Diazepam Photocatalytic Degradation in Laboratory- vs. Pilot-Scale Systems: Differences in Degradation Products and Reaction Kinetics
Abstract
1. Introduction
2. Materials and Methods
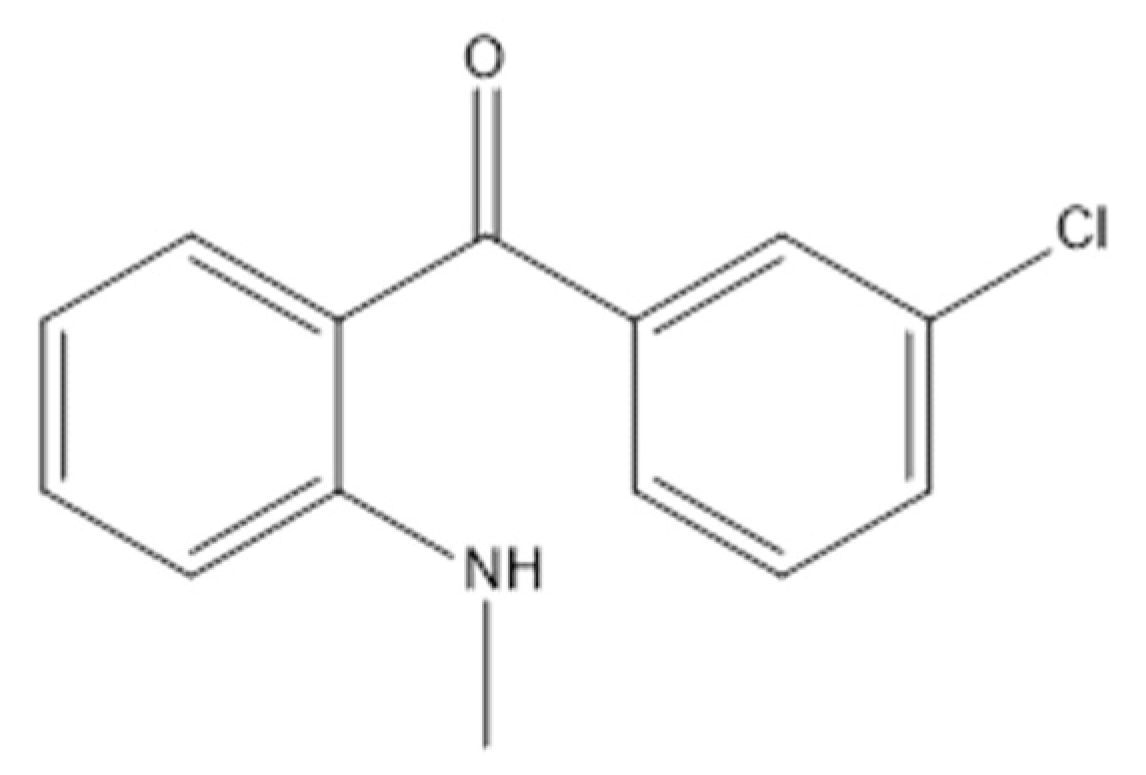
2.1. Materials and Reagents
2.2. Degradation Experiments
2.3. Analytical Procedure
2.4. Acute Toxicity Assessment
3. Results and Discussion
3.1. Photolysis of DIA
