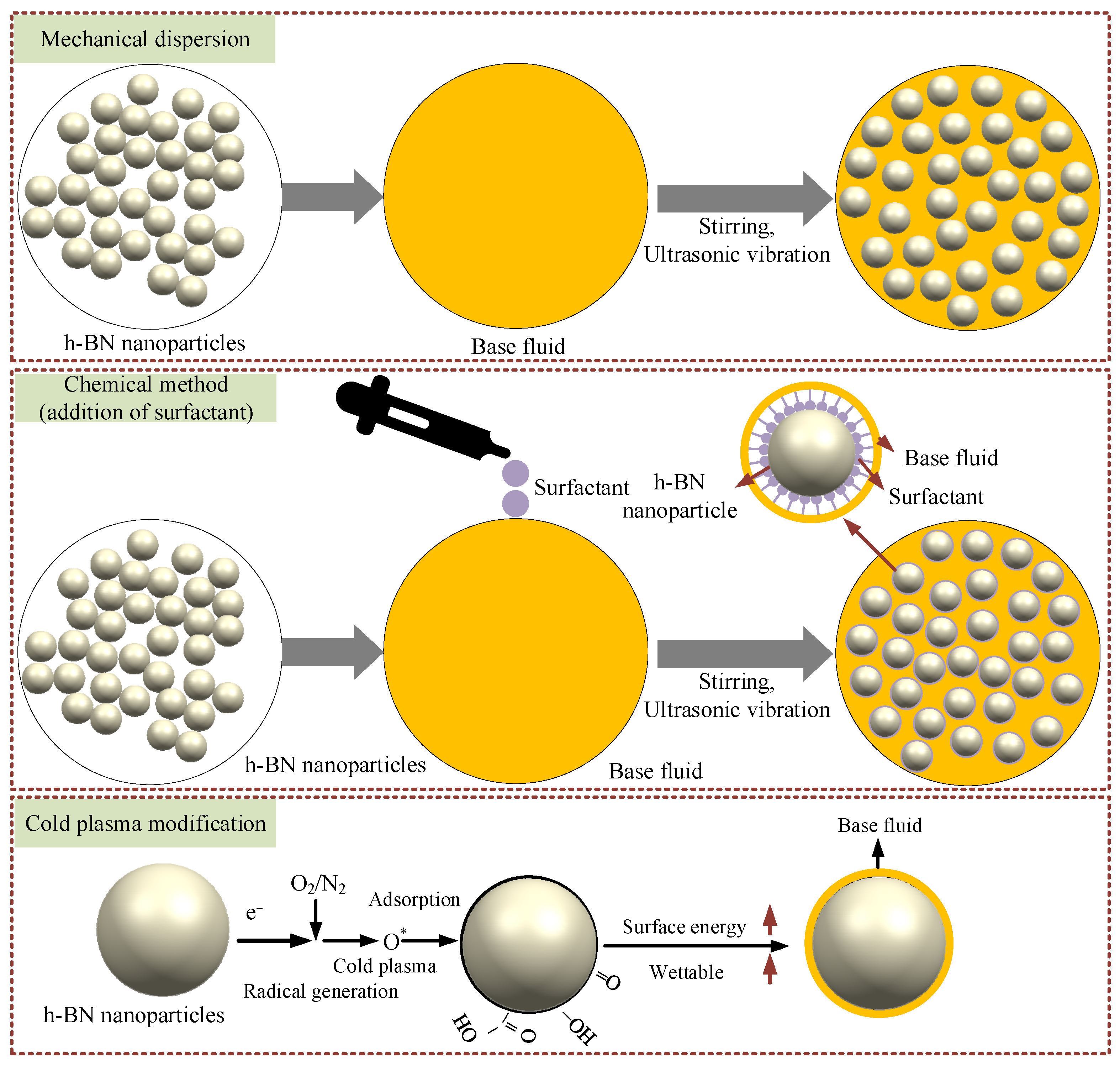
3. Dispersion Mechanism
4. Results and Discussion
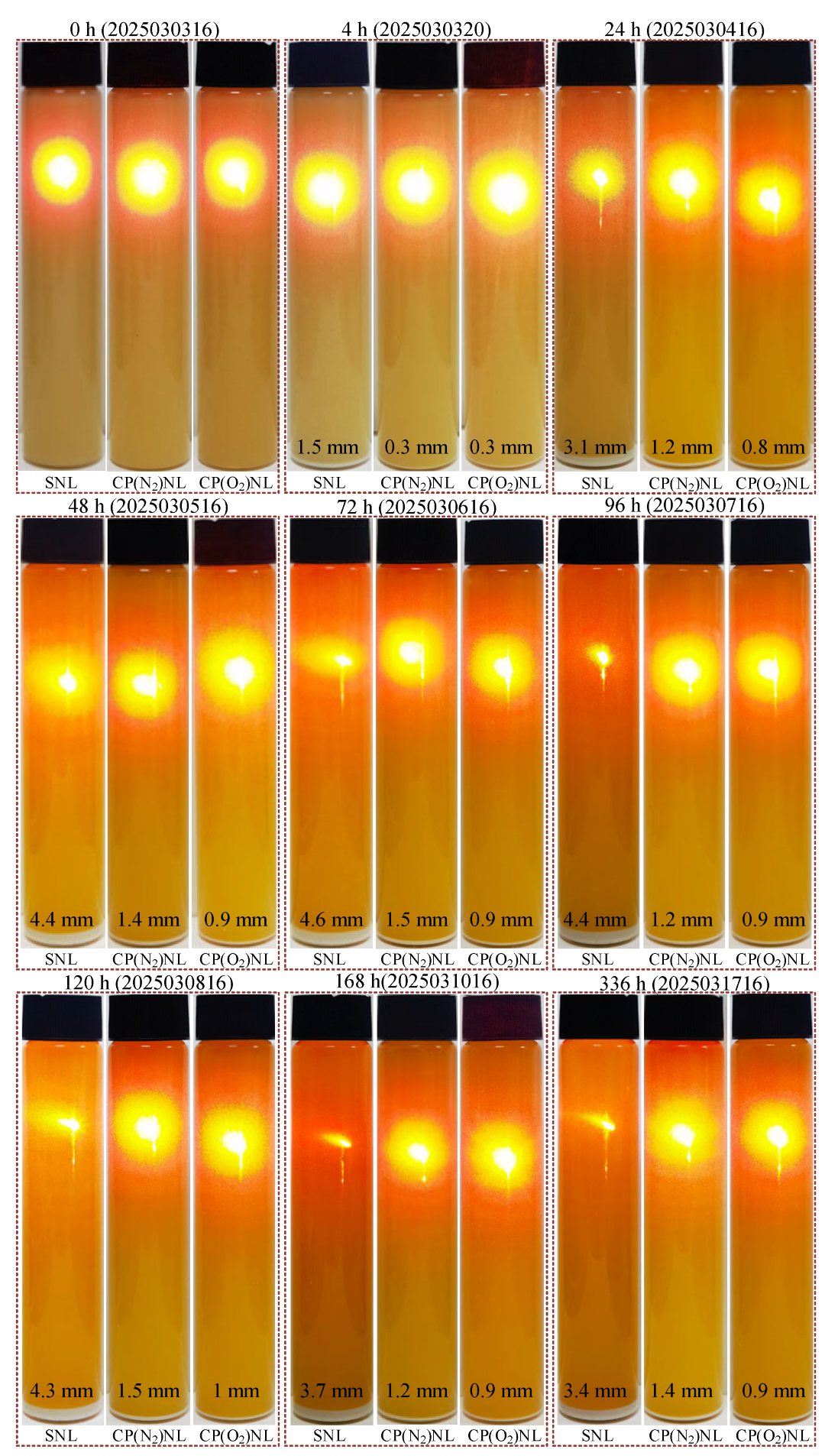
4.1. Stability
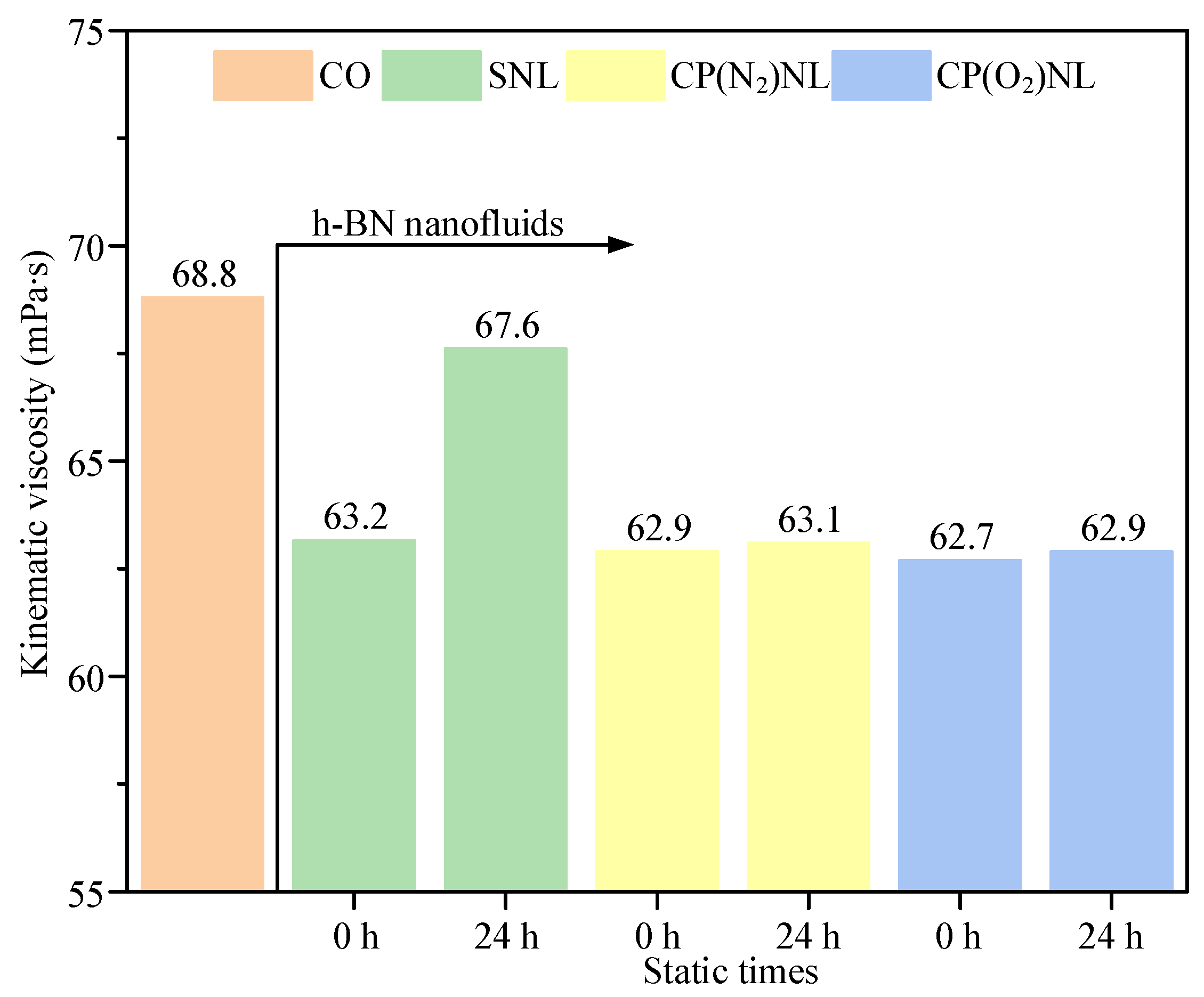
4.2. Kinematic Viscosity
