Combined Experimental and DFT Study of Alumina (α-Al2O3(0001))-Supported Fe Atoms in the Limit of a Single Atom
Abstract
1. Introduction
2. Experimental and Computational Details
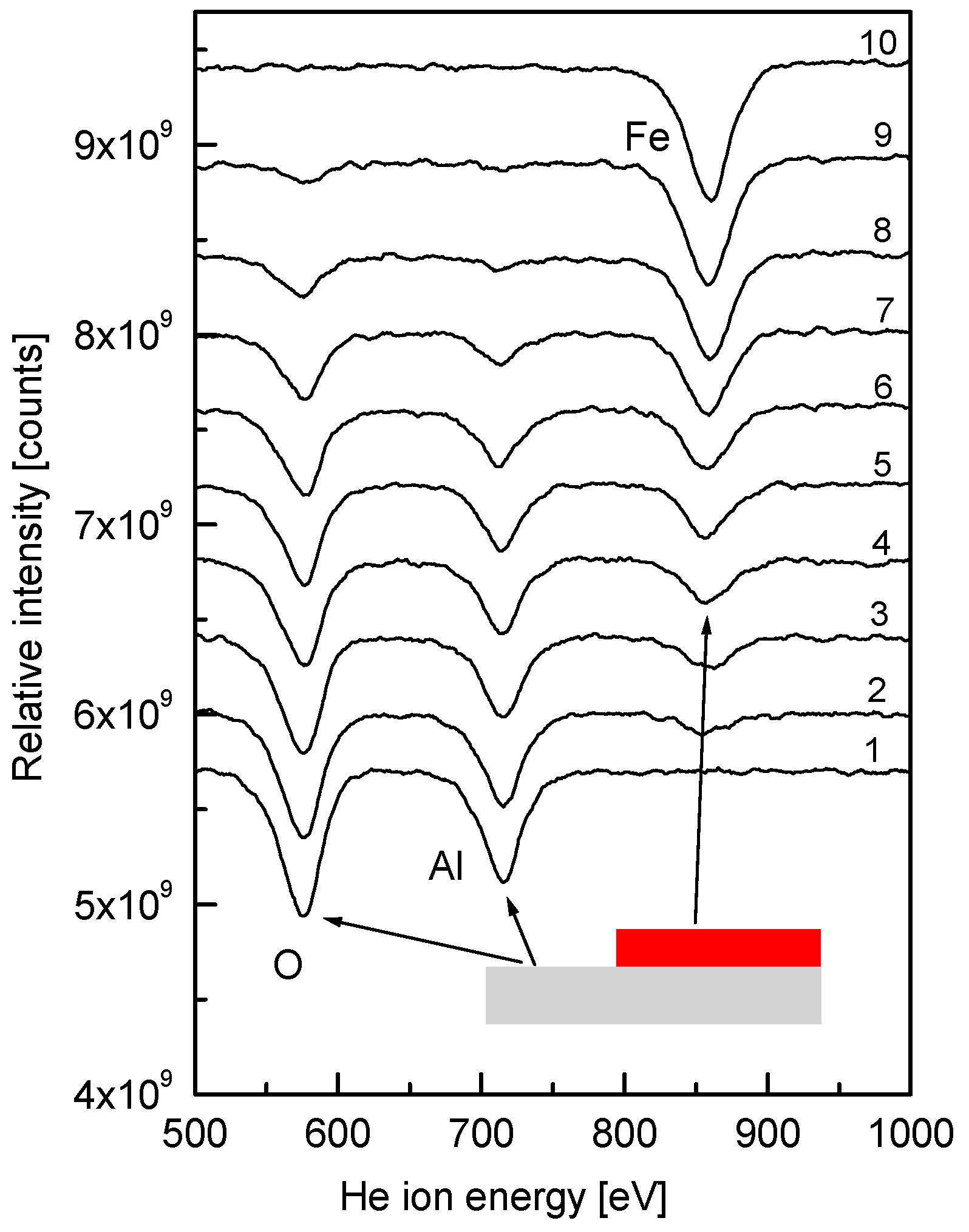
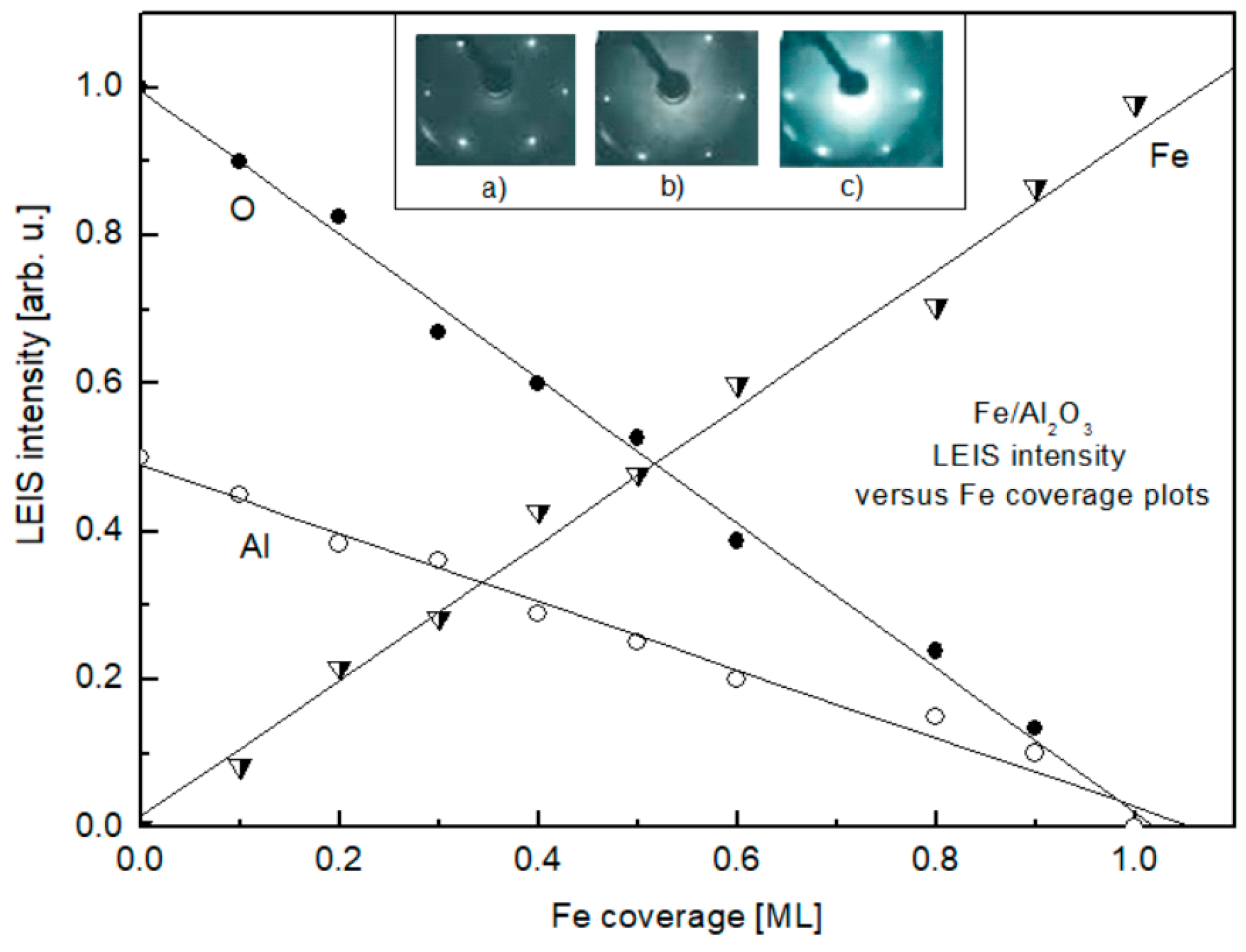
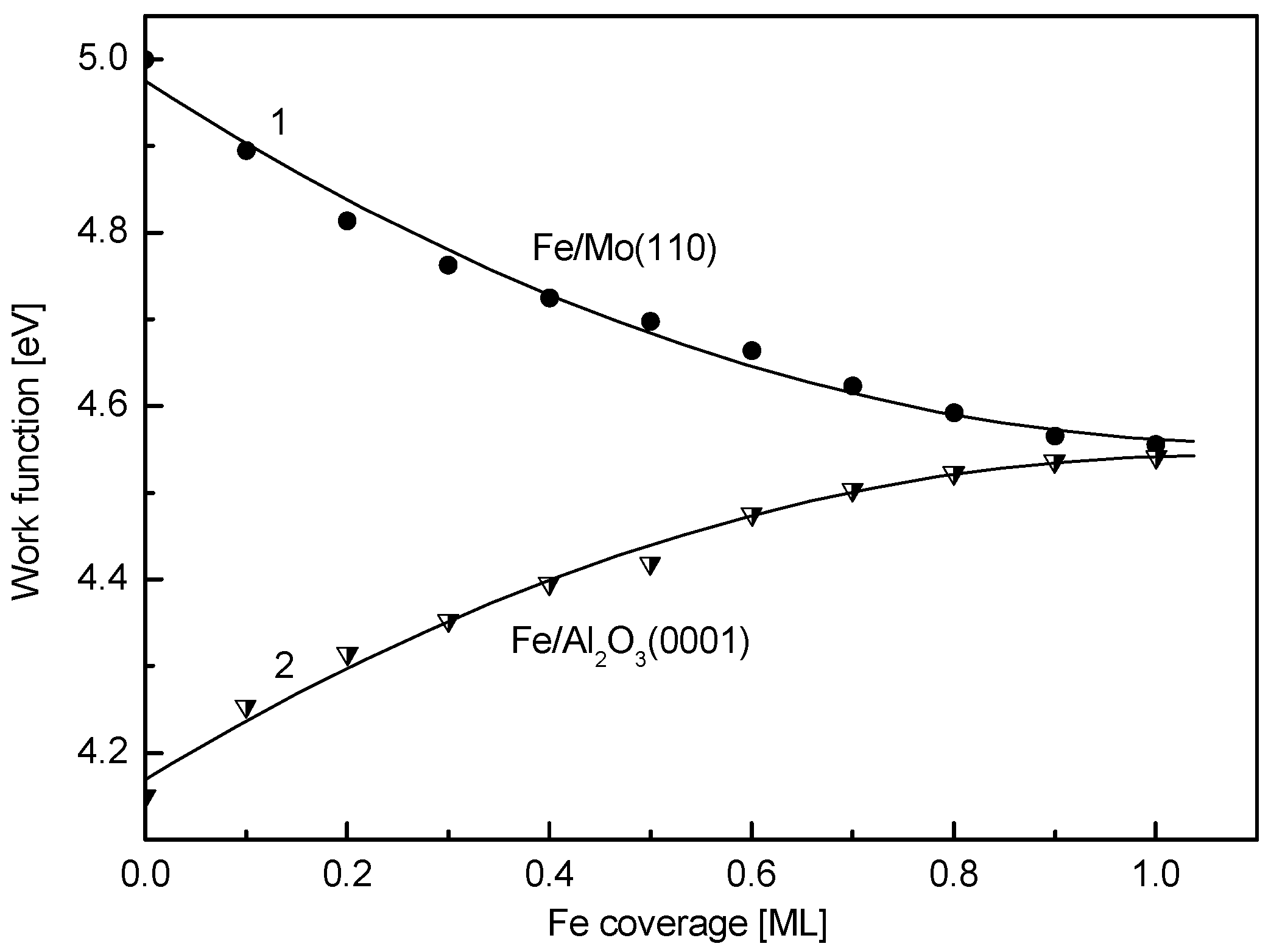
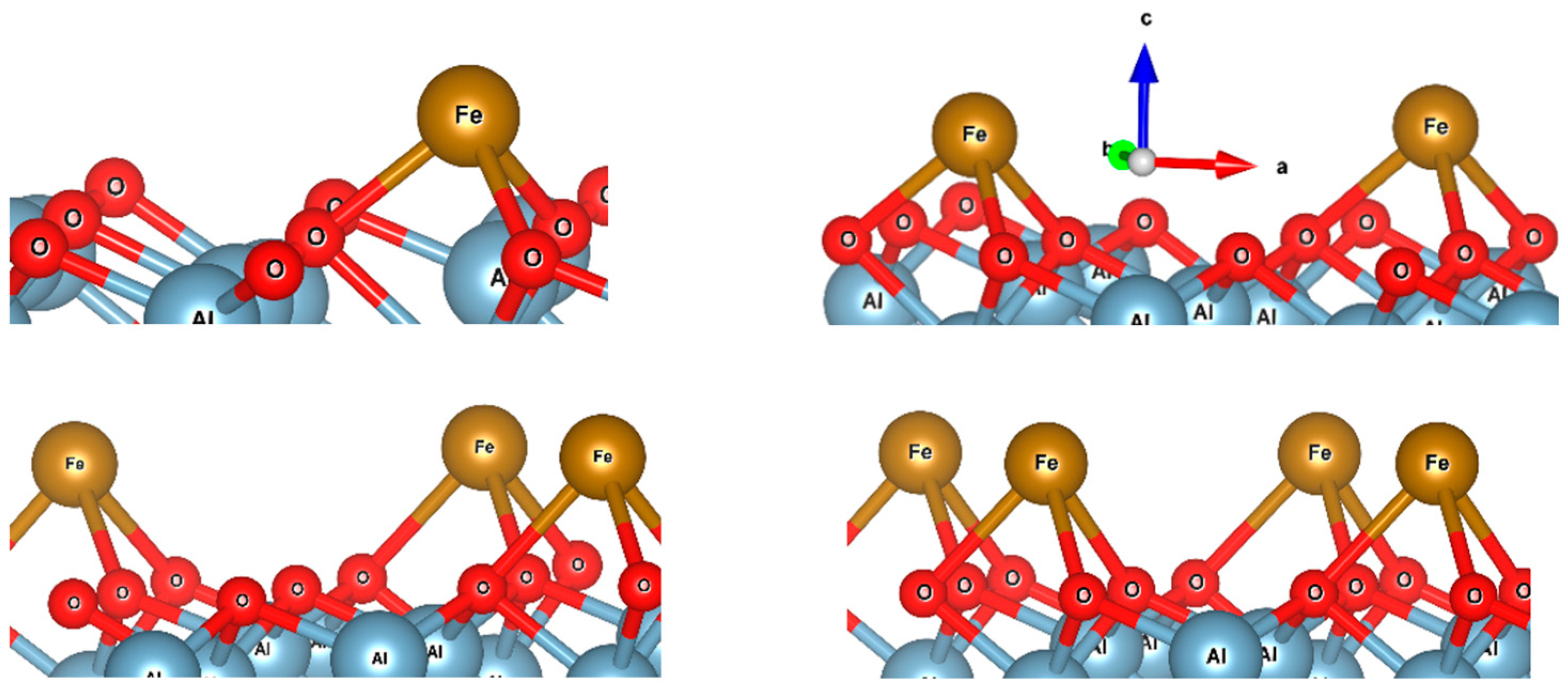
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravensburg, A.l.; Grassi, M.P.; Hjörvarsson, B.; Kapaklis, V. Effect of iron layer thickness on the interlayer exchange coupling in Fe/MgO (001) superlattices. Phys. Rev. B 2024, 109, 224404. [Google Scholar] [CrossRef]
- Reyes, A.; Herrera, G.; Capiod, P.; Le Roy, D.; Dupuis, V.; Cañero-Infante, I.; Saint-Girons, G.; Bachelet, R.; Resta, A.; Ohresser, P.; et al. Preferential orientations of FeRh nanomagnets deposited on a BaTiO3 epitaxial thin film. Phys. Rev. B 2024, 109, 245410. [Google Scholar] [CrossRef]
- Berwanger, J.; Polesya, S.; Mankovsky, S.; Ebert, H.; Giessibl, F.J. Atomically resolved chemical reactivity of small Fe clusters. Phys. Rev. Lett. 2020, 124, 096001. [Google Scholar] [CrossRef]
- Beyazit, Y.; Beckord, J.; Zhou, P.; Meyburg, J.P.; Kühne, F.; Diesing, D.; Ligges, M.; Bovensiepen, U. Local and nonlocal electron dynamics of Au/Fe/MgO(001) heterostructures analyzed by time-resolved two-photon photoemission spectroscopy. Phys. Rev. Lett. 2020, 125, 076803. [Google Scholar] [CrossRef]
- Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst. Science 2012, 338, 90. [Google Scholar] [CrossRef]
- Colonna, S.; Ronci, F.; Cricenti, A.; Luches, P.; Valeri, S.; Qi, J.; Xu, Y.; Miller, J.K.; Tolk, N. Ferromagnetic–antiferromagnetic Fe/NiO(100) interface studied by non-linear Kerr effect. Surf. Sci. 2007, 601, 4362–4365. [Google Scholar] [CrossRef]
