Optimizing the Size of Zr-Based Metal–Organic Frameworks for Enhanced Anticancer Efficacy
Abstract
1. Introduction
2. Materials and Methods
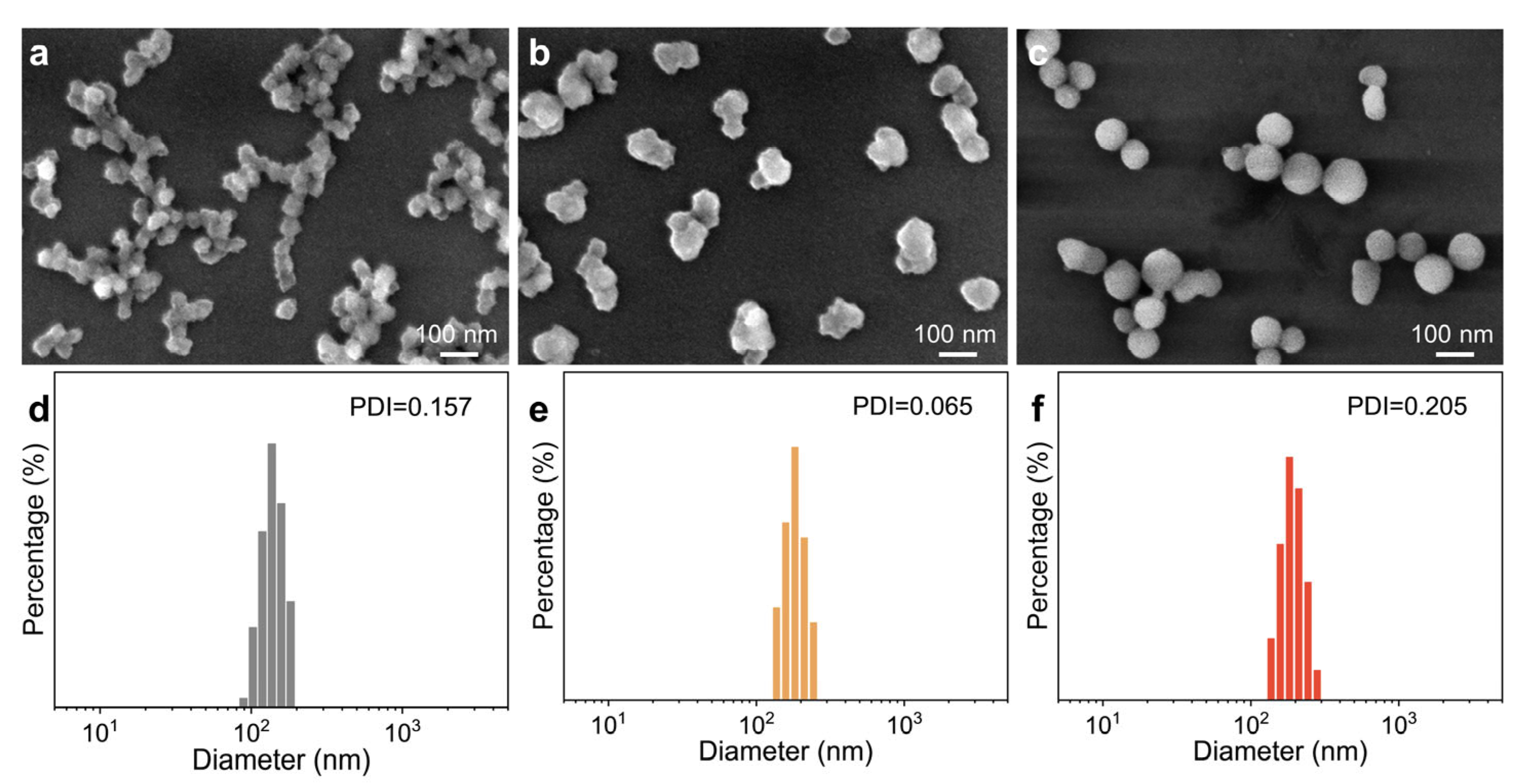
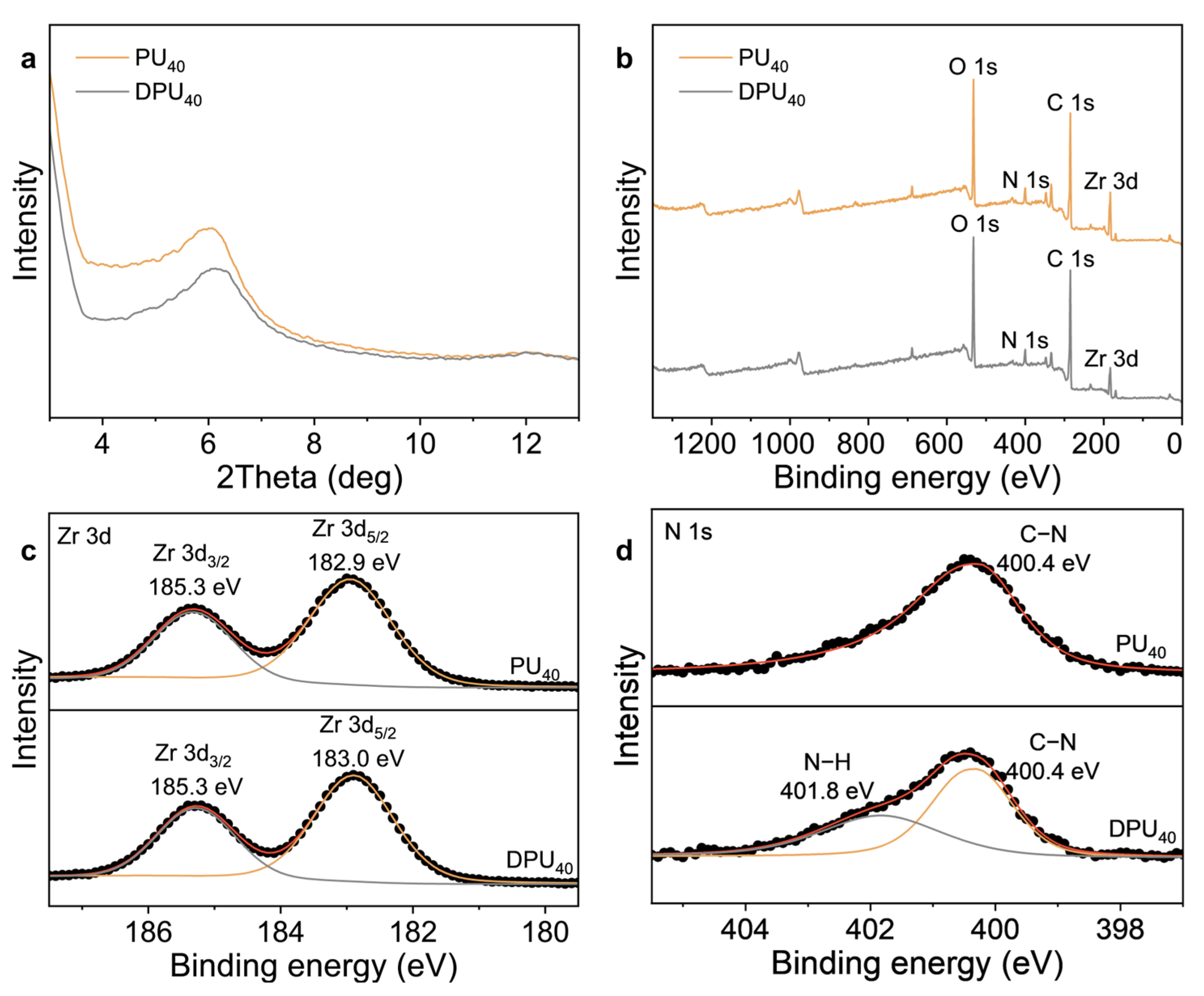
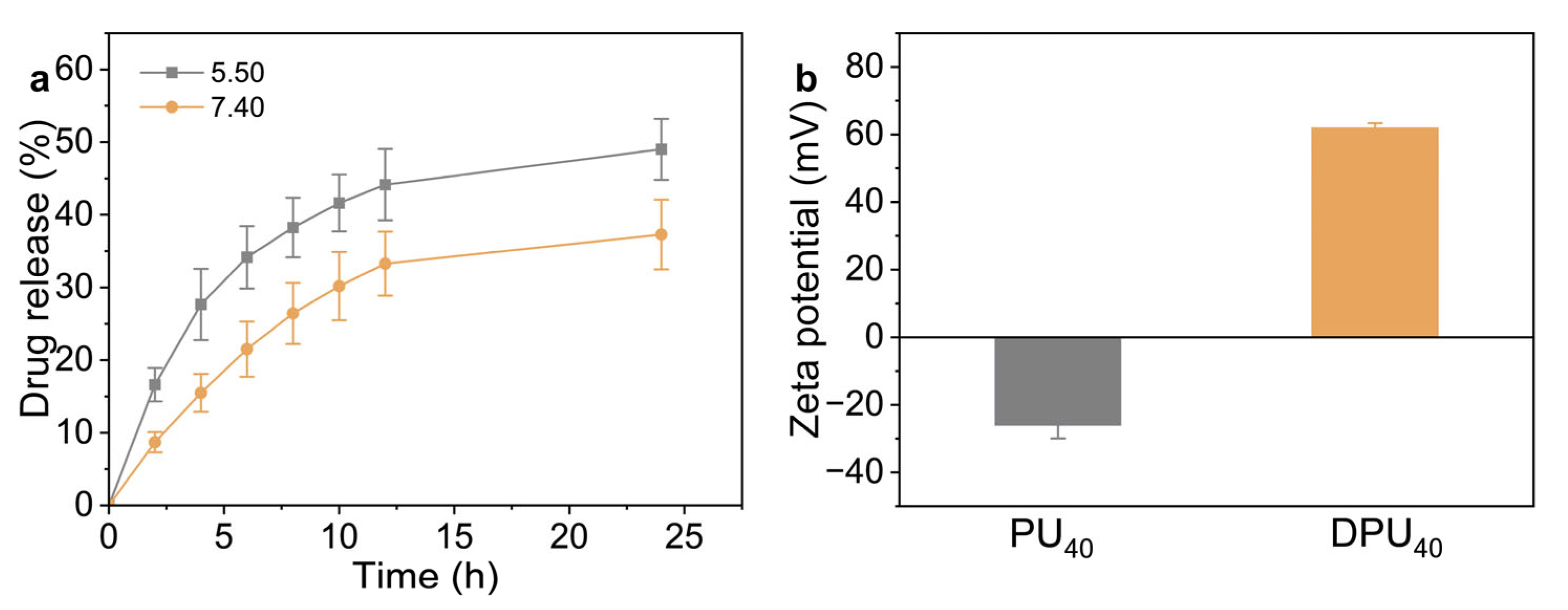
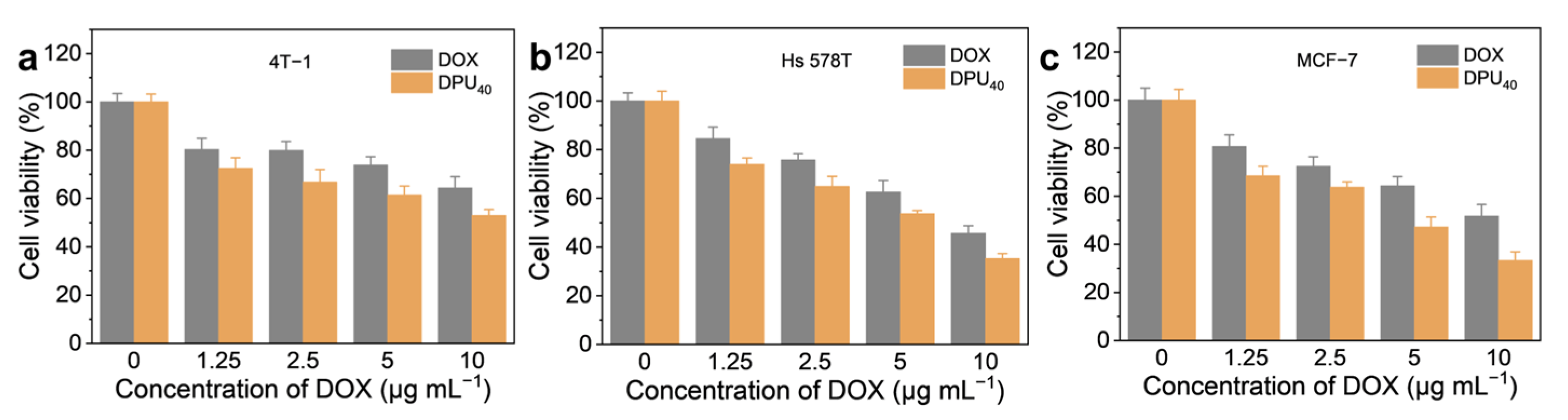
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Narvaez-Romero, A.M.; Rodriguez-Lozano, F.J.; Pecci-Lloret, M.P. Graphene-Based Materials for Bone Regeneration in Dentistry: A Systematic Review of In Vitro Applications and Material Comparisons. Nanomaterials 2025, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Saharan, R.; Paliwal, S.K.; Tiwari, A.; Tiwari, V.; Singh, R.; Beniwal, S.K.; Dahiya, P.; Sagadevan, S. Exploring graphene and its potential in delivery of drugs and biomolecules. J. Drug Deliv. Sci. Technol. 2023, 84, 104446. [Google Scholar] [CrossRef]
- Baumann, F.; Paul, T.; Wassersleben, S.; Regenthal, R.; Enke, D.; Aigner, A. Characterization of Drug Release from Mesoporous SiO2 -Based Membranes with Variable Pore Structure and Geometry. Pharmaceutics 2022, 14, 1184. [Google Scholar] [CrossRef]
- Shen, J.; He, Q.; Gao, Y.; Shi, J.; Li, Y. Mesoporous Silica Nanoparticles Loading Doxorubicin Reverse Multidrug Resistance: Performance And Mechanism. Nanoscale 2011, 3, 4314–4322. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Z.; Wang, H.; Chang, Y.; Xu, J.; Zhang, X. A Polymeric Nanoparticle to Co-Deliver Mitochondria-Targeting Peptides and Pt (IV) Prodrug: Toward High Loading Efficiency and Combination Efficacy. Angew. Chem. Int. Ed. Engl. 2024, 63, 8. [Google Scholar] [CrossRef]
- Liu, Q.; Zou, J.; Chen, Z.; He, W.; Wu, W. Current research trends of nanomedicines. Acta Pharm. Sin. B 2023, 13, 4391–4416. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Li, Z.; Zhang, F.; Chiarini, A.; Armato, U.; Wu, J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnol. 2022, 20, 395. [Google Scholar] [CrossRef]