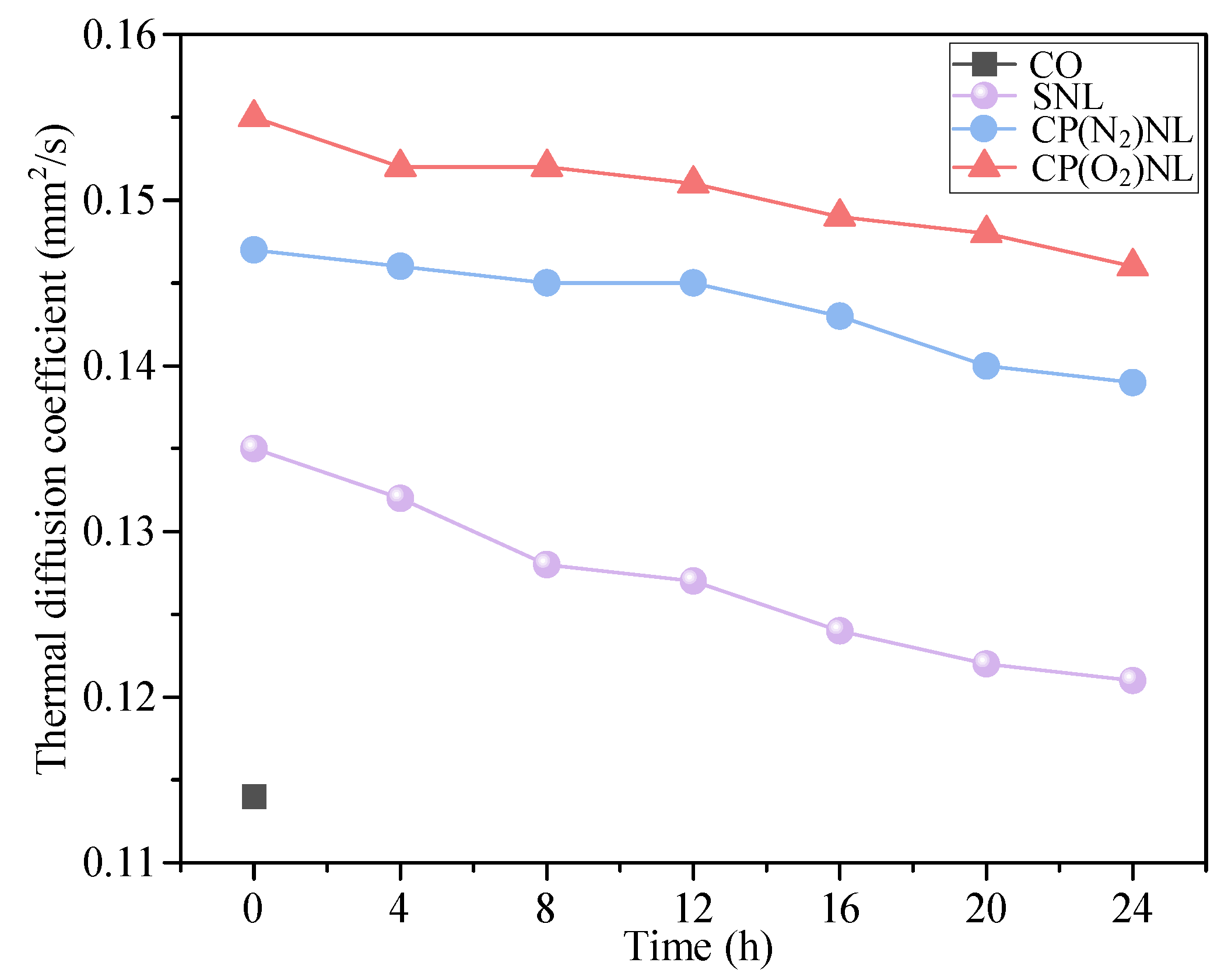
4.3. Heat Transfer Performance
4.4. Tribological Performance of h-BN Nanofluids
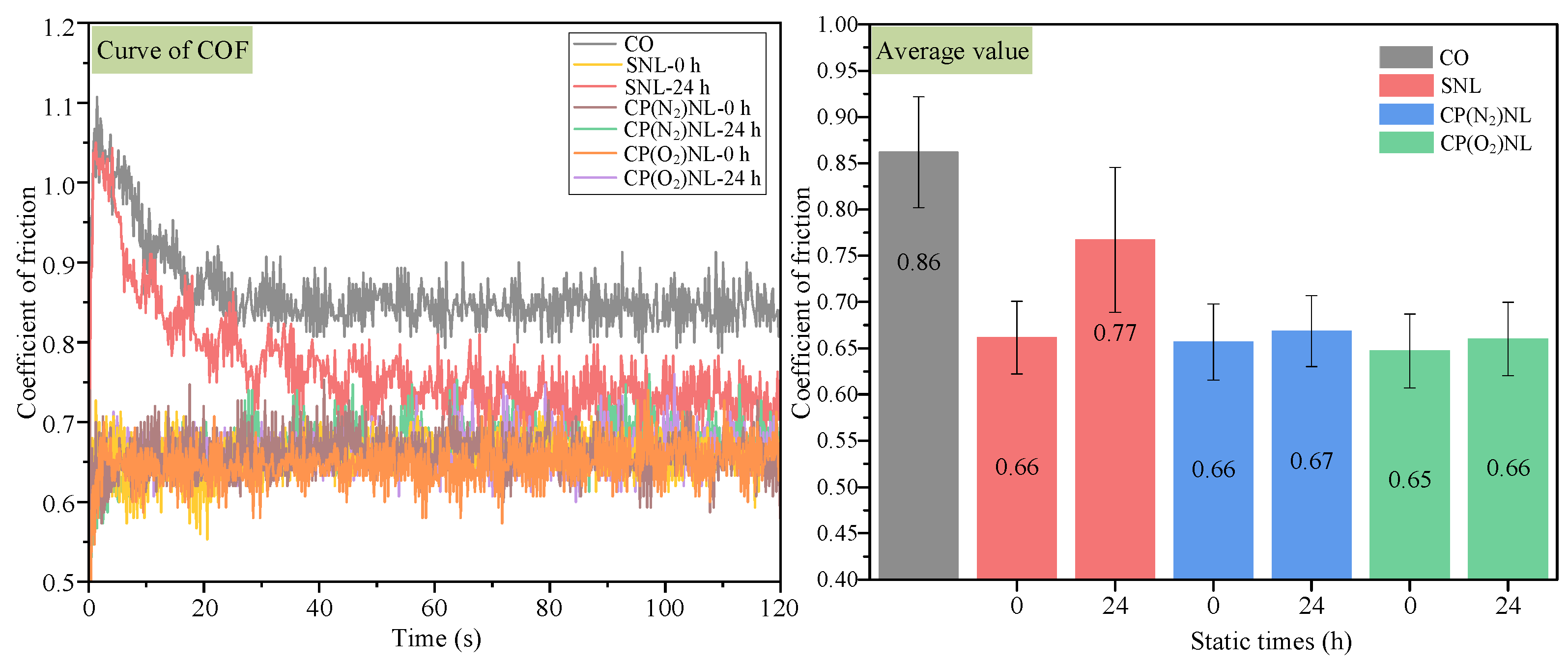
4.4.1. Coefficient of Friction