- Luque, R.; Lin, C.S.K.; Wilson, K.; Clark, J. (Eds.) Handbook of Biofuels: Production Processes and Technologies; Elsevier: Amsterdam, The Netherlands, 2016; 748p, ISBN 978-0-08-100455-5. [Google Scholar]
- Zhang, P.; Chen, H.-C.; Zhu, H.; Chen, K.; Li, T.; Zhao, Y.; Li, J.; Hu, R.; Huang, S.; Zhu, W.; et al. Inter-site structural heterogeneity induction of single atom Fe catalysts for robust oxygen reduction. Nat. Commun. 2024, 15, 2062. [Google Scholar] [CrossRef]
- Egawa, C.; Onawa, K.; Iwai, H.; Oki, S. Interaction of CO and NO with Fe thin films grown on Rh(100) surface. Surf. Sci. 2004, 557, 31–40. [Google Scholar] [CrossRef]
- Li, X.; Luo, Y.; Wu, S.; Lian, H.; Deng, X. The exceptional performance of the plasmonic Au-Fe/TiO2 nanocatalysts achieved by O plasma activation. Catal. Today 2023, 418, 114106. [Google Scholar] [CrossRef]
- Luches, P.; Torelli, P.; Benedetti, S.; Ferramola, E.; Gotter, R.; Valeri, S. Structure and electronic properties of Fe nanostructures on MgO(001). Surf. Sci. 2007, 601, 3902–3906. [Google Scholar] [CrossRef]
- Fetzer, C.; Dezsi, I.; Szucs, I.; Tancziko, F.; Balogh, A.G. The interaction of Fe on MgO(100) surfaces. Surf. Sci. 2009, 603, 3021–3023. [Google Scholar] [CrossRef]
- Chang, Q.; Li, J.; Suo, H.; Qing, M.; Wang, H.; Zhang, C.; Wen, X.; Xiang, H.; Yang, Y.; Li, Y. Unravelling the formation of Fe2SiO4 on Fischer-Tropsch Fe/SiO2 catalyst. Catal. Today 2024, 431, 114605. [Google Scholar] [CrossRef]
- Di Castro, V.; Ciampi, S. XPS study of the growth and reactivity of Fe/MnO thin films. Surf. Sci. 1995, 331–333, 294–299. [Google Scholar] [CrossRef]
- Kołaczkiewicz, J.; Bauer, E. V and Fe on the W(110) face. Surf. Sci. 2000, 450, 106–116. [Google Scholar] [CrossRef]