- Forgan, R.S. The surface chemistry of metal-organic frameworks and their applications. Dalton Trans. 2019, 48, 9037–9042. [Google Scholar] [CrossRef]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Peng, Y.; Gao, Z.; Qiao, B.; Li, D.; Pang, H.; Lai, X.; Pu, Q.; Zhang, R.; Zhao, X.; Zhao, G.; et al. Size-Controlled DNA Tile Self-Assembly Nanostructures Through Caveolae-Mediated Endocytosis for Signal-Amplified Imaging of MicroRNAs in Living Cells. Adv. Sci. 2023, 10, 2300614. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key Principles and Methods for Studying the Endocytosis of Biological and Nanoparticle Therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Petithory, T.; Pieuchot, L.; Josien, L.; Ponche, A.; Anselme, K.; Vonna, L. Size-Dependent Internalization Efficiency of Macrophages from Adsorbed Nanoparticle-Based Monolayers. Nanomaterials 2021, 11, 1963. [Google Scholar] [CrossRef]
- Kraus, A.; Kratzer, B.; Sehgal, A.N.A.; Trapin, D.; Khan, M.; Boucheron, N.; Pickl, W. Macropinocytosis is the Principal Uptake Mechanism of Antigen-Presenting Cells for Allergen-Specific Virus-like Nanoparticles. Eur. J. Immunol. 2024, 54, 797. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Ganjali, F.; Mirmohammadi, S.S.; Qazi, F.S.; Saeidirad, M.; Kashtiaray, A.; Zarei-Shokat, S.; Tian, Y.; Maleki, A. Effects of morphology and size of nanoscale drug carriers on cellular uptake and internalization process: A review. RSC Adv. 2022, 13, 80–114. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.J.; Burgers, T.C.Q.; Vlijm, R.; Roos, W.H.; Aberg, C. Rapid Internalization of Nanoparticles by Human Cells at the Single Particle Level. ACS Nano 2023, 17, 16517–16529. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent in Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Lalinia, M.; Nemati, N.H.; Mofazali, P.; Gross, J.D.; Samadi, A. Recent advances in metal-organic framework capabilities with machine learning innovations for enhanced drug release systems. Mater. Today Chem. 2025, 45, 102640. [Google Scholar] [CrossRef]
