Nanostructured Bubble Thin Films—From Simple Fabrication to Scalable Applications: A Review
Abstract
1. Introduction
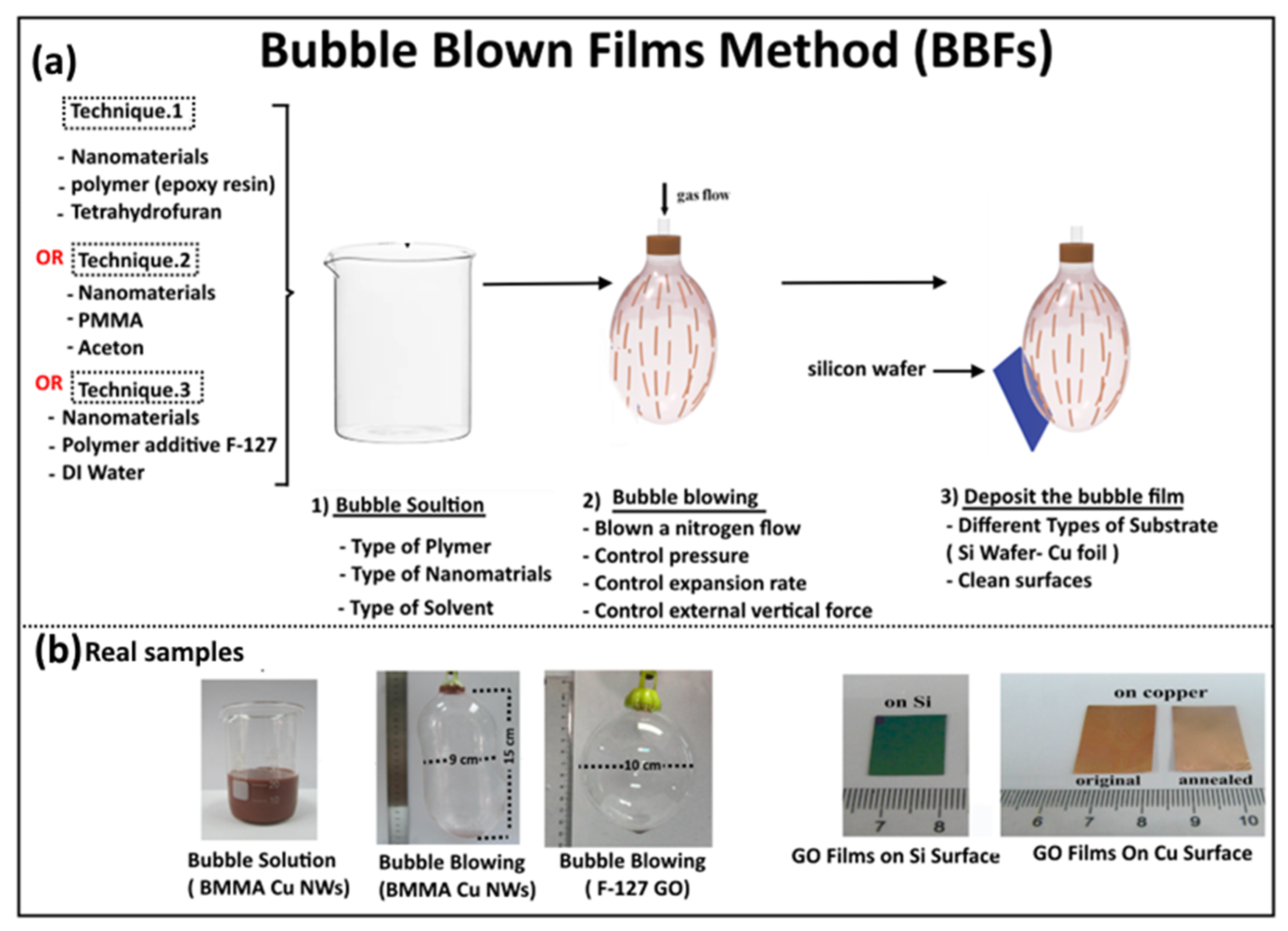
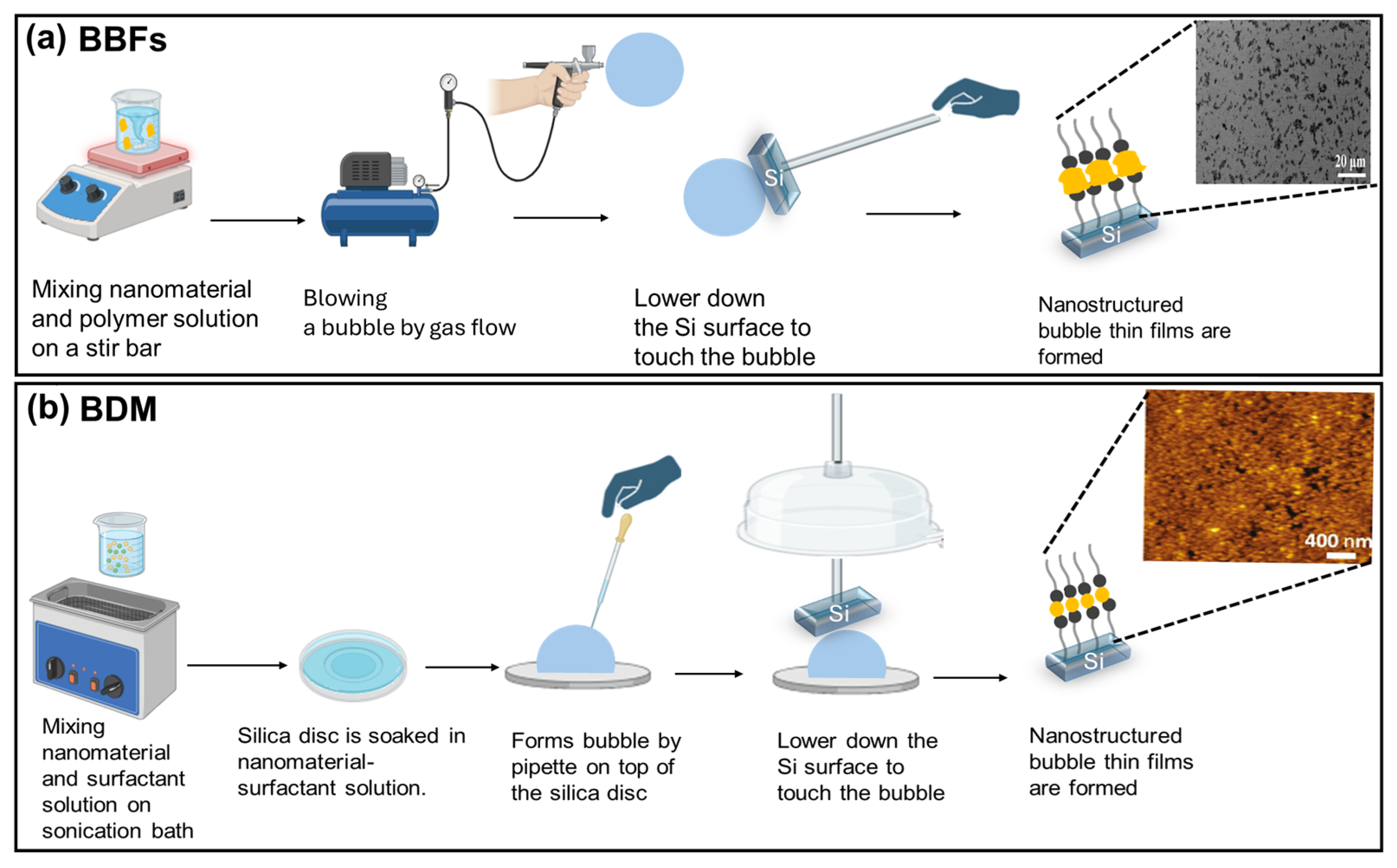
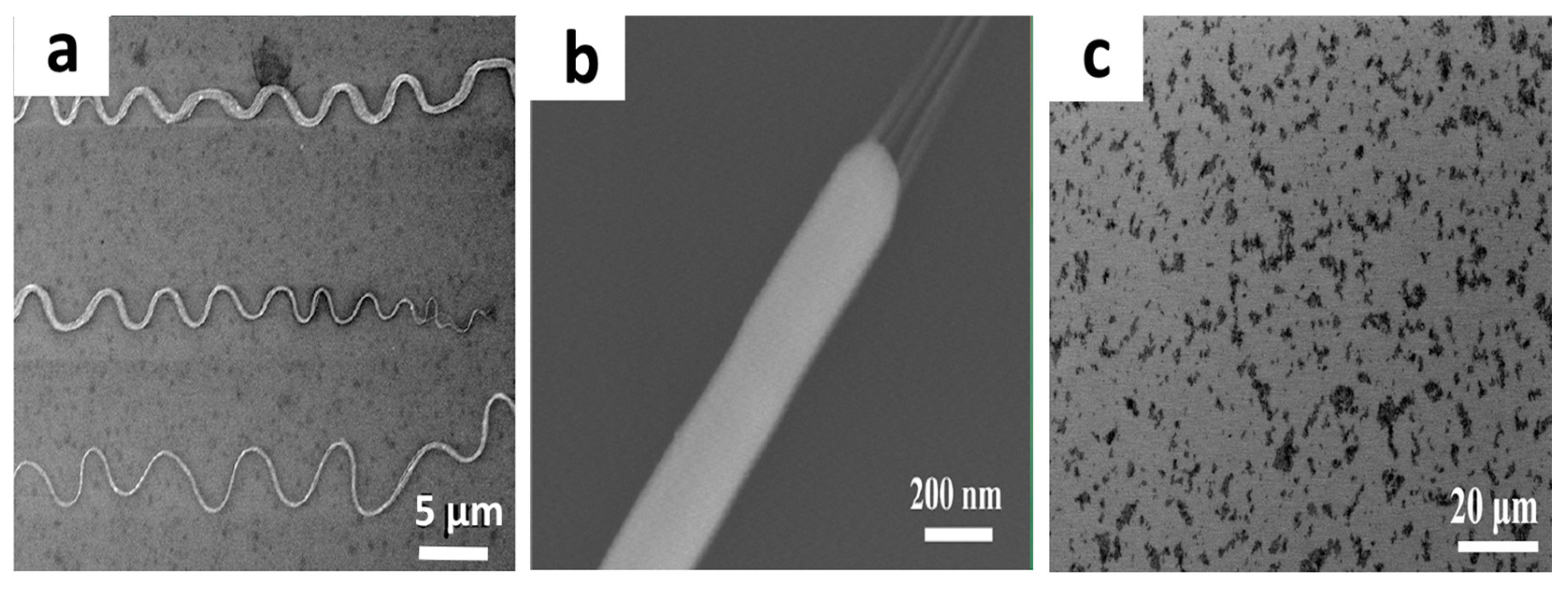
2. Bubble Blown Film (BBF) Method
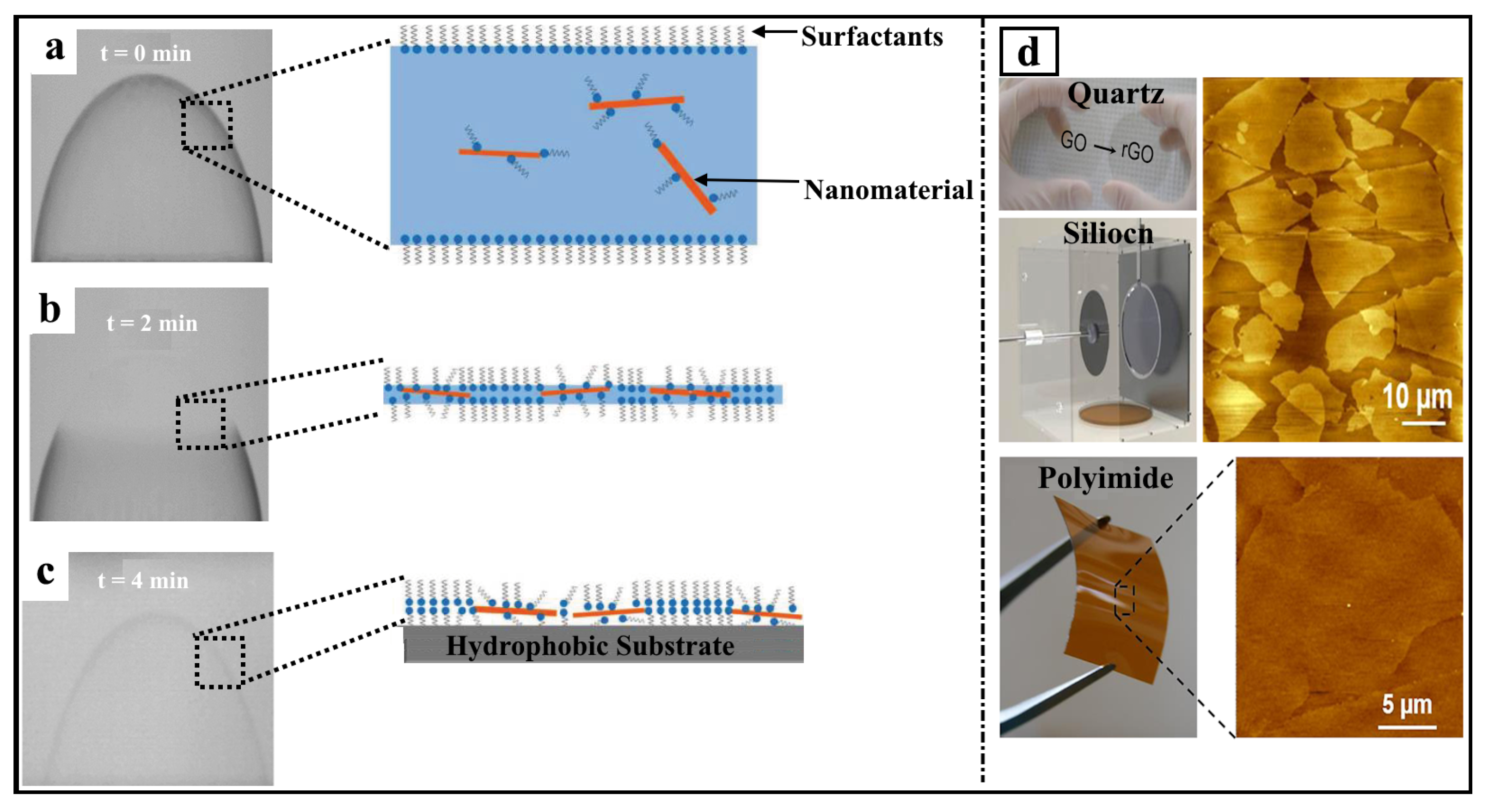
3. Bubble Deposition Method (BDM)
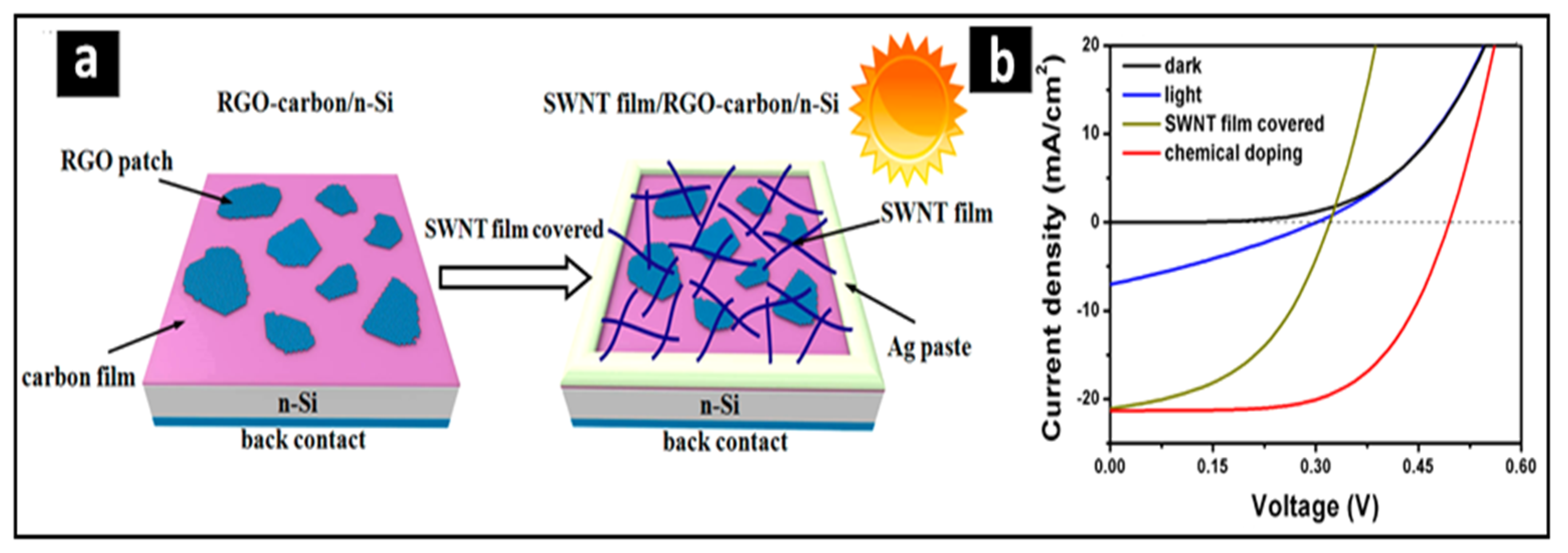
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBFs | bubble blown films |
| BDM | bubble deposition method |
| (NBFs) | Newton black films |
| CBFs | common black films |
| PMMA | poly (methylmethacrylate) polymer |
| Te NWs | tellurium nanowires |
| RGO | reduced graphene oxide |
| CNTs | carbon nanotubes |
| GO | graphene oxide |
| SWCNTs | single-wall carbon nanotubes |
| MWCNTs | multiwall carbon nanotubes |
| CdS NWs | cadmium sulfide nanowires |
| Si NWs | silicon nanowires |
| Te NWs | tellurium nanowires |
| NW-FETs | nanowire-filled effect transistor |
| CMC | critical micelle concentration |
| SDBS | sodium dodecyl benzene sulphonate |
| BOE | buffered oxide etch |
| HF | hydrogen fluoride |
| (NH4F) | ammonium fluoride |
| (H2SO4) | sulfuric acid |
| H2O2 | hydrogen peroxide |
| C12DMPO | fluorinated sulfoxide (C16F13SOTHAM) |
| CTAB | Dodecyldimethylpho-sphine oxide |
| (C16TAB) | hexadecyltrimethylammonium bromide |
| AFM | Atomic Force Microscope |
| SEM | Secanning electrcon micrsope |
References
- Schiavo, L.; Cammarano, A.; Carotenuto, G.; Longo, A.; Palomba, M.; Nicolais, L.J. An overview of the advanced nanomaterials science. Inorg. Chim. Acta 2024, 559, 121802. [Google Scholar] [CrossRef]
- Ustad, R.E.; Kundale, S.S.; Rokade, K.A.; Patil, S.L.; Chavan, V.D.; Kadam, K.D.; Patil, H.S.; Patil, S.P.; Kamat, R.K.; Kim, D.-k. Recent progress in energy, environment, and electronic applications of MXene nanomaterials. Nanoscale 2023, 15, 9891–9926. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.H.F.; Lai, C.W.; Leo, B.F. Nanomaterials and Its Classification: An Introduction. In Advanced Nanomaterials for Biological, Nutraceutical, and Medicinal Applications; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 1–23. [Google Scholar] [CrossRef]
- Iyer, K.A.; Tenchov, R.; Ralhan, K.; Bird, R.E.; Lotti Diaz, L.M.; Hughes, K.J.; Ganesan, M.; Ivanov, J.M.; Zhou, Q.A. Nanoscale Materials in Biomedical Applications of Sensors: Insights from a Comprehensive Landscape Analysis. ACS Appl. Nano Mater. 2025, 8, 1305–1331. [Google Scholar] [CrossRef]
- Khanna, V.K. Nanosensors: Physical, Chemical, and Biological, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021; p. 666. [Google Scholar] [CrossRef]
- Pozzi, M.; Jonak Dutta, S.; Kuntze, M.; Bading, J.; Rüßbült, J.S.; Fabig, C.; Langfeldt, M.; Schulz, F.; Horcajada, P.; Parak, W.J. Visualization of the High Surface-to-Volume Ratio of Nanomaterials and Its Consequences. J. Chem. Educ. 2024, 101, 3146–3155. [Google Scholar] [CrossRef]
