Abstract
To date, two-dimensional metal–organic frameworks (2D MOFs) have attracted much attention in many fields. Owing to their ultra-high porosity and specific surface area, great structural diversity and functional tunability, as well as feasible precision design at the molecular level, 2D MOFs have won rapid development in the field of energy storage. However, as a coordination compound, MOFs possess poor structural stability and are prone to structural collapse in electrochemical reactions, which seriously limits their electrochemical performance. Therefore, there is an urgent need to improve the structural stability of MOF electrode materials. In this study, a 2D MOF@Ti3C2TX hybrid was constructed, in which urea pyrimidinone isocyanate (UPy-NCO) units were introduced via a condensation reaction with the active functional groups on MOFs, thus forming multiple hydrogen bonds among MOF frameworks to strengthen their structural stability. Importantly, 2,6-diaminopyridine was utilized to modulate the structure and properties. Initially, the mono-coordination model of the N atom on a pyridine ring with metal ions could create defects and form further pores. Two −NH2 groups helped to improve the grafting reaction degree of UPy-NCO, leading to an increased ratio of forming quadruple hydrogen bonds (H-bonds), further strengthening the structure of the hybrid. As expected, the Cu-MOF@Ti3C2TX-20%DAP-UPy hybrid exhibited a specific capacitance of 148 F g−1 at 1 A g−1, which is 45% higher than that of Cu-MOF@Ti3C2TX-UPy (102 F g−1). A good capacitance retention of 88% was obtained as the current density increased from 0.2 to 5 A g−1. Moreover, excellent cycling stability (91.1%) was obtained at 1 A g−1 after 5000 cycles.
1. Introduction
With the rapid development of portable electronics, supercapacitors have attracted much attention due to their fast charging and discharging, high power density, green environmental protection, and long lifetime [1,2,3,4,5,6]. Due to their ultra-high porosity, large specific surface area, and structural tunability [7,8,9,10,11,12,13,14,15], MOFs have received widespread attention in supercapacitor and other energy-storage applications [16,17,18,19]. In particular, owing to their exposed active sites and higher electrical conductivity, as well as regular and well-ordered pore structure [20,21,22,23,24,25,26,27], 2D MOFs are more conducive to achieving high performance as electrode materials. However, the weak coordination bonds between metal ions and organic ligands make MOF frameworks unstable and even prone to collapse, leading to poor cycling stability [28,29,30,31,32,33,34], thus restricting their practical applications. Therefore, enhancing the cycling stability of MOF energy-storage materials needs to be addressed urgently.
Self-healing ability provides a novel design idea for improving the service life of energy storage devices. Currently, two self-healing categories are mainly investigated: externally assisted self-healing and intrinsic self-healing [35,36,37]. Externally assisted self-healing is realized by incorporating self-healing agents into the materials [38,39,40]. For example, Guo [41] et al. investigated epoxy matrix microencapsulated self-healing materials. However, most microcapsule-based self-repairs exhibit only onetime self-repair properties, which severely limits their practical applications. Intrinsic self-healing materials can repair by themselves through the introduction of reversible chemical bonds [37,41,42,43,44,45] (e.g., boronic acid diester bonds, hydrogen bonds, etc.). For example, in 2023, Wang [45] et al. sprayed hydrophobically modified CNT and NiO/CoO nanoparticles onto the surface of a hydrogel (PVA) to construct electrodes, which utilized the dynamic hydrogen bonds formed between PVA and HCl through physical cross-linking to provide the self-healing ability. The as-prepared supercapacitor achieved a capacitance retention of 75.4% after 2000 cycles. Both the introduction of self-healing agents and reversible chemical bonds can give energy storage devices self-healing abilities, which is important for prolonging their service life [46,47,48,49,50,51]. However, traditional self-healing materials are usually polymers, which are not electrochemically active and thus unfavorable for producing capacitance, leading to the decreased electrochemical performance of electrodes or energy storage devices [52,53,54,55,56,57,58].
We have proposed an innovative strategy to enhance the structural stability of MOFs by introducing quadruple hydrogen bonds directly into MOF frameworks [23]. Here, to further enhance the structural stability and performance of MOF-based electrodes, a modulated linker, 2,6-diaminopyridine, was utilized. On the one hand, the mono-coordination model of the N atom on a pyridine ring with metal ions could produce defects and form further pores. On the other hand, the two −NH2 groups help to improve the grafting reaction degree of UPy-NCO units, improving the ratio of forming quadruple hydrogen bonds, further strengthening their structure stability.
2. Materials and Methods
2.1. Preparation of Samples
The materials, reagents, and synthesis of Ti3C2TX, copper oxide nanosheets (Cu-ONS), Cu-MOF@Ti3C2TX, and UPy-NCO are displayed in the Supporting Information (SI).
Synthesis of Cu-MOF@Ti3C2TX-DAP: H2BDC-NH2 (30 mg), 2,6-diaminopyridine (0, 3, 6, 9 mg), and PVP (50 mg) were dissolved in a mixed solvent containing 1.1 mL of DMF, 1.1 mL of deionized (DI) water, and 1.1 mL of ethanol, obtaining a mixed solution. Cu-ONS (25 mg) was dispersed in 5 mL of DI water under stirring, and then Ti3C2TX (10 mg) was added, forming another solution. Then, the two solutions were together transferred to a reactor and remained at 100 °C for 20 h. Lastly, the sample was washed and dried, and denoted as Cu-MOF@Ti3C2TX-X%DAP (X = 10, 20 and 30).
Synthesis of Cu-MOF@Ti3C2TX-DAP-UPy: 40 mg of Cu-MOF@Ti3C2TX-DAP was dissolved in 20 mL of DMF and 30 mg of UPy-NCO was dissolved in 10 mL of DMF. Then, the two solutions were mixed, followed by the addition of 2 drops of dibutyltin dilaurate. The mixed solution was maintained at 90 °C for 20 h under stirring. Finally, the sample was washed with ethanol, dried at 40 °C, and denoted as Cu-MOF@Ti3C2TX-DAP-UPy, as described in Scheme 1.
