Abstract
Quickly detecting hydrogen leakage is crucial to provide early warning for taking emergency measures to avoid personnel casualties and explosion accidents in hydrogen energy fields. Here, a compact optical fiber hydrogen sensing system with high sensitivity and quick response rate is proposed in this work. A laser diode (LD) and two photodetectors (PD) are employed as light source and optical signal transformation devices, respectively. This sensing system employs single-mode optical fiber deposited with WO3-PdPt-Pt nanocomposite film system as sensing element. Under irrigating power of 6 mW, the sensing probe exhibits an ultra-fast response to hydrogen concentrations of 4000 ppm and 10,000 ppm, with response times of 0.44 s and 0.34 s, respectively. In addition, detection limit of 3 ppm can be achieved by using this sensing system. The sensor also shows good repeatability during hydrogen exposure of 3~10,000 ppm, demonstrating its great potential application for hydrogen leakage in hydrogen energy facilities.
1. Introduction
Hydrogen, as a clean and efficient energy carrier, has attracted considerable attention in these years. However, hydrogen leakage can pose serious accidents due to its highly flammable and explosive nature. In recent years, hydrogen explosion accidents have raised higher requirements for the safe use of hydrogen energy. Consequently, monitoring hydrogen leakage safely and reliably has become extremely important for various hydrogen energy locations.
At present, commercial hydrogen sensors are mainly electrical hydrogen sensors, including these on Pt-coated W18O49 nanowire networks [1] and hierarchical Pd-WO3 nanoflowers [2]. However, these sensors are not nature safe due to the potential electric sparks. Compared to electrical hydrogen sensor, optical fiber hydrogen sensors have attracted many researchers’ interests, owing to their nature safety, anti-electromagnetic interference, long-distance monitoring capabilities, and adaptability to harsh environments. In recent years, significant progress has been made in the research of optical fiber hydrogen sensors, with various sensor types being proposed, such as evanescent fields sensors based on Pd0.6Au0.4 [3,4], Pd thin films [5], micro-reflector sensors deposited with Pd thin film [6], nano-platelet WO3 film [7], interference sensor combined with Pt-loaded WO3/SiO2 coating [8], surface plasmon resonance (SPR) sensor utilizing Pd thin film [9], fiber Bragg gratings (FBG) sensors employing Pd films [10,11], Pt/Ni composite films [12], Pt-loaded WO3-SiO2 coating [13], sensitive layer made of WO3 doped with Pt [14], and stimulated Raman spectroscopy sensor consisting of nanofiber [15]. Among these fiber optic hydrogen sensors, micro-reflector hydrogen sensor shows more obvious advantages such as simple structure, low cost and ease for massive production.
However, most fiber optic hydrogen sensors still have some limitations in terms of sensitivity, response rate, and cost of sensing system. To overcome these problems, a compact and economic fiber optic sensing system is proposed in this paper. Firstly, sensing probes were prepared by depositing nano-WO3-based composite film on end face of single-mode fiber. As the diameter of single-mode fiber is about 125 μm, it is easy to deposit hydrogen sensing films on tips of multiple optical fibers. Then, the sensing probe is set in a gas room filled with hydrogen concentration about 10% to generate microcracks by utilizing phase transition of Pd [10]. During this process, a laser diode and two InGaAs photodetectors with diameters of 300 μm (operating temperature −40~80 °C, wavelength range 800~1700 nm) are used as light source and optic signal transformation elements, which can avoid using laser diode (LD) light source and optical spectrometer, resulting in significant decrease in the cost of the sensing system. Surprisingly, sensing probe deposited with nanocomposite films show quick response rate during hydrogen exposure. This sensing system can display fast response rates and high sensitivity, which can provide a safe and economic sensing platform for hydrogen leakage detection in various facilities.
2. Experimental Setup and Sensor Fabrication
As shown in Figure 1, the preparation of sensing probes mainly involves the evaporation of WO3 film and the sputtering of PdPt-Pt catalytic layer. Firstly, single-mode fibers were cut by an optical fiber cleaver to form a flat tip for depositing sensitive films. Then, 210 nm WO3 film were evaporated on the fiber tip by using the thermal evaporation method [16,17]. During the evaporation process, oxygen was injected into chamber at a flowing rate of 200 sccm to prevent defects in the WO3 film. 30 nm PdPt composite films and 5 nm Pt film were sputtered on surface of WO3 thin films with magnetron sputtering technology. During the depositing process, the thickness of hydrogen sensitive films was monitored by quartz crystal method, and the corresponding deposition rates for WO3, Pd, and Pt are 0.03, 0.10, and 0.05 nm/s, respectively. Meanwhile, sensitive films are prepared on Si substrate during the depositing process for further characterization.
