Abstract
Rapid industrialization has escalated environmental pollution caused by organic compounds, posing critical challenges for wastewater treatment. Advanced oxidation processes based on peroxymonosulfate (PMS) suffer from metal leaching and catalyst recycling challenges. To address these limitations, this study developed a nitrogen-doped biochar aerogel (NBA) derived from poplar wood powder as an eco-friendly and easily recoverable PMS activator. The NBA catalyst, optimized by tuning the calcination temperature to achieve a specific surface area of 297.5 m2 g−1, achieved 97% bisphenol A (BPA) removal within 60 min with a catalyst dosage of 0.3 g/L and 1.0 mM PMS under mild conditions. The material exhibited broad pH adaptability (pH 3.5–9), recyclability (>94% efficiency after thermal treatment), and versatility in degrading seven pollutants (BPA, phenol, 4-chlorophenol, 2,4-dichlorophenol, 2,4,6-trichlorophenol, rhodamine 6G, and levofloxacin) through synergistic radical (•OH, SO4•−, O2•−) and non-radical (1O2) pathways. X-ray photoelectron spectroscopy (XPS) analyses revealed that nitrogen doping enhanced PMS activation by optimizing electronic structures. This study highlights the potential of waste biomass-derived carbon aerogels as eco-friendly, efficient, and reusable catalysts for advanced oxidation processes in wastewater treatment.
1. Introduction
With the rapid development of industrialization, numerous types of industrial organic compounds enter the environment, leading to the pollution of aquatic ecosystems [1]. The treatment of refractory organic compounds is one of the most critical and challenging problems in the environmental remediation process [2]. Advanced oxidation processes (AOPs), which involve the in situ generation of highly reactive species, are widely used for the degradation of refractory organic compounds in industrial wastewater and drinking water [3]. As one of the prominent oxidants in AOPs, peroxymonosulfate (PMS, HSO5−) with an asymmetric structure can be activated by metal-based and metal-free materials to generate multiple reactive species, including sulfate radical (SO4•−), hydroxyl radical (•OH), superoxide radical (O2•−), singlet oxygen (1O2), catalyst-PMS complexes, among others [4,5,6,7]. AOPs based on PMS have been successfully applied to degrade various pollutants. However, there are still inherent drawbacks in practical applications, such as (i) short-lived radical species limiting mass transfer [8,9]; (ii) challenges in recycling powder catalysts; and (iii) secondary pollution caused by metal ion leaching [10,11].
Transition metal activation of PMS is highly effective in degrading organic pollutants [12,13]. However, inevitable metal leaching increases environmental risks, hindering the practical application of transition metal catalysts in environmental remediation. Compared to traditional metal-based catalysts such as iron and cobalt, carbon catalysts offer advantages, including environmental friendliness, corrosion resistance, and biocompatibility, while overcoming issues like sintering and metal leaching inherent to metal-based systems [14,15,16]. Despite these benefits, pure carbon materials exhibit limited activation ability. Consequently, heteroatom-doped carbon nanoparticles have garnered significant attention. Nitrogen doping, for instance, enhances PMS activation by modifying spin and charge distribution in carbon matrices [17]. Nitrogen-doped graphene, carbon nanotubes, and graphene–biochar aerogels have proven effective as PMS activators for pollutant degradation [17,18,19]. However, conventional carbon sources like graphene oxide (GO) or carbon nanotubes face limitations in scalability, cost, and sustainability. In contrast, biochar derived from waste biomass provides a low-cost, renewable, and easily processable alternative, making it an ideal precursor for carbon catalyst production [20,21].
The rapid global increase in solid waste generation has become one of the most pressing environmental challenges. As a renewable biomass material, poplar wood powder has attracted increasing attention as a cost-effective, widely available, and sustainable resource. Rich in cellulose and lignin, it offers excellent carbonization properties and a natural porous structure, making it an ideal precursor for the fabrication of functional biochar materials [22,23]. Additionally, studies indicate that wood powder can be utilized as a raw material to produce biochar aerogels. Biochar aerogels are unique porous materials consisting of interlinked carbon nanomaterials and interstitial mesopores (2–50 nm) [24]. This monolithic structure offers a high specific surface area, facile recyclability, and excellent mechanical performance, making it particularly advantageous for adsorption, filtration, and catalytic applications compared to traditional powder catalysts [25,26].
In this study, nitrogen-doped biochar aerogel (NBA) was prepared from poplar wood powder derived from forest waste and used as an activator for PMS. The prepared NBA catalyst was systematically characterized, and its ability to activate PMS for bisphenol A (BPA) degradation was tested. Meanwhile, optimal calcination conditions were determined by adjusting the calcination temperature. Key factors affecting BPA degradation were investigated, and the capability of NBA-activated PMS in pollutant degradation was further evaluated in terms of universality and reusability. Finally, the BPA degradation mechanism was elucidated, with potential reactive sites proposed.
2. Materials and Methods
2.1. Chemicals
Sodium hydroxide (NaOH), hydrogen peroxide (H2O2, ≥30.0%), sulfuric acid (H2SO4), p-chlorophenol (4-CP), rhodamine 6G (Rh6G), tert-butyl alcohol (TBA), 2,4-dichlorophenol (2,4-DCP), 2,4,6-trichlorophenol (2,4,6-TCP), levofloxacin, urea, and phenol were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Peroxymonosulfate (2KHSO5·KHSO4·K2SO4, PMS, ≥47.0% KHSO5 basis), bisphenol A (BPA), furfuryl alcohol (FFA), superoxide dismutase (SOD, ≥1400 units/mg) were purchased from Aladdin Chemical Co., Ltd. (Shanghai, China). Methanol and ethanol were obtained from Tianjin Fu Yu Fine Chemicals Co., Ltd. (Tianjin, China). All the chemicals were used without any further purification. Deionized water was used to prepare all aqueous solutions (18.25 MΩ·cm). Poplar sawdust was obtained from a wood processing factory in Jinan, Shandong, China, where it was generated as waste material from the mechanical cutting of poplar wood during board manufacturing. The resulting powder was then ground and sieved through a 120-mesh sieve prior to experimental use.