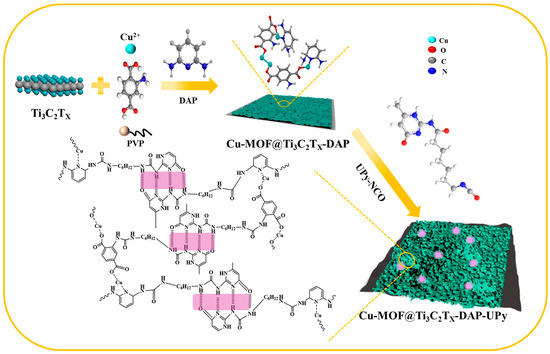
Scheme 1.
Schematic illustration of Cu-MOF@Ti3C2TX-DAP-UPy.
2.2. Electrochemical Characterization
The working electrode material consisted of acetylene black, polyvinylidene chloride (PVDF) and active material. The three materials were mixed (mass ratio of 8:1:1) with N-methylpyrrolidone to form a homogeneous paste. Then, the paste was coated on a piece of Ni foam (1 × 1 cm2) current collector, followed by a drying process at 80 °C for 12 h, thus obtaining a working electrode (the loading amount was 1.5~2 mg cm−2).
An electrochemical workstation (CHI760E) was used to perform electrochemical experiments including cyclic voltammetry (CV), constant current charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS). A three-electrode system was used to investigate the performance of single electrodes, in which the working electrode, counter electrode, and reference electrode were the as-prepared material, Pt plate, and Hg/HgO, respectively. A 1 M KOH aqueous solution was used as the electrolyte. The capacitance (C, F g−1) values were evaluated by calculating the integral of the area under the charging and discharging part of the curves as follows [48]:
where I (A g−1) is the current density, Δ t (s) is the discharge time, m (g) is the mass of the active material, and ΔV (V) is the potential.
In addition, the energy density (E, Wh kg−1), power density (P, W kg−1) and Coulombic Efficiency (CE) of the prepared device was investigated using the following formulae [56]:
where Δt (s) is the discharge time, ΔV (V) is the potential window, Id (A) is the discharge current, td (s) is the discharge time, Ic (A) is the charge current, and tc (s) is the charge time.
3. Results
3.1. Structural Characterization
The SEM images of the samples are shown in Figure 1 and Figure S1. As displayed in Figure S1a, lots of nanosheets with a size of about 1 μm were observed in Cu-MOF@Ti3C2TX. With the addition of 2,6-diaminopyridine, the microstructure of the samples changed obviously. The size of the lamellar structure became smaller with the increasing 2,6-diaminopyridine content. The mono-coordination model of the N atom on the pyridine ring with metal ions created defects and formed further pores, leading to the decrease in size of the nanosheets (Figure S1b,c). For Cu-MOF@Ti3C2TX-30%DAP, lots of irregular fragments can be seen (Figure S1d), caused by the large number of defects in the nanosheets due to the excess of 2,6-diaminopyridine [59]. After the UPy-NCO units were grafted via a condensation reaction with the −NH2 active groups on the MOFs, the morphology of the Cu-MOF@Ti3C2TX-DAP-UPy samples (Figure 1) were further changed. Especially, the Cu-MOF@Ti3C2TX-30%DAP-UPy (Figure 1c) exhibited a completely different morphology compared with Cu-MOF@Ti3C2TX-30%DAP (Figure S1d), showing a thinner layer and cross-linked network structure. As the amount of 2,6-diaminopyridine increased up to 30%, more −NH2 active groups were introduced onto the MOFs, leading to an improvement in the grafting reaction degree of UPy-NCO and a further increase in the ratio of forming quadruple hydrogen bonding, subsequently promoting the formation of a cross-linked network structure. The EDS elemental analysis (Figure 1d) shows that the elements, N, O, Cu, and Ti, are uniformly distributed in Cu-MOF@Ti3C2TX-20%DAP-UPy sample.
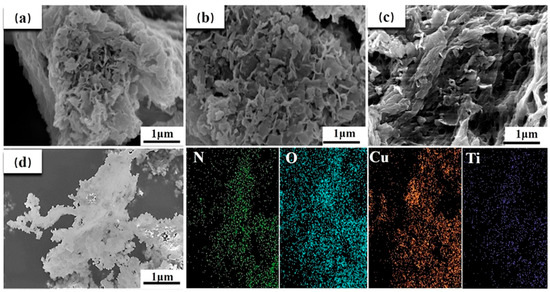
Figure 1.
SEM images of the samples. (a) Cu-MOF@Ti3C2TX-10%DAP-UPy, (b) Cu-MOF@Ti3C2TX- 20%DAP-UPy, (c) Cu-MOF@Ti3C2TX-30%DAP-UPy, and (d) EDS images of Cu-MOF@Ti3C2TX-20%DAP-UPy with the corresponding elemental distributions of N, O, Cu and Ti.
The N2 adsorption–desorption tests were performed to investigate the pore characteristics of the prepared materials, as shown in Figure 2. All the samples exhibit type IV curves (Figure 2a), indicating the co-existence of micropores and mesopores [60]. The hierarchical pores, including micropores (1.5 nm), mesopores (8, 17, 37 and 50 nm), and macropores (50–86 nm), are clearly displayed in Figure 2b,c. Most importantly, an 8 nm mesopore can be noticed in both Cu-MOF@Ti3C2TX-20%DAP and Cu-MOF@Ti3C2TX-20%DAP-UPy, which might be due to the defect originating from the mono-coordination model between 2,6-diaminopyridine and Cu2+. Moreover, the specific surface areas of Cu-MOF@Ti3C2TX, Cu-MOF@Ti3C2TX-20%DAP, and Cu-MOF@Ti3C2TX-20%DAP-UPy are 43.1, 27.4, and 14.6 m2 g−1, respectively. As a modulated linker, 2,6-diaminopyridine participated in the growth of MOFs, as well as the grafting reactions with UPy-NCO units, resulting in a decrease in the proportion of 1.6 nm micropores, thus causing a decrease in the specific surface area.
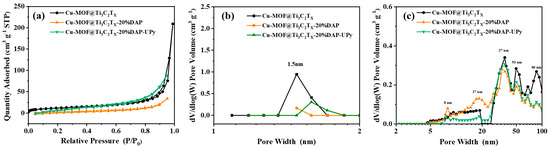
Figure 2.