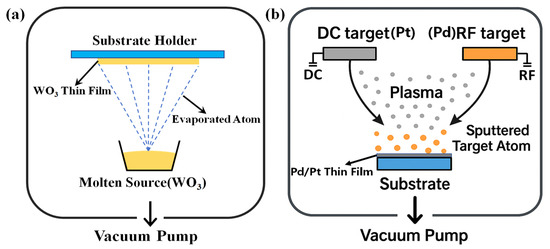
Figure 1.
Schematic of the (a) evaporating and (b) sputtering technologies for depositing hydrogen sensitive films.
As it is shown in Figure 2, an optical attenuator is used to connect the optical power of a laser diode (LD) light source and an optical coupler (95:5). The lower fraction (about 5%) power of the light source is detected by PD1, and the residual power of light source is guided to the sensing probe by an optical coupler (80:20). Electric signals of PD1 and PD2 are used to evaluate the optical power change of the LD light source and the optical power reflected by the sensing probe.
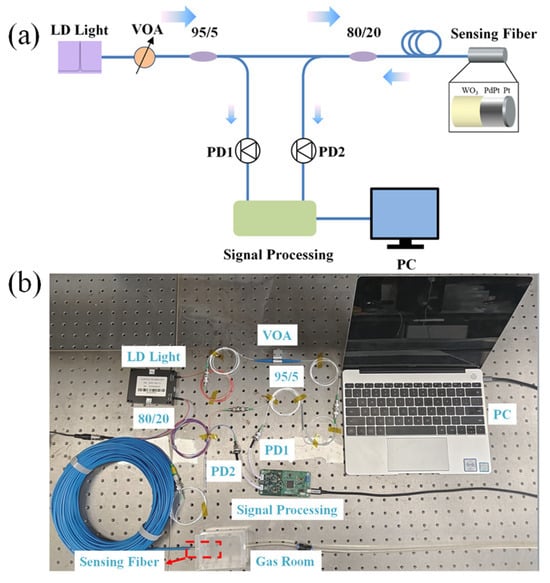
Figure 2.
(a) Schematic of the optical fiber hydrogen sensor system; (b) a photo of the hydrogen sensing system.
Figure 3a displays the spectrum of the LD light source. We can see that the central wavelength of the LD light source is about 1552.46 nm, which is located in low loss band for optical communication. This feature ensures long-distance detection capability of this sensing system. Figure 3b shows the fluctuations of IS/IR about 6000 s in air. It can be observed that the fluctuation of IS/IR is about 0.0003 during this process, and simultaneous resolution of this sensing system can be down to 0.0001. The fluctuation may be due to the variation of ambient temperature. However, utilizing IS/IR as a sensing parameter can significantly compensate power fluctuation [17] of light source and enhance stability of the hydrogen sensing system.
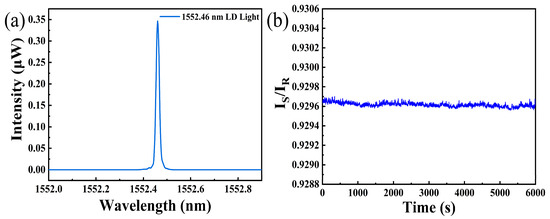
Figure 3.
(a) Spectrum of the LD light source; (b) noise level of hydrogen sensing system with IS/IR as a sensing parameter.
Hydrogen detection tests were conducted at a room temperature of 17 °C using air as carrier gas. A rectangular acrylic box with a capacity of approximately 200 mL is used as a gas room, and there are two circular holes with a diameter about 10 mm on the opposite side walls of the gas chamber as the inlet and outlet for the mixed gas. 0.1% H2 (H2/N2 = 1:99, volume ratio) and 99.99% H2 were employed as H2 supplying gas, and 21% O2 (O2/N2 = 21:79, volume ratio) was used as dry air for hydrogen sensing. Three mass flow controllers (Beijing Sevenstar, Inc., Beijing, China, CS200A, 0~30 sccm, 0~100 sccm, 0~1000 sccm) were utilized to adjust the flowing rate of three gases, respectively. In this work, hydrogen concentrations of 3~50 ppm were provided by mixing 0.1% H2 with 21% O2 mixed gas with a total flowing rate of 1000 sccm. Hydrogen concentrations above 50 ppm were prepared by mixing 99.99% H2 with 21% O2 mixed gas at the same flowing rate. During the hydrogen testing process, all data were recorded in real-time by a computer for further analysis.
