MXene/MOF-Derived Composites with Multidimensional Nanostructures: Synthesis Methods, Performance, and Applications in the Field of Energy Storage
Abstract
1. Introduction
2. Synthesis Method
2.1. Derived from MXene/MOF Composites
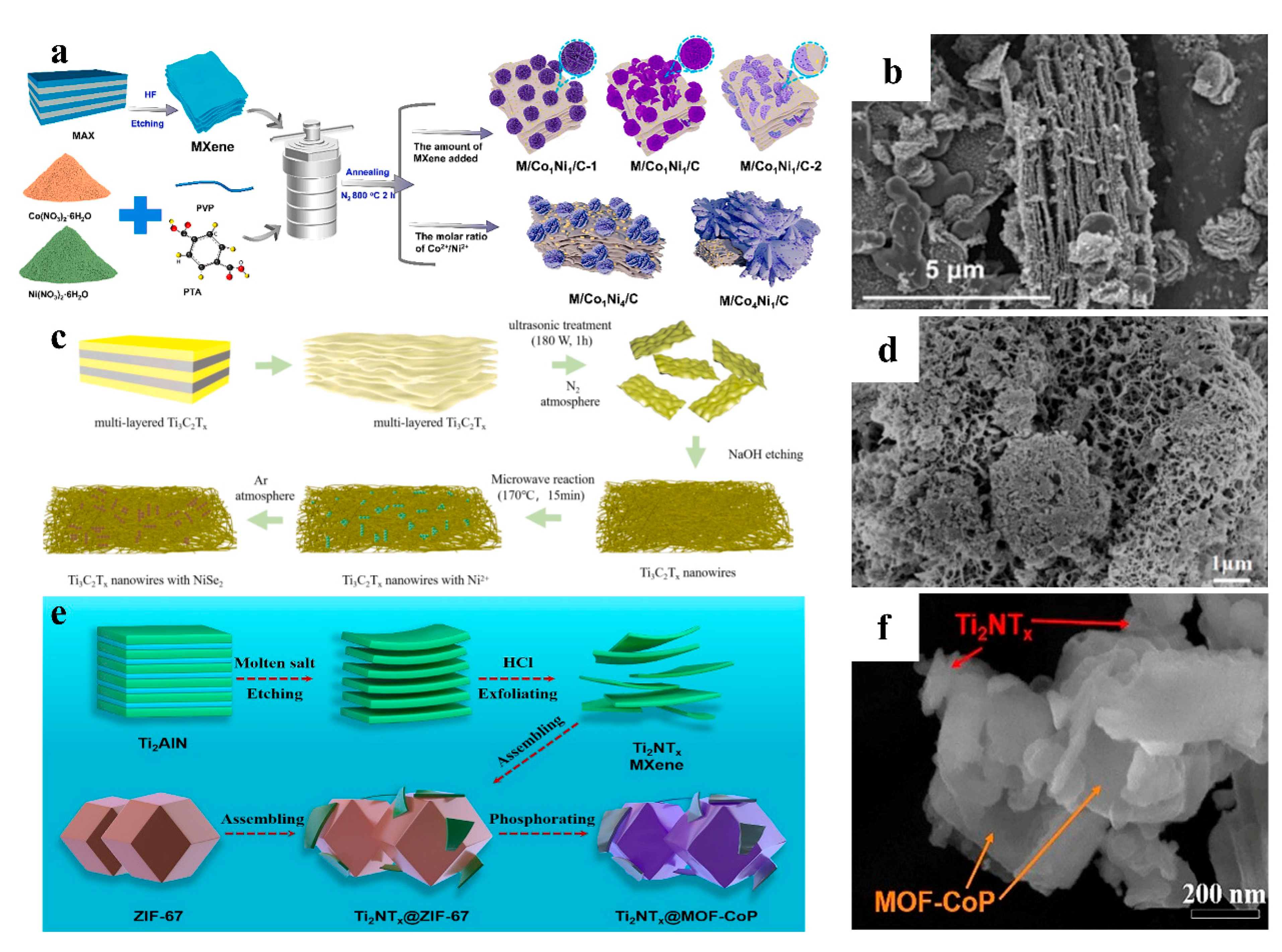
2.1.1. The In Situ Synthesis Method

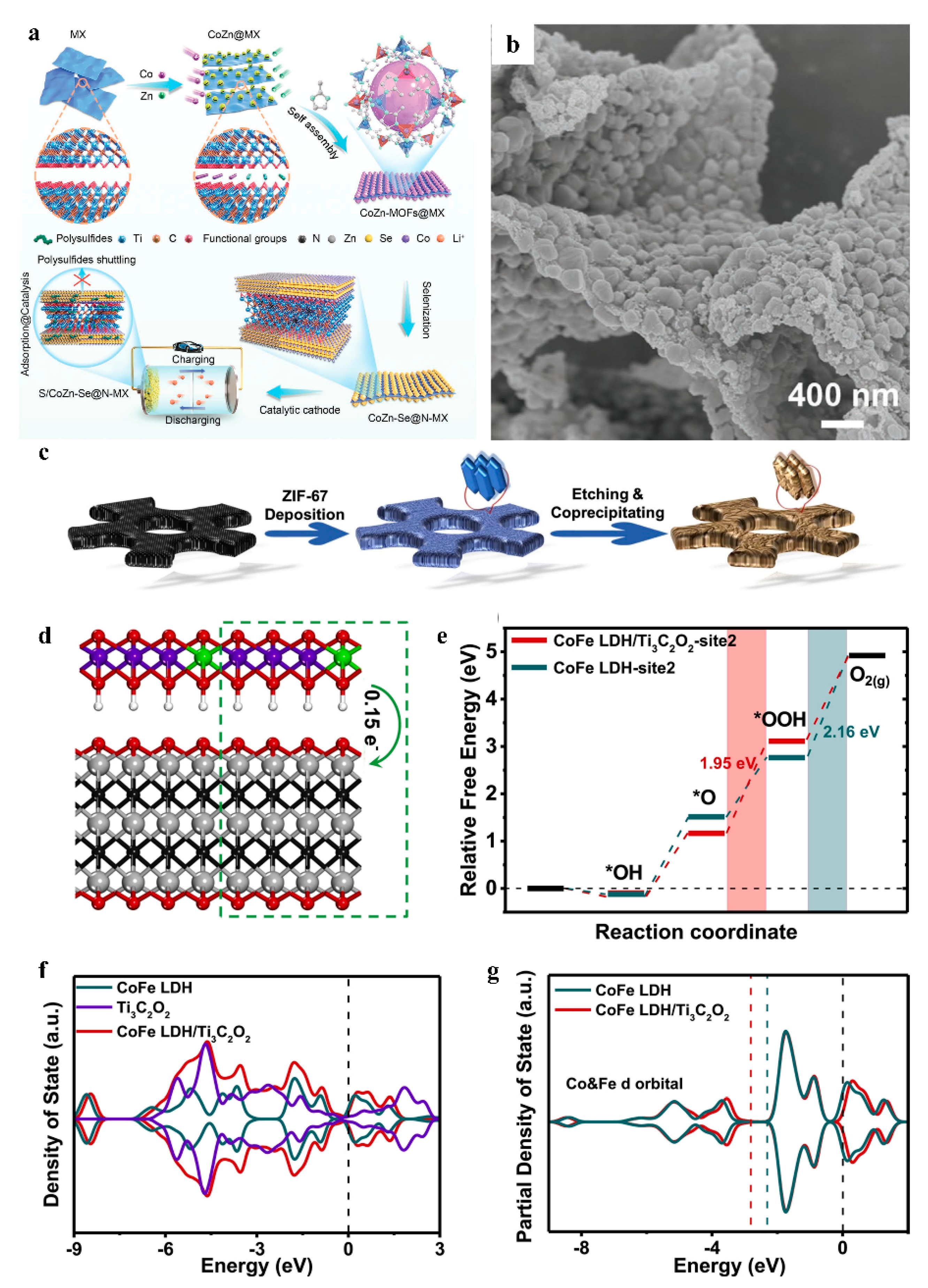
2.1.2. The Self-Assembly Method
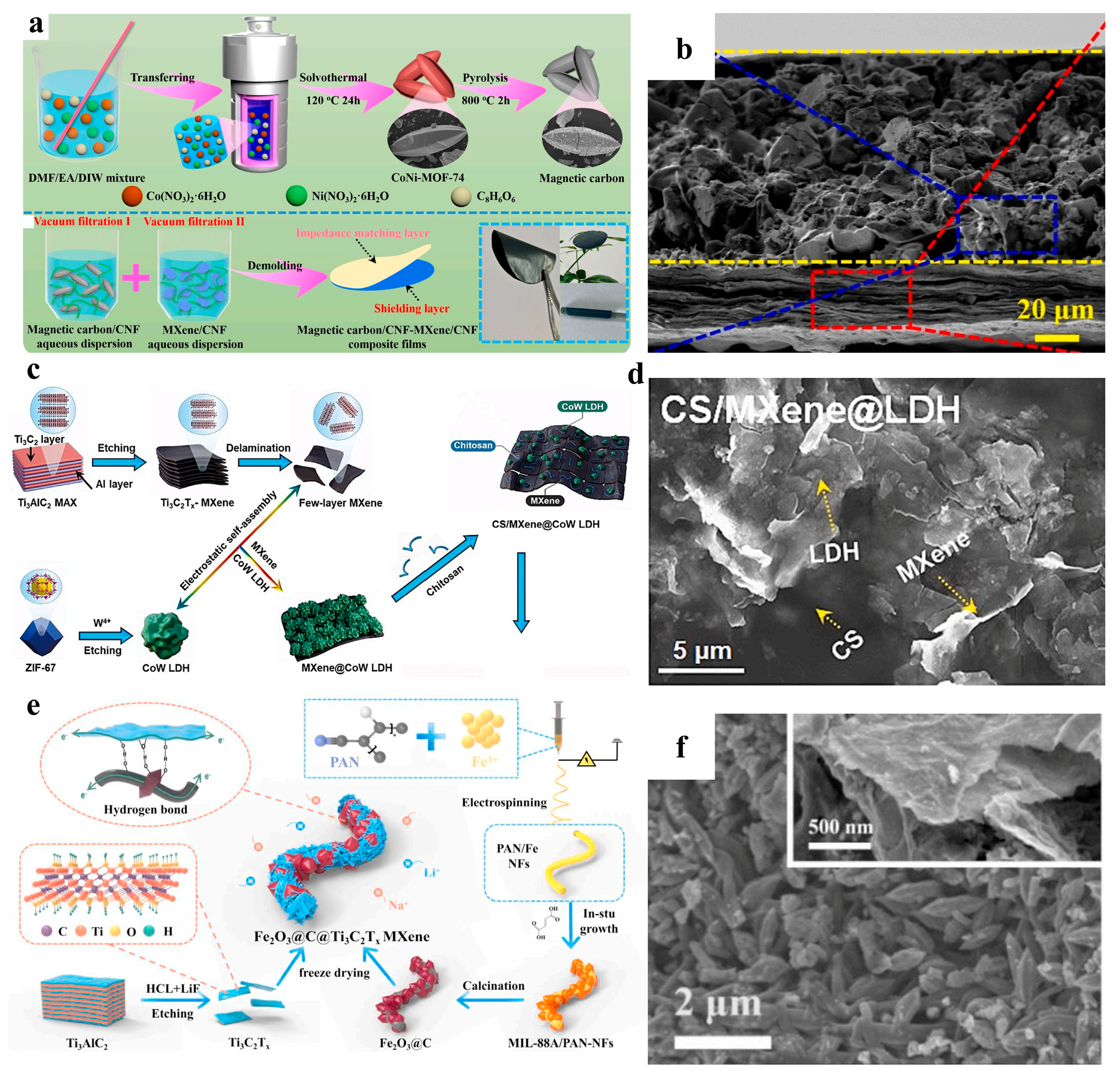
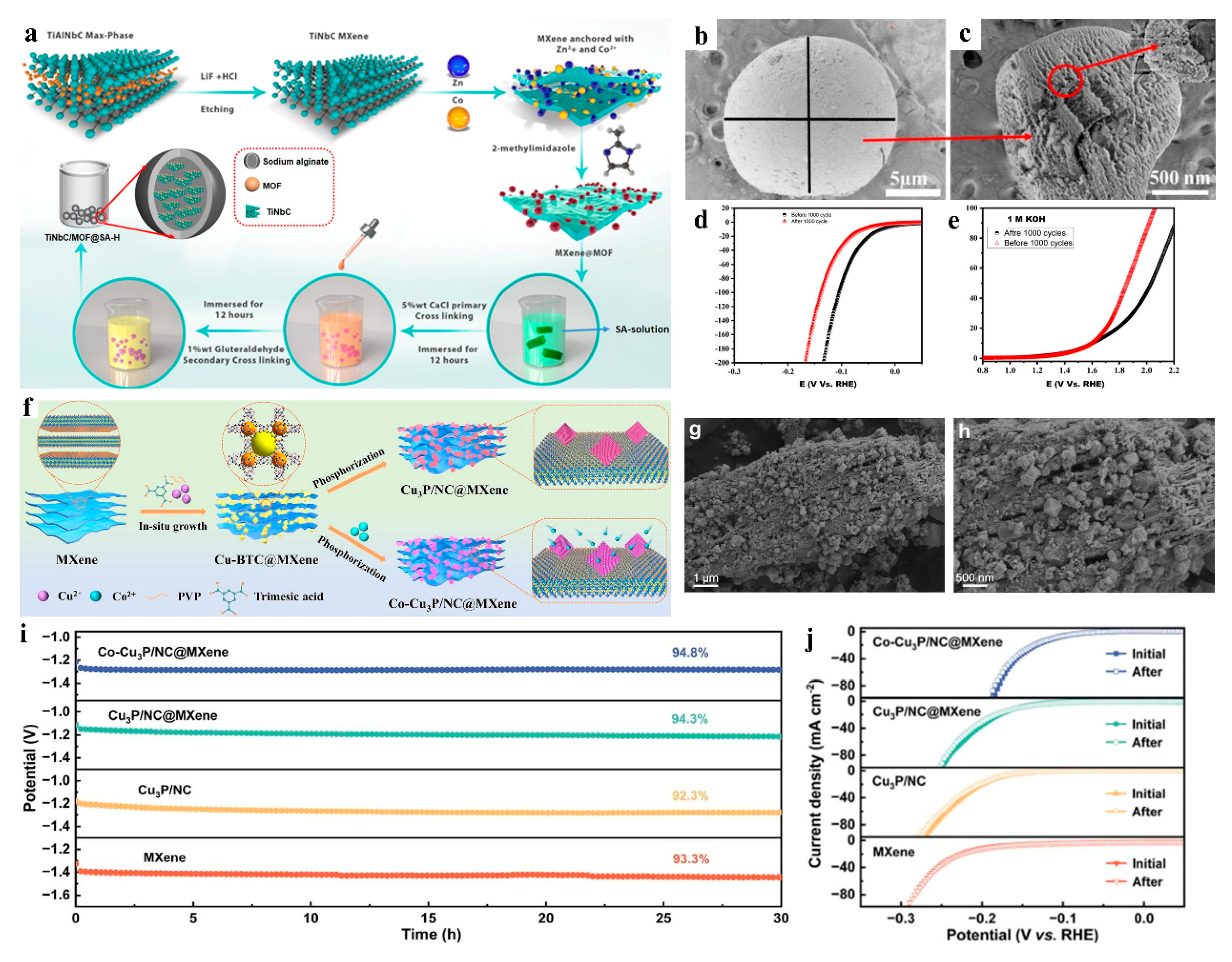
2.2. Integration of MOF-Derived Materials with MXene
| Material | Preparation | Assisting Method | Solvent | Ref. |
|---|---|---|---|---|
| TiO2/C/Co@CNTs | In situ synthesis | Hydrothermal Pyrolysis (800 °C, N2, 2 h) | DI | [33] |
| MXene/CoNi@C | In situ synthesis | Solvothermal Pyrolysis (700° C, Ar, 2 h) | Ethanol, DMF | [35] |
| NiSe2-CoSe2@C/Ti3C2Tx | In situ synthesis | Solvothermal Selenylation (400° C, Ar, 2 h) | MeOH | [37] |
| M/CoNi/C | Self-assembly | Solvothermal Pyrolysis (800° C, N2, 2 h) | Ethanol, DMF | [40] |
| NiSe2/NC/Ti3C2Tx | Self-assembly | Microwave-facilitated Solvothermal (600° C, Ar, 2 h) | DI, DMF | [41] |
| Ti2NTx@MOF-CoP | Self-assembly | Stirring Phosphating (300° C, Ar, 1.5 h) | DI | [42] |
| Carbon/CNF-MXene/CNF composite films | Direct mixing | Solvothermal Pyrolysis (800° C, N2, 2 h) vacuum-assisted filtration | DI, DMF, Ethanol | [44] |
| MXene@LDH hybrid NCs | Self-assembly | Stirring Solvothermal | DI, Ethanol | [45] |
| MOF-Fe2O3@carbon@Ti3C2Tx MXene | Direct mixing | Electrospinning Hydrothermal Pyrolysis (600° C, N2, 2 h) | DMF, DI | [46] |
3. Performance
3.1. Stabilities
3.2. Ion/Electron Transport Rate
3.3. Pseudocapacitance
3.4. Rate Performance
4. Applications
4.1. Electrocatalysts
4.2. Supercapacitors
4.3. Batteries
4.3.1. LIBs
4.3.2. SIBs
4.3.3. LSBs
4.3.4. ZABs
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.-E.; Li, Y.; Lin, X.; Wu, Y.; Zeb, A.; Zhang, S. Oxygen-deficient metal–organic framework derivatives for advanced energy storage: Multiscale design, application, and future development. Coord. Chem. Rev. 2023, 494, 215348. [Google Scholar] [CrossRef]
- Hong, A.N.; Yang, H.; Bu, X.; Feng, P. Pore space partition of metal-organic frameworks for gas storage and separation. Energy Chem. 2022, 4, 100080. [Google Scholar] [CrossRef]
- Wang, J.; Yue, X.; Xie, Z.; Abudula, A.; Guan, G. MOFs-derived transition metal sulfide composites for advanced sodium ion batteries. Energy Storage Mater. 2021, 41, 404–426. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, Y.; Zhang, X.; Zhang, S.; Xue, H.; Pang, H. MOF derived metal oxide composites and their applications in energy storage. Coord. Chem. Rev. 2023, 477, 214949. [Google Scholar] [CrossRef]
- Cao, W.; Chen, Z.; Chen, J.; Gao, J.; Cheng, X.; Zhang, M.; Guan, H.; Ahmad, W.; Lin, F.; Ling, M.; et al. Applications of MOF derivatives based on heterogeneous element doping in the field of electrochemical energy storage. Mater. Today 2024, 77, 118–141. [Google Scholar] [CrossRef]
- Ren, F.; Ma, L.; Li, C.; Wu, T.; Zhang, J.; Pei, L.; Jin, Y.; Sun, Z.; Guo, Z.; Song, P.; et al. Bimetallic MOF-derived Co/Ni@C and MXene co-decorated cellulose-derived carbon foams for absorption-dominated electromagnetic interference shielding. Carbohydr. Polym. 2025, 356, 123369. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, N.; Dong, M.; Jia, P.; Ma, C.; Zhang, T.; Jiao, T. MOF-Derived MnO/C Nanocomposites for High-Performance Supercapacitors. Nanomaterials 2022, 12, 4257. [Google Scholar] [CrossRef]
- Amrillah, T.; Abdullah, C.A.; Hermawan, A.; Sari, F.N.; Alviani, V.N. Towards Greener and More Sustainable Synthesis of MXenes: A Review. Nanomaterials 2022, 12, 4280. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Shen, H.; Man, Q.; Xiong, S.; Feng, J. Two-dimensional MXenes for flexible energy storage devices. Energy Environ. Sci. 2023, 16, 4191–4250. [Google Scholar] [CrossRef]