- Khulood, M.T.; Jijith, U.S.; Naseef, P.P.; Kallungal, S.M.; Geetha, V.S.; Pramod, K. Advances in metal-organic framework-based drug delivery systems. Int. J. Pharm. 2025, 673, 125380. [Google Scholar] [CrossRef]
- Nabipour, H.; Rohani, S. Metal-Organic Frameworks for Overcoming the Blood-Brain Barrier in the Treatment of Brain Diseases: A Review. Nanomaterials 2024, 14, 1379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Yan, L.; You, Y.; Zhao, F.; Cheng, J.; Yang, L.; Sun, Y.; Chang, Q.; Liu, R.; et al. Zeolitic Imidazolate Framework-8 (ZIF-8) as a Drug Delivery Vehicle for the Transport and Release of Telomerase Inhibitor BIBR 1532. Nanomaterials 2023, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Zhang, Y.; Qin, G.; Wei, Y.; Yang, J.; Huang, Y.; Ren, J.; Qu, X. A Neutrophil Mimicking Metal-Porphyrin-based Nanodevice Loaded with Porcine Pancreatic Elastase for Cancer Therapy. Nat. Commun. 2023, 14, 1974. [Google Scholar] [CrossRef]
- Xu, M.; Chen, H.; Zhu, G.; Zhu, X.; Gao, R.; Xu, B.; Song, X.; Han, X.; Shao, T.; Sun, Q.; et al. Spiky metal-organic framework nanosystem for enhanced cuproptosis-mediated cancer immunotherapy. Nano Today 2024, 56, 102231. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, R.; Gao, W.; Li, F.; Yang, M.; Feng, J.; Ji, Y.; Si, J.; Wang, X.; Dong, Y. An environment-responsive platform based on acid-resistant metal organic framework for efficient oral insulin delivery. J. Control Release 2025, 377, 540–552. [Google Scholar] [CrossRef]