3. Results and Discussion
The working principle of this sensor is based on the hydrogen-induced gasochromic effect of the WO3-PdPt-Pt composite film, which causes the reflectivity changes of the sensing probe during hydrogen response process. There are three models [18] that are widely accepted to explain the gasochoromic mechanism of WO3: “double injection of ions” [19], “generation of oxygen vacancies” [20], and “localized water molecules” [21]. As sensing mechanisms between the hydrogen sensitive film and hydrogen is still controversial, the change in reflectivity of the hydrogen sensitive film is mainly attributed to the photon absorption phenomenon within the defect band in HxWO3 [19] or WO3−x [20].
Figure 4a shows the SEM image of cross section of the hydrogen sensitive film deposited on a silicon substrate, with a thickness of 210 nm for the WO3 substrate layer and 35 nm for the PdPt-Pt catalytic layer. WO3 has excellent hydrogen induced discoloration performance [18], which can display remarkable absorption change of telecom wavelength band, resulting in obvious reflection change of optical signal in fiber core. Another advantage of using WO3 as basal layer is due to its good adhesion towards optical fibers. The catalytic layer consists of PdPt composite films and Pt thin film, which exhibits low volume expansion characteristics and good oxidation resistance in air environments. Stability of the hydrogen sensitive probe can be enhanced by employing a sensitive film with this membrane structure.
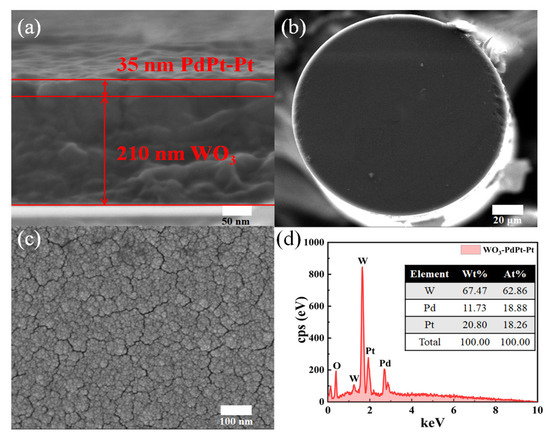
Figure 4.
(a) Cross-sectional SEM image of the hydrogen sensitive film deposited on a Si substrate. (b) SEM image of the fiber tip. (c) SEM image of the sensitive film deposited on the tip of the optical fiber. (d) EDAX pattern of hydrogen sensitive film.
Figure 4b presents the SEM image of the fiber tip deposited with hydrogen sensitive film. The hydrogen sensitive film adheres firmly to the fiber tip, confirming the good mechanical stability of the sensing probe. As shown in Figure 4c, the magnified image reveals numerous microcracks with widths ranging from 5 to 10 nm. These cracks may arise from hydrogen embrittlement caused by the accumulation of Pd catalyst during hydrogen exposure [10]. However, the reflectivity of the sensing probe is not significantly affected, which may be due to the much longer operating wavelength of the LD light source. Interestingly, these microcracks can provide extra diffusion channels, which can enhance the diffusion rate of hydrogen molecules. Figure 4d gives the elemental analysis of hydrogen sensitive film. Molar ratio of W, Pd, and Pt is about 10:3:3, which is approximately consistent with the W:Pd:Pt of 210 nm WO3, 30 nm PdPt composite film, and 5 nm pure Pt film.
From Figure 5a, partial Pd atoms are oxidized, which can be demonstrated by Pd 3d (Pd2+) spectra located at 334.46 eV and 340.06 eV. However, more than 50% of Pd atoms are Pd0 state. As shown in Figure 5b, the Pt 4f spectra of catalytic layer is deconvolved into Pt 4f7/2 and Pt 4f5/2 peaks, which proves their good antioxidant ability. This proves the effectiveness of the membrane structure design. The W element mainly coexists in the W6+ and W5+ states (Figure 5c), with more than 70% in the form of W6+ state. As shown in Figure 5d, the as-deposited PdPt-Pt film has relatively sharp peaks at 2θ = 40.6°, 47.8°, and 68.9°, confirming crystalline structure (JCPDS 65-6174) of the catalytic layer. However, WO3 mainly exists in an amorphous form, which is similar to the results we reported earlier [6]. Amorphous tungsten trioxide has a lower bandgap [18], which facilitates electron diffusion and may have a positive effect on faster response rates of hydrogen sensitive film [22].
