Nanoscale Cross-Sectional Characterization of Thin Layers in Material Assemblies
Abstract
1. Introduction
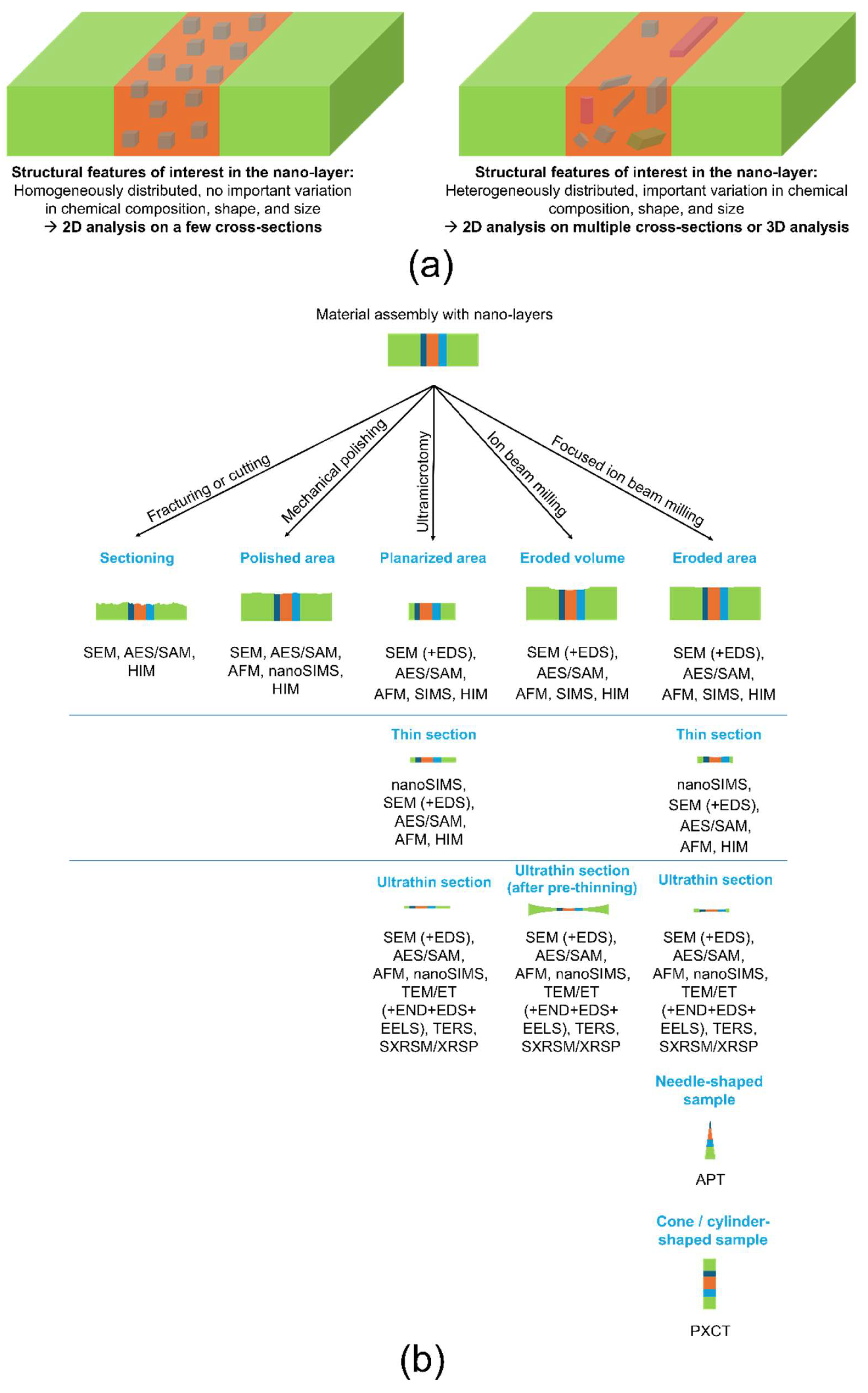
2. Sample Preparation Methods
3. Techniques Providing 2D Structural Information
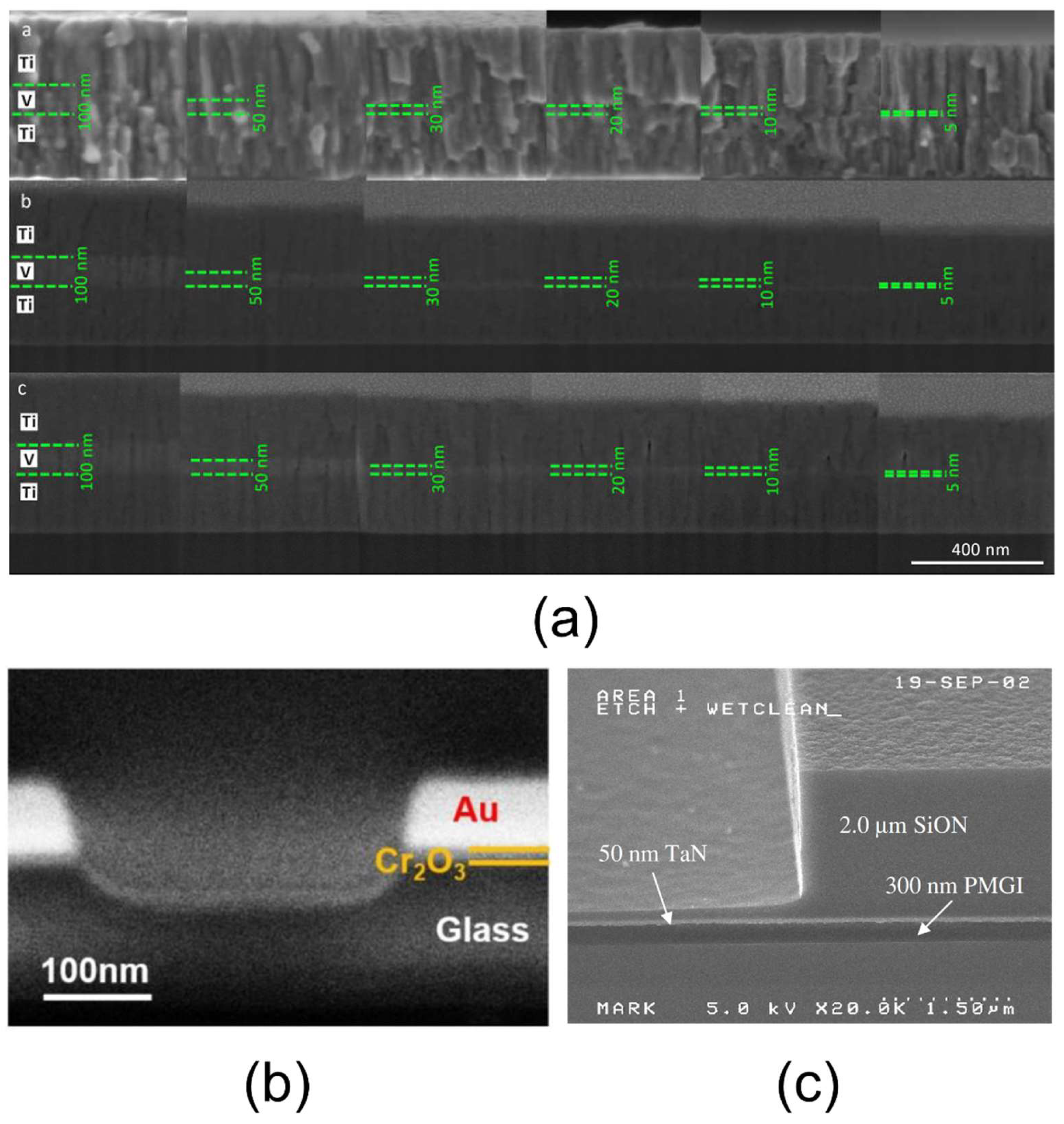
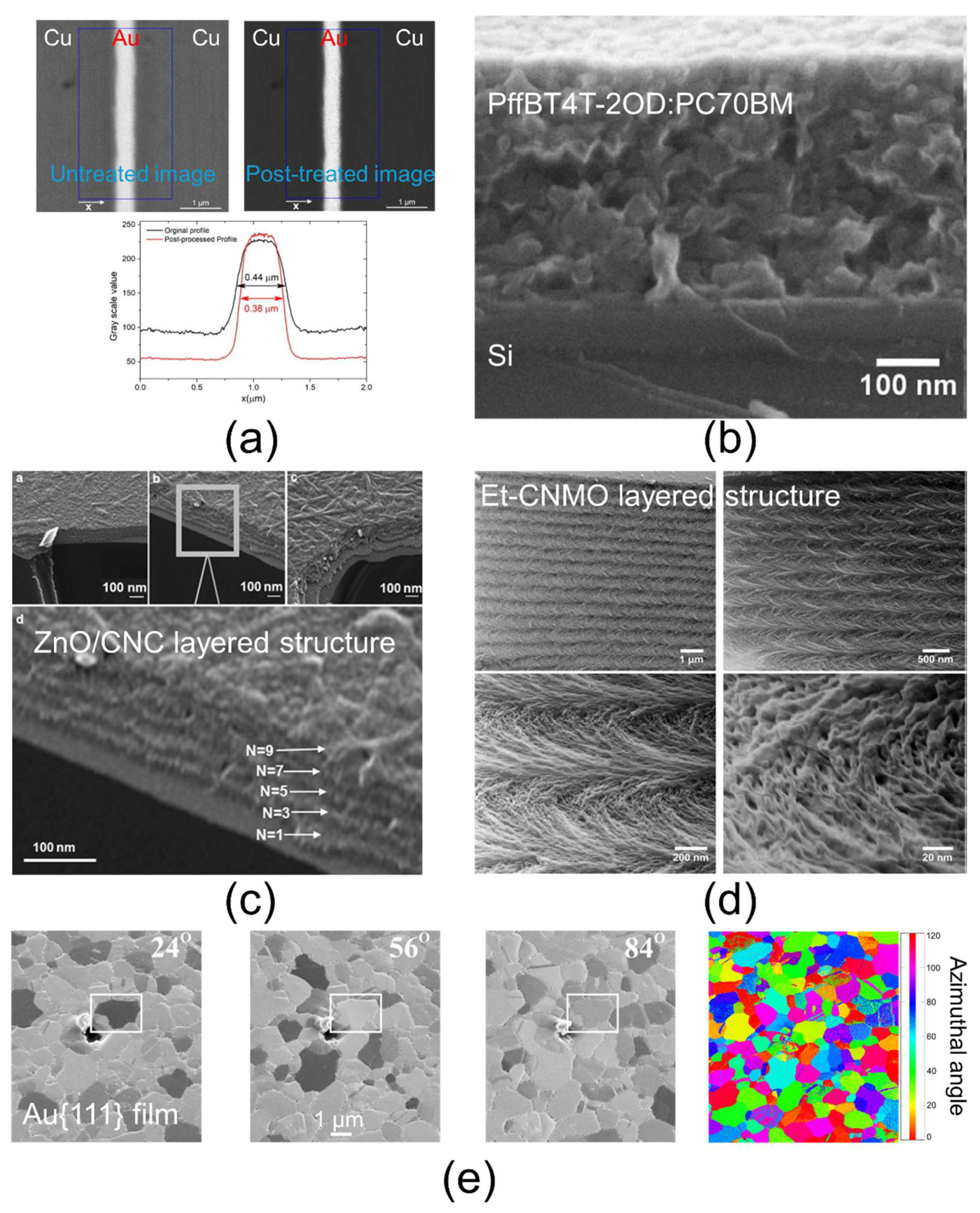
3.1. SEM
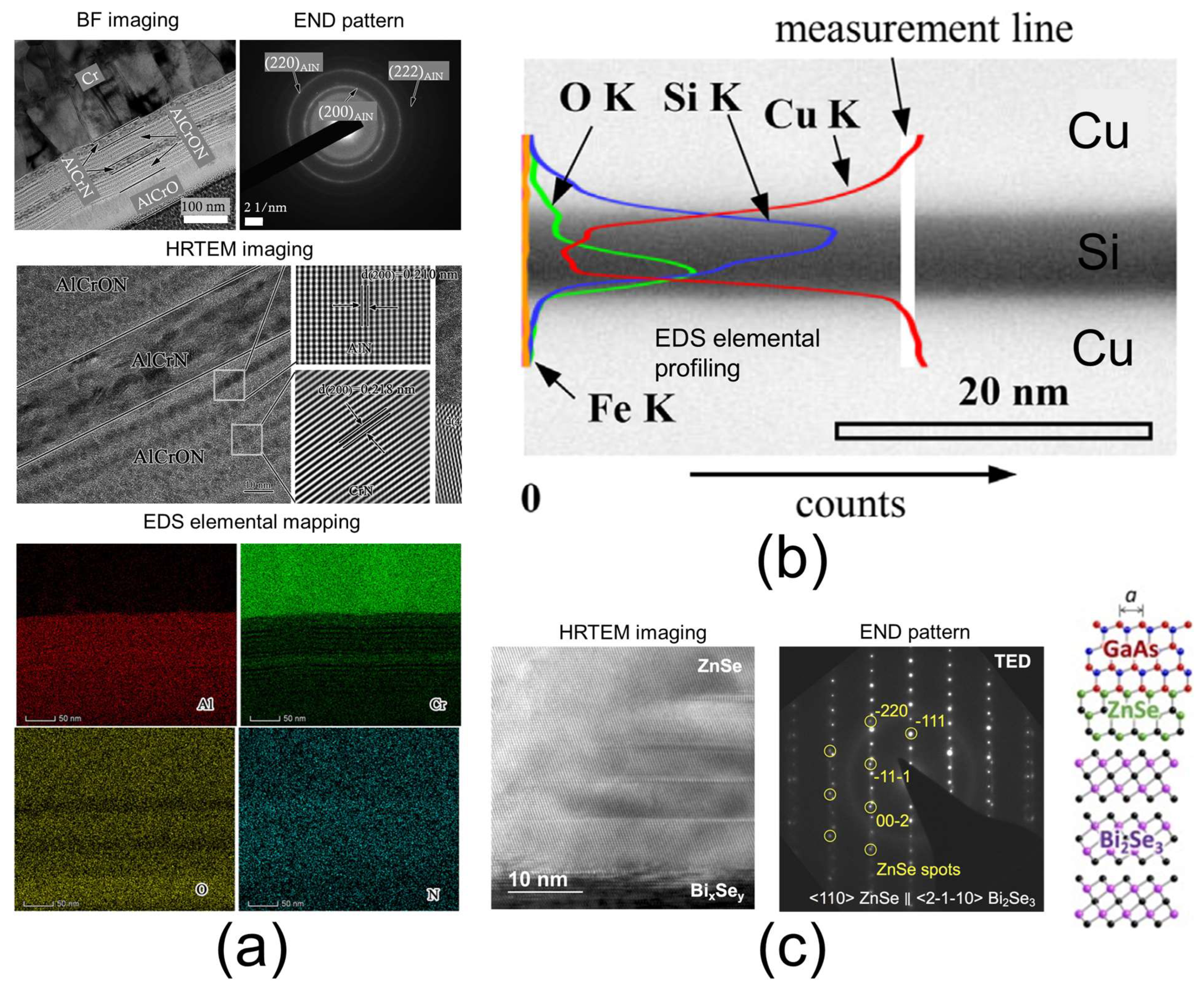
3.2. TEM
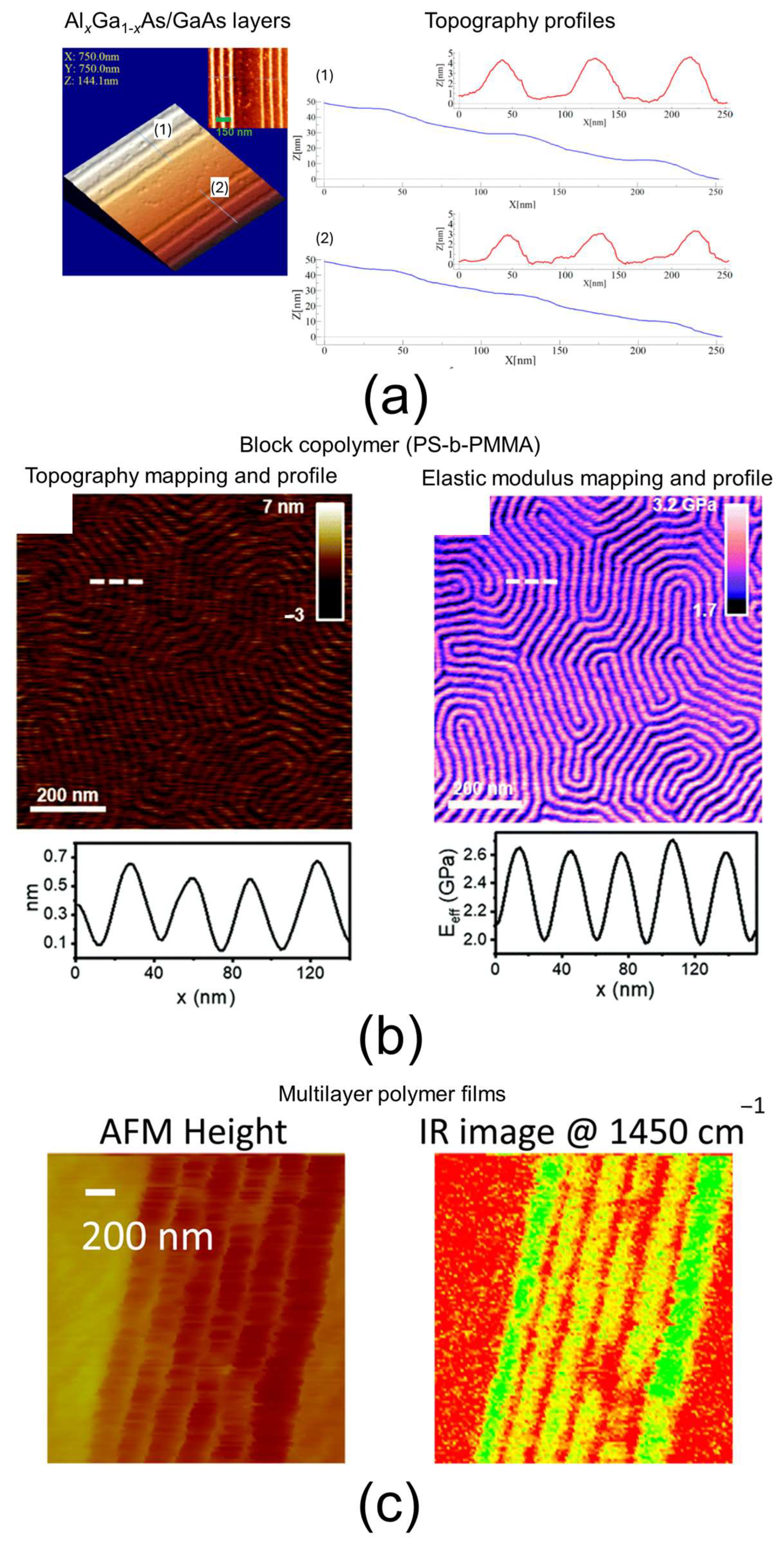
3.3. AFM
3.4. HIM
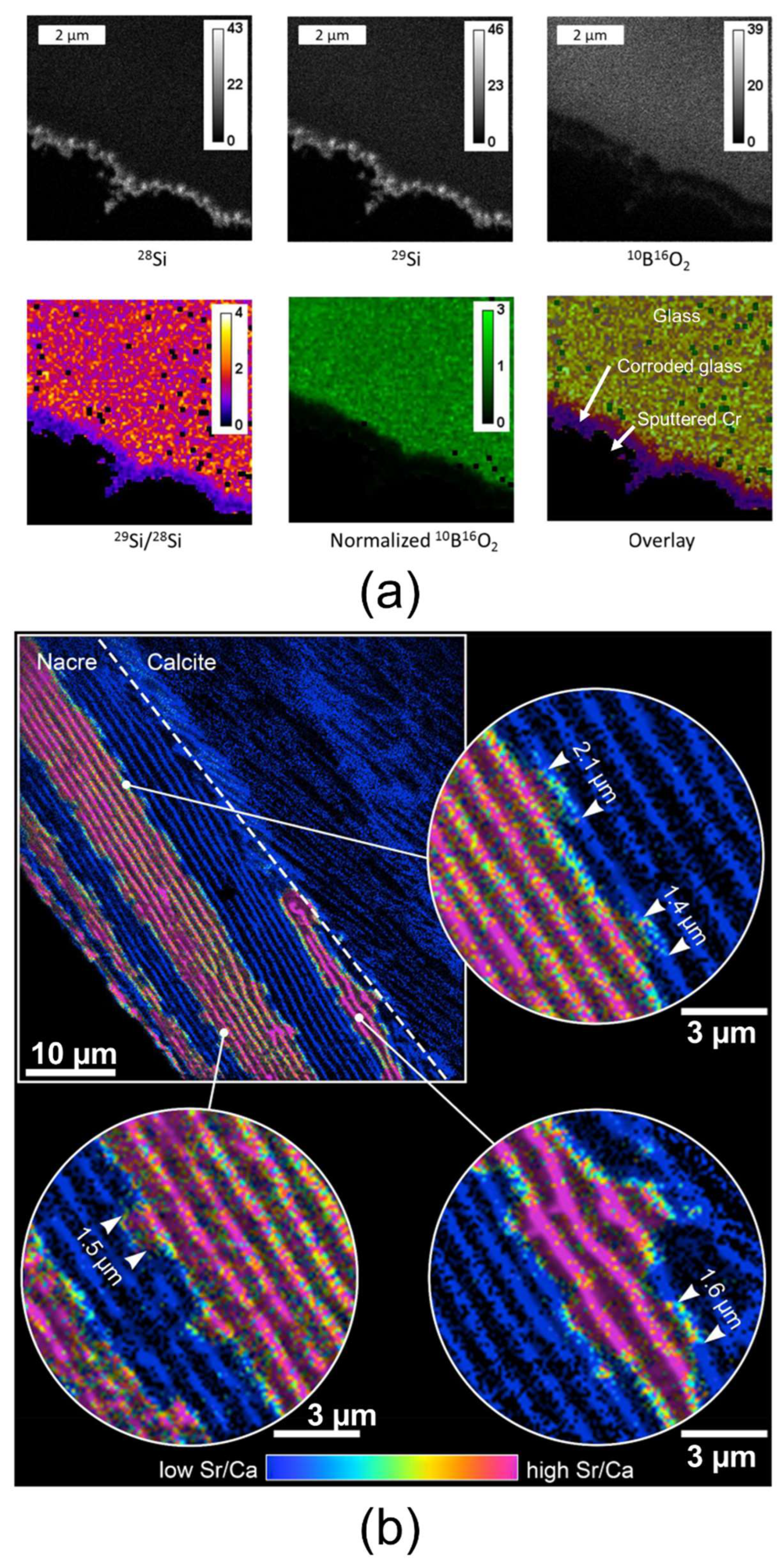
3.5. AES
3.6. nanoSIMS
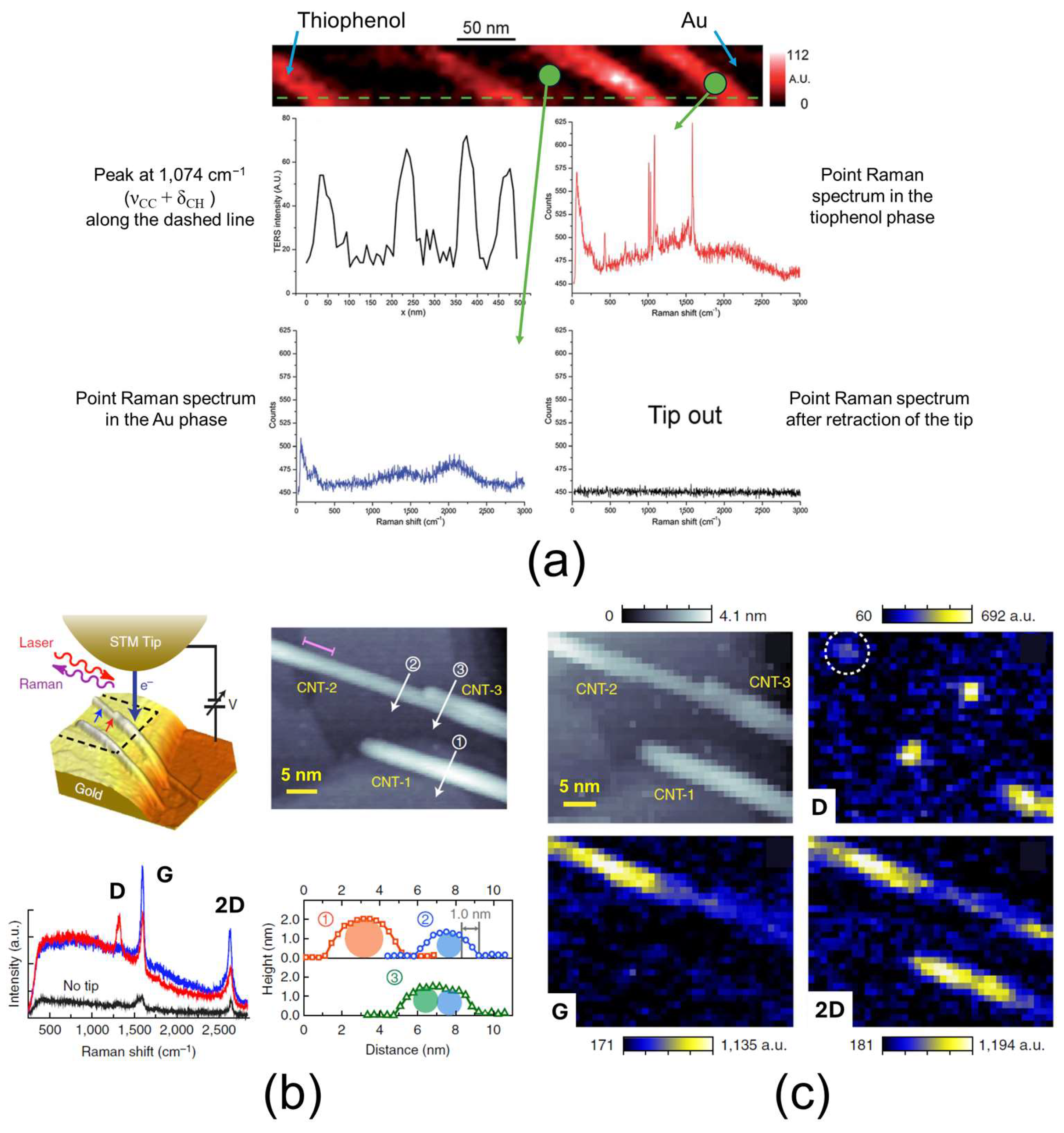
3.7. TERS
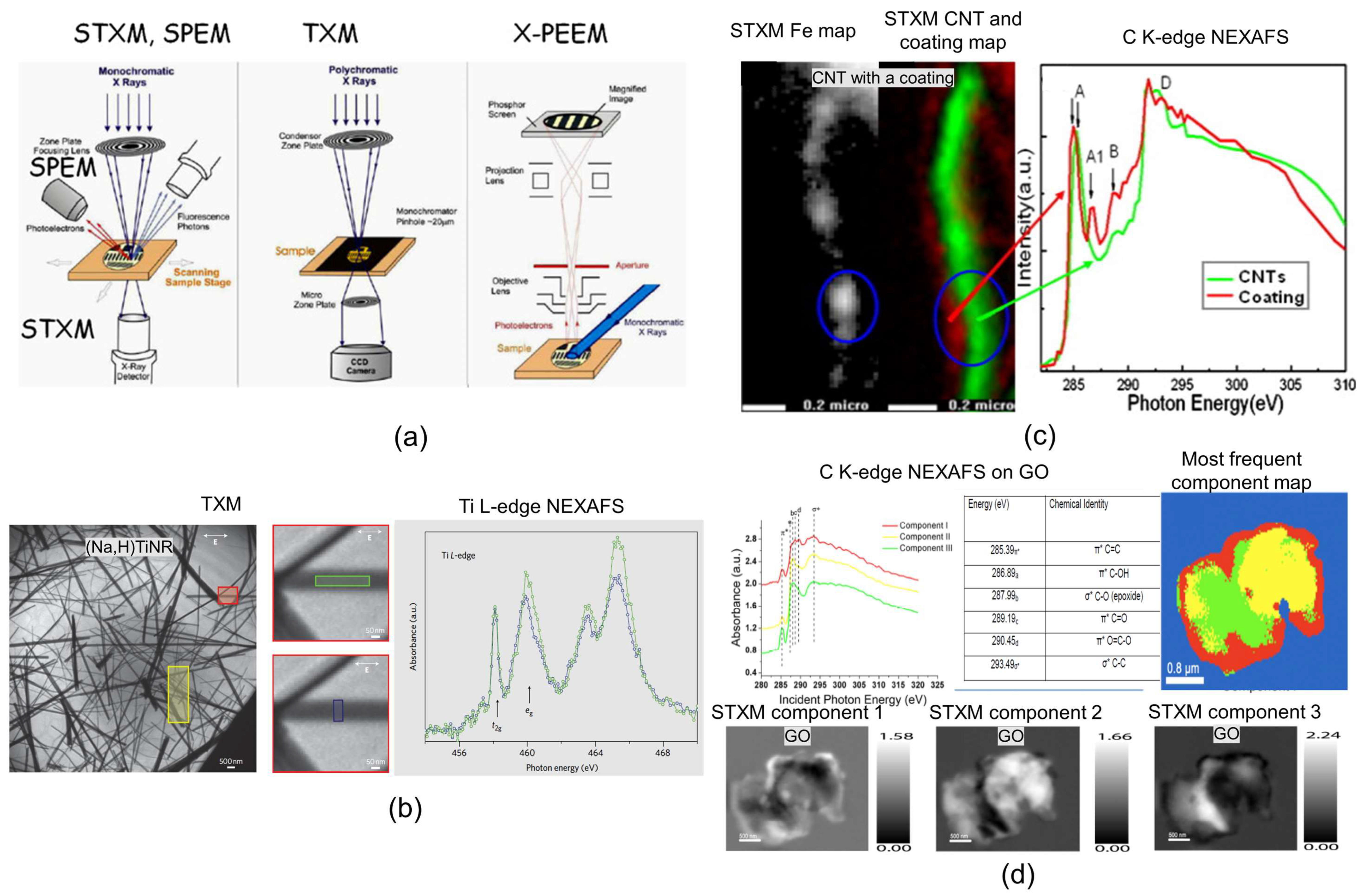
3.8. SXRSM
4. Techniques Providing 3D Structural Information
4.1. APT
4.2. ET
4.3. PXCT
5. Emerging Techniques and Methods
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gay, C. Stickiness: Some Fundamentals of Adhesion. Integr. Comp. Biol. 2002, 42, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Wang, F.; Li, J.; Liu, Q.; Zhang, G.; Li, W.; Yang, C.; Sun, R. Temporary Bonding and Debonding in Advanced Packaging: Recent Progress and Applications. Electronics 2023, 12, 1666. [Google Scholar] [CrossRef]
- Jong Ho, R.; Chang Yol, Y.; Joung Ah, K. Copper Foil Attached to the Carrier Foil, a Method for Preparing the Same and Printed Circuit Board Using the Same. US8530749B2, 10 September 2013. [Google Scholar]
- Montméat, P.; Enot, T.; Enyedi, G.; Pellat, M.; Thooris, J.; Fournel, F. Development and adhesion characterization of a silicon wafer for temporary bonding. Int. J. Adhes. Adhes. 2018, 82, 100–107. [Google Scholar] [CrossRef]
- Puligadda, R.; Pillalamarri, S.; Hong, W.; Brubaker, C.; Wimplinger, M.; Pargfrieder, S. High-Performance Temporary Adhesives for Wafer Bonding Applications. MRS Proc. 2006, 970, Y04–Y09. [Google Scholar] [CrossRef]
- Strepenne, F. Adhesion of Thin Films on Steel and Separation of the Energy Contributions. Ph.D. Thesis, Université Catholique de Louvain, Louvain-la-Neuve, Belgium, 2010. [Google Scholar]
- Beom, W.J.; Lee, S.H.; Choi, E.S.; Song, K.D.; Kim, H.C. Carrier-Foil-Attached Ultra-Thin Copper Foil. WO2018131962A1, 19 July 2018. [Google Scholar]
- Kaidi, Z.; Devahif, T.; Kersten, A.; Streel, M. Composite Copper Foil and Method of Fabricating the Same. US11639557B2, 12 April 2023. [Google Scholar]
- Guo, L.; Liu, J.; Xia, H.; Li, X.; Zhang, X.; Yang, H. Effects of Surface Treatment and Adhesive Thickness on the Shear Strength of Precision Bonded Joints. Polym. Test. 2021, 94, 107063. [Google Scholar] [CrossRef]
- Gleich, D.M.; Van Tooren, M.J.L.; Beukers, A. Analysis and evaluation of bondline thickness effects on failure load in adhesively bonded structures. J. Adhes. Sci. Technol. 2001, 15, 1091–1101. [Google Scholar] [CrossRef]
- Da Silva, L.F.M.; Rodrigues, T.N.S.S.; Figueiredo, M.A.V.; De Moura, M.F.S.F.; Chousal, J.A.G. Effect of Adhesive Type and Thickness on the Lap Shear Strength. J. Adhes. 2006, 82, 1091–1115. [Google Scholar] [CrossRef]
- Carbas, R.J.C.; Dantas, M.A.; Marques, E.A.S.; Da Silva, L.F.M. Effect of the adhesive thickness on butt adhesive joints under torsional loads. J. Adv. Join. Process. 2021, 3, 100061. [Google Scholar] [CrossRef]
- Kumar, S.; Gaire, B.; Persson, B.N.J.; Dhinojwala, A. Impact of Nanometer-Thin Stiff Layer on Adhesion to Rough Surfaces. Phys. Rev. Res. 2024, 6, 033015. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, J.; Sim, Y.; Lee, E. Minimization of residual layer thickness by using the optimized dispensing method in S-FILTM process. Microelectron. Eng. 2006, 83, 847–850. [Google Scholar] [CrossRef]
- Available online: https://scifinder-n.cas.org/ (accessed on 9 September 2024).
- Zhang, Z.; Wang, C.; Wang, X.; Reddy, K.M.; Liu, P.; Wang, Y.; Song, S. Precise quantification of the adhesion between metallic thin films and silicon wafer. Thin Solid Film. 2023, 784, 140081. [Google Scholar] [CrossRef]
- Matsumae, T.; Kurashima, Y.; Takagi, H.; Shirayanagi, Y.; Hiza, S.; Nishimura, K.; Higurashi, E. Room temperature bonding of GaN and diamond substrates via atomic layer. Scr. Mater. 2022, 215, 114725. [Google Scholar] [CrossRef]