2.2. Synthesis of Biochar Catalysts
The nitrogen-enriched biochar aerogel was fabricated from poplar-derived biomass through a multistep purification and carbonization process. Initially, the raw poplar powder underwent solvent dewaxing using anhydrous ethanol to eliminate lipophilic constituents. Subsequently, the delipidated biomass was subjected to alkaline-peroxide treatment in an aqueous solution containing 4 wt% NaOH and 0.7 wt% H2O2, achieving selective removal of lignin and hemicellulose components while preserving cellulose integrity. Subsequently, the material was immersed in a pre-cooled NaOH/urea mixed solution and vigorously stirred to oxidize cellulose. The reaction solution was ultrasonically dispersed and freeze-dried to form an aerogel. Finally, the aerogel was calcined under a N2 atmosphere at 600 °C for 2 h to obtain nitrogen-doped biochar aerogel (labeled NBA, Figure 1). For control samples, dewaxed poplar powder directly calcined under a N2 atmosphere at 600 °C was labeled PB. Non-ultrasonicated freeze-dried material calcined under a N2 atmosphere at 600 °C was labeled BC.
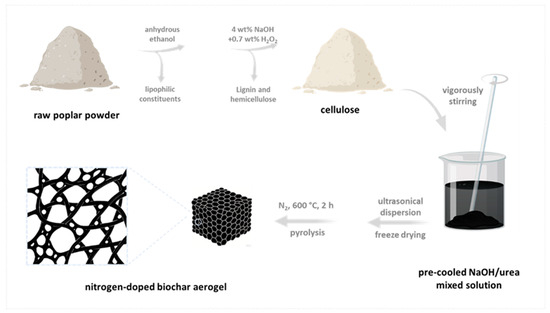
Figure 1.
Procedure of the preparation of nitrogen-doped biochar aerogel (NBA).
2.3. Catalyst Performance Evaluation
Degradation experiments were conducted in a 100 mL conical flask at 25 °C. In each test series, a predetermined amount of catalyst (15 mg) was dispersed in 50 mL of BPA solution (initial concentration: 10 mg/L) without adjusting the pH. The suspension was subjected to a 20 min dark-phase homogenization to establish thermal equilibrium and adsorption–desorption equilibrium. Then, 1 mL of 20 mM PMS was injected into the conical flask to initiate the reaction. At designated time points, 1 mL samples were extracted, quenched with 0.5 mL ethanol, and rapidly filtered through 0.22 μm PES membranes. Experimental variables, including catalytic loading, PMS concentration, pH conditions (modified using 0.1 M H2SO4/NaOH), and thermal parameters (25–45 °C), were systematically investigated to optimize degradation efficiency. Post-catalytic NBA was recovered and washed for reusability testing. All pollutant removal experiments were conducted in triplicate to obtain average values. Unless otherwise specified, standard conditions were maintained: [catalyst] = 0.3 g/L, [PMS] = 1 mM, [pollutant] = 10 mg/L, T = 25 °C, with unadjusted pH. The spent catalysts were regenerated through a sequential procedure: soaking in ethanol for 12 h to remove organic residues; repeated washing with deionized water until neutral pH was attained; and calcination at 500 °C for 1 h under a N2 atmosphere to restore catalytic activity.
2.4. Analytical Methods and Characterization
Concentrations of BPA, phenol, 4-CP, 2,4-DCP, and 2,4,6-TCP were determined by HPLC with C18 reversed-phase chromatography. For BPA, phenol, 4-CP, and 2,4-DCP, the mobile phases were mixed solutions of methanol and water with mixing ratios of 70:30, 80:20, 70:30, and 80:20, respectively. The detection wavelengths were 278 nm, 270 nm, 278 nm, and 284 nm, respectively. The concentration of 2,4,6-trichlorophenol was measured at 290 nm using a mixture of 80% methanol and 20% water (each containing 1% acetic acid) as the mobile phase. The concentrations of levofloxacin and Rh6G were measured at 292 nm and 552 nm using a Puxi UV–Vis spectrophotometer.
The morphology of the catalyst was observed by field emission scanning electron microscopy (FE-SEM, GeminiSEM 300, Carl Zeiss, Jena, Germany). The Brunauer–Emmett–Teller (BET) surface area was obtained by ASAP 2460-4MP (Micromeritics, Norcross, GA, USA) at 77 K, and the pore size distribution was calculated from the corresponding nitrogen absorption and desorption isotherm. The functional groups and surface composition of carbon materials were determined by X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher Scientific Ltd., Cheshire, UK). The Raman spectrometer (LabRAM HR 800, Horiba/Jobin Yvon, Palaiseau, France) with a 633 nm HeNe laser excitation source was used to analyze the defect structure of the catalyst.
3. Results and Discussion
3.1. Characterization of Catalysts
Under inert conditions, the thermochemical decomposition of lignocellulosic biomass initiates molecular fragmentation through bond cleavage mechanisms. This process facilitates progressive aromatization via cyclization reactions, ultimately resulting in the formation of graphitic carbon structures through solid-phase polycondensation. The resultant carbon-rich material exhibits characteristic features of biochar with enhanced structural ordering [27]. Direct carbonization of dewaxed poplar flour (PB) and purification of poplar flour (BC) resulted in irregular structures (Figure 2a,b). Figure 2c shows the macroscopic morphology and microstructure of NBA, with the inset displaying its macroscopic form. The biochar aerogel exhibits excellent mechanical properties and retains its structural integrity even under mechanical shock. The morphology and structure of the as-prepared samples were characterized using a field emission scanning electron microscope (FE-SEM). Following pyrolysis at 600 °C, the delignified PB demonstrated preservation of its inherent fibrous architecture while undergoing structural fragmentation characterized by the conversion of macroscopic constituents into micron-scale irregular particulates (Figure 2a). Alkali and H2O2 treatments effectively removed lignin and hemicellulose [28]. The NBA has a porous and interconnected three-dimensional net structure. As shown in Figure 2d, nitrogen (N) is uniformly distributed on the porous nanocarbon skeleton of NBA, confirming the successful preparation of this nitrogen-doped biochar aerogel material.
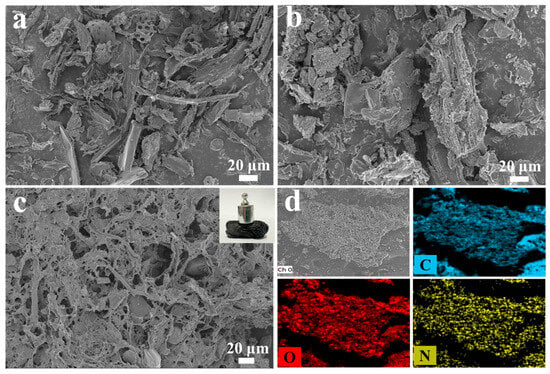
Figure 2.
FE-SEM images of (a) PB, (b) BC, (c) NBA, and (d) EDS element distribution of NBA.