- Planinšič, G.; Vollmer, M. The surface-to-volume ratio in thermal physics: From cheese cube physics to animal metabolism. Eur. J. Phys. 2008, 29, 369. [Google Scholar] [CrossRef]
- Ding, P.; Chen, D.; Ko, P.K.; Qammar, M.; Geng, P.; Guo, L.; Halpert, J.E. Nanomaterials for spin-based quantum information. Nanoscale 2025, 17, 1148–1170. [Google Scholar] [CrossRef]
- Ricciarelli, D.; Filice, S.; Calogero, G.; Scuderi, V.; Boscarino, S.; Iacono, V.; Ruffino, F.; Deretzis, I.; Fisicaro, G.; Scalese, S.; et al. Reduction and Quantum Confinement Effects in Graphene Oxide: Relevance for Electro-optical Applications. ACS Appl. Nano Mater. 2024, 7, 24969–24977. [Google Scholar] [CrossRef]
- Chauhan, P.; Pandit, J.; Harkhani, K.; Tripathi, A.; Kumar, N.; Patel, M.V.; Sharma, A.K. Nanomaterials a promising approach for environmental restoration. Nanoeng. Environ. Eng. 2025, 10, 14. [Google Scholar] [CrossRef]
- Xu, L.; Liang, H.-W.; Yang, Y.; Yu, S.-H. Stability and Reactivity: Positive and Negative Aspects for Nanoparticle Processing. Chem. Rev. 2018, 118, 3209–3250. [Google Scholar] [CrossRef]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Zeng, K.; Cheng, L.; Hu, W.; Li, J. Synthesis, Stability, and Tribological Performance of TiO2 Nanomaterials for Advanced Applications. Lubricants 2025, 13, 56. [Google Scholar] [CrossRef]
- Almeida, M.B.; Galdiano, C.M.R.; Silva Benvenuto, F.S.R.; Carrilho, E.; Brazaca, L.C. Strategies Employed to Design Biocompatible Metal Nanoparticles for Medical Science and Biotechnology Applications. ACS Appl. Mater. Interfaces 2024, 16, 67054–67072. [Google Scholar] [CrossRef] [PubMed]
- Hulugalla, K.; Shofolawe-Bakare, O.; Toragall, V.B.; Mohammad, S.A.; Mayatt, R.; Hand, K.; Anderson, J.; Chism, C.; Misra, S.K.; Shaikh, T.; et al. Glycopolymeric Nanoparticles Enrich Less Immunogenic Protein Coronas, Reduce Mononuclear Phagocyte Clearance, and Improve Tumor Delivery Compared to PEGylated Nanoparticles. ACS Nano 2024, 18, 30540–30560. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Lv, H.; Ju, Y.; Hao, J.; Cui, J. Zwitterionic Poly(ethylene glycol) Nanoparticles Minimize Protein Adsorption and Immunogenicity for Improved Biological Fate. ACS Appl. Mater. Interfaces 2025, 17, 6125–6133. [Google Scholar] [CrossRef]
- Shi, J.; Fu, W.; Zou, Q.; Ward, N.; Liao, S.; Wang, J.; Deng, X.; Zhao, R.C. Click Chemistry-Based Nanomaterial Modification for Cancer Targeting: A Review. ACS Appl. Nano Mater. 2025, 8, 10–21. [Google Scholar] [CrossRef]
- Xu, W.; Guan, G.; Yue, R.; Dong, Z.; Lei, L.; Kang, H.; Song, G. Chemical Design of Magnetic Nanomaterials for Imaging and Ferroptosis-Based Cancer Therapy. Chem. Rev. 2025, 125, 1897–1961. [Google Scholar] [CrossRef]
- Paramasivam, G.; Palem, V.V.; Meenakshy, S.; Suresh, L.K.; Gangopadhyay, M.; Antherjanam, S.; Sundramoorthy, A.K. Advances on carbon nanomaterials and their applications in medical diagnosis and drug delivery. Colloids Surf. B Biointerfaces 2024, 241, 114032. [Google Scholar] [CrossRef]