- Xue, Q.; Pei, Z.; Huang, Y.; Zhu, M.; Tang, Z.; Li, H.; Huang, Y.; Li, N.; Zhang, H.; Zhi, C. Mn3O4 nanoparticles on layer-structured Ti3C2 MXene towards the oxygen reduction reaction and zinc–air batteries. J. Mater. Chem. A 2017, 5, 20818–20823. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Xie, X.; Ren, C.E.; Makaryan, T.; Anasori, B.; Wang, G.; Gogotsi, Y. Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na-Ion Storage. Adv. Mater. 2017, 29, 1702410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Y.; Huang, Q.; Bi, Z.; Zhang, Z.; Guo, Z.; Wang, X.; Mei, T. A bifunctional VS2–Ti3C2 heterostructure electrocatalyst for boosting polysulfide redox in high performance lithium–sulfur batteries. J. Mater. Chem. A 2022, 10, 18866–18876. [Google Scholar] [CrossRef]
- Deng, B.; Xiang, Z.; Xiong, J.; Liu, Z.; Yu, L.; Lu, W. Sandwich-Like Fe&TiO2@C Nanocomposites Derived from MXene/Fe-MOFs Hybrids for Electromagnetic Absorption. Nano-Micro Lett. 2020, 12, 55. [Google Scholar]
- Shi, Y.; Song, G.; Yang, B.; Tang, Y.; Liu, Z.; Zhang, Z.; Shakouri, M.; Cheng, J.; Analogues, H.P.P.B. “Dressed” in MXene Nanosheets Tightly for High Performance Lithium-Ion Batteries. Adv. Mater. 2025, 37, 2416665. [Google Scholar] [CrossRef]
- Malaki, M.; Maleki, A.; Varma, R.S. MXenes and ultrasonication. J. Mater. Chem. A 2019, 7, 10843–10857. [Google Scholar] [CrossRef]
- Khalil, I.E.; Fonseca, J.; Reithofer, M.R.; Eder, T.; Chin, J.M. Tackling orientation of metal-organic frameworks (MOFs): The quest to enhance MOF performance. Coord. Chem. Rev. 2023, 481, 215043. [Google Scholar] [CrossRef]
- Rasheed, T.; Anwar, M.T. Metal organic frameworks as self-sacrificing modalities for potential environmental catalysis and energy applications: Challenges and perspectives. Coord. Chem. Rev. 2023, 480, 215011. [Google Scholar] [CrossRef]
- Hu, M.-L.; Masoomi, M.Y.; Morsali, A. Template strategies with MOFs. Coord. Chem. Rev. 2019, 387, 415–435. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Dutta, S.; Kamal, S.; Gómez-Graña, S.; Pastoriza-Santos, I.; Wuttke, S. State-of-the-Art, Insights, and Perspectives for MOFs-Nanocomposites and MOF-Derived (Nano)Materials. Adv. Mater. 2025, 2415399. [Google Scholar] [CrossRef]
- Otgonbayar, Z.; Yang, S.; Kim, I.-J.; Oh, W.-C. Recent Advances in Two-Dimensional MXene for Supercapacitor Applications: Progress, Challenges, and Perspectives. Nanomaterials 2023, 13, 919. [Google Scholar] [CrossRef]
- Cheng, L.; Tang, Y.; Xie, M.; Sun, Y.; Liu, H. 2D ultrathin NiMOF decorated by Ti3C2 MXene for highly improved photocatalytic performance. J. Alloys Compd. 2021, 864, 158913. [Google Scholar] [CrossRef]
- Hussain, I.; Bibi, F.; Pandiyarajan, S.; Hanan, A.; Chuang, H.-C.; Zhang, K. Partially oxidized MXenes for energy storage applications. Prog. Mater. Sci. 2025, 147, 101351. [Google Scholar] [CrossRef]
- Kim, D.; Ko, T.Y.; Kim, H.; Lee, G.H.; Cho, S.; Koo, C.M. Nonpolar Organic Dispersion of 2D Ti(3)C(2)T(x) MXene Flakes via Simultaneous Interfacial Chemical Grafting and Phase Transfer Method. ACS Nano 2019, 13, 13818–13828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Vashisth, A.; Blivin, J.W.; Tan, Z.; Holta, D.E.; Kotasthane, V.; Shah, S.A.; Habib, T.; Liu, S.; Lutkenhaus, J.L.; et al. Concentration, and Antioxidant Affect the Oxidation of Ti3C2T and Ti2CT MXene Dispersions. Adv. Mater. Interfaces 2020, 7, 2000845. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Yu, M.; Xiu, L.; Qiu, J. Stabilizing the MXenes by Carbon Nanoplating for Developing Hierarchical Nanohybrids with Efficient Lithium Storage and Hydrogen Evolution Capability. Adv. Mater. 2017, 29, 1607017. [Google Scholar] [CrossRef]