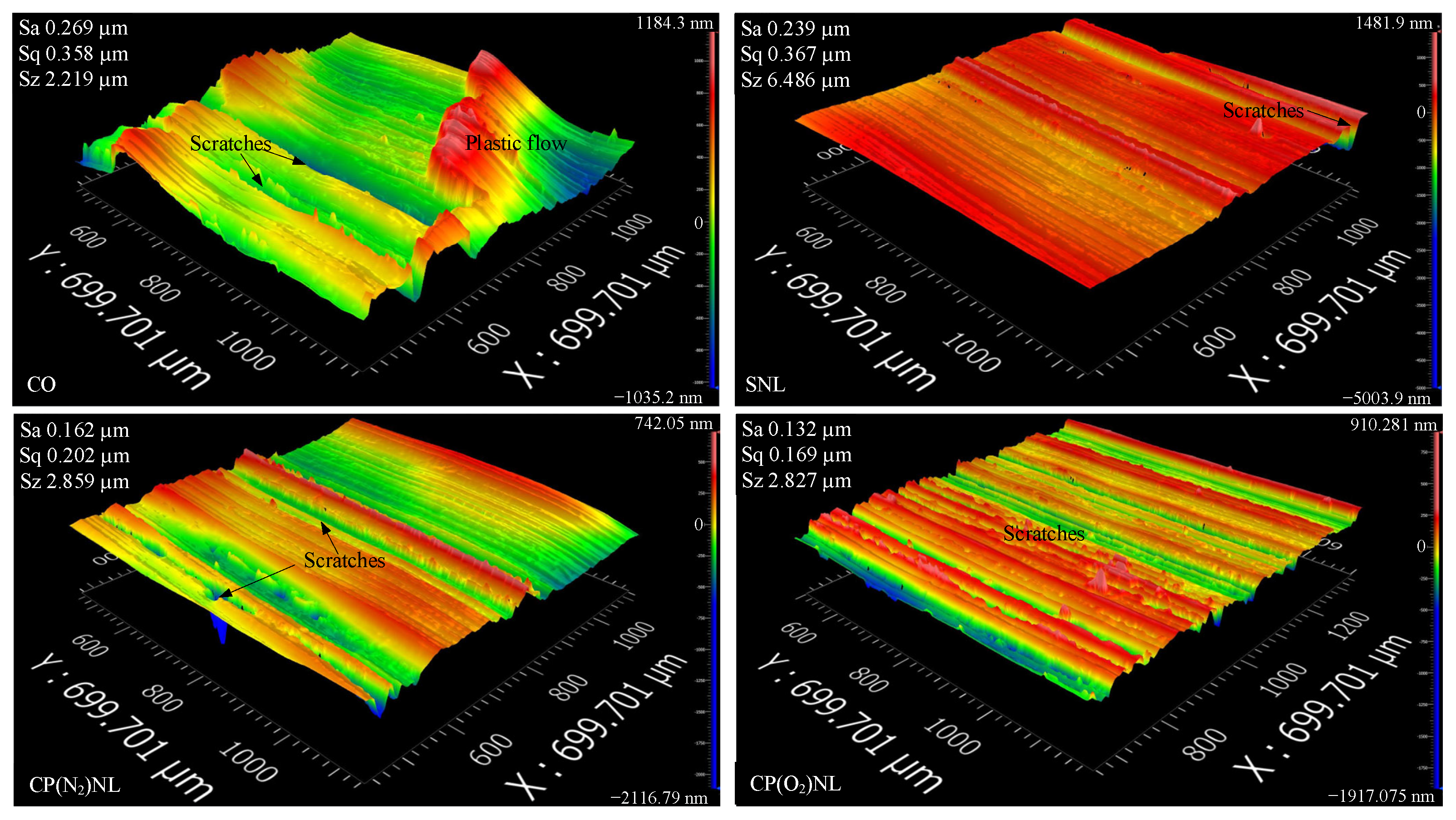
4.4.2. Three-Dimensional Surface Topography of the Sample Disk
4.4.3. Surface Roughness of the Friction Disc
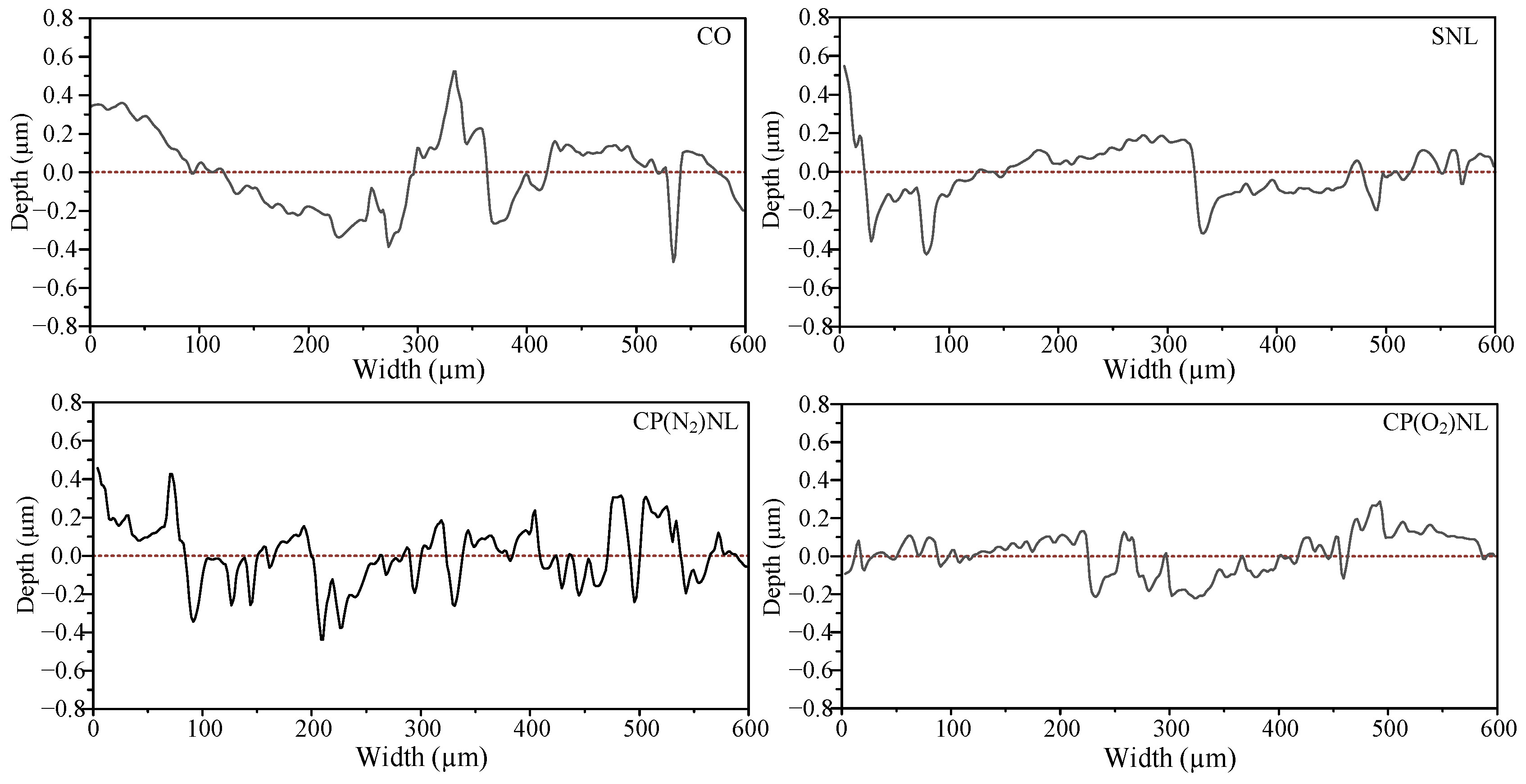