- Duman, F.D.; Monaco, A.; Foulkes, R.; Becer, C.R.; Forgan, R.S. Glycopolymer-Functionalized MOF-808 Nanoparticles as a Cancer-Targeted Dual Drug Delivery System for Carboplatin and Floxuridine. ACS Appl. Nano Mater. 2022, 12, 13862–13873. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Hu, C.; Liu, Y.; Pang, M.; Lin, J. Controllable Synthesis of Monodispersed NU-1000 Drug Carrier for Chemotherapy. ACS Appl. Bio Mater. 2019, 2, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Parsaei, M.; Akhbari, K. Magnetic UiO-66-NH 2 Core—Shell Nanohybrid as a Promising Carrier for Quercetin Targeted Delivery toward Human Breast Cancer Cells. ACS Omega 2023, 8, 41321–41338. [Google Scholar] [CrossRef]
- Chen, X.; Argandona, S.M.; Melle, F.; Rampal, N.; Fairen-Jimenez, D. Advances in surface functionalization of next-generation metal-organic frameworks for biomedical applications: Design, strategies, and prospects. Chem 2024, 10, 504–543. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Xia, L.; Cui, C.; Wan, S.; Jiang, Y.; Yang, Y.; Wu, Q.; Qiu, L.; Tan, W. ZrMOF nanoparticles as quenchers to conjugate DNA aptamers for target-induced bioimaging and photodynamic therapy. Chem. Sci. 2018, 9, 7505–7509. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, S.; Xiang, Y.; Xiong, Y.; Liu, G. UiO-67: A versatile metal-organic framework for diverse applications. Coord. Chem. Rev. 2025, 526, 216354. [Google Scholar] [CrossRef]
- Liu, W.C.; Pan, Y.; Zhong, Y.T.; Li, B.H.; Ding, Q.J.; XU, H.J.; Qu, Y.Z.; Ren, F.; Li, B.; Muddassir, M.; et al. A multifunctional aminated UiO-67 metal-organic framework for enhancing antitumor cytotoxicity through bimodal drug delivery. Chem. Eng. J. 2021, 412, 127899. [Google Scholar] [CrossRef]
- Yang, T.; Ahn, J.; Shi, S.; Qin, D. Understanding the Role of Poly (vinylpyrrolidone) in Stabilizing and Capping Colloidal Silver Nanocrystals. ACS Nano 2021, 15, 14242–14252. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Ma, J.; Zhao, X.; Li, Y.; Li, R. Polyvinylpyrrolidone (PVP) assisted in-situ construction of vertical metal-organic frameworks nanoplate arrays with enhanced electrochemical performance for hybrid supercapacitors. J. Colloid. Interface. Sci. 2021, 593, 32–40. [Google Scholar] [CrossRef]
