Organic Fluorescent Sensors for Environmental Analysis: A Critical Review and Insights into Inorganic Alternatives
Abstract
1. Introduction
2. Organic Materials
2.1. Small Molecule-Based Sensors
- (1)
- Pyrene and its derivatives are fluorescent probes used in sensors to (i) detect reactive oxygen species (ROS) in biological systems (as indicators of oxidative stress and cellular damage) [34]; (ii) enhance the electrochemical properties of materials, useful in energy storage applications [34]; and (iii) detect various analytes (metals [35], drugs [36], gas molecules [37]). In these sensors, sensing is allowed by changes in pyrene moiety fluorescence intensity. Interesting environmental applications are those in heavy metal detection, with an LOD of 2.9 nM for the detection of Ag+ (in DMSO–H2O, 1:1 v/v, HEPES = 50 mM, pH = 7.4) and 4 and 2 ppb for the detection of Hg+ and Pb2+ (in MeCN–H2O, 2:8 v/v), respectively [35].
- (2)
- Fluorescein, a synthetic organic compound with strong fluorescence, is commonly used in glucose sensors for medical diagnostics [38,39] and, along with its derivatives, for modern biochemical and biological studies. Derivatives (Eosin B, Rose Bengal, etc.) are obtained through the inclusion of heavy atoms or the substitution of the hydroxyl groups on the xanthene core in order to tune the fluorescent properties and achieve additional functionalities. The structural modification occurring at the carboxyl group by introducing different groups produces a spirolactam structure that is non-fluorescent. Various factors, like the pH, the temperature, and the presence of other molecules, can cause this modification, followed by quenching. In environmental analysis, fluoresceins have been used to detect different metal ions in aqueous solutions, with LOD values in the nanomolar range: Cu2+, noted for the toxic effect of its accumulation in Alzheimer’s or Parkinson’s diseases, was detected down to 6.32 nM [40]; Hg2+, which enters the food chain from the environment, was detected down to an LOD of 0.86 nM [41]; and even anions, such as ClO−, whose excessive intake can cause tissue damage, liver injury, arthritis, and cardiovascular diseases, was detected with an LOD of 56 pM [42].
- (3)
- Rhodamine represents a group of xanthene derivative dyes characterized by fine biocompatibility and near-infrared fluorescence, used in oxygen sensors for environmental monitoring. Structurally similar to fluoresceins, rhodamine sensing is based on the “closing–opening” of the rhodamine derivative’s ring structure, before and after target addition. Coordination between the sensor and target (mainly metal ions [43]) induces spirolactam ring opening and changes the colorimetric and/or fluorescent responses, which are usually absent before the coordination [44]. Optimal LOD values (0.107 µM, corresponding to 6.79 µg/L) have been recently achieved for Cu2+, reaching a concentration significantly below the drinking water quality standard of 2.0 mg/L of the World Health Organization (WHO) [43].
- (4)
- Cyanine dyes are a family of tetramethylindo(di)-carbocyanines that consist of a polymethine chain containing an odd number of carbon atoms between two tertiary amines, often represented by two aromatic nitrogen-containing heterocycles as charged chromophores, acting as both electron donors and acceptors [45]. Cyanine dyes have been studied widely and are some of the oldest [46] and brightest synthetic fluorophores [47]. They play significant roles in various scientific and technological fields:
- -
- Biological imaging and microscopy—as fluorescent probes they are widely used for labeling biomolecules such as DNA, RNA, and proteins, allowing researchers to visualize and track biological processes [48];
- -
- Chemical and biological sensors—due to their sensitive response to changes in their environment, they are employed in the development of sensors for analytes such as ions, H+, and biomolecules [48];
- -
- Photodynamic therapy (PDT)—certain cyanine dyes are used in PDT for cancer treatment, since they generate reactive oxygen species when exposed to light, which can destroy cancer cells [49];
- -
- Solar cells—cyanine dyes are explored as sensitizers in dye-sensitized solar cells, since they can absorb sunlight and convert it into electrical energy, contributing to the development of renewable energy sources [50].While less explored for environmental applications, Galhano et al. proposed silica platforms doped with cyanine derivatives for the detection of divalent metal ions in acetonitrile [51] (Zn2+, Cd2+, Co2+, Ni2+, and Hg2+), obtaining LOD values of 31 nM and 37 nM, with naked eye detection values of 2.9 ppm and 2.1 ppm, for Hg2+ and Cu2+ ions, respectively.
- (5)
- BODIPY (difluoroboron dipyrromethene, or 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) represents a class of fluorophores whose structure consists of boron difluoride and dipyrromethene. They possess high photostability and are stable under physiological conditions; moreover, their relative ease of functionalization allows for the extensive synthesis of derivatives, which have been used since the mid-1990s as fluorescent dyes and sensors [52]. A recent interesting application is reported in [53], which describes a fluorometric chemosensor based on mercaptoethanol and BODIPY constructed to detect dicofol, an organochlorine pesticide that is moderately toxic to humans. The chemosensor displayed turn-off fluorescence behavior under dicofol, with a detection limit of 200 ppb. In another study [54], a water-soluble near-infrared sensor based on aza-BODIPY was developed for the dual determination of Cd2+ in environmental and biological media. The sensor exhibited a color change from colorless to green along with a fluorescence enhancement in the near-infrared (NIR) region via photoinduced electron transfer (PET) after complexation with Cd2+, with an LOD of 2.8 ppb.
2.2. Nanoparticle-Based Sensors
- (1)
- Carbon quantum dots (CQDs) are a fascinating class of carbon-based nanomaterials known for their low toxicity, high biocompatibility, ease of synthesis (even from biomass and waste), and broad possibilities for surface modification or heteroatom doping [57]. These zero-dimensional nanoparticles have garnered significant attention for their potential applications in various fields, including biosensing for the detection of metal ions and (bio)molecules [58], bioimaging, and drug delivery [59]. As shown in Figure 3, belonging to the group of carbon dots, CQDs substantially differ from quantum dots (QDs): the latter have the same structure and atomic composition as bulk materials, while the former are quasi-spherical nanoparticles with both amorphous and crystalline cores, composed of graphitic or turbostratic carbon with an sp2 configuration, and may also consist of graphene or graphene oxide sheets fused or arranged through sp3-hybridized carbon atoms [60]. Due to the abundance of functional groups on their surfaces, CQDs possess high water solubility and provide active sites for functionalization, thus enhancing their versatility and applicability in different fields.The use of CQDs for environmental applications in the sensor and biosensor areas is widely reported in the literature. Most CQD-based sensors focuses on heavy metal detection, and, among them, mercury is the most frequently detected, with an LOD of 0.2 nM, useful for effective water quality control [62]. Meanwhile, most CQD-based biosensors targeting environmental pollutants focus on pesticides, such as diazinon [63], glyphosate [63], fenitrothion [64], malathion [65], and chlorpyrifos [65], with LODs ranging from 0.36 to 8.2 nM.An interesting environmental application is microplastic detection. In [66], N and Cl co-doped carbon quantum dots were synthesized and used to combine fluorescence and Rayleigh scattering, enabling the detection of polystyrene microplastics with three different particle sizes, with an LOD of 0.4 mg/L. Due to their remarkable photostability, CQDs are particularly suitable for real-time monitoring [67].
- (2)
- Polymeric nanoparticles are nanoscale particles composed of polymers, which have gained significant attention in various fields due to their numerous properties: they are biocompatible—and thus suitable for medical and biological applications—and can be designed in various shapes (spheres, rods, or capsules) depending on the intended application. Moreover, they can be engineered to minimize toxicity and immune responses and can be easily modified with various functional groups, targeting ligands, or therapeutic agents, enhancing their specificity and effectiveness. Examples of polymeric nanoparticles are polyethylene glycol (PEG) nanoparticles, polylactide-co-glycolide (PLGA) nanoparticles, or conductive polymer nanocomposites (CPNs), i.e., a combination of conductive polymers with materials like graphene, carbon nanotubes, and metal nanoparticles. Although most of the literature on polymeric nanoparticles centers on their use in drug delivery systems [68,69], imaging/diagnostics [70], gene therapy [71], and cancer treatment [69], compelling studies also demonstrate their effective utilization as chemical sensors [72]. In [73], a highly crosslinked polymer matrix, obtained by the mini-emulsion copolymerization of divinylbenzene (DVB) with an aggregation-induced emission (AIE) monomer composed of (4′-tetraphenyletheyl)pheny-3-butenylether (TPE-PBE), is designed for the detection of explosives based on the quenching of the polymer matrix (both in liquid and film states) observed after contact with the target (in solution or as a vapor) (Figure 4). The sensor showed very good sensitivity for the highly explosive picric acid, with an LOD value of 5.43 μM (or 1.24 ppm).Another notable application lies in the detection of Fe2+ and Fe3+ through a chemosensor based on fluorescent polymer nanoparticles bearing rhodamine B ethylenediamine acrylate, obtained by semicontinuous emulsion polymerization [74]. The color of the aqueous dispersion of polymer nanoparticles changed from violet to red–pink immediately after adding iron ions, with LOD values of 2.63 and 2.5 µM for Fe2+ and Fe3+, respectively.
- (3)
- Organic semiconducting nanoparticles (OSNs) are a class of nanomaterials derived from organic semiconductors, which are composed primarily of carbon and hydrogen atoms. They exhibit unique electronic and optical properties, which make them highly suitable for various applications, including biosensing, imaging, and optoelectronics [75,76]. Examples of OSNs are (i) poly(3-hexylthiophene) (P3HT) nanoparticles, used for applications in organic photovoltaics and biosensors [77,78]; (ii) polydiacetylene (PDA) nanoparticles, known for their colorimetric and fluorescence properties and useful in biosensing and environmental monitoring [79]; (iii) polyfluorene nanoparticles, used in optoelectronic devices, such as organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs) [80]; and (iv) poly(9,9-dioctylfluorene-co-bithiophene) (F8T2) nanoparticles, employed in organic solar cells and biosensors due to their excellent charge transport properties [81].
2.3. Polymer-Based Sensors
2.3.1. Conducting Polymers
- (1)
- Polypyrrole (PPy) is a conductive polymer known for its excellent electrical properties and environmental stability, which make it suitable for various optical sensing applications. PPy is widely used in chemical and biological sensors. Its ability to undergo reversible redox reactions makes it suitable for detecting gases, ions, and biomolecules [84] and metals with a negative impact on the environment [90].
- (2)
- Polyaniline (PANI) is valued for its tunable conductivity and ease of synthesis. It is used in sensors for detecting pH changes, gases, and humidity. Its conductive properties can be adjusted by doping with different acids [91], while, in the form of a nanocomposite based on electrostatic interactions between polyaniline and Ag-ZnO, it has been used for the detection of organophosphorous pesticides [92] (Figure 7 and Figure 8). Polypyrrole (PPy) and polyaniline (PANI) exhibit poor electrochemical stability when used as pure polymers [93]. However, researchers have been working to solve this issue and, for example, hydrogels based on these polymers have shown significantly improved stability. According to the literature, polyaniline- and polypyrrole-based hydrogels enhance performance by up to two orders of magnitude and maintain stability over thousands of working cycles [94].
- (3)
- Polyindol (PIN) is a polymer of an indole monomer that has a fused aromatic molecular structure consisting of a five-membered nitrogen-containing pyrrole ring and a six-membered benzene ring. In comparison to other conducting polymers, such as PANI, PIN shows relatively slow hydrolytic degradation, improved thermal stability, and excellent photoluminescent properties [95]. A noteworthy, albeit earlier, application of PIN is that reported by Faraz et al. [96] for the detection of picric acid. The fluorescence sensor, obtained from the in situ chemical oxidative polymerization of polyindole with CdS nanoparticles, worked based on a static/dynamic quenching mechanism. The comparison of the fluorescence intensities with those of diverse metal ions, such as Li+, Ca2+, Cd2+, Pb2+, Cr2+, Hg2+, Co2+, Ni2+, Cu2+, and Zn2+, confirmed the selectivity of the PIN/CdS nanocomposite for picric acid (Figure 9), showing a Stern–Volmer constant (Ksv) value of 30 × 103 M−1 (Figure 10) [96].
- (4)
- Polythiophene (PTh) is used with its derivatives for their excellent electrical properties and stability. PTh-based sensors are employed in applications such as gas detection and biosensing [97]. One of its derivatives (PEDOT), combined with polystyrene sulfonate, is particularly popular in flexible and wearable sensors [98], while malonic acid derivatives (PTMA) have been used for the detection of alkaloids [99].
- (5)
- Covalent organic polymers (COPs) are a class of materials characterized by highly stable, covalently bonded structures. These polymers are composed of organic building blocks linked together through strong covalent bonds, forming extended networks. Their high porosity and tunable properties make them suitable for a wide range of applications, such as gas storage [100], catalysis [101], and sensing [102], for the detection of heavy metal ions, explosives, and biological molecules. Due to their high thermal stability, easily adjustable functions, amplified responses, and superior sensitivity to analytes (compared with low-molar-mass congeners), COPs are promising candidates for fluorescent sensors. In [103], for instance, a COP containing ynone reaction sites (namely, COP-Ta) was used for detecting hydrazine (Figure 11).
- (6)
- Conjugated polymers with N-heterocyclic moieties are a class of polymers that incorporate N-heterocyclic moieties into their conjugated backbones. This enhances the electronic properties, such as conductivity and fluorescence, making them highly effective in detecting various analytes, such as environmental pollutants [104] and bioactive compounds [105]. This performance is further amplified by the molecular wire effect, a phenomenon first described by Zhou and Swager in the early 1990s [106]. It refers to the efficient transport of electronic excitations, or charge carriers, along the polymer backbone, facilitated by the delocalization of π-electrons across the conjugated system. This extended conjugation creates a quasi-one-dimensional pathway that enables rapid energy migration, allowing localized interactions, such as analyte binding or charge trapping, to influence the entire polymer chain. As a result, materials exhibiting this effect demonstrate enhanced sensitivity and signal amplification, particularly in applications such as fluorescent chemosensing and organic electronics. Examples of conjugated polymers with N-heterocyclic moieties are poly(2,5-bis(3-hexylthiophen-2-yl)thiazolo[5,4-d]thiazole) (PBTTT), whose thiazole units improve charge transport properties (useful in organic field-effect transistors (OFETs) and photovoltaic devices) [107]; poly(3-hexylthiophene) (P3HT), whose pyridine units enhance electron-accepting properties (useful for applications in organic solar cells and sensors) [108]; and poly(2,7-carbazole) derivatives, known for their high thermal stability and excellent optoelectronic properties, which make them useful for light-emitting diodes, photovoltaic devices [109], and optical sensors [110].
2.3.2. Non-Conducting Polymers
- (1)
- Polyvinyl alcohol (PVA) is a water-soluble polymer used in sensors for its excellent film-forming properties and biocompatibility. It is often used in combination with other materials to enhance the sensitivity and selectivity of sensors in detecting humidity, gases, biomolecules [86], and metals [113].
- (2)
- Polystyrene (PS) is a polymer with excellent hydrocarbon resistance, a high degree of dimensional stability, and superior mechanical performance at high temperatures. As nanobeads, microspheres, ultrathin films, or in association with other polymers (such as polyvinyl chloride, PVC), it has been used for the detection of alcohols, Fe3+, and chloroform [84], while, in association with fluorophores (like naphthalimide), it has been used for sensing heavy metals [114].
- (3)
- Poly (methyl methacrylate) (PMMA) is a synthetic polymer prepared from a methyl methacrylate monomer with the use of different polymerization methods. It is widely used for the sensing of pH [115] and gases such as NO2 [116] and, interestingly (as nanofibers), to detect volatile organic compounds (VOCs) [117]. In this study, electrospun nanofibers composed of PMMA doped with small amounts of polyfluorene (PFO) were used to realize a sensor, showing fluorescence quenching in the presence of various VOCs, particularly chloroform, with detection limits as low as 15.4 ppm. The sensing mechanism was attributed to conformational transitions of PFO from the glassy phase to the β-phase, induced by interactions with VOC molecules, resulting in changes in its photophysical behavior (Figure 12 and Figure 13).
- (4)
- Polyvinyl acetate (PVAc) is prepared by the polymerization of a vinyl acetate monomer; its reactivity depends on the triple-bond electronic density and the π-bond energy. It has been successfully employed for ammonia sensing [118] or as a filter (integrated with a complementary metal-oxide semiconductor imaging sensor) for the realization of a portable biochemical sensing device [119]. Moreover, it was recently used as randomly oriented nanofibers to realize a sensor with a surface-enhanced fluorescence factor of 1170 and an LOD as low as 7.24 fM for rhodamine 6G (used as a probe) [120].
2.4. Covalent Organic Framework (COF)-Based Sensors
- (1)
- TpPa-1 is composed of 1,3,5-triformylphloroglucinol (Tp) and p-phenylenediamine (Pa), known for its excellent chemical stability and high fluorescence efficiency and widely used in sensing applications, including the detection of metal ions and explosives [137].
- (2)
- COF-5 is constructed from benzene-1,4-diboronic acid and hexahydroxytriphenylene. It exhibits a three-dimensional structure with high thermal stability and is used in applications such as gas adsorption and catalysis, as well as fluorescent sensing [138].
- (3)
- (4)
- TzDa-1 is derived from 1,3,5-triformylphloroglucinol (Tz) and 2,5-diaminobenzene (Da). It is known for its high fluorescence quantum yield and is used in sensing applications for detecting environmental pollutants [140].
- (5)
2.5. Metal–Organic Framework (MOF)-Based Sensors
- (1)
- UiO-66 is known for its stability and high surface area. UiO-66 is widely used in optical sensing applications, particularly for detecting volatile organic compounds (VOCs), heavy metals, cancer, and SARS-CoV-2 [147].
- (2)
- ZIF-8 is known for its excellent chemical stability and large surface area. It is often used in gas sensing and detecting small organic molecules [148].
- (3)
- MIL-101(Cr) is favored for its large pore size and high surface area, making it suitable for sensing applications involving larger molecules and pollutants [148].
- (4)
- HKUST-1 is commonly used in optical sensing due to its unique luminescent properties and ability to detect various gases and organic compounds [149].
- (5)
- MOF-5 is known for its high porosity and tunable structure. MOF-5 is used in optical sensors for detecting gases and environmental pollutants [149].
2.6. Biomolecule-Based Sensors
- (1)
- Protein-based fluorescent sensors: These use fluorescent proteins such as green fluorescent protein (GFP) and its variants for the detection of ions, proteins, and nucleic acids [151] or mCherry (red fluorescent protein) and yellow fluorescent protein (YFP) to design sensors for live-cell imaging and the real-time monitoring of cellular processes [152]. In Sisila et al. [153], a Cu2+ sensor with high sensitivity and selectivity was developed by incorporating aminotyrosine (as an electron-donating group) into the GFP chromophore. The result was the development of an efficient red-shifted fluorescent protein-based biosensor that could also act as a bio-cleaner for the removal of Cu2+ ions from wastewater samples, with an estimated LOD of 1–5 μM, taking into account the calculated Kd of 5.25 ± 1.59 μM (see Cu2+ titration in Figure 23) [153].
- (2)
- Enzyme-based fluorescent sensors: These use enzymes that produce a fluorescent signal upon interaction with a target biomolecule. Enzymatic fluorescent biosensors are the main tools in biosensor technology, currently used in clinical diagnosis, environmental monitoring, and industrial applications. This group of sensors includes oxidoreductases (historically used to detect glucose in the blood), transferases, hydrolases, lyases, isomerases, and ligases [154], which are associated with quantum dots, fluorescent proteins, and nanomaterial-enhanced fluorophores and allow for enhanced signal stability, multiplexing capabilities, and reduced detection limits. In Cai et al. [155], an innovative dual-mode biosensor (fluorescent and colorimetric) is described for the detection of organophosphorous pesticides (OPs). The sensor is obtained by embedding gold nanoclusters (AuNCs) inside a ZIF-8 MOF, leading to the highly fluorescent composite AuNCs@ZIF-8. In the presence of active acetylcholinesterase (AChE) and choline oxidase (CHO), acetylcholine is enzymatically converted, producing H2O2, which degrades ZIF-8 and produces a fluorescence reduction. In such a system, when OPs are present, they inhibit AChE, so less H2O2 is formed and fluorescence is retained, while the system changes color (from blue to grey), as shown in Figure 24 [155].
- (3)
- Nucleic acid-based fluorescent sensors: These employ fluorescent probes designed to bind specific DNA or RNA sequences, but also distinct molecules, such as proteins; thus, they can be powerful tools to elucidate intracellular processes in genetic analysis and disease diagnostics [156]. This group includes (i) molecular beacons (MBs) (Figure 26A, extracted from [156]), which are pioneering nucleic acid-based sensors, developed as fluorescent “turn-on” detection systems [157]; (ii) aptamers (Figure 26B) [158], which are oligonucleotides binding to specific target compounds or proteins; and (iii) DNAzymes (Figure 26C) [159], which are synthetic single-stranded DNA molecules with catalytic activity, obtained by in vitro selection, having two complementary binding arms—one able to bind to the target sequence of a substrate and the second exhibiting phosphodiester bond cleavage activity [159].
- (4)
- Antibody-based fluorescent sensors, namely fluorescent immunosensors: These are the most commonly and widely used immunosensors for environmental and food monitoring, as well as disease diagnosis, owing to the high sensitivity and specificity of antibodies in the recognition of their substrates [161]. Fluorescence-based immunosensors offer several key advantages, including rapid response times and non-invasiveness due to the use of light-based excitation, the ability to perform multiplexed analyses via distinct fluorescence wavelengths, and superior sensitivity and selectivity relative to colorimetric or absorbance-based methods. They can be divided into two groups: (1) FRET-based immunosensors and (2) single-fluorophore immunosensors. The first group (Figure 27) [161] includes conventional FRET sensors (donor and acceptor fluorophores on antibodies or antigens), time-resolved FRET (TR-FRET, using long-lifetime donors to reduce background noise), open-sandwich FRET (using fluorescein- and rhodamine-labeled heavy-chain (VH) and light-chain (VL) fragments for small molecule detection), and pincer-type FRET (which uses DNA duplexes to stabilize fluorophore-labeled antibody pairs).
- (5)
- Fluorescent sensors based on living (micro)organisms or parts of them: This group of biosensors includes any living (micro)organism, or part of one (single cell, organelle, tissue), with unaltered vitality, naturally able to emit fluorescence or genetically engineered to produce fluorescent signals in response to specific stimuli. There are a number of articles in the literature reporting sensors of this type. Due to their large number, we only report here the latest trends for each mentioned subtype, considering (i) whole-cell biosensors, (ii) organelle-based biosensors, and (iii) tissue-based biosensors.
