A Workflow for Assessing Particle Counts of Mixed Micro- and Nanoplastics in Exposed Laboratory Animals
Abstract
1. Introduction
2. Results
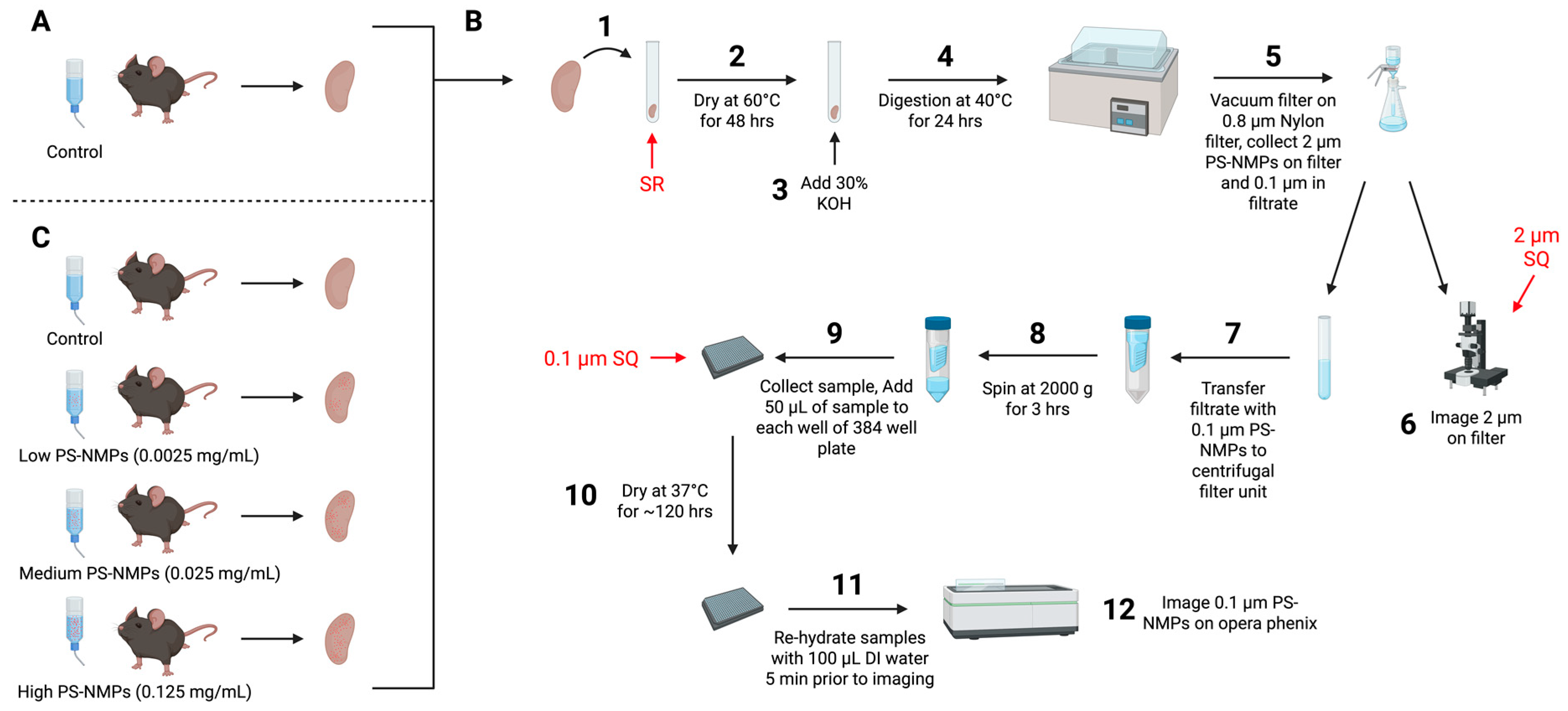
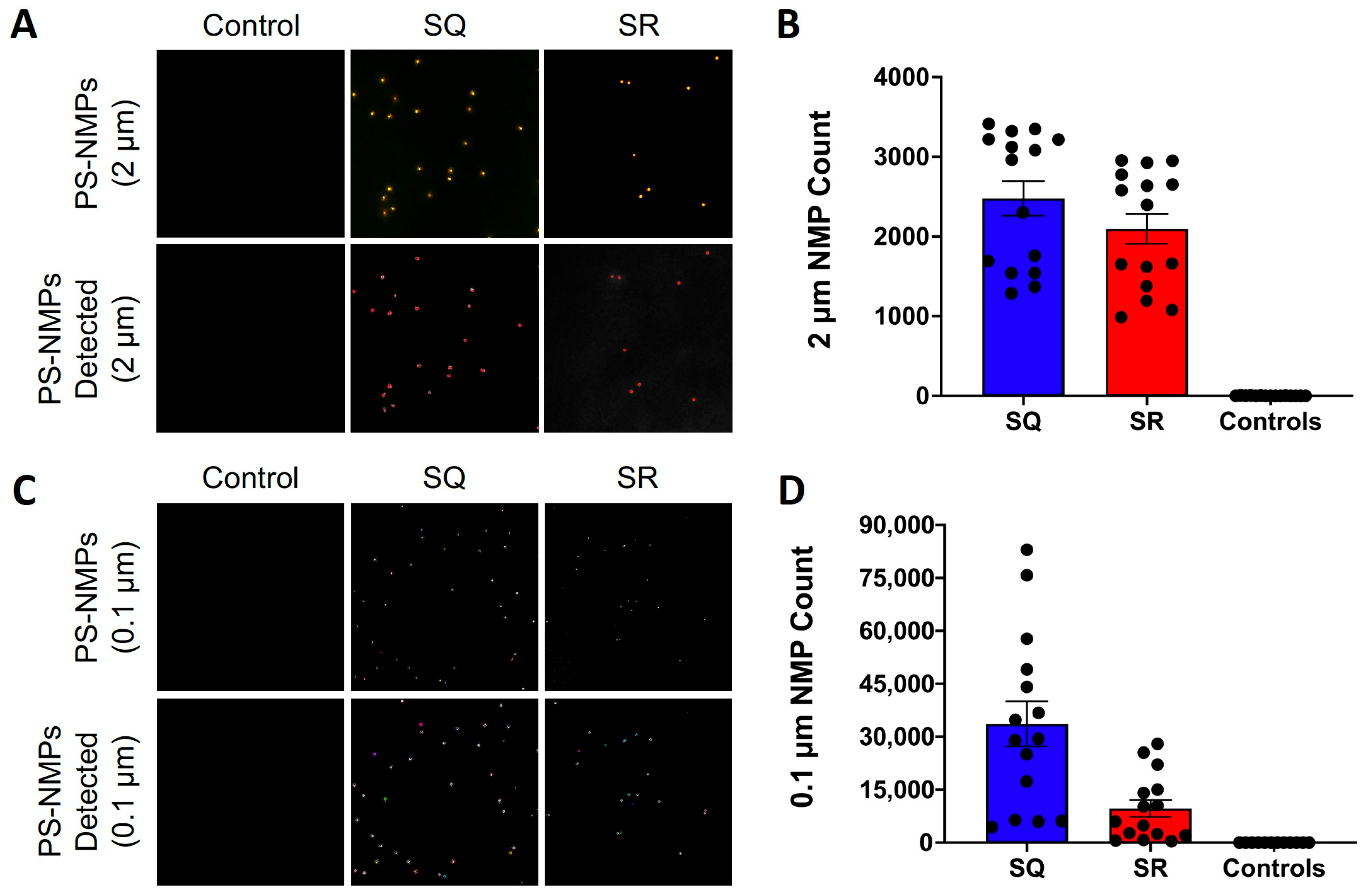
2.1. Recovery Rate Calculations
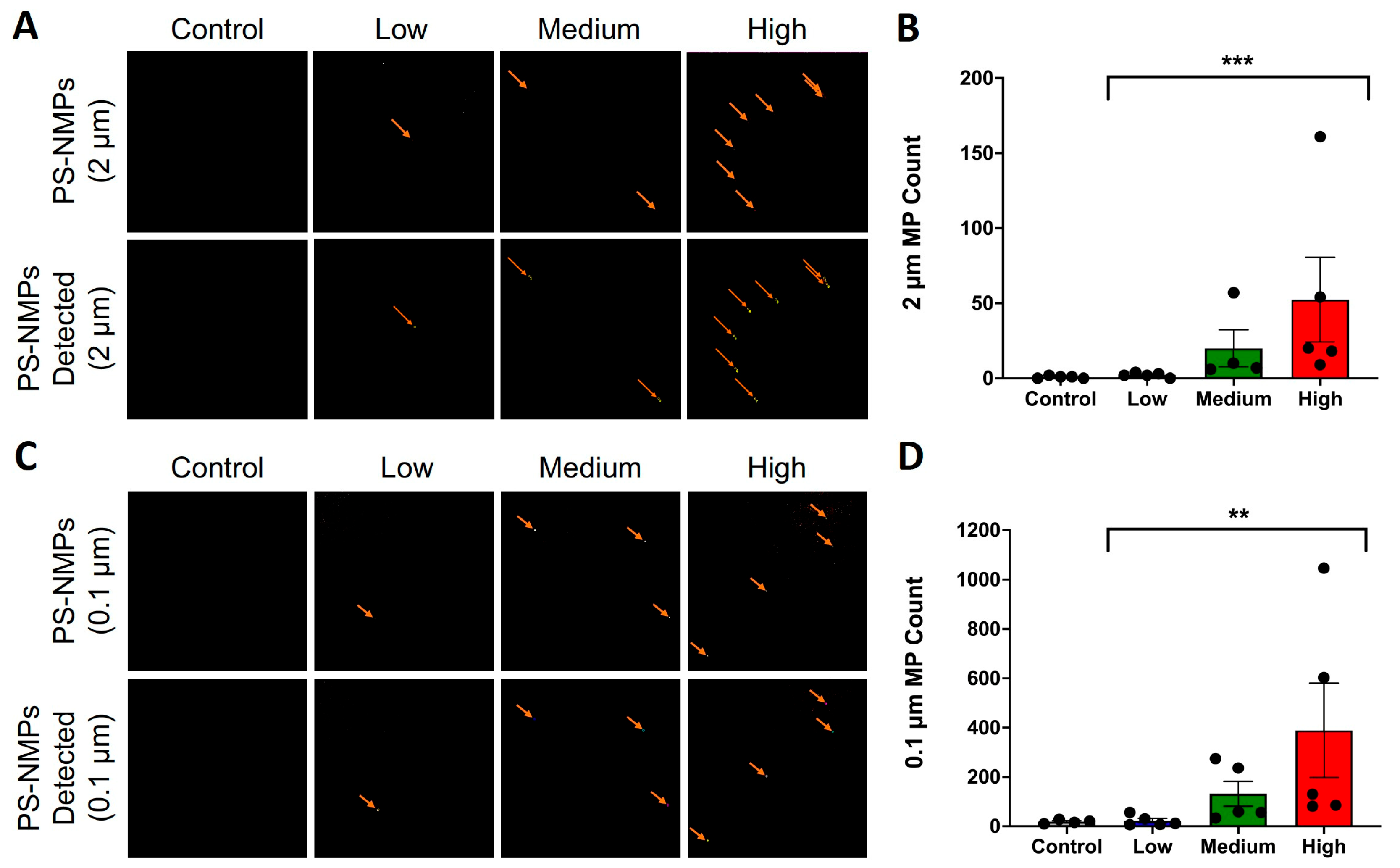
2.2. Proof-of-Concept Study
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. PS-NMPs Particle Characterization
5.2. Experimental Design
5.3. Recovery Rate Calculations
5.4. Proof-of-Concept Study
5.5. NMP Contamination Control
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| DI | Deionized |
| FTIR | Fourier Transform Infrared Spectroscopy |
| KOH | Potassium Hydroxide |
| NMPs | Nano- and Microplastics |
| PS-NMPs | Polystyrene Nano- and Microplastics |
| Py-GC/MS | Pyrolysis–Gas Chromatography/Mass Spectrometry |
| SEM | Scanning Electron Microscopy |
| SQ | Spike Quantification |
| SR | Spike Recovery |
References
- Stegmann, P.; Daioglou, V.; Londo, M.; van Vuuren, D.P.; Junginger, M. Plastic futures and their CO2 emissions. Nature 2022, 612, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Pilapitiya, P.G.C.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Andrady, A.L. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Gilliland, D. Definition of Micro and Nanoplastics & Analytical Challenges. Available online: https://www.efsa.europa.eu/sites/default/files/2021-05/4.definition-micro-nanoplastics-gilliland.pdf (accessed on 13 May 2025).