Biochar materials usually have good pore structures. Nitrogen adsorption–desorption analysis was implemented to characterize the BET-specific surface area (SSA) and porous architecture of the catalyst specimens. In accordance with IUPAC nomenclature, all synthesized materials manifested distinct Type IV physisorption isotherm profiles (Figure 3a). The specific surface areas of PB, BC, and NBA were 178.5 m2 g−1, 142.9 m2 g−1, and 297.5 m2 g−1, respectively. Obvious N2-type hysteresis loops belonging to the H4 type were observed in NBA, indicating a well-developed hierarchical porous structure [29,30]. NBA showed high N2 adsorption capacity at low relative pressure (P/P0), suggesting the presence of micropores in NBA. Hysteresis occurred in the range of P/P0 = 0.4–1.0, indicating the presence of mesoporous and macroporous. When the relative pressure P/P0 > 0.9, the adsorption amount continued to rise, attributed to N2 adsorption by macropores [31]. Compared to PB and BC, NBA exhibits advantages in both specific surface area and pore structure. The higher specific surface area and multi-pore structure reduce mass transfer and diffusion resistance, facilitating pollutant diffusion within carbon materials [32,33]. Additionally, the interlaced carbon fiber network structure enhances adsorption capacity and provides abundant PMS-active sites for NBA.
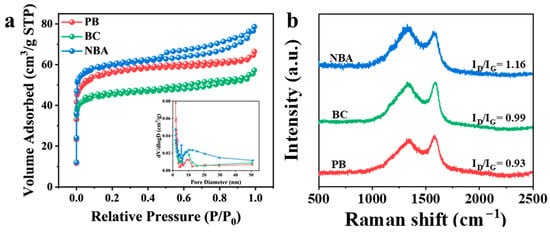
Figure 3.
Characterizations of NBA, PB, and BC: (a) N2 adsorption–desorption isotherms and aperture distribution, (b) Raman spectra.
Defects in materials have been demonstrated to enhance catalytic activity by providing abundant active sites for electron transfer during PMS activation, as evidenced in previous studies [34]. Through Raman spectral analysis, the ID/IG ratio (D-band: structural defects vs. G-band: graphitic structure) serves as a critical indicator for quantifying defect density and structural disorder in carbon-based materials [35]. Comparative analysis revealed progressively increasing ID/IG values of 0.93 (PB), 0.99 (BC), and 1.16 (NBA), confirming NBA’s superior defect density relative to PB and BC counterparts (Figure 3b). Abundant structural defects may promote the catalytic performance of NBA. The introduction of nitrogen precursors during biomass pyrolysis is conducive to increasing defects, resulting in NBA having the most defective structures. Many defects can alter electron transport pathways and provide sufficient active sites, thereby promoting rapid electron transfer and facilitating PMS activation.
3.2. Catalytic Performance Evaluation
The catalytic efficiencies of various PMS activators were assessed by measuring BPA degradation rates. As shown in Figure 4a,b, BPA degradation was negligible in the PMS-alone system, with only 2% removal within 60 min, indicating that unactivated PMS cannot efficiently oxidize BPA. In the NBA, BC, and PB systems, the BPA degradation efficiencies reached 97%, 64%, and 44% within 60 min, respectively. The activation of PMS and BPA degradation by these three catalysts all followed pseudo-first-order reaction kinetics (Equation (1)). The specific surface area-normalized apparent rate constant (kpseudo/SBET) of PB, BC, and NBA were 0.6, 2.3, and 10.8 × 10−3 g m−2 h−1, respectively (Figure 4c and Figure S1). Furthermore, a comparative analysis of NBA’s catalytic efficiency in PMS activation with various catalysts (Table S1) revealed that NBA demonstrated reactivity comparable to, or even exceeding, that of high-performance catalysts previously documented. This finding underscores NBA’s exceptional catalytic capability for PMS activation processes and its efficacy in BPA degradation. Notably, PMS (1 mM) alone exhibited minimal BPA degradation, suggesting that BPA cannot be effectively oxidized by PMS without a catalyst. When NBA was directly added without PMS, approximately 34% of BPA was removed from the solution, indicating that NBA has a certain adsorption capacity for BPA. Since PMS activation occurs on the catalyst surface, the adsorption and catalytic capabilities of the catalyst synergistically enhance degradation. The effective adsorption of BPA by NBA reduces the time required for target organic molecules to migrate to its surface and hierarchical pore structure, thereby facilitating attacks by reactive species [36].
where C0 and Ct are the concentrations of the dyes at time 0 and t, respectively, k = pseudo-first-order rate constant, and t = time in min.
ln(C0/Ct) = kpseudo t
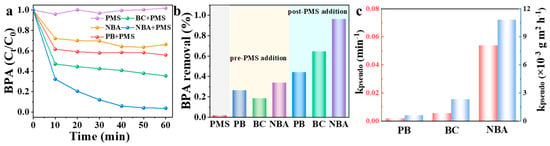
Figure 4.
(a) Kinetics of BPA removal, (b) BPA removal rate, and (c) the apparent rate constant (red: kpseudo, blue: normalized kpseudo) for different catalysts. Conditions: [catalyst] = 0.3 g/L, [PMS] = 1 mM, [BPA] = 10 mg/L, without pH adjustment.
According to previous studies on biochar, the catalytic performance is affected by calcination temperature [23]. Therefore, three different NBA samples were obtained by calcination at different temperatures in this study (Figure 5a). In the presence of PMS, 83% of BPA was removed by materials calcined at 500 °C within 60 min. Materials calcined at 600 °C and 700 °C showed removal rates of 68% and 84%, respectively, within 10 min, but both reached 97% within 60 min. Increasing the calcination temperature from 500 °C to 600 °C significantly improved the catalytic performance of NBA; however, further increasing the temperature to 700 °C did not enhance its ability to remove organic pollutants substantially. Temperature can regulate the structure, electrical conductivity, oxygen-containing functional groups, and nitrogen speciation in carbon materials [37]. As shown in Figure 5b, the ID/IG ratio of NBA gradually increases with rising calcination temperature due to chemical bond cleavage and carbon skeleton reconstruction [38]. Elevated temperatures promote defect site formation and nitrogen species modulation, thereby enhancing NBA’s catalytic activity [39]. However, excessive temperatures may induce carbon–nitrogen bond rupture and heteroatom loss, potentially degrading catalytic performance [37]. Additionally, higher calcination temperatures entail greater energy consumption. Consequently, materials calcined at 600 °C were selected for subsequent experiments. The experimental findings indicated that the NBA/PMS system exhibited enhanced versatility in eliminating diverse electron-rich contaminant species through degradation processes.
