Crystal Facet Engineering of 2D SnSe2 Photocatalysts for Efficient Degradation of Malachite Green Organic Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
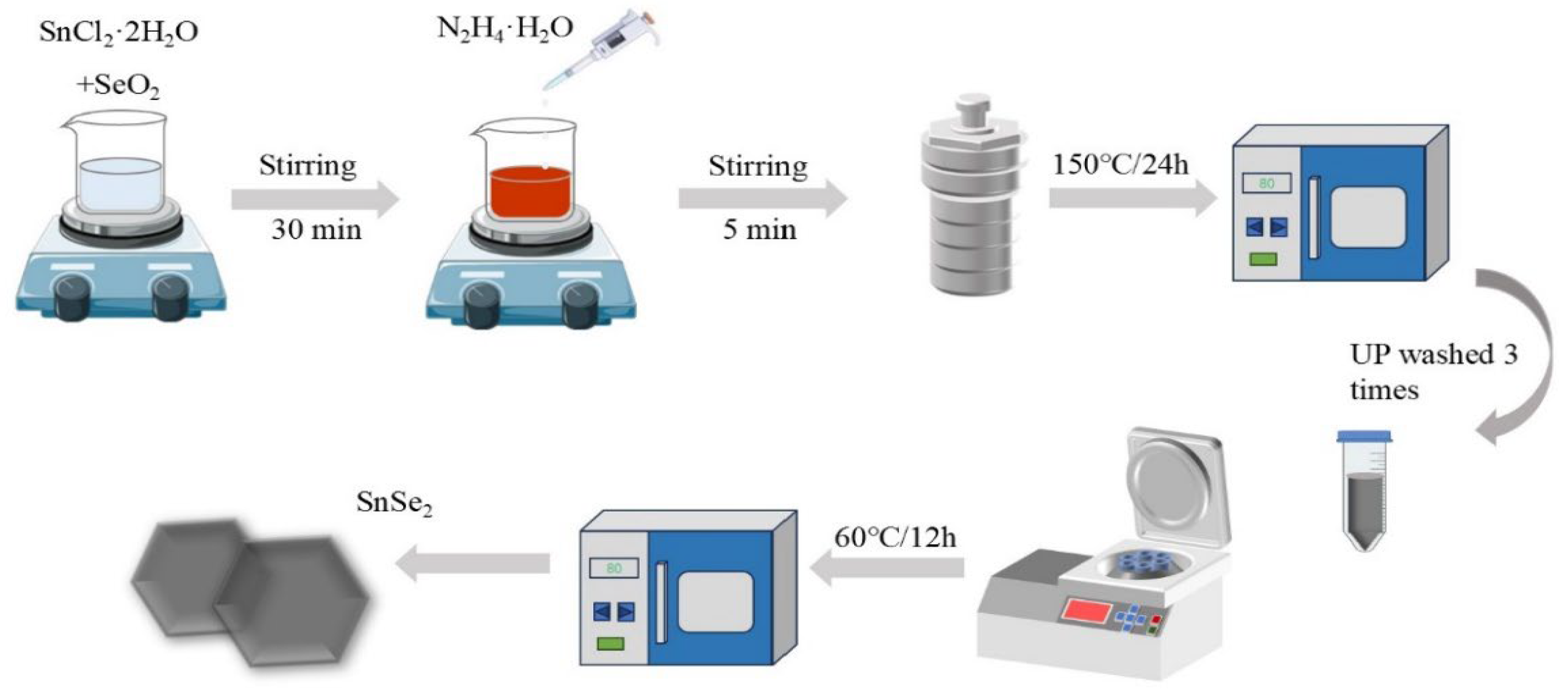
2.2. Preparation of SnSe2
2.3. Characterizations
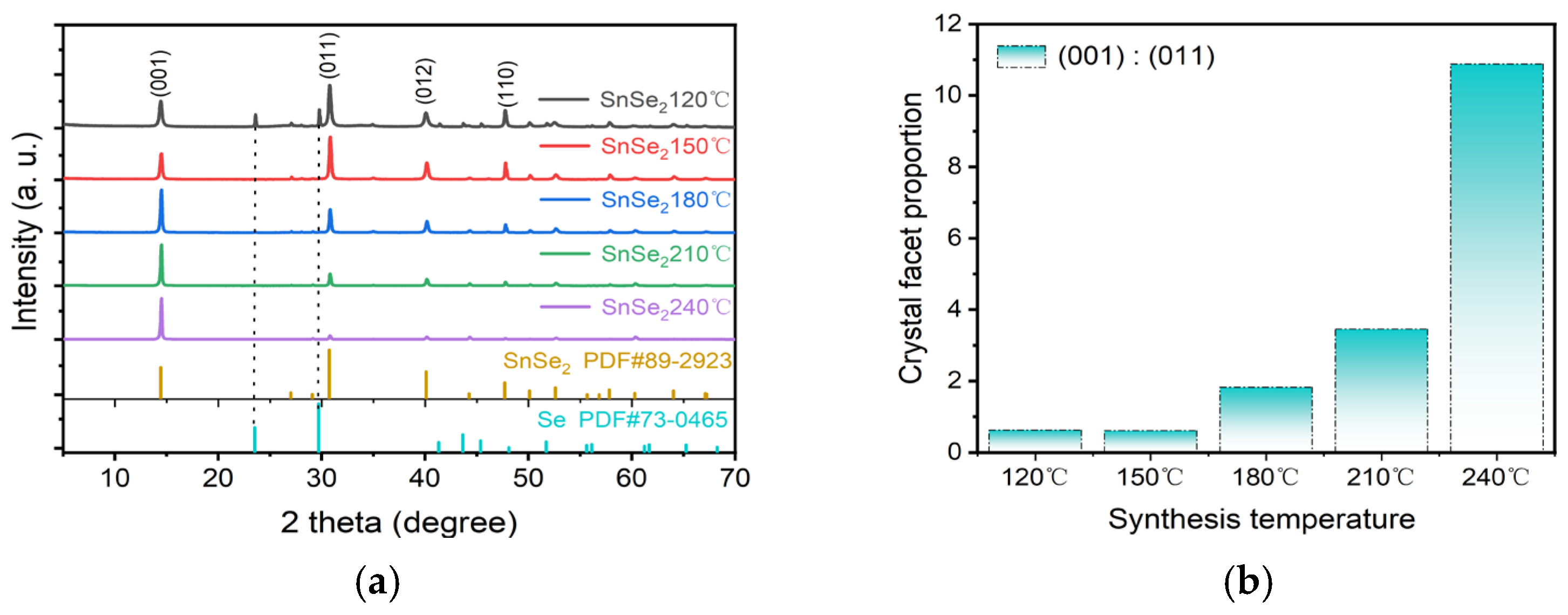
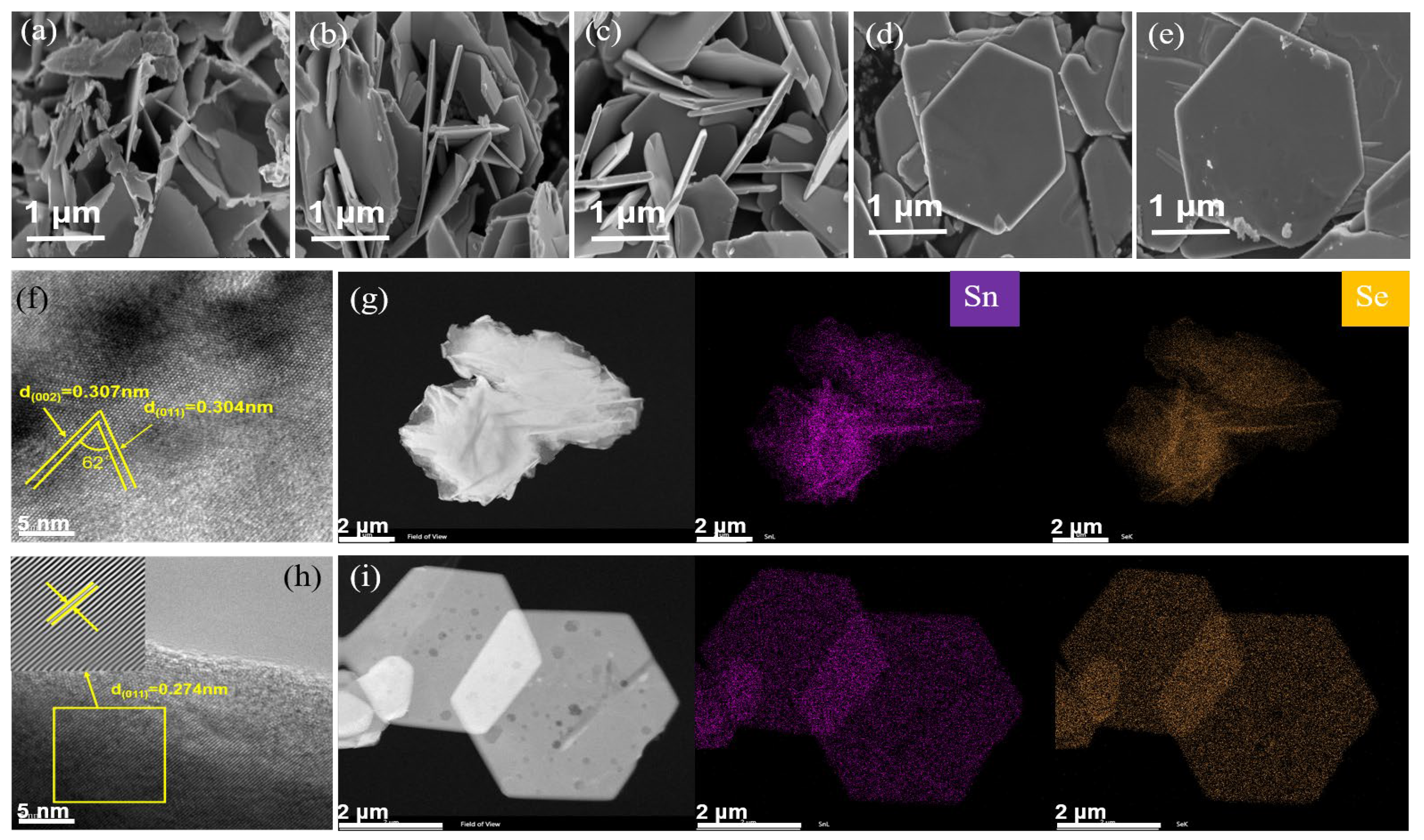
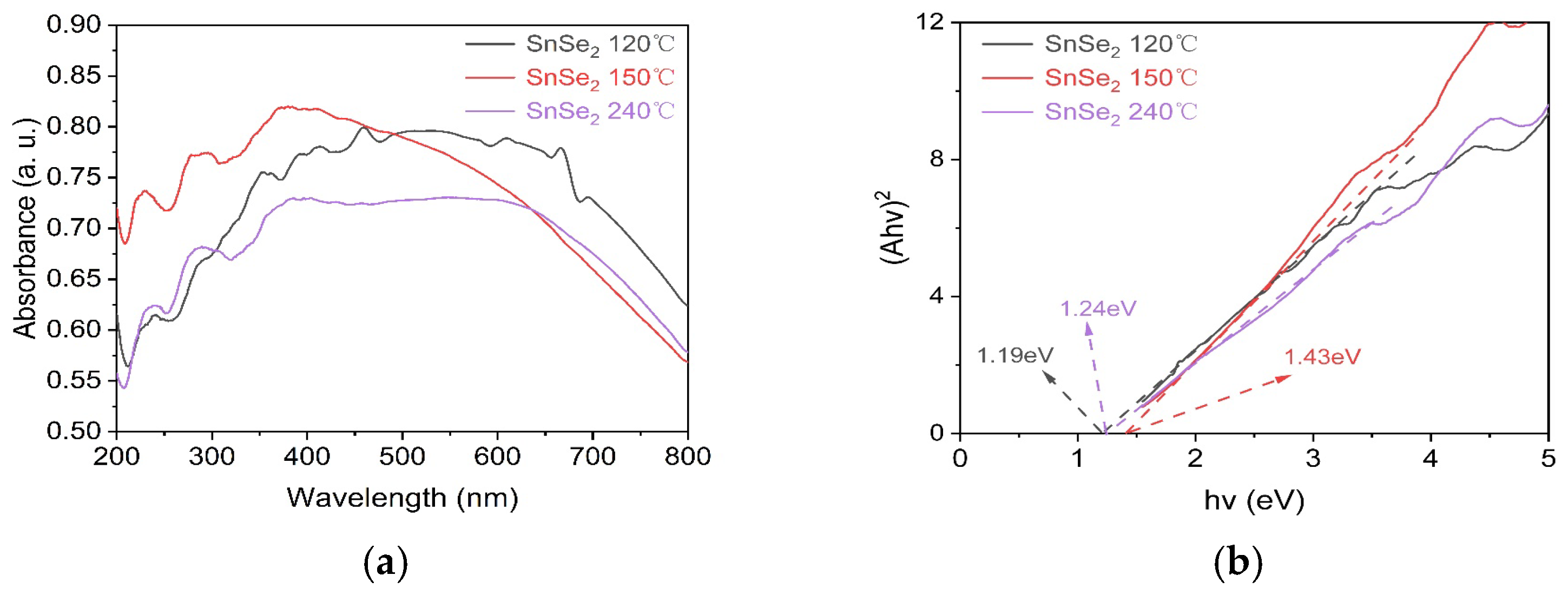
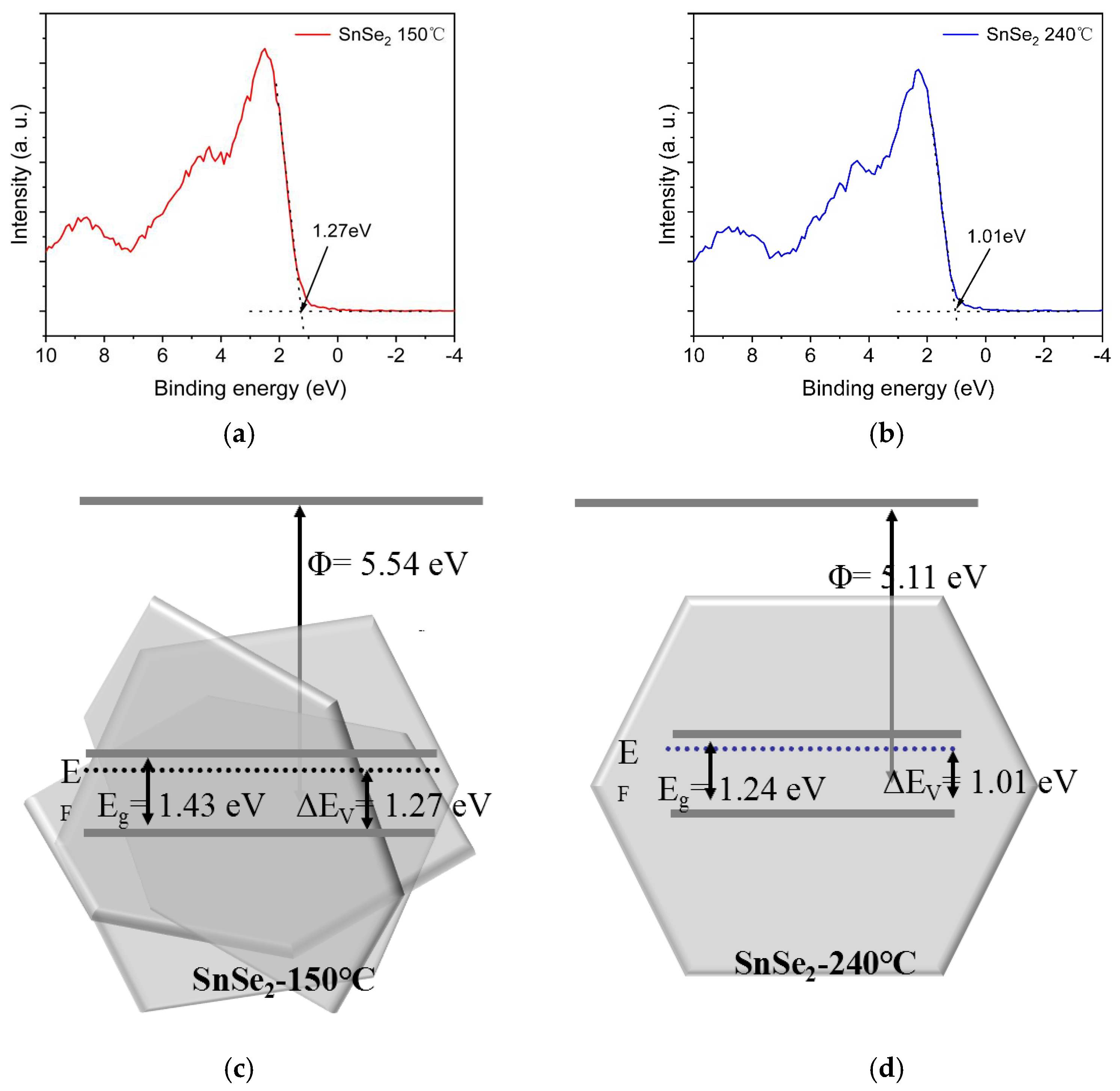
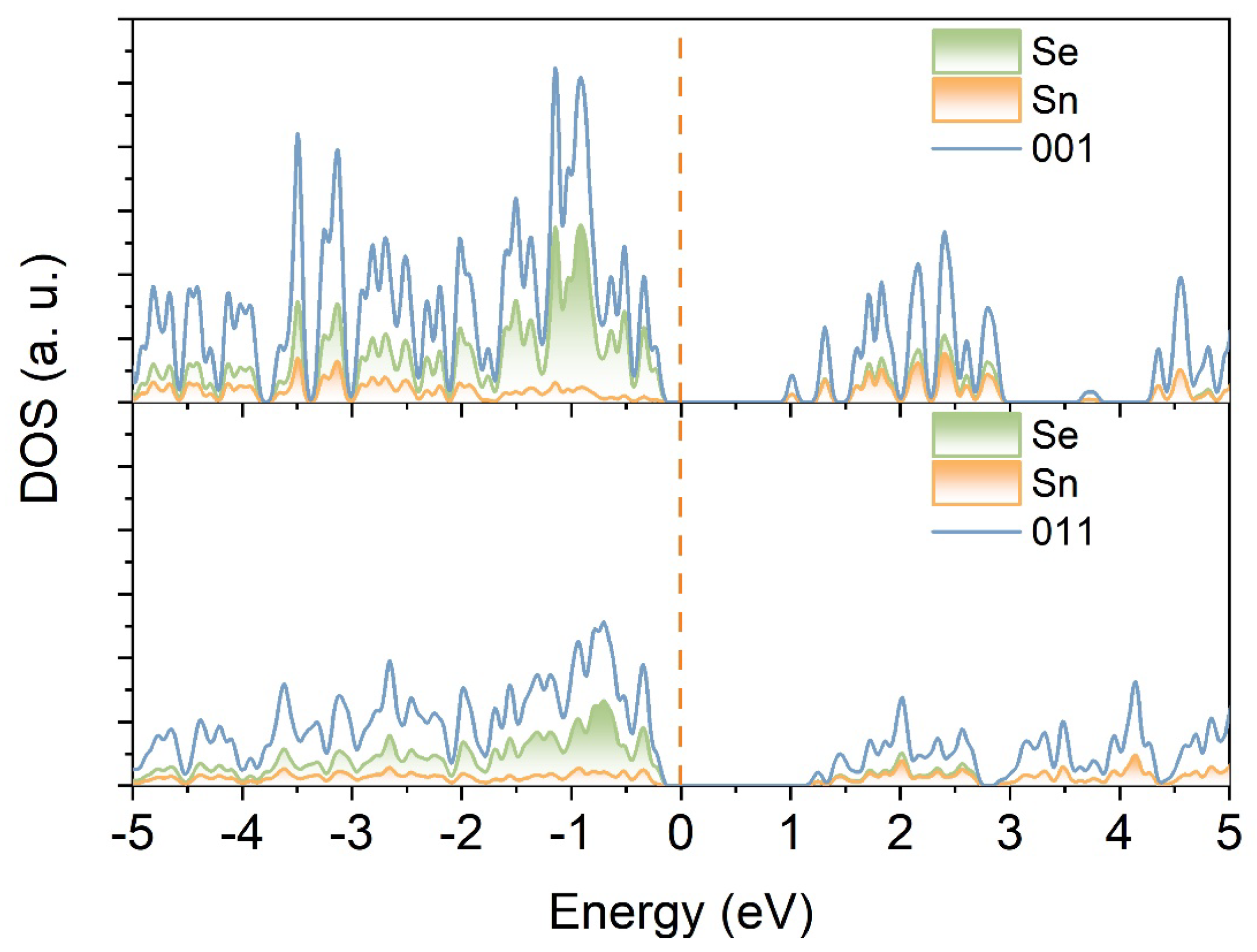
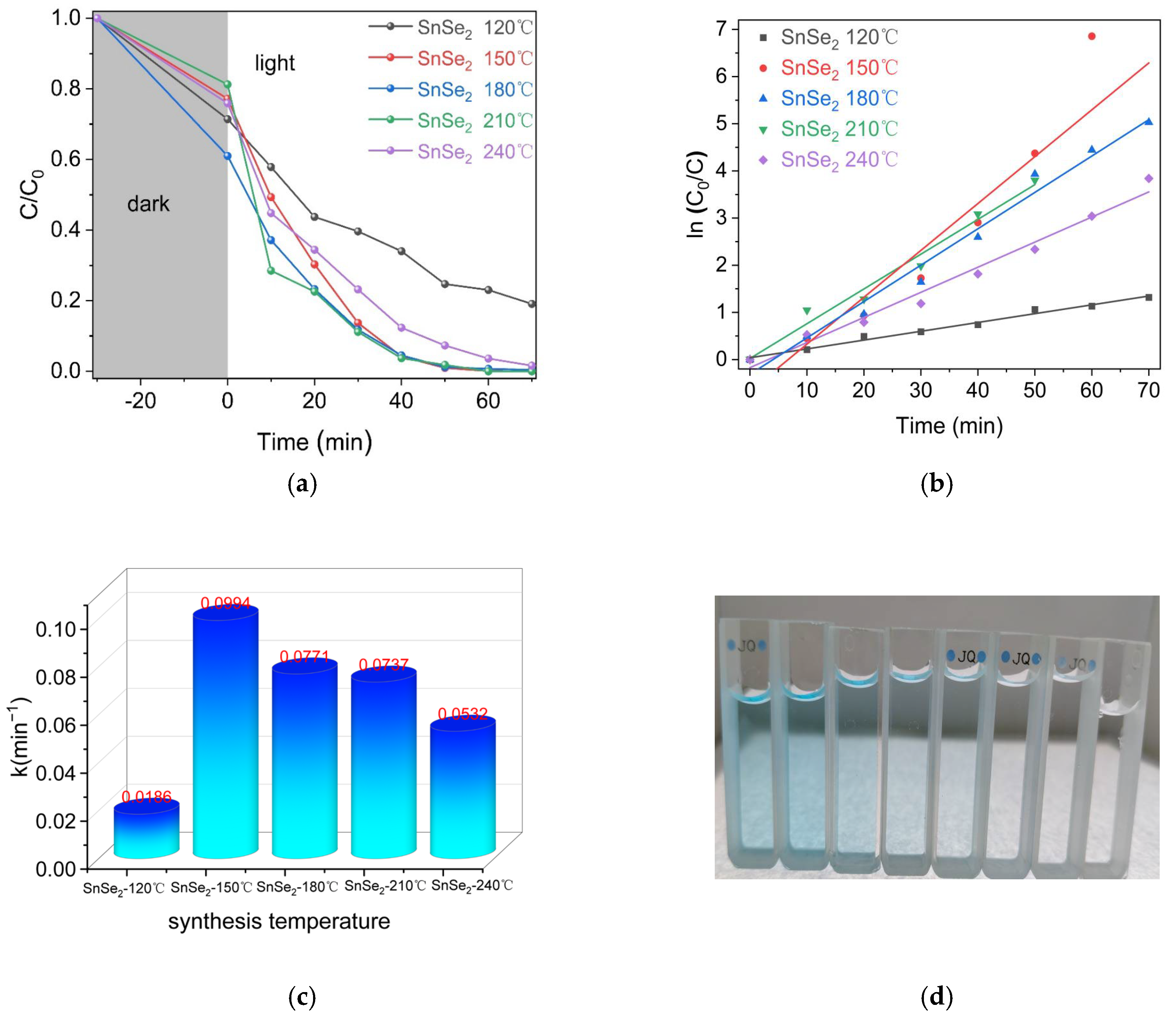
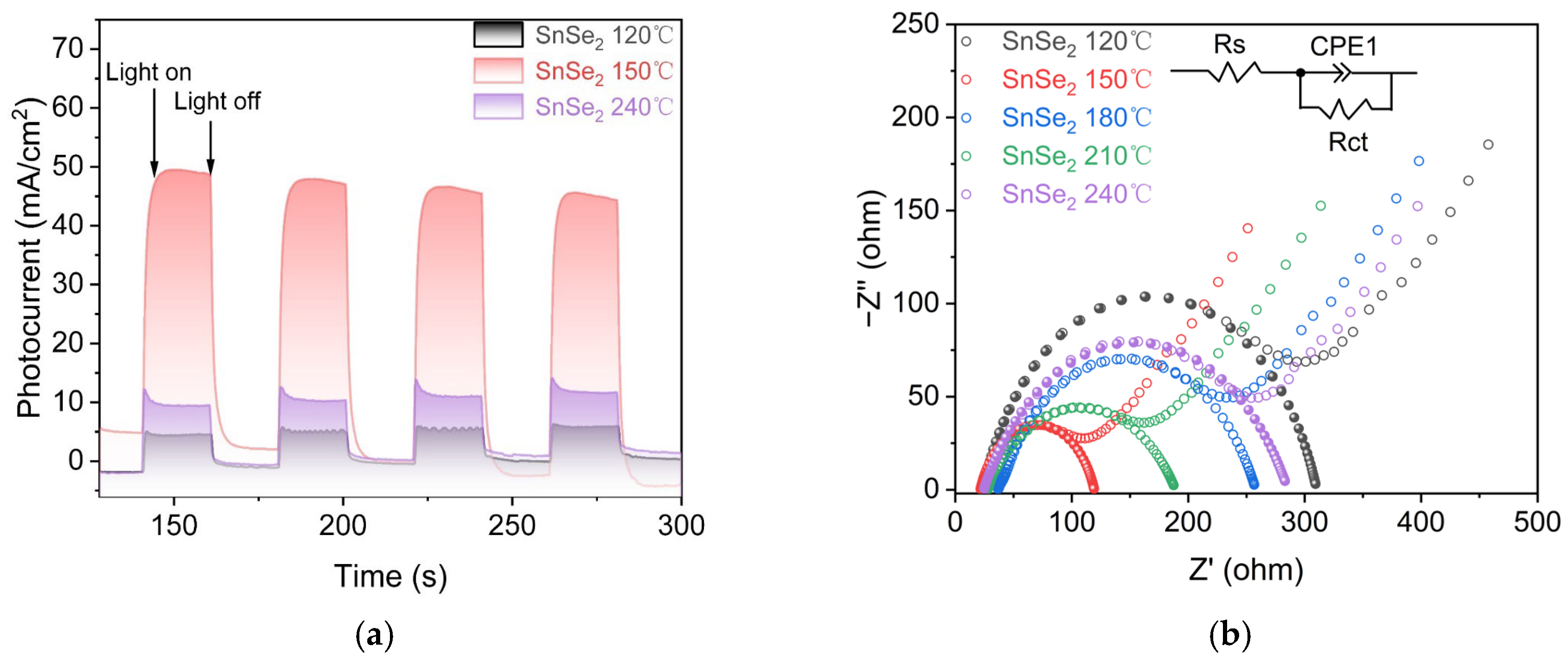
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meena, S.; Vaya, D.; Das, B.K. Photocatalytic Degradation of Malachite Green Dye by Modified ZnO Nanomaterial. Bull. Mater. Sci. 2016, 39, 1735–1743. [Google Scholar] [CrossRef]
- Ren, Q.; Kong, C.; Chen, Z.; Zhou, J.; Li, W.; Li, D.; Cui, Z.; Xue, Y.; Lu, Y. Ultrasonic Assisted Electrochemical Degradation of Malachite Green in Wastewater. Microchem. J. 2021, 164, 106059. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; He, J.; Liu, T.; Zhang, K.; Huang, X.; Kong, L.; Liu, J. EDTA-Fe(III) Fenton-like Oxidation for the Degradation of Malachite Green. J. Environ. Manag. 2018, 226, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Baeissa, E.S. Photocatalytic Degradation of Malachite Green Dye Using Au/NaNbO3 Nanoparticles. J. Alloys Compd. 2016, 672, 564–570. [Google Scholar] [CrossRef]