- Lu, B.; Cheng, H.; Qu, L. Inorganic Hydrogel Based on Low-Dimensional Nanomaterials. ACS Nano 2024, 18, 2730–2749. [Google Scholar] [CrossRef]
- Song, X.; Shan, X.; Xue, H.; Li, X.; Liu, R.; Kong, J.; Zuo, Z.; Su, X.; Zhang, Q.; Yin, Y.; et al. Advances in Photothermal Catalysis: Mechanisms, Materials, and Environmental Applications. ACS Appl. Nano Mater. 2024, 7, 26489–26514. [Google Scholar] [CrossRef]
- Das, S.; Chowdhury, S.; Tiwary, C.S. High-entropy-based nano-materials for sustainable environmental applications. Nanomaterials 2024, 16, 8256–8272. [Google Scholar] [CrossRef]
- Ananthi, P.; Hemkumar, K.; Pius, A. Antibacterial, Biodegradable Polymeric Films Loaded with Co-MOF/ZnS Nanoparticles for Food Packaging and Photo-Degradation Applications. ACS Food Sci. Technol. 2024, 4, 1462–1471. [Google Scholar] [CrossRef]
- Dhall, S.; Acharya, L. A Systematic Review of Basic Strategies for Enhancing Food Storage Life with Nanotechnology Products. ACS Food Sci. Technol. 2025, 5, 1239–1254. [Google Scholar] [CrossRef]
- Piluk, T.D.; Faccio, G.; Letsiou, S.; Liang, R.; Freire-Gormaly, M. A critical review investigating the use of nanoparticles in cosmetic skin products. Environ. Sci. Nano 2024, 11, 3674–3692. [Google Scholar] [CrossRef]
- Gohar, O.; Khan, M.Z.; Bibi, I.; Bashir, N.; Tariq, U.; Bakhtiar, M.; Karim, M.R.A.; Ali, F.; Hanif, M.B.; Motola, M.; et al. Nanomaterials for advanced energy applications: Recent advancements and future trends. Mater. Des. 2024, 241, 112930. [Google Scholar] [CrossRef]
- Cao, J.; Ji, K.-Y.; Du, M.-H.; Zhang, C.; Sun, Q.; Yi, Y.; Chai, Z.-F.; Yan, C.-J.; Deng, H. Ultrafast synthesis of porous Fe3C/carbon hybrid materials via a carbothermal shock reactor for advanced energy storage applications. J. Mater. Chem. A 2025, 13, 7515–7528. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart Materials for Flexible Electronics and Devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef]
- Xu, W.; Liu, J. Nanomaterials in Nonvolatile Resistive Memory Devices. In Handbook of Nanomaterials, Volume 1; Elsevier: Amsterdam, The Netherlands, 2024; pp. 57–79. [Google Scholar] [CrossRef]
- Fang, A.; Li, Q.; Liu, J.; Du, Z.; Tang, P.; Xu, H.; Xie, Y.; Song, J.; Zhang, K.; Yang, T. Enhanced Light Emission of Micro LEDs Using Graphene-Connected Micropillar Structures and Ag/SiO2 Nanoparticles. ACS Photonics 2025, 12, 1342–1350. [Google Scholar] [CrossRef]
- Mishra, R.K.; Verma, K.; Chianella, I.; Goel, S.; Nezhad, H.Y. Nanomaterial Integration in Micro LED Technology: Enhancing Efficiency and Applications. Next Nanotechnol. 2024, 5, 100056. [Google Scholar] [CrossRef]
- Lee, S.-M.; Jang, S.C.; Park, J.-M.; Park, J.; Choi, N.; Chung, K.-B.; Lee, J.W.; Kim, H.-S. High-Mobility Tellurium Thin-Film Transistor: Oxygen Scavenger Effect Induced by a Metal-Capping Layer. Nanomaterials 2025, 15, 418. [Google Scholar] [CrossRef]