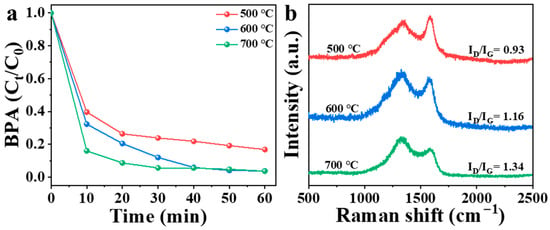
Figure 5.
(a) Influence of calcination temperature on NBA catalytic activity, (b) Raman spectra of materials at different calcination temperatures. Conditions: [catalyst] = 0.3 g/L, [PMS] = 1 mM, [BPA] = 10 mg/L, without pH adjustment.
3.3. Possible Activation Mechanism
The activation of PMS by NBA may degrade BPA through both free radical and non-free radical pathways. To identify the main active species, quenching experiments were conducted. Ethanol, tert-butanol (TBA), furfuryl alcohol (FFA), and superoxide dismutase (SOD) were employed as quenching agents to investigate the mechanism of pollutant degradation by PMS (Figure 6). Specifically, TBA and ethanol were used to probe the involvement of •OH and SO4•−. Ethanol can quench hydroxyl radicals (•OH, K = 1.2–2.8 × 109 m−1 s−1) and sulfate radicals (SO4•−, K = 1.6–7.7 × 107 m−1 s−1), while TBA primarily scavenges •OH (K = 3.8–7.6 × 108 m−1 s−1) [40]. As shown in Figure 6a, in the NBA/PMS/BPA system, the addition of 0.5 M and 1.0 M ethanol reduced the BPA degradation rate to 90% and 67% within 60 min, respectively. Similarly, 0.5 M and 1.0 M TBA decreased the removal efficiency to 78% and 63%. The stronger inhibitory effect of TBA compared to ethanol may be attributed to its high viscosity masking the active sites on the NBA surface [41].
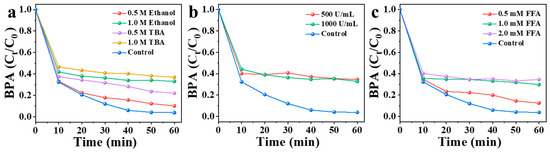
Figure 6.
(a) Influence of TBA and ethanol as radical scavengers for BPA degradation in NBA/PMS system. (b) Influence of SOD as radical scavengers for BPA degradation in NBA/PMS system. (c) Influence of different dosages of FFA for BPA degradation in NBA/PMS system. Conditions: [catalyst] = 0.3 g/L, [PMS] = 1 mM, [BPA] = 10 mg/L, without pH adjustment.
In addition, SOD and FFA were used as quenchers of superoxide anion radical (O2•−) and 1O2, respectively [42]. As illustrated in Figure 6b, the addition of SOD exhibited a significant quenching effect on BPA degradation. Specifically, the degradation efficiency of BPA was suppressed by 31% at an SOD concentration of 500 U/mL. Further increasing the SOD dosage to 1000 U/mL resulted in a comparable inhibition rate of 30%. These results suggest that SOD-mediated scavenging of O2•− contributed to approximately 30% of the overall BPA degradation process. 1O2, predominantly generated through non-radical pathways in carbon/PMS systems [14,42,43,44], demonstrated critical involvement in BPA degradation. This conclusion was supported by marked suppression of BPA removal efficiency (87% in 60 min) when adding 0.5 mM furfuryl alcohol (FFA), a selective 1O2 scavenger with a second-order rate constant of 1.2 × 108 M−1s−1 (Figure 6c) [42]. In the presence of 0.5 mM FFA, only 87% of BPA was removed within 60 min. As the dosage of FFA was increased from 0.5 mM to 1 mM and further to 2 mM, the inhibition rate of BPA degradation efficiency remained nearly constant at ~35%, with no significant enhancement observed (Figure 6c). This plateau suggests that the contribution of singlet oxygen to BPA degradation was approximately 33%. In conclusion, multiple active species, such as •OH, SO4•−, O2•−, and 1O2, were produced in the degradation of BPA by the NBA/PMS system.
The chemical states of C and N in NBA were analyzed by X-ray photoelectron spectroscopy (XPS). As shown in Figure 7a, the C 1s spectrum of NBA was deconvoluted into four peaks at 284.3 eV (C=C), 285.2 eV (C–O/C–N), 286.2 eV (C=O), and 288.6 eV (O–C=O) [35]. Among them, the percentage of C=O was 9.87%. In the activated PMS system, the presence of C=O contributes both to free radical oxidation (C=O acts as a Lewis base with lone pair electrons, supplying electrons to PMS to split the O–O bond, generating •OH and SO4•−) and to non-free radical oxidation (1O2 is produced via the formation of carbonyl-containing intermediates) [45,46,47]. At the same time, the existence of nitrogen species in NBA was analyzed (Figure 7b), and the N 1s via the formation of carbonyl-containing intermediates three peaks at 398.0 eV (pyridine N), 400.3 eV (pyrrole N), and 401.6 eV (graphite N) [35]. The proportions of pyridine N, pyrrolic N, and graphitic N in N species were 32.4%, 27.1%, and 40.5%, respectively. N doping can alter the properties of carbon by modifying the electron distribution and spin density, thereby improving the catalytic performance of carbon materials. Pyridine N and pyrrolic N, as Lewis bases with lone pair electrons, can directly activate PMS to generate •OH and SO4•−, which are beneficial to the free radical pathway [45]. In contrast, graphitic N tends to induce non-free radical pathways by forming positively charged carbon regions due to the electronegativity difference between N and C atoms [48]. The graphitic N exhibits higher charge density and electron density compared to other N configurations, enabling it to induce electron transfer from adjacent carbon atoms to nitrogen atoms, resulting in positively charged carbon atoms. Simultaneously, it enhances electron transfer between NBA and PMS, forming a metastable intermediate of NBA-PMS. This intermediate can decompose to produce 1O2 [5].
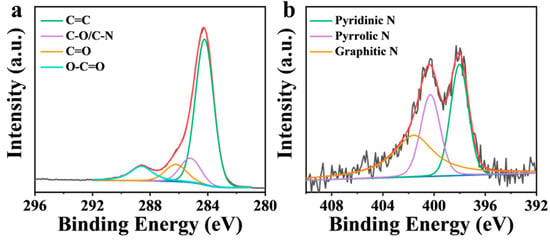
Figure 7.
XPS spectra of (a) NBA: C1s and (b) N1s.