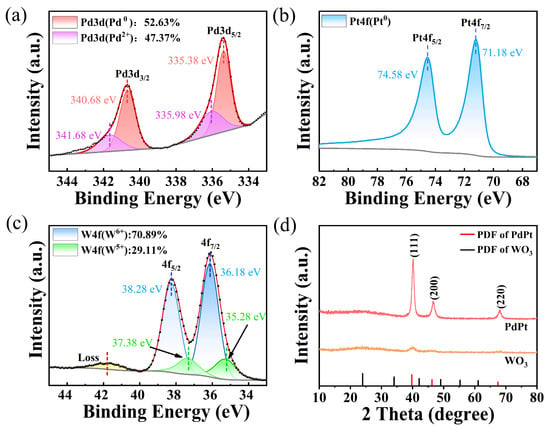
Figure 5.
XPS of (a) Pd, (b) Pt, and (c) W element of WO3-PdPt-Pt composite films; (d) XRD of WO3-PdPt-Pt composite films.
To investigate the sensing performance of the WO3-PdPt-Pt composite film at different irrigating power, an optical attenuator was used to adjust the optical power reaching the fiber tip. Since primary warning value of hydrogen leakage is 4000 ppm in many scenarios, response characteristics of the sensing probe exposed to this concentration were studied at different excitation powers. Figure 6a–d depicts the 4000 ppm response characteristics of the sensing probe irrigated at different optical powers. As shown in Figure 6, IS/IR decreased quickly as the sensing probe is exposed to 4000 ppm hydrogen at all irrigating power; then, it gradually reaches to an equilibrium in the following 5 min. Moreover, this sensing system shows a quicker response rate when the hydrogen sensitive film is irrigated at 6 mW and 7 mW. However, the stability of the sensor deteriorates as irrigating power is set to 7 mW. The reason for this phenomenon may be due to the stripping of catalysts, which can be attributed to much higher thermal expansion coefficient of Pd alloy [23]. Overall, the sensing probe exhibits better repeatability and response rate when the sensitive layer is irrigated at 6 mW. Therefore, the following hydrogen sensing test will be carried out under this power.
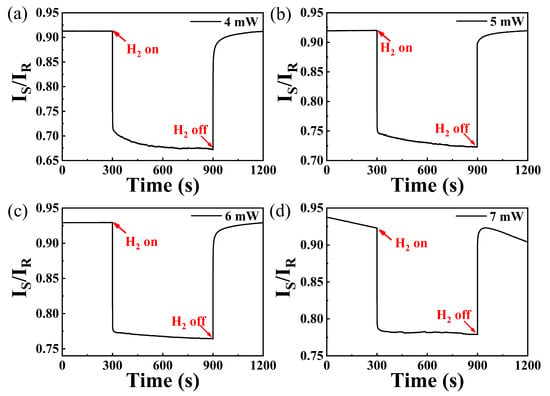
Figure 6.
Hydrogen response of sensing probe irrigated by different optical powers: (a) 4 mW; (b) 5 mW; (c) 6 mW; (d) 7 mW.
As hydrogen sensing systems with better responsibility can give earlier warnings, the threshold of this sensing system was explored in this paper. Figure 7a shows three cycles of 30 ppm and 50 ppm hydrogen response curves measured by this sensing system. Although the response time for 30 ppm is more than 35 s, this sensing system still has good repeatability in the following two cycles. In addition, this sensing system can display an obvious decrease of IS/IR during three cycles of 3 ppm hydrogen exposure (shown in Figure 7b), demonstrating its excellent ability for early warning.
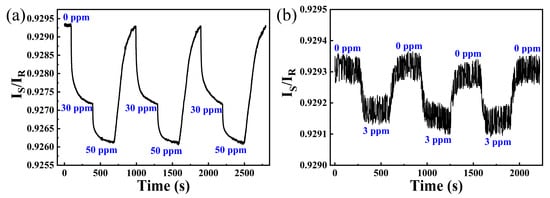
Figure 7.
(a) Response of the hydrogen sensing system towards 30 ppm and 50 ppm hydrogen; (b) response of the hydrogen sensing system towards 3 ppm hydrogen.