- Nebbia, C.; Girolami, F.; Carletti, M.; Gasco, L.; Zoccarato, I.; Giuliano Albo, A. In Vitro Interactions of Malachite Green and Leucomalachite Green with Hepatic Drug-Metabolizing Enzyme Systems in the Rainbow Trout (Onchorhyncus mykiss). Toxicol. Lett. 2017, 280, 41–47. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; He, J.; Chen, T.; Han, L.; Zhou, Q.; Zhao, D.; Wang, Y.; Wang, S. Effect of Size Tuning of Hexagonal SnS2 Nanosheet on the Efficiency of Photocatalytic Degradation Processes. Small 2024, 20, 2406002. [Google Scholar] [CrossRef]
- Singh, J.; Rishikesh; Kumar, S.; Verma, H.K.; Soni, R.K. Cost-Effective Scalable Synthesis of Few Layers MoS2 Based Thin Film for Sunlight Enforced Photocatalytic Activity. Opt. Mater. 2020, 110, 110506. [Google Scholar] [CrossRef]
- Dange, K.; Yogi, R.; Shukla, A. Two-Dimensional Transition-Metal Dichalcogenide–Based Bilayer Heterojunctions for Efficient Solar Cells and Photocatalytic Applications. Phys. Rev. Appl. 2025, 23, 014008. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ng, A.; Hu, Q.; Zhou, Q.; Li, X.; Liu, H. 2D Bi2Se3 van Der Waals Epitaxy on Mica for Optoelectronics Applications. Nanomaterials 2020, 10, 1653. [Google Scholar] [CrossRef]
- Shirvani, S.; Ghashghaee, M.; Smith, K.J. Two-Dimensional Nanomaterials in Thermocatalytic Reactions: Transition Metal Dichalcogenides, Metal Phosphorus Trichalcogenides and MXenes. Catal. Rev. 2023, 65, 1–51. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Huang, B.; Wang, X.; Niu, H.; Guo, Y.; Li, B.; Zheng, R.; Wu, H. Theoretical Study on the Photocatalytic Properties of 2D InX(X = S, Se)/Transition Metal Disulfide (MoS2 and WS2) van Der Waals Heterostructures. Nanoscale 2020, 12, 20025–20032. [Google Scholar] [CrossRef] [PubMed]