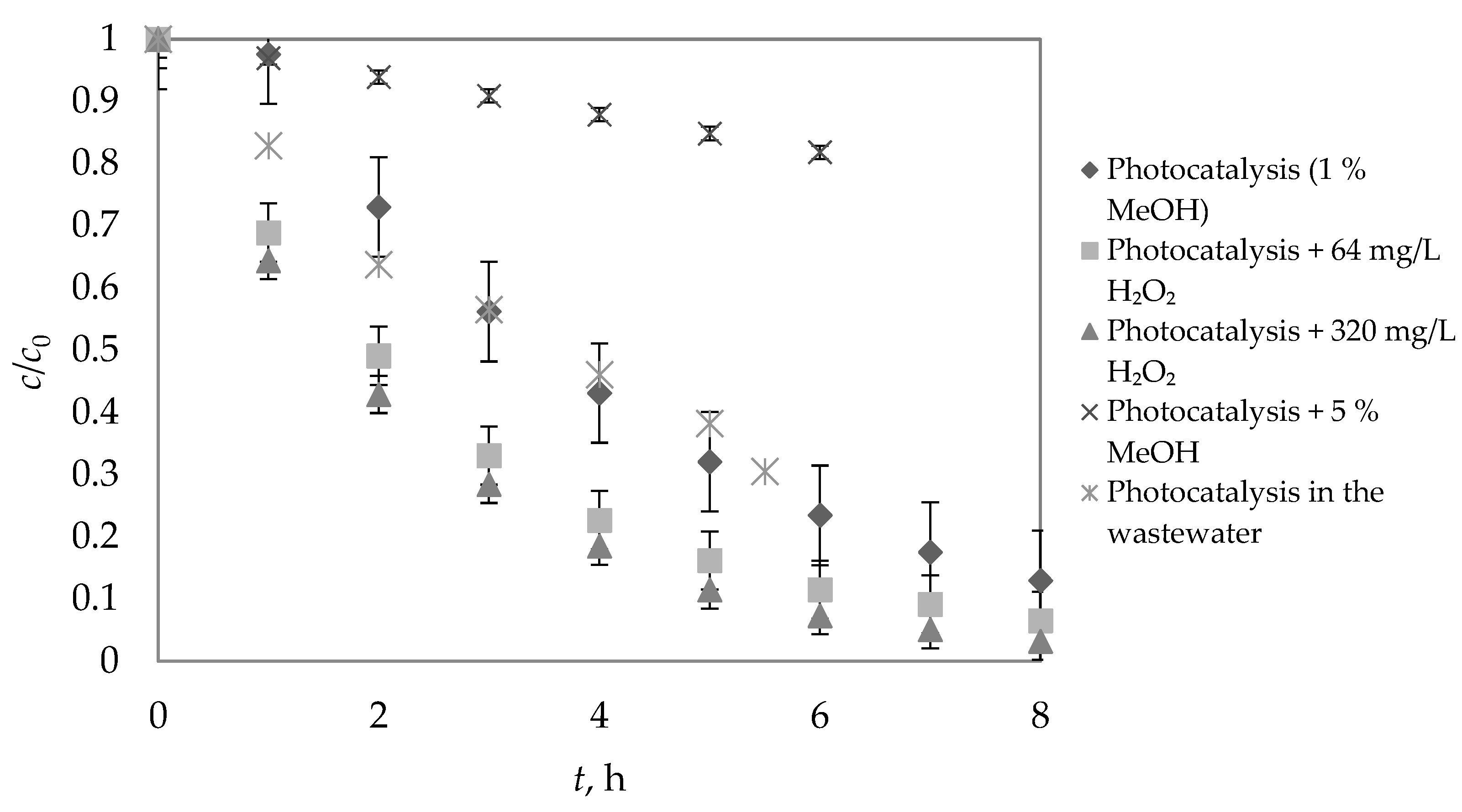
3.2. Photocatalytic Degradation of DIA at Laboratory Scale
3.3. Effect of Addition of H2O2
3.4. Effect of Methanol on Diazepam Photocatalysis
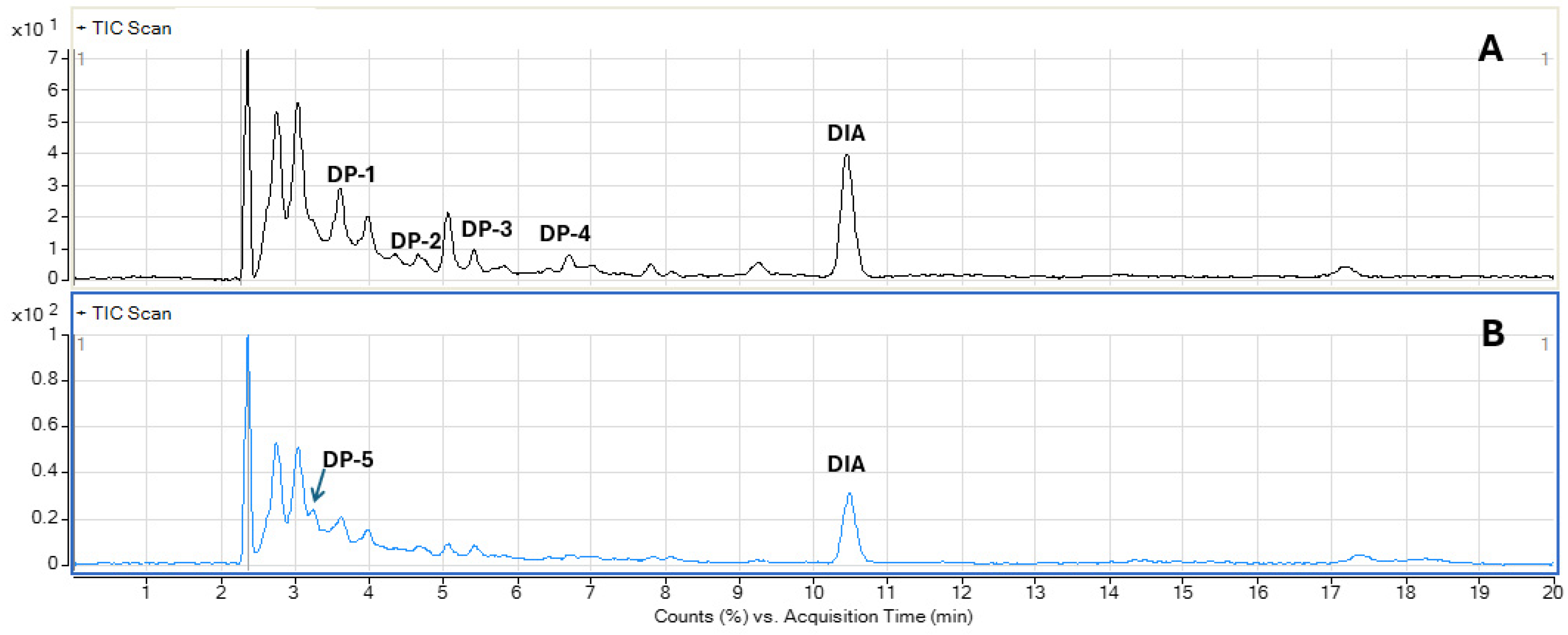
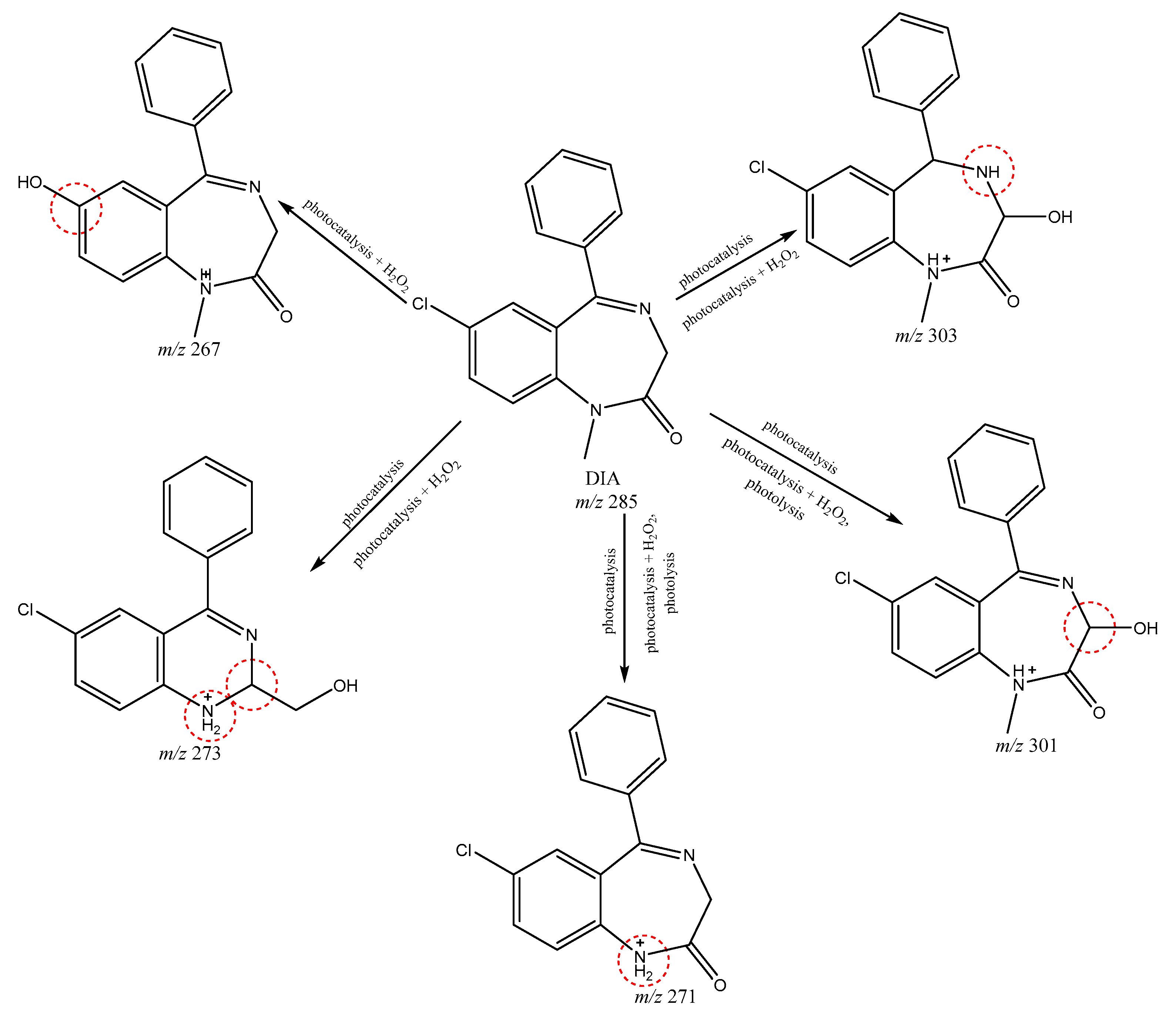
3.5. Identification of Photolytic and Photocatalytic Degradation Products of Diazepam
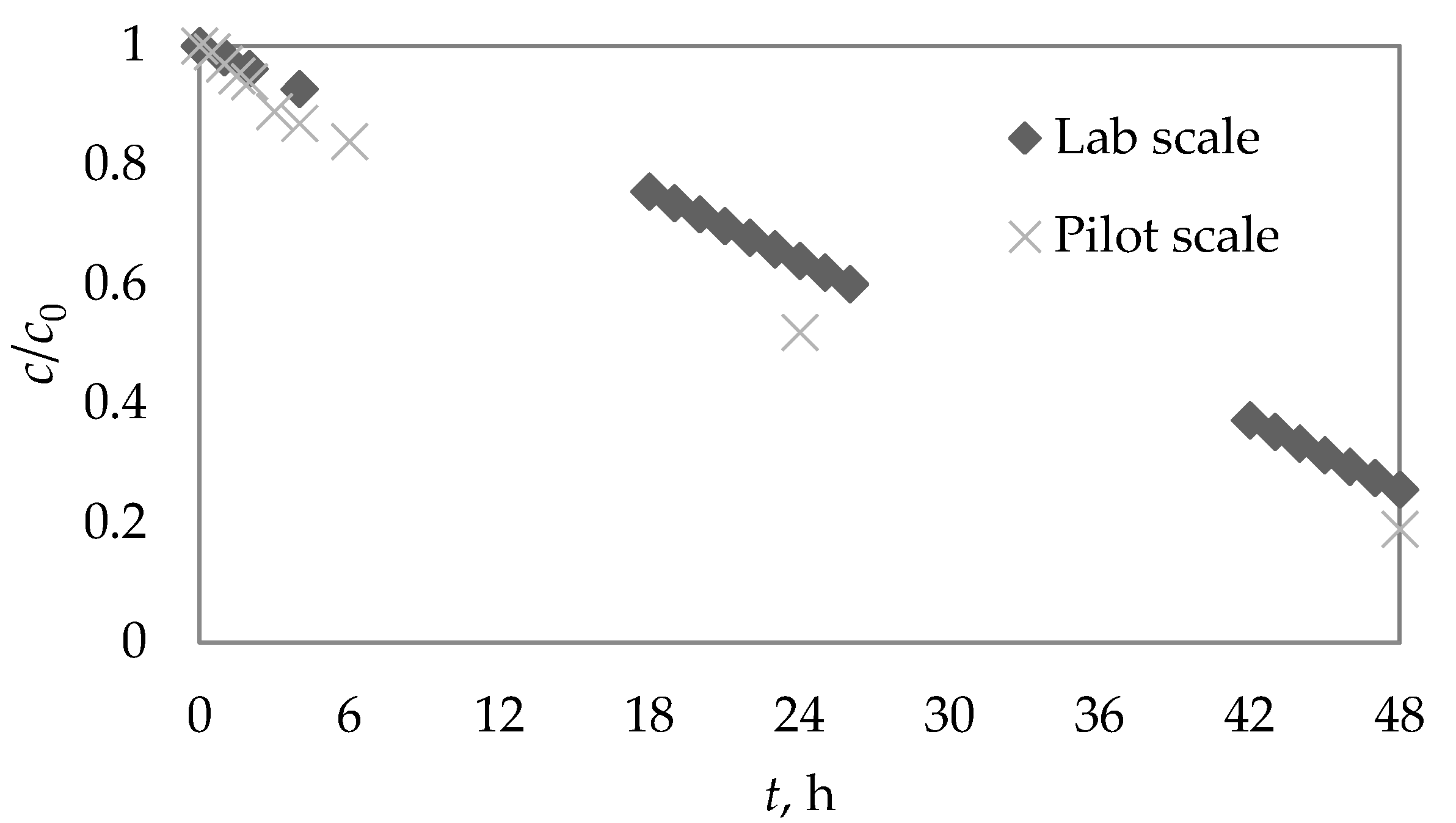