4.4.4. Scratch Depth
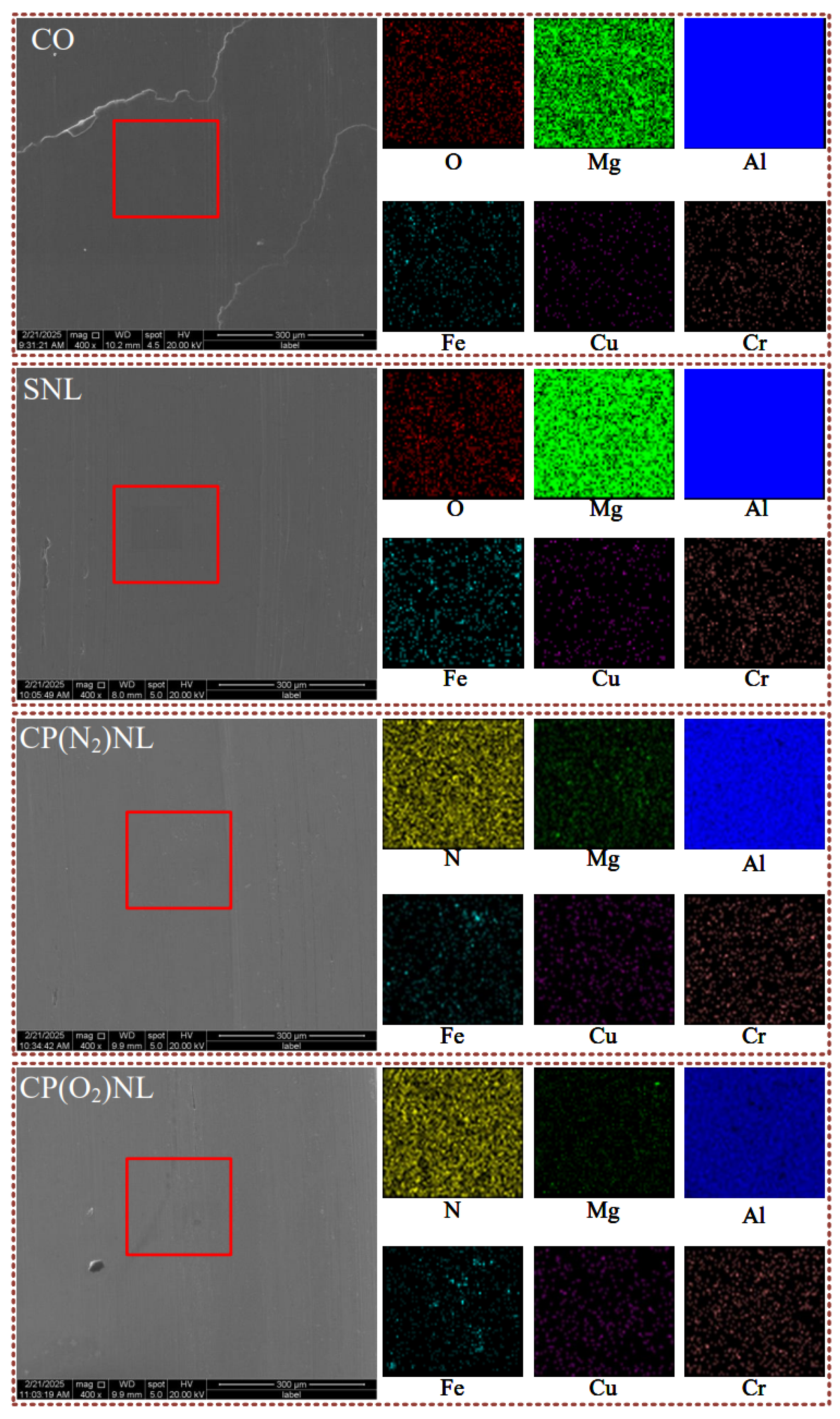
4.4.5. Micro-Morphology
5. Conclusions
- (1)
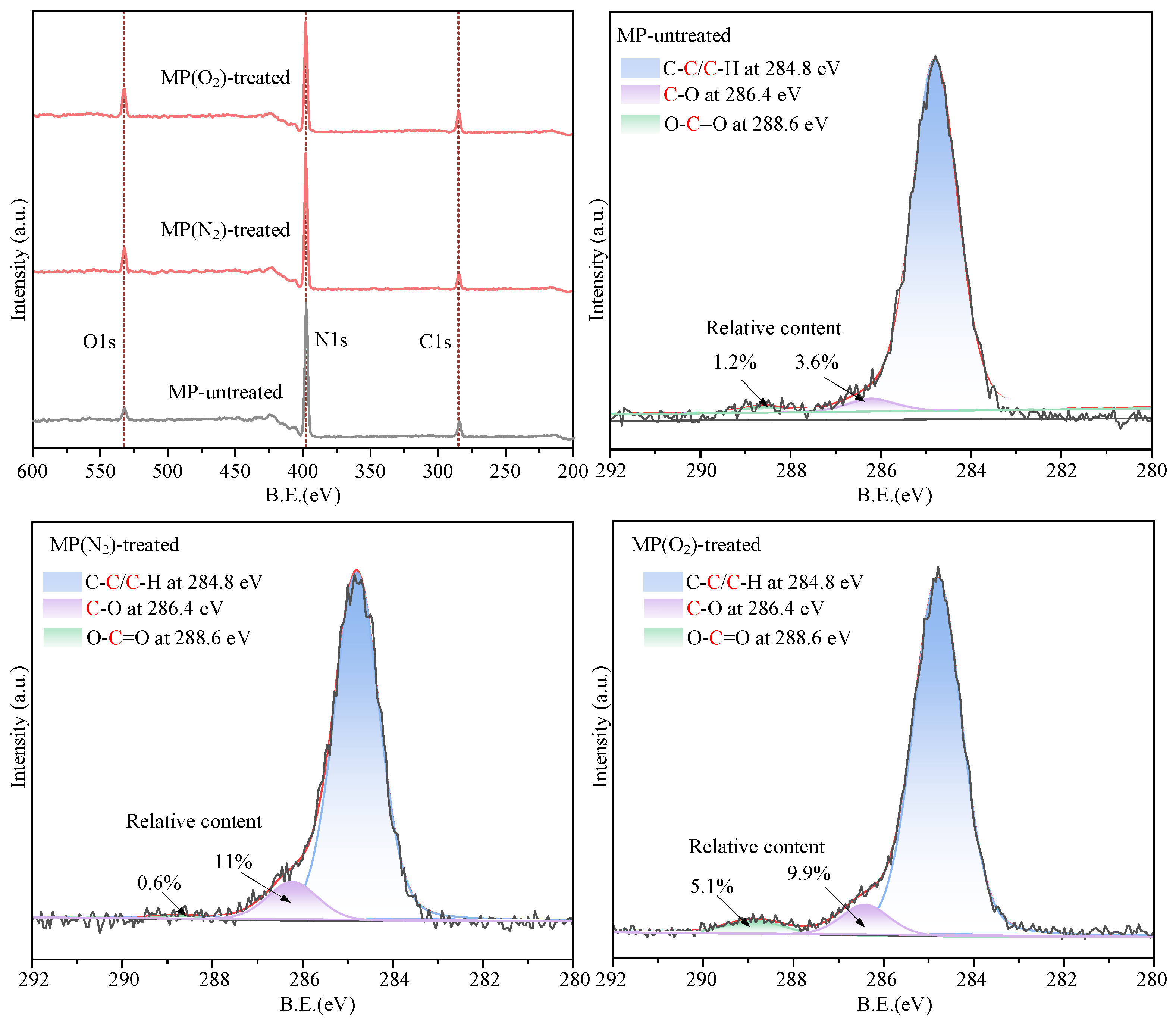
- The surface of h-BN nanoparticles after CP treatment shows an increase in polar groups and surface energy, improving the wettability of the base solution on the nanoparticles. Among them, the plasma modification using O2 as the working gas had the best effect.