- Fatima, R.; Yusuf, K.; Khan, M.S.; Nisa, M.U. Comparative Study of Zinc Sulfide, Tin Selenide, and Their Composite Electrocatalysts for Oxygen Evolution Reaction: Towards Efficient and Stable Water Splitting. Electrochim. Acta 2024, 508, 145279. [Google Scholar] [CrossRef]
- Moore, S.; Takahashi, M.; Sauerschnig, P.; Kobayashi, K.; Higashimine, K.; Miyata, M.; Baba, T.; Uzuhashi, J.; Ohta, M.; Mori, T.; et al. Mechanistic Insight into the Effect of Cu Doping on Thermoelectric Properties of Sintered Wet-Chemically Synthesized SnSe2 Nanosheets. ACS Appl. Energy Mater. 2024, 7, 7467–7477. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, T.; Qiu, P.; Zheng, W.; Li, Z.; Zhou, Z.; Shi, X. Mechanical and Thermoelectric Properties of Na-Intercalated SnSe2 Crystals. ACS Appl. Energy Mater. 2024, 7, 8973–8980. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, J.; Chen, C.; Yang, S.; Lin, J.; Zeng, J.; Xi, J.; Kong, Z.; Yuan, Y.-J. A Novel Photoelectrochemical Detector Based on 2D SnSSe Porous Nanoplates with Atom-Level Heterojunctions. J. Alloys Compd. 2022, 911, 165106. [Google Scholar] [CrossRef]
- Vemula, M.; Veeralingam, S.; Badhulika, S. Hybrid 2D/0D SnSe2-SnO2 Vertical Junction Based High Performance Broadband Photodetector. J. Alloys Compd. 2021, 883, 160826. [Google Scholar] [CrossRef]
- Lu, D.; Yue, C.; Luo, S.; Li, Z.; Xue, W.; Qi, X.; Zhong, J. Phase Controllable Synthesis of SnSe and SnSe2 Films with Tunable Photoresponse Properties. Appl. Surf. Sci. 2021, 541, 148615. [Google Scholar] [CrossRef]