- Suga, T. Cu-Cu Room Temperature Bonding-Current Status of Surface Activated Bonding (SAB). ECS Trans. 2006, 3, 155–163. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Chen, Y.; Kang, Q.; Luo, S.; Li, Z.; Xu, X.; Liu, Q.; Sui, X. Air atmosphere diffusion bonding of Al–Mg–Li alloy using Cu nano-coating interlayer: Microstructural characterization and formation mechanisms. Mater. Des. 2022, 215, 110431. [Google Scholar] [CrossRef]
- Chu, W.H.; Chin, R.; Huen, T.; Ferrari, M. Silicon membrane nanofilters from sacrificial oxide removal. J. Microelectromechanical Syst. 1999, 8, 34–42. [Google Scholar] [CrossRef]
- Lee, T.-H.; Huang, C.-H.; Yang, Y.Y.; Suryasindhu, T.; Li, P.W. Nanoscale thick layer transfer of hydrogen-implanted wafer by using polycrystalline silicon sacrificial layer. Appl. Phys. Lett. 2007, 91, 203119. [Google Scholar] [CrossRef]
- Stelling, C.; Retsch, M. Nanomeshes at Liquid Interfaces: From Free-Standing Hole Arrays toward Metal–Insulator–Metal Architectures. Adv. Mater. Interfaces 2018, 5, 1800154. [Google Scholar] [CrossRef]
- Hein, E. Lithography-free glass surface modification by self-masking during dry etching. J. Nanophotonics 2011, 5, 051703. [Google Scholar] [CrossRef]
- Liao, P.-Y.; Khot, K.; Alajlouni, S.; Snure, M.; Noh, J.; Si, M.; Zhang, Z.; Shakouri, A.; Ruan, X.; Ye, P.D. Alleviation of Self-Heating Effect in Top-Gated Ultrathin In 2 O 3 FETs Using a Thermal Adhesion Layer. IEEE Trans. Electron. Devices 2023, 70, 113–120. [Google Scholar] [CrossRef]
- Kim, A.; Ahn, Y.; Li, W.; Park, S.H.; Cho, M.J.; Choi, D.H.; Yang, H. Stretchable Semiconducting Polymers with Hydrogen-Bonding-Capable Conjugation Breakers: Synthesis and Application in Organic Thin-Film Transistors. ACS Appl. Mater. Interfaces 2023, 15, 58663–58672. [Google Scholar] [CrossRef]
- Montméat, P.; Dechamp, J.; Enyedi, G.; Fournel, F.; Zavvou, Z.; Jousseaume, V. Initiated Chemical Vapor Deposition of polysiloxane as adhesive nanolayer for silicon wafer bonding. Mater. Sci. Semicond. Process. 2022, 148, 106808. [Google Scholar] [CrossRef]
- Sutjianto, J.G.; Yoo, S.H.; Westerman, C.R.; Jackson, T.N.; Wilker, J.J.; Gomez, E.D. Blends of Conjugated and Adhesive Polymers for Sticky Organic Thin-Film Transistors. Adv. Electron. Mater. 2023, 9, 2300422. [Google Scholar] [CrossRef]
- Hao, L.; Cheng, H.; Ouyang, J.; Huan, Y.; Yan, J. Integration of Ferroelectric K0.5Na0.5NbO3 films on Si at 400 °C. Mater. Today Commun. 2022, 32, 104133. [Google Scholar] [CrossRef]
- Takakura, R.; Murakami, S.; Watanabe, K.; Takigawa, R. Room-temperature bonding of Al2O3 thin films deposited using atomic layer deposition. Sci. Rep. 2023, 13, 3581. [Google Scholar] [CrossRef]
- Xu, Z.; He, Z.; Yuan, Y.J. Investigation of sacrificial layer masking fabrication of dual-frequency quartz crystal microbalance. Sens. Actuators A Phys. 2018, 282, 157–162. [Google Scholar] [CrossRef]
- Yasuda, S.; Miyagawa, T.; Yonezu, A.; Ishibashi, K. Laser shock-wave adhesion test (LaSAT) and ab initio calculations for adhesive strength evaluation of thin metallic films. Mater. Today Commun. 2023, 35, 106237. [Google Scholar] [CrossRef]
- Amino, T.; Uomoto, M.; Shimatsu, T. Bonding performance in atomic diffusion bonding of wafers using amorphous Si thin films with smooth surface. Jpn. J. Appl. Phys. 2022, 61, SF1002. [Google Scholar] [CrossRef]
- Matsui, J.; Kubota, K.; Kado, Y.; Miyashita, T. Electroless Copper Plating onto Polyimide Using Polymer Nanosheet as a Nano-Adhesive. Polym. J. 2007, 39, 41–47. [Google Scholar] [CrossRef]
- Toru, H.; Akitoshi, T.; Tetsuhiro, M.; Ayumu, T. Ultrathin Copper Foil with Carrier and Method for Manufacturing Same. WO2016117587A1, 28 July 2016. [Google Scholar]