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef]
- Meaza, I.; Toyoda, J.H.; Wise Sr, J.P. Microplastics in Sea Turtles, Marine Mammals and Humans: A One Environmental Health Perspective. Front. Environ. Sci. 2021, 8, 575614. [Google Scholar] [CrossRef]
- Carlin, J.; Craig, C.; Little, S.; Donnelly, M.; Fox, D.; Zhai, L.; Walters, L. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. Pollut. 2020, 264, 114633. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Bi, R.; Guo, Q.; Yu, X.; Zeng, Q.; Huang, Z.; Liu, T.; Wu, H.; Chen, Y.; et al. Detection and Analysis of Microplastics in Human Sputum. Environ. Sci. Technol. 2022, 56, 2476–2486. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliviera, R.; Ribeiro Junior, G.; dos Santos Galvao, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Codrington, J.; Varnum, A.A.; Hildebrandt, L.; Profrock, D.; Bidhan, J.; Khodamoradi, K.; Hohme, A.L.; Held, M.; Evans, A.; Velasquez, D.; et al. Detection of microplastics in the human penis. Int. J. Impot. Res. 2025, 37, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.; Min, H. Identification and Characterization of Microplastics in Human Cervicovaginal Lavage Fluids Using Raman Spectroscopy: A Preliminary Study. Life 2025, 15, 357. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, H.; Wang, J.; Wu, B.; Guo, X. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance. Sci. Total Environ. 2022, 833, 155198. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, Y.; Liu, L.; Zhang, Q.; Xu, S.; Guo, M. Polystyrene microplastics induced inflammation with activating the TLR2 signal by excessive accumulation of ROS in hepatopancreas of carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2023, 251, 114539. [Google Scholar] [CrossRef]
- Medriano, C.A.; Bae, S. Acute exposure to microplastics induces metabolic disturbances and gut dysbiosis in adult zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 245, 114125. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Hong, Y.; Wu, S.; Wei, G. Adverse effects of microplastics and nanoplastics on the reproductive system: A comprehensive review of fertility and potential harmful interactions. Sci. Total Environ. 2023, 903, 166258. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhao, Y.; Yu, T.; Yao, Y.; Zhao, R.; Yu, R.; Liu, J.; Su, J. Reproductive toxicity of microplastics in female mice and their offspring from induction of oxidative stress. Environ. Pollut. 2023, 327, 121482. [Google Scholar] [CrossRef]
- Ripken, C.; Kotsifaki, D.G.; Chormaic, S.N. Analysis of small microplastics in coastal surface water samples of the subtropical island of Okinawa, Japan. Sci. Total Environ. 2021, 760, 143927. [Google Scholar] [CrossRef]
- Shen, M.; Zeng, Z.; Wen, X.; Ren, X.; Zeng, G.; Zhang, Y.; Xiao, R. Presence of microplastics in drinking water from freshwater sources: The investigation in Changsha, China. Environ. Sci. Pollut. Res. 2021, 28, 42313–42324. [Google Scholar] [CrossRef]
- Johnson, A.C.; Ball, H.; Cross, R.; Horton, A.A.; Jurgens, M.D.; Read, D.S.; Vollertsen, J.; Svendsen, C. Identification and Quantification of Microplastics in Potable Water and Their Sources within Water Treatment Works in England and Wales. Environ. Sci. Technol. 2020, 54, 12326–12334. [Google Scholar] [CrossRef] [PubMed]
- Fakour, H.; Lo, S.L.; Yoashi, N.T.; Massao, A.M.; Lema, N.N.; Mkhontfo, F.B.; Jomalema, P.C.; Jumanne, N.S.; Mbuya, B.H.; Mtweve, J.T.; et al. Quantification and Analysis of Microplastics in Farmland Soils: Characterization, Sources, and Pathways. Agriculture 2021, 11, 330. [Google Scholar] [CrossRef]