- Du, J.; Gu, Z.; Yan, L.; Yong, Y.; Yi, X.; Zhang, X.; Liu, J.; Wu, R.; Ge, C.; Chen, C.; et al. Poly(Vinylpyrollidone)- and Selenocysteine-Modified Bi(2) Se(3) Nanoparticles Enhance Radiotherapy Efficacy in Tumors and Promote Radioprotection in Normal Tissues. Adv. Mater. 2017, 29, 1701268. [Google Scholar] [CrossRef]
- Kaur, G.; Oien-Odegaard, S.; Lazzarin, A.; Chavan, S.M.; Bordiga, S.; Lillerud, K.P.; Olsbye, U. Controlling the Synthesis of Metal-Organic Framework UiO-67 by Tuning Its Kinetic Driving Force. Cryst. Growth Des. 2019, 19, 4246–4251. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Zhang, R.; Lu, G. PVP-assisted synthesis of monodisperse UiO-66 crystals with tunable sizes. Inorg. Chem. Commun. 2017, 82, 68–71. [Google Scholar] [CrossRef]
- Mei, L.; Xu, K.; Zhai, Z.; He, S.; Zhu, T.; Zhong, W. Doxorubicin-reinforced supramolecular hydrogels of RGD-derived peptide conjugates for pH-responsive drug delivery. Org. Biomol. Chem. 2019, 17, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Wang, Y.; Guo, X.; Gou, K.; Li, J.; Li, S.; Zhao, L.; Li, H. Biomimetic Synthesis and Evaluation of Interconnected Bimodal Mesostructured MSF@Poly(Ethyleneimine)s for Improved Drug Loading and Oral Adsorption of the Poorly Water-Soluble Drug, Ibuprofen. Int. J. Nanomed. 2020, 15, 7451–7468. [Google Scholar] [CrossRef]
- Lei, B.; Wang, M.; Jiang, Z.; Qi, W.; Su, R.; He, Z. Constructing Redox-Responsive Metal-Organic Framework Nanocarriers for Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 16698–16706. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Z.; Zhao, H.; Wan, S.; Zhai, X.; Zhou, J.; Liang, R.; Deng, Q.; Wu, Y.; Lin, G. Simple and Rapid Monitoring of Doxorubicin Using Streptavidin-Modified Microparticle-Based Time-Resolved Fluorescence Immunoassay. RSC Adv. 2018, 8, 15621–15631. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Castillo, J.E.; Gallo-Villanueva, R.C.; Madou, M.J.; Perez-Gonzalez, V.H. Anisotropic gold nanoparticles: A survey of recent synthetic methodologies. Coord. Chem. Rev. 2020, 425, 213489. [Google Scholar] [CrossRef]