- Wu, S.; Liu, C.; Wu, Z.; Miao, L.; Gao, J.; Hu, X.; Chen, J.; Zheng, Y.; Wang, X.; Shen, C.; et al. Realizing Tremendous Electrical Transport Properties of Polycrystalline SnSe2 by Cl-Doped and Anisotropy. Ceram. Int. 2019, 45, 82–89. [Google Scholar] [CrossRef]
- Tan, P.; Chen, X.; Wu, L.; Shang, Y.Y.; Liu, W.; Pan, J.; Xiong, X. Hierarchical Flower-like SnSe2 Supported Ag3PO4 Nanoparticles: Towards Visible Light Driven Photocatalyst with Enhanced Performance. Appl. Catal. B Environ. 2017, 202, 326–334. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.; Lei, Y.; Yang, Q.; Zheng, Z. Synthesis and Photocatalytic Properties of SnSe2/Se Heterojunction Films. NANO 2018, 13, 1850045. [Google Scholar] [CrossRef]
- Mu, J.; Luo, D.; Miao, H.; Fan, J.; Hu, X. Synergistic Wide Spectrum Response and Directional Carrier Transportation Characteristics of Se/SnSe2/TiO2 Multiple Heterojunction for Efficient Photoelectrochemical Simultaneous Degradation of Cr (VI) and RhB. Appl. Surf. Sci. 2021, 542, 148673. [Google Scholar] [CrossRef]
- Liu, G.; Liu, L.; Zhou, Y.; Wang, Y.; Sui, G.; Zhang, P. Photocatalytic Degradation of Malachite Green Based on PW12/MWCNTs/Bi2O3 Composite Photocatalyst. J. Iran. Chem. Soc. 2024, 21, 877–885. [Google Scholar] [CrossRef]
- Yao, D.; Xie, X.; Liang, X.; Lu, S.; Lai, H. Photocatalytic Degradation of Malachite Green by Titanium Dioxide/Covalent Organic Framework Composite: Characterization, Performance and Mechanism. ChemistryOpen 2024, 13, e202300209. [Google Scholar] [CrossRef]