- Wang, X.; Liao, S.; Huang, H.; Wang, Q.; Shi, Y.; Zhu, P.; Hu, Y.; Sun, R.; Wan, Y. Enhancing the Chemical Stability of MXene Through Synergy of Hydrogen Bond and Coordination Bond in Aqueous Solution. Small Methods 2023, 7, 2201694. [Google Scholar] [CrossRef]
- Liu, N.; Li, Q.; Wan, H.; Chang, L.; Wang, H.; Fang, J.; Ding, T.; Wen, Q.; Zhou, L.; Xiao, X. High-temperature stability in air of Ti3C2Tx MXene-based composite with extracted bentonite. Nat. Commun. 2022, 13, 5551. [Google Scholar] [CrossRef]
- Liu, L.-X.; Chen, W.; Zhang, H.-B.; Ye, L.; Wang, Z.; Zhang, Y.; Min, P.; Yu, Z.-Z. Super-Tough and Environmentally Stable Aramid. Nanofiber@MXene Coaxial Fibers with Outstanding Electromagnetic Interference Shielding Efficiency. Nano-Micro Lett. 2022, 14, 111. [Google Scholar] [CrossRef]
- Xu, H.; Yin, X.; Li, X.; Li, M.; Liang, S.; Zhang, L.; Cheng, L. Lightweight Ti2CTx MXene/Poly(vinyl alcohol) Composite Foams for Electromagnetic Wave Shielding with Absorption-Dominated Feature. ACS Appl. Mater. Interfaces 2019, 11, 10198–10207. [Google Scholar] [CrossRef]
- Tu, W.; Bai, Z.; Deng, Z.; Zhang, H.; Tang, H. In-Situ Synthesized Si@C Materials for the Lithium Ion Battery: A Mini Review. Nanomaterials 2019, 9, 432. [Google Scholar] [CrossRef]
- Li, R.; Fu, X.; Liu, G.; Li, J.; Zhou, G.; Liu, G.; Jin, W. Room-temperature in situ synthesis of MOF@MXene membrane for efficient hydrogen purification. J. Membr. Sci. 2022, 664, 121097. [Google Scholar] [CrossRef]
- Yao, L.; Gu, Q.; Yu, X. Three-Dimensional MOFs@MXene Aerogel Composite Derived MXene Threaded Hollow Carbon Confined CoS Nanoparticles toward Advanced Alkali-Ion Batteries. ACS Nano 2021, 15, 3228–3240. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lv, Y.; Zhang, L.; Chen, H.; Chen, S.; Wu, Y.; Ma, L.-A.; Wang, Q. Hierarchical carbon nanotubes-modified heterogeneous composites derived from melamine-mixed ZIF-67/MXene for broadband microwave absorption. Carbon 2024, 216, 118585. [Google Scholar] [CrossRef]
- Liu, M.; Cai, N.; Chan, V.; Yu, F. Development and Applications of MOFs Derivative One-Dimensional Nanofibers via Electrospinning: A Mini-Review. Nanomaterials 2019, 9, 1306. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, C.; Li, Z.; Gu, C.; Li, X.; Li, Z.; Li, Y.; Lou, G.; Chen, Y. Growing bimetallic CoNi-MOF derivatives between MXene layers with hierarchically coral-like interfaces for enhanced electromagnetic wave absorption. J. Mater. Chem. A 2024, 12, 29103–29112. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Xiao, A.; Qiu, H.; Liu, L. Preparation of Magnetic Fe3O4/MIL-88A Nanocomposite and Its Adsorption Properties for Bromophenol Blue Dye in Aqueous Solution. Nanomaterials 2019, 9, 51. [Google Scholar] [CrossRef]
- Hou, T.; Jia, Z.; Wang, B.; Li, H.; Liu, X.; Chi, Q.; Wu, G. Metal-organic framework-derived NiSe2-CoSe2@C/Ti3C2Tx composites as electromagnetic wave absorbers. Chem. Eng. J. 2021, 422, 130079. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.-J. Nanoparticle/Metal–Organic Framework Composites for Catalytic Applications: Current Status and Perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef]
- Feng, H.-T.; Lam, J.W.Y.; Tang, B.Z. Self-assembly of AIEgens. Coord. Chem. Rev. 2020, 406, 213142. [Google Scholar] [CrossRef]
- Lv, Y.; Tian, J.; Chen, Z.; Wang, J.; Ma, L.-A.; Zhang, L.; Chen, S.; Wang, Q.; Ye, X. MXene/bimetallic CoNi-MOF derived magnetic-dielectric balanced composites with multiple heterogeneous interfaces for excellent microwave absorption. Chem. Eng. J. 2023, 478, 147413. [Google Scholar] [CrossRef]
- Zhou, Y.; Duan, Y.; Liu, Y.; Geng, Y.; Jin, Z.; Yu, L.; Li, S. “Two birds with one stone” strategy enhances the high corrosion resistance and electromagnetic wave absorption of NiSe2/NC/MXene composites. Appl. Surf. Sci. 2025, 691, 162621. [Google Scholar] [CrossRef]