- Masi, R.; Reinicke, D.; Müller, F.; Steiner, P.; Hüfner, S. The interface of Mn, Fe, Co and Au metal films on NiO(001), investigated by photoemission and low energy electron diffraction. Surf. Sci. 2002, 515, 523–537. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, X.; Li, J.; An, J.; Zhu, X.; Li, X. Role of active oxygen species in Fe-doped ZrO2 catalyst during CO2 assisted ethane dehydrogenation reaction. J. Catal. 2023, 428, 115130. [Google Scholar] [CrossRef]
- Rodriguez, M.; Leonardi, S.A.; Hanon, F.; Miró, E.E.; Milt, V.G.; Gaigneaux, E.M. Plasma-assisted deposition of Mn and Fe phases on CeO2 biomorphic fibers for soot combustion and CO oxidation. Catal. Today 2024, 431, 114457. [Google Scholar] [CrossRef]
- Nakajima, N.; Kato, H.; Okazaki, T.; Sakisaka, Y. Photoemission study of the modification of the electronic structure of transition-metal overlayers on TiO2 surfaces: I. Fe onTiO2(110). Surf. Sci. 2004, 561, 79–86. [Google Scholar] [CrossRef]
- Arranz, A.; Perez-Dieste, V.; Palacio, C. Growth, electronic properties and thermal stability of the Fe/Al2O3 interface. Surf. Sci. 2002, 521, 77–83. [Google Scholar] [CrossRef]
- Li, S.; Wang, F.; Xie, Z.; Ng, D.; Shen, B. A novel core-shell structured Fe@CeO2-ZIF-8 catalyst for the reduction of NO by CO. J. Catal. 2023, 421, 240–251. [Google Scholar] [CrossRef]
- Luches, P.; Benedetti, S.; Liberati, M.; Boscherini, F.; Pronin, I.I.; Valeri, S. Absence of oxide formation at theFe/MgO(001) interface. Surf. Sci. 2005, 583, 191–198. [Google Scholar] [CrossRef]
- Fonin, M.; Dedkov, Y.S.; Rüdiger, U.; Güntherodt, G. Growth and morphology of the epitaxial Fe(110)/MgO(111)/Fe(110) trilayers. Surf. Sci. 2007, 601, 2166–2170. [Google Scholar] [CrossRef]