Due to the high specific surface area and hierarchical porous structure of NBA, the mass transfer and diffusion resistance of NBA are low, and the mass transfer rate is fast. This enables reactant molecules to be more easily adsorbed on their inner surface. In addition, the good adsorption capacity of NBA allows BPA to be enriched on its surface. With the addition of PMS, both free radical and non-free radical pathways are induced. Previous reports have confirmed that the catalytic activity of carbonaceous materials in heterogeneous systems is related to the complex electronic states of covalent carbon systems. Oxygen-containing functional groups and defect sites are the main active sites of carbonaceous catalysts [48,49]. In summary, Lewis bases possessing lone pair electrons, such as pyridine N, pyrrole N, and C=O, are conducive to the activation of PMS to generate free radicals during redox reactions. The non-free radical pathway operates by forming a surface complex between NBA and PMS to facilitate pollutant degradation. Multiple possible active sites exist on NBA, and the activation process is interdependent (Figure 8).
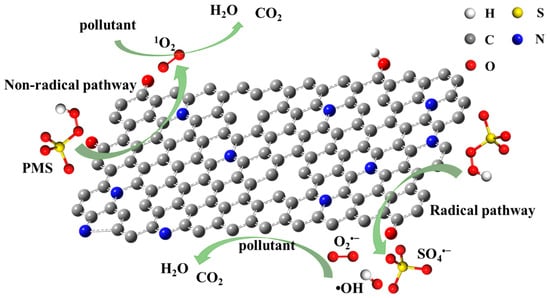
Figure 8.
Degradation mechanism of BPA by activation of PMS by NBA.
3.4. Influence of Operating Parameters on BPA Degradation
Key operational variables governing BPA degradation in the NBA/PMS system, including NBA dosage, PMS concentration, initial pH, and reaction temperature, were methodically examined through controlled parametric analysis. As shown in Figure 9, when the NBA dosage was increased from 0.1 g/L to 0.3 g/L, the degradation efficiency of BPA improved from 65% to 97%. The removal of BPA by the NBA/PMS system was significantly dependent on the NBA dosage. This is because a higher NBA dosage increases the number of active sites, thereby generating more reactive species [10]. However, when the NBA dosage was further raised to 0.4 g/L, only 95% of BPA was removed within 60 min, with no further improvement in the removal rate. This phenomenon may occur because excessive NBA dosage leads to an overproduction of active species, resulting in radical quenching reactions that interfere with the interaction between reactive species and pollutants (Equations (2)–(4)).
HSO5− + •OH → SO5•− + H2O
HSO5− + SO4•−→SO5•− + HSO4−
SO4•− + SO4•− → S2O82−
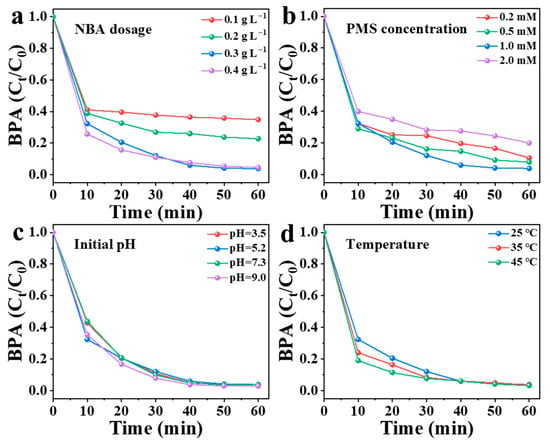
Figure 9.
Influence of different conditions in NBA/PMS system: (a) NBA dosage, (b) PMS concentration, (c) initial pH, and (d) temperature.
As shown in Figure 9b, PMS concentration was identified as the predominant factor influencing BPA degradation. The degradation efficiency of NBA increased by 7% when the PMS concentration was adjusted from 0.2 mM to 1 mM. This enhancement can be attributed to the activation of more PMS by NBA at higher concentrations, which generated additional active species for pollutant degradation. The BPA removal rate reached its maximum (specific value if available) at 1 mM PMS. However, further increasing the PMS concentration to 2 mM resulted in only an 80% removal efficiency within 60 min, indicating no significant improvement. This phenomenon is likely due to the quenching of excess free radicals under high PMS conditions [18], which reduces the availability of active species for pollutant degradation and may even inhibit the reaction. Therefore, maintaining PMS concentrations within an optimal range (e.g., 1 mM) is critical to minimizing these adverse effects.
When the pH is between 3 and 9, PMS exists in the form of HSO5− in the aqueous solution [50]. Figure 9c depicts that the NBA/PMS system can effectively remove BPA in a broad pH range of 3–9. Meanwhile, there was no significant change in solution pH during BPA degradation. The pH range of actual wastewater (5–9) [31] aligns with the operational feasibility of the NBA/PMS system, demonstrating its practicality for real wastewater treatment. As illustrated in Figure 9d, temperature-dependent oxidation kinetics analysis reveals enhanced BPA degradation efficiency in the NBA/PMS system with increasing temperature (25–45 °C). The increase in temperature improved the degradation efficiency of BPA. The reason may be twofold: (1) PMS activation is endothermic, and elevated temperature facilitates the rupture of the O–O bond in PMS [44]; (2) higher temperature accelerates the reaction between reactive species and pollutants, thereby enhancing the reaction rate.
3.5. Reusability and Regeneration Performance of NBA
Catalyst reusability represents a critical performance indicator for assessing industrial viability. To explore the reusability of NBA, four consecutive degradation experiments were conducted (Figure 10a). In the first cycle, NBA achieved 97% BPA removal within 60 min. The removal rates decreased to 86% and 62% in the second and third cycles, respectively. Thermal treatment has been recognized as an effective approach to restoring the catalytic activity of carbon materials [51]. In this study, NBA was regenerated through thermal treatment at 500 °C under a nitrogen atmosphere. Subsequent evaluation assessed the catalytic activity of thermally regenerated catalysts. Results demonstrated that the regenerated NBA regained its activity, achieving 94% BPA removal within 60 min. These findings indicate that NBA can serve as a novel green and sustainable material for wastewater treatment. Furthermore, to assess the universality of NBA, its efficiency in removing diverse organic pollutants was investigated at a concentration of 0.05 mM (Figure 10b). In the NBA/PMS system, the 60 min removal rates for BPA, phenol, 4-CP, 2,4-DCP, 2,4,6-TCP, Rh6G, and levofloxacin were 97%, 100%, 71%, 70%, 54%, 65%, and 88%, respectively. These results confirm that the NBA/PMS system exhibits outstanding catalytic performance for degrading various organic contaminants, positioning it as a promising strategy for pollutant removal.
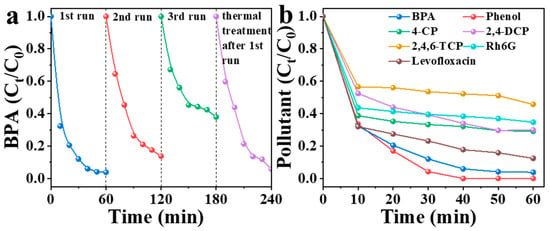
Figure 10.