- Zong, H.; Qi, R.; Yu, K.; Zhu, Z. Ultrathin Ti2NTx MXene-wrapped MOF-derived CoP frameworks towards hydrogen evolution and water oxidation. Electrochim. Acta 2021, 393, 139068. [Google Scholar] [CrossRef]
- Zhong, S.; Kang, B.; Cheng, X.; Chen, P.; Fang, B. Construction of a Co-MOF/MXene/BiVO4 Composite Photoanode for Efficient Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2024, 12, 1233–1246. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Y.; Luo, P.; Wang, J.; Pei, L.; Yu, H.; Song, P.; Ren, F.; Ren, P. Asymmetric and mechanically enhanced MOF derived magnetic carbon-MXene/cellulose nanofiber films for electromagnetic interference shielding and electrothermal/photothermal conversion. Chem. Eng. J. 2024, 497, 155707. [Google Scholar] [CrossRef]
- Kasinathan, K.; Park, Y.-K.; Ravindran, B.; Marimuthu, K.; Munuswamy-Ramanujam, G.; Chang, S.W.; Yim, J.-H. Synergistically self-assembled 2D nanosheets of MXene@MOF derived CoW-LDH into 3D frameworks functionalized with chitosan for improved skin wound healing. Chem. Eng. J. 2024, 482, 149088. [Google Scholar] [CrossRef]
- Li, Z.; Long, Z.; Dai, H.; Yan, Z.; Liu, K.; Qiao, H.; Wang, K.; Li, W. Ti3C2Tx MXene@metal-organic frameworks-derived bead-like carbon nanofibers heterostructure aerogel for enhanced performance lithium/sodium storage. J. Power Sources 2024, 606, 234586. [Google Scholar] [CrossRef]
- Raza, S.; Rehman, A.U.; Chen, C.; Zhao, T.; Hayat, A.; Bashir, T.; Shen, L.; Orooji, Y.; Lin, H. Synergistically self-assembled in situ growth of MXene@MOF derived sodium alginate hydrogel 3D frameworks as next-generation electrocatalysts for oxygen and hydrogen evolution. J. Mater. Chem. A 2025, 13, 4390–4403. [Google Scholar] [CrossRef]
- Zhang, M.; Yun, S.; Yang, T.; Yang, G.; Sun, M.; Dang, J.; Wang, Z.; Yang, H.; Yuan, S.; Arshad, A.; et al. 3D MOF-derived Co-doped Cu3P/NC octahedra embedded in 2D MXene nanosheets for efficient energy conversion. J. Mater. Chem. A 2024, 12, 29479–29492. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, H.; Hui, Z.; Sun, Y.; Yu, C.; Xue, J.; Zhou, R.; Wang, L.; Dai, H.; Zhao, Y.; et al. Electrostatically Assembling 2D Nanosheets of MXene and MOF-Derivatives into 3D Hollow Frameworks for Enhanced Lithium Storage. Small 2019, 15, 1904255. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Q.; Yan, Y.; Li, H.; Zhou, W.; Gu, T.; Shen, X.; Lu, C.; Zhao, Y.; Zhang, Y.; et al. Optimized Co–S bonds energy and confinement effect of hollow MXene@CoS2/NC for enhanced sodium storage kinetics and stability. Chem. Eng. J. 2022, 450, 137922. [Google Scholar] [CrossRef]
- Na, J.H.; Oh, H.G.; Lee, S.; Park, S.-K. Designing a 3D MXene microsphere encapsulating MOF-derived ZnSe nanoparticles as an anode for highly stable potassium-ion batteries. J. Mater. Chem. A 2024, 12, 2848–2855. [Google Scholar] [CrossRef]
- Saini, H.; Srinivasan, N.; Šedajová, V.; Majumder, M.; Dubal, D.P.; Otyepka, M.; Zbořil, R.; Kurra, N.; Fischer, R.A.; Jayaramulu, K. Emerging MXene@Metal–Organic Framework Hybrids: Design Strategies toward Versatile Applications. ACS Nano 2021, 15, 18742–18776. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Jiang, Y.; Li, L.; Wu, F.; Chen, R. Self-Assembly of 0D–2D Heterostructure Electrocatalyst from MOF and MXene for Boosted Lithium Polysulfide Conversion Reaction. Adv. Mater. 2021, 33, 2101204. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xiao, R.; Wang, X.; Wang, X.; Wang, C.; Wen, J.; Gu, W.; Zhu, C. MXene-induced electronic optimization of metal-organic framework-derived CoFe LDH nanosheet arrays for efficient oxygen evolution. Appl. Catal. B Environ. 2021, 298, 120599. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Xu, M.; Wang, M.; Yang, F.; Wu, N.; Zhang, T.; Sun, L.; Yang, W. Zeolite-imidazole framework derived nickel-cobalt hydroxide on ultrathin MXene nanosheets for long life and high performance supercapacitance. J. Alloys Compd. 2021, 888, 161250. [Google Scholar] [CrossRef]