3. Insights into Inorganic Alternatives
| Inorganic Material | Target | Technique | Type of Sample | Performance | Ref. |
|---|---|---|---|---|---|
| CdSe/ZnS QDs | pH | PL | Aqueous media | 3.2–6 (pH scale) | [217] |
| CdSe QDs | Cu, Co, Mg, Fe, Cr, Zn, Ni ions | PL restoring | Aqueous media | LOD 0.2 µM | [218] |
| CdTe/ZnSe QDs | Pb2+ | PL quenching | Real sample | LOD 31.8 nM 50 nM–10 µM (detection range) | [223] |
| CdSe/CdS QDs | Hg2+ | PL restoring | Real sample | LOD 0.01 nM 0.25–100 nM (detection range) | [224] |
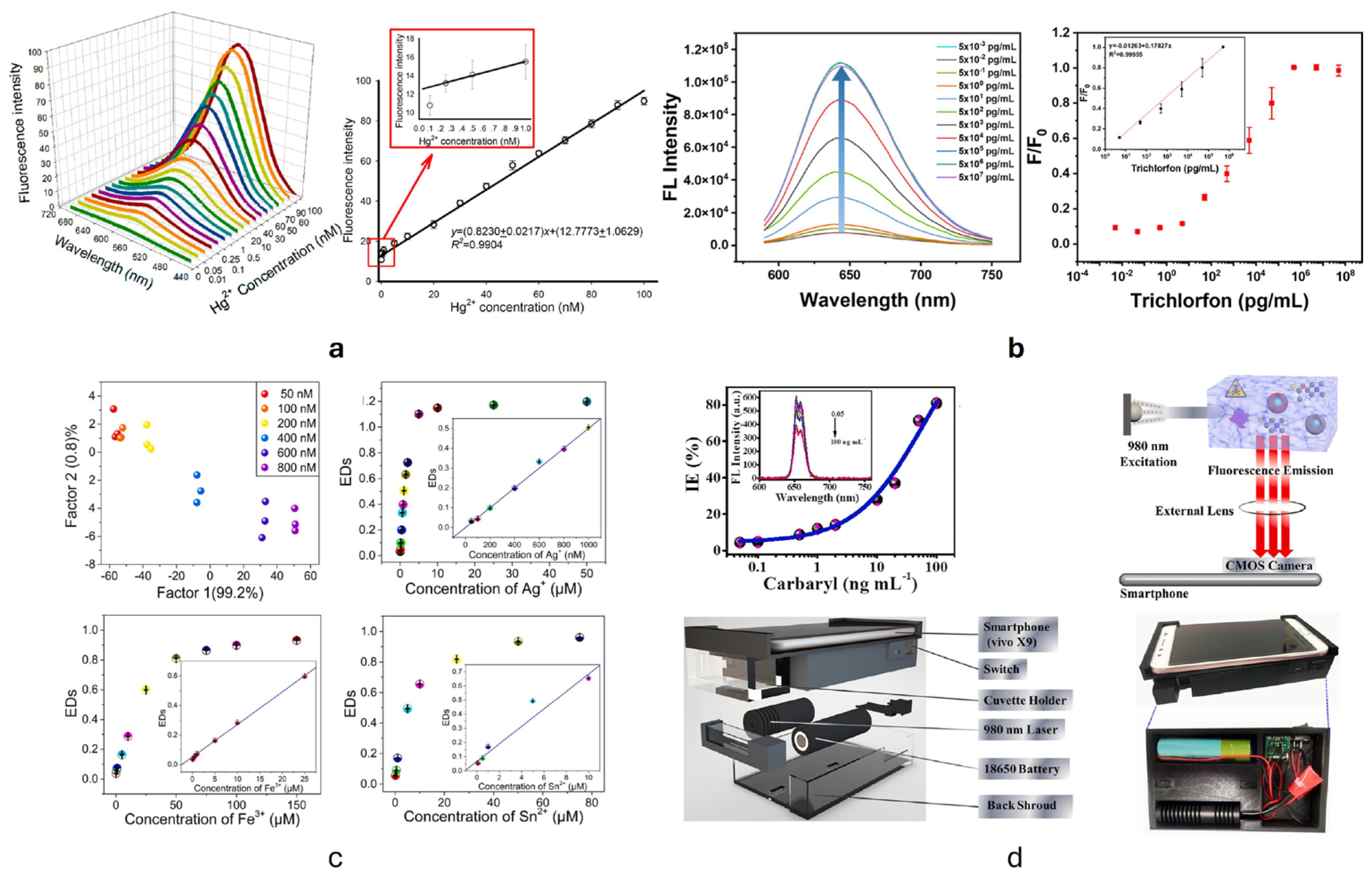
| CdSe/ZnS QDs | Trichlorfon | PL | Real sample | LOD 2.55 pg/mL 5–5 × 105 pg/mL (detection range) | [225] |
| Colorimetric analysis | LOD 1.06 pg/mL 0.05–5 × 1010 pg/mL (detection range) | ||||
| NaErF4-0.5% Tm3+/NaYF4 | Carbaryl | PL upconversion | Extracted sample | LOD 0.05 ng/mL 0.05–100 ng/mL | [235] |
| CuNPs | Hg2+ | PL restoring | Real sample | LOD 0.1 nM 0.5–100 nM (detection range) | [260] |
| Pb2+ | PL quenching | Real sample | LOD 5 nM 5–100 nM (detection range) | [261] | |
| Cu2+ | PL restoring | Real sample | LOD 0.3 ppm 0.95–6.35 ppm (detection range) | [262] | |
| Fe3+ | PL quenching | Real sample | LOD 10 nM 10 nM 10 μM (detection range) | [263] | |
| Cr(VI) | PL quenching | Aqueous media | LOD 65 nM 0.2–60 μM (detection range) | [264] | |
| Si QDs | Mg2+ | PL two-band ratio | Aqueous media | LOD 0.01 nM 0.02–10 nM (detection range) | [256] |
| Si QDs | Co2+ | PL quenching | Real sample | LOD 0.37 µM 1–120 µM (detection range) | [265] |
| S-Si QDs | Fe3+ | PL quenching | Real sample | LOD 0.21 μM 1–20 μM (detection range) | [266] |
| NaYF4 UCNPs * | Temperature | PL upconversion | Aqueous media | 20–45 °C | [267] |
| NaBiF4-Yb, Er NPs | Temperature | PL two-band ratio | Aqueous media | 303–523 K (detection range) | [268] |
4. Industrial Applications of Fluorescence-Based Optical Sensors
5. Advantages, Challenges, and Perspectives of Fluorescence-Based Optical Biosensors
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical Pollution: A Growing Peril and Potential Catastrophic Risk to Humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef] [PubMed]
- Xuan, V.N. Determinants of Environmental Pollution: Evidence from Indonesia. J. Open Innov. Technol. Mark. Complex. 2024, 10, 100386. [Google Scholar] [CrossRef]
- Kumar, P.; Saroj, D.P. Water–Energy–Pollution Nexus for Growing Cities. Urban Clim. 2014, 10, 846–853. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.-C. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 13147. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Eljarrat, E. Priority Lists for Persistent Organic Pollutants and Emerging Contaminants Based on Their Relative Toxic Potency in Environmental Samples. TrAC Trends Anal. Chem. 2003, 22, 655–665. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiang, L.; Zhou, Y.; Luo, Z.; Zhi, D.; Yang, J.; Lam, S.S. Microplastics and Environmental Pollutants: Key Interaction and Toxicology in Aquatic and Soil Environments. J. Hazard. Mater. 2022, 422, 126843. [Google Scholar] [CrossRef]
- Chatterjee, M.; Roy, K. Recent Advances on Modelling the Toxicity of Environmental Pollutants for Risk Assessment: From Single Pollutants to Mixtures. Curr. Pollut. Rep. 2022, 8, 81–97. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical Review on Hazardous Pollutants in Water Environment: Occurrence, Monitoring, Fate, Removal Technologies and Risk Assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef]
- Xu, H.; Jia, Y.; Sun, Z.; Su, J.; Liu, Q.S.; Zhou, Q.; Jiang, G. Environmental Pollution, a Hidden Culprit for Health Issues. Eco-Environ. Health 2022, 1, 31–45. [Google Scholar] [CrossRef]
- Aryal, N.; Wood, J.; Rijal, I.; Deng, D.; Jha, M.K.; Ofori-Boadu, A. Fate of Environmental Pollutants: A Review. Water Environ. Res. 2020, 92, 1587–1594. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Bani Mfarrej, M.F.; Alam, P.; Rinklebe, J.; Ahmad, P. Accumulation of Chromium in Plants and Its Repercussion in Animals and Humans. Environ. Pollut. 2022, 301, 119044. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Lufrano, E.; Curcuruto, G.; Zimbone, M.; Leonardi, A.A.; Sfuncia, G.; Nicotra, G.; Strano, V.; Buccheri, M.A.; Simari, C.; Carroccio, S.C.; et al. Contact Lenses–TiO2 Nanocomposites for a Sustainable Wastewater Remediation. ACS Appl. Nano Mater. 2025, 8, 147–156. [Google Scholar] [CrossRef]
- World Bank Group. A Better Bank for a Better World—Annual Report 2024; World Bank Group: Washington, DC, USA, 2024. [Google Scholar]
- European Central Bank. Economic, Financial and Monetary Developments. Econ. Bull. 2024. [Google Scholar]
- Madhav, S.; Ahamad, A.; Singh, A.K.; Kushawaha, J.; Chauhan, J.S.; Sharma, S.; Singh, P. Water Pollutants: Sources and Impact on the Environment and Human Health. In Sensors in Water Pollutants Monitoring: Role of Material; Springer: Berlin/Heidelberg, Germany, 2020; pp. 43–62. [Google Scholar]
- Weldeslassie, T.; Naz, H.; Singh, B.; Oves, M. Chemical Contaminants for Soil, Air and Aquatic Ecosystem. In Modern Age Environmental Problems and Their Remediation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human Health Effects of Air Pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Cachada, A.; Rocha-Santos, T.; Duarte, A.C. Soil and Pollution. In Soil Pollution; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar]
- Sun, Y.; Zhao, J.; Liang, L. Recent Development of Antibiotic Detection in Food and Environment: The Combination of Sensors and Nanomaterials. Microchim. Acta 2021, 188, 21. [Google Scholar] [CrossRef]
- Morganti, D.; Leonardi, A.A.; Lo Faro, M.J.; Leonardi, G.; Salvato, G.; Fazio, B.; Musumeci, P.; Livreri, P.; Conoci, S.; Neri, G.; et al. Ultrathin Silicon Nanowires for Optical and Electrical Nitrogen Dioxide Detection. Nanomaterials 2021, 11, 1767. [Google Scholar] [CrossRef]
- Walekar, L.; Dutta, T.; Kumar, P.; Ok, Y.S.; Pawar, S.; Deep, A.; Kim, K.-H. Functionalized Fluorescent Nanomaterials for Sensing Pollutants in the Environment: A Critical Review. TrAC Trends Anal. Chem. 2017, 97, 458–467. [Google Scholar] [CrossRef]
- Long, F.; Zhu, A.; Shi, H. Recent Advances in Optical Biosensors for Environmental Monitoring and Early Warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]
- Orellana, G. Fluorescence-Based Sensors. In Optical Chemical Sensors; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2008; pp. 99–116. [Google Scholar]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Liu, C.-W.; Chang, H.-T. Fluorescence Detection of Mercury(II) and Lead(II) Ions Using Aptamer/Reporter Conjugates. Talanta 2011, 84, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, S.-W.; Zhang, B.; Tu, Q.; Wang, J.; Yuan, M.-S. Non-Biological Fluorescent Chemosensors for Pesticides Detection. Talanta 2022, 240, 123200. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zou, L.; Wu, Z.; Sun, M. The Application of Quantum Dots in Aquaculture Pollution Detection. Toxicol. Environ. Chem. 2016, 98, 385–394. [Google Scholar] [CrossRef]
- Morganti, D.; Faro, M.J.L.; Leonardi, A.A.; Fazio, B.; Conoci, S.; Irrera, A. Luminescent Silicon Nanowires as Novel Sensor for Environmental Air Quality Control. Sensors 2022, 22, 8755. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ng, K.K.-H.; Hu, J.J.; Ye, S.; Yang, D. Small-Molecule-Based Fluorescent Sensors for Selective Detection of Reactive Oxygen Species in Biological Systems. Annu. Rev. Biochem. 2019, 88, 605–633. [Google Scholar] [CrossRef]
- Lukyanov, K.A.; Belousov, V.V. Genetically Encoded Fluorescent Redox Sensors. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 745–756. [Google Scholar] [CrossRef]
- Xue, W.; Mutlu, H.; Li, H.; Wenzel, W.; Theato, P. Structural Design of Pyrene-Functionalized TEMPO-Containing Polymers for Enhanced Electrochemical Storage Performance. Polym. Chem. 2021, 12, 2643–2650. [Google Scholar] [CrossRef]
- Paul, S.; Barman, P.; Dey, N.; Watkinson, M. Recent Developments in Pyrene-Based Fluorescence Recognition and Imaging of Ag+ and Pb2+ Ions: Synthesis, Applications and Challenges. Sens. Diagn. 2024, 3, 946–967. [Google Scholar] [CrossRef]
- Romano, G.M.; Di Menna, P.; Bencini, A.; Simonini Steiner, Y.T.; Innocenti, M.; Di Natale, C.; Paolesse, R.; Lvova, L. A Bis-Pyrene Polyamine Receptor for Fast Optical Detection of Ketoprofen: Synthesis, Characterization and Application in All-Solid-State Fluorescent Sensors. Analyst 2025, 150, 806–818. [Google Scholar] [CrossRef]
- Sengul, O.; Völkle, J.; Valli, A.; Stadler, R. Enhancing the Sensitivity and Selectivity of Pyrene-Based Sensors for Detection of Small Gaseous Molecules via Destructive Quantum Interference. Phys. Rev. B 2022, 105, 165428. [Google Scholar] [CrossRef]
- Yin, J.; Huang, L.; Wu, L.; Li, J.; James, T.D.; Lin, W. Small Molecule Based Fluorescent Chemosensors for Imaging the Microenvironment within Specific Cellular Regions. Chem. Soc. Rev. 2021, 50, 12098–12150. [Google Scholar] [CrossRef]
- Chen, L.; Hwang, E.; Zhang, J. Fluorescent Nanobiosensors for Sensing Glucose. Sensors 2018, 18, 1440. [Google Scholar] [CrossRef] [PubMed]
- Diwan, U.; Kumar, A.; Kumar, V.; Upadhyay, K.K.; Roychowdhury, P.K. A Water Compatible Turn ‘on’ Optical Probe for Cu2+ Based on a Fluorescein–Sugar Conjugate. Sens. Actuators B Chem. 2014, 196, 345–351. [Google Scholar] [CrossRef]
- Guang, S.; Tian, J.; Wei, G.; Yan, Z.; Pan, H.; Feng, J.; Xu, H. A Modified Fluorescein Derivative with Improved Water-Solubility for Turn-on Fluorescent Determination of Hg2+ in Aqueous and Living Cells. Talanta 2017, 170, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Xu, M.; Jiang, H.; Wang, W.; Wang, Q. A Simple Fluorescein Derived Colorimetric and Fluorescent ‘off–on’ Sensor for the Detection of Hypochlorite. Anal. Methods 2018, 10, 4562–4569. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, T.; Li, Y.; Li, T. A Novel Rhodamine B Derivative as a “Turn-on” Fluorescent Sensor for Cu2+ with High Selectivity and Sensitivity. J. Fluoresc. 2024, 35, 5807–5816. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Ma, W.; Lu, R.; Zhou, W.; Gao, H. Recent Developments in Rhodamine-Based Chemosensors: A Review of the Years 2018–2022. Chemosensors 2022, 10, 399. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Metal-Free Synthetic Organic Dyes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Levitus, M.; Ranjit, S. Cyanine Dyes in Biophysical Research: The Photophysics of Polymethine Fluorescent Dyes in Biomolecular Environments. Q. Rev. Biophys. 2011, 44, 123–151. [Google Scholar] [CrossRef]
- Wessendorf, M.W.; Brelje, T.C. Which Fluorophore Is Brightest? A Comparison of the Staining Obtained Using Fluorescein, Tetramethylrhodamine, Lissamine Rhodamine, Texas Red, and Cyanine 3.18. Histochemistry 1992, 98, 81–85. [Google Scholar] [CrossRef]
- Pronkin, P.; Tatikolov, A. Fluorescent Probes for Biomacromolecules Based on Monomethine Cyanine Dyes. Chemosensors 2023, 11, 280. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, H.; Huang, W.; Liu, S.; Zhang, H.; Zhang, X.; Peng, X. Design Strategies and Applications of Cyanine Dyes in Phototherapy. Chem. Soc. Rev. 2025, 54, 341–366. [Google Scholar] [CrossRef]
- Kaliramna, S.; Dhayal, S.S.; Chaudhary, R.; Khaturia, S.; Ameta, K.L.; Kumar, N. A Review And Comparative Analysis Of Different Types Of Dyes For Applications In Dye-Sensitized Solar Cells. Braz. J. Phys. 2022, 52, 136. [Google Scholar] [CrossRef]
- Galhano, J.; Marcelo, G.A.; Santos, H.M.; Capelo-Martínez, J.L.; Lodeiro, C.; Oliveira, E. Development of Cyanine 813@Imidazole-Based Doped Supported Devices for Divalent Metal Ions Detection. Chemosensors 2022, 10, 80. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators Based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Huangfu, C.; Zhu, M.; Li, C.; Feng, L. The BODIPY-Based Chemosensor for the Fluorometric Determination of Organochlorine Pesticide Dicofol. Food Chem. 2022, 370, 131033. [Google Scholar] [CrossRef]
- Piyanuch, P.; Patawanich, P.; Sirirak, J.; Suwatpipat, K.; Kamkaew, A.; Burgess, K.; Wanichacheva, N. Rapid and Visual Detection of Cd2+ Based on Aza-BODIPY near Infrared Dye and Its Application in Real and Biological Samples for Environmental Contamination Screening. J. Hazard. Mater. 2021, 409, 124487. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Foss, F.W.; Molina-Canteras, J.; Velazco-Cabral, I.; Marauri, A.; Larumbe, A.; Aparicio, B.; Vázquez, J.L.; Alberro, N.; Arrastia, I.; et al. Supramolecular Chemistry in Solution and Solid–Gas Interfaces: Synthesis and Photophysical Properties of Monocolor and Bicolor Fluorescent Sensors for Barium Tagging in Neutrinoless Double Beta Decay. RSC Appl. Interfaces 2025, 2, 185–199. [Google Scholar] [CrossRef]
- Roja, S.S.; Sneha Sunil, P.; Darussalam, M.M.; Manoharan, V.; Prakash, J.; Ranjith Kumar, R. Benzo[4,5]Imidazole[2,1-b]Quinazoline-1(2H)-One: An Efficient Fluorescent Probe for the Selective and Sensitive Detection of Cu(II) Ions. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2025, 333, 125853. [Google Scholar] [CrossRef]
- Gaviria-Arroyave, M.I.; Cano, J.B.; Peñuela, G.A. Nanomaterial-Based Fluorescent Biosensors for Monitoring Environmental Pollutants: A Critical Review. Talanta Open 2020, 2, 100006. [Google Scholar] [CrossRef]
- Šafranko, S.; Goman, D.; Stanković, A.; Medvidović-Kosanović, M.; Moslavac, T.; Jerković, I.; Jokić, S. An Overview of the Recent Developments in Carbon Quantum Dots—Promising Nanomaterials for Metal Ion Detection and (Bio) Molecule Sensing. Chemosensors 2021, 9, 138. [Google Scholar] [CrossRef]
- El-Shabasy, R.M.; Farouk Elsadek, M.; Mohamed Ahmed, B.; Fawzy Farahat, M.; Mosleh, K.N.; Taher, M.M. Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications. Processes 2021, 9, 388. [Google Scholar] [CrossRef]
- Huang, X.; Bao, R.; Yi, J. Improving Effect of Carbonized Quantum Dots (CQDs) in Pure Copper Matrix Composites. J. Cent. South Univ. 2021, 28, 1255–1265. [Google Scholar] [CrossRef]
- Rosales, S.; Medina, O.E.; Garzon, N.; Zapata, K.; Taborda, E.A.; Ordóñez, J.C.; Cortés, F.B.; Franco, C.A. Systematic Review of Carbon Quantum Dots (CQD): Definition, Synthesis, Applications and Perspectives. Renew. Sustain. Energy Rev. 2025, 219, 115854. [Google Scholar] [CrossRef]
- Gan, Z.; Hu, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J.; Zhang, W.; Li, Y.; Xu, Y. A Dual-Emission Fluorescence Sensor for Ultrasensitive Sensing Mercury in Milk Based on Carbon Quantum Dots Modified with Europium (III) Complexes. Sens. Actuators B Chem. 2021, 328, 128997. [Google Scholar] [CrossRef]
- Ashrafi Tafreshi, F.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive Fluorescent Detection of Pesticides in Real Sample by Using Green Carbon Dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-K.; Feng, J.-T.; Ma, Z.-Q. Nitrogen, Sulfur, Boron and Flavonoid Moiety Co-Incorporated Carbon Dots for Sensitive Fluorescence Detection of Pesticides. Carbon 2020, 161, 685–693. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Fatemi, F.; Rahmandoust, M.; Mirzajani, F.; Ranaei Siadat, S.O. Development of a Carbon Quantum Dot-Based Sensor for the Detection of Acetylcholinesterase and Organophosphate Pesticides. Heliyon 2023, 9, e19551. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Z.; Wang, C.; Li, Y. Preparation of N, Cl Co-Doped Lignin Carbon Quantum Dots and Detection of Microplastics in Water. Crystals 2023, 13, 983. [Google Scholar] [CrossRef]
- Praveena, U.; Raja, V.; Ragavan, K.V.; Anandharamakrishnan, C. Optical Detection Probes and Sensors for Micro-/Nano-Plastics. Rev. Environ. Sci. Bio/Technol. 2024, 23, 569–599. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Geszke-Moritz, M.; Moritz, M. Biodegradable Polymeric Nanoparticle-Based Drug Delivery Systems: Comprehensive Overview, Perspectives and Challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef] [PubMed]
- Criado-Gonzalez, M.; Marzuoli, C.; Bondi, L.; Gutierrez-Fernandez, E.; Tullii, G.; Lagonegro, P.; Sanz, O.; Cramer, T.; Antognazza, M.R.; Mecerreyes, D. Porous Semiconducting Polymer Nanoparticles as Intracellular Biophotonic Mediators to Modulate the Reactive Oxygen Species Balance. Nano Lett. 2024, 24, 7244–7251. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Zhong, H.; Zhao, B.; Deng, J. Synthesis and Application of Fluorescent Polymer Micro- and Nanoparticles. Small 2023, 19, 2300961. [Google Scholar] [CrossRef]
- Liang, X.; Wen, L.; Mi, Y.; Guo, J.; Yu, B.; Tao, M.; Cao, Z.; Zhao, Z. Highly Cross-Linked Polymeric Nanoparticles with Aggregation-Induced Emission for Sensitive and Recyclable Explosive Detection. Dye. Pigment. 2021, 191, 109369. [Google Scholar] [CrossRef]
- Ghezelsefloo, S.; Keyvan Rad, J.; Hajiali, M.; Mahdavian, A.R. Rhodamine-Based Fluorescent Polyacrylic Nanoparticles: A Highly Selective and Sensitive Chemosensor for Fe (II) and Fe (III) Cations in Water. J. Environ. Chem. Eng. 2021, 9, 105082. [Google Scholar] [CrossRef]
- Wang, Z.; Han, D.; Wang, H.; Zheng, M.; Xu, Y.; Zhang, H. Organic Semiconducting Nanoparticles for Biosensor: A Review. Biosensors 2023, 13, 494. [Google Scholar] [CrossRef]
- Hu, X.; Sun, F.; Zhu, C.; Yang, Z.; Huang, W. Repurposing Organic Semiconducting Nanomaterials to Accelerate Clinical Translation of NIR-II Fluorescence Imaging. Nano Res. 2023, 16, 5140–5154. [Google Scholar] [CrossRef]
- Zhu, M.; He, B.; Zhang, K.; Hussain, S.; Li, T. Recent Progress of Poly(3-Hexylthiophene)-Based Materials for Thermoelectric Applications. Mater. Chem. Front. 2024, 8, 2454–2492. [Google Scholar] [CrossRef]
- Eduardo, R. Investigation of P3HT Processing for In-Liquid EGOFET Biosensors: Impact of Solvent Choice and Surface Characteristics. J. Biosens. Bioelectron. 2024, 15, 1–2. [Google Scholar]
- Jang, H.; Jeon, J.; Shin, M.; Kang, G.; Ryu, H.; Kim, S.M.; Jeon, T.-J. Polydiacetylene (PDA) Embedded Polymer-Based Network Structure for Biosensor Applications. Gels 2025, 11, 66. [Google Scholar] [CrossRef]
- Bauri, J.; Choudhary, R.B.; Mandal, G. Recent Advances in Efficient Emissive Materials-Based OLED Applications: A Review. J. Mater. Sci. 2021, 56, 18837–18866. [Google Scholar] [CrossRef]
- Zhang, P.; Yi, W.; Xu, H.; Gao, C.; Hou, J.; Jin, W.; Lei, Y.; Hou, X. Supramolecular Interactions of Poly[(9,9-Dioctylfluorenyl-2,7-Diyl)-Co- Thiophene] with Single-Walled Carbon Nanotubes. Nanotechnol. Rev. 2018, 7, 487–495. [Google Scholar] [CrossRef]
- Thakuri, A.; Acharya, R.; Banerjee, M.; Chatterjee, A. Alendronate-Modified Polydiacetylene (PDA) Dual-Mode Sensor for the Selective Detection of Lead(II) Ions up to the NM Level in Solutions and Agarose Films. ACS Appl. Polym. Mater. 2024, 6, 1023–1032. [Google Scholar] [CrossRef]
- Suklabaidya, S.; Chakraborty, S.; Sarkar, S.; Paul, R.; Banik, H.; Chakraborty, A.; Bhattacharjee, D.; Majumdar, S.; Hussain, S.A. Polydiacetylene-N-1-Hexadecyl Imidazole Mixed Film and Its Application toward the Sensing of Volatile Organic Compounds, Gasoline, and Pollution Level in Car Exhaust. J. Phys. Chem. C 2021, 125, 15976–15986. [Google Scholar] [CrossRef]
- Shinde, S.J.; Waikar, M.R.; Sonker, R.K.; Sonkawade, R.G. Optical Sensors Based on Polymeric Materials. In Advanced Functional Materials for Optical and Hazardous Sensing; Springer: Berlin/Heidelberg, Germany, 2023; pp. 221–251. [Google Scholar]
- Alam, M.W.; Islam Bhat, S.; Al Qahtani, H.S.; Aamir, M.; Amin, M.N.; Farhan, M.; Aldabal, S.; Khan, M.S.; Jeelani, I.; Nawaz, A.; et al. Recent Progress, Challenges, and Trends in Polymer-Based Sensors: A Review. Polymers 2022, 14, 2164. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Losi, V.; Magnaghi, L.R.; Biesuz, R. Current Trends in Polymer Based Sensors. Chemosensors 2021, 9, 108. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and Sensing Applications of Polyaniline Nanocomposites: A Review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Yan, L.; Shi, H.; Sui, X.; Deng, Z.; Gao, L. MoS2-DNA and MoS2 Based Sensors. RSC Adv. 2017, 7, 23573–23582. [Google Scholar] [CrossRef]
- Do, J.S.; Wang, S.H. On the Sensitivity of Conductimetric Acetone Gas Sensor Based on Polypyrrole and Polyaniline Conducting Polymers. Sens. Actuators B Chem. 2013, 185, 39–46. [Google Scholar] [CrossRef]
- NIANG, M.; Lamine SALL, M.; FALL, B.; BA, S.; LO, M.; Alli, Y.A.; Diagne DIAW, A.K.; GNINGUE-SALL, D. Development of a Novel Fluorimetric Sensor Based on Polypyrrole Doped with 4-(Methylsulfonyl)-2-Nitrobenzoic Acid (MSNBA) for Sensitive Detection of Copper (Cu2+). Dye. Pigment. 2024, 225, 112028. [Google Scholar] [CrossRef]