- Jin, X.; Wu, J.-Y.; Wang, J.; Chen, C.-Y.; Kurioka, T.; Sone, M.; Hsu, Y.-J.; Okamoto, S.; Chang, T.-F.M. Sintered NiFe2 O4 Nanoparticles for the Photocatalytic Degradation of Malachite Green. ACS Appl. Nano Mater. 2025, 8, 9066–9076. [Google Scholar] [CrossRef]
- Ghosh, S.; Mallik, T.; Roy, M.N.; Ekka, D. Synthesis of Nanoceria from Salophen Metal Complex and Employed in Photocatalytic Degradation of Malachite Green. Chem. Pap. 2024, 78, 4329–4346. [Google Scholar] [CrossRef]
- Tang, K.; Qi, W.; Wei, Y.; Ru, G.; Liu, W. High-Throughput Calculation of Interlayer van Der Waals Forces Validated with Experimental Measurements. Research 2022, 2022, 9765121. [Google Scholar] [CrossRef]
- Guo, X.; Ding, Y.; Yang, X.; Du, B.; Zhao, C.; Liang, C.; Ou, Y.; Kuang, D.; Wu, Z.; He, Y. 2D SnSe2 Nanoflakes Decorated with 1D ZnO Nanowires for Ppb-Level NO2 Detection at Room Temperature. J. Hazard. Mater. 2022, 426, 128061. [Google Scholar] [CrossRef]
- Liu, K.; Liu, H.; Wang, J.; Feng, L. Synthesis and Characterization of SnSe2 Hexagonal Nanoflakes. Mater. Lett. 2009, 63, 512–514. [Google Scholar] [CrossRef]
- Yue, X.; Han, L.; Wang, S.; Dun, L.; Wang, J.; Wang, Y.; Du, C. In-situ Growth of MgO@rGO Core-shell Structure via CO2 Thermal Reaction for Enhanced Photocatalytic Performance. Adv. Mater. Interfaces 2024, 11, 2400073. [Google Scholar] [CrossRef]