As displayed in Figure 8a, the hydrogen sensor exhibits about 0.95 decrease and good repeatability during 20 cycles of 500 ppm hydrogen exposure. In the following five cycles of continuous increase of hydrogen concentration exposure (shown in Figure 8b), decreases of IS/IR for 1000, 2000, 3000, 4000, and 5000 ppm hydrogen are about 0.1268, 0.1390, 0.1495, 0.1587, and 0.1673, respectively. It can be observed in Figure 8c that the sensing probe shows faster response rates but worse repeatability with the increase in hydrogen concentration. This phenomenon is more obvious when hydrogen concentrations are above 4000 ppm. There are some hysteresis effects during the hydrogen exposure process, which gives a negative effect on hydrogen concentration monitoring. As water molecules can be generated during hydrogen reaction [21], some water molecules will be adsorbed on the surface of the sensitive film, which will hinder the reaction between the sensitive film and oxygen molecules.
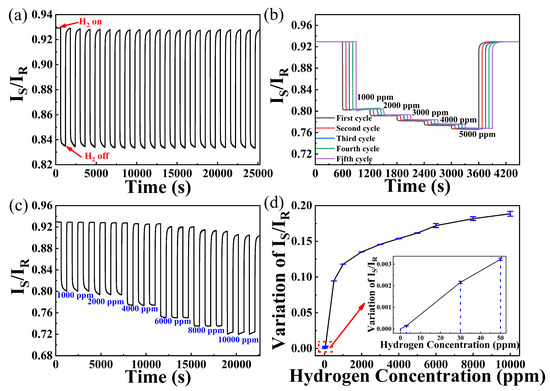
Figure 8.
(a) Twenty cycles of 500 ppm hydrogen exposure; (b) five cycles of hydrogen sensing probe under different hydrogen concentrations; (c) response of different hydrogen concentrations; (d) decrease of IS/IR under different hydrogen concentrations.
From Figure 8d, we can see that this sensing system displays a nonlinear response with increase in hydrogen concentrations. This sensing system can display high sensitivity in a low concentration hydrogen atmosphere, which is beneficial for early warning of hydrogen leakage. This excellent performance indicates that the sensor can monitor hydrogen over a wide concentration range, indicating its robust capability for hydrogen detection.
Figure 9a,b illustrates the response curves of the sensing probe during 4000 ppm hydrogen exposure. The response time is calculated by inserting the sensing probe into the gas chamber to the sensing system reaching 90% decrease of IS/IR. And response time for 10,000 ppm hydrogen can also be observed in Figure 9c,d. The response time of this sensing system for 4000 ppm and 10,000 ppm hydrogen are about 0.44 s and 0.34 s, respectively. The quick response rate can be attributed to the joint effect of optimized irrigating power and nanocomposite thin films with multiple transmission channels. Firstly, sensitive layer with microcracks can provide more diffusion channels [2], resulting in more active sites for adsorption of hydrogen molecular. Additionally, the PdPt-Pt layer displays outstanding catalytic properties [24], which can greatly accelerate and efficiently decompose hydrogen molecules into hydrogen atoms. Moreover, hydrogen atoms diffuse faster in nanocomposite films at higher irrigating power [17], which can further accelerate the gasochromic reaction. Nevertheless, cost of this sensing system is much lower than that of previous reported work [16,25], which is an obvious merit for hydrogen sensing.
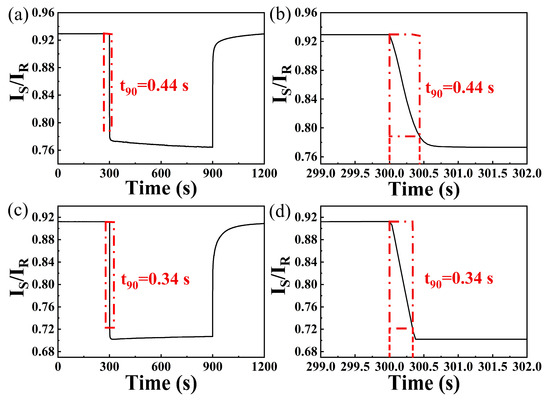
Figure 9.
(a) Response curve and (b) response time of the hydrogen sensing probe towards 4000 ppm hydrogen; (c) response curve and (d) response time of the hydrogen sensing probe towards 10,000 ppm hydrogen.
To explore the selectivity of this sensing system, different gases are mixed with air and injected into gas chamber by utilizing 0.5% CO/Ar, 99.99% CH4, and 0.1% NH3/air with total flowing rate of 1000 sccm. Figure 10 depicts the response of hydrogen sensing probe towards CO, CH4, NH3, and H2, respectively. It can be seen that the sensing probe displays obvious response during the exposure of 0.1% CO, 0.1% CH4, and NH3. Unfortunately, the selectivity of this sensing system is worse than our previous work [16]. The reason for this phenomenon can be attributed to the increase of Pt element in the catalytic layer and higher irrigating power, as complete combustion reaction of CO and CH4 can be achieved with Pt as catalyst in air [26]. Then, CO2 and H2O (in air) may react to form carbonic acid, which can cause double injection of H+ and e− in WO3 layer, leading to the increase in absorption of the optical single. The more obvious response of 0.1% NH3 may be due to the hydrogen bond between NH4+ and WO3 [27], which could also be demonstrated by the faster response rate of NH3 exposure.