(a) N2 adsorption–desorption isotherms, (b) DFT pore-size distribution curves and (c) BJH pore-size distribution curves of the samples.
The XRD patterns of the samples are displayed in Figure S2. Compared with Cu-MOF@Ti3C2TX-UPy, the samples containing 2,6-diaminopyridine exhibit new diffraction peaks at 2θ of 5.9°, 11.9°, 25.3°, 26.8°, and 43.2°, which is due to the formation of new crystal structures stemming from the coordinate reactions between Cu2+ and N atoms on the pyridine rings [61].
Figure 3a shows the FT-IR spectra of the samples. For Cu-MOF@Ti3C2TX, the two peaks at 3237 and 3142 cm−1 are ascribed to the −NH2 stretching vibrations [62], while the strong and sharp characteristic peak at 1689 cm−1 is caused by the C=O stretching vibrations. For the samples grafted with UPy-NCO, several obvious changes can be noticed in the peaks. First, the broad peak at 3454 cm−1 associated with the −OH is strengthened in intensity, which is attributed to the formation of intermolecular H-bonds (C=O···H−N). Second, the characteristic peaks at 2936 and 2861 cm−1 can be noticed, which are caused by the −CH2 stretching vibrations, implying that the UPy-NCO units were successfully grafted onto the MOFs. Most importantly, the characteristic peak corresponding to the C=O stretching vibrations exhibited a redshift in wavenumber and presented a blunt shape in profile, which is also associated with the intermolecular H−bonds (C=O···H−N). As the content of 2,6-diaminopyridine increased, the characteristic peak of C=O gradually became broader, proving that more hydrogen bonds were formed. Particularly, for Cu-MOF@Ti3C2TX-30%DAP-UPy a broad adsorption band at around 1640–1380 cm−1 was formed, caused by the peak shift of C=O, as well as the peak overlap of different groups including C=C and C=N. It is worth stressing that the −NH bonds are also an important component taking part in the formation of quadruple hydrogen bonds, which can be further identified by the variable FT-IR spectra (Figure 3b). When the temperature was increased from 25 °C to 125 °C, with the dissociation of hydrogen bonds, the peak associated with the stretching vibration of the −NH bonds shifted slightly from 3147.8 cm−1 to 3152.7 cm−1 [63,64].
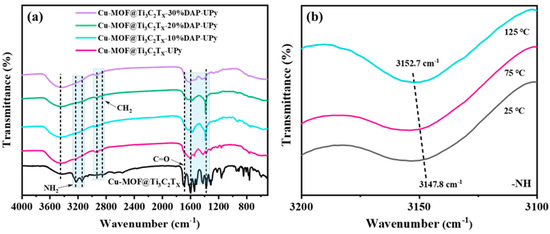
Figure 3.
(a) FT−IR spectra of samples and (b) temperature-variable FT-IR spectra of Cu-MOF@Ti3C2TX-20%DAP-UPy.
Figure 4 shows the XPS spectra of Cu-MOF@Ti3C2TX and Cu-MOF@Ti3C2TX-20%DAP-UPy. As displayed in Figure 4a, with the introduction of 2,6-diaminopyridine, the peak intensity of the N element in Cu-MOF@Ti3C2TX-20%DAP-UPy is highlighted compared to that of Cu-MOF@Ti3C2TX, which is attributed to the increase in grafting density of the UPy-NCO units. Moreover, in the N 1s spectra of Cu-MOF@Ti3C2TX-20%DAP-UPy (Figure 4b), the peak corresponding to the Cu−N (399.3–399.7 eV) can be observed, further confirming the coordinate reaction between the N atoms on the pyridine rings and Cu2+ ions. The other two peaks are assigned to C−N on pyridine (400.3–400.7 eV), and C−N formed on the amide bond (398.3–399 eV) [65].
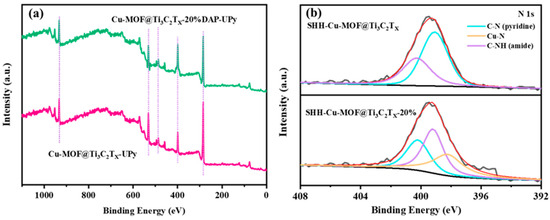
Figure 4.
XPS spectra of samples: (a) survey spectrum, (b) high-resolution XPS spectra of N 1s.
3.2. Electrochemical Performance
The electrochemical properties of the prepared samples are shown in Figure 5. The CV curves of different samples at a scan rate of 50 mV s−1 are shown in (Figure 5a). Obvious redox peaks can be observed in CV curves, which stems from the redox reactions of Cu−O clusters in the Cu-MOF as follows:
Cu−O + OH− ↔ CuOOH + e−
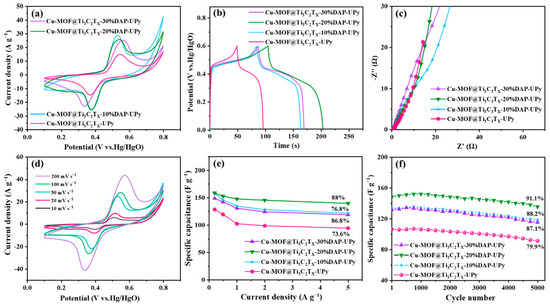
Figure 5.
Electrochemical performances of samples: (a) CV profiles at 50 mV s−1, (b) GCD profiles at 1 A g−1, (c) Nyquist plots, (d) CV profiles of Cu-MOF@Ti3C2TX-20%DAP-UPy, (e) specific capacitances at various current densities, and (f) cycling performances at 1 A g−1.
Moreover, the introduction of Ti3C2TX could also produce pseudo-capacitance [66,67]. The combination with non-ideal triangles in the GCD curves (Figure 5b) suggests the two electrochemical behaviors coexist: pseudo-capacitance and double-layer capacitance. Moreover, the longer discharging times indicate that the samples with the addition of 2,6-diaminopyridine exhibit higher capacitances compared with Cu-MOF@Ti3C2TX-UPy. As mentioned above, the mono-coordination model of the N atom on the pyridine ring with Cu2+ ions could create defects and form further pores, exposing more active sites and facilitating redox reactions, leading to enhanced capacitances. However, abundant defects and pores prolong the ion transportation path and reduce the ion diffusion rate, which can be demonstrated by the lower slope of the straight lines in the low-frequency region (Figure 5c). Notably, the Cu-MOF@Ti3C2TX-20%DAP-UPy exhibits the best electrochemical performance, delivering a specific capacitance of 148 F g−1 at 1 A g−1. With the increased scan rates (Figure 5d), similar shapes of CV curves were maintained, indicating good reversibility.