- Zhang, X.; Zhang, P.; Zhang, W.; Chen, J.; Hu, F. Preparation of UV Curable Optical Adhesive NOA81 Bionic Lotus Leaf Structure Films by Nanoimprint Technique and the Applications on Silicon Solar Cells. Coatings 2023, 13, 867. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Zhao, Z.; Mohamed, M.S.; Wang, J.; Zhu, X. FDTD simulation on transmittance of silica microsphere thin films with varying embedding in an optical adhesive. Optik 2021, 241, 166997. [Google Scholar] [CrossRef]
- She, S.; Zhang, Y.; Huang, Z.; Zhou, J.; Ke, S. Effect of the Thickness of the a-Si Bonding Layer at InGaAs/Si Bonded Interface on the Performance of InGaAs/Si Avalanche Photodiode. Acta Photonica Sin. 2022, 51, 0251218. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Liu, L.; Han, Y.; Tan, H.; Liu, L.; Lin, Z.; Yu, S.; Wang, R.; Cai, X. Heterogeneous integration of III–V semiconductor lasers on thin-film lithium niobite platform by wafer bonding. Appl. Phys. Lett. 2023, 122, 081103. [Google Scholar] [CrossRef]
- Yamada, Y.; Fukumoto, S.; Fujimoto, K. Mechanism for Adhesion of Epoxy Resin to Copper Substrate through C-H-Si Amorphous Thin Film. J. Smart Process. 2021, 10, 308–315. [Google Scholar] [CrossRef]
- Li, J.; Zhu, P.; Liu, L.; Huang, Y. Preparation Method of Nano Conductive Adhesive. CN116042131A, 23 July 2023. [Google Scholar]
- Howlader, M.M.R.; Zhang, F.; Deen, M.J.; Suga, T.; Yamauchi, A. Surface activated bonding of copper through silicon vias and gold stud bumps at room temperature. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 021007. [Google Scholar] [CrossRef]
- Chua, S.T.; Siow, K.S. Microstructural studies and bonding strength of pressureless sintered nano-silver joints on silver, direct bond copper (DBC) and copper substrates aged at 300 °C. J. Alloys Compd. 2016, 687, 486–498. [Google Scholar] [CrossRef]
- Tan, S.; Xiang, R. Thermally-Conductive Adhesive with High Thermal Conductivity and High Ageing Resistance as well as Preparation Method and Application Thereof. CN105199611A, 30 December 2015. [Google Scholar]
- Ding, Y.; Garland, S.; Howland, M.; Revzin, A.; Pan, T. Universal Nanopatternable Interfacial Bonding. Adv. Mater. 2011, 23, 5551–5556. [Google Scholar] [CrossRef]
- Ding, Y.; Garland, S.; Howland, M.; Revzin, A.; Pan, T. Universal Anisotropically Conductive Nano-adhesive of PDMS Oligomers. MRS Proc. 2013, 1553, 509. [Google Scholar] [CrossRef]
- Marques, A.; Mocanu, A.; Tomić, N.; Balos, S.; Stammen, E.; Lundevall, A.; Abrahami, S.T.; Günther, R.; de Kok, J.M.M.; de Freitas, S.T. Review on Adhesives and Surface Treatments for Structural Applications: Recent Developments on Sustainability and Implementation for Metal and Composite Substrates. Materials 2020, 13, 5590. [Google Scholar] [CrossRef]
- Şakalak, H.; Yilmaz, K.; Gürsoy, M.; Karaman, M. Roll-to-Roll Vapor Deposition of Hydrophobic and Transparent Nano-Adhesive Polymeric Thin Films on Rigid and Flexible Substrates. Ind. Eng. Chem. Res. 2022, 61, 8839–8846. [Google Scholar] [CrossRef]
- Hashemi Nasr, F.; Barikani, M.; Salehirad, M. Preparation of self-healing polyurethane/functionalized graphene nanocomposites as electro-conductive one part adhesives. RSC Adv. 2018, 8, 31094–31105. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Thundat, T.; Zeng, H. Polypyrrole-Doped Conductive Supramolecular Elastomer with Stretchability, Rapid Self-Healing, and Adhesive Property for Flexible Electronic Sensors. ACS Appl. Mater. Interfaces 2019, 11, 18720–18729. [Google Scholar] [CrossRef] [PubMed]
- Liu, H. Nano superpolymer high-flame-retardancy anti-aging adhesive and preparation method thereof. CN104974674A, 14 October 2015. [Google Scholar]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Sikora, M.; Wojcieszak, D.; Chudzyńska, A.; Zięba, A. Improved Methodology of Cross-Sectional SEM Analysis of Thin-Film Multilayers Prepared by Magnetron Sputtering. Coatings 2023, 13, 316. [Google Scholar] [CrossRef]
- Höflinger, G. Brief Introduction to Freeze Fracture and Etching. 2014. Available online: https://www.leica-microsystems.com/science-lab/life-science/brief-introduction-to-freeze-fracture-and-etching/ (accessed on 11 November 2024).