- Bhadra, J.; Popelka, A.; Abdulkareem, A.; Ahmad, Z.; Touati, F.; Al-Thani, N. Fabrication of Polyaniline–Graphene/Polystyrene Nanocomposites for Flexible Gas Sensors. RSC Adv. 2019, 9, 12496–12506. [Google Scholar] [CrossRef]
- Berhanu, S.; Habtamu, F.; Tadesse, Y.; Gonfa, F.; Tadesse, T. Fluorescence Sensor Based on Polyaniline Supported Ag-ZnO Nanocomposite for Malathion Detection. J. Sens. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Wang, J.; Zhu, J.; Bai, Y.; Xiong, L. Study on Capacitance Evolving Mechanism of Polypyrrole during Prolonged Cycling. J. Phys. Chem. B 2014, 118, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, M.A.; Vorobiov, V.K.; Kasatkin, I.A.; Vlasova, E.N.; Sokolova, M.P.; Bobrova, N.V. Long-Term Electrochemical Stability of Polyaniline- and Polypyrrole-Based Hydrogels. Chem. Pap. 2021, 75, 5103–5112. [Google Scholar] [CrossRef]
- Thadathil, A.; Pradeep, H.; Joshy, D.; Ismail, Y.A.; Periyat, P. Polyindole and Polypyrrole as a Sustainable Platform for Environmental Remediation and Sensor Applications. Mater. Adv. 2022, 3, 2990–3022. [Google Scholar] [CrossRef]
- Faraz, M.; Shakir, M.; Khare, N. Highly Sensitive and Selective Detection of Picric Acid Using a One Pot Biomolecule Inspired Polyindole/CdS Nanocomposite. New J. Chem. 2017, 41, 5784–5793. [Google Scholar] [CrossRef]
- Belhousse, S.; Tıghılt, F.-Z.; Bennıa, S.; Adjtoutah, S.; Sam, S.; Lasmı, K.; Hamdanı, K. Effect of Polythiophene Thickness on Hybrid Sensor Sensitivity. Rev. Adv. Mater. Sci. 2021, 60, 839–845. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, H.; Fu, Q. Recent Progress on PEDOT:PSS Based Polymer Blends and Composites for Flexible Electronics and Thermoelectric Devices. Mater. Chem. Front. 2020, 4, 3130–3152. [Google Scholar] [CrossRef]
- Gao, X.; Guo, M.; Liu, M.; Zhang, L.; Yao, Z. A Fluorometric and Colorimetric Approach for the Rapid Detection of Berberine Hydrochloride Based on an Anionic Polythiophene Derivative. Luminescence 2021, 36, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Jabin, S.; Abbas, S.; Gupta, P.; Jadoun, S.; Rajput, A.; Rajput, P. Recent Advances in Nanoporous Organic Polymers (NPOPs) for Hydrogen Storage Applications. Nanoscale 2025, 17, 4226–4249. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Du, Y.; Yang, Y.; Liu, K.; Zhao, J.; Xia, X.; Wang, L. Quenching to Optimize the Crystalline/Amorphous Ratio of CoPS Nanorods for Hydrazine-Assisted Total Water Decomposition at Ampere-Level Current Density. Chin. J. Catal. 2024, 62, 265–276. [Google Scholar] [CrossRef]
- Xue, M.; Ye, G.; Zhang, L.; Dong, Q.; Lee, C.-S.; Li, Z.; Zhang, Q. Preparation, Single-Crystal Structure and Room-Temperature Phosphorescence of a Covalent Organic Polymer Containing Te–O–P Bonds. Chem. Sci. 2025, 16, 5260–5265. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Jiang, S.; Ma, W.; Xu, B.; Liu, L.; Tian, W. A Covalent Organic Polymer for Turn-on Fluorescence Sensing of Hydrazine. J. Mater. Chem. C Mater. 2022, 10, 2807–2813. [Google Scholar] [CrossRef]
- Malik, A.H.; Habib, F.; Qazi, M.J.; Ganayee, M.A.; Ahmad, Z.; Yatoo, M.A. Correction to: A Short Review Article on Conjugated Polymers. J. Polym. Res. 2023, 30, 185. [Google Scholar] [CrossRef]
- Qadir, T.; Amin, A.; Sharma, P.K.; Jeelani, I.; Abe, H. A Review on Medicinally Important Heterocyclic Compounds. Open Med. Chem. J. 2022, 16. [Google Scholar] [CrossRef]
- Zhou, Q.; Swager, T.M. Fluorescent Chemosensors Based on Energy Migration in Conjugated Polymers: The Molecular Wire Approach to Increased Sensitivity. J. Am. Chem. Soc. 1995, 117, 12593–12602. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, N.; Bai, W.; Bao, Y. Fluorescent Chemosensors Based on Conjugated Polymers with N-Heterocyclic Moieties: Two Decades of Progress. Polym. Chem. 2020, 11, 3095–3114. [Google Scholar] [CrossRef]
- Mahesh, K.; Karpagam, S.; Pandian, K. How to Design Donor–Acceptor Based Heterocyclic Conjugated Polymers for Applications from Organic Electronics to Sensors. Top. Curr. Chem. 2019, 377, 12. [Google Scholar] [CrossRef]
- Nayana, V.; Kandasubramanian, B. Polycarbazole and Its Derivatives: Progress, Synthesis, and Applications. J. Polym. Res. 2020, 27, 285. [Google Scholar] [CrossRef]
- Guo, X.; Gao, B.; Cui, X.; Wang, J.; Dong, W.; Duan, Q.; Fei, T.; Su, Z. PL Sensor for Sensitive and Selective Detection of 2,4,6-Trinitrophenol Based on Carbazole and Tetraphenylsilane Polymer. Dye. Pigment. 2021, 191, 109379. [Google Scholar] [CrossRef]
- Hong, X.; Liu, Y.; Li, Y.; Wang, X.; Fu, J.; Wang, X. Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries. Polymers 2020, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J. Interaction of Conducting Polymers, Polyaniline and Polypyrrole, with Organic Dyes: Polymer Morphology Control, Dye Adsorption and Photocatalytic Decomposition. In Chemical Papers; Springer: Berlin/Heidelberg, Germany, 2020; Volume 74, pp. 1–54. [Google Scholar] [CrossRef]
- Yue, Y.; Gu, J.; Han, J.; Wu, Q.; Jiang, J. Effects of Cellulose/Salicylaldehyde Thiosemicarbazone Complexes on PVA Based Hydrogels: Portable, Reusable, and High-Precision Luminescence Sensing of Cu2+. J. Hazard. Mater. 2021, 401, 123798. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Feng, T.; Li, Y. Synthesis, Structure–Fluorescence Relationships and Density Functional Theory Studies of Novel Naphthalimide–Piperazine–Pyridine-Based Polystyrene Sensors for Hg(ii) Detection. RSC Adv. 2020, 10, 25281–25289. [Google Scholar] [CrossRef]
- Galhano, J.; Marcelo, G.A.; Kurutos, A.; Bértolo, E.; Capelo-Martinez, J.L.; Lodeiro, C.; Oliveira, E. Development of Low-Cost Colourimetric and PH Sensors Based on PMMA@Cyanine Polymers. Dye. Pigment. 2022, 200, 110154. [Google Scholar] [CrossRef]
- Peterson, S.R.; She, X.; Goodrich, M.J.; Gaikwad, A.; Cathcart, W.B.; Ainsworth, K.; Kolluru, P.V. A Fluorescence Microscopy Based Gas Sensing Approach to Detect NO2 with Perylene Doped PMMA Thin Films and Nanofibers. Sens. Actuators B Chem. 2024, 399, 134855. [Google Scholar] [CrossRef]
- Terra, I.A.A.; Sanfelice, R.C.; Valente, G.T.; Correa, D.S. Optical Sensor Based on Fluorescent PMMA/PFO Electrospun Nanofibers for Monitoring Volatile Organic Compounds. J. Appl. Polym. Sci. 2018, 135, 46128. [Google Scholar] [CrossRef]
- Khamidy, N.I.; Aflaha, R.; Nurfani, E.; Djamal, M.; Triyana, K.; Wasisto, H.S.; Rianjanu, A. Influence of Dopant Concentration on the Ammonia Sensing Performance of Citric Acid-Doped Polyvinyl Acetate Nanofibers. Anal. Methods 2022, 14, 4956–4966. [Google Scholar] [CrossRef]
- Yadav, N.; Kumari, P.; Yadav, S.; Paulraj, S.; Chaurasia, P.; Anwar, A.; Mahto, S.K. Optimizing Fabrication Method and Surface Modification of Polyvinyl Acetate-Benzophenone Emission Filters for Complementary Metal-Oxide-Semiconductor Imager Chips towards Biosensing Applications. J. Anal. Chem. 2024, 79, 1790–1799. [Google Scholar] [CrossRef]
- Sun, J.; Huang, T.; Wang, Z. Multiple Scattering-Enhanced Fluorescence Within Randomly Oriented Low-Index Polymer Nanofiber Sensors. Biosensors 2025, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G.; Sarhan, A. Über Die Anwendung von Enzymanalog Gebauten Polymeren Zur Racemattrennung. Angew. Chem. 1972, 84, 364. [Google Scholar] [CrossRef]
- Anderson, J.; Nelson, J.; Reynolds, C.; Ringelberg, D.; Tepper, G.; Pestov, D. Steady-State and Frequency-Domain Lifetime Measurements of an Activated Molecular Imprinted Polymer Imprinted to Dipicolinic Acid. J. Fluoresc. 2004, 14, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Cywinski, P. Fluorescent Molecularly Imprinted Polymers in Sensing of CAMP and CGMP. J. Phys. Chem. Biophys. 2013, 3, 1000111. [Google Scholar] [CrossRef]
- Chen, Y.C.; Wang, Z.; Yan, M.; Prahl, S.A. Fluorescence Anisotropy Studies of Molecularly Imprinted Polymers. Luminescence 2006, 21, 7–14. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Huang, R.F.; Ma, X.G.; Guo, L.H.; Wang, Y.; Fan, Y.M. Selective Fluorescence Sensor Based on Ion-Imprinted Polymer-Modified Quantum Dots for Trace Detection of Cr(VI) in Aqueous Solution. Anal. Bioanal. Chem. 2019, 411, 7165–7175. [Google Scholar] [CrossRef]
- Guo, L.; Yang, L.; Li, M.; Kuang, L.; Song, Y.; Wang, L. Covalent Organic Frameworks for Fluorescent Sensing: Recent Developments and Future Challenges. Coord. Chem. Rev. 2021, 440, 213957. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Zhen, D.; Liu, C.; Deng, Q.; Zhang, S.; Yuan, N.; Li, L.; Liu, Y. A Review of Covalent Organic Frameworks for Metal Ion Fluorescence Sensing. Chin. Chem. Lett. 2024, 35, 109249. [Google Scholar] [CrossRef]
- Nesterov, E.E.; Zhu, Z.; Swager, T.M. Conjugation Enhancement of Intramolecular Exciton Migration in Poly(p-Phenylene Ethynylene)s. J. Am. Chem. Soc. 2005, 127, 10083–10088. [Google Scholar] [CrossRef]
- Zhang, Y.; Sajjad, M.T.; Blaszczyk, O.; Parnell, A.J.; Ruseckas, A.; Serrano, L.A.; Cooke, G.; Samuel, I.D.W. Large Crystalline Domains and an Enhanced Exciton Diffusion Length Enable Efficient Organic Solar Cells. Chem. Mater. 2019, 31, 6548–6557. [Google Scholar] [CrossRef]
- Wu, C.; McNeill, J. Swelling-Controlled Polymer Phase and Fluorescence Properties of Polyfluorene Nanoparticles. Langmuir 2008, 24, 5855–5861. [Google Scholar] [CrossRef]
- Ge, S.; Wei, K.; Peng, W.; Huang, R.; Akinlabi, E.; Xia, H.; Shahzad, M.W.; Zhang, X.; Bin Xu, B.; Jiang, J. A Comprehensive Review of Covalent Organic Frameworks (COFs) and Their Derivatives in Environmental Pollution Control. Chem. Soc. Rev. 2024, 53, 11259–11302. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Zou, Y.; Wang, L.; Li, Z.; Wang, M.; Li, L.; Tian, M.; Wang, D.; Gao, D. Fluorescent Covalent Organic Frameworks for Environmental Pollutant Detection Sensors and Enrichment Sorbents: A Mini-Review. Anal. Methods 2023, 15, 5919–5946. [Google Scholar] [CrossRef]
- Xiu, J.; Zhang, N.; Li, C.; Salah, A.; Wang, G. Tetraphenylethylene-Based Covalent Organic Frameworks as Fluorescent Chemosensor for Rapid Sensitive Recognition and Selective “Turn-on” Fluorescence Detection of Trace-Level Al3+ Ion. Microporous Mesoporous Mater. 2021, 316, 110979. [Google Scholar] [CrossRef]
- Zhang, N.; Wei, B.; Ma, T.; Tian, Y.; Wang, G. A Carbazole-Grafted Covalent Organic Framework as Turn-on Fluorescence Chemosensor for Recognition and Detection of Pb2+ Ions with High Selectivity and Sensitivity. J. Mater. Sci. 2021, 56, 11789–11800. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G. A Hydroxyl-Rich Covalent Organic Framework for the Precisely Selective Fluorescence Sensing of Explosives with High Sensitivity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 318, 124483. [Google Scholar] [CrossRef] [PubMed]
- Shubham Kumar, R.J. Fluorescent Sensors Based on Covalent-Organic Frameworks. In Fluorescent Chemosensing and Bioimaging; Routledge: London, UK, 2024. [Google Scholar]
- Qian, Y.; Li, J.; Ji, M.; Li, J.; Ma, D.; Liu, A.; Zhao, Y.; Yang, C. Fluorescent Covalent Organic Frameworks: A Promising Material Platform for Explosive Sensing. Front. Chem. 2022, 10, 984313. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.F.; Serra, M.E.S.; Murtinho, D.; Valente, A.J.M.; Naushad, M. Covalent Organic Frameworks: Synthesis, Properties and Applications—An Overview. Polymers 2021, 13, 970. [Google Scholar] [CrossRef] [PubMed]
- Skorjanc, T.; Shetty, D.; Valant, M. Covalent Organic Polymers and Frameworks for Fluorescence-Based Sensors. ACS Sens. 2021, 6, 1461–1481. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mondal, P.; Chattopadhyay, P. Covalent Organic Framework as Fluorescent Turn-on/off Sensor and an Account of Operating Sensing Mechanism. Inorganica Chim. Acta 2023, 546, 121318. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.; Peng, J.; Du, Q.; He, H. Ratiometric Fluorescence Sensing of Metal-Organic Frameworks: Tactics and Perspectives. Coord. Chem. Rev. 2020, 404, 213113. [Google Scholar] [CrossRef]
- Wang, J.-X.; Yin, J.; Shekhah, O.; Bakr, O.M.; Eddaoudi, M.; Mohammed, O.F. Energy Transfer in Metal–Organic Frameworks for Fluorescence Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9970–9986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, L.; Ma, S.; Hu, T. Porous MB@Cd-MOF Obtained by Post-Modification: Self-Calibrated Fluorescent Turn-on Sensor for Highly Sensitive Detection of Carbaryl. Cryst. Growth Des. 2022, 22, 2662–2669. [Google Scholar] [CrossRef]
- Gong, X.; Tian, Y.; Tong, Y.; Yuan, Y.; Peng, S.; Wang, D.; Tao, H.; Tan, Q.; Gong, Z. Rapid Detection of Organophosphorus Pesticide Monocrotophos by Pore Size-Regulated Amplified Fluorescence “Turn-on” Response of Zr-MOF. Anal. Chim. Acta 2025, 1352, 343915. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Yao, Z.; Zhang, K.; Li, Q.; Fu, Z.; Zheng, R.; Li, W.; Xu, J.; Bu, X. Real-Time In Situ Volatile Organic Compound Sensing by a Dual-Emissive Polynuclear Ln-MOF with Pronounced LnIII Luminescence Response. Angew. Chem. Int. Ed. 2023, 62, e202217456. [Google Scholar] [CrossRef]
- Das, A.; Bej, S.; Pandit, N.R.; Banerjee, P.; Biswas, B. Recent Advancements of Metal–Organic Frameworks in Sensing Platforms: Relevance in the Welfare of the Environment and the Medical Sciences with Regard to Cancer and SARS-CoV-2. J. Mater. Chem. A. 2023, 11, 6090–6128. [Google Scholar] [CrossRef]
- Johari, R.; Kumar, P.; Samariya, U.; Budhiraja, N.; Siddhartha; Agrahari, K.; Pathak, C.S.; Singh, P.K.; Khan, Z.H.; Bhatia, M.; et al. Optical Sensors Based on Metal–Organic Frameworks. In Advanced Functional Materials for Optical and Hazardous Sensing; Springer: Berlin/Heidelberg, Germany, 2023; pp. 175–198. [Google Scholar]
- Shen, Y.; Tissot, A.; Serre, C. Recent Progress on MOF-Based Optical Sensors for VOC Sensing. Chem. Sci. 2022, 13, 13978–14007. [Google Scholar] [CrossRef]
- Funke, L.M.; Lund, A.; Zhuang, H.; Reimer, J.A. Metal–Organic Frameworks: Challenges Addressed via Magnetic Resonance Spectroscopy. Appl. Magn. Reson. 2023, 54, 1193–1220. [Google Scholar] [CrossRef]
- Tian, F.; Xu, G.; Zhou, S.; Chen, S.; He, D. Principles and Applications of Green Fluorescent Protein-Based Biosensors: A Mini-Review. Analyst 2023, 148, 2882–2891. [Google Scholar] [CrossRef]
- Dhakal, S.; Macreadie, I. The Use of Yeast in Biosensing. Microorganisms 2022, 10, 1772. [Google Scholar] [CrossRef]
- Sisila, V.; Janeena, A.; Prem, S.; Mohandass, P.; Narayanan, J.; Easwaramoorthi, S.; Ganesh, S.; Kamini, N.R.; Ayyadurai, N. Protein-based Metal Bio-cleaner for Detoxification of Wastewater. J. Chem. Technol. Biotechnol. 2022, 97, 2581–2591. [Google Scholar] [CrossRef]
- Kłos-Witkowska, A. Enzyme-Based Fluorescent Biosensors and Their Environmental, Clinical and Industrial Applications. Pol. J. Environ. Stud. 2015, 24, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhu, H.; Zhou, W.; Qiu, Z.; Chen, C.; Qileng, A.; Li, K.; Liu, Y. Capsulation of AuNCs with AIE Effect into Metal–Organic Framework for the Marriage of a Fluorescence and Colorimetric Biosensor to Detect Organophosphorus Pesticides. Anal. Chem. 2021, 93, 7275–7282. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Higashi, S.L.; Ikeda, M. Nucleic Acid-Based Fluorescent Sensor Systems: A Review. Polym. J. 2022, 54, 751–766. [Google Scholar] [CrossRef]
- Tyagi, S.; Kramer, F.R. Molecular Beacons: Probes That Fluoresce upon Hybridization. Nat. Biotechnol. 1996, 14, 303–308. [Google Scholar] [CrossRef]
- Yoo, H.; Jo, H.; Oh, S.S. Detection and beyond: Challenges and Advances in Aptamer-Based Biosensors. Mater. Adv. 2020, 1, 2663–2687. [Google Scholar] [CrossRef]
- Cepeda-Plaza, M.; Peracchi, A. Insights into DNA Catalysis from Structural and Functional Studies of the 8-17 DNA zyme. Org. Biomol. Chem. 2020, 18, 1697–1709. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Z.; Ma, T.; Bai, J. Aptamer Paper-Based Fluorescent Sensor for Determination of SARS-CoV-2 Spike Protein. Sensors 2025, 25, 1637. [Google Scholar] [CrossRef]
- Rani, A.Q.; Zhu, B.; Ueda, H.; Kitaguchi, T. Recent Progress in Homogeneous Immunosensors Based on Fluorescence or Bioluminescence Using Antibody Engineering. Analyst 2023, 148, 1422–1429. [Google Scholar] [CrossRef]
- Pan, Y.; Wei, X. A Novel FRET Immunosensor for Rapid and Sensitive Detection of Dicofol Based on Bimetallic Nanoclusters. Anal. Chim. Acta 2022, 1224, 340235. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, J.; Tao, Z.; Eremin, S.A.; Hua, X.; Wang, M. Development of Fluorescence Polarization Immunoassay for Imidacloprid in Environmental and Agricultural Samples. Front. Chem. 2020, 8, 615594. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.Y.; Rutter, J.W.; Barnes, C.P.; Dekker, L. Fundamental Building Blocks of Whole-Cell Biosensor Design. In Handbook of Cell Biosensors; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–23. [Google Scholar]
- William, F.S.; Budinger, F.E.; Mueller, P.K. Evaluation of Biological Effects of Air Pollutants by Use of Luminescent Bacteria. J. Bacteriol. 1965, 90, 832–833. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Thotapalli, S.; Sonali; Singh, J. Importance and Recent Aspects of Fungal-Based Biosensors. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 301–309. [Google Scholar]
- Buonasera, K.; Pezzotti, G.; Scognamiglio, V.; Tibuzzi, A.; Giardi, M.T. New Platform of Biosensors for Prescreening of Pesticide Residues To Support Laboratory Analyses †. J. Agric. Food Chem. 2010, 58, 5982–5990. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Bhunia, A.K. Mammalian Cell-Based Biosensors for Pathogens and Toxins. Trends Biotechnol. 2009, 27, 179–188. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, M.; Yang, Q.; Wei, H.; Xia, A.; Wang, L.; Ben, Y.; Zhou, Q.; Yang, Z.; Huang, X. A Living Plant Cell-Based Biosensor for Real-Time Monitoring Invisible Damage of Plant Cells under Heavy Metal Stress. Sci. Total. Environ. 2019, 697, 134097. [Google Scholar] [CrossRef]
- Aliakbar Ahovan, Z.; Hashemi, A.; De Plano, L.M.; Gholipourmalekabadi, M.; Seifalian, A. Bacteriophage Based Biosensors: Trends, Outcomes and Challenges. Nanomaterials 2020, 10, 501. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, V. Recent Advances in Synthetic Biology–Enabled and Natural Whole-Cell Optical Biosensing of Heavy Metals. Anal. Bioanal. Chem. 2021, 413, 73–82. [Google Scholar] [CrossRef]
- Yadav, S.; Saini, A.; Devi, R.; Lata, S. Transducers in Biosensors. In Biomaterials-Based Sensors; Springer Nature Singapore: Singapore, 2023; pp. 101–125. [Google Scholar]
- Buonasera, K.; Lambreva, M.; Rea, G.; Touloupakis, E.; Giardi, M.T. Technological Applications of Chlorophyll a Fluorescence for the Assessment of Environmental Pollutants. Anal. Bioanal. Chem. 2011, 401, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Merz, D.; Geyer, M.; Moss, D.A.; Ache, H.-J. Chlorophyll Fluorescence Biosensor for the Detection of Herbicides. Anal. Bioanal. Chem. 1996, 354, 299–305. [Google Scholar] [CrossRef]
- Huang, R.; Xu, Y.; Wan, P.; Zhu, T.; Heng, W.; Miao, W. Thylakoid-Based Green Preparation of Porous Microneedles for Antibiotic Residues Detection in Food Samples. Anal. Chim. Acta 2024, 1328, 343181. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Battaglino, B.; Sacco, A.; Saracco, G.; Pagliano, C. A Green and Easy-to-Assemble Electrochemical Biosensor Based on Thylakoid Membranes for Photosynthetic Herbicides Detection. Biosens. Bioelectron. 2022, 198, 113838. [Google Scholar] [CrossRef]
- Acha, V.; Andrews, T.; Huang, Q.; Sardar, D.K.; Hornsby, P.J. Tissue-Based Biosensors. In Recognition Receptors in Biosensors; Springer New York: New York, NY, USA, 2010; pp. 365–381. [Google Scholar]
- Bouchaala, R.; Mercier, L.; Andreiuk, B.; Mély, Y.; Vandamme, T.; Anton, N.; Goetz, J.G.; Klymchenko, A.S. Integrity of Lipid Nanocarriers in Bloodstream and Tumor Quantified by Near-Infrared Ratiometric FRET Imaging in Living Mice. J. Control. Release 2016, 236, 57–67. [Google Scholar] [CrossRef]
- Cui, W.-R.; Jiang, W.; Zhang, C.-R.; Liang, R.-P.; Liu, J.; Qiu, J.-D. Regenerable Carbohydrazide-Linked Fluorescent Covalent Organic Frameworks for Ultrasensitive Detection and Removal of Mercury. ACS Sustain. Chem. Eng. 2020, 8, 445–451. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Zheng, Q.-Q.; Xiao, S.-J.; Zhang, L.; Liang, R.-P.; Ouyang, G.; Qiu, J.-D. Construction of Two-Dimensional Fluorescent Covalent Organic Framework Nanosheets for the Detection and Removal of Nitrophenols. Anal. Chem. 2022, 94, 2517–2526. [Google Scholar] [CrossRef]
- Cui, R.; Li, R.; Li, Z.; Wei, M.; Wang, X.; Li, X. A Tb-MOF Anion, Porous Coordination Framework Constructed with Oxalate Ligand: Crystal Structure, Adsorption Properties, and Luminescence Sensing. Dye. Pigment. 2021, 195, 109669. [Google Scholar] [CrossRef]