- Aristote, N.T.; Zou, K.; Di, A.; Deng, W.; Wang, B.; Deng, X.; Hou, H.; Zou, G.; Ji, X. Methods of improving the initial Coulombic efficiency and rate performance of both anode and cathode materials for sodium-ion batteries. Chin. Chem. Lett. 2022, 33, 730–742. [Google Scholar] [CrossRef]
- An, R.; Niu, H.; Wang, C.; Liu, Z.; Gao, B. Constructing 0D-3D hollow MXene@CoSe2@NC heterostructures for superior sodium storage performance. J. Energy Storage 2024, 91, 111968. [Google Scholar] [CrossRef]
- Dai, H.; Long, Z.; Li, Z.; Yan, Z.; Wang, Q.; Wang, K.; Wei, Q.; Qiao, H. Metal-organic frameworks-derived CoFe2O4/Ti3C2Tx MXene/carbon nanofibers for high-rate lithium-ion batteries. J. Alloys Compd. 1007, 176489, 2024. [Google Scholar] [CrossRef]
- Feng, L.; Chen, J.; Li, Y.; Zhou, S.; Soomro, R.A.; Zhang, P.; Xu, B. MXene-reinforced Sb@C nanocomposites with synergizing spatial confinement architecture enabled ultra-stable and fast lithium ion storage. Chem. Eng. J. 2024, 489, 151396. [Google Scholar] [CrossRef]
- Li, F.; Huang, L.; Zaman, S.; Guo, W.; Liu, H.; Guo, X.; Xia, B.Y. Corrosion Chemistry of Electrocatalysts. Adv. Mater. 2022, 34, 2200840. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Chen, W.; Zou, Y.; Chen, R.; Zang, S.; Wang, Y.; Yao, X.; Wang, S. Insight into the design of defect electrocatalysts: From electronic structure to adsorption energy. Mater. Today 2019, 31, 47–68. [Google Scholar] [CrossRef]
- Shi, Q.; Hwang, S.; Yang, H.; Ismail, F.; Su, D.; Higgins, D.; Wu, G. Supported and coordinated single metal site electrocatalysts. Mater. Today 2020, 37, 93–111. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y. MXene chemistry, electrochemistry and energy storage applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef] [PubMed]
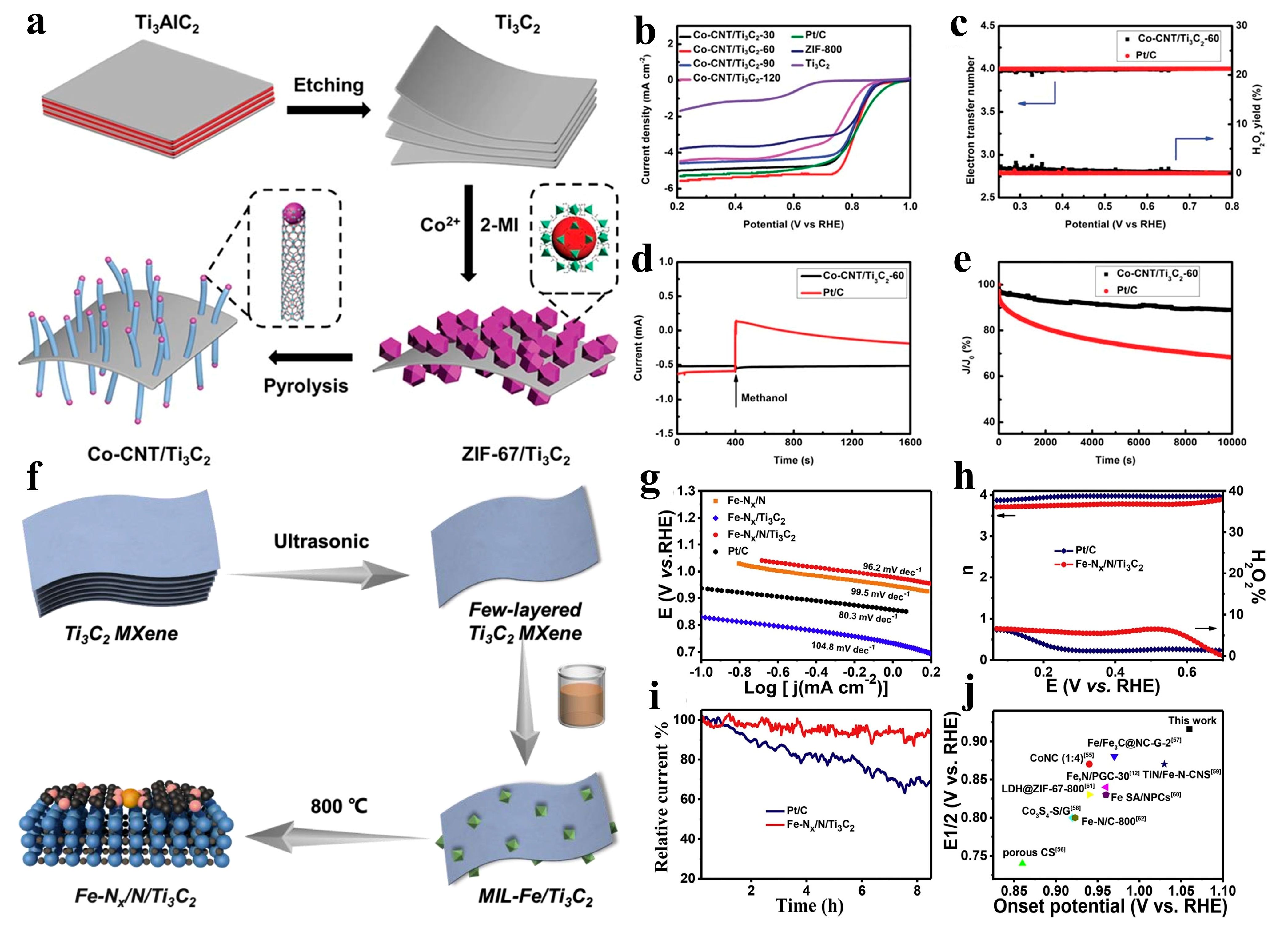
- Chen, J.; Yuan, X.; Lyu, F.; Zhong, Q.; Hu, H.; Pan, Q.; Zhang, Q. Integrating MXene nanosheets with cobalt-tipped carbon nanotubes for an efficient oxygen reduction reaction. J. Mater. Chem. A 2019, 7, 1281–1286. [Google Scholar] [CrossRef]
- Gu, W.; Wu, M.; Xu, J.; Zhao, T. MXene boosted metal-organic framework-derived Fe–N–C as an efficient electrocatalyst for oxygen reduction reactions. Int. J. Hydrog. Energy 2022, 47, 17224–17232. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, D.; Cheng, M.; Zhou, J.; Owusu, K.A.; Mai, L.; Yu, Y. A New View of Supercapacitors: Integrated Supercapacitors. Adv. Energy Mater. 2019, 9, 1901081. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Chettiannan, B.; Dhandapani, E.; Arumugam, G.; Rajendran, R.; Selvaraj, M. Metal-organic frameworks: A comprehensive review on common approaches to enhance the energy storage capacity in supercapacitor. Coord. Chem. Rev. 2024, 518, 216048. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Gao, M.; Wang, F.; Yang, S.; Ricciardulli, A.G.; Yu, F.; Li, J.; Sun, J.; Wang, R.; Huang, Y.; Zhang, P. Engineered 2D MXene-based materials for advanced supercapacitors and micro-supercapacitors. Mater. Today 2024, 72, 318–358. [Google Scholar] [CrossRef]
- Shingte, S.; Chavan, V.; Dhavale, R.; Kim, D.-K.; Park, H.-H.; Dongale, T.; Patil, P. Optimizing supercapacitive performance of MXene through MOF-derived nickel ferrite nanoparticle integration. J. Energy Storage 2024, 92, 112169. [Google Scholar] [CrossRef]
- Liu, C.; Bai, Y.; Li, W.; Yang, F.; Zhang, G.; Pang, H. In Situ Growth of Three-Dimensional MXene/Metal–Organic Framework Composites for High-Performance Supercapacitors. Angew. Chem. Int. Ed. 2022, 61, e202116282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Hou, Z.; Mei, H.; Feng, Y.; Xu, B.; Sun, D. Self-assembly of MOF on MXene nanosheets and in-situ conversion into superior nickel phosphates/MXene battery-type electrode. Chem. Eng. J. 2021, 425, 130602. [Google Scholar] [CrossRef]