- (2)
- Among SNL, CP(N2)NL, and CP(O2)NL, the nanofluid, CP(O2)NL, prepared using CP(O2)-modified h-BN, is stable for more than 336 h. It has excellent dispersion stability with a viscosity of 62.9 mPa∙s and a thermal diffusion coefficient of 0.155 mm2/s.
- (3)
- For the friction wear test, the coefficient of friction under the cotton seed oil condition was 0.86, and the Sa value of the disc was 0.244 µm, with a scratch depth higher than 0.4 µm. The tribological performance of SNL was not much different from that of CO after static placement for 24 h. In contrast, the nanofluids prepared from CP-modified h-BN have a friction coefficient of about 0.66 before and after 24 h of static observation, and the Sa values of the discs are 31.6–49.2% lower than those of the CO.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SNL | h-BN nanofluid with added surfactant |
| CP(N2)NL | Cold plasma-modified h-BN nanofluid with N2 as the working gas |
| CP(O2)NL | Cold plasma-modified h-BN nanofluid with O2 as the working gas |
| CP | Cold plasma |
| XPS | X-ray photoelectron spectroscopy |
| Sa | Surface roughness |
| CO | Cottonseed oil |
| PVP | Polyvinylpyrrolidone |
| CTAB | Cetyltrimethylammonium bromide |
| GA | Gum arabic |
| SDS | Sodium dodecyl sulfate |
| SDOC | Sodium deoxycholate |
| SLS | Sodium dodecyl sulfate |
| MP | Microwave plasma |
References
- Akram, S.; Athar, M.; Saeed, K.; Razia, A.; Alghamdi, M.; Muhammad, T. Impact of Partial Slip on Double Diffusion Convection of Sisko Nanofluids in Asymmetric Channel with Peristaltic Propulsion and Inclined Magnetic Field. Nanomaterials 2022, 12, 2736. [Google Scholar] [CrossRef] [PubMed]
- Wohld, J.; Beck, J.; Inman, K.; Palmer, M.; Cummings, M.; Fulmer, R.; Vafaei, S. Hybrid Nanofluid Thermal Conductivity and Optimization: Original Approach and Background. Nanomaterials 2022, 12, 2847. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Lin, J.; Yang, H. Particle Distribution and Heat Transfer of SiO2/Water Nanofluid in the Turbulent Tube Flow. Nanomaterials 2022, 12, 2803. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, H.; Hao, J.C.; Li, Z.H.; Li, R.Z.; Zhou, Z.M.; Gao, T.; Liu, M.Z.; Cui, X.; Wang, X.M.; et al. Droplet size distribution model of needle electrode electrostatic atomization and milling nickel-based alloy performance evaluation. J. Manuf. Process. 2024, 119, 682–698. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Pourpasha, H.; Heris, S.Z. High-temperature lubricity and physicochemical behaviors of synthesized Cu/TiO2/MnO2-doped GO nanocomposite in high-viscosity index synthetic biodegradable PAO oil. Int. Commun. Heat Mass Transf. 2024, 156, 107642. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Experimental comparison between ZnO and MoS2 nanoparticles as additives on performance of diesel oil-based nano lubricant. Sci. Rep. 2020, 10, 5813. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z. Experimental investigation of ZnO nanoparticles effects on thermophysical and tribological properties of diesel oil. Int. J. Hydrogen Energy 2020, 45, 23603–23614. [Google Scholar] [CrossRef]
- Duan, Z.; Xi, S.; Wang, S.; Wang, Z.; Bian, P.; Li, C.; Song, J.; Liu, X. Identification of Cutting Workpiece Surface Defects Based on an Improved Single Shot Multibox Detector. Intell. Sustain. Manuf. 2024, 2, 10020. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Hosseini, M.G. Experimental investigation of MoS2/diesel oil nanofluid thermophysical and rheological properties. Int. Commun. Heat Mass Transf. 2019, 108, 104298. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, Y.B.; Wang, C.J.; Cui, X.; Li, R.Z.; Sharma, S.; Liu, M.Z.; Gao, T.; Zhou, Z.M.; Wang, X.M.; et al. Residual stress generation in grinding: Mechanism and modeling. J. Mater. Process. Tech. 2024, 324, 118262. [Google Scholar] [CrossRef]
- Yang, M.; Kong, M.; Li, C.H.; Long, Y.Z.; Zhang, Y.B.; Sharma, S.; Li, R.Z.; Gao, T.; Liu, M.Z.; Cui, X.; et al. Temperature field model in surface grinding: A comparative assessment. Int. J. Extreme. Manuf. 2023, 5, 042011. [Google Scholar] [CrossRef]
- Duan, Z.J.; Wang, Z.H.; Wang, S.S.; Zhang, B.Z.; Bian Peng, B.; Li, Y.H.; Liu, J.Y.; Song, J.L.; Li, C.H.; Liu, X. Tool wear in enhanced minimum quantity lubrication assisted milling: From mechanism to application. Chin. J. Aeronaut. 2025, 103597. [Google Scholar] [CrossRef]
- Gao, T.; Xu, P.M.; Wang, W.; Zhang, Y.B.; Xu, W.H.; Wang, Y.Q.; An, Q.L.; Li, C.H. Force model of ultrasonic empowered minimum quantity lubrication grinding CFRP. Int. J. Mech. Sci. 2024, 280, 109522. [Google Scholar] [CrossRef]
- Anand, N.; Kumar, A.S.; Paul, S. Effect of cutting fluids applied in MQCL mode on machinability of Ti-6Al-4V. J. Manuf. Process. 2019, 43, 154–163. [Google Scholar] [CrossRef]
- Talib, N.; Rahim, E.A. Performance of modified jatropha oil in combination with hexagonal boron nitride particles as a bio-based lubricant for green machining. Tribol. Int. 2018, 118, 89–104. [Google Scholar] [CrossRef]
- Boukheit, A.; Chabert, F.; Otazaghine, B.; Taguet, A. h-BN Modification Using Several Hydroxylation and Grafting Methods and Their Incorporation into a PMMA/PA6 Polymer Blend. Nanomaterials 2022, 12, 2735. [Google Scholar] [CrossRef]