- Li, X.; Mu, Z.; Song, X. Microstructure and Stress Evolution of W Nanofilms Prepared by Arc Ion Plating under Different Deposition Time and Substrate Bias. J. Mater. Sci. 2024, 59, 19254–19272. [Google Scholar] [CrossRef]
- Jebali, S.; Vayer, M.; Belal, K.; Mahut, F.; Sinturel, C. Dip-Coating Deposition of Nanocomposite Thin Films Based on Water-Soluble Polymer and Silica Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132688. [Google Scholar] [CrossRef]
- Buzea, C.; Beydaghyan, G.; Elliott, C.; Robbie, K. Control of Power Law Scaling in the Growth of Silicon Nanocolumn Pseudo-Regular Arrays Deposited by Glancing Angle Deposition. Nanotechnology 2005, 16, 1986–1992. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Dražić, G. Influence of Growth Defects on the Oxidation Resistance of Sputter-Deposited TiAlN Hard Coatings. Coatings 2021, 11, 123. [Google Scholar] [CrossRef]
- Hayles, M.F.; De Winter, D.A.M. An Introduction to cryo-FIB-SEM Cross-sectioning of Frozen, Hydrated Life Science Samples. J. Microsc. 2021, 281, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Biddut, A.Q.; Zhang, L.C.; Ali, Y.M.; Liu, Z. Damage-Free Polishing of Monocrystalline Silicon Wafers without Chemical Additives. Scr. Mater. 2008, 59, 1178–1181. [Google Scholar] [CrossRef]
- Vlcak, P.; Jirka, I. Protective Sliding Carbon-Based Nanolayers Prepared by Argon or Nitrogen Ion-Beam Assisted Deposition on Ti6Al4V Alloy. J. Nanomater. 2016, 5, 1–9. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Bark, C.W. Effect of Surface Treatment by Chemical-Mechanical Polishing for Transparent Electrode of Perovskite Solar Cells. Energies 2020, 13, 585. [Google Scholar] [CrossRef]
- Samim, P.M.; Fattah-alhosseini, A.; Elmkhah, H.; Imantalab, O. Nanoscale Architecture of ZrN/CrN Coatings: Microstructure, Composition, Mechanical Properties and Electrochemical Behavior. J. Mater. Res. Technol. 2021, 15, 542–560. [Google Scholar] [CrossRef]
- Cairney, J.M.; Munroe, P.R.; Hoffman, M. The Application of Focused Ion Beam Technology to the Characterization of Coatings. Surf. Coat. Technol. 2005, 198, 165–168. [Google Scholar] [CrossRef]
- Keim, E.G.; Bijker, M.D.; Lodder, J.C. Preparation of Cross-Sectional Transmission Electron Microscopy Specimens of Obliquely Deposited Magnetic Thin Films on a Flexible Tape. J. Vac. Sci. Technol. A Vac. Surf. Film. 2001, 19, 1191–1194. [Google Scholar] [CrossRef]
- Cha, H.-W.; Kang, M.-C.; Shin, K.; Yang, C.-W. Transmission Electron Microscopy Specimen Preparation of Delicate Materials Using Tripod Polisher. Appl. Microsc. 2016, 46, 110–115. [Google Scholar] [CrossRef]
- Enganati, S.K.; Addiego, F.; Fernandes, J.P.C.; Koutsawa, Y.; Zielinski, B.; Ruch, D.; Mertz, G. Multiscale Characterization of the Interfacial Region in Flexible Rubber Composites: Initial Structure and Evolution upon Thermal Treatment. Polym. Test. 2021, 98, 107203. [Google Scholar] [CrossRef]
- Ranner, R.; DeRose, J. Introduction to Ultramicrotomy. 2019. Available online: https://www.leica-microsystems.com/science-lab/life-science/introduction-to-ultramicrotomy/ (accessed on 13 November 2024).
- Zhang, H.; Wang, C.; Zhou, G. Ultra-Microtome for the Preparation of TEM Specimens from Battery Cathodes. Microsc. Microanal. 2020, 26, 867–877. [Google Scholar] [CrossRef]
- Li, J.; Lu, W.; Gibson, J.; Zhang, S.; Chen, T.; Korte-Kerzel, S.; Raabe, D. Eliminating Deformation Incompatibility in Composites by Gradient Nanolayer Architectures. Sci. Rep. 2018, 8, 16216. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-H.; Yoo, S.M.; Sohn, B.-H.; Jung, J.C.; Zin, W.-C.; Kwak, S.-Y.; Lee, T.S. Electrically Anisotropic Thin Films Consisting of Polymeric and Metallic Nanolayers from Self-Assembled Lamellae of Diblock Copolymers. Langmuir 2005, 21, 3625–3628. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, J.-I.; Seo, W.-S. Exfoliation Route to Nanostructured Cobalt Oxide with Enhanced Thermoelectric Performance. Appl. Phys. Express 2011, 4, 065201. [Google Scholar] [CrossRef]
- Montana, J.-S.; Roland, S.; Richaud, E.; Miquelard-Garnier, G. From Equilibrium Lamellae to Out-of-Equilibrium Cylinders in Triblock Copolymer Nanolayers Obtained via Multilayer Coextrusion. Polymer 2018, 136, 27–36. [Google Scholar] [CrossRef]
- De Samber, B.; De Rycke, R.; De Bruyne, M.; Kienhuis, M.; Sandblad, L.; Bohic, S.; Cloetens, P.; Urban, C.; Polerecky, L.; Vincze, L. Effect of Sample Preparation Techniques upon Single Cell Chemical Imaging: A Practical Comparison between Synchrotron Radiation Based X-Ray Fluorescence (SR-XRF) and Nanoscopic Secondary Ion Mass Spectrometry (Nano-SIMS). Anal. Chim. Acta 2020, 1106, 22–32. [Google Scholar] [CrossRef]
- Timmermans, N.; Van Meer, M.; Okhuijsen, R.; Chen, Q. Process Optimization of Broad Ion Beam Milling for Preparation of Coating Cross-Sections. Ultramicroscopy 2024, 255, 113858. [Google Scholar] [CrossRef]
- Sharma, P.; Maya, P.N.; Satyaprasad, A.; Deshpande, S.P. Characterization of Vacancy Defects Using TEM in Heavy-Ion-Irradiated Tungsten Foils. Met. Mater. Trans. A 2024, 55, 2932–2939. [Google Scholar] [CrossRef]
- Dieterle, L.; Butz, B.; Müller, E. Optimized Ar+-Ion Milling Procedure for TEM Cross-Section Sample Preparation. Ultramicroscopy 2011, 111, 1636–1644. [Google Scholar] [CrossRef]
- Santos, A.J.; Lacroix, B.; Maudet, F.; Paumier, F.; Hurand, S.; Dupeyrat, C.; Gómez, V.J.; Huffaker, D.L.; Girardeau, T.; García, R.; et al. Application of Advanced (S)TEM Methods for the Study of Nanostructured Porous Functional Surfaces: A Few Working Examples. Mater. Charact. 2022, 185, 111741. [Google Scholar] [CrossRef]
- Kant, K.; Losic, D. Focused Ion Beam (FIB) Technology for Micro- and Nanoscale Fabrications. In FIB Nanostructures; Wang, Z.M., Ed.; Springer International Publishing: Cham, Switzerland, 2013; Volume 20, pp. 1–22. ISBN 9783319028736. [Google Scholar]
- Practical Electron Microscopy and Database—An Online Book. Available online: https://www.globalsino.com/EM/ (accessed on 14 November 2024).
- Hofmann, F.; Harder, R.J.; Liu, W.; Liu, Y.; Robinson, I.K.; Zayachuk, Y. Glancing-Incidence Focussed Ion Beam Milling: A Coherent X-Ray Diffraction Study of 3D Nano-Scale Lattice Strains and Crystal Defects. Acta Mater. 2018, 154, 113–123. [Google Scholar] [CrossRef]
- Huang, Z. Combining Ar Ion Milling with FIB Lift-out Techniques to Prepare High Quality Site-specific TEM Samples. J. Microsc. 2004, 215, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.R.P.; Chawla, N.; Shen, Y.-L. Focused Ion Beam (FIB) Tomography of Nanoindentation Damage in Nanoscale Metal/Ceramic Multilayers. Mater. Charact. 2010, 61, 481–488. [Google Scholar] [CrossRef]
- Lévy, D.; Aléon, J.; Aléon-Toppani, A.; Troadec, D.; Duhamel, R.; Gonzalez-Cano, A.; Bureau, H.; Khodja, H. NanoSIMS Imaging of D/H Ratios on FIB Sections. Anal. Chem. 2019, 91, 13763–13771. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S. Which Method of Sectioning Is Best for My Sample? Available online: https://www.eag.com/app-note/which-method-of-sectioning-is-best-for-my-sample/ (accessed on 18 November 2024).