- Anderson, M.D.; Carr, D.S. Battery energy storage technologies. Proc. IEEE 1993, 81, 475–479. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, J.; Xiao, P.; Nie, S.; Luo, F.; Chen, Y. Exploring metal-organic frameworks in battery electrodes, separators, and electrolytes: A comprehensive review. Coord. Chem. Rev. 2025, 532, 216501. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef]
- Cai, J.; Liu, C.; Tao, S.; Cao, Z.; Song, Z.; Xiao, X.; Deng, W.; Hou, H.; Ji, X. MOFs-derived advanced heterostructure electrodes for energy storage. Coord. Chem. Rev. 2023, 479, 214985. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Wu, L.; Li, W.; Yin, Y.; Liu, Z.; Zheng, B.; Zhang, Q.; Hao, Z. In-situ construction of Ti3C2TX/Ni-HHTP heterostructure as anode for lithium-ion batteries. Mater. Today Commun. 2024, 38, 108160. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ma, L.; Zhang, X.; Li, J.; Li, J.; Lu, T.; Pan, L. Heteroatomic interface engineering of an octahedron VSe2–ZrO2/C/MXene composite derived from a MXene-MOF hybrid as a superior-performance anode for lithium-ion batteries. J. Mater. Chem. A 2023, 11, 2836–2847. [Google Scholar] [CrossRef]
- Schäfer, D.; Hankins, K.; Allion, M.; Krewer, U.; Karcher, F.; Derr, L.; Schuster, R.; Maibach, J.; Mück, S.; Kramer, D.; et al. Multiscale Investigation of Sodium-Ion Battery Anodes: Analytical Techniques and Applications. Adv. Energy Mater. 2024, 14, 2302830. [Google Scholar] [CrossRef]
- Chen, L.; Fiore, M.; Wang, J.E.; Ruffo, R.; Kim, D.; Longoni, G. Readiness Level of Sodium-Ion Battery Technology: A Materials Review. Adv. Sustain. Syst. 2018, 2, 1700153. [Google Scholar] [CrossRef]
- Yang, X.; Rogach, A.L. Anodes and Sodium-Free Cathodes in Sodium Ion Batteries. Adv. Energy Mater. 2020, 10, 2000288. [Google Scholar] [CrossRef]
- Li, C.; Ji, Y.; Wang, Y.; Liu, C.; Chen, Z.; Tang, J.; Hong, Y.; Li, X.; Zheng, T.; Jiang, Q.; et al. Applications of Metal–Organic Frameworks and Their Derivatives in Electrochemical CO2 Reduction. Nano-Micro Lett. 2023, 15, 113. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, F.; Liu, R.; Liu, W.; Sun, Y.; Dong, C.; Cheng, G.; Ding, J. Deep etching assisted hollow-carbon-skeleton structured porous Co0.85Se nanocubes anchored on MXene nanosheets for superior sodium storage toward ultrahigh initial Coulombic efficiency. J. Alloys Compd. 2023, 968, 172216. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, D.; Xie, Y.; Wu, K.; Feng, Z.; Wen, K.; Li, Z.; He, M. Co3C/Mxene composites wrapped in N-rich carbon as stable-performance anodes for potassium/sodium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130332. [Google Scholar] [CrossRef]
- Raza, H.; Bai, S.; Cheng, J.; Majumder, S.; Zhu, H.; Liu, Q.; Zheng, G.; Li, X. Li-S Batteries: Challenges, Achievements and Opportunities. Electrochem. Energy Rev. 2023, 6, 2023. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, Q.; Pei, F.; Wang, Q.; Zhang, Y.; Du, L.; Zhang, Y.; Zhang, Y.; Zhang, Y.; Fan, L.; et al. Interface engineering toward stable lithium–sulfur batteries. Energy Environ. Sci. 2024, 17, 1330–1367. [Google Scholar] [CrossRef]
- Hu, B.; Xu, J.; Fan, Z.; Xu, C.; Han, S.; Zhang, J.; Ma, L.; Ding, B.; Zhuang, Z.; Kang, Q.; et al. Covalent Organic Framework Based Lithium–Sulfur Batteries: Materials, Interfaces, and Solid-State Electrolytes. Adv. Energy Mater. 2023, 13, 2203540. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q. Designing high-energy lithium–sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zheng, N.; Chen, X.; Ding, G.; Li, Y.; Sun, F.; Li, Y. In-situ decoration of MOF-derived carbon on nitrogen-doped ultrathin MXene nanosheets to multifunctionalize separators for stable Li-S batteries. Chem. Eng. J. 2019, 373, 1309–1318. [Google Scholar] [CrossRef]