- Busool, R.; Haick, H. Nanomaterial-Based Transistors for Chemical and Biological Sensing. Org. Inorg. Mater. Based Sens. 2024, 2, 479–493. [Google Scholar] [CrossRef]
- Liao, L.; Kovalska, E.; Regner, J.; Song, Q.; Sofer, Z. Two-Dimensional Van Der Waals Thin Film and Device. Small 2024, 20, 2303638. [Google Scholar] [CrossRef] [PubMed]
- Mo, R. Functional Nanomaterial-Based Flexible Electronics. Coatings 2022, 12, 809. [Google Scholar] [CrossRef]
- Phogat, P.; Jha, R.; Singh, S. Emerging Advances and Future Prospects of Two-Dimensional Nanomaterials Based Solar Cells. J. Appl. Compd. 2024, 1001, 175063. [Google Scholar] [CrossRef]
- Sharma, P.; Mishra, R.K.; Panda, A.; Palodhi, K. The Effect of Indium Tin Oxide (ITO) Nanowire on Perovskite Thin Film Solar Cells. J. Electron. Mater. 2025, 54, 2797–2809. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, W.; Zhang, Z.; Feng, Y.; Li, J.; Zheng, M.; Chao, Z.; Fan, J. Porous Dodecahedral NiS2/NiSe2 Nanomaterials as Cathode Material for Aluminum Batteries. ACS Appl. Nano Mater. 2025, 8, 6318–6326. [Google Scholar] [CrossRef]
- Saini, H.; Vishwanathan, S.; Sil, S.; Kumar, N.S.; Matte, H.S.S.R. Micro/Nanostructured Co3O4 as an Anode Material for Lithium-Ion Batteries. ACS Appl. Nano Mater. 2025, 8, 917–923. [Google Scholar] [CrossRef]
- Nargatti, K.I.; Ahankari, S.S.; Dizon, J.R.C.; Subramaniam, R.T. Flexible, Interdigitated Shape Memory Supercapacitor Based on Reduced Graphene Oxide/Nanocellulose Aqueous Ink. ACS Appl. Nano Mater. 2025, 8, 7642–7652. [Google Scholar] [CrossRef]
- Kamar, E.; Qasim, K.; Mousa, M. Supercapacitor and Oxygen Evolution Reaction Performances Based on rGO and Mn2V2O7 Nanomaterials. Electrochim. Acta 2022, 430, 141106. [Google Scholar] [CrossRef]
- Yu, G.; Cao, A.; Lieber, C.M. Large-Area Blown Bubble Films of Aligned Nanowires and Carbon Nanotubes. Nat. Nanotechnol. 2007, 2, 372–377. [Google Scholar] [CrossRef]
- Jin, S.; Whang, D.; McAlpine, M.C.; Friedman, R.S.; Wu, Y.; Lieber, C.M. Scalable Interconnection and Integration of Nanowire Devices Without Registration. Nano Lett. 2004, 4, 915–919. [Google Scholar] [CrossRef]
- Liu, H.; Takagi, D.; Chiashi, S.; Homma, Y. Transfer and Alignment of Random Single-Walled Carbon Nanotube Films by Contact Printing. ACS Nano 2010, 4, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Nordquist, C.D.; Jackson, T.N.; Mayer, T.S.; Martin, B.R.; Mbindyo, J.; Mallouk, T.E. Electric-Field Assisted Assembly and Alignment of Metallic Nanowires. Appl. Phys. Lett. 2000, 77, 1399–1401. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, X.; Wei, Q.; Lieber, C.M. Directed Assembly of One-Dimensional Nanostructures into Functional Networks. Science 2001, 291, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Briston, J.H.; Katan, L.L. Plastic Films; Wiley & Sons, Inc.: New York, NY, USA, 1974; ISBN 9780470104729. [Google Scholar]