- Huang, L.; Gan, Y. A Review on SEM Imaging of Graphene Layers. Micron 2024, 187, 103716. [Google Scholar] [CrossRef]
- Suga, M.; Asahina, S.; Sakuda, Y.; Kazumori, H.; Nishiyama, H.; Nokuo, T.; Alfredsson, V.; Kjellman, T.; Stevens, S.M.; Cho, H.S.; et al. Recent Progress in Scanning Electron Microscopy for the Characterization of Fine Structural Details of Nano Materials. Prog. Solid State Chem. 2014, 42, 1–21. [Google Scholar] [CrossRef]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning Electron Microscopy: Principle and Applications in Nanomaterials Characterization. In Handbook of Materials Characterization; Sharma, S.K., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 113–145. ISBN 9783319929552. [Google Scholar]
- Bernstein, G.H.; Carter, A.D.; Joy, D.C. Do SEII Electrons Really Degrade SEM Image Quality? Scanning 2013, 35, 1–6. [Google Scholar] [CrossRef]
- Jiang, Q.; Rogez, B.; Claude, J.-B.; Moreau, A.; Lumeau, J.; Baffou, G.; Wenger, J. Adhesion Layer Influence on Controlling the Local Temperature in Plasmonic Gold Nanoholes. Nanoscale 2020, 12, 2524–2531. [Google Scholar] [CrossRef]
- Young, S.; Weston, D.; Dauksher, B.; Mancini, D.; Pacheco, S.; Zurcher, P.; Miller, M. A Novel Low-Temperature Method to Fabricate MEMS Resonators Using PMGI as a Sacrificial Layer. J. Micromech. Microeng. 2005, 15, 1824–1830. [Google Scholar] [CrossRef]
- Egerton, R.F. Physical Principles of Electron Microscopy; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319398761. [Google Scholar]
- Dwyer, C. Quantitative Annular Dark-Field Imaging in the Scanning Transmission Electron Microscope—A Review. J. Phys. Mater. 2021, 4, 042006. [Google Scholar] [CrossRef]
- MacLaren, I.; Fraser, A.T.; Lipsett, M.R.; Ophus, C. Digital Dark Field—Higher Contrast and Greater Specificity Dark Field Imaging Using a 4DSTEM Approach. Microsc. Microanal. 2024, 31, ozae104. [Google Scholar] [CrossRef]
- Luo, R.; Gao, M.; Wang, C.; Zhu, J.; Guzman, R.; Zhou, W. Probing Functional Structures, Defects, and Interfaces of 2D Transition Metal Dichalcogenides by Electron Microscopy. Adv. Funct. Mater. 2024, 34, 2307625. [Google Scholar] [CrossRef]
- Mayeen, A.; Shaji, L.K.; Nair, A.K.; Kalarikkal, N. Morphological Characterization of Nanomaterials. In Characterization of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 335–364. ISBN 9780081019733. [Google Scholar]
- Klenov, D.; Freitag, B.; Von Harrach, H.; D’Alfonso, A.; Allen, L. Chemical Mapping at the Atomic Level Using Energy Dispersive X-Ray Spectroscopy. Microsc. Microanal. 2011, 17, 598–599. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, X.; Gong, D.; Cheng, X.; Li, K. Optical Properties and Thermal Stability of AlCrON-Based Multilayer Solar Selective Absorbing Coating for High Temperature Applications. J. Mater. Res. Technol. 2021, 15, 6162–6174. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Performing Elemental Microanalysis with High Accuracy and High Precision by Scanning Electron Microscopy/Silicon Drift Detector Energy-Dispersive X-Ray Spectrometry (SEM/SDD-EDS). J. Mater. Sci. 2015, 50, 493–518. [Google Scholar] [CrossRef]
- Von Harrach, H.; Klenov, D.; Freitag, B.; Schlossmacher, P.; Collins, P.; Fraser, H. Comparison of the Detection Limits of EDS and EELS in S/TEM. Microsc. Microanal. 2010, 16, 1312–1313. [Google Scholar] [CrossRef]
- Bosman, M.; Watanabe, M.; Alexander, D.T.L.; Keast, V.J. Mapping Chemical and Bonding Information Using Multivariate Analysis of Electron Energy-Loss Spectrum Images. Ultramicroscopy 2006, 106, 1024–1032. [Google Scholar] [CrossRef]
- Utsumi, J.; Ide, K.; Ichiyanagi, Y. Cu/SiO2 Hybrid Bonding Obtained by Surface-Activated Bonding Method at Room Temperature Using Si Ultrathin Films. Micro Nano Eng. 2019, 2, 1–6. [Google Scholar] [CrossRef]
- McMahon, W.E.; Melamed, C.L.; Tamboli, A.E.; Toberer, E.S.; Norman, A.G. Growth of GaAs on Single-Crystal Layered-2D Bi2Se3. J. Cryst. Growth 2020, 534, 125457. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, H.; Zhang, D.; Liu, L.; Han, Y. Low-Dose Electron Microscopy Imaging of Electron Beam-Sensitive Crystalline Materials. Acc. Mater. Res. 2022, 3, 552–564. [Google Scholar] [CrossRef]
- Brodusch, N.; Gauvin, R. Low Voltage Scanning Transmission Electron Microscopy as a Viable Tool for Routine Analysis of Materials Science Specimens. Microsc. Microanal. 2024, 30, ozae044.323. [Google Scholar] [CrossRef]
- Nordström, R.; Zhu, L.; Härmark, J.; Levi-Kalisman, Y.; Koren, E.; Barenholz, Y.; Levinton, G.; Shamrakov, D. Quantitative Cryo-TEM Reveals New Structural Details of Doxil-Like PEGylated Liposomal Doxorubicin Formulation. Pharmaceutics 2021, 13, 123. [Google Scholar] [CrossRef]
- Wang, D.; Russell, T.P. Advances in Atomic Force Microscopy for Probing Polymer Structure and Properties. Macromolecules 2018, 51, 3–24. [Google Scholar] [CrossRef]
- Collinson, D.W.; Sheridan, R.J.; Palmeri, M.J.; Brinson, L.C. Best Practices and Recommendations for Accurate Nanomechanical Characterization of Heterogeneous Polymer Systems with Atomic Force Microscopy. Prog. Polym. Sci. 2021, 119, 101420. [Google Scholar] [CrossRef]
- Bian, K.; Gerber, C.; Heinrich, A.J.; Müller, D.J.; Scheuring, S.; Jiang, Y. Scanning Probe Microscopy. Nat. Rev. Methods Primers 2021, 1, 36. [Google Scholar] [CrossRef]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J.M. Local Infrared Microspectroscopy with Subwavelength Spatial Resolution with an Atomic Force Microscope Tip Used as a Photothermal Sensor. Opt. Lett. 2005, 30, 2388. [Google Scholar] [CrossRef]
- Hoogenboom, B.W. Stretching the Resolution Limit of Atomic Force Microscopy. Nat. Struct. Mol. Biol. 2021, 28, 629–630. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, X.G. What Do Different Modes of AFM-IR Mean for Measuring Soft Matter Surfaces? Langmuir 2023, 39, 17593–17599. [Google Scholar] [CrossRef]
- Robson, A.J.; Grishin, I.; Young, R.J.; Sanchez, A.M.; Kolosov, O.V.; Hayne, M. High-Accuracy Analysis of Nanoscale Semiconductor Layers Using Beam-Exit Ar-Ion Polishing and Scanning Probe Microscopy. ACS Appl. Mater. Interfaces 2013, 5, 3241–3245. [Google Scholar] [CrossRef]
- Benaglia, S.; Amo, C.A.; Garcia, R. Fast, Quantitative and High Resolution Mapping of Viscoelastic Properties with Bimodal AFM. Nanoscale 2019, 11, 15289–15297. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and Applications in Nanoscale Infrared Spectroscopy and Chemical Imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef]
- Yang, C.; Dang, C.-Q.; Zhu, W.-L.; Ju, B.-F. High-Speed Atomic Force Microscopy in Ultra-Precision Surface Machining and Measurement: Challenges, Solutions and Opportunities. Surf. Sci. Technol. 2023, 1, 7. [Google Scholar] [CrossRef]
- Petrov, Y.V.; Vyvenko, O.F. Secondary Electron Generation in the Helium Ion Microscope: Basics and Imaging. In Helium Ion Microscopy; Hlawacek, G., Gölzhäuser, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 119–146. ISBN 9783319419886. [Google Scholar]
- Joy, D.C. Helium Ion Microscopy: Principles and Applications; Springer Briefs in Materials; Springer: New York, NY, USA, 2013; ISBN 9781461486596. [Google Scholar]
- Wirtz, T.; De Castro, O.; Audinot, J.-N.; Philipp, P. Imaging and Analytics on the Helium Ion Microscope. Annu. Rev. Anal. Chem. 2019, 12, 523–543. [Google Scholar] [CrossRef]
- Boden, S.A.; Franklin, T.M.W.; Scipioni, L.; Bagnall, D.M.; Rutt, H.N. Ionoluminescence in the Helium Ion Microscope. Microsc. Microanal. 2012, 18, 1253–1262. [Google Scholar] [CrossRef]
- Veligura, V.; Hlawacek, G.; Van Gastel, R.; Zandvliet, H.J.W.; Poelsema, B. Channeling in Helium Ion Microscopy: Mapping of Crystal Orientation. Beilstein J. Nanotechnol. 2012, 3, 501–506. [Google Scholar] [CrossRef]
- Klingner, N.; Heller, R.; Hlawacek, G.; Facsko, S.; Von Borany, J. Time-of-Flight Secondary Ion Mass Spectrometry in the Helium Ion Microscope. Ultramicroscopy 2019, 198, 10–17. [Google Scholar] [CrossRef]
- Giurlani, W.; Berretti, E.; Innocenti, M.; Lavacchi, A. Measuring the Thickness of Metal Coatings: A Review of the Methods. Coatings 2020, 10, 1211. [Google Scholar] [CrossRef]
- Masters, R.C.; Wan, Q.; Zhang, Y.; Dapor, M.; Sandu, A.M.; Jiao, C.; Zhou, Y.; Zhang, H.; Lidzey, D.G.; Rodenburg, C. Novel Organic Photovoltaic Polymer Blends: A Rapid, 3-Dimensional Morphology Analysis Using Backscattered Electron Imaging in the Scanning Electron Microscope. Sol. Energy Mater. Sol. Cells 2017, 160, 182–192. [Google Scholar] [CrossRef]
- Spiliopoulos, P.; Gestranius, M.; Zhang, C.; Ghiyasi, R.; Tomko, J.; Arstila, K.; Putkonen, M.; Hopkins, P.E.; Karppinen, M.; Tammelin, T.; et al. Cellulose-Inorganic Hybrids of Strongly Reduced Thermal Conductivity. Cellulose 2022, 29, 8151–8163. [Google Scholar] [CrossRef]
- Terpstra, A.S.; Shopsowitz, K.E.; Gregory, C.F.; Manning, A.P.; Michal, C.A.; Hamad, W.Y.; Yang, J.; MacLachlan, M.J. Helium Ion Microscopy: A New Tool for Imaging Novel Mesoporous Silica and Organosilica Materials. Chem. Commun. 2013, 49, 1645. [Google Scholar] [CrossRef]
- Gunawardane, R.P.; Arumainayagam, C.R. Auger Electron Spectroscopy. In Handbook of Applied Solid State Spectroscopy; Vij, D.R., Ed.; Springer: Boston, MA, USA, 2006; pp. 451–483. ISBN 9780387324975. [Google Scholar]
- Martinez, E.; Yadav, P.; Bouttemy, M.; Renault, O.; Borowik, Ł.; Bertin, F.; Etcheberry, A.; Chabli, A. Scanning Auger Microscopy for High Lateral and Depth Elemental Sensitivity. J. Electron. Spectrosc. Relat. Phenom. 2013, 191, 86–91. [Google Scholar] [CrossRef]