The calculated capacitances investigating the effect of 2,6-diaminopyridine on the performances of the samples are shown in Figure 5e. With the current density increased from 0.2 to 5 A g−1, the capacitance retentions of Cu-MOF@Ti3C2TX-UPy, Cu-MOF@Ti3C2TX-10%DAP-UPy, Cu-MOF@Ti3C2TX-20%DAP-UPy, and Cu-MOF@Ti3C2TX-30%DAP-UPy are 73.6%, 76.8%, 88%, and 86.8%, respectively. Furthermore, the cycling performances were investigated, as shown in Figure 5f. After 5000 cycles, the capacitance retentions of the above samples are 79.9%, 88.2%, 91.1% and 87.1%, respectively. The participation of 2,6-diaminopyridine not only improves the storage capacitance of the samples, but also optimizes their rate performance and cycle stability. The −NH2 group on 2,6-diaminopyridine helps to facilitate the grafting reaction of UPy-NCO, and promotes the formation of quadruple hydrogen bonding, thus strengthening the structural stability of the hybrid. However, excessive 2,6-diaminopyridine accordingly produces more defects, weakening the crystal structure of the MOF; thus, the Cu-MOF@Ti3C2TX-30%DAP-UPy shows a decrease in the rate and cycle performances. As shown in Figure 6, compared with the other two samples, the Cu-MOF@Ti3C2TX-20%DAP-UPy presents a relatively complete surface structure. The Cu-MOF@Ti3C2TX-20%DAP-UPy exhibits a high capacitance retention of 91.1% after 5000 cycles, which is superior to some other MOF-based electrodes in the literature (e.g., Table 1).

Figure 6.
SEM images of (a) Cu-MOF@Ti3C2TX-10%DAP-UPy, (b) Cu-MOF@Ti3C2TX-20%DAP-UPy, and (c) Cu-MOF@Ti3C2TX-30%DAP-UPy electrodes after cycling tests.

Table 1.
Cycle stability of MOF-based electrode materials.
The kinetic mechanism of the as-obtained hybrid was investigated by using the b-value model as follows [69]:
where i is the peak current and v is the scan rate. Especially, b is an adjustable parameter and has two critical values of 0.5 and 1.0, referring to the diffusive-controlled behavior and surface capacitive-controlled behavior, respectively.
i = aνb
In addition, to reveal the energy-storage mechanism in detail the Dunn’s model was also used, in which the contributions of diffusive-controlled behavior and surface capacitive-controlled behavior are descripted as follows [70]:
where k1ν and k2ν0.5 correspond to the capacitive and diffusion-controlled currents, respectively. As shown in Figure 7a, for the Cu-MOF@Ti3C2TX-20%DAP-UPy, the calculated b values of the oxidation and reduction peaks are 0.66 and 0.74, respectively, indicating that the charge storage process includes both diffusive-controlled behavior and surface capacitive behavior. Combining the calculated contributions of the two charge storage behaviors (Figure 7b,c), it is obvious that the diffusive contribution is superior to the surface capacitive contribution even at 50 mV s−1. The formation of defects derived from the coordination reaction between 2,6-diaminopyridine and Cu2+ ions could promote ions to thoroughly penetrate materials, making full use of active sites to produce more capacitance. With the increased scan rates, the ions cannot diffuse sufficiently inside active materials in time, thus the diffusive-controlled proportion decreased while the surface capacitive contribution increased.
i(V) = k1ν + k2ν0.5
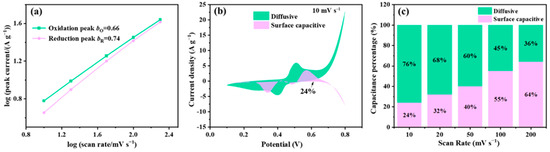
Figure 7.
(a) Plots of log(i) against log(ν), (b) surface capacitive and diffusive contributions at scan rate of 10 mV s−1, and (c) capacitance contribution ratios.
3.3. Electrochemical Properties of ASC Device
An asymmetric supercapacitor (Cu-MOF@Ti3C2TX-20%DAP-UPy//AC) was assembled using Cu-MOF@Ti3C2TX-20%DAP-UPy as the positive electrode, activated carbon (AC) (Figure S3) as the negative electrode, and 1 M KOH aqueous solution as the electrolyte, respectively.
The CV curves of the device under different voltage windows from 0–1.0 to 0–1.8 V are displayed in Figure 8a. When the voltage window was set as 1.7–1.8 V, a slight polarization phenomenon can be observed, thus the 0–6 V was chosen as the appropriate working voltage. The CV curves at different scan rates are shown in Figure 8b, exhibiting similar shapes without obvious deformation, indicating that the device possesses good capacitive behavior even under fast charging/discharging. Based on the GCD curves of the device (Figure 8c), the specific capacitances were calculated and are displayed in Figure 8d. At 0.5 A g−1, the device delivers a specific capacitance of 50 F g−1, exhibiting a capacitance retention of 85% as the current density was increased to 5 A g−1. A maximum energy density of 17.9 W h kg−1 was obtained (Figure 8e), which is superior to that of some reported MOF-based devices [64,74,75,76,77,78]. The cycle stability of the device is shown in Figure 8f: a capacitance retention of 85% was delivered after 5000 cycles at 2 A g−1. Moreover, the calculated coulombic efficiency was 90%.
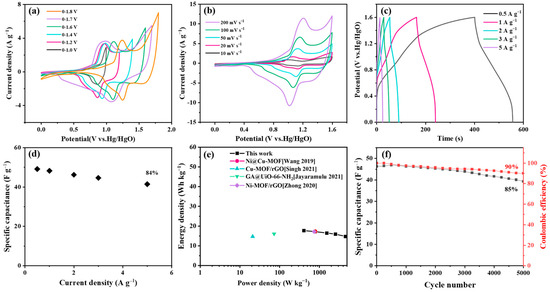
Figure 8.