- Silva, F.E.; Salim, V.; Rodrigues, T. Controlled Nickel Nanoparticles: A Review on How Parameters of Synthesis Can Modulate Their Features and Properties. AppliedChem 2024, 4, 7. [Google Scholar] [CrossRef]
- Usman, K.A.S.; Maina, J.W.; Seyedin, S.; Conato, M.T.; Payawan, L.M., Jr.; Dumée, L.F.; Razal, J.M. Downsizing Metal–organic Frameworks by Bottom-Up and Top-Down Methods. NPG Asia Mater. 2020, 12, 58. [Google Scholar] [CrossRef]
- Wang, F.; Guo, H.; Chai, Y.; Li, Y.; Liu, C. The controlled regulation of morphology and size of HKUST-1 by “coordination modulation method”. Microporous Mesoporous Mat. 2013, 173, 181–188. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, Y.; Qiu, S.; Lercher, J.A.; Zhang, H. Coordination modulation induced synthesis of nanoscale Eu(1-x)Tb(x)-metal-organic frameworks for luminescent thin films. Adv. Mater. 2010, 22, 4190–4192. [Google Scholar] [CrossRef]
- Tsuruoka, T.; Furukawa, S.; Takashima, Y.; Yoshida, K.; Isoda, S.; Kitagawa, S. Nanoporous nanorods fabricated by coordination modulation and oriented attachment growth. Angew. Chem. Int. Ed. Engl. 2009, 48, 4739–4743. [Google Scholar] [CrossRef]
- Luczak, J.; Kroczewska, M.; Baluk, M.; Sowik, J.; Mazierski, P.; Zaleska-Medynska, A. Morphology Control Through the Synthesis of Metal-Organic Frameworks. Adv. Colloid. Interface. Sci. 2023, 314, 102864. [Google Scholar] [CrossRef]
- Song, J.; Cui, N.; Sun, S.; Lu, X.; Wang, Y.; Shi, H.; Lee, E.; Jiang, H. Controllability of Graphene Oxide Doxorubicin Loading Capacity Based on Density Functional Theory. Nanomaterials 2022, 12, 479. [Google Scholar] [CrossRef]