- Robinson, K.J.; Thissen, H. Selecting the Best Surface Analysis Method for Your Materials/Samples. J. Vac. Sci. Technol. A 2024, 42, 040801. [Google Scholar] [CrossRef]
- De Hosson, J.T.M.; Carvalho, N.J.M.; Pei, Y.; Galvan, D. Electron Microscopy Characterization of Nanostructured Coatings. In Nanostructured Coatings; Cavaleiro, A., De Hosson, J.T.M., Eds.; Springer: New York, NY, USA, 2006; pp. 143–215. ISBN 9780387256429. [Google Scholar]
- Fu, J.; Fan, Z.; Nakabayashi, M.; Ju, H.; Pastukhova, N.; Xiao, Y.; Feng, C.; Shibata, N.; Domen, K.; Li, Y. Interface Engineering of Ta3N5 Thin Film Photoanode for Highly Efficient Photoelectrochemical Water Splitting. Nat. Commun. 2022, 13, 729. [Google Scholar] [CrossRef]
- Weber, P.K.; Debliqui, M.; Defouilloy, C.; Mayali, X.; Liu, M.-C.; Hestrin, R.; Pett-Ridge, J.; Stuart, R.; Morris, M.; Ramon, C.; et al. The NanoSIMS-HR: The Next Generation of High Spatial Resolution Dynamic SIMS. Anal. Chem. 2024, 96, 19321–19329. [Google Scholar] [CrossRef]
- Sangely, L.; Boyer, B.; De Chambost, E.; Valle, N.; Audinot, J.-N.; Ireland, T.; Wiedenbeck, M.; Aléon, J.; Jungnickel, H.; Barnes, J.-P.; et al. Secondary Ion Mass Spectrometry. In Sector Field Mass Spectrometry for Elemental and Isotopic Analysis; Prohaska, T., Irrgeher, J., Zitek, A., Jakubowski, N., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 439–499. ISBN 9781849733922. [Google Scholar]
- Nuñez, J.; Renslow, R.; Cliff, J.B.; Anderton, C.R. NanoSIMS for Biological Applications: Current Practices and Analyses. Biointerphases 2018, 13, 03B301. [Google Scholar] [CrossRef]
- Wacey, D.; Kilburn, M.R.; Mcloughlin, N.; Parnell, J.; Stoakes, C.A.; Grovenor, C.R.M.; Brasier, M.D. Use of NanoSIMS in the Search for Early Life on Earth: Ambient Inclusion Trails in a c. 3400 Ma Sandstone. J. Geol. Soc. 2008, 165, 43–53. [Google Scholar] [CrossRef]
- Wang, Y.; Schreiber, D.K.; Neeway, J.J.; Thevuthasan, S.; Evans, J.E.; Ryan, J.V.; Zhu, Z.; Wei, W.D. NanoSIMS Imaging Alteration Layers of a Leached SON68 Glass via a FIB-made Wedged Crater. Surf. Interface Anal. 2014, 46, 233–237. [Google Scholar] [CrossRef]
- Otter, L.M.; Eder, K.; Kilburn, M.R.; Yang, L.; O’Reilly, P.; Nowak, D.B.; Cairney, J.M.; Jacob, D.E. Growth Dynamics and Amorphous-to-Crystalline Phase Transformation in Natural Nacre. Nat. Commun. 2023, 14, 2254. [Google Scholar] [CrossRef]
- Höppener, C.; Schacher, F.H.; Deckert, V. Multimodal Characterization of Resin Embedded and Sliced Polymer Nanoparticles by Means of Tip-Enhanced Raman Spectroscopy and Force–Distance Curve Based Atomic Force Microscopy. Small 2020, 16, 1907418. [Google Scholar] [CrossRef]
- Kumar, N.; Mignuzzi, S.; Su, W.; Roy, D. Tip-Enhanced Raman Spectroscopy: Principles and Applications. EPJ Tech. Instrum. 2015, 2, 9. [Google Scholar] [CrossRef]
- Sacco, A.; Imbraguglio, D.; Giovannozzi, A.M.; Portesi, C.; Rossi, A.M. Development of a Candidate Reference Sample for the Characterization of Tip-Enhanced Raman Spectroscopy Spatial Resolution. RSC Adv. 2018, 8, 27863–27869. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Dong, Z.C.; Jiang, S.; Zhang, C.; Chen, L.G.; Zhang, L.; Liao, Y.; Aizpurua, J.; Luo, Y.; et al. Chemical Mapping of a Single Molecule by Plasmon-Enhanced Raman Scattering. Nature 2013, 498, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, S.M.; Tronco, M.F.; Ponçot, M.; C.S, C.; Guigo, N.; Malfois, M.; Kalarikkal, N.; Thomas, S.; Royaud, I.; Rouxel, D. Quantifying the Crystalline Polymorphism in PVDF: Comparative Criteria Using DSC, WAXS, FT-IR, and Raman Spectroscopy. ACS Appl. Polym. Mater. 2024, 6, 8291–8305. [Google Scholar] [CrossRef]
- Chen, C.; Hayazawa, N.; Kawata, S. A 1.7 Nm Resolution Chemical Analysis of Carbon Nanotubes by Tip-Enhanced Raman Imaging in the Ambient. Nat. Commun. 2014, 5, 3312. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, A.P. Soft X-Ray Spectromicroscopy and Ptychography. J. Electron. Spectrosc. Relat. Phenom. 2015, 200, 49–63. [Google Scholar] [CrossRef]
- Andrews, J.C.; Meirer, F.; Liu, Y.; Mester, Z.; Pianetta, P. Transmission X-ray Microscopy for Full-field Nano Imaging of Biomaterials. Microsc. Res. Tech. 2011, 74, 671–681. [Google Scholar] [CrossRef]
- Watts, B.; McNeill, C.R.; Raabe, J. Imaging Nanostructures in Organic Semiconductor Films with Scanning Transmission X-Ray Spectro-Microscopy. Synth. Met. 2012, 161, 2516–2520. [Google Scholar] [CrossRef]
- Scholl, A. Applications of Photoemission Electron Microscopy (PEEM) in Magnetism Research. Curr. Opin. Solid State Mater. Sci. 2003, 7, 59–66. [Google Scholar] [CrossRef]
- Barinov, A.; Dudin, P.; Gregoratti, L.; Locatelli, A.; Onur Menteş, T.; Ángel Niño, M.; Kiskinova, M. Synchrotron-Based Photoelectron Microscopy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 601, 195–202. [Google Scholar] [CrossRef]
- Watts, B.; Thomsen, L.; Dastoor, P.C. Methods in Carbon K-Edge NEXAFS: Experiment and Analysis. J. Electron. Spectrosc. Relat. Phenom. 2006, 151, 105–120. [Google Scholar] [CrossRef]
- Hieulle, J. Structures and Electronic Properties of Organic Self-Assembled Monolayers Characterized by STM and XPS. Ph.D. Thesis, Ecole Polytechnique, Paris, France, 2014. [Google Scholar]
- Newville, M. Fundamentals of XAFS; Consortium for Advanced Radiation Sources, University of Chicago: Chicago, IL, USA, 2004. [Google Scholar]
- Watts, B.; Schuettfort, T.; McNeill, C.R. Mapping of Domain Orientation and Molecular Order in Polycrystalline Semiconducting Polymer Films with Soft X-Ray Microscopy. Adv. Funct. Mater. 2011, 21, 1122–1131. [Google Scholar] [CrossRef]
- Foetisch, A.; Filella, M.; Watts, B.; Vinot, L.-H.; Bigalke, M. Identification and Characterisation of Individual Nanoplastics by Scanning Transmission X-Ray Microscopy (STXM). J. Hazard. Mater. 2022, 426, 127804. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, S.G. X-Ray Spectroptychography. ACS Omega 2022, 7, 11521–11529. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, A.P.; Zhang, C.; Eraky, H.; Higgins, D.; Belkhou, R.; Millle, N.; Swaraj, S.; Stanescu, S.; Sun, T.; Wang, J. Comparison of Soft X-Ray Spectro-Ptychography and Scanning Transmission X-Ray Microscopy. J. Electron. Spectrosc. Relat. Phenom. 2024, 276, 147487. [Google Scholar] [CrossRef]
- Guttmann, P.; Bittencourt, C.; Rehbein, S.; Umek, P.; Ke, X.; Van Tendeloo, G.; Ewels, C.P.; Schneider, G. Nanoscale Spectroscopy with Polarized X-Rays by NEXAFS-TXM. Nat. Photonics 2012, 6, 25–29. [Google Scholar] [CrossRef]
- Xie, T.; Bai, L.; Liu, J.; Zhao, G.; Sun, X.; Zhong, J. X-Ray Induced Carbon Coating on Carbon Nanotubes. Carbon 2013, 56, 385–388. [Google Scholar] [CrossRef]
- De Jesus, L.R.; Dennis, R.V.; Depner, S.W.; Jaye, C.; Fischer, D.A.; Banerjee, S. Inside and Outside: X-Ray Absorption Spectroscopy Mapping of Chemical Domains in Graphene Oxide. J. Phys. Chem. Lett. 2013, 4, 3144–3151. [Google Scholar] [CrossRef]
- Atom Probe Tomography—Introduction to the Technique n.d. Available online: https://www.cameca.com/products/apt/technique (accessed on 30 May 2024).
- Miller, M.K.; Kelly Thomas, F.; Rajan, K.; Ringer, S.P. The future of atom probe tomography. Mater. Today 2012, 15, 158–165. [Google Scholar] [CrossRef]
- Gault, B.; Chiaramonti, A.; Cojocaru-Mirédin, O.; Stender, P.; Dubosq, R.; Freysoldt, C.; Makineni, S.K.; Li, T.; Moody, M.; Cairney, J.M. Atom probe tomography. Nat. Rev. Methods Primers 2021, 1, 51. [Google Scholar] [CrossRef]
- Thompson, K.; Lawrence, D.; Larson, D.J.; Olson, J.D.; Kelly, T.F.; Gorman, B. In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 2007, 107, 131–139. [Google Scholar] [CrossRef]
- Yang, T.; Zhao, Y.L.; Li, W.P.; Yu, C.Y.; Luan, J.H.; Lin, D.Y.; Fan, L.; Jiao, Z.B.; Liu, W.H.; Liu, X.J.; et al. Ultrahigh-Strength and Ductile Superlattice Alloys with Nanoscale Disordered Interfaces. Science 2020, 369, 427–432. [Google Scholar] [CrossRef]
- Brognara, A.; Jung, C.; Poltronieri, C.; Djemia, P.; Dehm, G.; Ghidelli, M.; Best, J.P. Thermal Modification of ZrCu Metallic Glass Nanolaminates: Structure and Mechanical Properties. arXiv 2024, arXiv:2407.13272. [Google Scholar] [CrossRef]
- Weyland, M.; Midgley, P.A. Electron Tomography. Mater. Today 2004, 7, 32–40. [Google Scholar] [CrossRef]
- Ersen, O.; Hirlimann, C.; Drillon, M.; Werckmann, J.; Tihay, F.; Pham-Huu, C.; Crucifix, C.; Schultz, P. 3D-TEM Characterization of Nanometric Objects. Solid State Sci. 2007, 9, 1088–1098. [Google Scholar] [CrossRef]
- Ercius, P.; Alaidi, O.; Rames, M.J.; Ren, G. Electron Tomography: A Three-Dimensional Analytic Tool for Hard and Soft Materials Research. Adv. Mater. 2015, 27, 5638–5663. [Google Scholar] [CrossRef]
- Chang, S.; Li, L.; Hong, B.; Liu, J.; Xu, Y.; Pang, K.; Zhang, L.; Han, H.; Chen, X. An Intelligent Workflow for Sub-Nanoscale 3D Reconstruction of Intact Synapses from Serial Section Electron Tomography. BMC Biol. 2023, 21, 198. [Google Scholar] [CrossRef]
- Bals, S.; Van Tendeloo, G.; Kisielowski, C. A New Approach for Electron Tomography: Annular Dark-Field Transmission Electron Microscopy. Adv. Mater. 2006, 18, 892–895. [Google Scholar] [CrossRef]
- Hata, S.; Furukawa, H.; Gondo, T.; Hirakami, D.; Horii, N.; Ikeda, K.-I.; Kawamoto, K.; Kimura, K.; Matsumura, S.; Mitsuhara, M.; et al. Electron Tomography Imaging Methods with Diffraction Contrast for Materials Research. Microscopy 2020, 69, 141–155. [Google Scholar] [CrossRef]
- Haberfehlner, G.; Orthacker, A.; Albu, M.; Li, J.; Kothleitner, G. Nanoscale Voxel Spectroscopy by Simultaneous EELS and EDS Tomography. Nanoscale 2014, 6, 14563–14569. [Google Scholar] [CrossRef]