Electrochemical performance of Cu-MOF@Ti3C2TX-20%DAP-UPy//AC device. (a) CV profiles at various voltage windows at scan rate of 50 mV s−1, (b) CV profiles at various scan rates, (c) GCD curves under different current densities, (d) specific capacitances at different current densities, (e) Ragone plots [72,73,74,75], (f) cycling stability and coulombic efficiency at current density of 2 A g−1.
4. Discussion
To enhance the structural stability of MOF-based electrodes, we have explored a strategy of introducing quadruple hydrogen bonds onto MOFs. Here, to further promote the introduction of quadruple hydrogen bonds and optimize the performance of 2D MOF electrodes, 2,6-diaminopyridine was used as a modulated linker to participate in the growth of MOF and the grafting reaction of UPy-NCO. Due to the mono-coordination reaction between the pyridine ring and Cu2+ ions, new mesopores (8–20 nm) were introduced, enriching the pore architecture of the MOF@Ti3C2TX hybrid. Profiting from the enhanced grafting reaction between the −NH2 groups and UPy-NCO units, quadruple hydrogen bonds among frameworks were increased, leading to the formation of hierarchical cross-linked networks. The as-obtained Cu-MOF@Ti3C2TX-20%DAP-UPy presented a capacitance of 158 F g−1 at 0.2 A g−1, delivering a capacitance retention of 88% at 5 A g−1, while the Cu-MOF@Ti3C2TX-UPy exhibited a capacitance of 128 F g−1 at 0.2 A g−1, presenting a 73.6% retention at 5 A g−1. Furthermore, the Cu-MOF@Ti3C2TX-20%DAP-UPy delivered a 91.1% capacitance retention after 5000 cycles, which is much superior to that of Cu-MOF@Ti3C2TX-UPy (79.9%), presenting an apparent increase in cycling performance. The prepared ASC device presented a specific capacitance of 50 F g−1 at 0.2 A g−1, an energy density of 17.9 Wh kg−1, and a capacitance retention of 85% after 5000 cycles at 2 A g−1. Our strategy not only provides ideas for designing novel and efficient MOFs, but also provides innovative ideas for exploring novel porous frameworks with self-healing ability.
5. Conclusions
In this study, the structural stability and electrochemical performance of two-dimensional MOF electrodes were optimized by introducing a quadruple hydrogen bonding strategy; 2,6-diaminopyridine (DAP) was employed as a moderating ligand involved in MOF growth, and the quadruple hydrogen bonding network was strengthened by the grafting reaction of its amino group with UPy-NCO. It was found that the mono-coordination of the pyridine ring with Cu2⁺ formed 8–20 nm new mesopores, which enriched the pore structure of MOF@Ti3C2TX composites; the modified material, Cu-MOF@Ti3C2TX-20% DAP-UPy, reached a specific capacity of 15.5 mm at 0.2 A g−1 with a specific capacity of 158 F g−1 (23% enhancement), 88% capacity retention at 5 A g−1 (control 73.6%), and 91.1% capacity retention after 5000 cycles (control 79.9%); and the assembled asymmetric supercapacitor (ASC) had an energy density of 17.9 Wh kg−1 and 85% capacity retention after 5000 cycles. This strategy not only improves the conductivity and cycling stability of MOF electrodes by constructing a hierarchical cross-linking network, but also provides an innovative idea for the design of porous framework materials with a self-healing function.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15110864/s1, Figure S1. SEM images of the samples. Figure S2. XRD patterns of the samples. Figure S3. (a) CV curves of AC and Cu-MOF@Ti3C2TX-20%DAP-UPy at 50 mV s−1 and (b) GCD curve of AC at 1 A g−1.
Author Contributions
Methodology, F.L. and P.Y.; Validation, B.Y., Q.C., M.Y., Y.N. and M.Z.; Writing—original draft, X.Q.; Writing—review & editing, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (52073126, 52173020).
Data Availability Statement
The data presented in the study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Du, M.; Li, Q.; Zhao, Y.; Liu, C.-S.; Pang, H. A review of electrochemical energy storage behaviors based on pristine metal–organic frameworks and their composites. Coord. Chem. Rev. 2020, 416, 41. [Google Scholar] [CrossRef]
- Khalil, I.E.; Fonseca, J.; Reithofer, M.R.; Eder, T.; Chin, J.M. Tackling orientation of metal-organic frameworks (MOFs): The quest to enhance MOF performance. Coord. Chem. Rev. 2023, 481, 215043. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Li, F.; Xiao, X.; Xu, Q. Metal-organic-framework-based materials as platforms for energy applications. Chem 2024, 10, 86–133. [Google Scholar] [CrossRef]
- Kang, L.; Liang, Q.; Abdul, Q.; Rashid, A.; Ren, X.; Ma, H. Preparation technology and preservation mechanism of γ-CD-MOFs biaological packaging film loaded with curcumin. Food Chem. 2023, 420, 136142. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, X.; Xu, X.; Zhang, W.; Zou, X.; Shi, J.; Zheng, K.; Arslan, M. A portable test strip based on fluorescent europium-based metal–organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Wang, X.; Guo, Y.; Ni, C.; Wu, J.; Hao, C. High performance hybrid supercapacitors assembled with multi-cavity nickel cobalt sulfide hollow microspheres as cathode and porous typha-derived carbon as anode. Ind. Crops Prod. 2022, 189, 115863. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Zhang, X.; Li, W.; Guo, Z.; Huang, X.; Li, Z.; Zhang, R.; Shen, T.; Zou, X.; et al. Ratiometric Sensing for Ultratrace Tetracycline Using Electrochemically Active Metal–Organic Frameworks as Response Signals. J. Agric. Food Chem. 2023, 71, 7584–7592. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Li, Y.; Zhang, L.; Bi, N.; Gou, J.; Zhu, T.; Jia, L. A novel intelligently integrated MOF-based ratio fluorescence sensor for ultra-sensitive monitoring of TC in water and food samples. Food Chem. 2023, 405, 134899. [Google Scholar] [CrossRef]