(a) Reusability of NBA and (b) removal capacity of NBA for different pollutants. Conditions: [catalyst] = 0.3 g/L, [PMS] = 1 mM, [BPA] = 10 mg/L, without pH adjustment.
4. Conclusions
In this study, nitrogen-doped biochar aerogel (NBA) was prepared from poplar powder as a biomass source. Under conditions of 0.3 g/L NBA and 1 mM PMS, the NBA/PMS system exhibited excellent degradation efficiency toward diverse pollutants (10 mg/L each), achieving 60 min removal rates of 97% (BPA), 100% (phenol), 71% (4-CP), 70% (2,4-DCP), 54% (2,4,6-TCP), 65% (Rh6G), and 88% (levofloxacin). Moreover, the system showed a good removal rate over a wide pH range. Quenching experiments demonstrated that the degradation of BPA proceeds through radical and non-radical pathways dominated by •OH, SO4•−, O2•− and 1O2. XPS analysis was also used to further investigate the possible active sites of NBA in activating PMS. The NBA/PMS system exhibits good recyclability and broad applicability to various pollutants, suggesting its potential for practical wastewater treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15110865/s1, Figure S1: Kinetics of BPA degradation in the different systems; Table S1: Comparison of catalytic performance of NBA with other catalysts on PMS/PS activation for BPA degradation [52,53,54,55,56,57,58,59,60,61,62,63,64,65].
Author Contributions
Software: L.K. and M.Z.; Formal analysis, L.K.; Investigation, L.K. and M.Z.; Data curation, M.Z.; Writing—original draft, L.K.; Writing—review and editing, L.K.; Funding acquisition, L.K. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Shandong Provincial Natural Science Foundation (No. ZR2024QB281), the National Natural Science Foundation of China (No. 22276110), and the Youth Innovation Team Development Project of Shandong Universities (No. 2023KJE297).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Mingshuo Zhu is from Shandong Energy Group Co., Ltd. and there is no conflict of interest.
References
- Rockström, J.; Gupta, J.; Qin, D.; Lade, S.J.; Abrams, J.F.; Andersen, L.S.; Armstrong McKay, D.I.; Bai, X.; Bala, G.; Bunn, S.E. Safe and just Earth system boundaries. Nature 2023, 619, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-J.; Huang, G.-X.; Wang, Z.-H.; Duan, Y.; Zhang, Y.-J.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q.; Elimelech, M. Dual-substrate synergistic catalysis for highly efficient water purification. Nat. Water 2025, 3, 345–353. [Google Scholar] [CrossRef]
- Kong, L.; Liu, G.; Liu, Y.; Cai, B.; Zhan, S.; Zhan, J. A bioinspired iron-peroxy species of feroxyhyte for micropollutants oxidation with ultrahigh peroxymonosulfate utilization efficiency. Chem. Eng. J. 2024, 480, 148084. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Qian, J.; Pan, B. Are Free Radicals the Primary Reactive Species in Co(II)-Mediated Activation of Peroxymonosulfate? New Evidence for the Role of the Co(II)–Peroxymonosulfate Complex. Environ. Sci. Technol. 2021, 55, 6397–6406. [Google Scholar] [CrossRef]
- Fang, Q.; Yang, H.; Ye, S.; Zhang, P.; Dai, M.; Hu, X.; Gu, Y.; Tan, X. Generation and identification of 1O2 in catalysts/peroxymonosulfate systems for water purification. Water Res. 2023, 245, 120614. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Fu, L.; Peng, X.; Pan, C.; Mao, Q.; Wang, C.; Yan, J. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective. Sci. Total Environ. 2021, 765, 142794. [Google Scholar] [CrossRef]
- Zhang, S.; Hedtke, T.; Zhu, Q.; Sun, M.; Weon, S.; Zhao, Y.; Stavitski, E.; Elimelech, M.; Kim, J.-H. Membrane-Confined Iron Oxychloride Nanocatalysts for Highly Efficient Heterogeneous Fenton Water Treatment. Environ. Sci. Technol. 2021, 55, 9266–9275. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, M.; Hedtke, T.; Deshmukh, A.; Zhou, X.; Weon, S.; Elimelech, M.; Kim, J.-H. Mechanism of Heterogeneous Fenton Reaction Kinetics Enhancement under Nanoscale Spatial Confinement. Environ. Sci. Technol. 2020, 54, 10868–10875. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Liu, J.; Mine, S.; Matsuoka, M.; Zhang, J.; Xing, M. Designing 3D-MoS2 Sponge as Excellent Cocatalysts in Advanced Oxidation Processes for Pollutant Control. Angew. Chem. Int. Ed. 2020, 59, 13968–13976. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Liu, H.; Qu, J. Confining Free Radicals in Close Vicinity to Contaminants Enables Ultrafast Fenton-like Processes in the Interspacing of MoS2 Membranes. Angew. Chem. Int. Ed. 2019, 58, 8134–8138. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Xu, M.; Feng, S.; Qiu, C.; Li, X.; Li, J. Iron-doped ordered mesoporous Co3O4 activation of peroxymonosulfate for ciprofloxacin degradation: Performance, mechanism and degradation pathway. Sci. Total Environ. 2019, 658, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.; Guo, L.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Hu, P.; Su, H.; Chen, Z.; Yu, C.; Li, Q.; Zhou, B.; Alvarez, P.J.; Long, M. Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation. Environ. Sci. Technol. 2017, 51, 11288–11296. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, Y.; Kang, J.; Wang, S. N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Lim, T.-T. Graphene-and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Fan, Y.; Tong, T.; Han, X.; Du, Y. Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate. Chem. Eng. J. 2018, 336, 721–731. [Google Scholar] [CrossRef]
- Ren, X.; Guo, H.; Feng, J.; Si, P.; Zhang, L.; Ci, L. Synergic mechanism of adsorption and metal-free catalysis for phenol degradation by N-doped graphene aerogel. Chemosphere 2018, 191, 389–399. [Google Scholar] [CrossRef]