- Lehnert, A.; Krupski, A.; Degen, S.; Franke, K.; Decker, R.; Rusponi, S.; Kralj, M.; Becker, C.; Brune, H.; Wandelt, K. Nucleation of ordered Fe islands on Al2O3/Ni3Al(111). Surf. Sci. 2006, 600, 1804–1808. [Google Scholar] [CrossRef]
- Di Castro, V.; Polzonetti, G.; Ciampi, S.; Contini, G.; Sakho, O. Photoemission study of the Fe/MnO interface formation. Surf. Sci. 1993, 293, 41–46. [Google Scholar] [CrossRef]
- Magkoev, T.T.; Vladimirov, G.G.; Remar, D.; Moutinho, A.M.C. Comparative study of metal adsorption on the metal and the oxide surfaces. Solid State Commun. 2002, 122, 341–346. [Google Scholar] [CrossRef]
- Magkoev, T.T.; Men, Y.; Behjatmanesh-Ardakani, R.; Takaev, A.A.; Khekilaev, R.A. The value of charge of Fe atoms adsorbed on the surface of a-Al2O3(0001). Pis’ma Zhurnal Tekhnicheskoi Fiz. 2025, 51, 29–32. [Google Scholar] [CrossRef]
- Yates, J.T., Jr. Experimental Innovations in Surface Science: A Guide to Practical Laboratory Methods and Instruments; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–655. ISBN 9783319176673. [Google Scholar]
- Berge, S.; Gartland, P.O.; Slagsvold, B.J. Photoelectric work function of a molybdenum single crystal for the (100), (110), (111), (112), (114), and (332) faces. Surf. Sci. 1974, 43, 275–292. [Google Scholar] [CrossRef]
- Goodman, D.W. Chemical and spectroscopic studies of metal oxide surfaces. J. Vac. Sci. Technol. A 1996, 14, 1526–1531. [Google Scholar] [CrossRef]
- Magkoev, T.T.; Men, Y.; Behjatmanesh-Ardakani, R.; Elahifard, M.; Abaev, V.T.; Chalikidi, P.N.; Zaalishvili, V.B.; Magkoev, T.T., Jr.; Ashkhotov, O.G. The value of charge of Fe single to multiple atoms doped in Ge: Combined experimental and density functional theory study. Solid State Commun. 2024, 378, 115409. [Google Scholar] [CrossRef]