- Gu, Z.; Zhu, S.; Yan, L.; Zhao, F.; Zhao, Y. Graphene-Based Smart Platforms for Combined Cancer Therapy. Adv. Mater. 2019, 31, e1800662. [Google Scholar] [CrossRef]
- Dou, R.; Du, Z.; Bao, T.; Dong, X.; Zheng, X.; Yu, M.; Yin, W.; Dong, B.; Yan, L.; Gu, Z. The polyvinylpyrrolidone functionalized rGO/Bi2S3 nanocomposite as a near-infrared light-responsive nanovehicle for chemo-photothermal therapy of cancer. Nanoscale 2016, 8, 11531–11542. [Google Scholar] [CrossRef] [PubMed]
- Chudoba, D.; Ludzik, K.; Jazdzewska, M.; Woloszczuk, S. Kinetic and Equilibrium Studies of Doxorubicin Adsorption onto Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 8230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Meng, H.; Yan, L.; Wang, B.; Zhao, Y. Nanosurface chemistry and dose govern the bioaccumulation and toxicity of carbon nanotubes, metal nanomaterials and quantum dots in vivo. Sci. Bull. 2015, 60, 3–20. [Google Scholar] [CrossRef]
- Fatima, S.F.; Sabouni, R.; Husseini, G.; Paul, V.; Gomaa, H.; Radha, R. Microwave-Responsive Metal-Organic Frameworks (MOFs) for Enhanced In Vitro Controlled Release of Doxorubicin. Nanomaterials 2024, 14, 1081. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, N.; Liao, J.; Tian, G.; Mei, L.; Yang, G.; Wang, Q.; Yin, W. Ag-Activated Metal-Organic Framework with Peroxidase-like Activity Synergistic Ag(+) Release for Safe Bacterial Eradication and Wound Healing. Nanomaterials 2022, 12, 4058. [Google Scholar] [CrossRef]
- Sang, X.; Hu, X.; Tao, R.; Zhang, Y.; Zhu, H.; Wang, D. A Zirconium Indazole Carboxylate Coordination Polymer as an Efficient Catalyst for Dehydrogenation-Cyclization and Oxidative Coupling Reactions. ChemPlusChem 2020, 85, 123–129. [Google Scholar] [CrossRef]
- Lackner, P.; Zou, Z.; Mayr, S.; Diebold, U.; Schmid, M. Using photoelectron spectroscopy to observe oxygen spillover to zirconia. Phys. Chem. Chem. Phys. 2019, 21, 17613–17620. [Google Scholar] [CrossRef]