- Ma, L.-N.; Zhang, B.; Wang, Z.-H.; Hou, L.; Zhu, Z.; Wang, Y.-Y. Efficient Gas and VOC Separation and Pesticide Detection in a Highly Stable Interpenetrated Indium–Organic Framework. Inorg. Chem. 2021, 60, 10698–10706. [Google Scholar] [CrossRef]
- Bai, W.; Qin, G.; Wang, J.; Li, L.; Ni, Y. 2-Aminoterephthalic Acid Co-Coordinated Co-MOF Fluorescent Probe for Highly Selective Detection of the Organophosphorus Pesticides with p-Nitrophenyl Group in Water Systems. Dye. Pigment. 2021, 193, 109473. [Google Scholar] [CrossRef]
- Wiwasuku, T.; Boonmak, J.; Burakham, R.; Hadsadee, S.; Jungsuttiwong, S.; Bureekaew, S.; Promarak, V.; Youngme, S. Turn-on Fluorescent Probe towards Glyphosate and Cr3+ Based on Cd(ii)-Metal Organic Framework with Lewis Basic Sites. Inorg. Chem. Front 2021, 8, 977–988. [Google Scholar] [CrossRef]
- Wang, L.; He, K.; Quan, H.; Wang, X.; Wang, Q.; Xu, X. A Luminescent Method for Detection of Parathion Based on Zinc Incorporated Metal-Organic Framework. Microchem. J. 2020, 153, 104441. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W. A Novel 2D Eu-MOF as a Dual-Functional Fluorescence Sensor for Detection of Benzaldehyde and Fe3+. Dalton Trans. 2024, 53, 11850–11857. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Zhao, Y.; Fan, Y.; Li, Y.; Gao, H.; Li, H.; Gao, D.; Ning, Z. A Ratiometric Fluorescent Probe Dye-Functionalized MOFs Integrated with Logic Gate Operation for Efficient Detection of Acetaldehyde. Molecules 2024, 29, 2970. [Google Scholar] [CrossRef]
- Mengwen, L.; Ao, S.; Yueqi, L.; Hao, Z.; Xiaohui, H.; Xueliang, L.; Xinchao, S.; Yunxu, Y. The Selective and Sensitive Detection of Formaldehyde by ZIF-90-LW via Aza-Cope Rearrangement. Anal. Methods 2020, 12, 3748–3755. [Google Scholar] [CrossRef]
- Moscoso, F.G.; Almeida, J.; Sousaraei, A.; Lopes-Costa, T.; Silva, A.M.G.; Cabanillas-Gonzalez, J.; Cunha-Silva, L.; Pedrosa, J.M. A Lanthanide MOF Immobilized in PMMA Transparent Films as a Selective Fluorescence Sensor for Nitroaromatic Explosive Vapours. J. Mater. Chem. C 2020, 8, 3626–3630. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Dong, J.; Xu, F.; Li, S. Amino-Functionalized Cu Metal–Organic Framework Nanosheets as Fluorescent Probes for Detecting TNP. Anal. Methods 2021, 13, 5328–5334. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhao, J. Loading Fluorescent Dyes into MOF Pores for the Optical Sensing and Adsorption of Heavy Metal Ions: Synthesis, Characterization, and Performance. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 340, 126323. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Wang, J.; Zhang, L.; Wang, S.; Yang, C.; Xie, L.; Liu, Z.; Ni, Y.; Xie, X.; Sun, J.; et al. Missing-Linker Engineering of Eu (III)-Doped UiO-MOF for Enhanced Detection of Heavy Metal Ions. Chem. Eng. J. 2022, 431, 134050. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M.; Ahmad, M. Amine- and Imine-Functionalized Mn-Based MOF as an Unusual Turn-On and Turn-Off Sensor for d10 Heavy Metal Ions and an Efficient Adsorbent to Capture Iodine. Cryst. Growth Des. 2022, 22, 3277–3294. [Google Scholar] [CrossRef]
- Wang, Q.; Ke, W.; Lou, H.; Han, Y.; Wan, J. A Novel Fluorescent Metal-Organic Framework Based on Porphyrin and AIE for Ultra-High Sensitivity and Selectivity Detection of Pb2+ Ions in Aqueous Solution. Dye. Pigment. 2021, 196, 109802. [Google Scholar] [CrossRef]
- Agrawal, P.; Nair, M.S. Explicit Fluorescence Sensing Method for Sensitive Detection of Pb2+ Ions Based on DNA Aptamer Folding as a Molecular Probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 333, 125882. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, H.; Lin, G.; Ye, S.; Yang, B.; Xu, W. DNA Nanosphere-Enhanced DNAzyme System for Sensitive and Rapid Detection of Lead Contamination in Water and Living Cells. J. Hazard. Mater. 2025, 488, 137437. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Ding, W.; Bao, X.; Wang, S.; Lin, Q.; Xu, Y.; Lu, J.; Lyu, M.; Wang, S. An Efficient DNAzyme for the Fluorescence Detection of Vibrio Cholerae. Food Sci. Nutr. 2023, 11, 3235–3245. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, R.; Chen, B.; Yu, X.; Wang, X.; Zuo, X.; Liang, B.; Yang, J. A Transcription Factor-Based Bacterial Biosensor System and Its Application for on-Site Detection of Explosives. Biosens. Bioelectron. 2024, 244, 115805. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Du, R.; Liu, Y.; Chi, J.; He, X.; Huang, K.; Luo, Y.; Xu, W. A Test Strip Platform Based on a Whole-Cell Microbial Biosensor for Simultaneous on-Site Detection of Total Inorganic Mercury Pollutants in Cosmetics without the Need for Predigestion. Biosens. Bioelectron. 2020, 150, 111899. [Google Scholar] [CrossRef]
- Bae, J.; Lim, J.-W.; Kim, T. Reusable and Storable Whole-Cell Microbial Biosensors with a Microchemostat Platform for in Situ on-Demand Heavy Metal Detection. Sens. Actuators B Chem. 2018, 264, 372–381. [Google Scholar] [CrossRef]
- Chamas, A.; Pham, H.T.M.; Jähne, M.; Hettwer, K.; Uhlig, S.; Simon, K.; Einspanier, A.; Baronian, K.; Kunze, G. Simultaneous Detection of Three Sex Steroid Hormone Classes Using a Novel Yeast-based Biosensor. Biotechnol. Bioeng. 2017, 114, 1539–1549. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum Dots: Synthesis, Bioapplications, and Toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef]
- Baba, K.; Nishida, K. Single-Molecule Tracking in Living Cells Using Single Quantum Dot Applications. Theranostics 2012, 2, 655–667. [Google Scholar] [CrossRef]
- Cassette, E.; Helle, M.; Bezdetnaya, L.; Marchal, F.; Dubertret, B.; Pons, T. Design of New Quantum Dot Materials for Deep Tissue Infrared Imaging. Adv. Drug Deliv. Rev. 2013, 65, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Chandan, H.R.; Schiffman, J.D.; Balakrishna, R.G. Quantum Dots as Fluorescent Probes: Synthesis, Surface Chemistry, Energy Transfer Mechanisms, and Applications. Sens. Actuators B Chem. 2018, 258, 1191–1214. [Google Scholar] [CrossRef]
- Han, A.; Hao, S.; Yang, Y.; Li, X.; Luo, X.; Fang, G.; Liu, J.; Wang, S. Perspective on Recent Developments of Nanomaterial Based Fluorescent Sensors: Applications in Safety and Quality Control of Food and Beverages. J. Food Drug. Anal. 2020, 28, 487–508. [Google Scholar] [CrossRef] [PubMed]
- Vo-Dinh, T.; Wang, H.N.; Scaffidi, J. Plasmonic Nanoprobes for SERS Biosensing and Bioimaging. J. Biophotonics 2010, 3, 89–102. [Google Scholar] [CrossRef]
- He, H.; Sun, D.-W.; Wu, Z.; Pu, H.; Wei, Q. On-off-on Fluorescent Nanosensing: Materials, Detection Strategies and Recent Food Applications. Trends Food Sci. Technol. 2022, 119, 243–256. [Google Scholar] [CrossRef]
- Reiss, P.; Protière, M.; Li, L. Core/Shell Semiconductor Nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Loukanov, A.R.; Dushkin, C.D.; Papazova, K.I.; Kirov, A.V.; Abrashev, M.V.; Adachi, E. Photoluminescence Depending on the ZnS Shell Thickness of CdS/ZnS Core-Shell Semiconductor Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2004, 245, 9–14. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Sun, Y.; Vernier, P.T.; Liang, C.-H.; Chong, S.Y.C.; Gundersen, M.A. PH-Sensitive Photoluminescence of CdSe/ZnSe/ZnS Quantum Dots in Human Ovarian Cancer Cells. J. Phys. Chem. C 2007, 111, 2872–2878. [Google Scholar] [CrossRef]
- Ast, S.; Rutledge, P.J.; Todd, M.H. PH-Responsive Quantum Dots (RQDs) That Combine a Fluorescent Nanoparticle with a PH-Sensitive Dye. Phys. Chem. Chem. Phys. 2014, 16, 25255–25257. [Google Scholar] [CrossRef]
- Ji, X.; Palui, G.; Avellini, T.; Bin Na, H.; Yi, C.; Knappenberger, K.L.; Mattoussi, H. On the PH-Dependent Quenching of Quantum Dot Photoluminescence by Redox Active Dopamine. J. Am. Chem. Soc. 2012, 134, 6006–6017. [Google Scholar] [CrossRef]
- Jin, T.; Sasaki, A.; Kinjo, M.; Miyazaki, J. A Quantum Dot-Based Ratiometric PH Sensor. Chem. Commun. 2010, 46, 2408. [Google Scholar] [CrossRef]
- Tomasulo, M.; Yildiz, I.; Raymo, F.M. PH-Sensitive Quantum Dots. J. Phys. Chem. B 2006, 110, 3853–3855. [Google Scholar] [CrossRef]
- Li, D.; Xu, H.; Li, D.; Wang, Y. P -Aminothiophenol-Coated CdSe/ZnS Quantum Dots as a Turn-on Fluorescent Probe for PH Detection in Aqueous Media. Talanta 2017, 166, 54–62. [Google Scholar] [CrossRef]
- Marukhyan, S.S.; Hovhannisyan, V.A.; Gasparyan, V.K. Urease Combined with CdSe Quantum Dots as an Enzyme Sensor for Quantitative Detection of Some Metals in Water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 326, 125186. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, S.; Rinnert, H.; Balan, L.; Jasniewski, J.; Medjahdi, G.; Ben Chaabane, R.; Schneider, R. Aqueous Synthesis of Core/Shell/Shell ZnSeS/Cu:ZnS/ZnS Quantum Dots and Their Use as a Probe for the Selective Photoluminescent Detection of Pb2+ in Water. J. Photochem. Photobiol. A Chem. 2022, 431, 114050. [Google Scholar] [CrossRef]
- Zhou, J.; Li, B.; Qi, A.; Shi, Y.; Qi, J.; Xu, H.; Chen, L. ZnSe Quantum Dot Based Ion Imprinting Technology for Fluorescence Detecting Cadmium and Lead Ions on a Three-Dimensional Rotary Paper-Based Microfluidic Chip. Sens. Actuators B Chem. 2020, 305, 127462. [Google Scholar] [CrossRef]
- Xie, L.; Yang, Y.; Gong, G.; Feng, S.; Liu, D. One-Step Hydrothermal Synthesis of Highly Fluorescent MoS2 Quantum Dots for Lead Ion Detection in Aqueous Solutions. Nanomaterials 2022, 12, 3329. [Google Scholar] [CrossRef]
- Sharma, P.; Mehata, M.S. Rapid Sensing of Lead Metal Ions in an Aqueous Medium by MoS2 Quantum Dots Fluorescence Turn-Off. Mater. Res. Bull. 2020, 131, 110978. [Google Scholar] [CrossRef]
- Farahmandzadeh, F.; Kermanshahian, K.; Molahosseini, E.; Molaei, M.; Karimipour, M. Highly Fluorescent CdTe/ZnSe Quantum Dot-Based “Turn-off” Sensor for the on-Site Rapid Detection of Lead Ions in Aqueous Medium. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 324, 124914. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.-Y.; Zhu, Y.; Tian, L.-J.; Wang, W.-K.; Zhu, T.-T.; Li, W.-W.; Yu, H.-Q. Fluorescence Sensor Based on Biosynthetic CdSe/CdS Quantum Dots and Liposome Carrier Signal Amplification for Mercury Detection. Anal. Chem. 2020, 92, 3990–3997. [Google Scholar] [CrossRef]
- Kang, X.; Yuan, J.; Gu, X.; Li, X.; Wen, T.; He, M.; He, Y.; Yang, X.; Li, Y.; Guo, C.; et al. DNA-Templated Ag Nanocluster/CdSe@ZnS Quantum Dot-Based Platform for Colorimetric and Fluorescence Detection of Organophosphorus Pesticide. ACS Appl. Nano Mater. 2025, 8, 1663–1672. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, T.; Tao, T. ZnO Quantum Dots for Fluorescent Detection of Environmental Contaminants. J. Environ. Chem. Eng. 2021, 9, 106800. [Google Scholar] [CrossRef]
- Mello, G.P.C.; Simões, E.F.C.; Crista, D.M.A.; Leitão, J.M.M.; Pinto da Silva, L.; Esteves da Silva, J.C.G. Glucose Sensing by Fluorescent Nanomaterials. Crit. Rev. Anal. Chem. 2019, 49, 542–552. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, W.; Zhang, X.; Mei, L.; Liu, J.; Wang, F. Machine Learning-Driven Fluorescent Sensor Array Using Aqueous CsPbBr3 Perovskite Quantum Dots for Rapid Detection and Sterilization of Foodborne Pathogens. J. Hazard. Mater. 2025, 483, 136655. [Google Scholar] [CrossRef]
- Kang, X.; Zhu, M. Tailoring the Photoluminescence of Atomically Precise Nanoclusters. Chem. Soc. Rev. 2019, 48, 2422–2457. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Yao, Q.; Luo, Z.; Li, J.; Chen, T.; Xie, J. Luminescent Metal Nanoclusters with Aggregation-Induced Emission. J. Phys. Chem. Lett. 2016, 7, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Hu, G.; Fung, V.; Jiang, D. Insights into Interfaces, Stability, Electronic Properties, and Catalytic Activities of Atomically Precise Metal Nanoclusters from First Principles. Acc. Chem. Res. 2018, 51, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Li, Q.; Zhou, M.; Zhao, S.; Li, Y.; Li, S.; Jin, R. Toward the Tailoring Chemistry of Metal Nanoclusters for Enhancing Functionalities. Acc. Chem. Res. 2018, 51, 2764–2773. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, H.; Zhou, H.; Zhao, Y.; Wu, Y.; Zhang, J.; Zhang, S. A Novel Fluorescent Sensor Array to Identify Baijiu Based on the Single Gold Nanocluster Probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 284, 121787. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Huang, K.-Y.; He, S.-B.; Peng, H.-P.; Xia, X.-H.; Chen, W.; Deng, H.-H. Single Gold Nanocluster Probe-Based Fluorescent Sensor Array for Heavy Metal Ion Discrimination. J. Hazard. Mater. 2021, 405, 124259. [Google Scholar] [CrossRef]
- Su, D.; Zhao, X.; Yan, X.; Han, X.; Zhu, Z.; Wang, C.; Jia, X.; Liu, F.; Sun, P.; Liu, X.; et al. Background-Free Sensing Platform for on-Site Detection of Carbamate Pesticide through Upconversion Nanoparticles-Based Hydrogel Suit. Biosens. Bioelectron. 2021, 194, 113598. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, F.; Lei, X.; Mang, L.; Cheng, S.; Song, J. Fluorescent Copper Nanoparticles: Recent Advances in Synthesis and Applications for Sensing Metal Ions. Nanoscale 2016, 8, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Gorris, H.H. Perspectives and Challenges of Photon-Upconversion Nanoparticles—Part I: Routes to Brighter Particles and Quantitative Spectroscopic Studies. Anal. Bioanal. Chem. 2017, 409, 5855–5874. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, A.J.; Pope, S.J.A. Using Lanthanide Ions in Molecular Bioimaging. Chem. Soc. Rev. 2015, 44, 4723–4742. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Li, H. Lanthanide Organic/Inorganic Hybrid Systems: Efficient Sensors for Fluorescence Detection. Dye. Pigment. 2020, 178, 108386. [Google Scholar] [CrossRef]
- Wang, M.; Abbineni, G.; Clevenger, A.; Mao, C.; Xu, S. Upconversion Nanoparticles: Synthesis, Surface Modification and Biological Applications. Nanomedicine 2011, 7, 710–729. [Google Scholar] [CrossRef]
- Ng, S.M.; Koneswaran, M.; Narayanaswamy, R. A Review on Fluorescent Inorganic Nanoparticles for Optical Sensing Applications. RSC Adv. 2016, 6, 21624–21661. [Google Scholar] [CrossRef]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical Sensor Arrays for Chemical Sensing: The Optoelectronic Nose. Chem. Soc. Rev. 2013, 42, 8649. [Google Scholar] [CrossRef]
- Vennerberg, D.; Lin, Z. Upconversion Nanocrystals: Synthesis, Properties, Assembly and Applications. Sci. Adv. Mater. 2011, 3, 26–40. [Google Scholar] [CrossRef]
- Chien, Y.; Chan, K.K.; Yap, S.H.K.; Yong, K. NIR-responsive Nanomaterials and Their Applications; Upconversion Nanoparticles and Carbon Dots: A Perspective. J. Chem. Technol. Biotechnol. 2018, 93, 1519–1528. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An Overview of Nanoparticles Commonly Used in Fluorescent Bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Sciuto, E.L.; Faro, M.J.L.; Fazio, B.; Rizzo, M.G.; Calabrese, G.; Francioso, L.; Picca, R.; Nastasi, F.; Mancuso, G.; et al. SARS-CoV-2 and Omicron Variant Detection with a High Selectivity, Sensitivity, and Low-Cost Silicon Bio-Nanosensor. Nano Sel. 2023, 4, 160–169. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Battaglia, R.; Morganti, D.; Faro, M.J.L.; Fazio, B.; De Pascali, C.; Francioso, L.; Palazzo, G.; Mallardi, A.; Purrello, M.; et al. A Novel Silicon Platform for Selective Isolation, Quantification, and Molecular Analysis of Small Extracellular Vesicles. Int. J. Nanomed. 2021, 16, 5153–5165. [Google Scholar] [CrossRef] [PubMed]
- Giurlani, W.; Dell’Aquila, V.; Vizza, M.; Calisi, N.; Lavacchi, A.; Irrera, A.; Lo Faro, M.J.; Leonardi, A.A.; Morganti, D.; Innocenti, M. Electrodeposition of Nanoparticles and Continuous Film of CdSe on N-Si (100). Nanomaterials 2019, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.A.; Lo Faro, M.J.; Fazio, B.; Spinella, C.; Conoci, S.; Livreri, P.; Irrera, A. Fluorescent Biosensors Based on Silicon Nanowires. Nanomaterials 2021, 11, 2970. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, G.; Galletta, M.; Ielo, I.; Irrera, A.; Lo Faro, M.J.; Leonardi, A.A.; Nastasi, F.; Puntoriero, F. Hybrid Light Harvesting Antenna Based on Si NWs and RuOs2 Dendrons for Near IR Light-Emission. ACS Omega 2025, 10, 39606–39614. [Google Scholar] [CrossRef]
- Fujii, M.; Yamaguchi, Y.; Takase, Y.; Ninomiya, K.; Hayashi, S. Control of Photoluminescence Properties of Si Nanocrystals by Simultaneously Doping N- and p-Type Impurities. Appl. Phys. Lett. 2004, 85, 1158–1160. [Google Scholar] [CrossRef]
- Wu, X.L.; Xiong, S.J.; Siu, G.G.; Huang, G.S.; Mei, Y.F.; Zhang, Z.Y.; Deng, S.S.; Tan, C. Optical Emission from Excess Si Defect Centers in Si Nanostructures. Phys. Rev. Lett. 2003, 91, 157402. [Google Scholar] [CrossRef]
- Djurabekova, F.; Nordlund, K. Atomistic Simulation of the Interface Structure of Si Nanocrystals Embedded in Amorphous Silica. Phys. Rev. B 2008, 77, 115325. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, G.; Fermi, A.; Mazzaro, R.; Morandi, V.; Ceroni, P.; Smilgies, D.-M.; Korgel, B.A. Size-Dependent Photoluminescence Efficiency of Silicon Nanocrystal Quantum Dots. J. Phys. Chem. C 2017, 121, 23240–23248. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Dong, D.; You, T. Microwave-Assisted Synthesis of Fluorescent Silicon Quantum Dots for Ratiometric Sensing of Hg (II) Based on the Regulation of Energy Transfer. Talanta 2021, 226, 122093. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Acosta, T.B.; Dang, H.; Liu, S.; Yao, C.; Torrisi, F. Sprayed Graphene-Based Coating Enabling Antifog and Smart Features. ACS Appl. Mater. Interfaces 2025, 17, 37012–37024. [Google Scholar] [CrossRef]
- Das, P.; Marvi, P.K.; Ganguly, S.; Tang, X.; Wang, B.; Srinivasan, S.; Rajabzadeh, A.R.; Rosenkranz, A. MXene-Based Elastomer Mimetic Stretchable Sensors: Design, Properties, and Applications. Nano-Micro Lett. 2024, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Perala, R.S.; Chandrasekar, N.; Balaji, R.; Alexander, P.S.; Humaidi, N.Z.N.; Hwang, M.T. A Comprehensive Review on Graphene-Based Materials: From Synthesis to Contemporary Sensor Applications. Mater. Sci. Eng. R Rep. 2024, 159, 100805. [Google Scholar] [CrossRef]
- Wang, H.-B.; Chen, Y.; Li, Y.; Zhang, H.-D.; Cao, J.-T. A Rapid, Sensitive and Label-Free Sensor for Hg(ii) Ion Detection Based on Blocking of Cysteine-Quenching of Fluorescent Poly(Thymine)-Templated Copper Nanoparticles. RSC Adv. 2015, 5, 94099–94104. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Fang, Z.; Zeng, L. Random DsDNA-Templated Formation of Copper Nanoparticles as Novel Fluorescence Probes for Label-Free Lead Ions Detection. Chem. Commun. 2012, 48, 1057–1059. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Yang, S.I. A Selective Fluorescence Turn-on Sensing System for Evaluation of Cu 2+ Polluted Water Based on Ultra-Fast Formation of Fluorescent Copper Nanoclusters. Anal. Methods 2015, 7, 2278–2282. [Google Scholar] [CrossRef]
- Cao, H.; Chen, Z.; Zheng, H.; Huang, Y. Copper Nanoclusters as a Highly Sensitive and Selective Fluorescence Sensor for Ferric Ions in Serum and Living Cells by Imaging. Biosens. Bioelectron. 2014, 62, 189–195. [Google Scholar] [CrossRef]
- Cui, M.; Song, G.; Wang, C.; Song, Q. Synthesis of Cysteine-Functionalized Water-Soluble Luminescent Copper Nanoclusters and Their Application to the Determination of Chromium(VI). Microchim. Acta 2015, 182, 1371–1377. [Google Scholar] [CrossRef]
- Sullam, E.M.; Adam, K.M.; Liu, J.; Chen, H.; Xiao, J. Silicon Quantum Dots-Based Fluorescent Sensor for the Detection of Cobalt with High Sensitivity and Selectivity. Chin. Chem. Lett. 2024, 35, 108476. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Zhao, S.; Pang, H.; Han, Y.; Luo, X.; Tang, W.; Li, Z. S Doped Silicon Quantum Dots with High Quantum Yield as a Fluorescent Sensor for Determination of Fe3+ in Water. Opt. Mater. 2020, 110, 110461. [Google Scholar] [CrossRef]
- Sedlmeier, A.; Achatz, D.E.; Fischer, L.H.; Gorris, H.H.; Wolfbeis, O.S. Photon Upconverting Nanoparticles for Luminescent Sensing of Temperature. Nanoscale 2012, 4, 7090. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, M.A.; Zelewski, S.J.; Oliva, R.; Żak, A.; Kudrawiec, R.; Nyk, M. Combined Temperature and Pressure Sensing Using Luminescent NaBiF4:Yb,Er Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 4209–4217. [Google Scholar] [CrossRef]
- Joe, H.-E.; Yun, H.; Jo, S.-H.; Jun, M.B.G.; Min, B.-K. A Review on Optical Fiber Sensors for Environmental Monitoring. Int. J. Precis. Eng. Manuf.-Green Technol. 2018, 5, 173–191. [Google Scholar] [CrossRef]
- A Beginner’s Guide to Fiber Optic Sensing & Industrial Applications|Hifi. Available online: https://hifieng.com/blog/fiber-optic-sensing-a-beginners-guide/ (accessed on 2 July 2025).