- Wei, A.; Wang, L.; Li, Z. Metal-organic framework derived binary-metal oxide/MXene composite as sulfur host for high-performance lithium-sulfur batteries. J. Alloys Compd. 2022, 899, 163369. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Lee, J.-H.; Kim, C.-H.; Gautam, J.; Heo, K.; Hussain, S.; Ikram, M.; AlObaid, A.A.; Lee, S.-Y.; et al. A Review of Rechargeable Zinc–Air Batteries: Recent Progress and Future Perspectives. Nano-Micro Lett. 2024, 16, 138. [Google Scholar] [CrossRef]
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y. Rechargeable Zn-air batteries: Recent trends and future perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, M.; Wang, K.; Wang, H.; Zuo, Y.; Zhang, M. Performance optimization of zinc-air batteries via nanomaterials. Energy Storage Mater. 2025, 75, 104109. [Google Scholar] [CrossRef]
- Liu, J.-N.; Zhao, C.-X.; Wang, J.; Ren, D.; Li, B.-Q.; Zhang, Q. A brief history of zinc–air batteries: 140 years of epic adventures. Energy Environ. Sci. 2022, 15, 4542–4553. [Google Scholar] [CrossRef]
- Qiao, J.; Bao, Z.; Kong, L.; Liu, X.; Lu, C.; Ni, M.; He, W.; Zhou, M.; Sun, Z. MOF-derived heterostructure CoNi/CoNiP anchored on MXene framework as a superior bifunctional electrocatalyst for zinc-air batteries. Chin. Chem. Lett. 2023, 34, 108318. [Google Scholar] [CrossRef]
- Zou, H.; He, B.; Kuang, P.; Yu, J.; Fan, K. Metal–Organic Framework-Derived Nickel–Cobalt Sulfide on Ultrathin Mxene Nanosheets for Electrocatalytic Oxygen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 22311–22319. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Vallem, S.; Umer, M.; Jyothi, N.; Babu, A.G.; Govindaraju, S.; Son, Y.; Kim, M.J.; Yoon, M. Recent progress on MOF/MXene nanoarchitectures: A new era in coordination chemistry for energy storage and conversion. J. Energy Chem. 2023, 86, 409–436. [Google Scholar] [CrossRef]
- Liang, J.; Liang, K. Nano-bio-interface engineering of metal-organic frameworks. Nano Today 2021, 40, 101256. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Dong, H.; Gui, Q.; Hu, Z.; Li, Y.; Liu, J. Surface and Interface Engineering of Nanoarrays toward Advanced Electrodes and Electrochemical Energy Storage Devices. Adv. Mater. 2021, 33, 2004959. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Hong, J.; Lee, D.; Lee, S.-Y.; Zheng, H. In Situ TEM Characterization of Battery Materials. Chem. Rev. 2025, 125, 1840–1896. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, Q.; Lan, S.; Feng, H.; Zhao, Y.; Li, Q.; Li, X.; Huang, T. Microwave-Assisted Efficient Intercalation for Fast Fabrication of High-Quality and Large-Size Single-Layer Ti3C2T Nanosheets. Adv. Sci. 2024, 11, 2405686. [Google Scholar] [CrossRef]


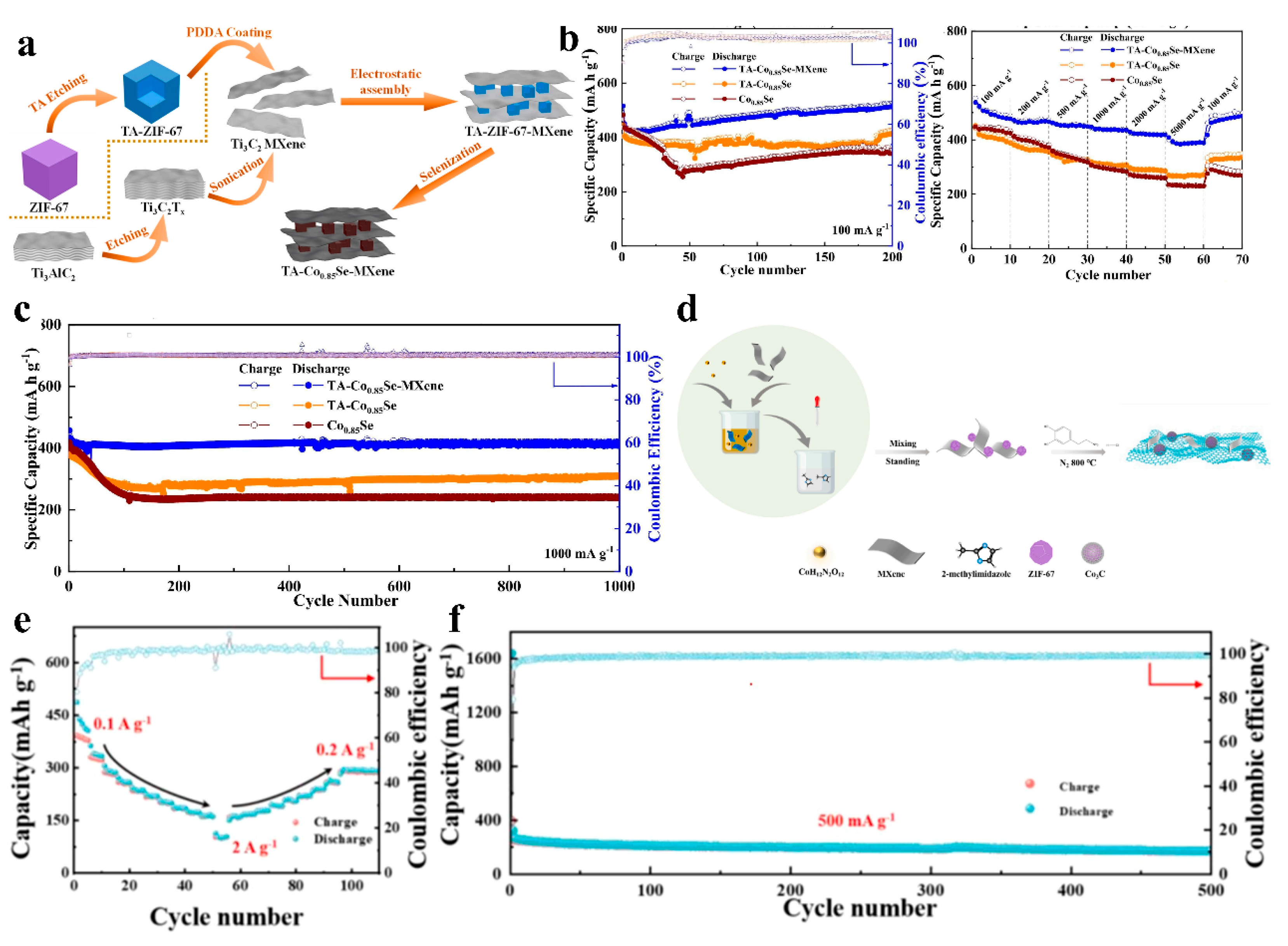
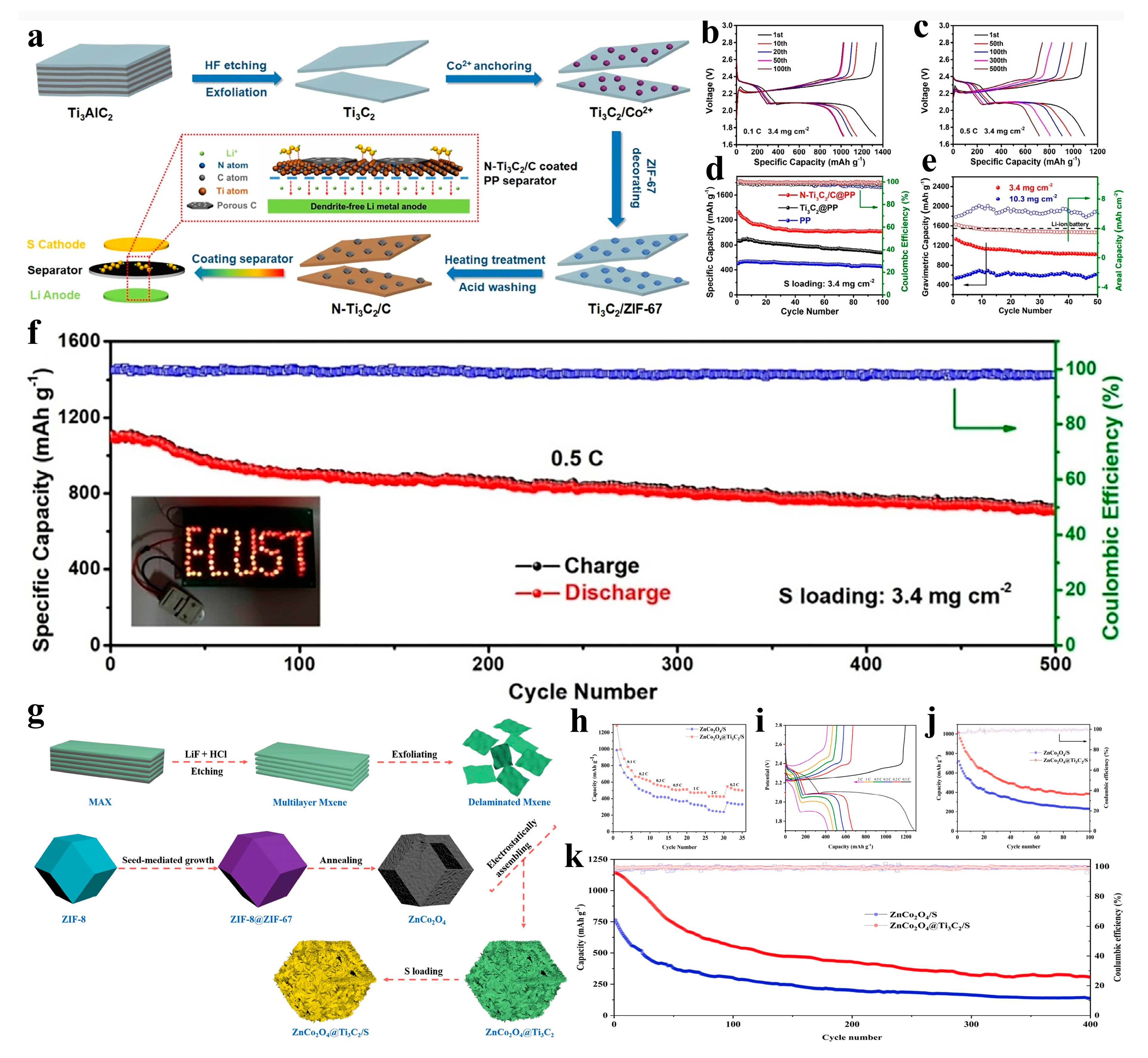
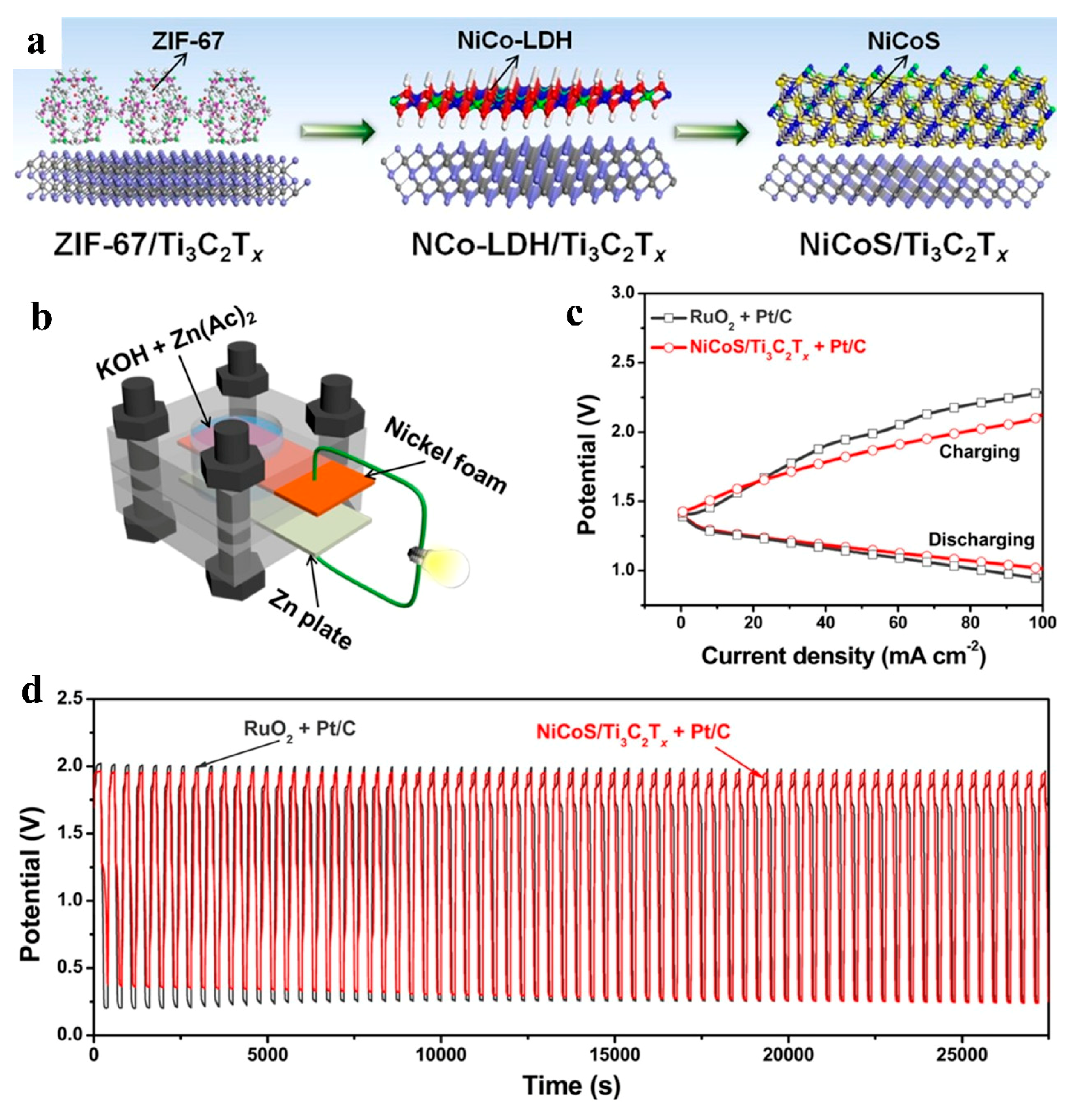
| Material | Performance | Comparison with Single Components | Testing Conditions | Application | Ref. |
|---|---|---|---|---|---|
| MXene@MOF/SA@H hydrogel framework | HER Tafel slope: 61.8 mV dec−1; OER Tafel slope: 84 mV dec−1; no degradation after 1000 cycles | — | Alkaline electrolyte, 25 °C | Electrocatalyst | [47] |
| 3D@2D Co-Cu3P/NC@MXene | HER stability: No LSV curve decay after 1000 CV cycles | Cyclic stability outperformed non-hybridized Co-Cu3P/NC | 0.5 M H2SO4 5 mV s−1 | Electrocatalyst | [48] |
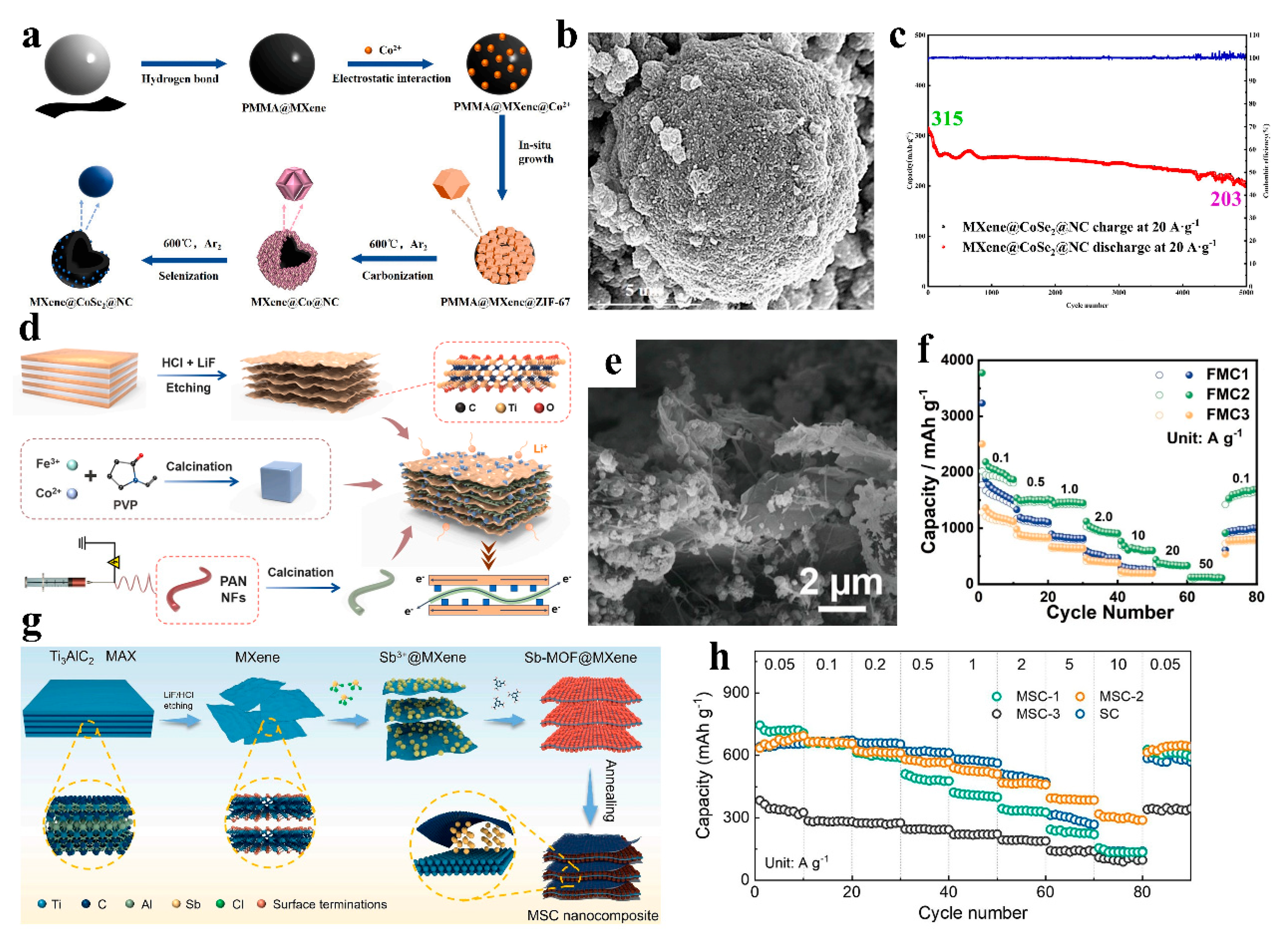
| MXene@CoSe2@NC | 203 mAh g−1 (20 A g−1, 5000 cycles) | Retention rate significantly higher than CoSe2@NC (Figure 8c) | 0.01–3.0 V | SIBs | [50] |
| CoZnSe@N-MX | Ionic conductivity increased by 2.3× (vs. pristine MXene-based counterparts) | Direct comparison with pristine MXene-based materials | 1 A g−1 | SIBs | [53] |
| CoFe MLDH/Ti3C2/NF | OER overpotential optimization (DFT-confirmed interfacial charge redistribution) | Lower overpotential than pure CoFe LDH | 1 M KOH 5 mV s−1 | Electrocatalyst | [54] |