- Rafique, A.; Irfan, M.; Mumtaz, M.; Qadir, A. Spatial distribution of microplastics in soil with context to human activities: A case study from the urban center. Environ. Monit. Assess. 2020, 192, 671. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Luo, Y.; Gibson, C.T.; Tang, Y.; Naidu, R.; Megharaj, M.; Fang, C. Collecting Microplastics in Gardens: Case Study (i) of Soil. Front. Environ. Sci. 2021, 9, 739775. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Ullah, R.; Tsui, M.T.K.; Chen, H.; Chow, A.; Williams, C.; Ligaba-Osena, A. Microplastics interaction with terrestrial plants and their impacts on agriculture. J. Environ. Qual. 2021, 50, 1024–1041. [Google Scholar] [CrossRef]
- McIlwraith, H.K.; Kim, J.; Helm, P.; Bhavsar, S.P.; Metzger, J.S.; Rochman, C.M. Evidence of Microplastic Translocation in Wild-Caught Fish and Implications for Microplastic Accumulation Dynamics in Food Webs. Environ. Sci. Technol. 2021, 55, 12372–12382. [Google Scholar] [CrossRef]
- Bilal, M.; Yaqub, A.; Hassan, H.U.; Akhtar, S.; Rafiq, N.; Ali Shah, M.I.; Hussain, I.; Salman Khan, M.; Nawaz, A.; Manoharadas, S.; et al. Microplastic Quantification in Aquatic Birds: Biomonitoring the Environmental Health of the Panjkora River Freshwater Ecosystem in Pakistan. Toxics 2023, 11, 972. [Google Scholar] [CrossRef]
- Tarricone, S.; Colonna, M.A.; Freschi, P.; Cosentino, C.; La Gioia, G.; Carbonara, C.; Ragni, M. The Presence of Microplastics in the Gastrointestinal Tracts of Song Thrushes (Turdus philomelos) Wintering in Apulia (Southern Italy)—Preliminary Results. Animals 2024, 14, 2050. [Google Scholar] [CrossRef]
- Borriello, L.; Scivicco, M.; Cacciola, N.A.; Esposito, F.; Severino, L.; Cirillo, T. Microplastics, a Global Issue: Human Exposure through Environmental and Dietary Sources. Foods 2023, 12, 3396. [Google Scholar] [CrossRef]
- Espiritu, E.Q.; Pauco, J.L.R.; Bareo, R.S.; Palaypayon, G.B.; Capistrano, H.A.M.; Jabar, S.R.; Coronel, A.S.O.; Rodolfo, R.S.; Enriquez, E.P. Microplastics contamination in selected staple consumer food products. J. Food Sci. Technol. 2024, 61, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Saikumar, S.; Mani, R.; Ganesan, M.; Dhinakarasamy, I.; Palanisami, T.; Gopal, D. Trophic transfer and their impact of microplastics on estuarine food chain model. J. Hazard. Mater. 2024, 464, 132927. [Google Scholar] [CrossRef] [PubMed]
- Qualhato, G.; Cirquiera Dias, F.; Lopes Rocha, T. Hazardous effects of plastic microfibres from facial masks to aquatic animal health: Insights from zebrafish model. Sci. Total Environ. 2024, 951, 175555. [Google Scholar] [CrossRef]
- Kehinde, S.A.; Fatokun, T.P.; Olajide, A.T.; Praveena, S.M.; Sokan-Adeaga, A.A.; Adekunle, A.P.; Fouad, D.; Papadakis, M. Impact of polyethylene microplastics exposure on kallikrein-3 levels, steroidal-thyroidal hormones, and antioxidant status in murine model: Protective potentials of naringin. Sci. Rep. 2024, 14, 23664. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Li, D.; Yuan, X.; Wang, Z.; Shao, Z.; Feng, X.; Yang, C.; Liu, H.; Zhang, G.; Wang, Y.; et al. Potential Effects of Orally Ingesting Polyethylene Terephthalate Microplastics on the Mouse Heart. Cardiovasc. Toxicol. 2024, 24, 291–301. [Google Scholar] [CrossRef]
- Wang, X.; Bolan, N.; Tsang, D.C.W.; Sarkar, B.; Bradney, L.; Li, Y. A review of microplastics aggregation in aquatic environment: Influence factors, analytical methods, and environmental implications. J. Hazard. Mater. 2021, 402, 123496. [Google Scholar] [CrossRef]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Kumar Awasthi, M.; Kumar, S.; Singh, L.; Zhang, Z.; Bolan, N.; et al. Current research trends on micro- and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspotter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Cai, M.; He, H.; Liu, M.; Li, S.; Tang, G.; Wang, W.; Huang, P.; Wei, G.; Lin, Y.; Chen, B.; et al. Lost but can’t be neglected: Huge quantities of small microplastics hide in the South China Sea. Sci. Total Environ. 2018, 633, 1206–1216. [Google Scholar] [CrossRef]