- Principle and Analysis Method of Fluorescence Immunoassay (FIA)|AxisPharm. Available online: https://axispharm.com/principle-and-analysis-method-of-fluorescence-immunoassay-fia (accessed on 2 July 2025).
- Sistani, S.; Shekarchizadeh, H. Applications of Quantum Dots in the Food Industry. In Quantum Dots—Recent Advances, New Perspectives and Contemporary Applications; IntechOpen: Hamilton, NJ, USA, 2023. [Google Scholar]
- Deka, M.J.; Chowdhury, D.; Nath, B.K. Recent Development of Modified Fluorescent Carbon Quantum Dots-Based Fluorescence Sensors for Food Quality Assessment. Carbon Lett. 2022, 32, 1131–1149. [Google Scholar] [CrossRef]
- Yu, H.; Feng, R.; Chen, F.; Wu, Z.; Li, D.; Qiu, X. Rapid FRET Assay for the Early Detection of Alpha-Synuclein Aggregation in Parkinson’s Disease. ACS Chem. Neurosci. 2024, 15, 1378–1387. [Google Scholar] [CrossRef]
- Verma, A.K.; Noumani, A.; Yadav, A.K.; Solanki, P.R. FRET Based Biosensor: Principle Applications Recent Advances and Challenges. Diagnostics 2023, 13, 1375. [Google Scholar] [CrossRef]
- Ye, X.W.; Dong, C.Z.; Liu, T. A Review of Machine Vision-Based Structural Health Monitoring: Methodologies and Applications. J. Sens. 2016, 2016, 7103039. [Google Scholar] [CrossRef]
- Optical Sensors Market Size, Share|Industry Forecast—2032. Available online: https://www.skyquestt.com/report/optical-sensors-market (accessed on 2 July 2025).
- Wang, X.D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2008–2012). Anal. Chem. 2013, 85, 487–508. [Google Scholar] [CrossRef]
- Optical Sensors Market Size, Share & Growth Trends, 2024–2032. Available online: https://www.gminsights.com/industry-analysis/optical-sensor-market (accessed on 2 July 2025).
- Yu, Y.; Shi, F.; Zhang, Y.; Li, F.; Han, J. Optical Sensor Array for the Discrimination of Liquors. J. Future Foods 2024, 4, 48–60. [Google Scholar] [CrossRef]
- Kersey, A.D. A Review of Recent Developments in Fiber Optic Sensor Technology. Opt. Fiber Technol. 1996, 2, 291–317. [Google Scholar] [CrossRef]
- Simone Soares, M.; Singh, R.; Kumar, S.; Jha, R.; Nedoma, J.; Martinek, R.; Marques, C. The Role of Smart Optical Biosensors and Devices on Predictive Analytics for the Future of Aquaculture Systems. Opt. Laser Technol. 2024, 177, 111049. [Google Scholar] [CrossRef]
- Srisruthi, S.; Swarna, N.; Susmitha Ros, G.M.; Elizabeth, E. Sustainable Agriculture Using Eco-Friendly and Energy Efficient Sensor Technology. In Proceedings of the 2016 IEEE International Conference on Recent Trends in Electronics, Information and Communication Technology, RTEICT, Bangalore, India, 20–21 May 2016; pp. 1442–1446. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Fargher, H.A.; d’Oelsnitz, S.; Diaz, D.J.; Anslyn, E.V. Pushing Differential Sensing Further: The Next Steps in Design and Analysis of Bio-Inspired Cross-Reactive Arrays. Anal. Sens. 2023, 3, e202200095. [Google Scholar] [CrossRef]
- Khan, J. Synthesis and Applications of Fluorescent Chemosensors: A Review. J. Fluoresc. 2024, 34, 2485–2494. [Google Scholar] [CrossRef]
- Fichera, L.; Li-Destri, G.; Tuccitto, N. Fluorescent Nanoparticle-Based Internet of Things. Nanoscale 2020, 12, 9817–9823. [Google Scholar] [CrossRef]
- Pizzoferrato, R. Optical Chemical Sensors: Design and Applications. Sensors 2023, 23, 5284. [Google Scholar] [CrossRef]