3.6. Photocatalytic Degradation of DIA at Pilot Scale
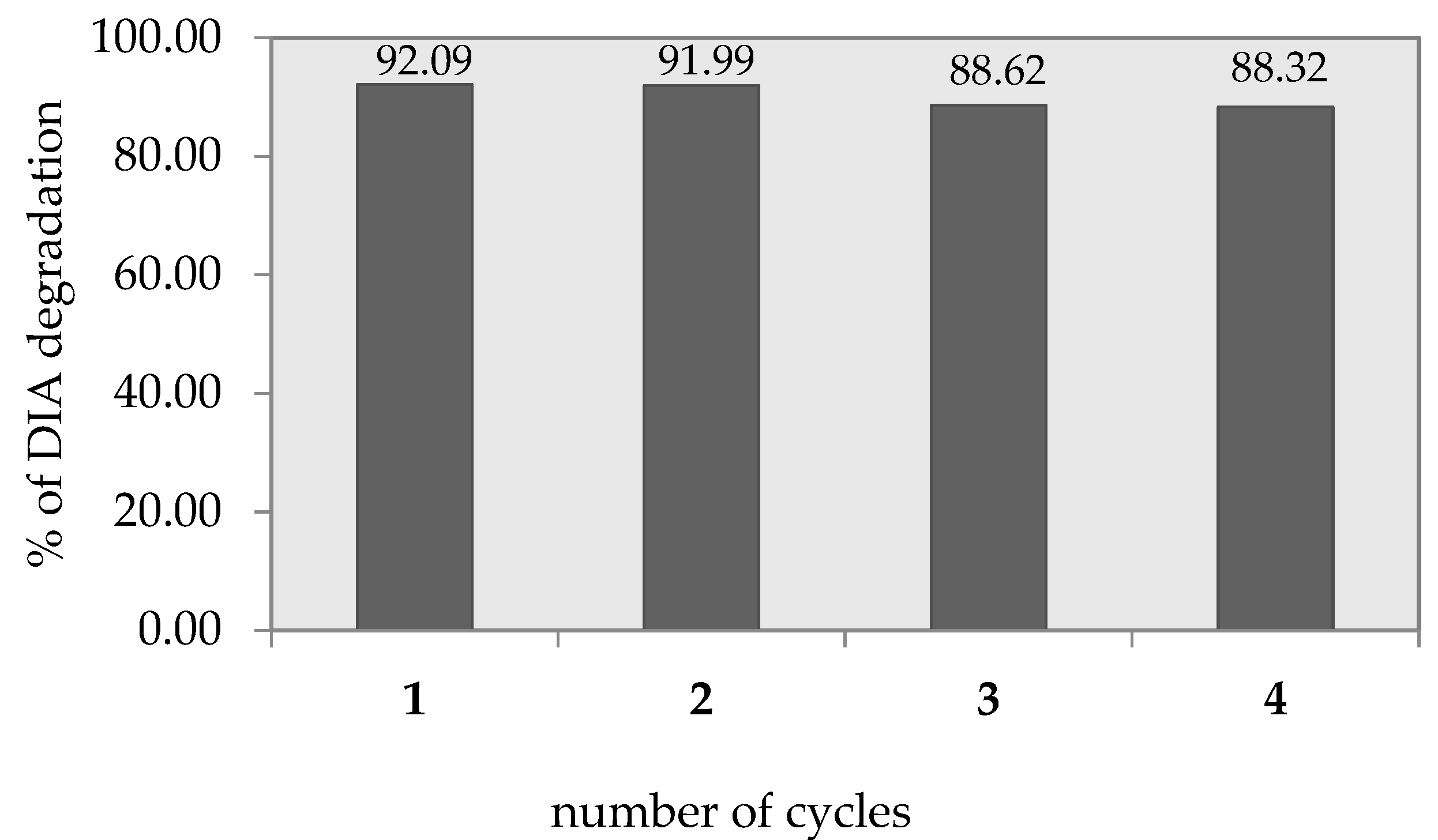
3.7. Reusability of the Photocatalyst
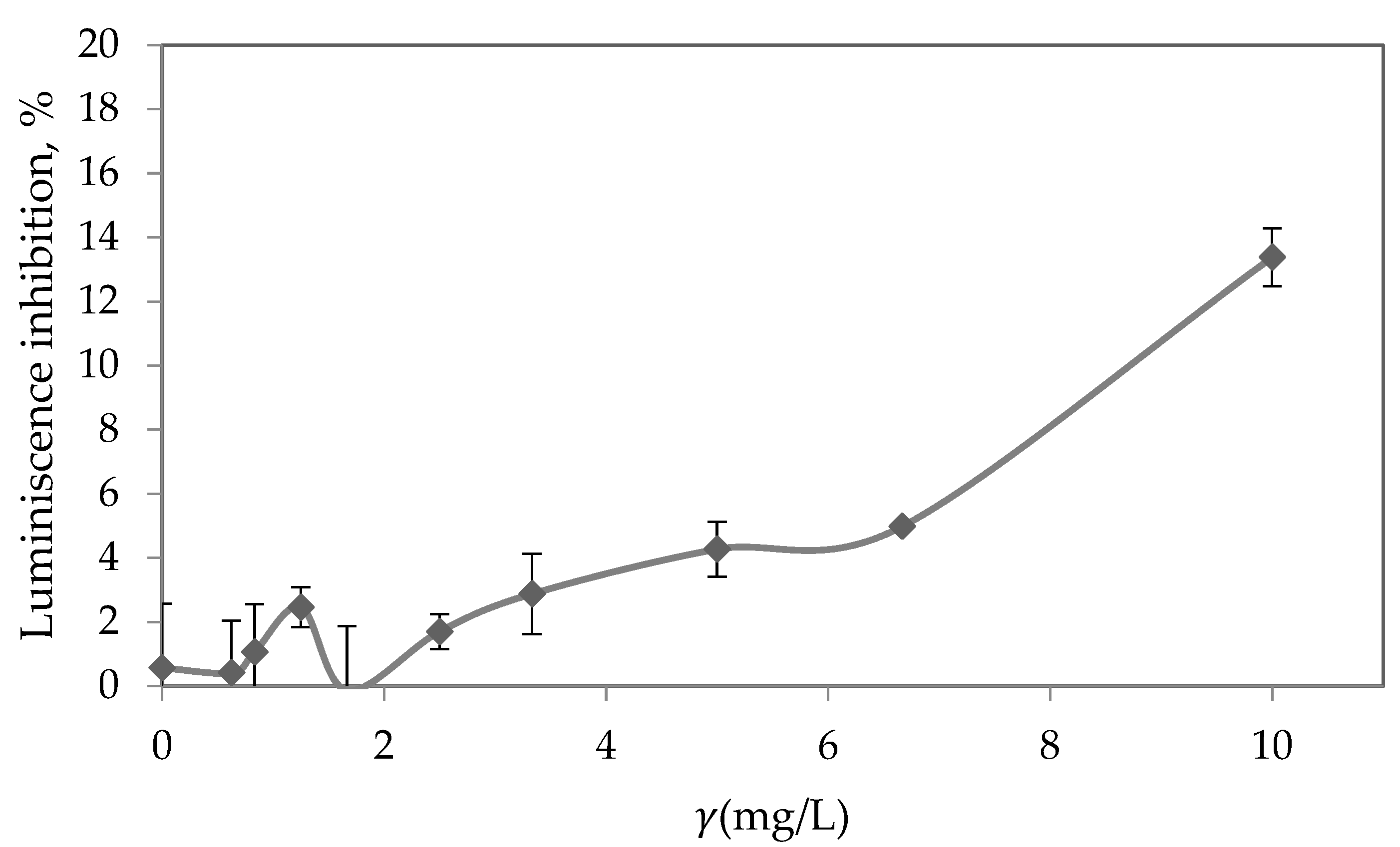
3.8. Toxicity Assessment
3.9. Comparison of Diazepam Removal with Other Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marks, J.L. Benzodiazepines-Withdrawal & Side Effects. 2015. Available online: https://www.everydayhealth.com/benzodiazepines/guide/ (accessed on 4 June 2024).
- Torres-Bondia, F.; de Batlle, J.; Galván, L.; Buti, M.; Barbé, F.; Piñol-Ripoll, G. Trends in the consumption rates of benzodiazepines and benzodiazepine related drugs in the health region of Lleida from 2002 to 2015. BMC Public Health 2020, 20, 818. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547871/ (accessed on 25 April 2023).