- Ruiz-Velducea, H.A.; Moreno-Vásquez, M.d.J.; Guzmán, H.; Esquer, J.; Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Santos-Sauceda, I.; Quintero-Reyes, I.E.; Barreras-Urbina, C.G.; Vásquez-López, C. Valorization of Agave Angustifolia Bagasse Biomass from the Bacanora Industry in Sonora, Mexico as a Biochar Material: Preparation, Characterization, and Potential Application in Ibuprofen Removal. Sustain. Chem. 2024, 5, 196–214. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, C.; Zhao, Y.; Song, C.; Lai, J.; Song, M. Singlet oxygen in biochar-based catalysts-activated persulfate process: From generation to detection and selectivity removing emerging contaminants. Chem. Eng. J. 2024, 485, 149724. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Z.; Zhang, H.; Di, G.; Qiu, Y.; Yin, D.; Wang, S. Hydrochars from pinewood for adsorption and nonradical catalysis of bisphenols. J. Hazard. Mater. 2020, 385, 121548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, X.; Geng, M.; Chen, D.; Lin, H.; Zhang, H. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: Effect of biochar pyrolysis temperature, performance and mechanism. Chem. Eng. J. 2019, 374, 1253–1263. [Google Scholar] [CrossRef]
- Hui, B.; Chen, H.; Zhou, C.; Cai, L.; Zhang, K.; Quan, F.; Yang, D. Biochar aerogel-based electrocatalyst towards efficient oxygen evolution in acidic media. Biochar 2022, 4, 39. [Google Scholar] [CrossRef]
- Ding, C.; Liu, Y.; Xie, P.; Lan, J.; Yu, Y.; Fu, X.; Yang, X.; Zhong, W.-H. A novel carbon aerogel enabling respiratory monitoring for bio-facial masks. J. Mater. Chem. A 2021, 9, 13143–13150. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, S.-J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Chen, D.; Ren, W.; Lin, H.; Zhang, H. Wood-based biochar as an excellent activator of peroxydisulfate for Acid Orange 7 decolorization. Chemosphere 2019, 231, 32–40. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, X.; Huang, C.; Zhou, X.; Lyu, Y.; Lin, Y.; Huang, C.; Ma, W.; Xie, Z.; Fang, G. Investigation of the alkaline hydrogen peroxide pretreatment: From cellulose saccharification to lignin isolation. Ind. Crops Prod. 2024, 214, 118533. [Google Scholar] [CrossRef]
- Wang, C.; Wu, X.; Wang, F.; Xie, F.; Yao, Y. Pine-derived porous carbon for efficient capacitive deionization and the role of its hierarchical pore structure. Sep. Purif. Technol. 2024, 342, 126865. [Google Scholar] [CrossRef]
- Zhu, P.; Yu, Z.; Sun, H.; Zheng, D.; Zheng, Y.; Qian, Y.; Wei, Y.; Lee, J.; Srebnik, S.; Chen, W. 3D Printed cellulose nanofiber aerogel scaffold with hierarchical porous structures for fast solar-driven atmospheric water harvesting. Adv. Mater. 2024, 36, 2306653. [Google Scholar] [CrossRef]
- Shao, P.; Tian, J.; Yang, F.; Duan, X.; Gao, S.; Shi, W.; Luo, X.; Cui, F.; Luo, S.; Wang, S. Identification and regulation of active sites on nanodiamonds: Establishing a highly efficient catalytic system for oxidation of organic contaminants. Adv. Funct. Mater. 2018, 28, 1705295. [Google Scholar] [CrossRef]
- Lu, K.; Min, Z.; Qin, J.; Shi, P.; Wu, J.; Fan, J.; Min, Y.; Xu, Q. Preparation of nitrogen self-doped hierarchical porous carbon with rapid-freezing support for cooperative pollutant adsorption and catalytic oxidation of persulfate. Sci. Total Environ. 2021, 752, 142282. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, C.; Wang, Z.; Ding, H.; Deng, H.; Yang, G.; Li, J.; Zheng, H. Urea-assisted one-step fabrication of a novel nitrogen-doped carbon fiber aerogel from cotton as metal-free catalyst in peroxymonosulfate activation for efficient degradation of carbamazepine. Chem. Eng. J. 2020, 386, 124015. [Google Scholar] [CrossRef]
- Tao, Y.; Hou, Y.; Yang, H.; Gong, Z.; Yu, J.; Zhong, H.; Fu, Q.; Wang, J.; Zhu, F.; Ouyang, G. Interlayer synergistic reaction of radical precursors for ultraefficient 1O2 generation via quinone-based covalent organic framework. Proc. Natl. Acad. Sci. USA 2024, 121, e2401175121. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Zhang, Y.; Han, S.; Ni, S.Q.; Wang, Y.; Boczkaj, G.; Kong, L.; Zhan, J. Probing the Active Nitrogen Species in Nitrogen-Doped Carbon Nanozymes for Enhanced Oxidase-Like Activity. Small 2025, 21, 2411273. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Zhou, X.; Zhang, C.; Chen, L.; Yan, H.; Qin, L.; Huang, D.; Ye, H.; Chen, W. Peroxydisulfate activation by sulfur-doped ordered mesoporous carbon: Insight into the intrinsic relationship between defects and 1O2 generation. Water Res. 2022, 221, 118797. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Nitrogen doping sludge-derived biochar to activate peroxymonosulfate for degradation of sulfamethoxazole: Modulation of degradation mechanism by calcination temperature. J. Hazard. Mater. 2021, 418, 126309. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; Yang, C.; Wu, S.; Fu, X.; Li, X. Calcination temperature regulates non-radical pathways of peroxymonosulfate activation via carbon catalysts doped by iron and nitrogen. Chem. Eng. J. 2023, 451, 138468. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, Y.; Sun, Y.; Ma, J.; Qiao, S.; Wu, Y.; Zhao, B.; Wang, L.; Xu, M.; Wu, Y. N-doped hollow spherical composite derived from coal gasification fine slag through spatial reconstruction for peroxymonosulfate activation. Sep. Purif. Technol. 2025, 354, 129106. [Google Scholar] [CrossRef]
- Kong, L.; Fang, G.; Fang, Z.; Zou, Y.; Zhu, F.; Zhou, D.; Zhan, J. Peroxymonosulfate activation by localized electrons of ZnO oxygen vacancies for contaminant degradation. Chem. Eng. J. 2021, 416, 128996. [Google Scholar] [CrossRef]
- Huang, G.-X.; Wang, C.-Y.; Yang, C.-W.; Guo, P.-C.; Yu, H.-Q. Degradation of bisphenol A by peroxymonosulfate catalytically activated with Mn1. 8Fe1. 2O4 nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Fang, G.; Xi, X.; Wen, Y.; Chen, Y.; Xie, M.; Zhu, F.; Zhou, D.; Zhan, J. A novel peroxymonosulfate activation process by periclase for efficient singlet oxygen-mediated degradation of organic pollutants. Chem. Eng. J. 2021, 403, 126445. [Google Scholar] [CrossRef]