- Cantor, K. Blown Film Extrusion, 3rd ed.; Hanser: Munich, Germany, 2018; ISBN 9781569906965. [Google Scholar] [CrossRef]
- Benattar, J.J.; Nedyalkov, M.; Lee, F.K.; Tsui, O.K. Adhesion of a Free-Standing Newton Black Film onto a Solid Substrate. Angew. Chem. Int. 2006, 45, 4186–4188. [Google Scholar] [CrossRef]
- Lalli, N.S.; Giusti, A. Thin Film Modelling of Magnetic Soap Films. J. Fluid. Mech. 2024, 986, A7. [Google Scholar] [CrossRef]
- Pessanha, T.M.; Varade, S.; Salonen, A.; Langevin, D. Coalescence Frequency in O/W Emulsions: Comparisons of Experiments with Models. Langmuir 2024, 40, 23695–23705. [Google Scholar] [CrossRef]
- Wu, S.; Huang, K.; Shi, E.; Xu, W.; Fang, Y.; Yang, Y.; Cao, A. Soluble Polymer-Based, Blown Bubble Assembly of Single-and Double-Layer Nanowires with Shape Control. ACS Nano 2014, 8, 3522–3530. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Li, Y.; Wang, C.; Xu, W.; Shi, E.; Zou, M.; Yang, L.; Yang, X.; Li, Y. Blown Bubble Assembly of Graphene Oxide Patches for Transparent Electrodes in Carbon–Silicon Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 28330–28336. [Google Scholar] [CrossRef]
- Wu, S.; Shi, E.; Yang, Y.; Xu, W.; Li, X.; Cao, A. Direct Fabrication of Carbon Nanotube-Graphene Hybrid Films by a Blown Bubble Method. Nano Res. 2015, 8, 1746–1754. [Google Scholar] [CrossRef]
- Wu, S.; Yang, L.; Zou, M.; Yang, Y.; Du, M.; Xu, W.; Yang, L.; Fang, Y.; Cao, A. Blown-Bubble Assembly and in Situ Fabrication of Sausage-Like Graphene Nanotubes Containing Copper Nanoblocks. Nano Lett. 2016, 16, 4917–4924. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, G.; Yang, S.; Benattar, J.-J. Two-dimensional self-assemblies of silica nanoparticles formed using the “bubble deposition technique”. Langmuir 2010, 26, 16828–16832. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, G.; Jian Wang, Y.; Kay Lee, F.; Polidori, A.; Tong, P.; Pucci, B.; Benattar, J.-J. Molecular Transfer of Surfactant Bilayers: Widening the Range of Substrates. Langmuir 2008, 24, 6072–6078. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, G.; Benattar, J.-J.; Petkova, R.; Wang, J.; Tong, P.; Polidori, A.; Pucci, B. Deposition of Organized Surfactant Films on Solid Substrates. Colloids Surf. A Physicochem. Eng. Asp. 2008, 321, 211–217. [Google Scholar] [CrossRef]
- Alshehri, N.; Bennett, T.; Metha, G.F.; Andersson, G.G. Transfer of Ordered Phospholipid Films onto Solid Substrates from a Drained Foam Film. J. Phys. Chem. C 2015, 119, 22496–22503. [Google Scholar] [CrossRef]
- Alharbi, T.M.; Andersson, G.G. Ordering in Surfactant Foam Films Transferred onto Hydrophilic and Hydrophobic Substrates. J. Phys. Chem. C 2017, 121, 25967–25977. [Google Scholar] [CrossRef]