- Yin, L.; Wen, X.; Du, C.; Jiang, J.; Wu, L.; Zhang, Y.; Hu, Z.; Hu, S.; Feng, Z.; Zhou, Z.; et al. Comparison of the abundance of microplastics between rural and urban areas: A case study from East Dongting Lake. Chemosphere 2020, 244, 125486. [Google Scholar] [CrossRef] [PubMed]
- Lindeque, P.K.; Cole, M.; Coppock, R.L.; Lewis, C.N.; Miller, R.Z.; Watts, A.J.R.; Wilson-McNeal, A.; Wright, S.L.; Galloway, T.S. Are we underestimating microplastic abundance in the marine environment? A comparison of microplastic capture with nets of different mesh-size. Environ. Pollut. 2020, 265, 114721. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef] [PubMed]
- Pikuda, O.; Roubeau Dumont, E.; Chen, Q.; Macairan, J.R.; Robinson, S.A.; Berk, D.; Tufenkji, N. Toxicity of microplastics and nanoplastics to Daphnia magna: Current status, knowledge gaps and future directions. TrAC Trends Anal. Chem. 2023, 167, 117208. [Google Scholar] [CrossRef]
- Gaspar, L.; Bartman, S.; Coppotelli, G.; Ross, J.M. Acute Exposure to Microplastics Induced Changes in Behavior and Inflammation in Young and Old Mice. Int. J. Mol. Sci. 2023, 24, 12308. [Google Scholar] [CrossRef]
- The C57BL 6 (Black 6) Mouse Strain|Lifespan.io. Available online: https://www.lifespan.io/topic/the-c57bl-6-black-6-mouse-strain/ (accessed on 2 September 2024).
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Zhang, W.; Shen, T.; Li, H.; Wu, J.; Zhang, L.; Qin, L.; Chen, R.; Gu, W.; et al. Lipidomics and transcriptomics insight into impacts of microplastics exposure on hepatic lipid metabolism in mice. Chemosphere 2022, 308, 136591. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Z.; Zhou, X.; Su, L.; Wang, M.; Wang, T.; Zhang, H. Nanoplastic-induced vascular endothelial injury and coagulation dysfunction in mice. Sci. Total Environ. 2023, 865, 161271. [Google Scholar] [CrossRef]
- Du, B.; Li, T.; He, H.; Xu, X.; Zhang, C.; Lu, X.; Wang, Y.; Cao, J.; Lu, Y.; Liu, Y.; et al. Analysis of Biodistribution and in vivo Toxicity of Varying Sized Polystyrene Micro and Nanoplastics in Mice. Int. J. Nanomed. 2024, 19, 7617–7630. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef]
- Zeng, L.; Zhou, C.; Xu, W.; Huang, Y.; Wang, W.; Ma, Z.; Huang, J.; Li, J.; Hu, L.; Xue, Y.; et al. The ovarian-related effects of polystyrene nanoplastics on human ovarian granulosa cells and female mice. Ecotoxicol. Environ. Saf. 2023, 257, 114941. [Google Scholar] [CrossRef] [PubMed]
- Maheswaran, B.; Karmegam, N.; Al-Ansari, M.; Subbaiya, R.; Al-Humaid, L.; Sebastin Raj, J.; Govarthanan, M. Assessment, characterization, and quantification of microplastics from river sediments. Chemosphere 2022, 298, 134268. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, D.; Pei, J.; Fei, Y.; Ouyang, D.; Zhang, H.; Luo, Y. Identification and quantification of microplastics using Fourier-transform infrared spectroscopy: Current status and future prospects. Curr. Opin. Environ. Sci. Health 2020, 18, 14–19. [Google Scholar] [CrossRef]
- Hiltunen, A.; Huopalainen, J.; Makila, E.; Hakkila, S.; Damlin, P.; Hanninen, J. Expanding sample volume for microscopical detection of nanoplastics. Mar. Environ. Res. 2024, 202, 106806. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Guan, B. Separation and identification of nanoplastics in tap water. Environ. Res. 2022, 204, 112134. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Genbo Xu, E.; Du, F.; Li, R.; Liu, J.; Shi, H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Matsui, K.; Ishimura, T.; Mattonai, M.; Iwai, I.; Watanabe, A.; Teramae, N.; Ohtani, H.; Watanabe, C. Identification algorithm for polymer mixtures based on Py-GC/MS and its application for microplastic analysis in environmental samples. J. Anal. Appl. Pyrolysis 2020, 149, 104834. [Google Scholar] [CrossRef]