- Yun, H.; Kwak, M.-G.; Park, K.; Kim, Y. Fabrication, Thermal Conductivity, and Mechanical Properties of Hexagonal-Boron-Nitride-Pattern-Embedded Aluminum Oxide Composites. Nanomaterials 2022, 12, 2815. [Google Scholar] [CrossRef]
- Yildirim, C.V. Experimental comparison of the performance of nanofluids, cryogenic and hybrid cooling in turning of Inconel 625. Tribol. Int. 2019, 137, 366–378. [Google Scholar] [CrossRef]
- Said, Z.; Sundar, L.S.; Tiwari, A.K.; Ali, H.M.; Sheikholeslami, M.; Bellos, E.; Babar, H. Recent advances on the fundamental physical phenomena behind stability, dynamic motion, thermophysical properties, heat transport, applications, and challenges of nanofluids. Phys. Rep. 2022, 946, 1–94. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Saha, B.B.; Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 2020, 305, 112787. [Google Scholar] [CrossRef]
- Xia, G.D.; Jiang, H.M.; Liu, R.; Zhai, Y.L. Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Altun, A.; Sara, O.N.; Simsek, B. A comprehensive statistical approach for determining the effect of two non-ionic surfactants on thermal conductivity and density of Al2O3-water-based nanofluids. Colloids Surf. A 2021, 626, 127099. [Google Scholar] [CrossRef]
- Mostafizur, R.M.; Rasul, M.G.; Nabi, M.N. Effect of surfactant on stability, thermal conductivity, and viscosity of aluminium oxide-methanol nanofluids for heat transfer applications. Therm. Sci. Eng. Prog. 2022, 31, 101302. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. Stabilization of the aqueous dispersion of carbon nanotubes using different approaches. Therm. Sci. Eng. Prog. 2018, 8, 411–417. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Manasrah, A.D.; Al-Mubaiyedh, U.A.; Al-Ansari, T.; Malaibari, Z.O.; Atieh, M.A. An experimental study on stability and thermal conductivity of water/CNTs nanofluids using different surfactants: A comparison study. J. Mol. Liq. 2020, 304, 111025. [Google Scholar] [CrossRef]
- Ebrahim, S.A.; Pradeep, E.; Mukherjee, S.; Ali, N. Rheological behavior of dilute graphene-water nanofluids using various surfactants: An experimental evaluation. J. Mol. Liq. 2023, 370, 120987. [Google Scholar] [CrossRef]
- Ibrahim, A.M.M.; Li, W.; Zeng, Z.X.; Bedairi, B.H.; Elsheikh, A. Graphene nanoplatelets-water nanofluids: A sustainable approach to enhancing Ti-6Al-4V grinding performance through minimum quantity lubrication. Tribol. Int. 2025, 201, 110145. [Google Scholar] [CrossRef]
- Gao, T.; Li, C.H.; Zhang, Y.B.; Yang, M.; Jia, D.Z.; Jin, T.; Hou, Y.L.; Li, R.Z. Dispersing mechanism and tribological performance of vegetable oil-based CNT nanofluids with different surfactants. Tribol. Int. 2019, 131, 51–63. [Google Scholar] [CrossRef]
- Musavi, S.H.; Davoodi, B.; Niknam, S.A. Effects of reinforced nanoparticles with surfactant on surface quality and chip formation morphology in MQL-turning of superalloys. J. Manuf. Process. 2019, 40, 128–139. [Google Scholar] [CrossRef]
- Sirin, E.; Kivak, T.; Yildirim, Ç.V. Effects of mono/hybrid nanofluid strategies and surfactants on machining performance in the drilling of Hastelloy X. Tribol. Int. 2021, 157, 106894. [Google Scholar] [CrossRef]
- Wang, R.T.; Yang, C.A.; Chen, S.L.; Chang, T.L.; Wang, J.C. Investigations of thermoelectric properties in polymer-based hybrid nanofluids with various surfactants. J. Phys. Chem. Solids 2025, 199, 112557. [Google Scholar] [CrossRef]
- Yang, B.; Shi, Y.; Ma, X.Q.; Yu, X.H. Effects of mixed anionic/cationic surfactants on ZnO nanofluid. J. Mol. Liq. 2023, 377, 121530. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Kaladgi, A.R.; Halemani, B.S.; Buradi, A.; Afzal, A.; Saleel, C.A. Performance enhancement in tribological properties of lubricants by dispersing TiO2 nanoparticles. Mater. Today Proc. 2021, 47, 6180–6184. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.Q.; Li, Y.H.; Zhou, Y.Y.; Zhang, J.H.; Wang, Z.H.; Yan, J.C.; Gu, X.L.; Yuan, Z.Z.; Chen, Y.; et al. Improving machinability of single-crystal silicon by cold plasma jet. J. Manuf. Process. 2023, 99, 581–591. [Google Scholar] [CrossRef]
- Wang, Z.H.; Duan, Z.J.; Wang, S.S.; Li, Y.H.; Zhou, Y.Y.; Liu, J.Y.; Liu, X. Influence of cold plasma on material removal behavior during diamond grit scratching single crystal silicon. Colloid Surf. A 2025, 705, 135630. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, W.; Duan, Z.; Wang, S.; Li, Y.-h.; Zhou, Y.; Liu, J.; Song, J.; Liu, X. Experimental Study on Cold Plasma Jet (CPJ) Assisted Micro-Milling of 30CrMnSiNi2A. Intell. Sustain. Manuf. 2024, 1, 10017. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, Y.H.; Wang, S.S.; Duan, Z.J.; Cao, X.M.; Zhou, Y.Y.; Liu, X.; Liu, J.Y. Feasibility and mechanism of atmospheric pressure cold plasma jet (APCPJ) assisted micro-milling of bulk metallic glasses (BMGs). Ceram. Int. 2024, 50, 11094–11105. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, S.S.; Li, Y.H.; Duan, Z.J.; Ning, L.J.; Wang, Z.H.; Chen, Y.; Liu, X. High-efficiency and low-damage modification of engineering metal materials by oxygen-mixing atmospheric pressure cold plasma jets. Appl. Surf. Sci. 2024, 662, 160142. [Google Scholar] [CrossRef]
- Duan, Z.J.; Wang, S.S.; Li, C.H.; Wang, Z.H.; Bian, P.; Sun, J.; Song, J.L.; Liu, X. Cold plasma and different nano-lubricants multi-energy field coupling-assisted micro-milling of Al-Li alloy 2195-T8 and flow rate optimization. J. Manuf. Process. 2024, 127, 218–237. [Google Scholar] [CrossRef]