- Cabrera, C.G.; de Waisbaum, R.G.; Nudelman, N.S. Kinetic and mechanistic studies on the hydrolysis and photodegradation of diazepam and alprazolam. J. Phys. Org. Chem. 2005, 18, 156–161. [Google Scholar] [CrossRef]
- Trawiński, J.; Skibiński, R. Studies on photodegradation process of psychotropic drugs: A review. Environ. Sci. Pollut. Res. 2017, 24, 1152–1199. [Google Scholar] [CrossRef]
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the aquatic environment: A review on eco-toxicology and the remediation potential of algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef]
- West, E.; Rowland, S.J. Aqueous phototransformation of diazepam and related human metabolites under simulated sunlight. Environ. Sci. Technol. 2012, 46, 4749–4756. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiang, J.; Que, C.; Chen, F.; Xu, G. Occurrence and fate of psychiatric pharmaceuticals in the urban water system of Shanghai, China. Chemosphere 2015, 138, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Lamaczová, A.; Malina, T.; Maršálková, E.; Odehnalová, K.; Opatřilová, R.; Přibilová, P.; Zezulka, Š.; Maršálek, B. Anxiety in duckweed–metabolism and effect of diazepam on lemna minor. Water 2022, 14, 1484. [Google Scholar] [CrossRef]
- da Silva, A.Q.; Nilin, J.; Loureiro, S.; Costa-Lotufo, L.V. Acute and chronic toxicity of the benzodiazepine diazepam to the tropical crustacean Mysidopsis juniae. An. Acad. Bras. Cienc. 2020, 92, e20180595. [Google Scholar] [CrossRef]
- Bu, Q.; Shi, X.; Yu, G.; Huang, J.; Wang, B. Assessing the persistence of pharmaceuticals in the aquatic environment: Challenges and needs. Emerg. Contam. 2016, 2, 145–147. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abdallah, M.A.E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive review of emerging contaminants: Detection technologies, environmental impact, and management strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef] [PubMed]
- Shemer, H.; Wald, S.; Semiat, R. Challenges and solutions for global water scarcity. Membranes 2023, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment-A review. Environ. Res. 2022, 204 Pt C, 112298. [Google Scholar] [CrossRef]
- Marinho, A.; Suhadolnik, L.; Likozar, B.; Huš, M.; Marinko, Ž.; Čeh, M. Photocatalytic, electrocatalytic and photoelectrocatalytic degradation of pharmaceuticals in aqueous media: Analytical methods, mechanisms, simulations, catalysts and reactors. J. Clean. Prod. 2022, 343, 131061. [Google Scholar] [CrossRef]
- Papagiannaki, D.; Belay, M.H.; Gonçalves, N.P.F.; Robotti, E.; Bianco-Prevot, A.; Binetti, R.; Calza, P. From monitoring to treatment, how to improve water quality: The pharmaceuticals case. Chem. Eng. J. Adv. 2022, 10, 100245. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.Y. Removal of cytostatic drugs from aquatic environment: A review. Sci Total. Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpä, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Antonopoulou, M. Homogeneous and heterogeneous photocatalysis for the treatment of pharmaceutical industry wastewaters: A Review. Toxics 2022, 10, 539. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ángeles Ferro-García, M.; Prados-Joya, G.; Ocampo-Pére, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Achak, M.; Sillanp, M.; Barka, N. Comparative overview of advanced oxidation processes and biological approaches for the removal pharmaceuticals. J. Environ. Manag. 2021, 288, 112404. [Google Scholar] [CrossRef] [PubMed]
- Zaiha Arman, N.; Salmiati, S.; Aris, A.; Razman Salim, M.; Hassan Nazifa, T.; Suliza Muhamad, M.; Marpongahtun, M. A Review on emerging pollutants in the water environment: Existences, health effects and treatment processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Chong, M.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Yue, C.; He, L.; Li, H.; Zhang, H.; Yang, S.; Ma, T. Photocatalytic degradation by TiO2-conjugated/coordination polymer heterojunction: Preparation, mechanisms, and prospects. Appl. Catal. B Environ. 2024, 344, 12360. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-infrared light responsive TiO2 for efficient solar energy utilization. Adv. Funct. Mater. 2022, 32, 2108977. [Google Scholar] [CrossRef]
- Barakat, M.A.; Tseng, J.M.; Huang, C.P. Hydrogen peroxide-assisted photocatalytic oxidation of phenolic compounds. Appl. Catal. B Environ. 2005, 59, 99–104. [Google Scholar] [CrossRef]
- Kang, S.; Do, J.Y.; Jo, S.W.; Kim, K.M.; Jeong, K.M.; Park, S.-M.; Kang, M. Efficient removal of bisphenol A by an advanced photocatalytic oxidation-type UV/H2O2/Fe-loaded TiO2 System. Bull. Korean Chem. Soc. 2015, 36, 2006–2014. [Google Scholar] [CrossRef]
- Cabrera-Reina, A.; Salazar-González, R.; Marugán, J.; Sánchez Pérez, J.A.; Miralles-Cuevas, S. Comprehensive evaluation of a novel pilot-scale UVA-LED photoreactor for water treatment applications: Characterization, catalytic efficiency, energy performance and economic viability. J. Water Process. Eng. 2024, 63, 105483. [Google Scholar] [CrossRef]
- Mohadesi, M.; Sanavi Fard, M.; Shokri, A. The application of modified nano-TiO2 photocatalyst for wastewater treatment: A review. J. Environ. Anal. Chem. 2022, 104, 2571–2592. [Google Scholar] [CrossRef]
- Othman, S.H.; Abdul Rashid, S.; Mohd Ghazi, T.I.; Abdullah, N. Dispersion and stabilization of photocatalytic TiO2 nanoparticles in aqueous suspension for coatings applications. J. Nanomater. 2012, 2012, 718214. [Google Scholar] [CrossRef]
- Sousa, M.A.; Gonçalves, C.; Vilar, V.J.P.; Boaventura, R.A.R.; Alpendurada, M.F. Suspended TiO2-assisted photocatalytic degradation of emerging contaminants in a municipal WWTP effluent using a solar pilot plant with CPCs. Chem. Eng. J. 2012, 198–199, 301–309. [Google Scholar] [CrossRef]
- Chang, C.; Li, Z.; Rad, S.; Gan, L.; Dai, J.; Shahab, A. Preparation and application of nano TiO2 film coated recycled low-iron crushed glass in a novel packed-bed photocatalytic reactor for efficient removal of biodegradable contaminants. Environ. Technol. Innov. 2024, 33, 103541. [Google Scholar] [CrossRef]
- Rachel, A.; Subrahmanyam, M.; Boule, P. Comparison of photocatalytic efficiencies of TiO2 in suspended and immobilized form for the photocatalytic degradation of nitrobenzene sulfonic acids. Appl. Catal. B Environ. 2002, 37, 301–308. [Google Scholar] [CrossRef]
- Grčić, I.; Marčec, J.; Radetić, L.; Radovan, A.M.; Melnjak, I.; Jajčinović, I.; Brnardić, I. Ammonia and methane oxidation on TiO2 supported on glass fiber mesh under artificial solar irradiation. Environ. Sci. Pollut. Res. 2020, 28, 18354–18367. [Google Scholar] [CrossRef]