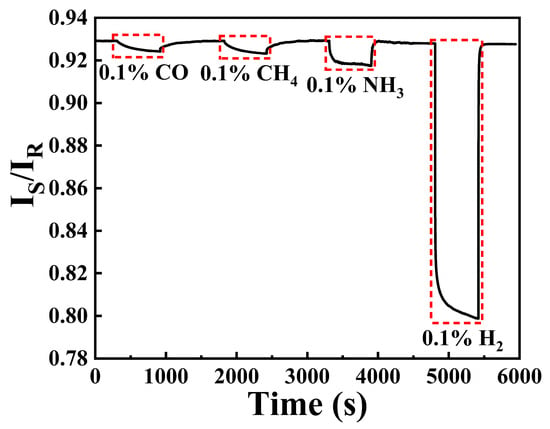
Figure 10.
Response curve of the hydrogen sensing probe towards different gases.
After the exposure of CO, CH4, and NH3, the sensing probe still shows remarkable response (more than 10 times) towards 0.1% H2, demonstrating its better selectivity towards H2. However, the selectivity of this sensing system may be improved by optimizing the constitute catalytic layer and irrigating power. For oil and gas industry, utilizing hydrogen sensors based on Pt alloys [12] or Raman spectroscopy [15] maybe be more feasible, as these sensors can show repetitive response towards hydrogen in an aerobic environment.
Table 1 shows the performance comparison of several recently reported hydrogen sensors. From Table 1, we can see that the sensing system based on the WO3-PdPt-Pt composite film can display quicker response rate capability when compared to that of the recently reported work [25,28,29,30,31,32,33,34]. Although the detection limit of this sensing system is higher than that of recently organic hydrogen sensor [34], it can detect hydrogen far below the lower explosive limit (4%) of hydrogen in air. These merits can provide economic and reliable hydrogen leakage detecting technology for the hydrogen energy field. Furthermore, this paper can provide a clue for developing a gas sensor with excellent responsibility.

Table 1.
Comparison of reported hydrogen sensors.
4. Conclusions
This work presents a novel optical fiber hydrogen sensing system based on a WO3-PdPt-Pt nanocomposite film. The system features a simple structure and relatively low cost. At an optimal irrigating power of 6 mW, the sensor shows ultra-fast responses to hydrogen concentrations of 4000 ppm and 10,000 ppm, with response times of 0.44 s and 0.34 s, respectively. The sensor also exhibits good repeatability during hydrogen exposure of 1000 to 10,000 ppm. Moreover, it achieves a remarkably low detection limit of 3 ppm. With its simple and compact design, the sensor can detect hydrogen concentrations far below the hydrogen explosion limit, demonstrating an economic technology for early warning of hydrogen leakage. This compact hydrogen sensing system shows advantages such as high sensitivity, rapid response speed, good repeatability, and wide monitoring range, making it promising for detecting hydrogen leakage in hydrogen energy facilities.