- Azevedo, J.; Campidelli, S.; He, D.; Cornut, R.; Bertucchi, M.; Sorgues, S.; Benattar, J.-J.; Colbeau-Justin, C.; Derycke, V. Versatile Wafer-Scale Technique for the Formation of Ultrasmooth and Thickness-Controlled Graphene Oxide Films Based on Very Large Flakes. ACS Appl. Mater. Interfaces 2015, 7, 21270–21277. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, X.; Yang, S.; Derycke, V.; Benattar, J.J. New confinement method for the formation of highly aligned and densely packed single-walled carbon nanotube monolayers. Small 2010, 6, 1488–1491. [Google Scholar] [CrossRef]
- Houston, M.R.; Maboudian, R. Stability of ammonium fluoride-treated Si (100). J. Appl. Phys. 1995, 78, 3801–3808. [Google Scholar] [CrossRef]
- Costa-Coquelard, C.; Jegou, P.; Benattar, J.-J. Role of substrate wettability in the “bubble deposition method” applied to the CeVO4 nanowire films. Langmuir 2011, 27, 4397–4402. [Google Scholar] [CrossRef]
- Andreatta, G.; Cousty, J.; Benattar, J.-J. Line tension action on 2D networks of gold nanoparticles obtained by the bubble deposition method. Chem. Commun. 2011, 47, 3571–3573. [Google Scholar] [CrossRef]
- Azevedo, J.; Costa-Coquelard, C.; Jegou, P.; Yu, T.; Benattar, J.-J. Highly ordered monolayer, multilayer, and hybrid films of graphene oxide obtained by the bubble deposition method. J. Phys. Chem. C 2011, 115, 14678–14681. [Google Scholar] [CrossRef]
- Costa-Coquelard, C.; Azevedo, J.; Ardiaca, F.; Benattar, J.-J. Spontaneous and dense assemblies of nanoparticles within micro-channels by the bubble deposition method. Appl. Surf. Sci. 2013, 264, 364–367. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lu, W.; Hu, Y.; Wu, Y.; Yan, H.; Lieber, C.M. Ge/Si nanowire heterostructures as high-performance field-effect transistors. Nature 2006, 441, 489–493. [Google Scholar] [CrossRef]
- Hobbie, E.K.; Wang, H.; Kim, H.; Lin-Gibson, S.; Grulke, E.J. Orientation of carbon nanotubes in a sheared polymer melt. Phys. Fluids 2003, 15, 1196–1202. [Google Scholar] [CrossRef]
- Khan, W.A.; Kazi, S.N.; Chowdhury, Z.Z.; Zubir, M.N.M.; Wong, Y.H.; Shaikh, K.; Nawaz, R.; Hasnain, S.U. Carbon nanomaterials in coatings: A review focusing thin film photovoltaic solar cells. Mater. Sci. Semicond. Process 2025, 185, 108929. [Google Scholar] [CrossRef]
- Bhise, S.; Kathwate, L.; Umadevi, G.; Krishna, K.; Mote, V.; Dole, B. Structural, optical and gas sensing properties of Zn-doped CuO nanostructure thin films for benzene gas sensing applications. Mater. Sci. Mater. Electron. 2024, 35, 66. [Google Scholar] [CrossRef]
- Franklin, A.D. Nanomaterials in transistors: From high-performance to thin-film applications. Science 2015, 349, aab2750. [Google Scholar] [CrossRef]
- Patolsky, F.; Timko, B.P.; Zheng, G.; Lieber, C.M. Nanowire-based nanoelectronic devices in the life sciences. MRS Bull. 2007, 32, 142–149. [Google Scholar] [CrossRef]
- Babazadeh, N.; Ershad-Langroudi, A.; Mousaei, S.M.; Alizadegan, F. Nanotechnology in semiconductors: Role of nano-dimensions and thin film structure. In Handbook of Semiconductors; CRC Press: Boca Raton, FL, USA, 2025; pp. 109–121. [Google Scholar] [CrossRef]