- Malinowski, S.; Presečki, I.; Jajčinović, I.; Brnardić, I.; Mandić, V.; Grčić, I. Intensification of dyhidroxybenzenes degradation over immobilized TiO2 based photocatalysts under simulated solar light. Appl. Sci. 2020, 10, 7571. [Google Scholar] [CrossRef]
- Tolić Čop, K.; Mutavdžić Pavlović, D.; Duić, K.; Pranjić, M.; Fereža, I.; Jajčinović, I.; Brnardić, I.; Špada, V. Sorption potential of different forms of TiO2 for the removal of two anticancer drugs from water. Appl. Sci. 2022, 12, 4113. [Google Scholar] [CrossRef]
- Jajčinović, I.; Brnardić, I.; Kožuh, S.; Tolić, K. The impact of multiwall carbon nanotubes on the photocatalytic properties of imobilizied TiO2. In Proceedings of the 18th International Foundrymen Conference, Sisak, Croatia, 15–17 May 2019. [Google Scholar]
- OECD. Guidelines for the Testing of Chemicals: Test No. 111: Hydrolysis as a 722 Function of pH 1–15; OECD: Washington, DC, USA, 2004. [Google Scholar]
- Grčić, I.; Radetić, L.; Miklec, K.; Presečki, I.; Leskovar, K.; Meaški, H.; Čizmić, M.; Brnardić, I. Solar photocatalysis application in UWWTP outlets-simulations based on predictive models in flat-plate reactors and pollutant degradation studies with in silico toxicity assessment. J. Hazard. Mater. 2024, 461, 132589. [Google Scholar] [CrossRef]
- Biošić, M.; Dabić, D.; Škorić, I.; Babić, S. Effects of environmental factors on nitrofurantoin photolysis in water and its acute toxicity assessment. Environ. Sci. Process. Impacts 2021, 23, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K. Mechanism of alkaline hydrolysis of diazepam. J. Chin. Chem. Soc. 1998, 45, 277–284. [Google Scholar] [CrossRef]
- Sulaiman, S.; Khamis, M.; Nir, S.; Scrano, L.; Bufo, S.A.; Karaman, R. Diazepam stability in wastewater and removal by advanced membrane technology, activated carbon, and micelle–clay complex. Desalination Water Treat. 2014, 57, 3098–3106. [Google Scholar] [CrossRef]
- Paumo, H.K.; Dalhatou, S.; Katata-Seru, L.M.; Kamdem, B.P.; Tijani, J.O.; Vishwanathan, V.; Kane, A.; Bahadu, I. TiO2 assisted photocatalysts for degradation of emerging organic pollutants in water and wastewater. J. Mol. Liq. 2021, 331, 115458. [Google Scholar] [CrossRef]
- Rioja, N.; Zorita, S.; Peñas, F.J. Effect of water matrix on photocatalytic degradation and general kinetic modelling. Appl. Catal. B Environ. 2016, 180, 330–335. [Google Scholar] [CrossRef]
- Fattahi, A.; Jaciw-Zurakowsky, I.; Srikantha, N.; Bragg, L.; Liang, R.; Zhou, N.; Servos, M.; Arlos, M. Effect of background water matrices on pharmaceutical and personal care product removal by UV-LED/TiO2. Catalysts 2021, 11, 576. [Google Scholar] [CrossRef]
- Heredia Deba, S.A.; Wols, B.A.; Yntema, D.R.; Lammertink, R.G.H. Effects of the water matrix on the degradation of micropollutants by a photocatalytic ceramic membrane. Membranes 2022, 12, 1004. [Google Scholar] [CrossRef]
- Cunha, D.L.; Kuznetsov, A.; Achete, C.A.; Machado, A.E.D.H.; Marques, M. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. PeerJ 2018, 6, e4464. [Google Scholar] [CrossRef]
- da Silva, J.C.; Teodoro, J.A.; Afonso, R.J.; Aquino, S.F.; Augusti, R. Photolysis and photocatalysis of ibuprofen in aqueous medium: Characterization of by-products via liquid chromatography coupled to high-resolution mass spectrometry and assessment of their toxicities against Artemia salina. J. Mass Spectrom. 2014, 49, 145–153. [Google Scholar] [CrossRef]
- Petala, A.; Mantzavinos, D.; Frontistis, Z. Impact of water matrix on the photocatalytic removal of pharmaceuticals by visible light active materials. Curr. Opin. Green Sustain. Chem. 2021, 28, 100445. [Google Scholar] [CrossRef]
- Pesqueira, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T. A life cycle assessment of solar-based treatments (H2O2, TiO2 photocatalysis, circumneutral photo-Fenton) for the removal of organic micropollutants. Sci. Total Environ. 2021, 761, 143258. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic degradation of an organic pollutant by zinc oxide–solar process. Arab. J. Chem. 2016, 9, S1858–S1868. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Ueda, Y.; Soramoto, A.; Hinokuma, S.; Hirai, T. Photocatalytic hydrogen peroxide splitting on metal-free powders assisted by phosphoric acid as a stabilizer. Nat. Commun. 2020, 11, 3386. [Google Scholar] [CrossRef]
- Rosa, D.; Lattanzio, S.; Bavasso, I.; Di Palma, L. Investigation of the synergistic effect of hydrogen peroxide and ultrasound on the photocatalytic treatment under visible light of dyes wastewater. Chem. Eng. Sci. 2023, 282, 119290. [Google Scholar] [CrossRef]
- El-Morsi, T.; Budakowski, W.; Abd-El-Aziz, A.; Friesen, K. Photocatalytic degradation of 1,10-dichlorodecane in aqueous suspensions of TiO2: A reaction of adsorbed chlorinated alkane with surface hydroxyl radicals. Environ. Sci. Technol. 2000, 34, 1018–1022. [Google Scholar] [CrossRef]
- Puga, F.; Navío, J.A.; Hidalgo, M.C. A critical view about use of scavengers for reactive species in heterogeneous photocatalysis. Appl. Catal. A Gen. 2024, 685, 119879. [Google Scholar] [CrossRef]
- Xia, S.; Wang, T.; Ren, Z.; Yang, X.; Guo, Q.; Zhou, C. Adsorption structure-activity correlation in the photocatalytic chemistry of methanol and water on TiO2(110). Acc. Chem. Res. 2024, 57, 3407–3418. [Google Scholar] [CrossRef]
- Schneider, J.T.; Scheres Firak, D.; Rocha Ribeiro, R.; Peralta-Zamora, P. Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef]
- Secrétan, P.H.; Karoui, M.; Sadou-Yaye, H.; Levi, Y.; Tortolano, L.; Solgadi, A.; Yagoubi, N.; Do, B. Imatinib: Major photocatalytic degradation pathways in aqueous media and the relative toxicity of its transformation products. Sci. Total Environ. 2019, 655, 547–556. [Google Scholar] [CrossRef]
- Sulaiman, S. Diazepam TiO2 photodegradation along with metabolites obtained from the kinetic study in sludge. J. Water Environ. Technol. 2017, 15, 174–185. [Google Scholar] [CrossRef][Green Version]
- Jakimska-Nagórska, A.; Śliwka-Kaszyńska, M.; Nagórski, P.; Kot-Wasik, A.; Namieśnik, J. Environmental fate of two psychiatric drugs, diazepam and sertraline: Phototransformation and investigation of their photoproducts in natural waters. J. Chromatogr. Sep. Tech. 2014, 5, 253–264. [Google Scholar]
- Oztekin, R.; Sponza, D.T. Treatment of wastewaters from the olive mill industry by sonication. J. Chem. Technol. Biotechnol. 2012, 88, 212–225. [Google Scholar] [CrossRef]
- User’s Guide for T.E.S.T.; version 5.1; Toxicity Estimation Software Tool: A Program to Estimate Toxicity from Molecular Structure; U.S. EPA: Washington, DC, USA, 2020. Available online: https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test (accessed on 15 March 2025).