- Collins, S.M.; Midgley, P.A. Progress and Opportunities in EELS and EDS Tomography. Ultramicroscopy 2017, 180, 133–141. [Google Scholar] [CrossRef]
- Koneti, S.; Roiban, L.; Dalmas, F.; Langlois, C.; Gay, A.-S.; Cabiac, A.; Grenier, T.; Banjak, H.; Maxim, V.; Epicier, T. Fast Electron Tomography: Applications to Beam Sensitive Samples and in Situ TEM or Operando Environmental TEM Studies. Mater. Charact. 2019, 151, 480–495. [Google Scholar] [CrossRef]
- Xia, W.; Yang, Y.; Meng, Q.; Deng, Z.; Gong, M.; Wang, J.; Wang, D.; Zhu, Y.; Sun, L.; Xu, F.; et al. Bimetallic Nanoparticle Oxidation in Three Dimensions by Chemically Sensitive Electron Tomography and in Situ Transmission Electron Microscopy. ACS Nano 2018, 12, 7866–7874. [Google Scholar] [CrossRef] [PubMed]
- Eijsbouts, S.; Li, X.; Juan-Alcaniz, J.; Van Den Oetelaar, L.C.A.; Bergwerff, J.A.; Loos, J.; Carlsson, A.; Vogt, E.T.C. Electron Tomography Reveals the Active Phase–Support Interaction in Sulfidic Hydroprocessing Catalysts. ACS Catal. 2017, 7, 4817–4821. [Google Scholar] [CrossRef]
- Holler, M.; Diaz, A.; Guizar-Sicairos, M.; Karvinen, P.; Färm, E.; Härkönen, E.; Ritala, M.; Menzel, A.; Raabe, J.; Bunk, O. X-Ray Ptychographic Computed Tomography at 16 Nm Isotropic 3D Resolution. Sci. Rep. 2014, 4, 3857. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, M.; Sala, S.; Johansson, U.; Björling, A.; Jiang, Z.; Kalbfleisch, S.; Lenrick, F.; Pikul, J.H.; Thånell, K. First Ptychographic X-Ray Computed Tomography Experiment on the NanoMAX Beamline. J. Appl. Crystallogr. 2020, 53, 1444–1451. [Google Scholar] [CrossRef]
- Górecki, R.; Polo, C.C.; Kalile, T.A.; Miqueles, E.X.S.; Tonin, Y.R.; Upadhyaya, L.; Meneau, F.; Nunes, S.P. Ptychographic X-Ray Computed Tomography of Porous Membranes with Nanoscale Resolution. Commun. Mater. 2023, 4, 68. [Google Scholar] [CrossRef]
- Pedersen, E.B.L.; Angmo, D.; Dam, H.F.; Thydén, K.T.S.; Andersen, T.R.; Skjønsfjell, E.T.B.; Krebs, F.C.; Holler, M.; Diaz, A.; Guizar-Sicairos, M.; et al. Improving Organic Tandem Solar Cells Based on Water-Processed Nanoparticles by Quantitative 3D Nanoimaging. Nanoscale 2015, 7, 13765–13774. [Google Scholar] [CrossRef]
- Yuan, H.; Yuan, H.; Casagrande, T.; Shapiro, D.; Yu, Y.-S.; Enders, B.; Lee, J.R.I.; Van Buuren, A.; Biener, M.M.; Gammon, S.A.; et al. 4D Imaging of ZnO-Coated Nanoporous Al2 O3 Aerogels by Chemically Sensitive Ptychographic Tomography: Implications for Designer Catalysts. ACS Appl. Nano Mater. 2021, 4, 621–632. [Google Scholar] [CrossRef]
- Ponomaryov, S.S.; Yukhymchuk, V.O.; Lytvyn, P.M.; Valakh, M.Y. Direct Determination of 3D Distribution of Elemental Composition in Single Semiconductor Nanoislands by Scanning Auger Microscopy. Nanoscale Res. Lett. 2016, 11, 103. [Google Scholar] [CrossRef][Green Version]
- Li, K.; Liu, J.; Grovenor, C.R.M.; Moore, K.L. NanoSIMS Imaging and Analysis in Materials Science. Annu. Rev. Anal. Chem. 2020, 13, 273–292. [Google Scholar] [CrossRef]
- Michelson, A.; Minevich, B.; Emamy, H.; Huang, X.; Chu, Y.S.; Yan, H.; Gang, O. Three-Dimensional Visualization of Nanoparticle Lattices and Multimaterial Frameworks. Science 2022, 376, 203–207. [Google Scholar] [CrossRef]
- Aidukas, T.; Phillips, N.W.; Diaz, A.; Poghosyan, E.; Müller, E.; Levi, A.F.J.; Aeppli, G.; Guizar-Sicairos, M.; Holler, M. High-Performance 4-Nm-Resolution X-Ray Tomography Using Burst Ptychography. Nature 2024, 632, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Mille, N.; Yuan, H.; Vijayakumar, J.; Stanescu, S.; Swaraj, S.; Desjardins, K.; Favre-Nicolin, V.; Belkhou, R.; Hitchcock, A.P. Ptychography at the Carbon K-Edge. Commun. Mater. 2022, 3, 8. [Google Scholar] [CrossRef]
- Mathurin, J.; Deniset-Besseau, A.; Bazin, D.; Dartois, E.; Wagner, M.; Dazzi, A. Photothermal AFM-IR Spectroscopy and Imaging: Status, Challenges, and Trends. J. Appl. Phys. 2022, 131, 010901. [Google Scholar] [CrossRef]
- Wieland, K.; Ramer, G.; Weiss, V.U.; Allmaier, G.; Lendl, B.; Centrone, A. Nanoscale Chemical Imaging of Individual Chemotherapeutic Cytarabine-Loaded Liposomal Nanocarriers. Nano Res. 2019, 12, 197–203. [Google Scholar] [CrossRef]
- dos Santos, A.C.V.D.; Hondl, N.; Ramos-Garcia, V.; Kuligowski, J.; Lendl, B.; Ramer, G. AFM-IR for Nanoscale Chemical Characterization in Life Sciences: Recent Developments and Future Directions. ACS Meas. Sci. Au 2023, 3, 301–314. [Google Scholar] [CrossRef]
- Dazzi, A.; Mathurin, J.; Leclere, P.; Nickmilder, P.; De Wolf, P.; Wagner, M.; Hu, Q.; Deniset-Besseau, A. Photothermal AFM-IR Depth Sensitivity: An Original Pathway to Tomographic Reconstruction. Anal. Chem. 2024, 96, 17931–17940. [Google Scholar] [CrossRef]
- Jakob, D.S.; Wang, H.; Zeng, G.; Otzen, D.E.; Yan, Y.; Xu, X.G. Peak Force Infrared–Kelvin Probe Force Microscopy. Angew. Chem. Int. Ed. 2020, 59, 16083–16090. [Google Scholar] [CrossRef]
- Rutherford, D.; Kolářová, K.; Čech, J.; Haušild, P.; Kuliček, J.; Ukraintsev, E.; Stehlík, Š.; Dao, R.; Neuman, J.; Rezek, B. Correlative Atomic Force Microscopy and Scanning Electron Microscopy of Bacteria-Diamond-Metal Nanocomposites. Ultramicroscopy 2024, 258, 113909. [Google Scholar] [CrossRef]
- Hughes, L.A.; Savitzky, B.H.; Deng, H.D.; Jin, N.L.; Lomeli, E.G.; Yu, Y.-S.; Shapiro, D.A.; Herring, P.; Anapolsky, A.; Chueh, W.C.; et al. Correlative Analysis of Structure and Chemistry of LixFePO4 Platelets Using 4D-STEM and X-Ray Ptychography. Mater. Today 2022, 52, 102–111. [Google Scholar] [CrossRef]
- Jia, H.; Wang, C.; Wang, C.; Clancy, P. Machine Learning Approach to Enable Spectral Imaging Analysis for Particularly Complex Nanomaterial Systems. ACS Nano 2023, 17, 453–460. [Google Scholar] [CrossRef]
- Aoyagi, S. Application of Machine Learning to Spectrum and Image Data. J. Vac. Sci. Technol. A 2023, 41, 063202. [Google Scholar] [CrossRef]

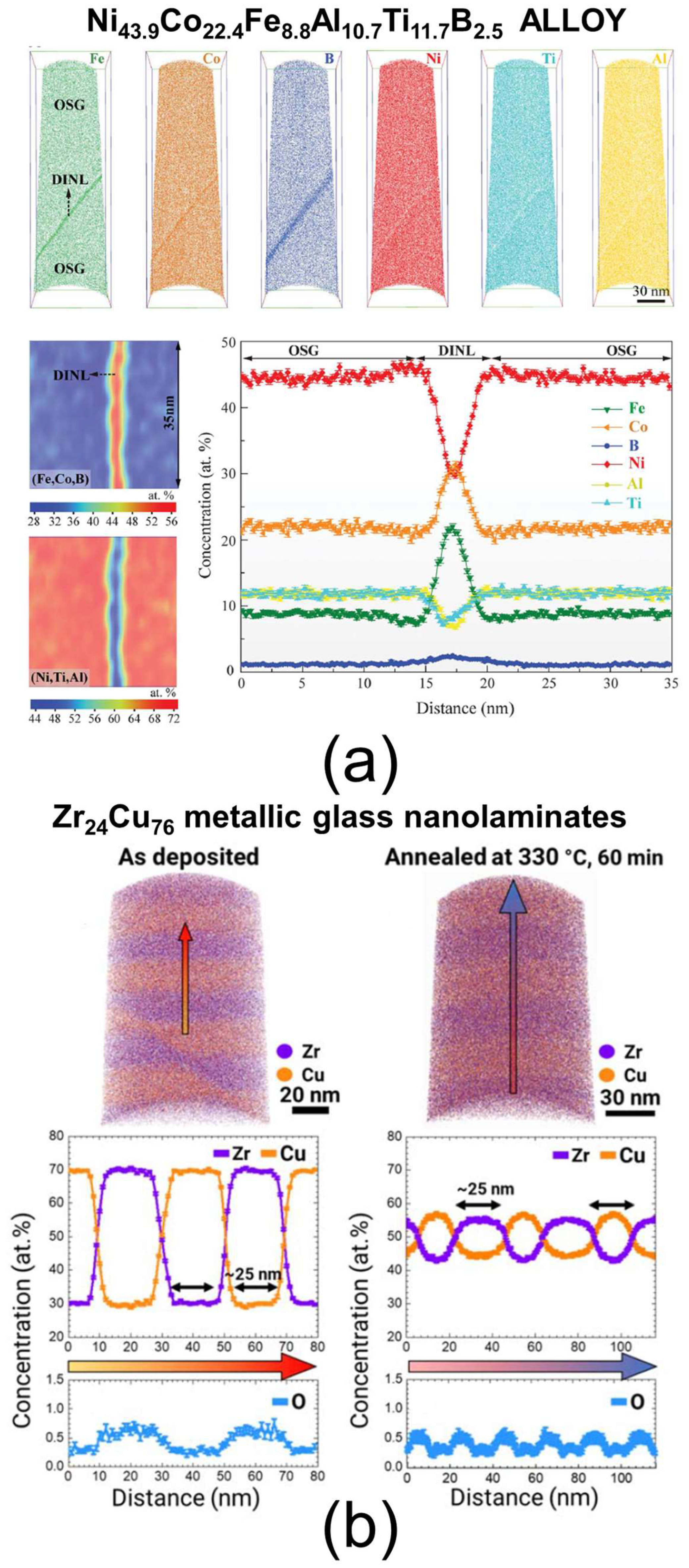
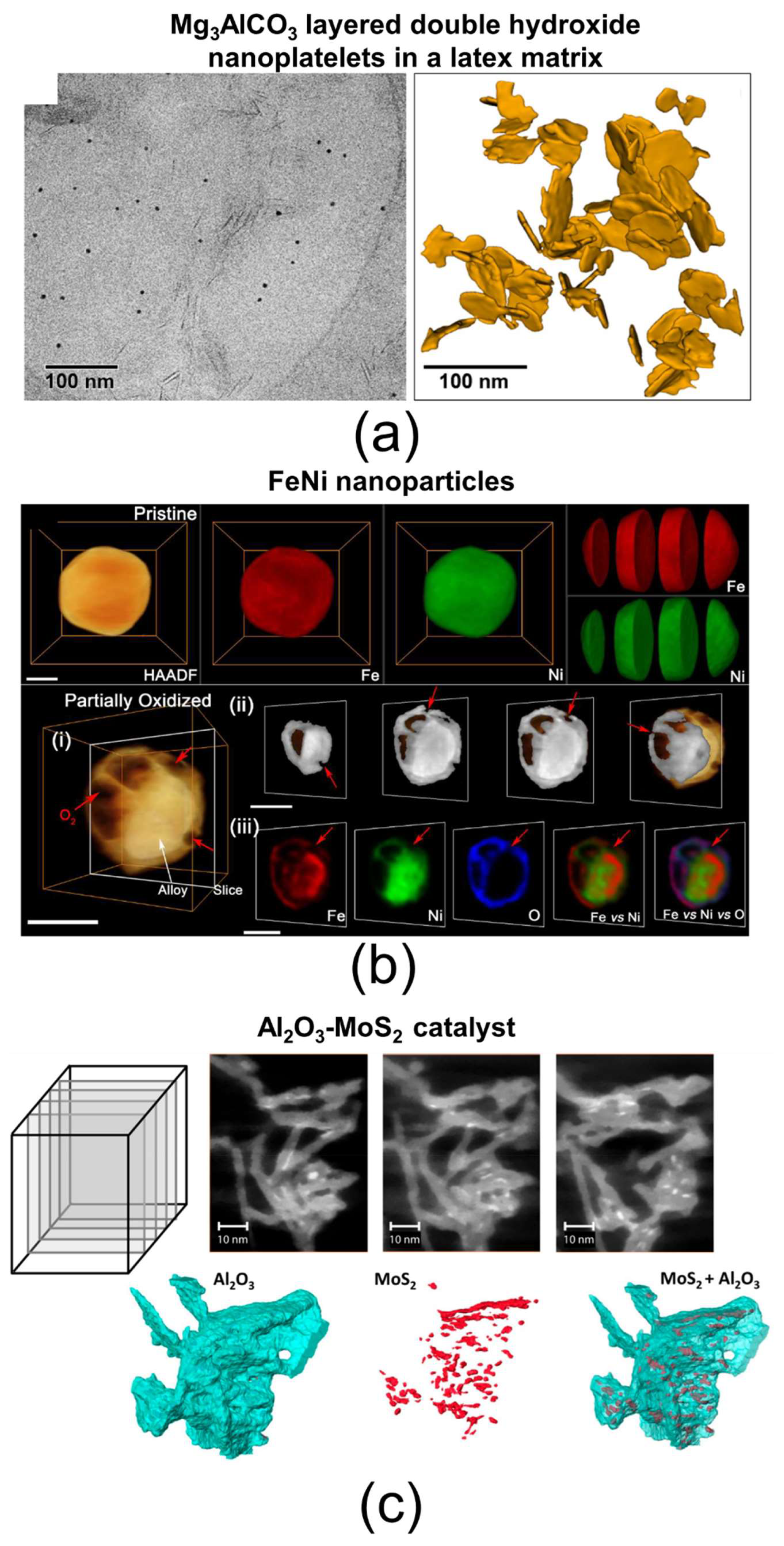
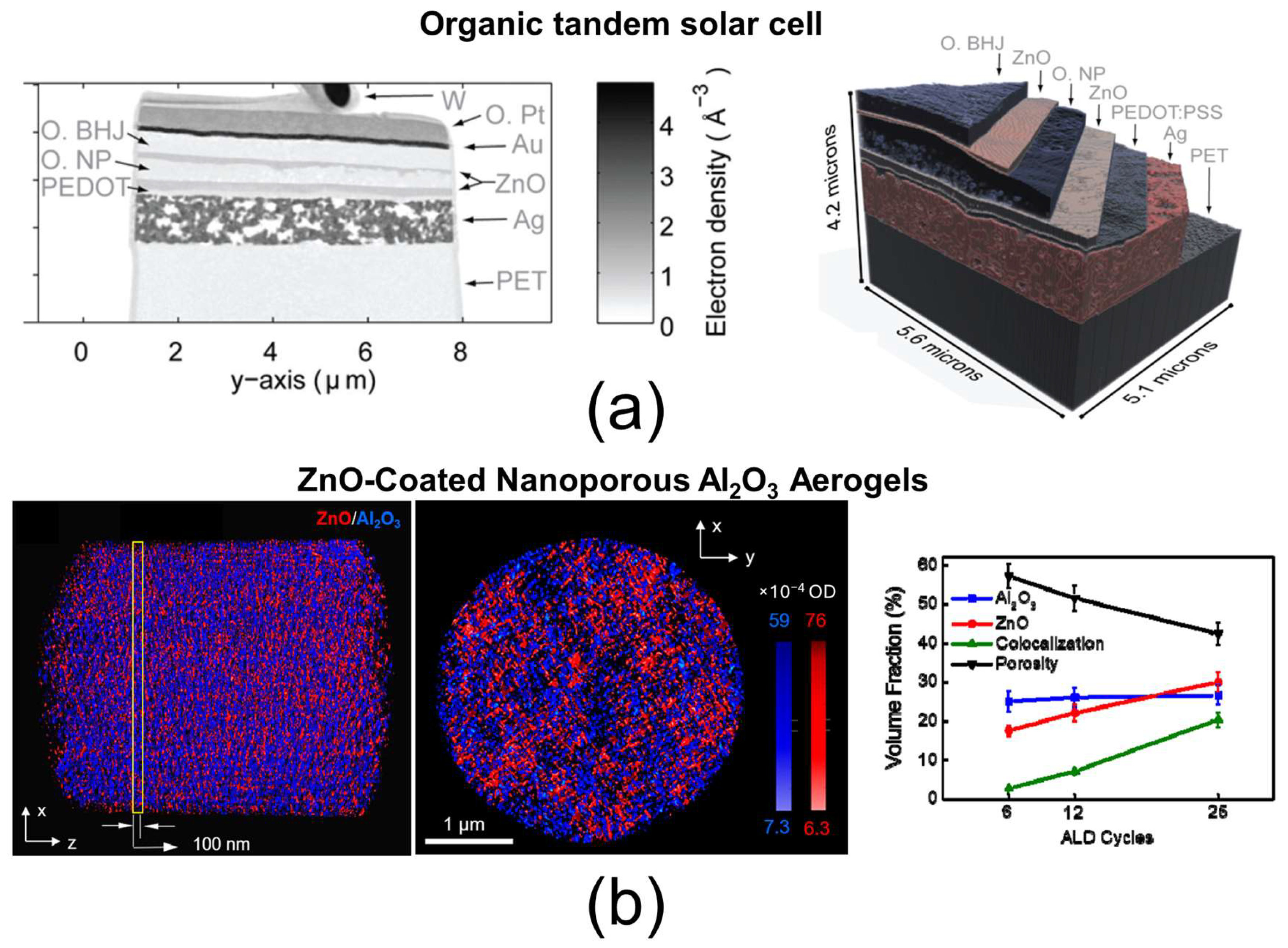
| Techniques | Typical Spatial Resolution * | Structural Information | Section |
|---|---|---|---|
| Scanning electron microscopy (SEM) + energy-dispersive X-ray spectrometry (EDS) | 1 nm for morphological imaging | Morphological imaging, thickness of the layer, layer homogeneity and bonding quality (qualitative) by scanning large samples, element presence (qualitative) | 3.1 |
| Transmission electron microscopy (TEM) + Electron nanodiffraction (END) + energy-dispersive X-ray spectrometry (EDS) + electron energy loss spectroscopy (EELS) | 0.1 nm for morphological imaging 1 nm–20 nm for chemical analysis | Morphological imaging, thickness of the layer and interfacial region, 2D elemental composition/profiling/mapping, elemental bonding, crystal structure and defects, interfacial region chemistry and morphology | 3.2 |
| Atomic force microscopy (AFM) AFM + nanomechanical mode Conductive AFM (c-AFM) AFM + infrared spectroscopy (AFM-IR) | 1 nm–15 nm for AFM, AFM + nanomechanical mode, and c-AFM 20 nm–100 nm for AFM-IR | Morphological imaging, layer thickness, layer homogeneity and bonding quality (qualitative) by scanning large samples, interfacial region morphology, viscoelastic properties of the layer and interfacial region, electrical conductivity of the layer and interfacial region, identifying a material thanks to the recorded IR spectrum (qualitative), verifying the presence or not of a given chemical bond in the layer (qualitative) | 3.3 |
| Scanning helium ion microscopy (HIM) | 0.5 nm | Morphological imaging of the layer and interfacial region, thickness of the layer, layer homogeneity and bonding quality (qualitative) by scanning large samples, ionoluminescence, crystallographic texture | 3.4 |
| Auger electron spectroscopy (AES)/scanning Auger microscope (SAM) | 10 nm | Two-dimensional elemental composition/profiling/mapping, in-depth elemental profiling if combined with an ions milling sputtering method | 3.5 |
| Nanoscale secondary ion mass spectrometry (nanoSIMS) | 30 nm | Two-dimensional elemental composition/profiling/mapping, in-depth elemental profiling, including isotope analysis, detection of trace elements | 3.6 |
| Tip-enhanced Raman spectroscopy (TERS) | 10 nm–50 nm | Topographic imaging and 2D chemical composition/profiling/mapping based on Raman spectroscopy (including crystalline phase identification, and crystallinity assessment), information about structural rearrangements during the application of stress and/or heat | 3.7 |
| Soft X-ray spectromicroscopy (SXRSM)/X-ray spectro-ptychography (XRSP) | 10 nm–100 nm for morphological imaging with SXRSM, 3 nm for morphological imaging with XRSP 30 nm for chemical analysis with SXRSM, 20 nm for chemical analysis with XRSP | Absorption and phase-contrast morphological imaging, for a given atom the oxidation state/coordination chemistry, neighbor distances/coordination number/species, the electronic structure is sensitive to spatial orientation (induced by the application of stress) | 3.8 |
| Atom probe tomography (APT) | 0.3–0.5 nm | Three-dimensional elemental composition, including mapping and profiling, interfacial region chemistry | 4.1 |
| Electron tomography (ET) | 0.7 nm for mass–thickness imaging 1 nm for chemical analysis | Three-dimensional mass–thickness imaging, three-dimensional elemental composition, including mapping and profiling, layer thickness, interfacial region morphology and chemistry | 4.2 |
| X-ray ptychographic computed tomography (PXCT) | 16 nm | Three-dimensional mass–thickness imaging, layer thickness | 4.3 |
| Structural Parameter | Technique |