- Yun, E.-T.; Lee, J.H.; Kim, J.; Park, H.-D.; Lee, J. Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation Versus Mediated Electron Transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, M.; Wang, C.; Zhang, M.; Khan, M.A.N.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res. 2019, 148, 416–424. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Zhang, P.-H.; Webster, R.D.; Lim, T.-T. Surface construction of nitrogen-doped chitosan-derived carbon nanosheets with hierarchically porous structure for enhanced sulfacetamide degradation via peroxymonosulfate activation: Maneuverable porosity and active sites. Chem. Eng. J. 2020, 382, 122908. [Google Scholar] [CrossRef]
- Gerritz, L.; Wei, J.; Fang, T.; Wong, C.; Klodt, A.L.; Nizkorodov, S.A.; Shiraiwa, M. Reactive oxygen species formation and peroxide and carbonyl decomposition in aqueous photolysis of secondary organic aerosols. Environ. Sci. Technol. 2024, 58, 4716–4726. [Google Scholar] [CrossRef]
- Zhou, H.; Xiao, L.; Deng, Y.; Chen, C.; Pei, X.; Li, Q.; Ye, Y.; Pan, F. Revisiting the multipath elimination of contaminants by carbonyl-containing manganese-carbon composites in the peroxymonosulfate system: A new way of constructing C-Mn-PMS complexes to distinguish the stages of active species production. Chem. Eng. J. 2023, 471, 144685. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Sun, H.; Ao, Z.; Wang, S.; Liu, S. Understanding of the oxidation behavior of benzyl alcohol by peroxymonosulfate via carbon nanotubes activation. ACS Catal. 2020, 10, 3516–3525. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G. Activation of peroxymonosulfate by chemically modified sludge biochar for the removal of organic pollutants: Understanding the role of active sites and mechanism. Chem. Eng. J. 2020, 392, 123681. [Google Scholar] [CrossRef]
- Zhu, M.; Kong, L.; Xie, M.; Lu, W.; Liu, H.; Li, N.; Feng, Z.; Zhan, J. Carbon aerogel from forestry biomass as a peroxymonosulfate activator for organic contaminants degradation. J. Hazard. Mater. 2021, 413, 125438. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Zhou, J.; Nie, C.; Li, W.; Li, D.; Zhang, Y.; Ao, Z. Insights into Mn-doped biochar induce peroxymonosulfate activation for phenol degradation: The overlooked significance of C-O-Mn. J. Hazard. Mater. 2025, 492, 138031. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, C.; Chen, C.; Liu, B.; Du, J.; Wu, Q.; Feng, X.; Zhan, S.; Guo, W.-Q. Synergistic adsorption and singlet oxygenation of humic acid on alkali-activated biochar via peroxymonosulfate activation. Chin. Chem. Lett. 2025, 36, 110244. [Google Scholar] [CrossRef]
- Tang, F.; Dai, H.; Yang, X.; Li, W.; Wang, B. Nitrogen and sulfur co-doped watermelon rind as an ordered mesoporous biochar activated peroxymonosulfate (PMS) for efficient tetracycline degradation. J. Environ. Chem. Eng. 2024, 12, 112302. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Yuan, M.; Ni, B.-J.; Xia, S.; Zhao, J. Insights into the removal of sulfamethazine and sulfonamide-resistant bacteria from wastewater by Fe-Mn spinel oxide modified cow manure biochar activated peroxymonosulfate: A nonradical pathway regulated by enhanced adsorption and 3d orbital electron reconstruction. Appl. Catal. B Environ. Energy 2025, 361, 124652. [Google Scholar]
- Wang, C.; Tian, J.; Cui, Y.; Li, N.; Cui, X.; Yan, B.; Chen, G. Synergy of CoN3 and CuN3O2 sites in single atom-decorated biochar for peroxymonosulfate activation: Accelerating the production of SO4•− and •OH. Chem. Eng. J. 2024, 496, 154133. [Google Scholar] [CrossRef]
- Akaniro, I.R.; Zhang, R.; Chai, X.; Tsang, C.H.M.; Wang, P.; He, S.; Yang, Z.; Zhao, J. Engineered digestate-derived biochar mediated peroxymonosulfate activation for oxytetracycline removal in sustainable wastewater remediation. Environ. Pollut. 2024, 360, 124640. [Google Scholar] [CrossRef]
- Fang, J.; He, F.; Yan, Z.; Wang, J.; Yu, R.; Zhou, H. Pyrite/biochar-activated peroxymonosulfate strengthens tetracycline degradation: Important roles of surface functional groups and Fe(II)/Fe(III) redox cycling. J. Environ. Chem. Eng. 2024, 12, 112923. [Google Scholar] [CrossRef]
- Pan, M.; He, Z.; Yang, X. Functional biochar accelerates peroxymonosulfate activation for organic contaminant degradation via the specific B–C–N configuration. Chemosphere 2024, 365, 143202. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, T.; Wang, Z.; Cheng, W.; Li, L.; Wang, Y.; Xie, X. Activation of peroxymonosulfate with natural pyrite-biochar composite for sulfamethoxazole degradation in soil: Organic matter effects and free radical conversion. J. Hazard. Mater. 2024, 469, 133895. [Google Scholar] [CrossRef]
- Cheng, L.; Lu, H.; Xu, C.; Meng, J.; Luo, J.; Jiang, J.; Qin, H. The efficient degradation of high concentration norfloxacin by nitrogen, nickel dual-site biochar activated peroxymonosulfate: Performance and mechanism. J. Environ. Chem. Eng. 2025, 13, 116950. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Jiang, Z.; Zhu, K.; An, Q.; Xiao, Z.; Dong, X.; Zhai, S. Photothermal-synergistic peroxymonosulfate activation promoting carbamazepine degradation by Porphyra-derived porous biochar composites: Performance, mechanism, transformation pathway and practical application. Chem. Eng. J. 2024, 489, 151263. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Liu, P.; Bai, X.; Du, X.; Jin, P.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Shi, Y.; Zhang, H.; Deng, H.; Xia, D. Efficient activation of peroxymonosulfate by N-doped waste herb senna obtusifolia biochar for degrading NPX: Synergistic effect of carbonyl and nitrogen sites. J. Environ. Manag. 2024, 371, 123207. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, X.; Ye, X.; Li, Q.; Wang, J.; Wu, L.; Huang, Z.-H.; Wang, M.-X. Activating peroxymonosulfate by high nitrogen-doped biochar from lotus pollen for efficient degradation of organic pollutants from water: Performance, kinetics and mechanism investigation. Sep. Purif. Technol. 2024, 346, 127456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).