- Wen, L.; Cai, Q.; Du, Y.; Han, L.; Li, X.; Wang, Y.; Wang, S. Fabrication of Cs0.33WO3@TiO2 Heterostructure with Enhanced Light Modulation for Energy Saving Coating. Ceram. Int. 2025, 51, 2597–2606. [Google Scholar] [CrossRef]
- Manjunatha, K.; Alagarasan, D.; Das, S.; Ganesan, R.; Naik, R.; Purohit, D.; Ramudu, M. Influence of Substrate Temperature on SnSe2/TiO2 Heterostructure for Photodetector Applications. Sens. Actuators A Phys. 2025, 391, 116641. [Google Scholar] [CrossRef]
- Duong, D.L.; Yun, S.J.; Lee, Y.H. Van Der Waals Layered Materials: Opportunities and Challenges. ACS Nano 2017, 11, 11803–11830. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.Q.P.; Liu, S.; Van Der Lee, A.; Cuscó, R.; Artús, L.; Michel, T.; Valvin, P.; Edgar, J.H.; Cassabois, G.; Gil, B. Isotope Engineering of van Der Waals Interactions in Hexagonal Boron Nitride. Nat. Mater. 2018, 17, 152–158. [Google Scholar] [CrossRef]
- Jayaraman, P.; Pourzolfaghar, H.; Li, Y.-Y.; Therese, H.A. Accelerated Ion Switching and Its Impact on Performance in Pillared Interdigitated SnSe2‖PVA-LiClO4‖SnSe2 Flexible Supercapacitors. Mater. Today Energy 2025, 49, 101819. [Google Scholar] [CrossRef]
- Yao, J.; Liu, B.; Ozden, S.; Wu, J.; Yang, S.; Rodrigues, M.-T.F.; Kalaga, K.; Dong, P.; Xiao, P.; Zhang, Y.; et al. 3D Nanostructured Molybdenum Diselenide/Graphene Foam as Anodes for Long-Cycle Life Lithium-Ion Batteries. Electrochim. Acta 2015, 176, 103–111. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Lv, X.; Jia, J.; Du, X.; He, J.; Xie, F.; Din, Z.; Cai, J. Aptamers Adsorbed on WSe2 Nanosheets in a Label-Free Colorimetric Aptasensor for Ochratoxin a. ACS Appl. Nano Mater. 2024, 7, 4835–4842. [Google Scholar] [CrossRef]