- Magkoev, T.T. Effect of electron tunneling through the oxide film grown on metal substrate upon the efficiency of molecular reaction over the oxide supported metal nanoparticles: A case of CO oxidation on Au/Al2O3/Mo(110). Vacuum 2021, 189, 110220. [Google Scholar] [CrossRef]
- García, A.; Papior, N.; Akhtar, A.; Artacho, E.; Blum, V.; Bosoni, E.; Brandimarte, P.; Brandbyge, M.; Cerdá, J.I.; Corsetti, F.; et al. Siesta: Recent developments and applications. J. Chem. Phys. 2020, 152, 204108. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.H.; Mortensen, J.J.; Blomqvist, J.; Castelli, I.E.; Christensen, R.; Dułak, M.; Friis, J.; Groves, M.N.; Hammer, B.; Hargus, C. The atomic simulation environment-a Python library for working with atoms. J. Phys. Condens. Matter 2017, 29, 273002. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.H.; Almlof, J. General methods for geometry and wave function optimization. J. Phys. Chem. 1992, 96, 9768–9774. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Van Setten, M.J.; Giantomassi, M.; Bousquet, E.; Verstraete, M.J.; Hamann, D.R.; Gonze, X.; Rignanese, G.M. The PseudoDojo: Training and grading a 85 element optimized norm-conserving pseudopotential table. Comp. Phys. Commun. 2018, 226, 39–54. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and efficient algorithm for Bader charge integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comp. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Blum, V.; Gehrke, R.; Hanke, F.; Havu, P.; Havu, V.; Ren, X.; Reuter, K.; Scheffler, M. Ab initio molecular simulations with numeric atom-centered orbitals. Comp. Phys. Commun. 2009, 180, 2175–2196. [Google Scholar] [CrossRef]