|---|---|
| 2D morphological/topographical imaging | SEM, TEM, AFM, HIM, TERS, SXRSM/XRSP |
| 3D morphological imaging | ET, PXCT |
| Thickness of the layer | SEM, TEM, AFM, HIM, ET, PXCT |
| Homogeneity of the layer by scanning large samples | SEM, AFM, HIM |
| Bonding quality (qualitative) by scanning large samples | SEM, AFM, HIM |
| Element presence (qualitative) | SEM+EDS |
| Material identification (qualitative) | AFM-IR |
| 2D elemental composition/profiling/mapping | TEM+EDS, TEM+EELS, AES/SAM, nanoSIMS, TERS, APT, ET |
| 3D elemental composition | APT, ET |
| In-depth elemental profiling | AES/SAM + ion milling sputtering, nanoSIMS |
| Detection of trace elements | nanoSIMS |
| Elemental bonding (qualitative) | AFM-IR |
| Elemental bonding | TEM+EELS |
| Crystal structure and defects | TEM+END |
| Interfacial region morphology | TEM, AFM, HIM, ET |
| Interfacial region chemistry | TEM+EDS, TEM+EELS, APT, ET |
| Interfacial region viscoelastic properties | AFM + nanomechanical mode |
| Viscoelastic properties of the layer | AFM + nanomechanical mode |
| Electrical conductivity of the layer | c-AFM |
| Interfacial region electrical conductivity | c-AFM |
| Crystallographic texture | HIM |
| Ionoluminescence | HIM |
| Crystalline phase identification, crystallinity calculation | TERS |
| Structural rearrangements during the application of stress and/or heat | TERS, SXRSM/XRSP |
| For a given atom: oxidation state/coordination chemistry | SXRSM/XRSP |
| For a given atom: neighbor distances/coordination number/species | SXRSM/XRSP |
| Method | Fracturing or Cutting | Mechanical Polishing | Ultramicrotome (UM) Planarization or Thin/Ultrathin Sectioning | Ion Beam (IB) Milling and Polishing | Focused Ion Beam (FIB) Milling and Polishing |
|---|---|---|---|---|---|
| Sample area/volume | Overall sample section. | Polished area: few mm2 to few tens of mm2. | Planarized area: <few mm2. Thin section: few mm × few mm × submicron thickness. Ultrathin section: <0.1 mm2 × <100 nm (thickness). | Eroded volume: few hundreds of µm × few hundreds of µm × few tens of µm (thickness). Ultrathin section: few mm × few mm × <100 nm (thickness) at the center. | Eroded area: few tens of µm × few µm. Thin section: few µm × few mm × submicron to few µm for the thickness. Ultrathin section: few tens of µm × few µm × <100 nm (thickness). Needle-shaped sample: tip radius at the apex in the range of 50 nm–150 nm, length in the range of few µm to few tens of µm. Cone/cylinder-shaped sample: maximum diameter < 6 µm, height < 10 µm. |
| Advantages | This method is fast, very easy to implement, and cost-effective, making it ideal for quickly visualizing the overall sample cross-section. | This is an easy and cost-effective method that does not require advanced technical expertise. Mechanical polishing can be combined with chemical etching to further reveal the material’s internal structure or with chemical polishing to remove residual scratches and/or weaken the bonds between surface atoms. This enhances the removal of surface material during subsequent mechanical polishing. Typically, mechanical polishing serves as a preparatory step for ion beam milling or FIB, helping to accelerate the overall sample preparation process. Depending on specific needs, various systems can be employed, such as a precision disk grinder (for fine mechanical polishing with micrometer steps), a tripod polisher (for reducing sample thickness to electron transparency), or a dimple grinder (for thinned central areas of the sample). | This method allows for sample preparation in a clean environment, minimizing contamination. Recent advancements include the integration of an ultramicrotome stage within an SEM, enabling in situ imaging during sample planarization. This facilitates in-depth imaging of the cross-section with enhanced precision. | There is no pressure applied to the sample, thereby avoiding the introduction of mechanical artifacts. Ion beam (IB) milling can also be employed to effectively remove surface artifacts produced during FIB milling, resulting in a cleaner, more accurate surface. | FIB is particularly useful when precise positioning below the micron scale or specific orientation for milling is required within a sample. Since there is no pressure applied to the sample, this method avoids mechanical artifacts often associated with other preparation techniques. Multiple milling steps, typically in the order of 0.1 µm, can be performed to analyze the structure as a function of cross-section depth. When combined with an SEM column (FIB-SEM), the system allows for the integration of additional instruments, such as an atomic force microscope (AFM) head or a secondary ion mass spectrometer (SIMS), enabling in situ correlative analyses. Additionally, FIB can be used to deposit materials on the sample surface through the dissociation of precursor gases, allowing for various site-specific modifications or enhancements. |
| Drawbacks | Applying pressure to break the sample can induce artifacts such as local plastic deformation, damage, and debris. The fracture or cut path is difficult to control, resulting in low reproducibility and a very rough surface. Consequently, interpreting the structure becomes complex, and elemental analysis is less reliable due to the need for a very flat, smooth surface. | The application of pressure during sample preparation may induce artifacts. Additionally, performing multiple steps to finely study the structure as a function of cross-section depth is challenging due to significant material loss during polishing. | A high level of expertise is required to effectively utilize this method. It also necessitates an initial trimming step, during which the exact position of the region of interest must be precisely known and controlled. The diamond knives used to cut hard materials are very expensive to replace if damaged; however, they can often be resharpened if damage is limited. This method may induce local deformation or damage to the sample due to the applied compression by the knife. Additionally, ultrathin sections cannot be obtained from very hard, very soft, very brittle, or highly heterogeneous samples using this technique. | Due to the limited milling depth, the region of interest must be located near the edge of the sample. To address this, areas outside the region of interest can be removed through mechanical polishing. For ultrathin section preparation, a pre-thinning step—using techniques such as mechanical polishing, magnetorheological polishing, or dimpling—is typically performed. The final thinning with an ion beam (IB) is usually carried out on the opposite surface of the pre-thinned area. It is important to note that ion beam milling can induce surface amorphization and alter the surface topography of the sample. Additionally, IB milling is generally not suitable for isolating regions of interest below the micron scale. | Only small volumes can be prepared using this method. In the case of electrically insulating samples, charging effects may occur due to the positive ion beam, leading to deflection of the beam and potential inaccuracies. For thermally insulating materials, the ion beam can generate local heating, causing damage; using cryogenic FIB is often suitable to mitigate this issue. FIB milling can induce surface artifacts such as amorphization, Ga implantation, lattice strains, and crystal defects, with a higher production of artifacts compared to ion beam (IB) techniques. Due to limited milling depth, the region of interest must be close to the edge of the sample. To address this, the non-interesting areas can be removed through mechanical polishing. Additionally, to decrease the milling time needed to prepare ultrathin sections, pre-thinning of the sample via mechanical methods is often employed. |
| Targeted characterization techniques | SEM, AES/SAM, HIM | SEM, AES/SAM, AFM, nanoSIMS, HIM | Planarized area: SEM (+EDS), AES/SAM, AFM, SIMS, HIM. Thin section: nanoSIMS, SEM (+EDS), AES/SAM, AFM, HIM. Ultrathin section: SEM (+EDS), AES/SAM, AFM, nanoSIMS. TEM/ET (+END+EDS+EELS), TERS, SXRSM/XRSP. | Eroded volume: SEM (+EDS), AES/SAM, AFM, SIMS, HIM. Ultrathin section: SEM (+EDS), AES/SAM, AFM, nanoSIMS, TEM/ET (+END+EDS+EELS), TERS, SXRSM/XRSP. | Eroded area: SEM (+EDS), AES/SAM, AFM, SIMS, HIM. Thin section: nanoSIMS, SEM (+EDS), AES/SAM, AFM, HIM. Ultrathin section: SEM (+EDS), AES/SAM, AFM, nanoSIMS, TEM/ET (+END, +EDS+EELS), TERS, SXRSM/XRSP. Needle-shaped sample: APT Cone/cylinder-shaped sample: PXCT. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addiego, F.; Bhusari, R.; Bardon, J.; Scholzen, S.; Kaidi, Z. Nanoscale Cross-Sectional Characterization of Thin Layers in Material Assemblies. Nanomaterials 2025, 15, 840. https://doi.org/10.3390/nano15110840
Addiego F, Bhusari R, Bardon J, Scholzen S, Kaidi Z. Nanoscale Cross-Sectional Characterization of Thin Layers in Material Assemblies. Nanomaterials. 2025; 15(11):840. https://doi.org/10.3390/nano15110840
Chicago/Turabian StyleAddiego, Frédéric, Rutuja Bhusari, Julien Bardon, Sascha Scholzen, and Zainhia Kaidi. 2025. "Nanoscale Cross-Sectional Characterization of Thin Layers in Material Assemblies" Nanomaterials 15, no. 11: 840. https://doi.org/10.3390/nano15110840
APA StyleAddiego, F., Bhusari, R., Bardon, J., Scholzen, S., & Kaidi, Z. (2025). Nanoscale Cross-Sectional Characterization of Thin Layers in Material Assemblies. Nanomaterials, 15(11), 840. https://doi.org/10.3390/nano15110840