- Wu, R.; Yan, K.; Zhao, J.; Cai, Z.; Jian, S.; Qiu, L. 2D/2D SnS2/SnSe2 van Der Waals Heterostructure for Highly Sensitive Room-Temperature NO2 Sensor: Key Role of Interface Contact. Chem. Eng. J. 2023, 466, 143369. [Google Scholar] [CrossRef]
- Verma, N.; Chundawat, T.S.; Chandra, H.; Vaya, D. An Efficient Time Reductive Photocatalytic Degradation of Carcinogenic Dyes by TiO2-GO Nanocomposite. Mater. Res. Bull. 2023, 158, 112043. [Google Scholar] [CrossRef]
- Ming, J.; Ni, S.-Q.; Guo, Z.; Wang, Z.-B.; Xie, L. Photocatalytic Material–Microorganism Hybrid Systems in Water Decontamination. Trends Biotechnol. 2024, 43, 1031–1047. [Google Scholar] [CrossRef]
- Cheng, Y.; Shah, N.H.; Yang, J.; Zhang, K.; Cui, Y.; Wang, Y. Bi-Based Z-Scheme Nanomaterials for the Photocatalytic Degradation of Organic Dyes. ACS Appl. Nano Mater. 2019, 2, 6418–6427. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, J.; Yu, X.; Du, R.; Zhang, T.; Wang, X.; Llorca, J.; Cabot, A. A SnS2 Molecular Precursor for Conformal Nanostructured Coatings. Chem. Mater. 2020, 32, 2097–2106. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An Overview on the Photocatalytic Degradation of Azo Dyes in the Presence of TiO2 Doped with Selective Transition Metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Kaneva, N.; Bojinova, A.; Papazova, K. Enhanced Removal of Organic Dyes Using Co-Catalytic Ag-Modified ZnO and TiO2 Sol-Gel Photocatalysts. Catalysts 2023, 13, 245. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Nonempirical Meta-Generalized Gradient Approximations for Modeling Chemisorption at Metal Surfaces. J. Chem. Theory Comput. 2018, 14, 3083–3090. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, M.; Han, K.; Liang, Y.; Zhao, X.; Han, L.; Wang, J.; Wang, S.; Li, Y. Ti3+ Self-Doping of TiO2 Boosts Its Photocatalytic Performance: A Synergistic Mechanism. Molecules 2024, 29, 5385. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, L.; Cheng, F.; Zhao, X.; Han, L.; Zhao, D.; Wang, S. Crystal Facet Engineering of 2D SnSe2 Photocatalysts for Efficient Degradation of Malachite Green Organic Dyes. Nanomaterials 2025, 15, 850. https://doi.org/10.3390/nano15110850
Wen L, Cheng F, Zhao X, Han L, Zhao D, Wang S. Crystal Facet Engineering of 2D SnSe2 Photocatalysts for Efficient Degradation of Malachite Green Organic Dyes. Nanomaterials. 2025; 15(11):850. https://doi.org/10.3390/nano15110850
Chicago/Turabian StyleWen, Liying, Fangfang Cheng, Xinyu Zhao, Lin Han, Dongye Zhao, and Shifeng Wang. 2025. "Crystal Facet Engineering of 2D SnSe2 Photocatalysts for Efficient Degradation of Malachite Green Organic Dyes" Nanomaterials 15, no. 11: 850. https://doi.org/10.3390/nano15110850
APA StyleWen, L., Cheng, F., Zhao, X., Han, L., Zhao, D., & Wang, S. (2025). Crystal Facet Engineering of 2D SnSe2 Photocatalysts for Efficient Degradation of Malachite Green Organic Dyes. Nanomaterials, 15(11), 850. https://doi.org/10.3390/nano15110850