- Loiseau, A.; Boudon, J.; Oudot, A.; Moreau, M.; Boidot, R.; Chassagnon, R.; Said, N.M.; Roux, S.; Mirjolet, C.; Millot, N. Titanate Nanotubes Engineered with Gold Nanoparticles and Docetaxel to Enhance Radiotherapy on Xenografted Prostate Tumors. Cancers 2019, 11, 1962. [Google Scholar] [CrossRef]
- Racles, C.; Zaltariov, M.; Peptanariu, D.; Vasiliu, T.; Cazacu, M. Functionalized Mesoporous Silica as Doxorubicin Carriers and Cytotoxicity Boosters. Nanomaterials 2022, 12, 1823. [Google Scholar] [CrossRef]
- Ugur, E.; Tidim, G.; Gundogdu, D.; Alemdar, C.; Oral, G.; Husnugil, H.H.; Banerjee, S.; Erel-Goktepe, I. Effect of Periodate-Induced Cross-linking on Dual Anticancer Drug Release from Poly(2-isopropyl-2-oxazoline)/Tannic Acid-Based Layer-by-Layer Microparticles. ACS Omega 2024, 9, 39626–39642. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Yu, M.; Wan, Y.; Xiang, H.; Wei, H.; Zu, X.; Li, X.; Zhang, R.; Li, F.; Wang, S.; et al. Optimizing the Size of Zr-Based Metal–Organic Frameworks for Enhanced Anticancer Efficacy. Nanomaterials 2025, 15, 826. https://doi.org/10.3390/nano15110826
Cheng Z, Yu M, Wan Y, Xiang H, Wei H, Zu X, Li X, Zhang R, Li F, Wang S, et al. Optimizing the Size of Zr-Based Metal–Organic Frameworks for Enhanced Anticancer Efficacy. Nanomaterials. 2025; 15(11):826. https://doi.org/10.3390/nano15110826
Chicago/Turabian StyleCheng, Zan, Mei Yu, Yilong Wan, Huandong Xiang, Haoran Wei, Xu Zu, Xin Li, Ruiting Zhang, Fangshu Li, Shanshan Wang, and et al. 2025. "Optimizing the Size of Zr-Based Metal–Organic Frameworks for Enhanced Anticancer Efficacy" Nanomaterials 15, no. 11: 826. https://doi.org/10.3390/nano15110826
APA StyleCheng, Z., Yu, M., Wan, Y., Xiang, H., Wei, H., Zu, X., Li, X., Zhang, R., Li, F., Wang, S., & She, Y. (2025). Optimizing the Size of Zr-Based Metal–Organic Frameworks for Enhanced Anticancer Efficacy. Nanomaterials, 15(11), 826. https://doi.org/10.3390/nano15110826