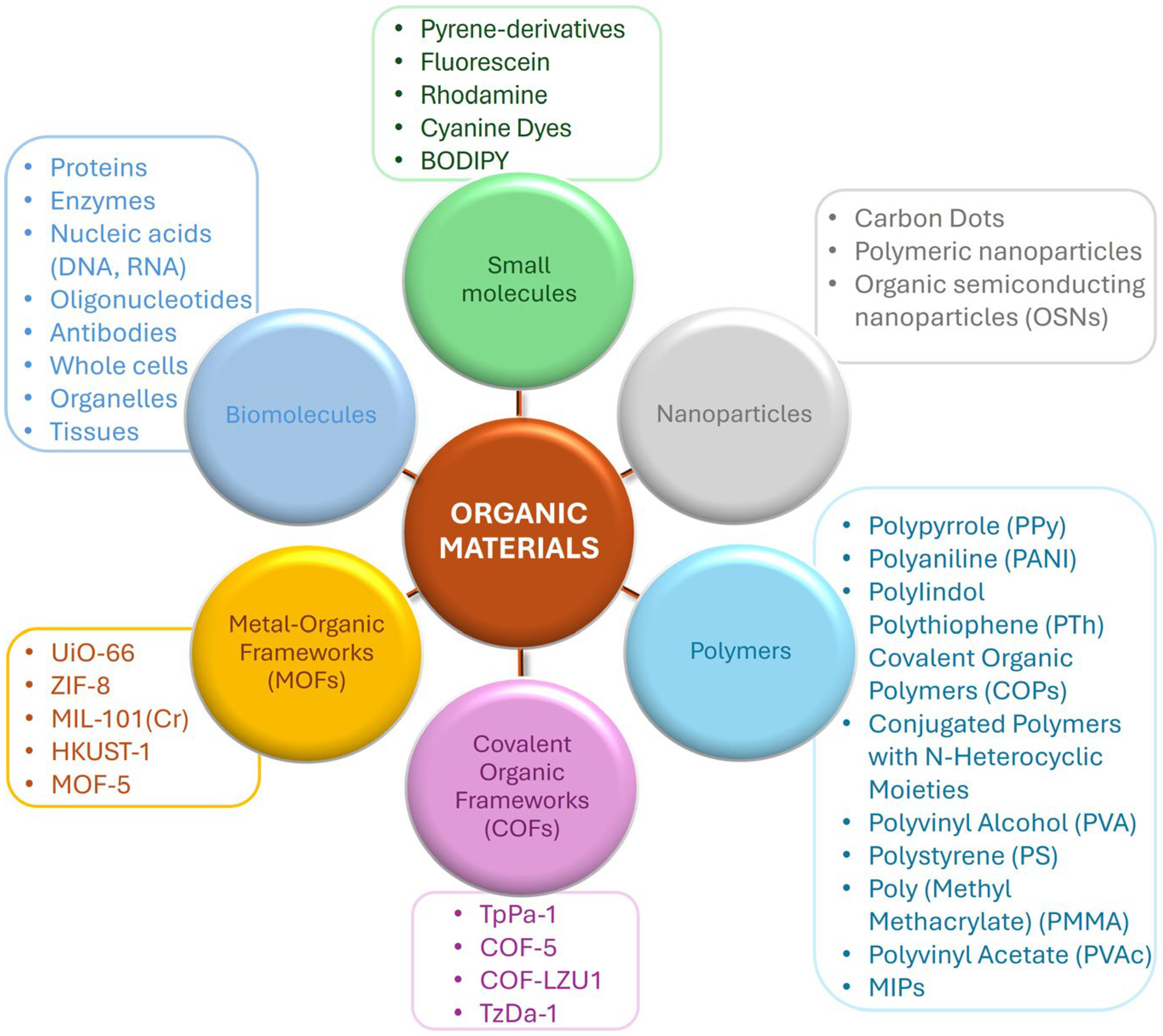

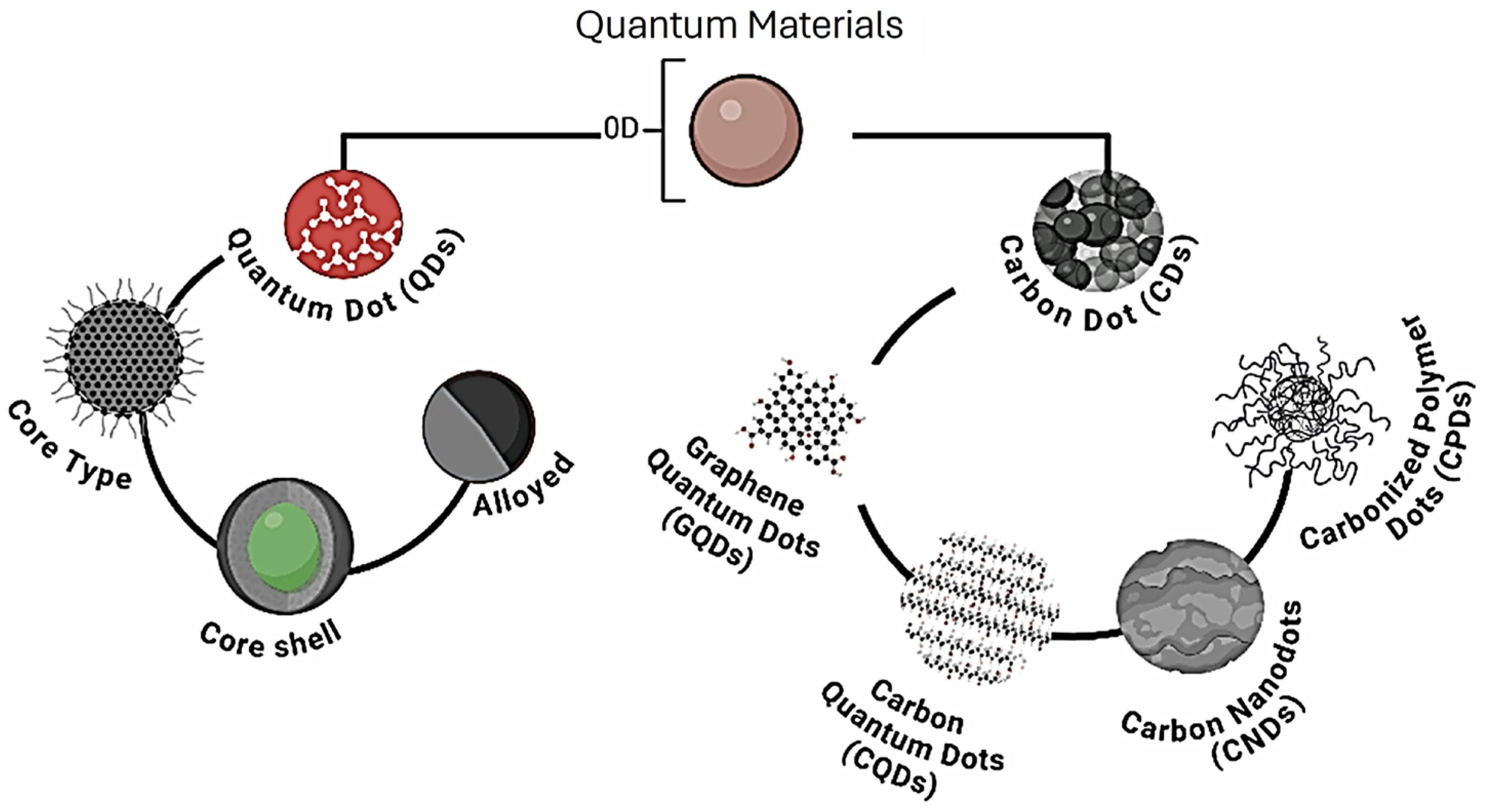
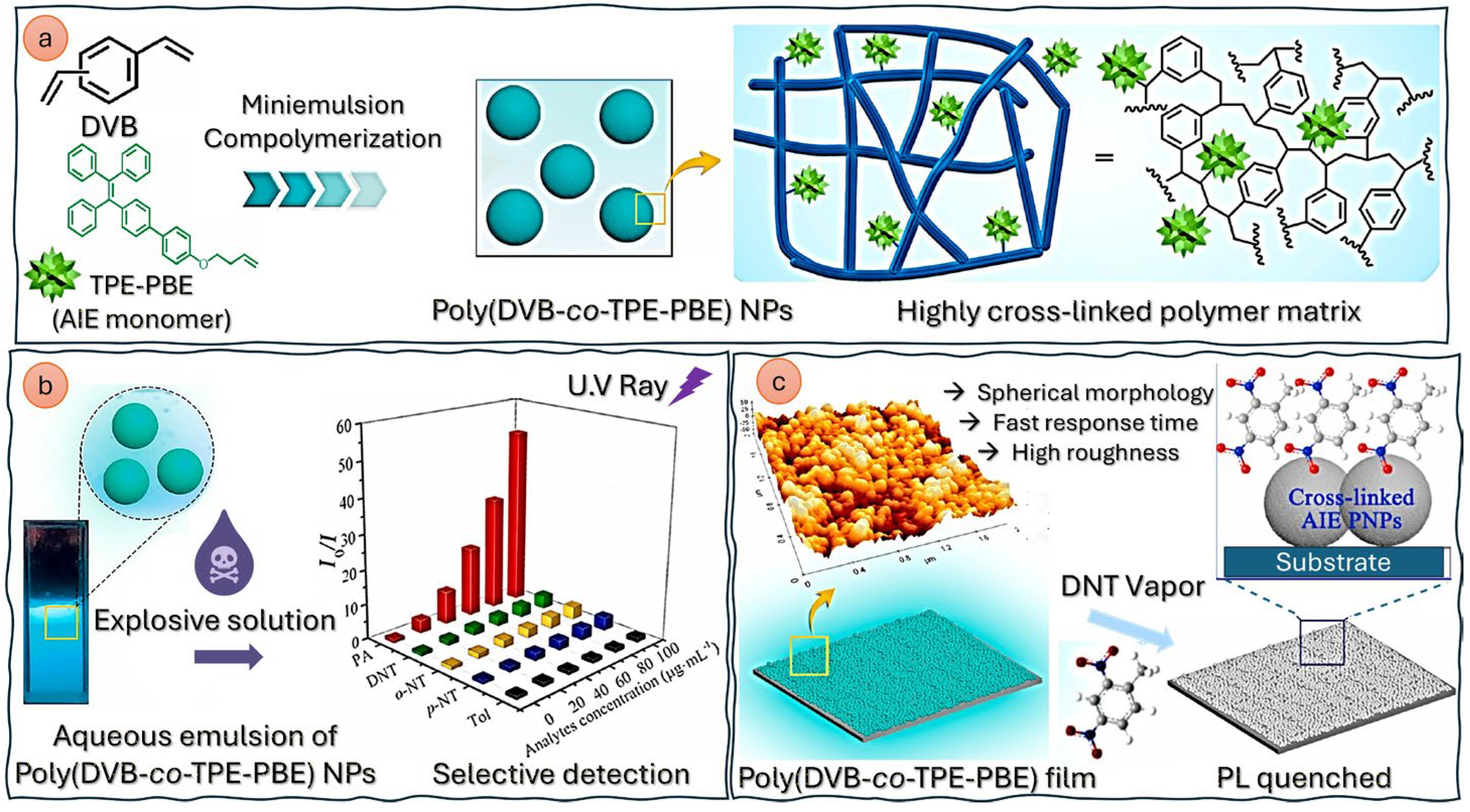

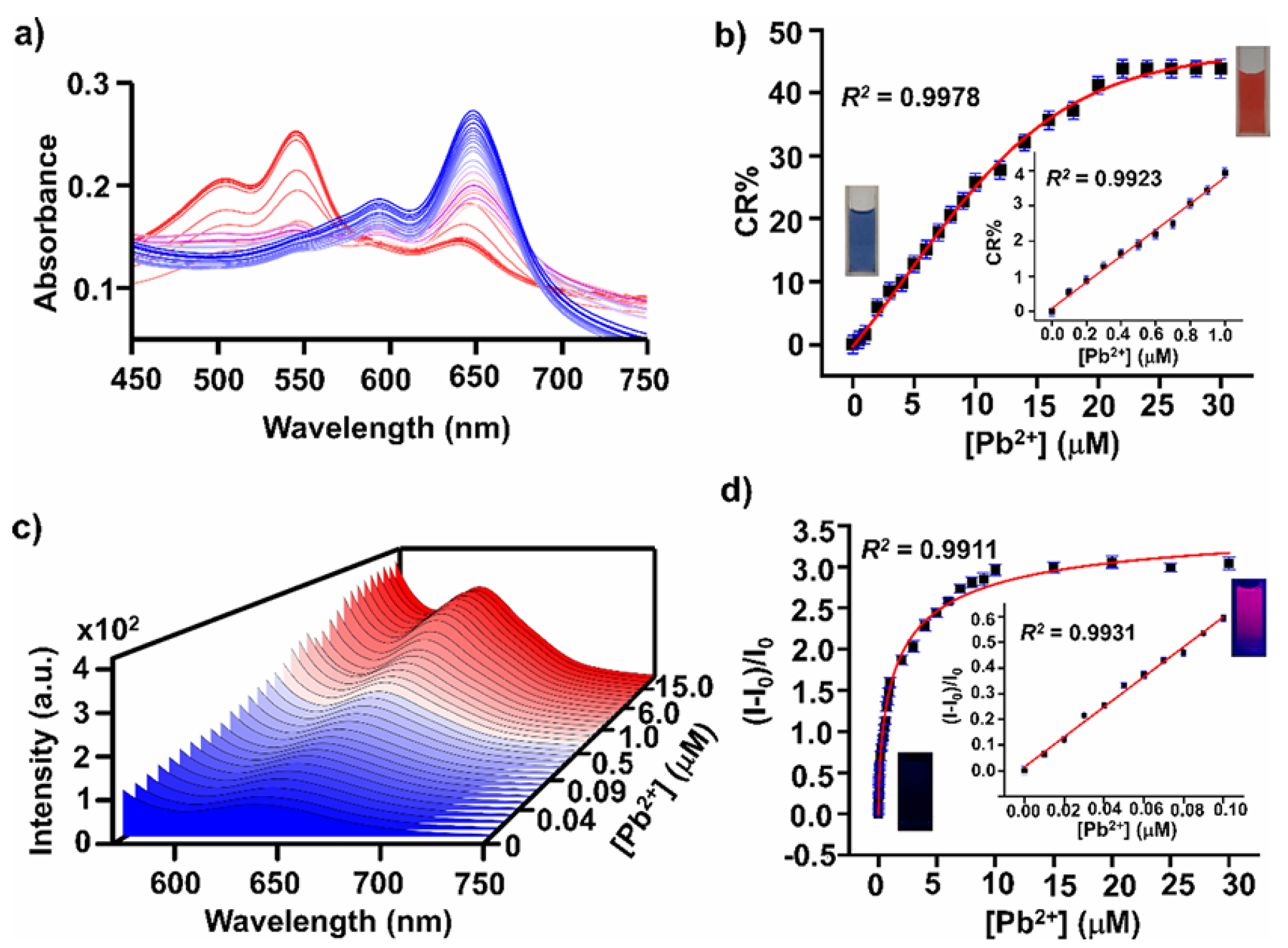

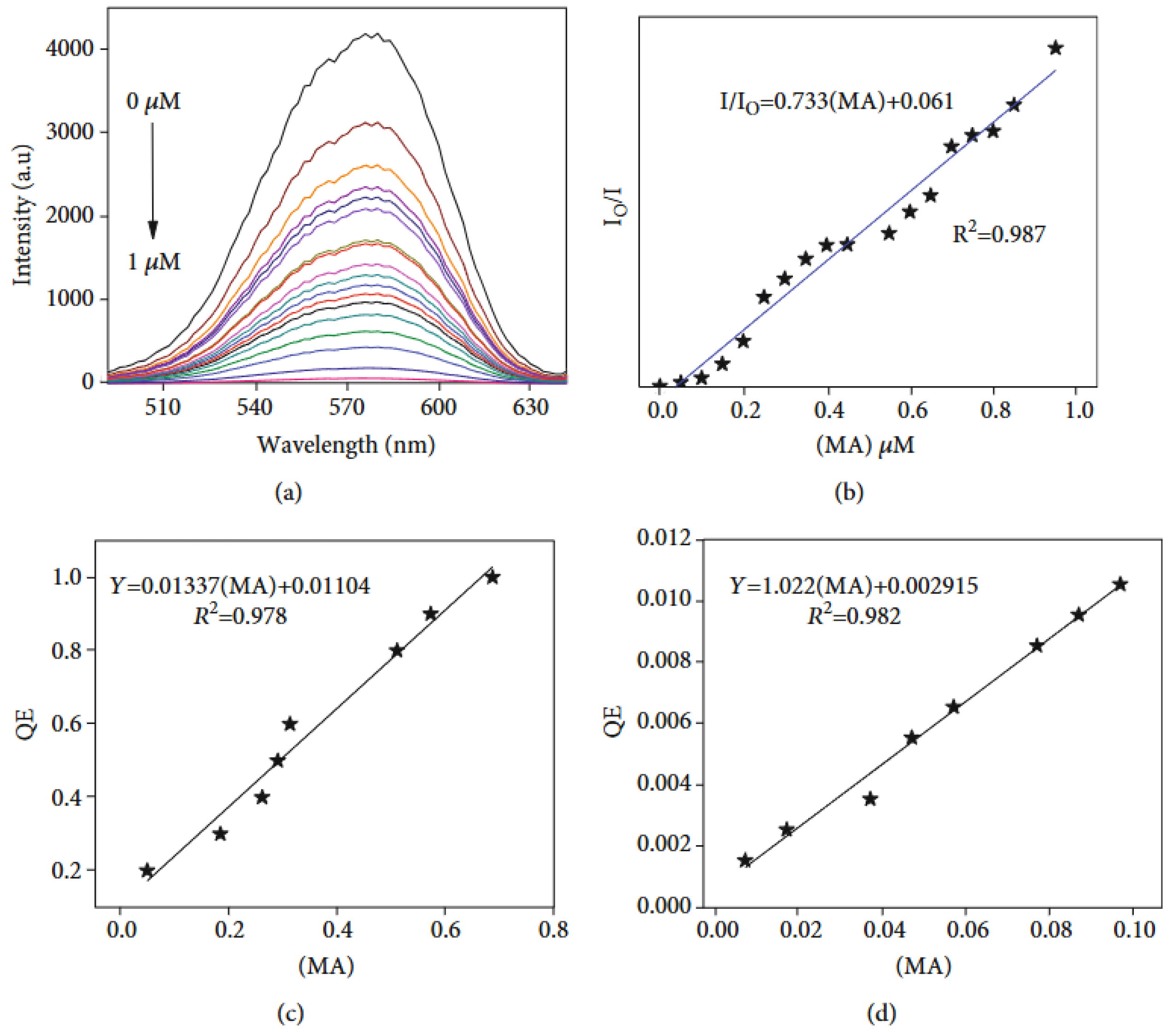


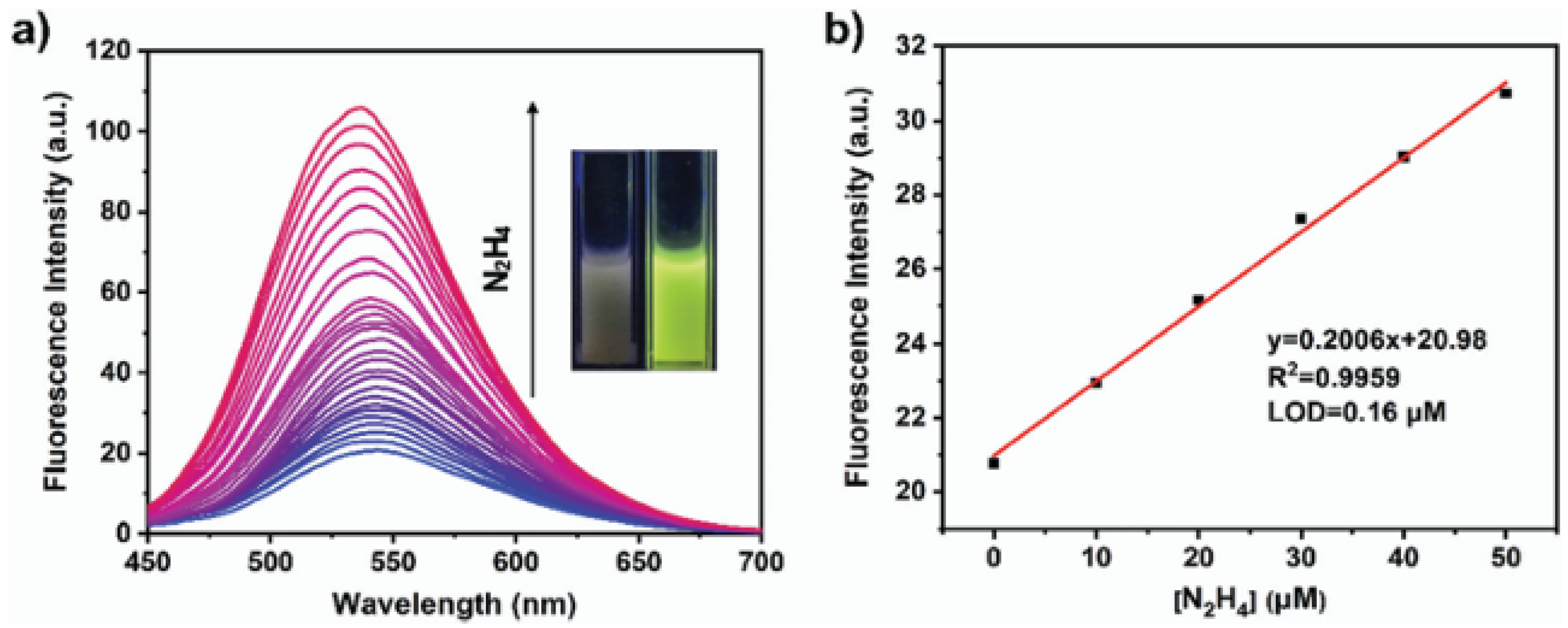
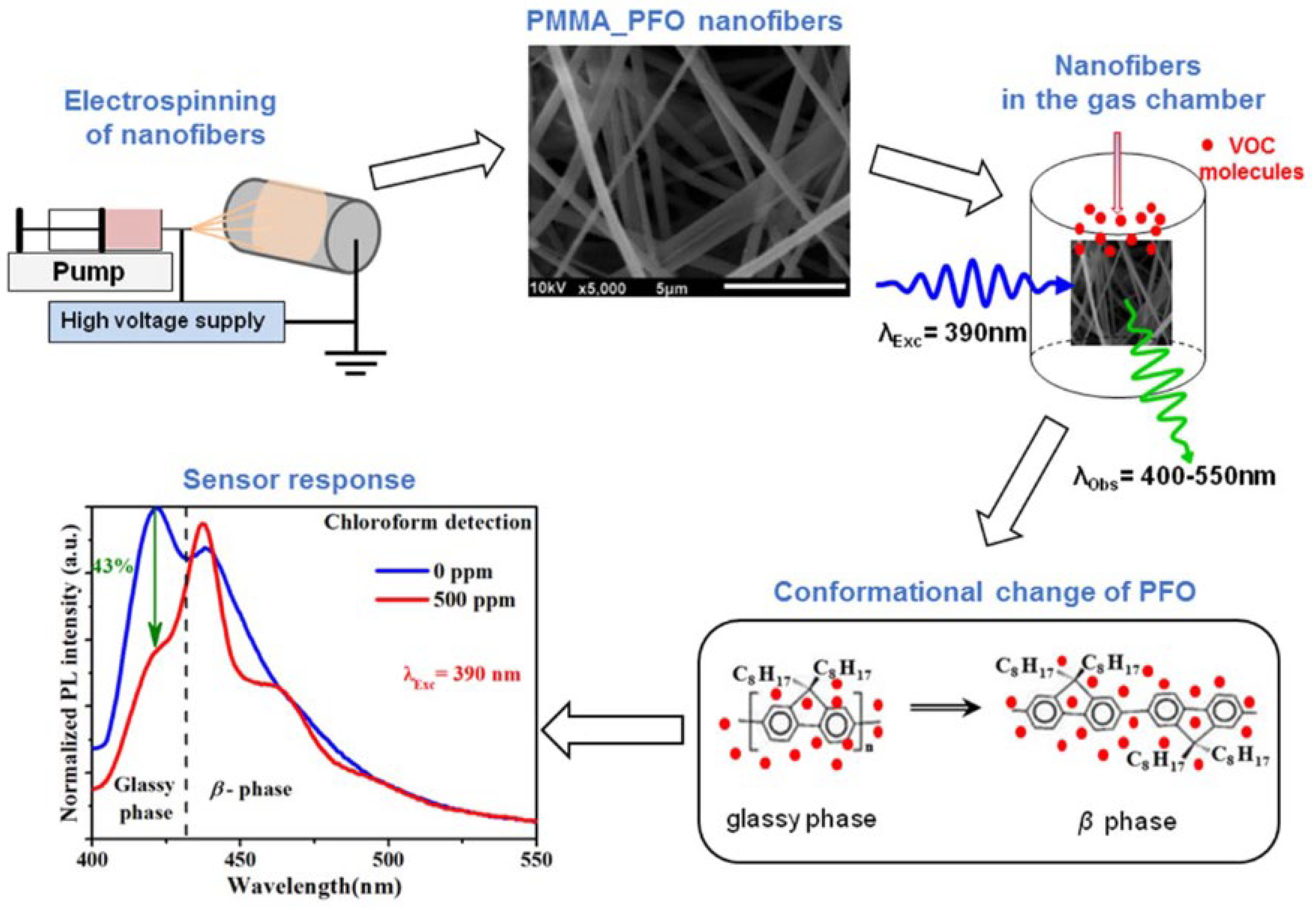
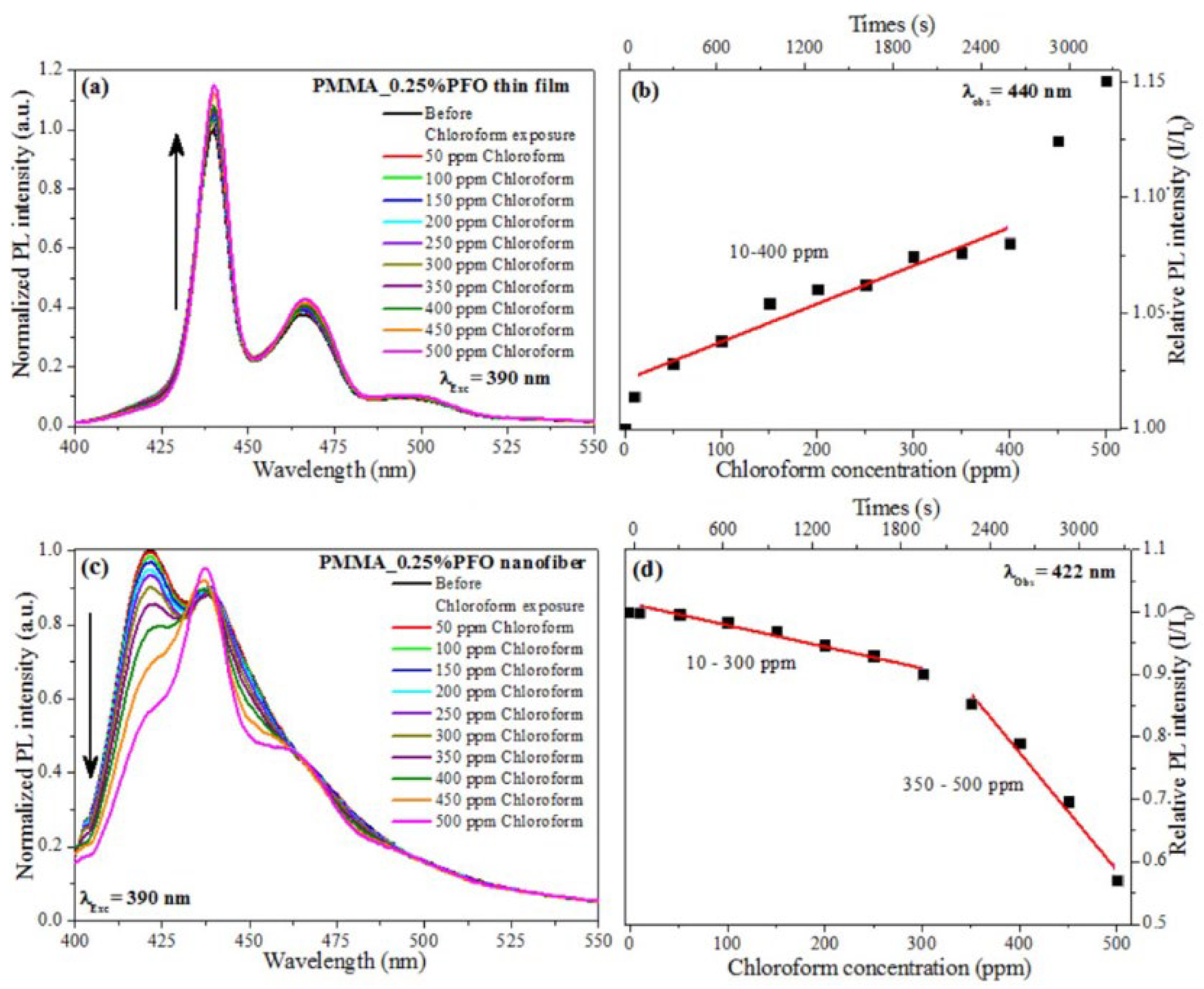
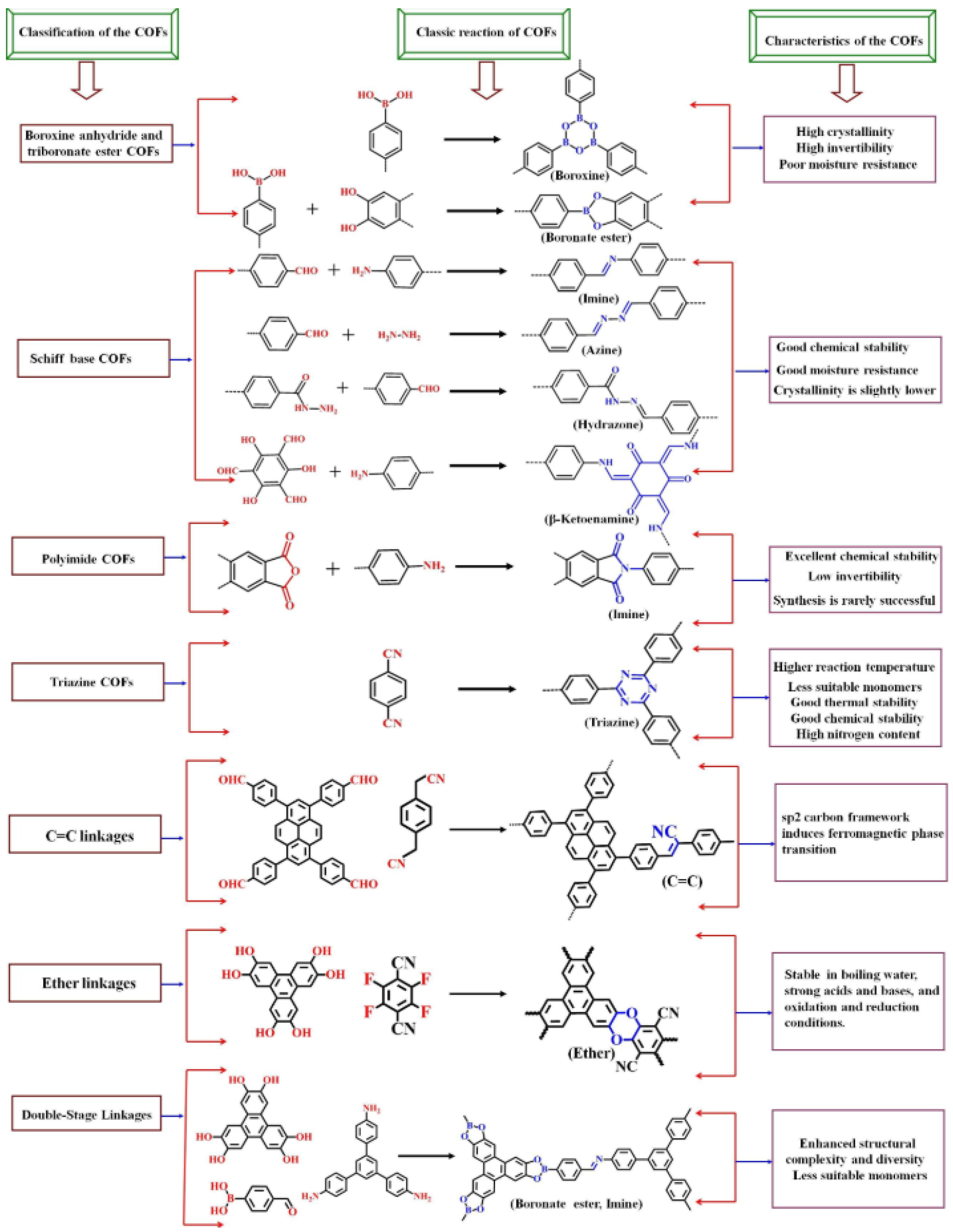
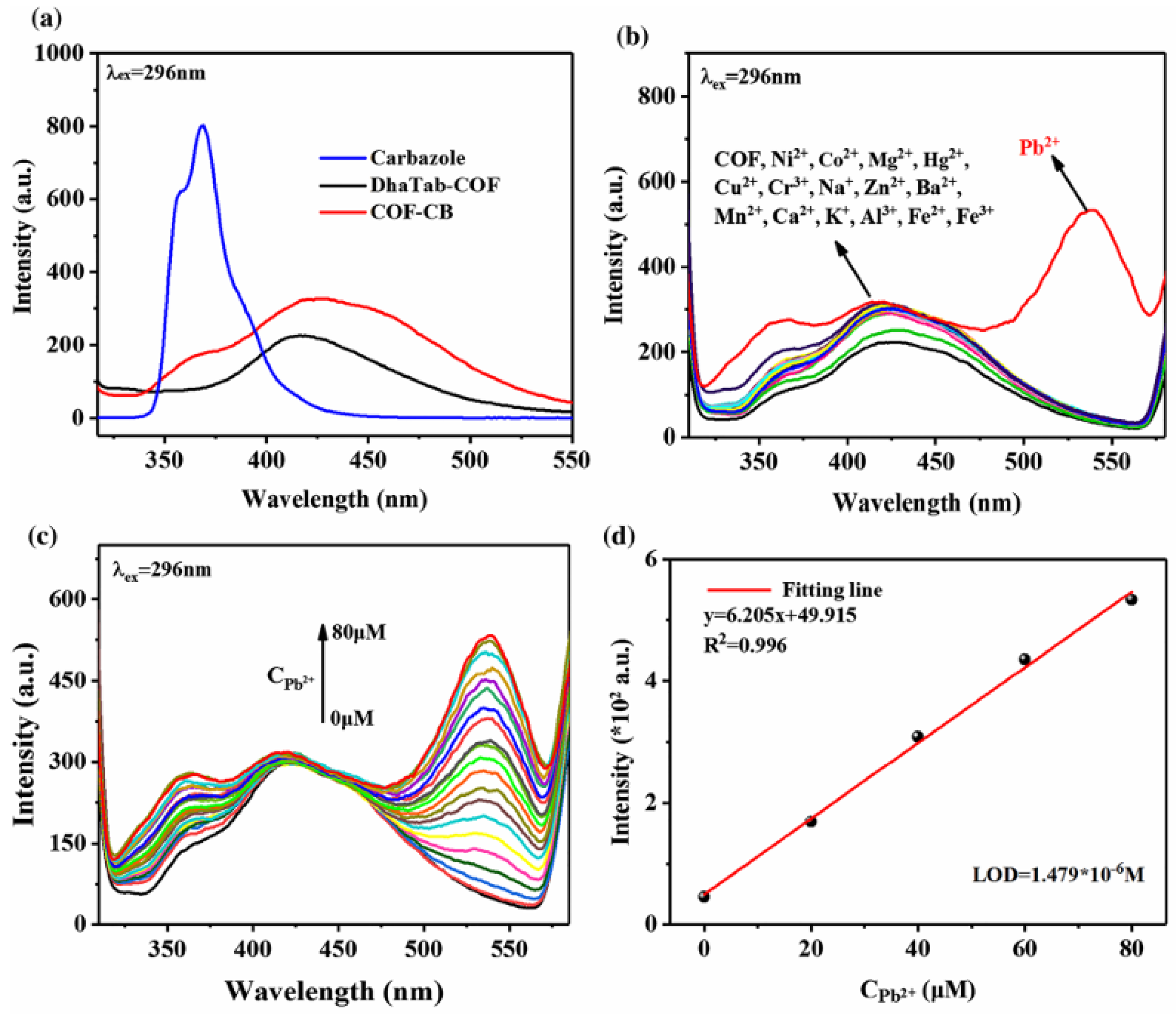
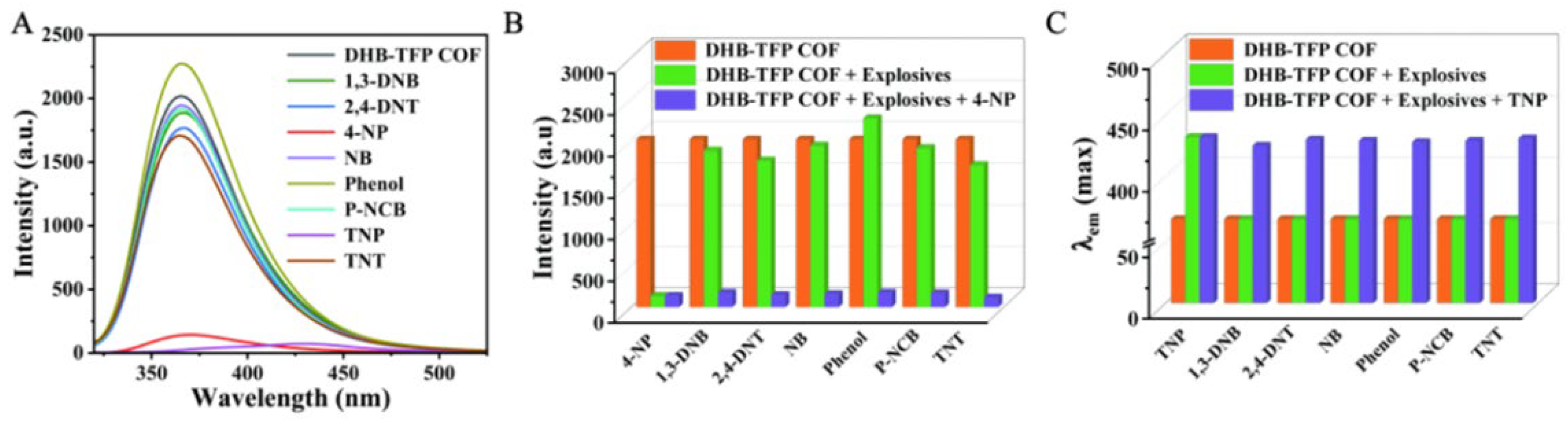
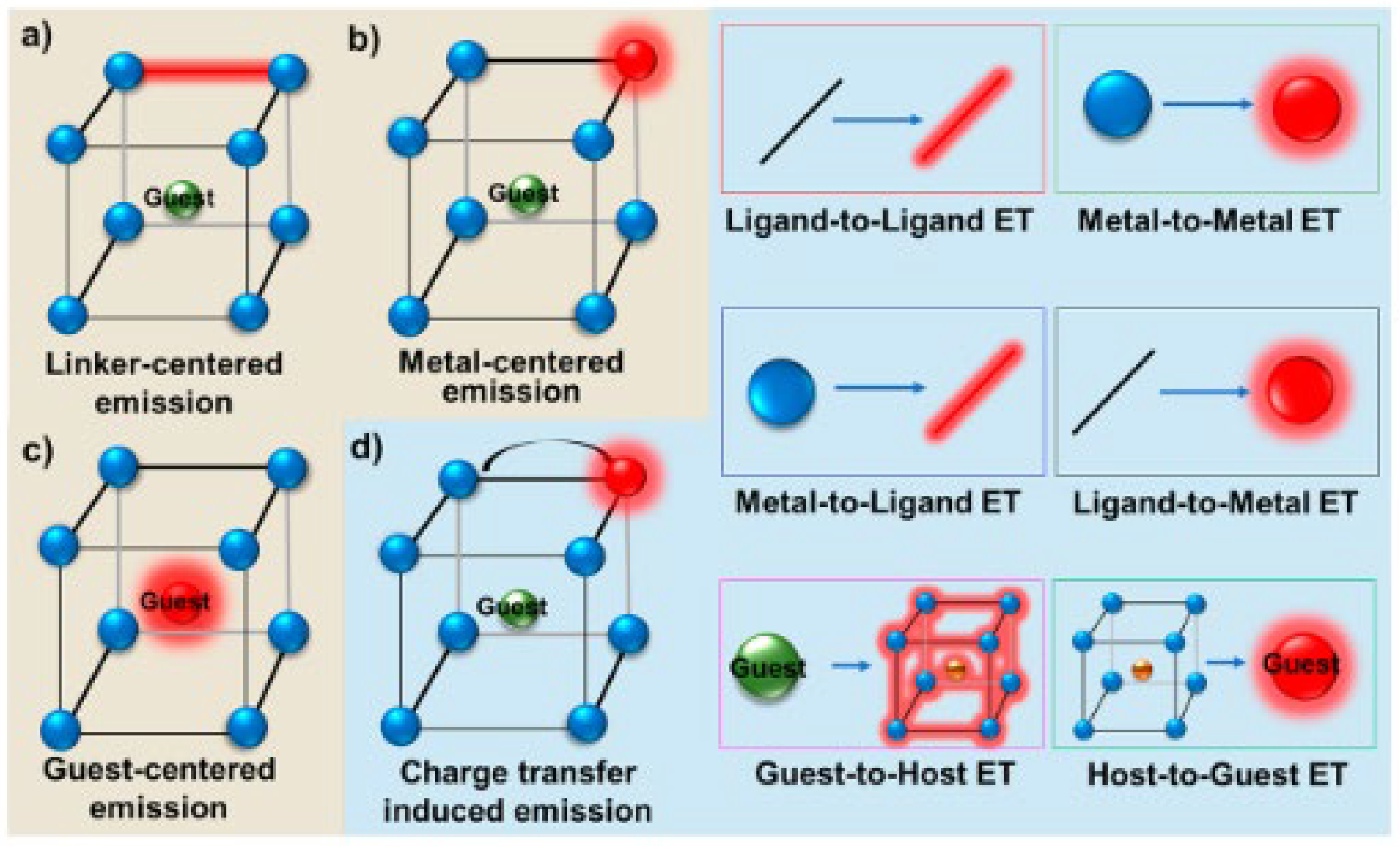

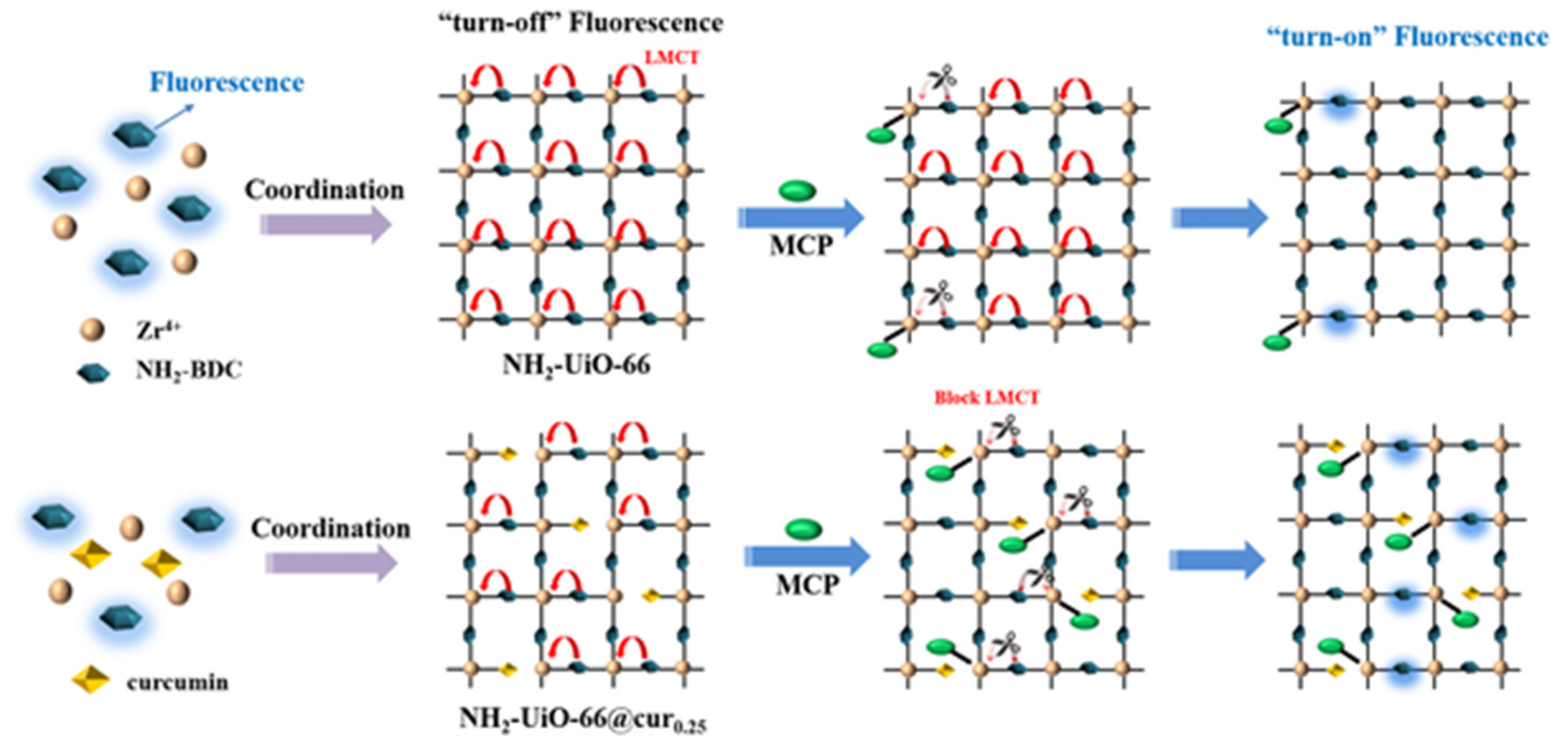
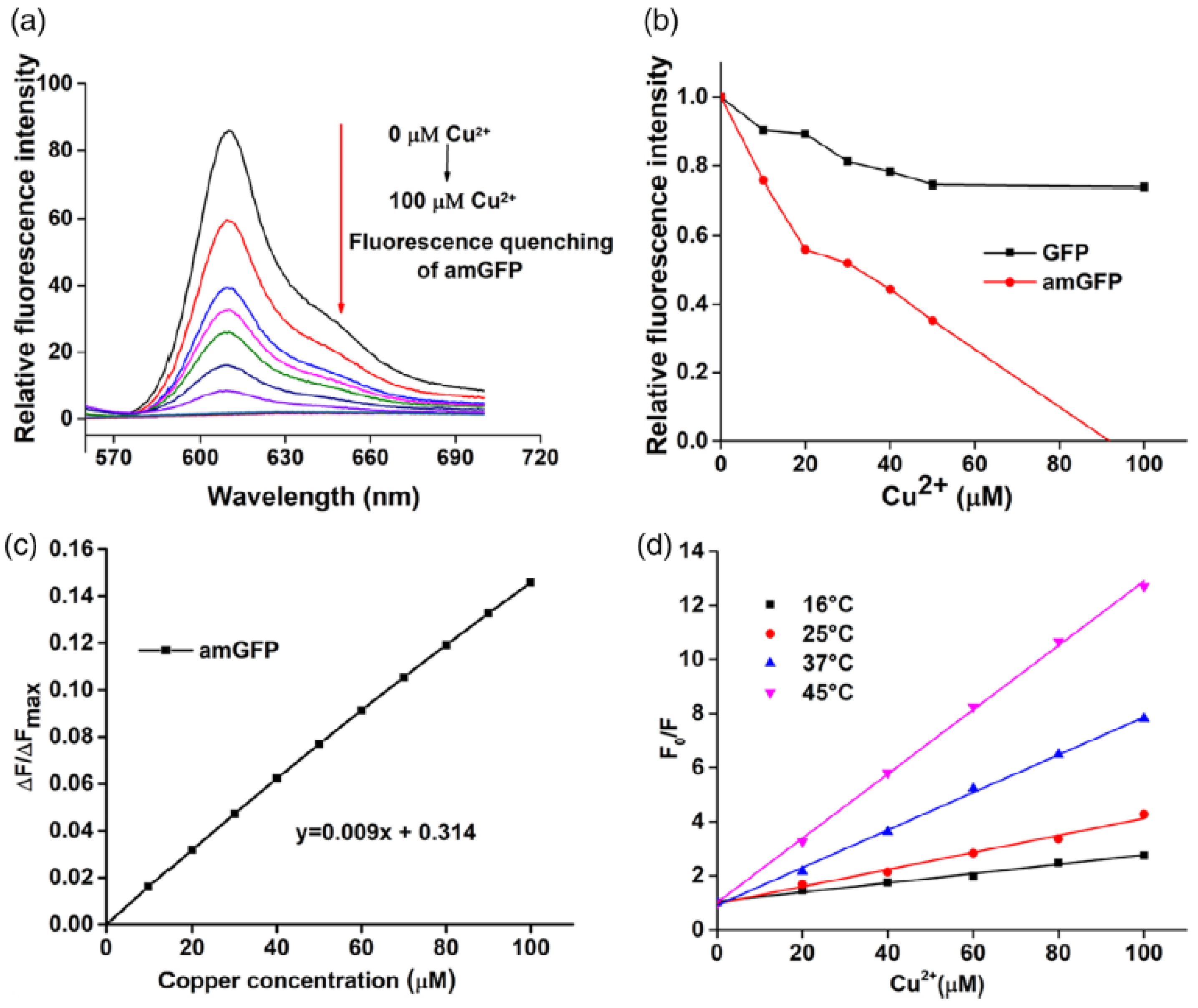
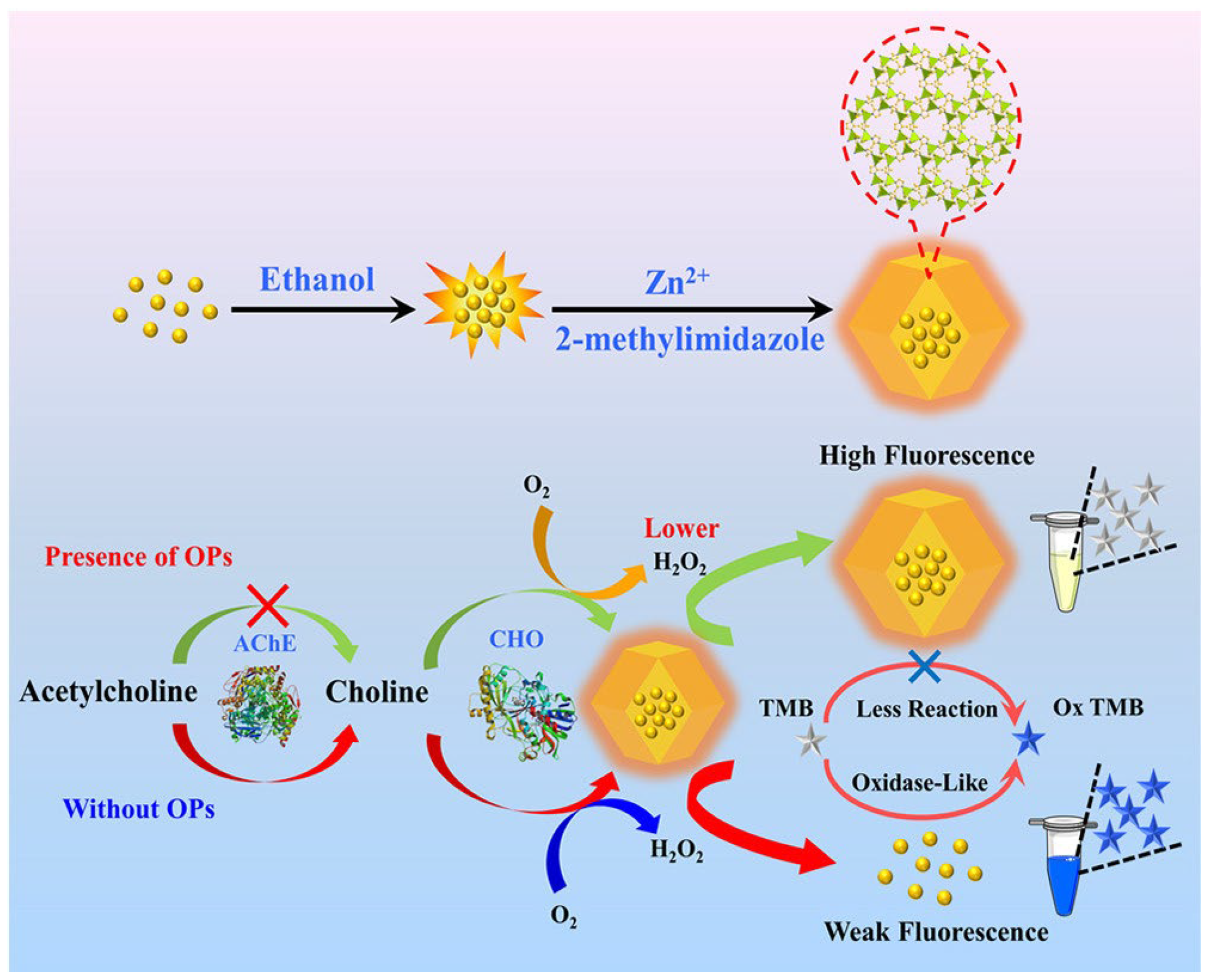
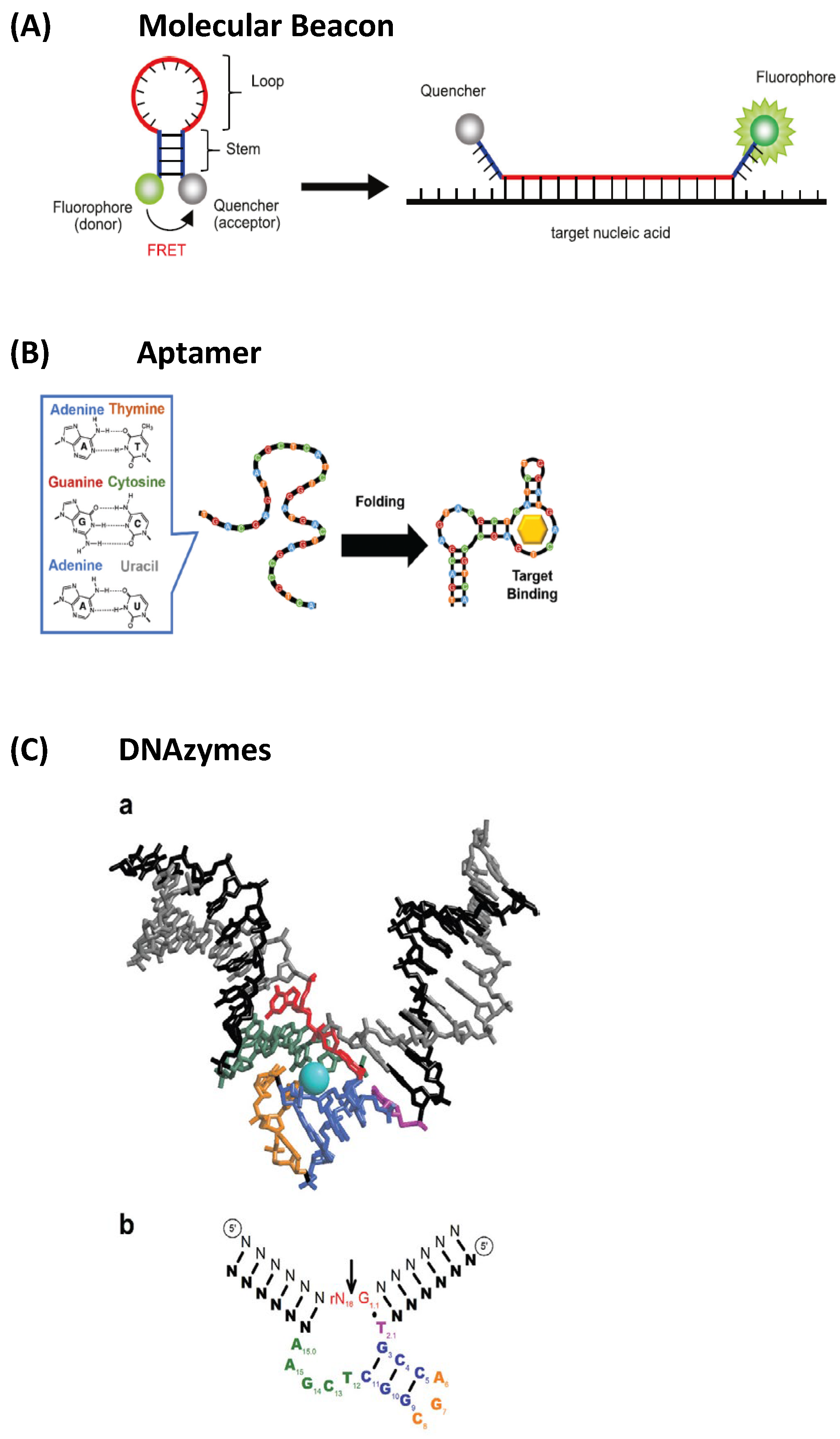
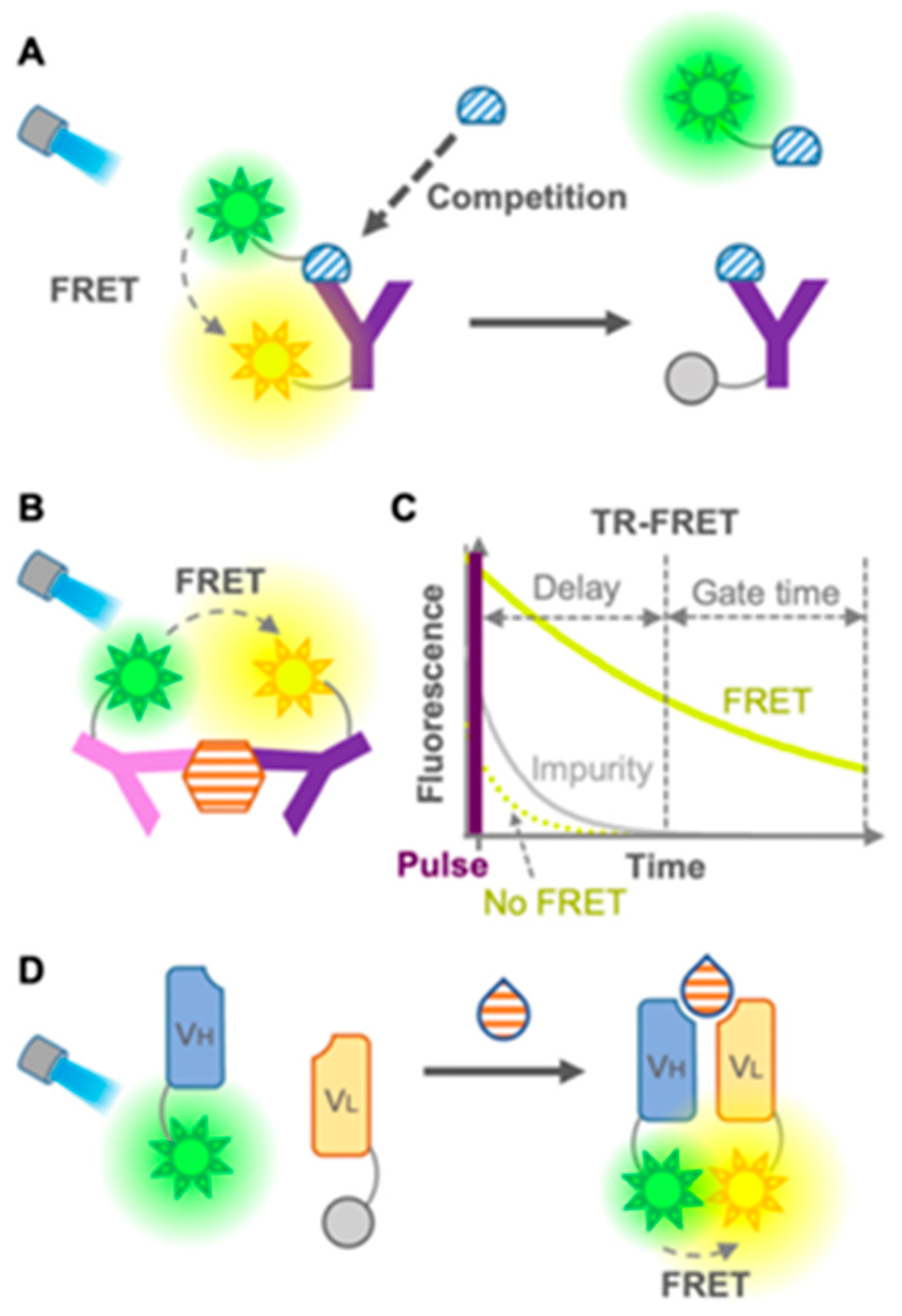
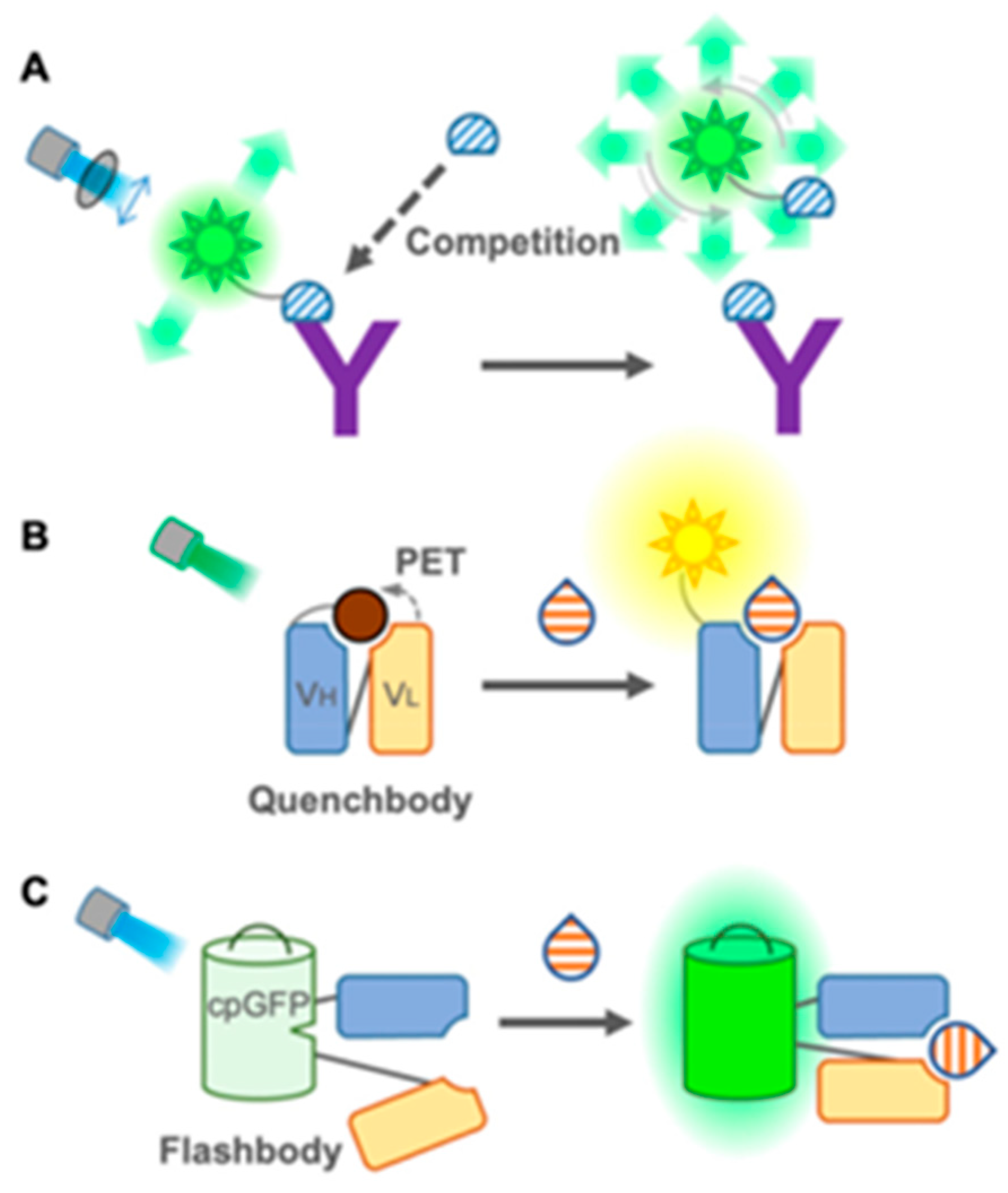
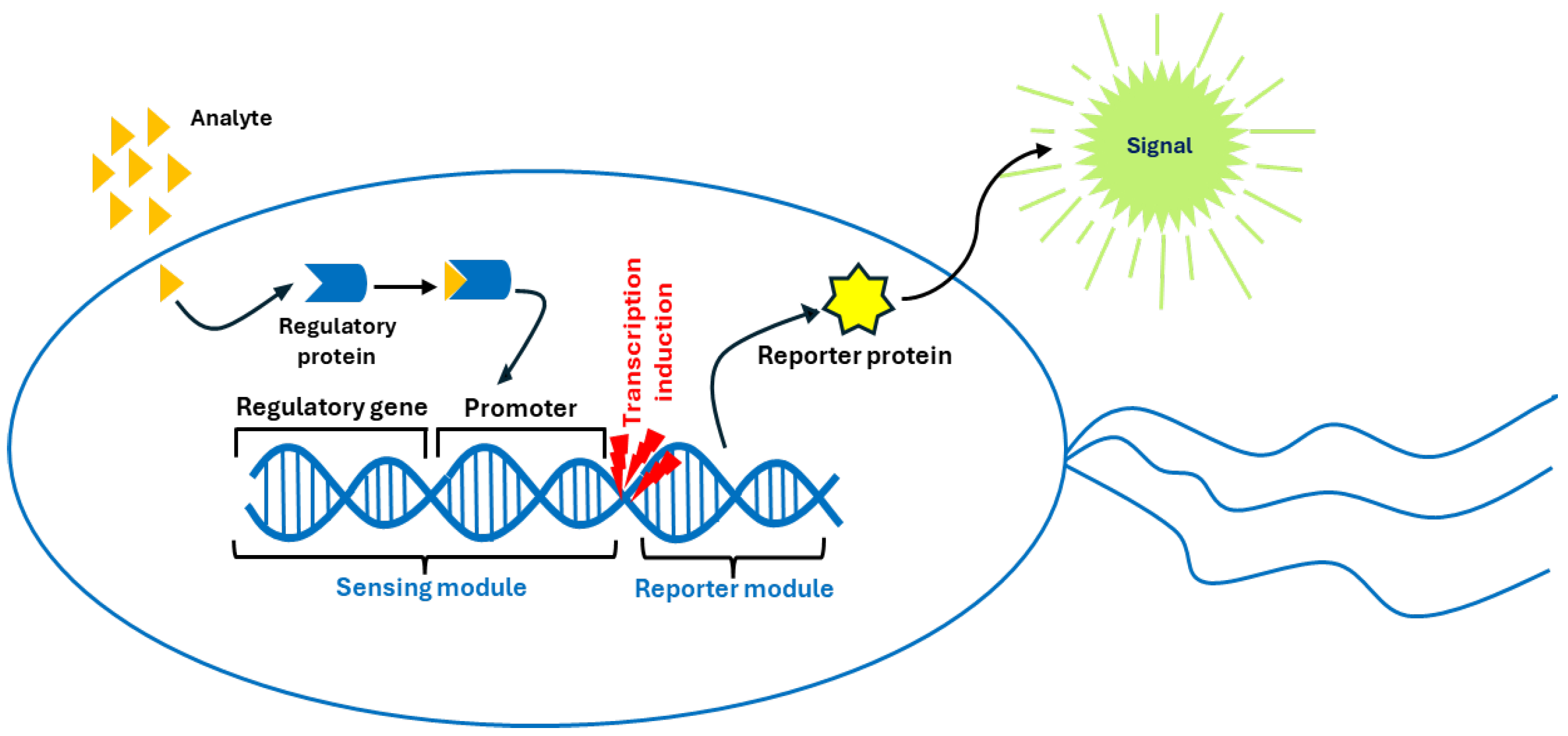
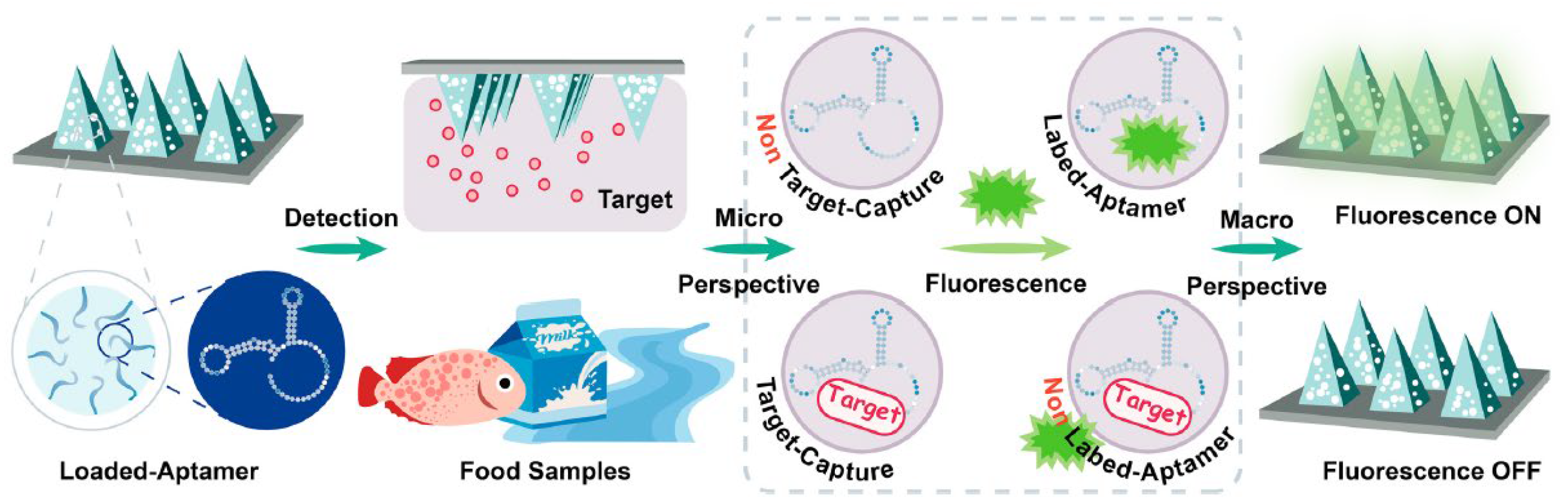
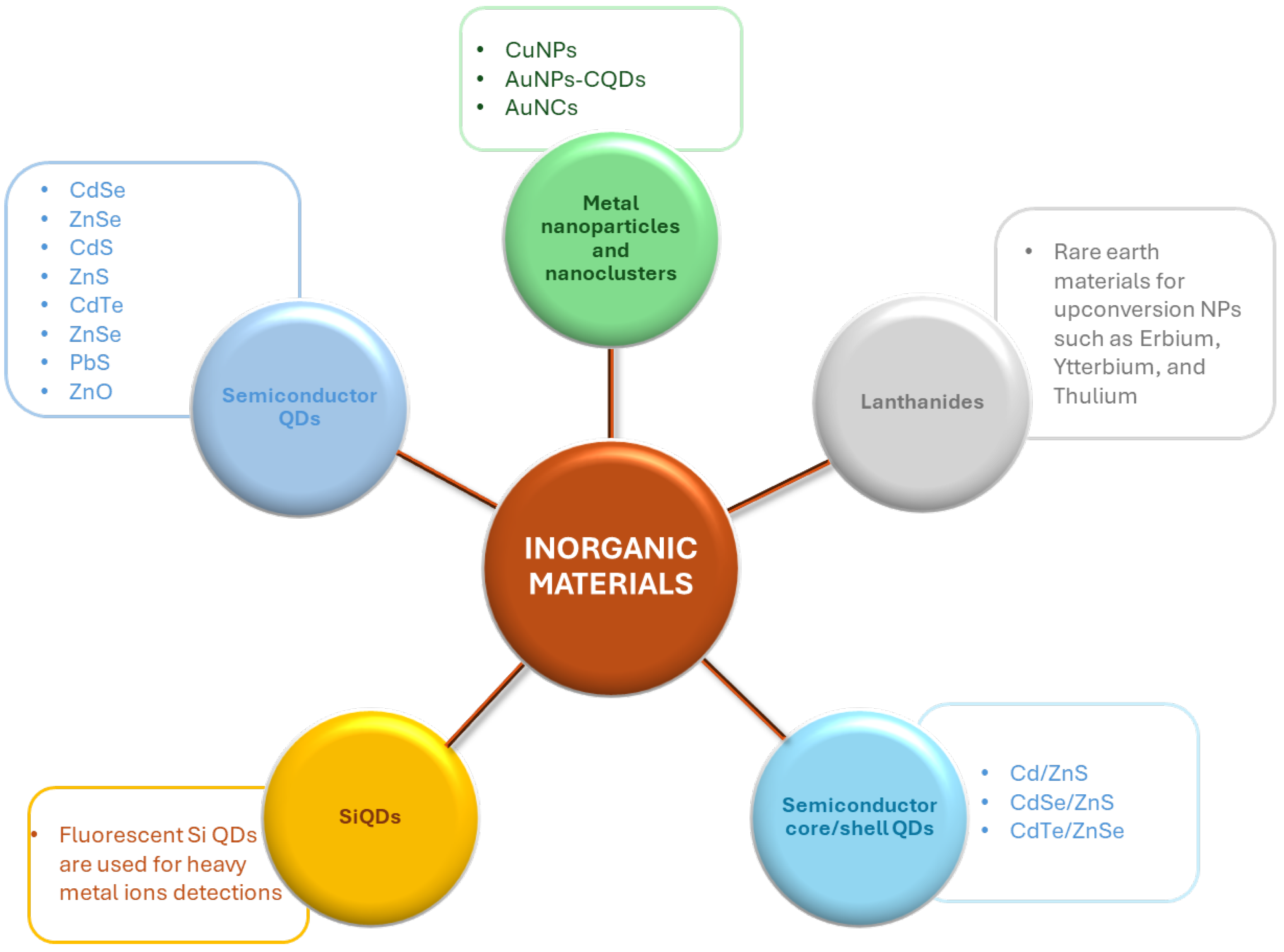
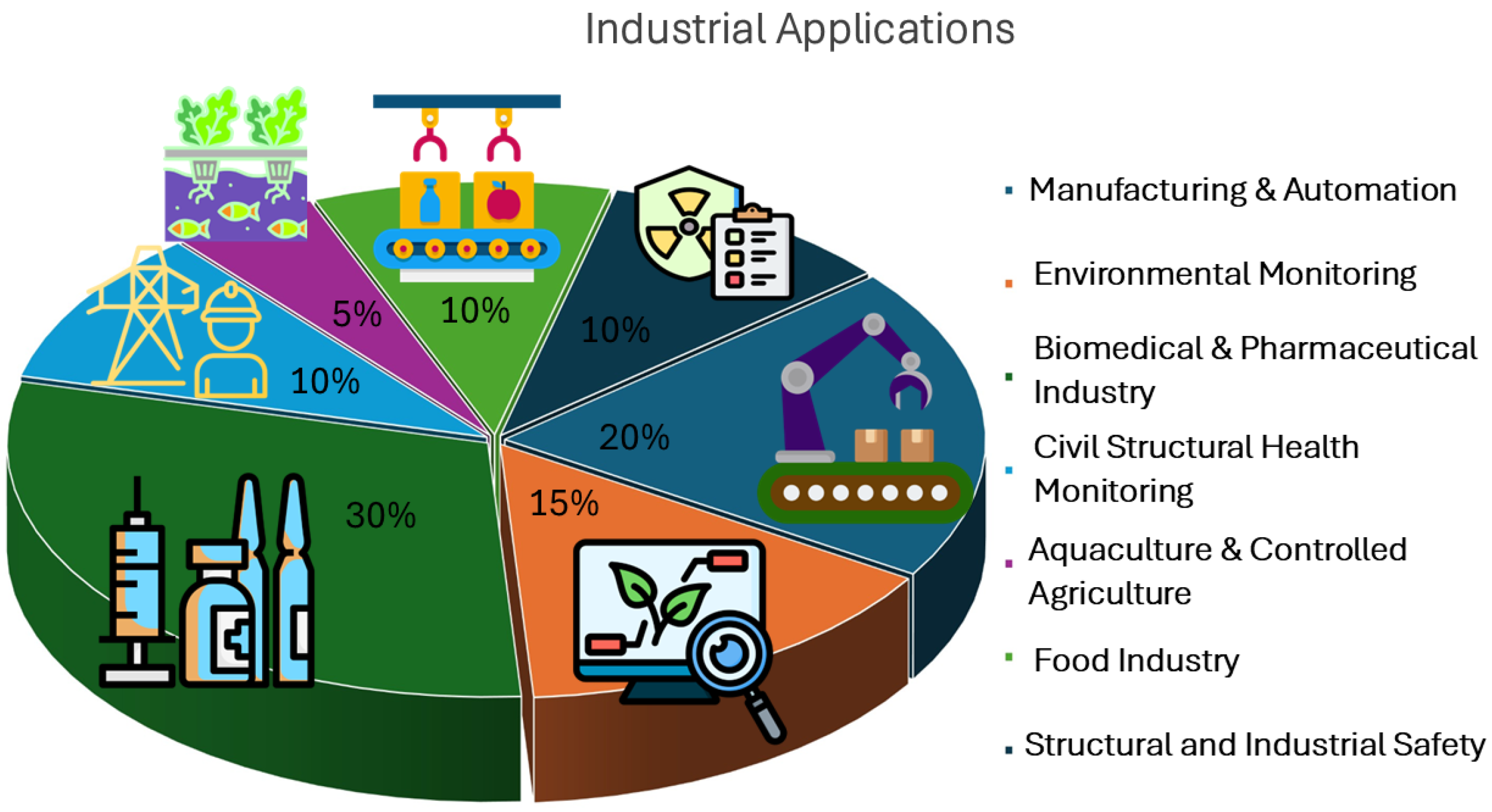
| Organic Material | Target | Technique | Type of Sample | LOD | [Ref.] |
|---|---|---|---|---|---|
| Small molecules | |||||
| Pyrene-derivatives | Ag+ | Turn-off |
HEPES-buffered DMSO/H2O | 2.9 nM | [35] |
| Hg+ | Quenching | MeCN/H2O | 4 ppb | ||
| Pb2+ | Quenching | MeCN/H2O | 2 ppb | ||
| Fluorescein | Cu2+ | Turn-on | Aqueous solution | 6.32 nM | [40] |
| Hg2+ | Turn-on | Aqueous solution | 0.86 nM | [41] | |
| ClO− | Turn-on–turn-off | Aqueous solution | 56 pM | [42] | |
| Rhodamine | Cu2+ | Turn-on | MeCN/H2O | 0.107 µM | [43] |
| Cyanine dyes | Hg2+ | Color change | Acetonitrile | 31 nM (naked eye detection: 2.9 ppm) | [51] |
| Cu2+ | Quenching | Acetonitrile | 37 nM, (naked eye detection: 2.1 ppm) | ||
| BODIPY | Dicofol | turn-off fluorescence | Water and tea | 200 ppb | [53] |
| Cd2+ | color change | 2.8 ppb | [54] | ||
| Nanoparticles | |||||
| Carbon quantum dots | Diazinon | Quenching | Cherry tomato juice | 0.00821 µM | [63] |
| Glyphosate | Quenching | Cherry tomato juice | 0.00296 µM | [63] | |
| Fenitrothion | Quenching | Rice samples | 0.00036 µM | [64] | |
| Malathion | Quenching | Water | 0.00514 µM | [65] | |
| Chlorpyrifos | Quenching | Water | 0.00427 µM | [65] | |
| CQDs modified with Eu(III) complexes | Hg2+ | Quenching | Milk | 0.2 nM | [62] |
| N and Cl co-doped lignin CQDs | Polystyrene microplastics | Fluorescence emission | Water | 0.4 mg/L | [66] |
| Polymeric nanoparticles | |||||
| Poly(DVB-co-TPE-PBE) | Picric acid | Quenching | Water solutions | 5.43 µM | [73] |
| Poly(methyl methacrylate-co-glycidyl methacrylate) NPs | Fe2+ | Color change and fluorescence emission enhancement | 2.63 µM | [74] | |
| Fe3+ | 2.5 µM | ||||
| Organic semiconducting nanoparticles (OSNs) | |||||
| PCDA-Alen (based on PDA) | Pb2+ | Color change (from blue to red) with increase in fluorescence output | Water from various natural bodies | 16.3 nM (3.2 ppb) | [82] |
| TCDA-clay-N-1-hexadecyl imidazole | Toluene | Color change (from blue to red) with increase in fluorescence output | VOC atmosphere in sealed chamber (25 mL) | 0.02% | [83] |
| THF | 0.08% | ||||
| Benzene | 0.04% | ||||
| Polymers | |||||
| PPy | Cu2+ | Quenching | Water | 1.2 μM | [90] |
| PANI | Malathion | Quenching | Potatoes, tomatoes | 0.132 μM | [92] |
| PIN | Picric acid | Quenching | Organic media | 30 × 103 M−1 (*) | [96] |