- Franke, G.; Studinger, G.S.; Berger, S.; Böhling, U.; Bruckmann, D.; Cohors-Fresenborg, U.; Jöhncke, U. The assessment of bioaccumulation. Chemosphere 1994, 29, 1501–1514. [Google Scholar] [CrossRef]
- Yang, B.; Peng, T.; Cai, W.; Ying, G. Transformation of diazepam in water during UV/chlorine and simulated sunlight/chlorine advanced oxidation processes. Sci. Total Environ. 2020, 746, 141332. [Google Scholar] [CrossRef] [PubMed]
- Bautitz, I.R.; Velosa, A.C.; Nogueira, R.F.P. Zero valent iron mediated degradation of the pharmaceutical diazepam. Chemosphere 2012, 88, 688–692. [Google Scholar] [CrossRef] [PubMed]
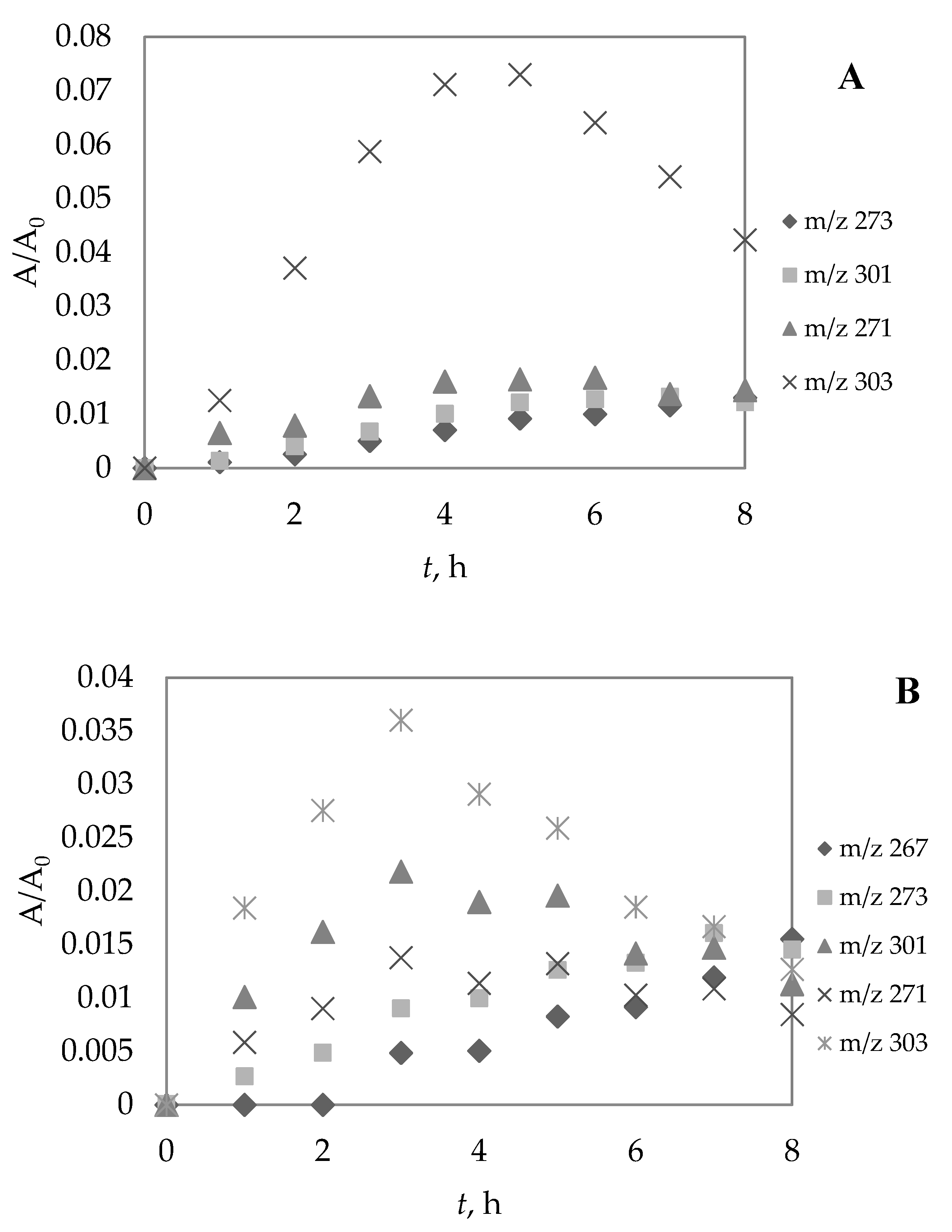
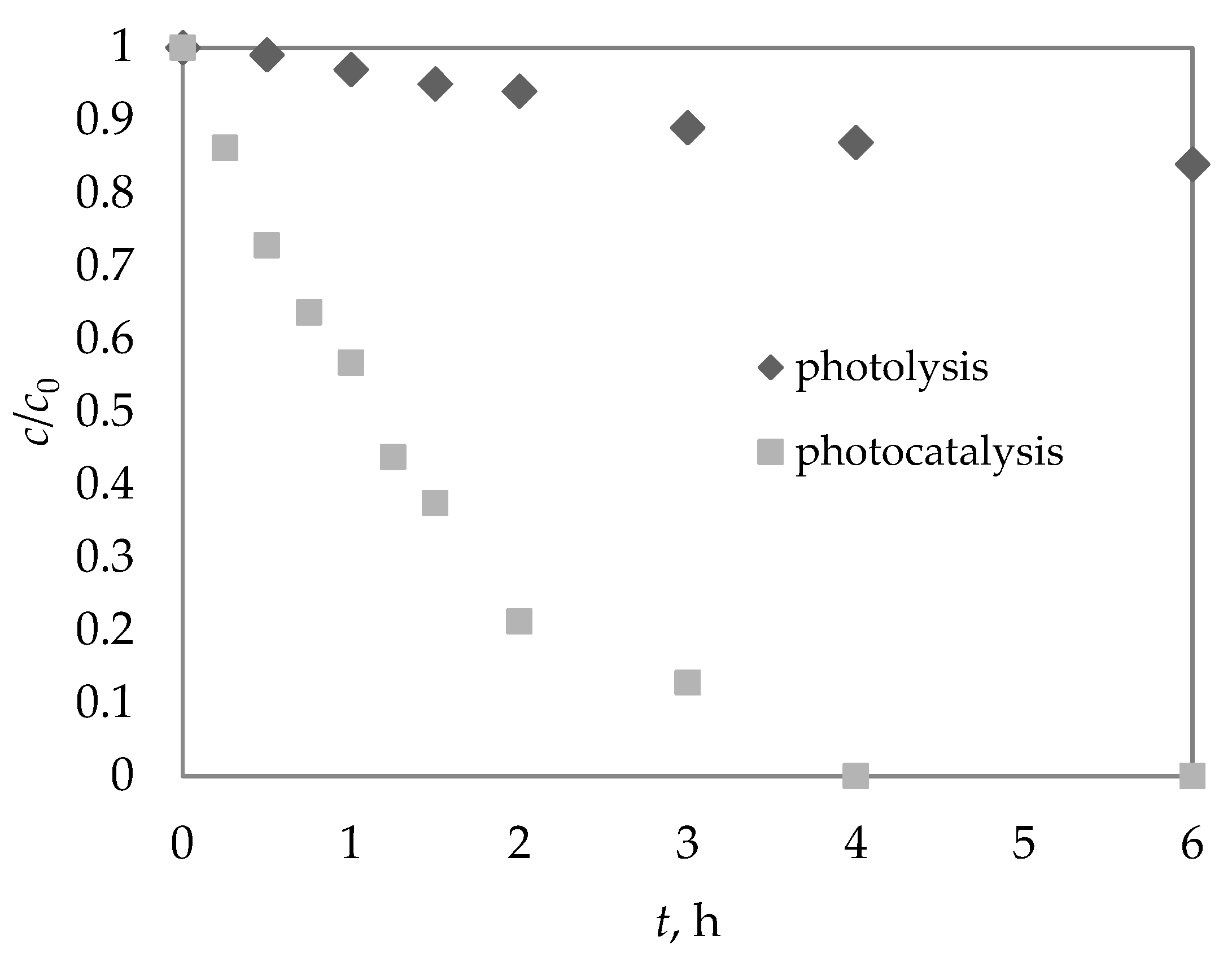
| t1/2, h | k, h−1 | R2 | |
|---|---|---|---|
| Photolysis | 25.11 | 0.0276 (±0.0026) | 0.9570 |
| Photocatalysis | 2.58 | 0.2691 (±0.12) | 0.9884 |
| Photocatalysis + 64 ppm H2O2 | 2.02 | 0.3433 (±0.047) | 0.9969 |
| Photocatalysis + 320 ppm H2O2 | 1.61 | 0.4298 (±0.030) | 0.9996 |
| Photocatalysis in wastewater | 3.40 | 0.2037 | 0.9650 |
| Photocatalysis + 5% MeOH | 20.75 | 0.0334 (±0.011) | 0.9992 |
| Pilot-scale photocatalysis | 1.01 | 0.6888 | 0.9908 |
| Laboratory Scale Photocatalysis | Pilot-Scale Photocatalysis in FPCR | |
|---|---|---|
| μUVB, m−1 | 2.0 × 10 [36,41] | |
| μUVA, m−1 | 7.3 × 106 | |
| I0, UVA, Wm−2 | 7.5 | 15.4 * |
| I0, UVB, Wm−2 | 0 | 3.6 * |
| ki, h−1 W−0.5 m1.5 | 3.65 × 10−5 | 3.61 × 10−5 |
| Analyte | Developmental Toxicity | BAF Log 10 | Mutagenicity | Daphnia Magna LC50 (48 h), mol/L | Oral Rat LC50 (48 h) mol/kg | Fathead Minnow LC50 (96 h) mol/L | Method |
|---|---|---|---|---|---|---|---|
| DIA | +(0.83) | 26.64 | −(0.00) | 4.87 | 2.21 | 5.77 | Consensus |
| m/z 267 | +(1.00) | n/a | n/a | 2.47 | 5.02 | 6.12 | Nearest neighbor |
| m/z 273 | +(1.00) | 87.84 | −(0.00) | 5.02 | 2.36 | 5.66 | Nearest neighbor |
| m/z 303 | +(1.00) | n/a | n/a | 4.21 | 2.10 | 7.01 | Nearest neighbor |