- Wang, K.-B.; Xun, Q.; Zhang, Q. Recent progress in metal-organic frameworks as active materials for supercapacitors. EnergyChem 2020, 2, 100025. [Google Scholar] [CrossRef]
- Shao, G.; Yu, R.; Chen, N.; Ye, M.; Liu, X.Y. Stretchable Supercapacitors: From Materials and Structures to Devices. Small Methods 2021, 5, 2000853. [Google Scholar] [CrossRef] [PubMed]
- Linares-Moreau, M.; Brandner, L.A.; Velásquez-Hernández, M.d.J.; Fonseca, J.; Benseghir, Y.; Chin, J.M.; Maspoch, D.; Doonan, C.; Falcaro, P. Fabrication of Oriented Polycrystalline MOF Superstructures. Adv. Mater. 2024, 36, 2309645. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Geng, P.; Zhang, G.; Li, X.; Pang, H. Synthesis of Conductive MOFs and Their Electrochemical Application. Small 2024, 20, 2308264. [Google Scholar] [CrossRef]
- Pramanik, B.; Sahoo, R.; Das, M.C. pH-stable MOFs: Design principles and applications. Coord. Chem. Rev. 2023, 493, 215301. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Z.; Wang, X.; Liu, X.; Kapteijn, F. Water Adsorption in MOFs: Structures and Applications. Adv. Funct. Mater. 2024, 34, 2304788. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, L.; Wang, Y.; Qiu, S.; Luo, Y.; Zou, Y.; Xu, F.; Sun, L.; Chu, H. Recent progress on construction and applications of metal-organic frameworks-based materials for lithium-ion batteries and supercapacitors. Carbon Neutralization 2024, 3, 396–414. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Guo, X.; Pang, H. Electrospun metal–organic framework nanofiber membranes for energy storage and environmental protection. Adv. Fiber Mater. 2022, 4, 1463–1485. [Google Scholar] [CrossRef]
- Sun, R.; Dou, M.; Chen, Z.; Wang, R.; Zheng, X.; Zhang, Y.; Zhou, C.; Menezes, P.W. Engineering strategies of metal-organic frameworks toward advanced batteries. Battery Energy 2023, 2, 20220064. [Google Scholar] [CrossRef]
- Wu, X.-M.; Liu, M.-M.; Guo, H.-X.; Ying, S.-M.; Chen, Z.-X. Polyoxovanadate-based MOFs microsphere constructed from 3-D discrete nano-sheets as supercapacitor. Chin. J. Struct. Chem. 2021, 40, 994–998. [Google Scholar]
- Kumar, Y.A.; Vignesh, S.; Ramachandran, T.; Kumar, K.D.; Al-Sehemi, A.G.; Moniruzzaman, M.; Oh, T.H. Solidifying the future: Metal-organic frameworks in zinc battery development. J. Energy Storage 2024, 97, 112826. [Google Scholar] [CrossRef]
- Huang, M.; Liang, Z.; Huang, J.; Wen, Y.; Zhu, Q.-L.; Wu, X. Introduction of Multicomponent Dyes into 2D MOFs: A Strategy to Fabricate White Light-Emitting MOF Composite Nanosheets. ACS Appl. Mater. Interfaces 2023, 15, 11131–11140. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.Z.; Shaheen, M.; Khan, M.W.; Siddique, S.; Farid, S.; Aftab, S.; Wabaidur, S.M. The rise of 2D conductive metal-organic framework: Cu3(HHTP)2 d-π MOF for integrated battery-supercapacitor hybrids. Mater. Today Sustain. 2023, 22, 100331. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Ye, P.; Zhu, M.; Nie, Y.; Dai, Y.; Yang, F. Construction of Battery-Like Hierarchical MOF@MXene Heterostructures for Hybrid Supercapacitors. Cryst. Growth Des. 2024, 24, 7445–7454. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Que, M.; Zheng, H.; Yang, L.; Yuan, H.; Ma, Y.; Li, Y.; Yang, X. 2D Ti3C2T MXene/MOFs composites derived CoNi bimetallic nanoparticles for enhanced microwave absorption. Chem. Eng. J. 2022, 450, 138442. [Google Scholar] [CrossRef]
- Hussain, N.; Abbas, Z.; Ansari, S.N.; Kedarnath, G.; Mobin, S.M. Phosphorization Engineering on a MOF-Derived Metal Phosphide Heterostructure (Cu/Cu3P@NC) as an Electrode for Enhanced Supercapacitor Performance. Inorg. Chem. 2023, 62, 17083–17092. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Chen, Q.; Ouyang, Q. Multifunctional Metal–Organic Frameworks Driven Three-Dimensional Folded Paper-Based Microfluidic Analysis Device for Chlorpyrifos Detection. J. Agric. Food Chem. 2024, 72, 14375–14385. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, D.; Liu, W.; Su, J. Recent advances in all-in-one flexible supercapacitors. Sci. China Mater. 2020, 64, 27–45. [Google Scholar] [CrossRef]
- Li, S.; Cheng, Q.; Ye, P.; Zhang, Y.; Zhang, L.; Liu, F.; Qiu, H.; Qu, X.; Nie, Y. Hierarchical two-dimensional Ti-MOF derived from MXene for hybrid supercapacitor electrodes. Appl. Organomet. Chem. 2024, 38, e7547. [Google Scholar] [CrossRef]
- Ghosh, A.; Fathima Thanutty Kallungal, S.; Ramaprabhu, S. 2D Metal-Organic Frameworks: Properties, Synthesis, and Applications in Electrochemical and Optical Biosensors. Biosensors 2023, 13, 123. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, F.-Z.; Han, X.; Xu, J.; Bu, X.-H. Recent Progress in 2D Metal-Organic Frameworks for Optical Applications. Adv. Opt. Mater. 2020, 8, 2000110. [Google Scholar] [CrossRef]