Author Contributions
Conceptualization, J.D. and M.Y.; methodology, Z.C. and W.H.; software, Z.T.; validation, Z.C., R.Y. and Z.W.; formal analysis, C.C.; investigation, X.W.; resources, R.Y.; data curation, Z.W.; writing—original draft preparation, J.D.; writing—review and editing, M.Y.; visualization, Z.T.; supervision, M.Y.; project administration, X.W.; funding acquisition, M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Joint Fund for Regional Innovation and Development of NSFC (U23A20374), National Science Found for Distinguished Young Scholars of China (62025505), State Key Laboratory of Maritime Technology and Safety (WUT104972024KFYd0033), Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (2021JJLH0058).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, L.; She, J.; Luo, J.; Deng, S.; Chen, J.; Ji, X.; Xu, N. Self-heated hydrogen gas sensors based on Pt-coated W18O49 nanowire networks with high sensitivity, good selectivity and low power consumption. Sens. Actuators B Chem. 2011, 153, 354–360. [Google Scholar] [CrossRef]
- Wang, X.; Meng, X.; Gao, W. Ultrahigh-response sensor based on hierarchical Pd-WO3 nanoflowers for rapid hydrogen detection. Sens. Actuators B Chem. 2023, 387, 133790. [Google Scholar] [CrossRef]
- Zhao, Z.; Carpenter, M.; Xia, H.; Welch, D. All-optical hydrogen sensor based on a high alloy content palladium thin film. Sens. Actuators B Chem. 2006, 113, 532–538. [Google Scholar] [CrossRef]
- Zhao, Z.; Knight, M.; Kumar, S.; Eisenbraun, E.; Carpenter, M. Humidity effects on Pd/Au-based all-optical hydrogen sensors. Sens. Actuators B Chem. 2008, 129, 726–733. [Google Scholar] [CrossRef]
- Tabib-Azar, M.; Sutapun, B.; Petrick, R.; Kazemi, A. Highly sensitive hydrogen sensors using palladium coated fiber optics with exposed cores and evanescent field interactions. Sens. Actuators B Chem. 1999, 56, 158–163. [Google Scholar] [CrossRef]
- Butler, M. Micromirror optical-fiber hydrogen sensor. Sens. Actuators B Chem. 1994, 22, 155–163. [Google Scholar] [CrossRef]
- Ou, J.; Yaacob, M.; Campbell, J.; Breedon, M.; Kalantar-zadeh, K.; Wlodarski, W. H2 sensing performance of optical fiber coated with nano-platelet WO3 film. Sens. Actuators B Chem. 2012, 166, 1–6. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W.; Zhao, C.; Xu, B.; Wang, D.; Yang, M. Optical hydrogen sensor based on PDMS-formed double-C cavities with embedded Pt-loaded WO3-SiO2. Sens. Actuators B Chem. 2018, 276, 23–30. [Google Scholar] [CrossRef]
- Lin, K.; Lu, Y.; Chen, J.; Zheng, R.; Wang, P.; Ming, H. Surface plasmon resonance hydrogen sensor based on metallic grating with high sensitivity. Opt. Express 2008, 16, 18599–18604. [Google Scholar] [CrossRef]
- Sutapun, B.; Tabib-Azar, M.; Kazemi, A. Pd-coated elastooptic fiber optic Bragg grating sensors for multiplexed hydrogen sensing. Sens. Actuators B Chem. 1999, 60, 27–34. [Google Scholar] [CrossRef]
- Ma, G.; Li, C.; Luo, Y.; Mu, R.; Wang, L. High sensitive and reliable fiber Bragg grating hydrogen sensor for fault detection of power transformer. Sens. Actuators B Chem. 2012, 169, 195–198. [Google Scholar] [CrossRef]
- Kilinc, N.; Sanduvac, S.; Erkovan, M. Platinum-Nickel alloy thin films for low concentration hydrogen sensor application. J. Alloys Compd. 2022, 892, 162237. [Google Scholar] [CrossRef]
- Kefer, S.; Dai, J.; Yang, M.; Schmauss, B.; Hellmann, R. Hypersensitive H2 sensor based on polymer planar Bragg gratings coated with Pt-loaded WO3-SiO2. Opt. Lett. 2020, 45, 3601–3604. [Google Scholar] [CrossRef]
- Caucheteur, C.; Debliquy, M.; Lahem, D.; Mégret, P. Hybrid fiber gratings coated with a catalytic sensitive layer for hydrogen sensing in air. Opt. Express 2008, 16, 16854–16859. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, Y.; Bao, H.; Jin, W.; Ho, H. Nanofiber enhanced stimulated Raman spectroscopy for ultra-fast, ultra-sensitive hydrogen detection with ultra-wide dynamic range. Optica 2019, 6, 570–576. [Google Scholar] [CrossRef]