| PTh | Berberine hydrochloride (alkaloid) | Decrease in fluorescence emission and color change (from pale yellow to red) | Water, urine samples | 0.27 μM | [99] |
| COPs | Hydrazine | Fluorescence turn-on | Aqueous solutions | 0.16 μM | [103] |
| Conjugated polymers with N-heterocyclic moieties | 2,4,6-trinitrophenol (TNP) or picric acid solution and vapor | PL quenching | Aqueous solutions | 147 nM (38 ppb) | [110] |
| PVA | Cu2+ | Luminescence quenching | Aqueous environments | 0.086 μM | [113] |
| PS | Hg2+ | Increase in fluorescence emission | Real water samples | 1.01 μM | [114] |
| PMMA | Chloroform | Luminescence quenching | Gas chamber | 15.4 ppm | [117] |
| PVAc | |||||
| COFs | |||||
| COF-DHTA | Al3+ | Turn-on | DMF suspension | 0.93 μmol/L | [134] |
| COF-CB | Pb2+ | Turn-on | DMF solution | 1.48 µmol/L | [135] |
| Hg2+ | Quenching | Aqueous solution | 17 nM | [180] | |
| DHB-TFP COF | NP | Turn-off | 0.40 μmol/L | [136] | |
| TNP (picric acid) | Turn-off | 11.15 μmol/L | |||
| TFPB-TTA COF | DNP | Quenching | Aqueous solution | 18 nM | [181] |
| TNP | 16 nM | ||||
| MOFs | |||||
| MB@Cd-MOF | Carbaryl | Fluorescence enhancement | Tap and river water, fruit juices | 6.7 ng·mL–1 | [144] |
| TbMOF | Imidacloprid | Quenching | Water | 1.3 × 10−5 M−1 | [182] |
| Thiamethoxam | Quenching | 7.3 × 10−6 M−1 | |||
| {(Me2 NH2)[In(BDPO)]·DMF·2H 2O}n | 2,6-Dichloro-4-nitroaniline (DCN) | Quenching | Water | 0.14 μmol L−1 3.85 ppm | [183] |
| Co-MOF | p-Nitrophenyl phosphate (PNPP) | Quenching | Food, fruits, and domestic water | 352 nM | [184] |
| Cd(II)-MOF | Glyphosate | Turn-on fluorescence | Drinking water | 0.025 μmol L−1 | [185] |
| Cr3+ | Turn-on fluorescence | 0.6 μM | |||
| ZnMOF | Parathion | Quenching | Irrigation water | 1.95 mg L−1 | [186] |
| Zr-MOF | Monocrotophos (MCP) | “Turn-on” fluorescence | Tap water, lake water, wastewater | 1.84 nM | [145] |
| Eu-MOF | Tetrahydrofuran (THF) vapor | Turn-on | THF-saturated air | 17.33 Pa | [146] |
| Eu-MOF | Benzaldehyde solution | Benzyl alcohol | 9.3 × 10−6 M | [187] | |
| Fe3+ | DMF solution | 5.8 × 10−6 M | |||
| Dye@Eu-MOFs) | Acetaldehyde vapor | Quenching | Dye@Eu-MOF hydrogel plate | 8.12 × 10−4 mg/L | [188] |
| ZIF-90 MOF | Formaldehyde | Turn-on | Liquid and gas phases | 2.3 µM | [189] |
| Tb(BTC)-MOF | TNP vapor | Quenching | Saturated glass vial | <1 ppb | [190] |
| CuMOF | 2,4,6-Trinitrophenol (TNP) | Quenching | River and tap water samples | 0.08 μmol L−1 | [191] |
| P1@BMOF | Cu2+ | Quenching | Deionized water | 0.22 μM | [192] |
| P2@BMOF |
0.20 μM | ||||
| Eu@UiO-MOF-X | Cd2+ | IEu3+/IBPYDC (663 nm/426 nm) reduction | Rice, grape juice, and liquor samples | 5.67 × 10−7 M (114 ppb) | [193] |
|
{[H-Phen]2[Mn3(FDA)4(H2O)2]·2H2O}n (Mn-based MOF) | Ag+ | Turn-on | Water | 0.023 ppb | [194] |
| Cd2+ | Turn-on | 0.05 ppb | |||
| Hg2+ | Turn-off | 0.10 ppb | |||
| ZnTCPP-MOF | Pb2+ | Quenching (from bright red to colorless under UV light) | Water | 4.99 × 10−8 M | [195] |
| Biomolecule-based sensors | |||||
| Protein-based fluorescent sensors | |||||
| amGFP (sensor and bio-cleaner) | Cu2+ | Quenching | Wastewater | 1–5 μM (estimated) | [153] |
| Enzyme-based fluorescent sensors | |||||
| AuNC@ZIF-8 | Acephate | Turn-on | Water, lettuce | 0.67 μg/L | [155] |
| Glyphosate | - | ||||
| Malathion | - | ||||
| Parathion | - | ||||