- Hussain, S.A.; Dey, B.; Bhattacharjee, D.; Mehta, N. Unique supramolecular assembly through Langmuir–Blodgett (LB) technique. Heliyon 2018, 4, e01012. [Google Scholar] [CrossRef]
- Gu, W.; Li, Q.; Wang, R.; Zhang, L.; Liu, Z.; Jiao, T. Recent progress in the applications of Langmuir–Blodgett film technology. Nanomaterials 2024, 14, 1039. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, M.M.; Alejo, T.; López-Díaz, D.; Martín-García, B.; Merchán, M.D. Langmuir-Blodgett methodology: A versatile technique to build 2D material films. In Two-Dimensional Materials—Synthesis, Characterization and Potential Applications; Makarov, V., Ed.; InTech: Rijeka, Croatia, 2016; p. 280. [Google Scholar] [CrossRef]
- Mabudi, A.; Naseri, M.; Zamzami, S.M.; Khosravi Nessiani, R. Enhanced wastewater treatment using metal-based nanoparticles: A comprehensive study. Int. J. Min. Geol. Eng. 2025, 59, 97–113. [Google Scholar]
- Lv, J.; Zhang, C.; Qu, G.; Pan, K.; Qin, J.; Wei, K.; Liang, Y. Modification strategies for semiconductor metal oxide nanomaterials applied to chemiresistive NOx gas sensors: A review. Talanta 2024, 273, 125853. [Google Scholar] [CrossRef] [PubMed]
- Begi, A.N.; Hussain, S.; Amu-Darko, J.N.O.; Shah, S.; Junhao, W.; Zhang, X.; Yusuf, K.; Manavalan, R.K.; Qiao, G.; Liu, G. Low-concentration H2S gas sensors based on MOF-derived Co3O4 nanomaterials. Sens. Actuators A Phys. 2024, 378, 115776. [Google Scholar] [CrossRef]
- Patil, M.K.; Shaikh, S.; Ganesh, I. Recent advances on TiO2 thin film-based photocatalytic applications (a review). Curr. Nanosci. 2015, 11, 271–285. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Rebelo, R.; Reis, R.L.; Bhattacharya, M.; Correlo, V.M. Current nanotechnology advances in diagnostic biosensors. Med. Devices Sens. 2021, 4, e10156. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Tavares Ferreira, J.; Pinto, L.A.; Ferreira, Q. Graphene oxide thin films with drug delivery function. Nanomaterials 2022, 12, 1149. [Google Scholar] [CrossRef]
- Vannozzi, L.; Iacovacci, V.; Menciassi, A.; Ricotti, L. Nanocomposite thin films for triggerable drug delivery. Expert Opin. Drug Deliv. 2018, 15, 509–522. [Google Scholar] [CrossRef]
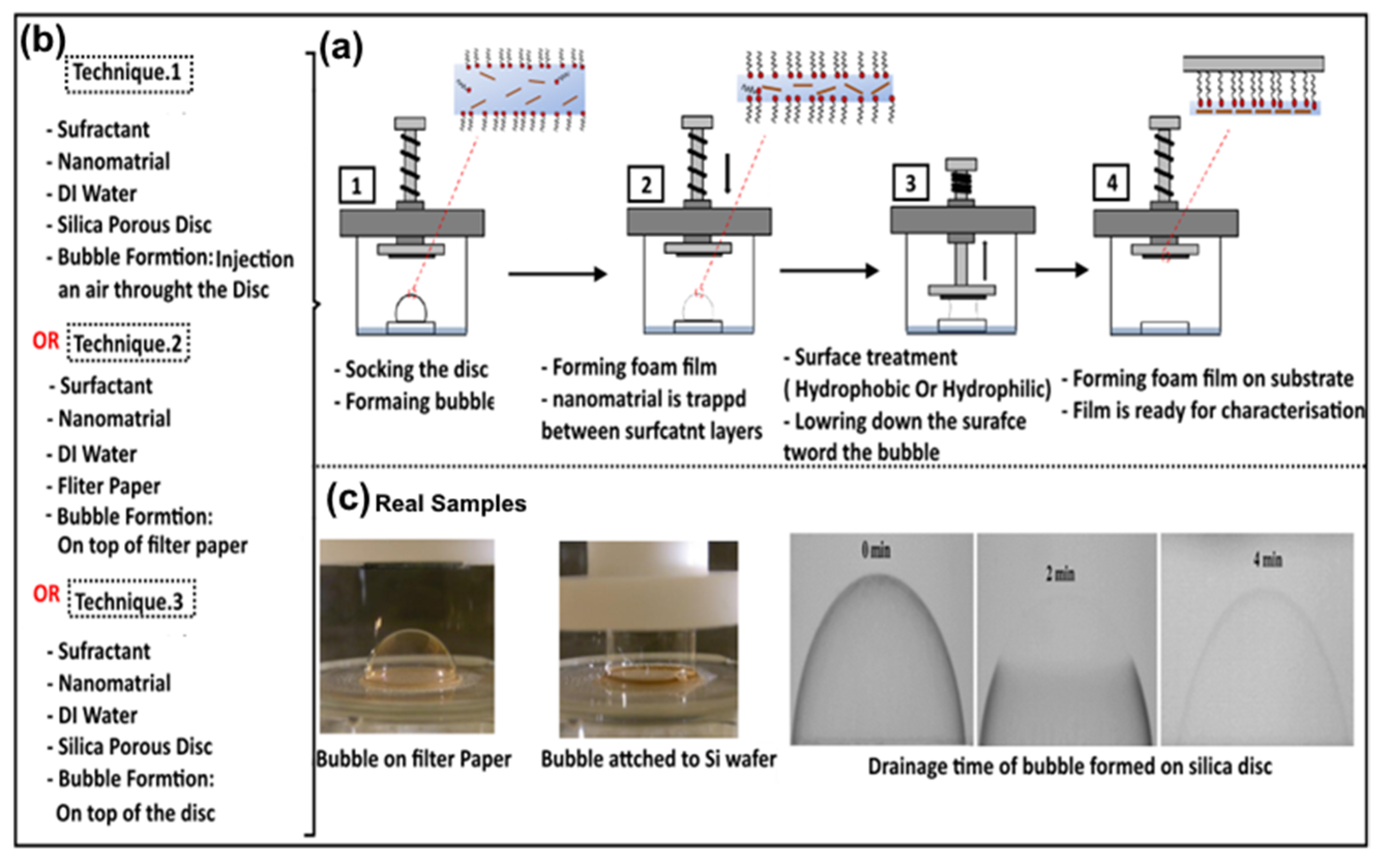
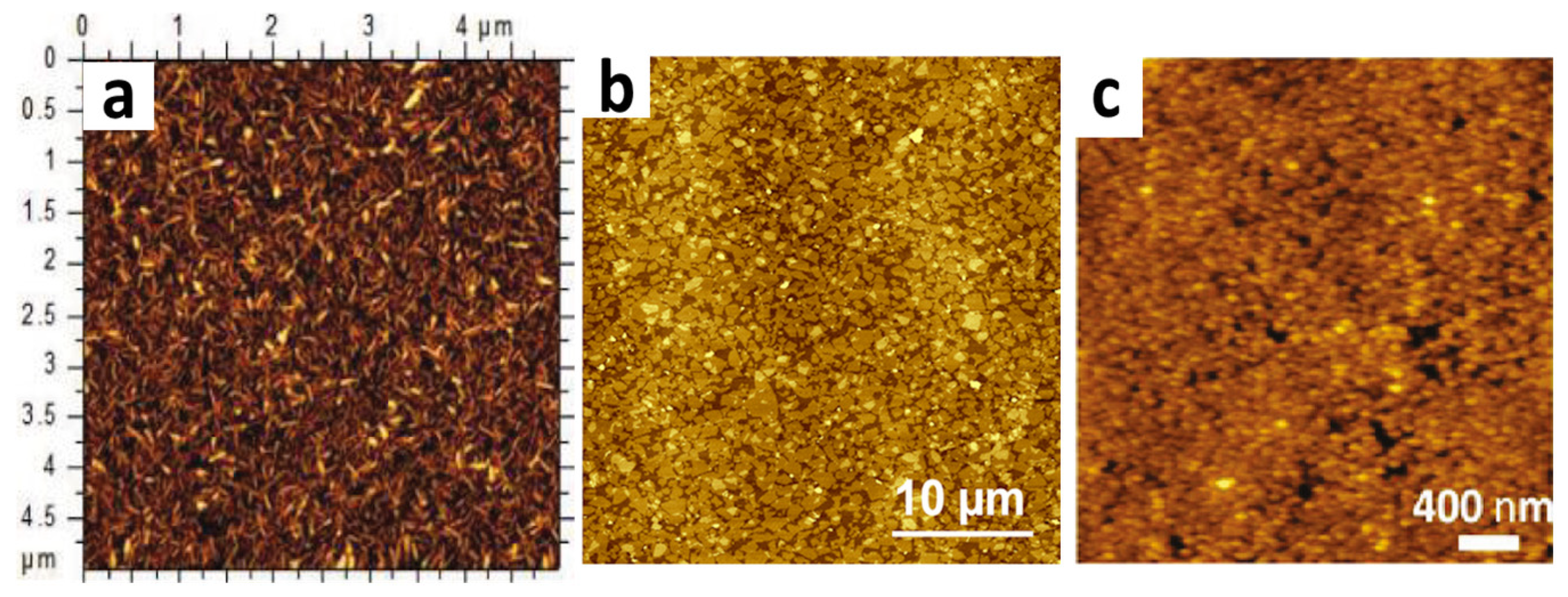
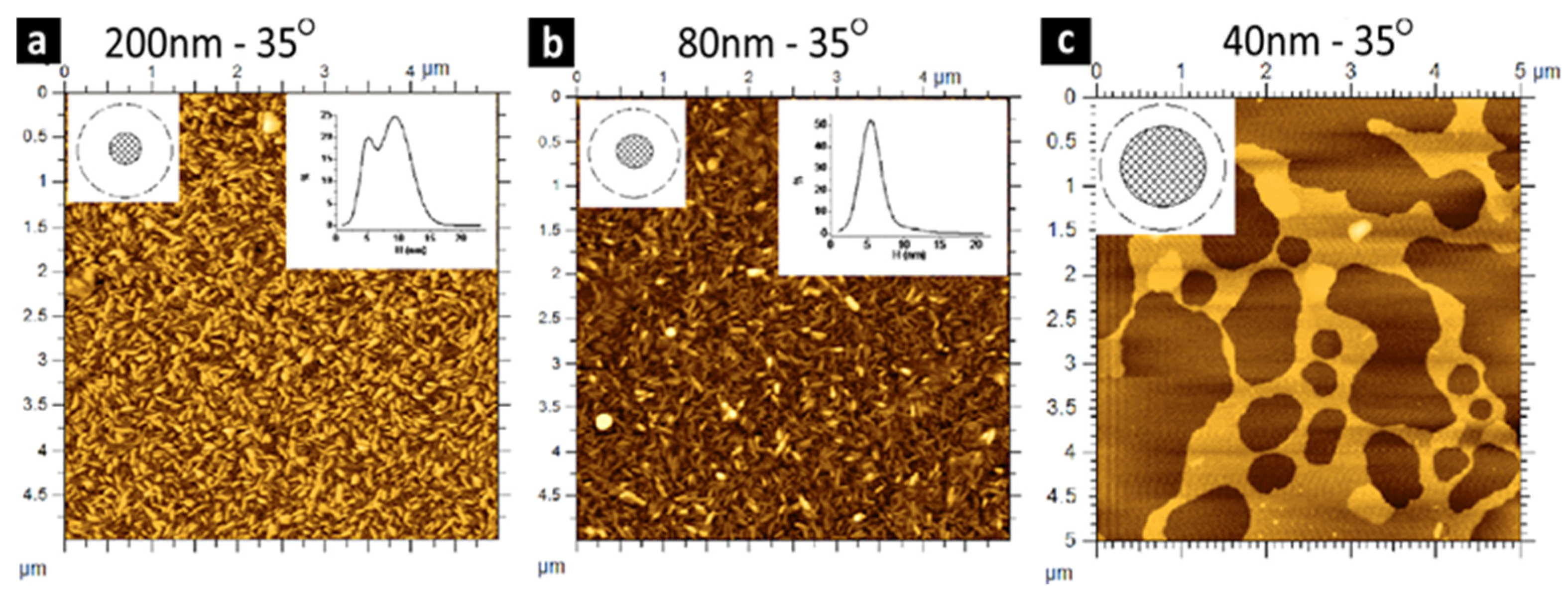
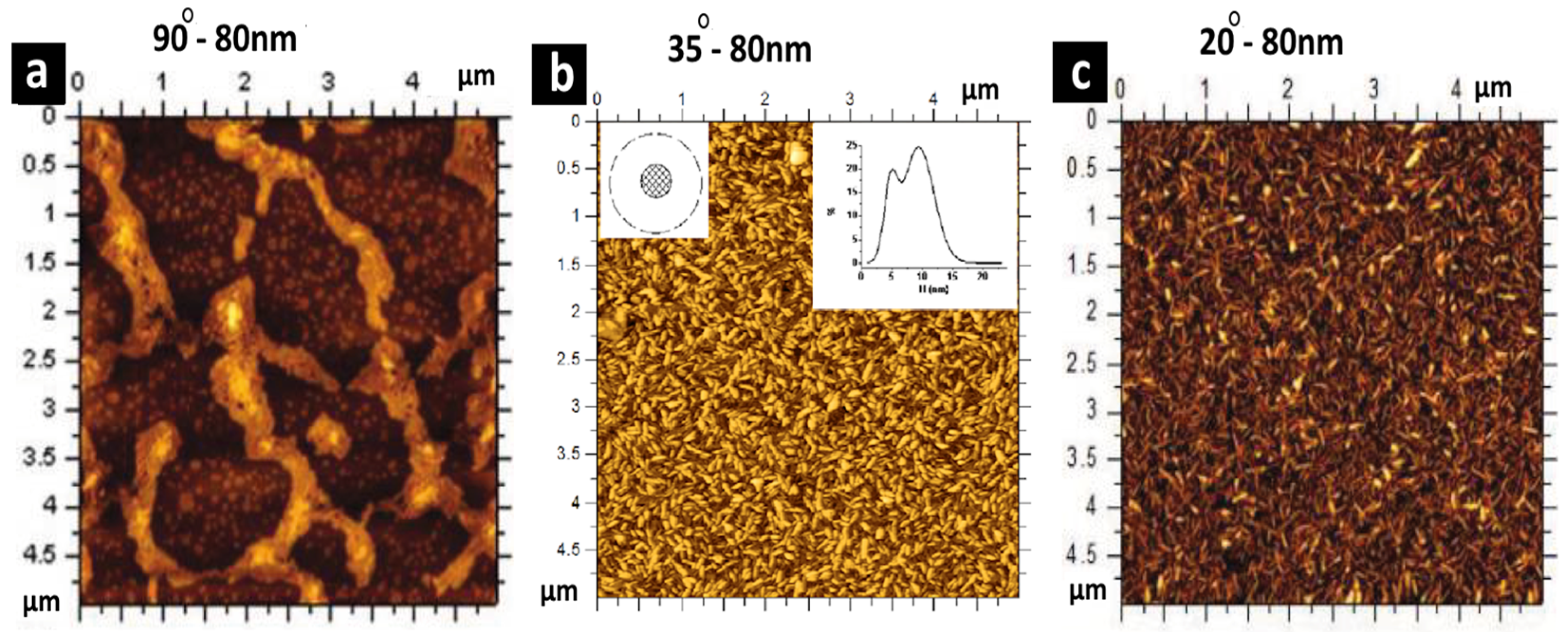
| Comparison Criteria | Blown Bubble Films (BBFs) | Bubble Deposition Method (BDM) | Langmuir–Blodgett Method |
|---|---|---|---|
| Advantages | (1) The method assembles 3D nanomaterial thin films onto solid and flexible substrates. | (1) The method deposits 2D nanomaterial thin films onto solid and flexible substrates. | (1) The method is able to assemble an individual molecule into 2D and 3D systems [76]. |
| (2) The method allows for precise control over film thickness at the molecular level. | (2) The method allows for precise control over film thickness at the molecular level. | (2) The method enables precise control over the film’s thickness and arrangement and it is suitable for creating uniform layers and complex multilayer structures [77]. | |