- Zhang, H.; Mei, H.; Qin, D.; Li, Z.; Hou, Z.; Lu, X.; Xu, B.; Sun, D. Conversion of Amorphous MOF Microspheres into a Nickel Phosphate Battery-Type Electrode Using the “Anticollapse” Two-Step Strategy. Inorg. Chem. 2021, 60, 17094–17102. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, X.; Li, W.; Liang, N.; Hu, X.; Xiao, J.; Wang, D.; Zou, X.; Shi, J. An intrinsic dual-emitting fluorescence sensing toward tetracycline with self-calibration model based on luminescent lanthanide-functionalized metal-organic frameworks. Food Chem. 2023, 400, 133995. [Google Scholar] [CrossRef]
- Odarczenko, M.; Thakare, D.; Li, W.; Venkateswaran, S.P.; Sottos, N.R.; White, S.R. Sunlight-Activated Self-Healing Polymer Coatings. Adv. Eng. Mater. 2020, 22, 1901223. [Google Scholar] [CrossRef]
- Platonova, E.O.; Vlasov, E.; Pavlov, A.A.; Kireynov, A.; Nelyub, V.A.; Polezhaev, A.V. Self-healing polyurethane based on a difuranic monomer from biorenewable source. J. Appl. Polym. Sci. 2019, 136, 47869. [Google Scholar] [CrossRef]
- Chang, K.; Jia, H.; Gu, S.-Y. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur. Polym. J. 2019, 112, 822–831. [Google Scholar] [CrossRef]
- Guo, M.; Pitet, L.M.; Wyss, H.M.; Vos, M.; Dankers, P.Y.W.; Meijer, E.W. Tough Stimuli-Responsive Supramolecular Hydrogels with Hydrogen-Bonding Network Junctions. J. Am. Chem. Soc. 2014, 136, 6969–6977. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Ahmed, J.; Tian, W.; Li, L. Flexible perovskite photodetector with room-temperature self-healing capability without external trigger. InfoMat 2024, 6, e12594. [Google Scholar] [CrossRef]
- Liu, H.; Huang, H.; Wu, X.; Peng, H.; Li, Z.; Hu, J.; Yu, Q. Effects of external multi-ions and wet-dry cycles in a marine environment on autogenous self-healing of cracks in cement paste. Cem. Concr. Res. 2019, 120, 198–206. [Google Scholar] [CrossRef]
- Guo, M.; Li, W.; Han, N.; Wang, J.; Su, J.; Li, J.; Zhang, X. Novel Dual-Component Microencapsulated Hydrophobic Amine and Microencapsulated Isocyanate Used for Self-Healing Anti-Corrosion Coating. Polymers 2018, 10, 319. [Google Scholar] [CrossRef]
- Li, H.; Xin, L.; Gao, J.; Shao, Y.; Zhang, Z.; Ren, L. Underwater Bionic Self-Healing Superhydrophobic Coating with the Synergetic Effect Of Hydrogen Bonds and Self-Formed Bubbles. Small 2024, 20, 2309012. [Google Scholar] [CrossRef]
- Das, M.; Baran Bhattacharya, A.; Rahman Parathodika, A.; Naskar, K. Room temperature Self-healable and extremely stretchable elastomer with improved mechanical Properties: Exploring a simplistic Metal-Ligand interaction. Eur. Polym. J. 2022, 174, 111341. [Google Scholar] [CrossRef]
- Rahimpour, S.; Luo, L.; Teimuri-Mofrad, R. Preparation of ferrocenyl-furan modified graphene oxide via Diels-Alder click reaction and using of its polypyrrole nanocomposites as supercapacitor electrode material. Electrochim. Acta 2022, 416, 140285. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Ye, F.; Wang, W.; Wang, G.; Zhang, Z.; Li, S.; Zhou, Y.; Cai, J. Vulcanization treatment: An effective way to improve the electrochemical cycle stability of polyaniline in supercapacitors. J. Power Sources 2019, 443, 227246. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Z.; Zhang, X.; Liao, Y.; Duan, W.; Yue, Y.; Zhang, Y. Stretchable Superhydrophobic Supercapacitor with Excellent Self-Healing Ability. Energy Fuels 2023, 37, 5567–5576. [Google Scholar] [CrossRef]
- Tee, B.C.K.; Wang, C.; Allen, R.; Bao, Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.; Xue, Y.; Cheng, Y.; Du, M.; Du, L.; Pang, H. From Co-MOF to CoNi-MOF to Ni-MOF: A Facile Synthesis of 1D Micro-/Nanomaterials. Inorg. Chem. 2021, 60, 13168–13176. [Google Scholar] [CrossRef]
- Mashkoor, F.; Lee, S.J.; Yi, H.; Noh, S.M.; Jeong, C. Self-Healing Materials for Electronics Applications. Int. J. Mol. Sci. 2022, 23, 622. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Wu, G.; Zhang, Y.; Zhang, R.; Li, X.J. A Fusion Parameter Method for Classifying Freshness of Fish Based on Electrochemical Impedance Spectroscopy. J. Food Qual. 2021, 2021, 6664291. [Google Scholar] [CrossRef]
- Li, Y.; Meng, S.; Dong, N.; Wei, Y.; Wang, Y.; Li, X.; Liu, D.; You, T. Space-Confined Electrochemical Aptasensing with Conductive Hydrogels for Enhanced Applicability to Aflatoxin B1 Detection. J. Agric. Food Chem. 2023, 71, 14806–14813. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, S.; Wang, M.; Li, W.; Dong, N.; Liu, D.; Li, Y.; You, T. In-depth interpretation of aptamer-based sensing on electrode: Dual-mode electrochemical-photoelectrochemical sensor for the ratiometric detection of patulin. Food Chem. 2023, 410, 135450. [Google Scholar] [CrossRef] [PubMed]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Bi, S.; Banda, H.; Chen, M.; Niu, L.; Chen, M.; Wu, T.; Wang, J.; Wang, R.; Feng, J.; Chen, T.; et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat. Mater. 2020, 19, 552–558. [Google Scholar] [CrossRef]
- Acharya, D.; Pathak, I.; Dahal, B.; Lohani, P.C.; Bhattarai, R.M.; Muthurasu, A.; Kim, T.; Ko, T.H.; Chhetri, K.; Kim, H.Y. Immoderate nanoarchitectures of bimetallic MOF derived Ni–Fe–O/NPC on porous carbon nanofibers as freestanding electrode for asymmetric supercapacitors. Carbon 2023, 201, 12–23. [Google Scholar] [CrossRef]