- Pico, Y.; Barceló, D. Pyrolysis gas chromatography-mass spectrometry in environmental analysis: Focus on organic matter and microplastics. TrAC Trends Anal. Chem. 2020, 130, 115964. [Google Scholar] [CrossRef]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Maurizi, L.; Iordachescu, L.; Kirstein, I.V.; Nielsen, A.H.; Vollertsen, J. It matters how we measure-Quantification of microplastics in drinking water by μFTIR and μRaman. Heliyon 2023, 9, e20119. [Google Scholar] [CrossRef]
- Tsering, T.; Viitala, M.; Hyvönen, M.; Reinikainen, S.-P.; Mänttäri, M. The assessment of particle selection and blank correction to enhance the analysis of microplastics with Raman microspectroscopy. Sci. Total Environ. 2022, 842, 156804. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef] [PubMed]
- Bouadil, O.; Benomar, M.; El Ouarghi, H.; Abdelazize Aboulhassan, M.; Benbrahim, S. Identification and quantification of microplastics in surface water of a southwestern Mediterranean Bay (Al Hoceima, Morocco). Waste Manag. Bull. 2024, 2, 142–151. [Google Scholar] [CrossRef]
- Hansen, J.; Hildebrandt, L.; Zimmermann, T.; El Gareb, F.; Fischer, E.K.; Profrock, D. Quantification and characterization of microplastics in surface water samples from the Northeast Atlantic Ocean using laser direct infrared imaging. Mar. Pollut. Bull. 2023, 190, 114880. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.R.; Caraher, M.C.; Cummins, N.M.; O’Driscoll, O.M.; Eastell, R.; Ralston, S.H.; Towler, M.R. Raman spectral variation for human fingernails of postmenopausal women is dependent on fracture risk and osteoporosis status. J. Raman Spectrosc. 2017, 48, 813–821. [Google Scholar] [CrossRef]
- Yang, W.; Li, Y.; Wang, X.; Zheng, Y.; Li, D.; Zhao, X.; Yang, X.; Shan, J. One-stop quantification of microplastics and nanoparticles based on meniscus self-assembly technology. Sci. Total Environ. 2024, 949, 174946. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.G.; Wu, X.F.; Zhao, Z.G.; Yang, H.Y.; Sun, H.M. Identification and quantification of micro–nano-plastics in polypropylene-bottled injections. Heliyon 2024, 10, e35101. [Google Scholar] [CrossRef]
- Xie, D.; Fang, H.; Zhao, X.; Lin, Y.; Su, Z. Identification of microplastics and nanoplastics in environmental water by AFM-IR. Anal. Chim. Acta 2025, 1354, 343992. [Google Scholar] [CrossRef]
- Xie, J.; Gowen, A.; Xu, W.; Xu, J. Analysing micro- and nanoplastics with cutting-edge infrared spectroscopy techniques: A critical review. Anal. Methods 2024, 16, 2177–2197. [Google Scholar] [CrossRef]
- Kooi, M.; Primpke, S.; Mintenig, S.M.; Lorenz, C.; Gerdts, G.; Koelmans, A.A. Characterizing the multidimensionality of microplastics across environmental compartments. Water Res. 2021, 202, 117429. [Google Scholar] [CrossRef]
- Ahrendt, C.; Perez-Venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M.; Pulgar, J.; Galban-Malagon, C. Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar. Pollut. Bull. 2020, 151, 110795. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lai, Y.; Zheng, R.; Li, Q.; Sheng, X.; Yu, S.; Hao, Z.; Cai, Y.; Liu, J. Extraction of Common Small Microplastics and Nanoplastics Embedded in Environmental Solid Matrices by Tetramethylammonium Hydroxide Digestion and Dichloromethane Dissolution for Py-GC-MS Determination. Environ. Sci. Technol. 2023, 57, 12010–12018. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9. [Google Scholar] [CrossRef]
- Lekše, N.; Žgajnar Gotvajn, A.; Zupančič, M.; Griessler Bulc, T. Oil-based extraction as an efficient method for the quantification of microplastics in environmental samples. Environ. Sci. Eur. 2024, 36, 68. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Keong Choo, C.; Romano, N.; Bin Ho, Y.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Yan, P.; Hao, X.; Zhang, S. Extraction and decontamination of microplastics from high organic matter soils: A simple, cost-saving and high efficient method. J. Environ. Manag. 2023, 344, 118381. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Ronsmans, S.; Vanoirbeek, J.A.J.; Hoet, P.H.M.; Ghosh, M. Challenges in Raman spectroscopy of (micro)Plastics: The interfering role of colourants. Environ. Pollut. 2024, 363, 125250. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Shim, W.J. A comparison of spectroscopic analysis methods for microplastics: Manual, semi-automated, and automated Fourier transform infrared and Raman techniques. Mar. Pollut. Bull. 2021, 173, 113101. [Google Scholar] [CrossRef]