| m/z 301 | +(1.00) | n/a | n/a | 5.02 | 2.36 | 7.18 | Nearest neighbor |
| m/z 271 | +(1.00) | n/a | −(0.00) | 5.02 | 2.47 | 5.84 | Nearest neighbor |
| Removal Process | Media/Conditions | Removal | Reference |
|---|---|---|---|
| Photolysis | Milli-Q Water humic acids | 103 ± 13.20 h 28 ± 12.20 h | [7] |
| Advanced membrane technology | Wastewater; GAC effluent UF-SW effluent | 93.7% 90.4% | [44] |
| UV/chlorine simulated sunlight/chlorine | pH 7.0 phosphate buffer solutions | 90.1% 72.4% | [66] |
| Photo-Fenton | Distilled water Fe(NO3)3 | t1/2 11.55 min | [67] |
| Photolysis | Wastewater effluent–solar irradiation River water–xenon lamp radiation | t1/2 102.1 day 540.2 min | [62] |
| Photocatalysis | TiO2 MilliQ Pilot scale | t1/2 2.56 h 1.01 h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolić Čop, K.; Gotovuša, M.; Mutavdžić Pavlović, D.; Dabić, D.; Grčić, I. Diazepam Photocatalytic Degradation in Laboratory- vs. Pilot-Scale Systems: Differences in Degradation Products and Reaction Kinetics. Nanomaterials 2025, 15, 827. https://doi.org/10.3390/nano15110827
Tolić Čop K, Gotovuša M, Mutavdžić Pavlović D, Dabić D, Grčić I. Diazepam Photocatalytic Degradation in Laboratory- vs. Pilot-Scale Systems: Differences in Degradation Products and Reaction Kinetics. Nanomaterials. 2025; 15(11):827. https://doi.org/10.3390/nano15110827
Chicago/Turabian StyleTolić Čop, Kristina, Mia Gotovuša, Dragana Mutavdžić Pavlović, Dario Dabić, and Ivana Grčić. 2025. "Diazepam Photocatalytic Degradation in Laboratory- vs. Pilot-Scale Systems: Differences in Degradation Products and Reaction Kinetics" Nanomaterials 15, no. 11: 827. https://doi.org/10.3390/nano15110827
APA StyleTolić Čop, K., Gotovuša, M., Mutavdžić Pavlović, D., Dabić, D., & Grčić, I. (2025). Diazepam Photocatalytic Degradation in Laboratory- vs. Pilot-Scale Systems: Differences in Degradation Products and Reaction Kinetics. Nanomaterials, 15(11), 827. https://doi.org/10.3390/nano15110827