- Kim, M.; Xin, R.; Earnshaw, J.; Tang, J.; Hill, J.P.; Ashok, A.; Nanjundan, A.K.; Kim, J.; Young, C.; Sugahara, Y.; et al. MOF-derived nanoporous carbons with diverse tunable nanoarchitectures. Nat. Protoc. 2022, 17, 2990–3027. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J.; Yamauchi, Y. New strategies for novel MOF-derived carbon materials based on nanoarchitectures. Chem 2020, 6, 19–40. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, Y.; Xue, H.; Braunstein, P.; Huang, W.; Pang, H. Dual-ligand and hard-soft-acid-base strategies to optimize metal-organic framework nanocrystals for stable electrochemical cycling performance. Natl. Sci. Rev. 2022, 9, nwab197. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, J.; Gong, M.; Li, K.; Gu, J. Specific Screening of Prostate Cancer Individuals Using an Enzyme-Assisted Substrate Sensing Platform Based on Hierarchical MOFs with Tunable Mesopore Size. J. Am. Chem. Soc. 2021, 143, 15145–15151. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; Zhou, Z.; Pang, H.; Yang, Z. Synthesis of nickel-metal organic framework nanoplates with pyridine modulation and application to supercapacitors. J. Energy Storage 2021, 38, 102528. [Google Scholar] [CrossRef]
- Li, Z.-X.; Yang, B.-L.; Zou, K.-Y.; Kong, L.; Yue, M.-L.; Duan, H.-H. Novel porous carbon nanosheet derived from a 2D Cu-MOF: Ultrahigh porosity and excellent performances in the supercapacitor cell. Carbon 2019, 144, 540–548. [Google Scholar] [CrossRef]
- Otun, K.O.; Zong, S.; Hildebrandt, D.; Liu, X. Self-assembled Zn-functionalized Ni-MOF as an efficient electrode for electrochemical energy storage. J. Phys. Chem. Solids 2022, 167, 110779. [Google Scholar] [CrossRef]
- Jo, Y.H.; Zhou, B.; Jiang, K.; Li, S.; Zuo, C.; Gan, H.; He, D.; Zhou, X.; Xue, Z. Self-healing and shape-memory solid polymer electrolytes with high mechanical strength facilitated by a poly (vinyl alcohol) matrix. Polym. Chem. 2019, 10, 6561–6569. [Google Scholar] [CrossRef]
- Saraf, M.; Rajak, R.; Mobin, S.M. A fascinating multitasking Cu-MOF/rGO hybrid for high performance supercapacitors and highly sensitive and selective electrochemical nitrite sensors. J. Mater. Chem. A 2016, 4, 16432–16445. [Google Scholar] [CrossRef]
- Hussain, I.; Kathiresan, M.; Singh, K.; Kalidasan, B.; Mendhe, A.C.; Islam, M.N.; Meng, K.; Aslam, M.K.; Hanif, M.B.; Al Zoubi, W.; et al. Interface and surface engineering of MXenes and COFs for energy storage and conversion. InfoMat 2025, e70011. [Google Scholar] [CrossRef]
- Wang, J.; Du, C.F.; Xue, Y.; Tan, X.; Kang, J.; Gao, Y.; Yu, H.; Yan, Q. MXenes as a versatile platform for reactive surface modification and superior sodium-ion storages. Exploration 2021, 1, 20210024. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Schepisi, I.M.; Amir, F.Z. Extraordinary cycling stability of Ni3(HITP)2 supercapacitors fabricated by electrophoretic deposition: Cycling at 100,000 cycles. Chem. Eng. J. 2019, 378, 122150. [Google Scholar] [CrossRef]
- Li, W.-H.; Ding, K.; Tian, H.-R.; Yao, M.-S.; Nath, B.; Deng, W.-H.; Wang, Y.; Xu, G. Conductive Metal–Organic Framework Nanowire Array Electrodes for High-Performance Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, N.; Yang, S.; Lei, D.; Liu, A.; Zhou, Q. Cobalt induced growth of hollow MOF spheres for high performance supercapacitors. Mater. Chem. Front. 2021, 5, 482–491. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, H.; Liu, W.; Li, Y.; Zhang, J.; Hou, H.; Yang, J. Ultrathin NiCo-MOF Nanosheets for High-Performance Supercapacitor Electrodes. ACS Appl. Energy Mater. 2019, 2, 2063–2071. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, S.; Liu, Y.; Yan, W.; Lin, S.; Cheng, G.; Yang, H.; Luo, J. Room-Temperature Fabrication of a Nickel-Functionalized Copper Metal–Organic Framework (Ni@Cu-MOF) as a New Pseudocapacitive Material for Asymmetric Supercapacitors. Polymers 2019, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Gupta, A.K.; Krishnan, S.; Guha, N.; Marimuthu, S.; Rai, D.K. A new hierarchically porous Cu-MOF composited with rGO as an efficient hybrid supercapacitor electrode material. J. Energy Storage 2021, 43, 103301. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Horn, M.; Schneemann, A.; Saini, H.; Bakandritsos, A.; Ranc, V.; Petr, M.; Stavila, V.; Narayana, C.; Scheibe, B.; et al. Covalent Graphene-MOF Hybrids for High-Performance Asymmetric Supercapacitors. Adv. Mater. 2021, 33, 2004560. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Cao, X.; Ying, L.; Cui, L.; Barrow, C.; Yang, W.; Liu, J. Homogeneous nickel metal-organic framework microspheres on reduced graphene oxide as novel electrode material for supercapacitors with outstanding performance. J. Colloid Interface Sci. 2020, 561, 265–274. [Google Scholar] [CrossRef]
- Ren, Y.-F.; He, Z.-L.; Zhao, H.-Z.; Zhu, T. Fabrication of MOF-derived mixed metal oxides with carbon residues for pseudocapacitors with long cycle life. Rare Met. 2022, 41, 830–835. [Google Scholar] [CrossRef]
- Han, Y.; Hou, X.-Y.; Wang, X.; Fu, F.; Tang, L.; Wang, J.-J. A Capacity Supercapacitor Electrode Material of Ni-MOF with High Surface Area and Porosity. Chin. J. Struct. Chem. 2019, 38, 1779–1786. [Google Scholar]
- Kim, M.; Nara, H.; Asakura, Y.; Hamada, T.; Yan, P.; Earnshaw, J.; An, M.; Eguchi, M.; Yamauchi, Y. End-to-End Pierced Carbon Nanosheets with Meso-Holes. Adv. Sci. 2025, 12, 2409546. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).