- Thiele, C.J.; Hudson, M.D.; Russell, A.E. Evaluation of existing methods to extract microplastics from bivalve tissue: Adapted KOH digestion protocol improves filtration at single-digit pore size. Mar. Pollut. Bull. 2019, 142, 384–393. [Google Scholar] [CrossRef]
- Geppner, L.; Karaca, J.; Wegner, W.; Rados, M.; Gutwald, T.; Werth, P.; Henjakovic, M. Testing of Different Digestion Solutions on Tissue Samples and the Effects of Used Potassium Hydroxide Solution on Polystyrene Microspheres. Toxics 2023, 11, 790. [Google Scholar] [CrossRef]
- Ortiz, D.; Munoz, M.; Nieto-Sandoval, J.; Romera-Castillo, C.; de Pedro, Z.M.; Casas, J.A. Insights into the degradation of microplastics by Fenton oxidation: From surface modification to mineralization. Chemosphere 2022, 309, 136809. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 4528. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Bennett, A.; Triantafyllou, M. Characterization of Nile Red-Stained Microplastics through Fluorescence Spectroscopy. J. Mar. Sci. Eng. 2024, 12, 1403. [Google Scholar] [CrossRef]
- Nava, V.; Frezzotti, M.L.; Leoni, B. Raman Spectroscopy for the Analysis of Microplastics in Aquatic Systems. Appl. Spectrosc. 2021, 75, 1341–1357. [Google Scholar] [CrossRef]
- CMDR|Products and Services. Available online: https://www.hpu.edu/cncs/cmdr/products-and-services.html (accessed on 17 April 2025).
- Thaysen, C.; Munno, K.; Hermabessiere, L.; Rochman, C.M. Towards Raman Automation for Microplastics: Developing Strategies for Particle Adhesion and Filter Subsampling. Appl. Spectrosc. 2020, 74, 976–988. [Google Scholar] [CrossRef]
- Haskell, H.; Suckling, C.C.; Gomez-Chiarri, M.; McMahon, K.W.; Preziosi, B.; Davies, A.J. Eastern oyster (Crassostrea virginica) shows physiological tolerance to polyester microfibers at environmental concentrations. J. Exp. Mar. Biol. Ecol. 2024, 578, 152032. [Google Scholar] [CrossRef]
- Provencher, J.F.; Covernton, G.A.; Moore, R.C.; Horn, D.A.; Conkle, J.L.; Lusher, A.L. Proceed with caution: The need to raise the publication bar for microplastics research. Sci. Total Environ. 2020, 748, 141426. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Silva Fernandes, A.J.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Selection of microplastics by Nile Red staining increases environmental sample throughput by micro-Raman spectroscopy. Sci. Total Environ. 2021, 783, 146979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspar, L.; Davis, S.; Coppotelli, G.; Davies, A.J.; Suckling, C.C.; Ross, J.M. A Workflow for Assessing Particle Counts of Mixed Micro- and Nanoplastics in Exposed Laboratory Animals. Nanomaterials 2025, 15, 812. https://doi.org/10.3390/nano15110812
Gaspar L, Davis S, Coppotelli G, Davies AJ, Suckling CC, Ross JM. A Workflow for Assessing Particle Counts of Mixed Micro- and Nanoplastics in Exposed Laboratory Animals. Nanomaterials. 2025; 15(11):812. https://doi.org/10.3390/nano15110812
Chicago/Turabian StyleGaspar, Lauren, Sarah Davis, Giuseppe Coppotelli, Andrew J. Davies, Coleen C. Suckling, and Jaime M. Ross. 2025. "A Workflow for Assessing Particle Counts of Mixed Micro- and Nanoplastics in Exposed Laboratory Animals" Nanomaterials 15, no. 11: 812. https://doi.org/10.3390/nano15110812
APA StyleGaspar, L., Davis, S., Coppotelli, G., Davies, A. J., Suckling, C. C., & Ross, J. M. (2025). A Workflow for Assessing Particle Counts of Mixed Micro- and Nanoplastics in Exposed Laboratory Animals. Nanomaterials, 15(11), 812. https://doi.org/10.3390/nano15110812