|
Pirimiphos-methyl, fenitrothion | - | ||||
| (OPs) | - | ||||
| Nucleic acid-based fluorescent sensors | |||||
| G-CD@Apt | SARS-CoV-2 spike protein | Turn-on | Synthetic saliva, river water | 0.067 ng/mL (equivalent to 0.335 pg per test) | [160] |
| Thrombin-binding aptamer–crystal violet (TBA-CV) | Pb2+ | Fluorescence intensity decrease | Pond water | 1.18 nM (0.32 ppb) | [196] |
| DNA nanosphere-enhanced substrate strand–DNAzyme (DS-Sub-Dz) | Pb2+ | Fluorescence intensity increase | Drinking water, tap water, rainwater, and lake water | 2.0 nM | [197] |
| DVc1 (DNAzyme) | Vibrio cholerae | Turn-on | Raw choking sea crab forceps, raw choking oysters, and cold jellyfish | 7.2 × 103 CFU/mL | [198] |
| Antibody-based fluorescent sensors | |||||
| Au-Ag bimetallic nanoclusters (NCs) | Dicofol | Fluorescence recovery | Green tea, black tea, and dark tea | 0.185 ng/mL in liquid system | [162] |
| Gold nanoflowers (NFs) | 0.170 ng/mL in paper-based analytical devices | ||||
| FPIA (composed of anti-IMI monoclonal antibody and fluorescein isothiocyanate ethylenediamine (EDF)) | Imidacloprid (IMI) | Fluorescence polarization | Paddy water, corn, and cucumber | 1.7 µg/L | [163] |
| Fluorescent sensors based on living (micro)organisms or parts of them | |||||
| Whole-cell biosensors | |||||
| E. coli S17-1 (donor) | 1,3-Dinitrobenzene (1,3-DNB) | Liquid solution | 0.1 μg/mL | [199] | |
| P. putida (recipient) | Sand | 0.5 mg/kg | |||
| Escherichia coli DH5α | Hg(II) | Increase in red fluorescence | Cosmetics | 0.03 μM | [200] |
| Escherichia coli DH5α | Pb (II) | Synthetic wastewater | 2 nM | [201] | |
| Arxula adeninivorans | 17b-Estradiol | Fluorescence emission | Serum samples | 1 ng/L | [202] |
| Progesterone | 6 ng/L | ||||
| 5a-Dihydrotestosterone | 25 ng/L | ||||
| Chlamydomonas reinardtii Organelle-based biosensors Thylakoid membranes | Atrazine |
Fluorescence yield increase Fluorescence decrease | Water Fish, milk, river water | 7.3 × 10−10 M | [167] |
| Diuron | 2.3 × 10−10 M | ||||
|
Prometryn Netilmicin | 3.5 × 10−10 M 5.99 nM [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonasera, K.; Galletta, M.; Calvo, M.R.; Pezzotti Escobar, G.; Leonardi, A.A.; Irrera, A. Organic Fluorescent Sensors for Environmental Analysis: A Critical Review and Insights into Inorganic Alternatives. Nanomaterials 2025, 15, 1512. https://doi.org/10.3390/nano15191512
Buonasera K, Galletta M, Calvo MR, Pezzotti Escobar G, Leonardi AA, Irrera A. Organic Fluorescent Sensors for Environmental Analysis: A Critical Review and Insights into Inorganic Alternatives. Nanomaterials. 2025; 15(19):1512. https://doi.org/10.3390/nano15191512
Chicago/Turabian StyleBuonasera, Katia, Maurilio Galletta, Massimo Rosario Calvo, Gianni Pezzotti Escobar, Antonio Alessio Leonardi, and Alessia Irrera. 2025. "Organic Fluorescent Sensors for Environmental Analysis: A Critical Review and Insights into Inorganic Alternatives" Nanomaterials 15, no. 19: 1512. https://doi.org/10.3390/nano15191512
APA StyleBuonasera, K., Galletta, M., Calvo, M. R., Pezzotti Escobar, G., Leonardi, A. A., & Irrera, A. (2025). Organic Fluorescent Sensors for Environmental Analysis: A Critical Review and Insights into Inorganic Alternatives. Nanomaterials, 15(19), 1512. https://doi.org/10.3390/nano15191512