| (3) The method allows for organizing molecules in a specific orientation and it is localized. | (3) The method allows for organizing molecules in a specific orientation and it is localized. | (3) The method allows for organizing molecules in a specific orientation and it is localized. | |
| (4) The method is low-cost and highly scalable, making it suitable for industrial processes. | (4) The method is low-cost and highly scalable, making it suitable for industrial processes. | (4) The method allows different types of materials to create films, such as organic and inorganic compounds [76]. | |
| (5) The method allows for making films with high-density materials at a fast operation speed with good alignment and arrangement to control the interparticle distances with only one bubble thin film’s deposition. | |||
| (6) The method is easy to operate and does not require hard equipment or control of environmental conditions. | |||
| Disadvantages | (1) The method organizes the nanomaterials with controlled alignment over large areas, but controlling the space between nanomaterials with high density to define interconnections requires multiple deposited films. | (1) The method uses hydrogen fluoride (HF) chemicals during the experimental procedure, which is dangerous. | (1) The method is expensive, making it less accessible for some research and industrial applications [78]. |
| (2) The method is complex to operate and requires precise control of nitrogen pressure flow, bubble expansion rate and control of external vertical force. | (2) It remains uncertain whether this method can be used for scaling up nanomaterials films onto large solid and flexible substrates. | ||
| (3) The method is complex and requires precise control of environmental conditions [78]. | |||
| (4) Monolayer film structures are often modified, making it difficult to obtain a high-quality film [78]. | |||
| (5) Film defects are possibly produced during their transfer process, especially for softer or unstable molecular films [77]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, N.A. Nanostructured Bubble Thin Films—From Simple Fabrication to Scalable Applications: A Review. Nanomaterials 2025, 15, 868. https://doi.org/10.3390/nano15110868
Alshehri NA. Nanostructured Bubble Thin Films—From Simple Fabrication to Scalable Applications: A Review. Nanomaterials. 2025; 15(11):868. https://doi.org/10.3390/nano15110868
Chicago/Turabian StyleAlshehri, Naif Ahmed. 2025. "Nanostructured Bubble Thin Films—From Simple Fabrication to Scalable Applications: A Review" Nanomaterials 15, no. 11: 868. https://doi.org/10.3390/nano15110868
APA StyleAlshehri, N. A. (2025). Nanostructured Bubble Thin Films—From Simple Fabrication to Scalable Applications: A Review. Nanomaterials, 15(11), 868. https://doi.org/10.3390/nano15110868