| MXene/NiCoZDH | Specific capacitance: 658 F g−1 (1 A g−1); 92% capacity retention after 5000 cycles | Higher capacitance than pristine MXene, ZIF-67, and non-composited NiCo-ZIF-67 (Figure 7d,e) | 6 M KOH 0–0.5 V | Supercapacitor | [55] |
| Ti3C2 MXene/NFO-8 | Specific capacitance: 660 F g−1 (1 A g−1); energy density: 17.36 Wh kg−1; 70% retention after 5000 cycles | Superior to pristine MXene and NFO-free electrodes | PVA-HOH gel electrolyte | Supercapacitor | [72] |
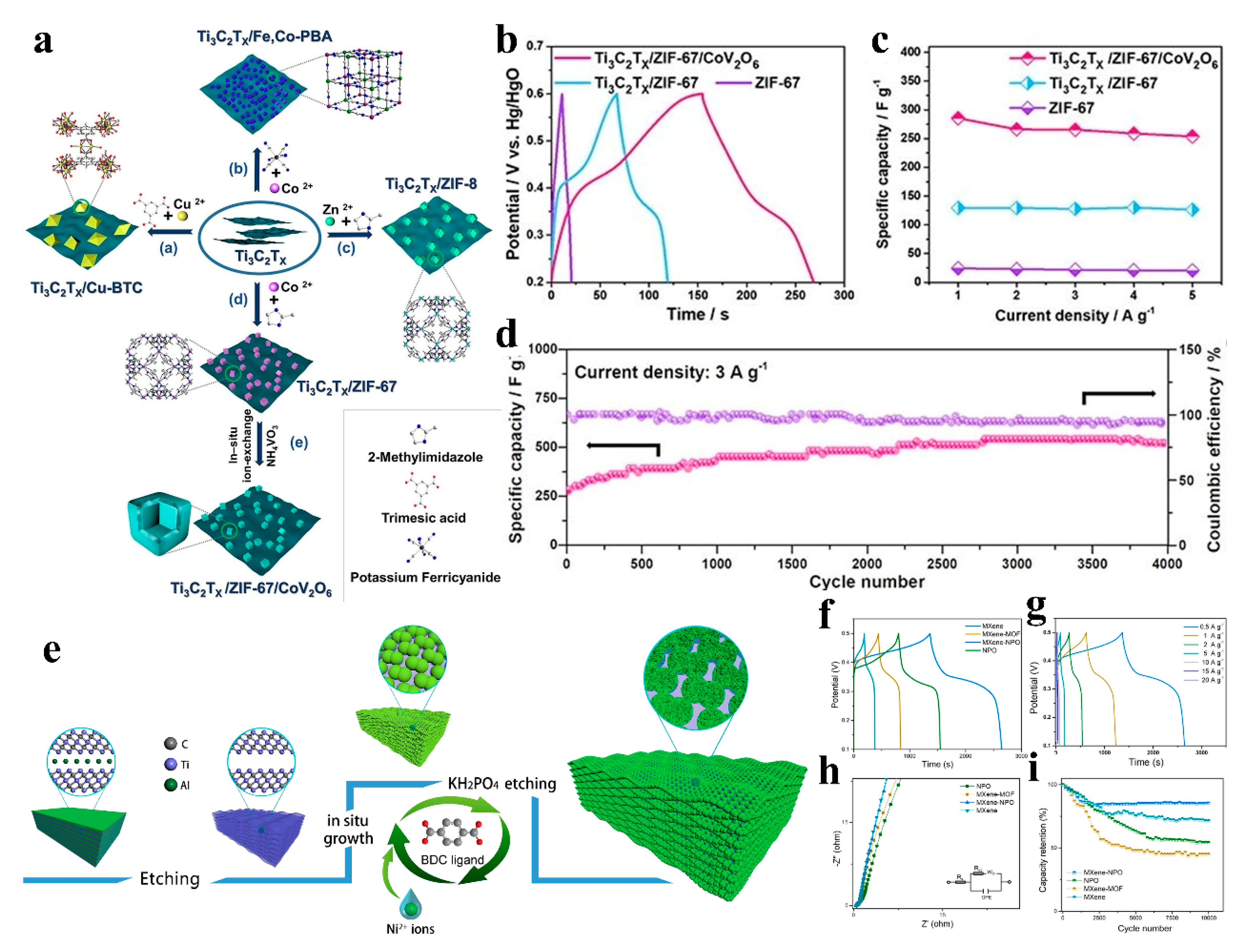
| Ti3C2Tx/ZIF-67/CoV2O6 | Specific capacitance: 285.5 F g−1 (1 A g−1); 94.4% retention after 4000 cycles | Higher capacitance than pristine ZIF-67 and CoV2O6-free composites (Figure 10b,c) | 3 A g−1, KOH/PVA gel electrolyte | Supercapacitor | [73] |
| MXene/NPO | Specific capacity: 639 C g−1 (1 A g−1); energy density: 72.6 Wh kg−1 | Higher capacity than non-composited NPO and MXene | KOH/PVA gel electrolyte, 1.6 V | Supercapacitor | [74] |
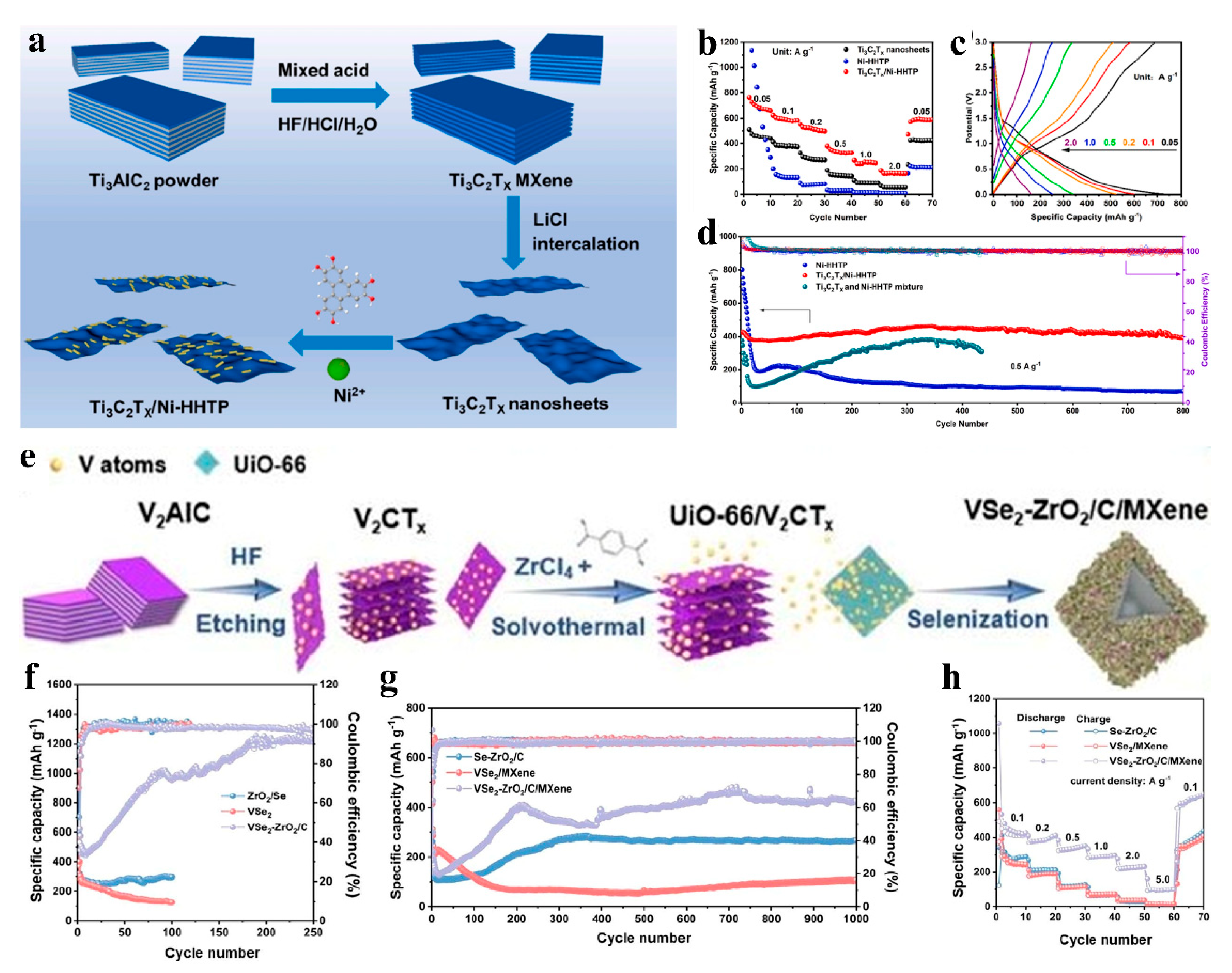
| Ti3C2Tx/Ni-HHTP | Reversible capacity: 390.2 mAh g−1 (0.5 A g−1); 92% retention after 800 cycles | Outperformed pure Ni-HHTP and MXene (rate capability enhanced as shown in Figure 11b) | 0.01–3.0 V | LIBs | [81] |
| VSe2-ZrO2/C/MXene | Reversible capacity: 1238.5 mAh g−1 (100 mA g−1); 430 mAh g−1 retained after 1000 cycles | Superior capacity and cyclability to non-composited VSe2 and ZrO2/C (Figure 11f–h) | 1.0 A g−1, 0.01–3.0 V | LIBs | [82] |
| TA-Co0.85Se-MXene | Rate capability: 389 mAh g−1 (5 A g−1); 418 mAh g−1 retained after 1000 cycles (1 A g−1) | 2×improved cyclability vs. pure Co0.85Se (Figure 12b) | 0.01–3.0 V | SIBs | [87] |
| Co3C/MXene@C | Initial Coulombic efficiency: 98.43%; 89% capacity retention after 500 cycles | Initial Coulombic efficiency: 98.43%; 89% capacity retention after 500 cycles | 500 mA g−1, 0.01–3.0 V | SIBs | [88] |
| N-Ti3C2/C@PP | Reversible capacity: 716 mAh g−1 (0.5 C); >80% retention after 500 cycles | Performance exceeds unmodified Ti3C2@PP and pure PP separators (Figure 13d–f) | Sulfur loading: 3.4 mg cm−2 | LSBs | [93] |
| ZnCo2O4@Ti3C2/S | Initial discharge capacity: 1320 mAh g−1 (0.1 C); >85% retention after 100 cycles | Higher capacity retention than ZnCo2O4/S without MXene (Figure 13j,k) | 0.2 C, 1.7–2.8 V | LSBs | [94] |
| NiCoS/Ti3C2Tx | Voltage gap: 0.7 V (50 mA cm−2); no decay after 8 h of constant-current cycling | Lower overpotential than non-composited NiCoS | 6 M KOH/ 0.2 M Zn(CH3COO)2 electrolyte | ZABs | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Wen, S.; Wang, R.; Yang, X.; Yuan, X.; Liu, Y.; Ma, J.; Li, Z. MXene/MOF-Derived Composites with Multidimensional Nanostructures: Synthesis Methods, Performance, and Applications in the Field of Energy Storage. Nanomaterials 2025, 15, 841. https://doi.org/10.3390/nano15110841
Feng S, Wen S, Wang R, Yang X, Yuan X, Liu Y, Ma J, Li Z. MXene/MOF-Derived Composites with Multidimensional Nanostructures: Synthesis Methods, Performance, and Applications in the Field of Energy Storage. Nanomaterials. 2025; 15(11):841. https://doi.org/10.3390/nano15110841
Chicago/Turabian StyleFeng, Shufan, Shilong Wen, Rutao Wang, Xiaokun Yang, Xiangsen Yuan, Yuxuan Liu, Jingyun Ma, and Zhaoqiang Li. 2025. "MXene/MOF-Derived Composites with Multidimensional Nanostructures: Synthesis Methods, Performance, and Applications in the Field of Energy Storage" Nanomaterials 15, no. 11: 841. https://doi.org/10.3390/nano15110841
APA StyleFeng, S., Wen, S., Wang, R., Yang, X., Yuan, X., Liu, Y., Ma, J., & Li, Z. (2025). MXene/MOF-Derived Composites with Multidimensional Nanostructures: Synthesis Methods, Performance, and Applications in the Field of Energy Storage. Nanomaterials, 15(11), 841. https://doi.org/10.3390/nano15110841