- Havu, V.; Blum, V.; Havu, P.; Scheffler, M. Efficient O(N) integration for all-electron electronic structure calculation using numeric basis functions. J. Comp. Phys. 2009, 228, 8367–8379. [Google Scholar] [CrossRef]
- Marek, A.; Blum, V.; Johanni, R.; Havu, V.; Lang, B.; Auckenthaler, T.; Heinecke, A.; Bungartz, H.-J.; Lederer, H. The ELPA library: Scalable parallel eigenvalue solutions for electronic structure theory and computational science. J. Phys. Condens. Matter 2014, 26, 213201. [Google Scholar] [CrossRef]
- Yu, V.W.; Corsetti, F.; García, A.; Huhn, W.P.; Jacquelin, M.; Jia, W.; Lange, B.; Lin, L.; Lu, J.; Mi, W.; et al. ELSI: A unified software interface for Kohn–Sham electronic structure solvers. Comp. Phys. Commun. 2018, 222, 267–285. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, Y.; Chung, T.F.; Tai, C.-L.; Chen, C.-Y.; Yang, J.-R.; Li, D.Y. Electron work function: An indicative parameter towards a novel material design methodology. Sci. Rep. 2021, 11, 11565. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.C.; Tucker, P.M.; Wild, R.K. High resolution LMM auger electron spectra of some first-row transition elements. Surf. Sci. 1977, 68, 469–478. [Google Scholar] [CrossRef]
- Esser, N.; Benne, I.; Srama, R.; Richter, W. The surface dipole contribution to the work function for Sb on GaAs(110): A comparative study by Kelvin probe and Raman spectroscopy. Surf. Sci. 1992, 269–270, 1037–1040. [Google Scholar] [CrossRef]
- Ishizawa, N.; Miyata, T.; Minato, I.; Marumo, F.; Iwai, S. A structural investigation of [alpha]-Al2O3 at 2170 K. Acta Cryst. B 1980, 36, 228–230. [Google Scholar] [CrossRef]