- Dai, J.; Peng, W.; Wang, G.; Xiang, F.; Qin, Y.; Wang, M.; Yang, M. Improved performance of fiber optic hydrogen sensor based on WO3-Pd2Pt-Pt composite film and self-referenced demodulation method. Sens. Actuators B Chem. 2017, 249, 210–216. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Ruan, H.; Ye, Z.; Chai, N.; Wang, X.; Qiu, S.; Bai, W.; Yang, M. Fiber optical hydrogen sensor based on WO3-Pd2Pt-Pt nanocomposite films. Nanomaterials 2021, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Guo, X.; Nie, L.; Wu, X.; Peng, L.; Chen, J. A review on WO3 gasochromic film: Mechanism, preparation and properties. Int. J. Hydrogen Energy 2023, 48, 2442–2465. [Google Scholar] [CrossRef]
- Lee, S.; Cheong, H.; Liu, P.; Smith, D.; Tracy, C.; Mascarenhas, A.; Pitts, J.; Deb, S. Raman spectroscopic studies of gasochromic a-WO3 thin films. Electrochim. Acta 2001, 46, 1995–1999. [Google Scholar] [CrossRef]
- Wittwer, V.; Datz, M.; Ell, J.; Georg, A.; Graf, W.; Walze, G. Gasochromic windows. Sol. Energy Mater. Sol. Cells 2004, 84, 305–314. [Google Scholar] [CrossRef]
- Luo, J.; Deng, S.; Tao, Y.; Zhao, F.; Zhu, L.; Gong, L.; Chen, J.; Xu, N. Evidence of localized water molecule and their role in the gasochromic effect of WO3 nanowire films. J. Phys. Chem. 2009, 113, 15877–15881. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, X.; Yu, S.; Yang, H.; Guo, W.; Li, S.; Zheng, J. Review on amorphous WO3 for eletrochromic devices: Structure, optimization strategies and applications. Mater. Today Chem. 2025, 43, 102513. [Google Scholar] [CrossRef]
- Ievlev, V.; Dontsov, A.; Kannykin, S.; Prizhimov, A.; Solntsev, K.; Roshan, N.; Gorbunov, S. Thermal expansion coefficient of a Pd-Cu solid solution. Inorg. Mater. 2020, 56, 1225–1228. [Google Scholar] [CrossRef]
- Lebon, A.; García-Fuente, A.; Vega, A.; Aguilera-Granja, F. Hydrogen interaction in Pd-Pt alloy nanoparticles. J. Phys. Chem. C 2012, 116, 126–133. [Google Scholar] [CrossRef]
- Dai, J.; Ruan, H.; Zhou, Y.; Yin, K.; Hu, X.; Ye, Z.; Wang, X.; Yang, M.; He, P.; Yang, H. Ultra-high sensitive fiber optic hydrogen sensor in air. J. Light. Technol. 2022, 40, 6583–6589. [Google Scholar] [CrossRef]
- Shuk, P.; Mcguire, C.; Brosha, E. Methane gas sensing technologies in combustion: Comprehensive Review. Sens. Transducers J. 2019, 229, 1–10. [Google Scholar]
- Zhong, J.; Huang, B.; Song, J.; Zhang, X.; Du, L.; Gao, Y.; Liu, W.; Kang, L. Stable WO3 electrochromic system based on NH4+ hydrogen bond chemistry. Chem. Eng. J. 2023, 480, 148098. [Google Scholar] [CrossRef]
- Abdalwareth, A.; Flachenecker, G.; Angelmahr, M.; Schade, W. Optical fiber evanescent hydrogen sensor based on palladium nanoparticles coated Bragg gratings. Sens. Actuators A Phys. 2023, 361, 114594. [Google Scholar] [CrossRef]
- Dissananyake, K.; Dewi, H.; Schreuders, H.; Bannerberg, L.; Abdalwareth, A.; Flachenecker, G.; Angelmahr, M.; Schade, W. Advancing hydrogen sensing for sustainable aviation: A metal hydride coated TFBG optical fibre hydrogen sensor. e-J. Nondestruct. Test. 2024, 29, 1–8. [Google Scholar]
- Khanikar, T.; Karki, D.; Su, Y.; Hong, J.; Wang, Y.; Naeem, K.; Ohodnicki, P. Pd/PMMA nanocomposite-coated optical fiber hydrogen sensor operating at room temperature with humidity tolerance. IEEE Sens. J. 2024, 24, 34498–34506. [Google Scholar] [CrossRef]
- Wang, C.; Han, Z.; Wang, C.; Peng, G.; Rao, Y.; Gong, Y. Highly sensitive fiber grating hydrogen sensor based on hydrogen-doped Pt/WO3. Sens. Actuators B Chem. 2024, 404, 135250. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, L.; Wei, X.; Liu, Q.; Liang, Y.; Wang, J.; Peng, W. Thermo-optic nanomaterial fiber hydrogen sensor. Nanomaterials 2025, 15, 440. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Liu, S.; Chen, P.; Liu, B.; Xiao, H.; Ding, X.; Wang, Y.; Wang, Y. Optically driven nano-beam resonator for hydrogen sensing. J. Light. Technol. 2024, 42, 2611–2616. [Google Scholar] [CrossRef]
- Mandal, S.; Marsh, A.; Faber, H.; Ghoshal, T.; Goswami, D.; Tsetseris, L.; Heeney, M.; Anthopoulos, T. A robust organic hydrogen sensor for distributed monitoring applications. Nat. Electron. 2025, 8, 343–352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).