- Duan, Z.J.; Wang, S.S.; Wang, Z.H.; Li, C.H.; Li, Y.H.; Song, J.L.; Liu, J.Y.; Liu, X. Tool wear mechanisms in cold plasma and nano-lubricant multi-energy field coupled micro-milling of Al-Li alloy. Tribol. Int. 2024, 192, 109337. [Google Scholar] [CrossRef]
- Put, S.; Bertels, C.; Vanhulsel, A. Atmospheric pressure plasma treatment of polymeric powders. Surf. Coat. Tech. 2013, 234, 76–81. [Google Scholar] [CrossRef]
- Yildirim, C.V.; Sarikaya, M.; Kivak, T.; Sirin, S. The effect of addition of hBN nanoparticles to nanofluid-MQL on tool wear patterns, tool life, roughness and temperature in turning of Ni-based Inconel 625. Tribol. Int. 2019, 134, 443–456. [Google Scholar] [CrossRef]
- Bian, P.; Duan, Z.J.; Jia, Y.S.; Wang, Z.H.; Wang, S.S.; Tan, J.; Zhou, Y.Y.; Song, J.L.; Liu, X. Evaluation of Surface Integrity of Multi-Energy Field Coupling-Assisted Micro-Grinding Hastelloy Alloy. Micromachines 2025, 16, 565. [Google Scholar] [CrossRef]
- Duan, Z.J.; Wang, S.S.; Li, C.H.; Wang, Z.H.; Bian, P.; Song, J.L.; Liu, X. Tribological and micro-milling performance of surfactant-free microwave plasma-modified Al2O3 nanoparticles biodegradable lubricants. J. Clean. Prod. 2025, 493, 144969. [Google Scholar] [CrossRef]
- Yang, M.; Hao, J.C.; Wu, W.T.; Li, Z.H.; Ma, Y.Q.; Zhou, Z.M.; Gao, T.; Liu, M.Z.; Cui, X.; Zhang, Y.B.; et al. Critical cutting thickness model considering subsurface damage of zirconia grinding and friction—wear performance evaluation applied in simulated oral environment. Tribol. Int. 2024, 198, 109881. [Google Scholar] [CrossRef]
- Gao, T.; Liu, J.X.; Sun, X.F.; Zhang, Y.B.; Yang, M.; Liu, M.Z.; Xu, W.H.; An, Q.L.; Wang, D.Z.; Xu, P.M.; et al. Enhanced permeation mechanism and tribological assessment of ultrasonic vibration nanolubricants grinding CFRP. Tribol. Int. 2025, 204, 110494. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Viscosity, tribological and physicochemical features of ZnO and MoS2 diesel oil-based nanofluids: An experimental study. Fuel 2021, 293, 120481. [Google Scholar] [CrossRef]
- Kai, X.U.; Yun, Y.; Wei, F.; Min, W.A.N.; Weihong, Z. Internal cooling techniques in cutting process: A review. J. Adv. Manuf. Sci. Technol. 2024, 4, 2024013. [Google Scholar] [CrossRef]
- Danni, L.U.; Kaining, S.H.I.; Jiale, L.I.; Huhu, L.I.; Yuchang, F.A.N.; Zhen, C.; Yaoyao, S.H.I. Surface generation mechanism and efficiency improvement in ultrasonic vibration assisted belt flapwheel flexible polishing GH4169. J. Adv. Manuf. Sci. Technol. 2024, 4, 2024017. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.R.; Huang, Y.M.; Gong, S.Q.; Sun, M.S.; Liu, M. Study on developing predicted system model of cutting-edge trajectory for micro-milling process based on tool runout error, chip thickness and force signal. Mech. Syst. Signal Process. 2025, 228, 112410. [Google Scholar] [CrossRef]
- Qu, S.S.; Wei, C.X.; Yang, Y.Y.; Yao, P.; Chu, D.K.; Gong, Y.D.; Zhao, D.; Zhang, X.P. Grinding mechanism and surface quality evaluation strategy of single crystal 4H-SiC. Tribol. Int. 2024, 194, 109515. [Google Scholar] [CrossRef]
- Zhao, G.L.; Zhao, B.; Ding, W.F.; Xin, L.J.; Nian, Z.W.; Peng, J.H.; He, N.; Xu, J.H. Nontraditional energy-assisted mechanical machining of difficult-to-cut materials and components in aerospace community: A comparative analysis. Int. J. Extreme. Manuf. 2024, 6, 022007. [Google Scholar] [CrossRef]
- Xu, Q.H.; Wang, J.L.; Wang, Y.Q.; Gao, H. Exploring the anisotropic damage behaviour during the scratching process of SiCf/SiC composites. Compos. Part A Appl. Sci. Manuf. 2025, 190, 108717. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |




| Element | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| Measured value | 0.66 | 0.4 | 0.27 | 0.12 | 1.1 | 0.33 | 0.03 | 0.03 | Remainder |
| Parameter | Value |
|---|---|
| Power frequency of CP (MHz) | 2450 |
| Power of CP (W) | 150 |
| O2/N2 flow of CP (mL/min) | 80 |
| CP treatment time (min) | 10 |
| Lubricant | Kinematic Viscosity (mPa∙s) | Thermal Diffusion Coefficient (mm2/s) | Sa (µm) |
|---|---|---|---|
| CO | 68.8 | 0.117 | 0.244 |
| SNL | 63.2–67.6 (0–24 h) | 0.135–121 (0–24 h) | 0.237 |
| CO(N2)NL | 62.9–63.1 (0–24 h) | 0.147–0.139 (0–24 h) | 0.162 |
| CO(O2)NL | 62.7–62.9 (0–24 h) | 0.155–0.146 (0–24 h) | 0.124 |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Z.; Wang, Z.; Jia, Y.; Wang, S.; Bian, P.; Tan, J.; Song, J.; Liu, X. Dispersion Stability and Tribological Properties of Cold Plasma-Modified h-BN Nanofluid. Nanomaterials 2025, 15, 874. https://doi.org/10.3390/nano15110874
Duan Z, Wang Z, Jia Y, Wang S, Bian P, Tan J, Song J, Liu X. Dispersion Stability and Tribological Properties of Cold Plasma-Modified h-BN Nanofluid. Nanomaterials. 2025; 15(11):874. https://doi.org/10.3390/nano15110874
Chicago/Turabian StyleDuan, Zhenjing, Ziheng Wang, Yishuai Jia, Shuaishuai Wang, Peng Bian, Ji Tan, Jinlong Song, and Xin Liu. 2025. "Dispersion Stability and Tribological Properties of Cold Plasma-Modified h-BN Nanofluid" Nanomaterials 15, no. 11: 874. https://doi.org/10.3390/nano15110874
APA StyleDuan, Z., Wang, Z., Jia, Y., Wang, S., Bian, P., Tan, J., Song, J., & Liu, X. (2025). Dispersion Stability and Tribological Properties of Cold Plasma-Modified h-BN Nanofluid. Nanomaterials, 15(11), 874. https://doi.org/10.3390/nano15110874