| n | Mean Coverage | Charge | Binding Energy |
|---|---|---|---|
| 1 | 0 | +1.15 | −6.76 |
| 2 | 0.14 | +1.08 | −5.12 |
| 3 | 0.21 | +0.86 | −4.47 |
| 4 | 0.28 | +0.69 | −3.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magkoev, R.T.; Men, Y.; Behjatmanesh-Ardakani, R.; Elahifard, M.; Silaev, I.V.; Bliev, A.P.; Pukhaeva, N.E.; Turiev, A.M.; Zaalishvili, V.B.; Takaev, A.A.; et al. Combined Experimental and DFT Study of Alumina (α-Al2O3(0001))-Supported Fe Atoms in the Limit of a Single Atom. Nanomaterials 2025, 15, 804. https://doi.org/10.3390/nano15110804
Magkoev RT, Men Y, Behjatmanesh-Ardakani R, Elahifard M, Silaev IV, Bliev AP, Pukhaeva NE, Turiev AM, Zaalishvili VB, Takaev AA, et al. Combined Experimental and DFT Study of Alumina (α-Al2O3(0001))-Supported Fe Atoms in the Limit of a Single Atom. Nanomaterials. 2025; 15(11):804. https://doi.org/10.3390/nano15110804
Chicago/Turabian StyleMagkoev, Ramazan T., Yong Men, Reza Behjatmanesh-Ardakani, Mohammadreza Elahifard, Ivan V. Silaev, Aleksandr P. Bliev, Nelli E. Pukhaeva, Anatolij M. Turiev, Vladislav B. Zaalishvili, Aleksandr A. Takaev, and et al. 2025. "Combined Experimental and DFT Study of Alumina (α-Al2O3(0001))-Supported Fe Atoms in the Limit of a Single Atom" Nanomaterials 15, no. 11: 804. https://doi.org/10.3390/nano15110804
APA StyleMagkoev, R. T., Men, Y., Behjatmanesh-Ardakani, R., Elahifard, M., Silaev, I. V., Bliev, A. P., Pukhaeva, N. E., Turiev, A. M., Zaalishvili, V. B., Takaev, A. A., Magkoev, T. T., Khekilaev, R. A., & Ashkhotov, O. G. (2025). Combined Experimental and DFT Study of Alumina (α-Al2O3(0001))-Supported Fe Atoms in the Limit of a Single Atom. Nanomaterials, 15(11), 804. https://doi.org/10.3390/nano15110804









