Acoustic Enhancement Performance of Hierarchical ZSM-5 Zeolites with Different Si/Al Ratios
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of Zeolite
2.2. Methods
2.2.1. Characterization
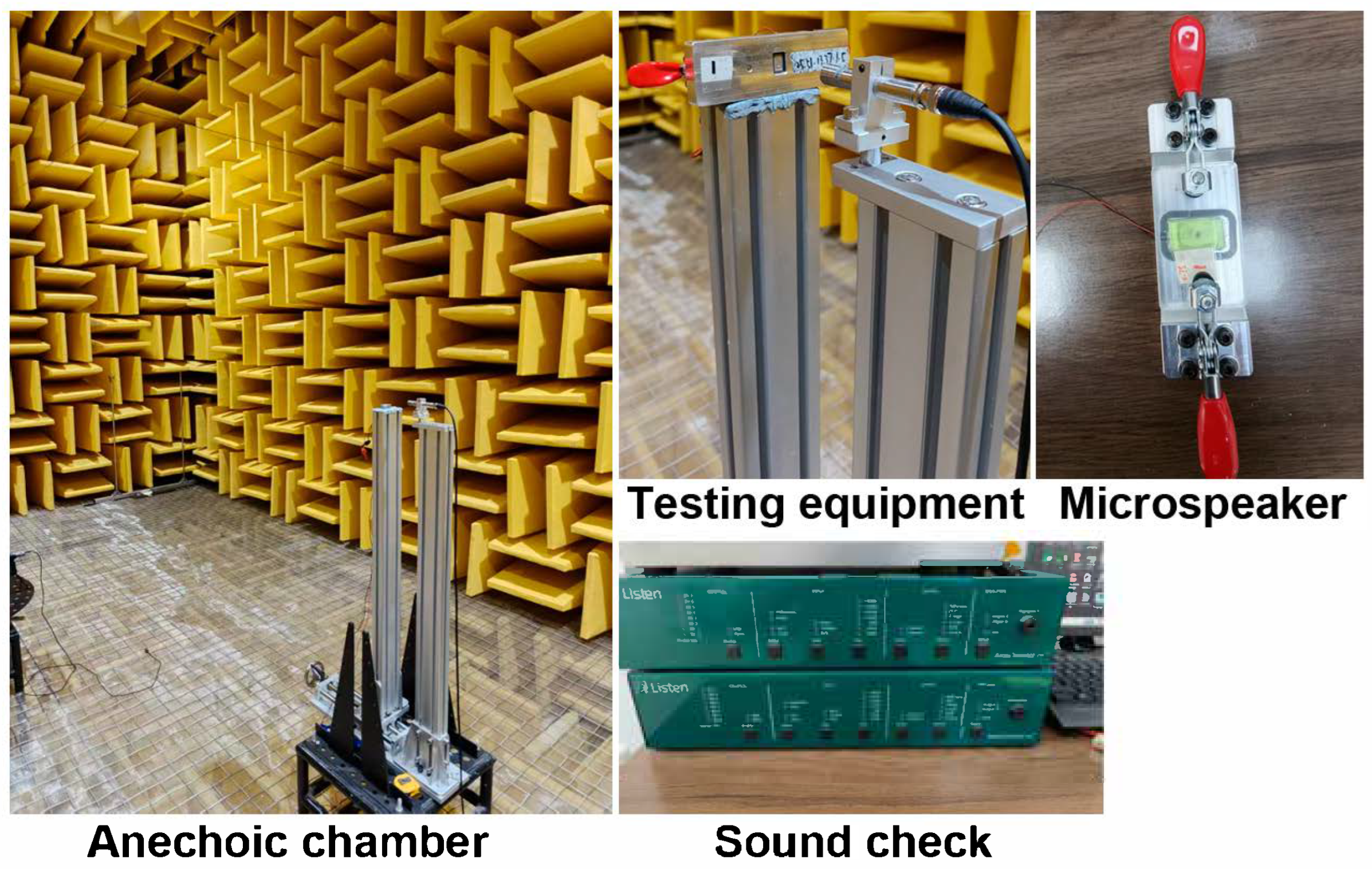
2.2.2. Acoustic Performance Measurement
3. Results
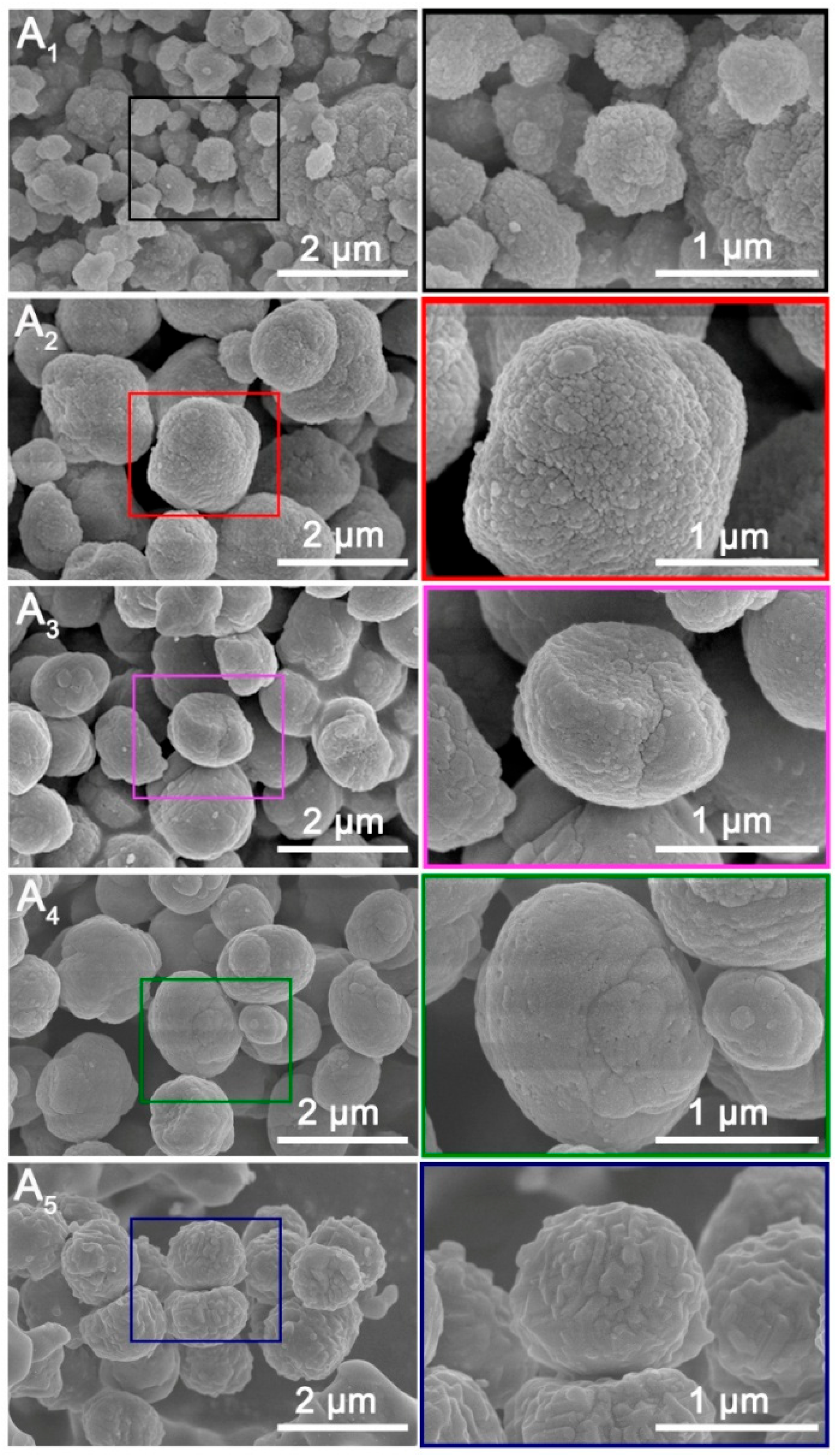
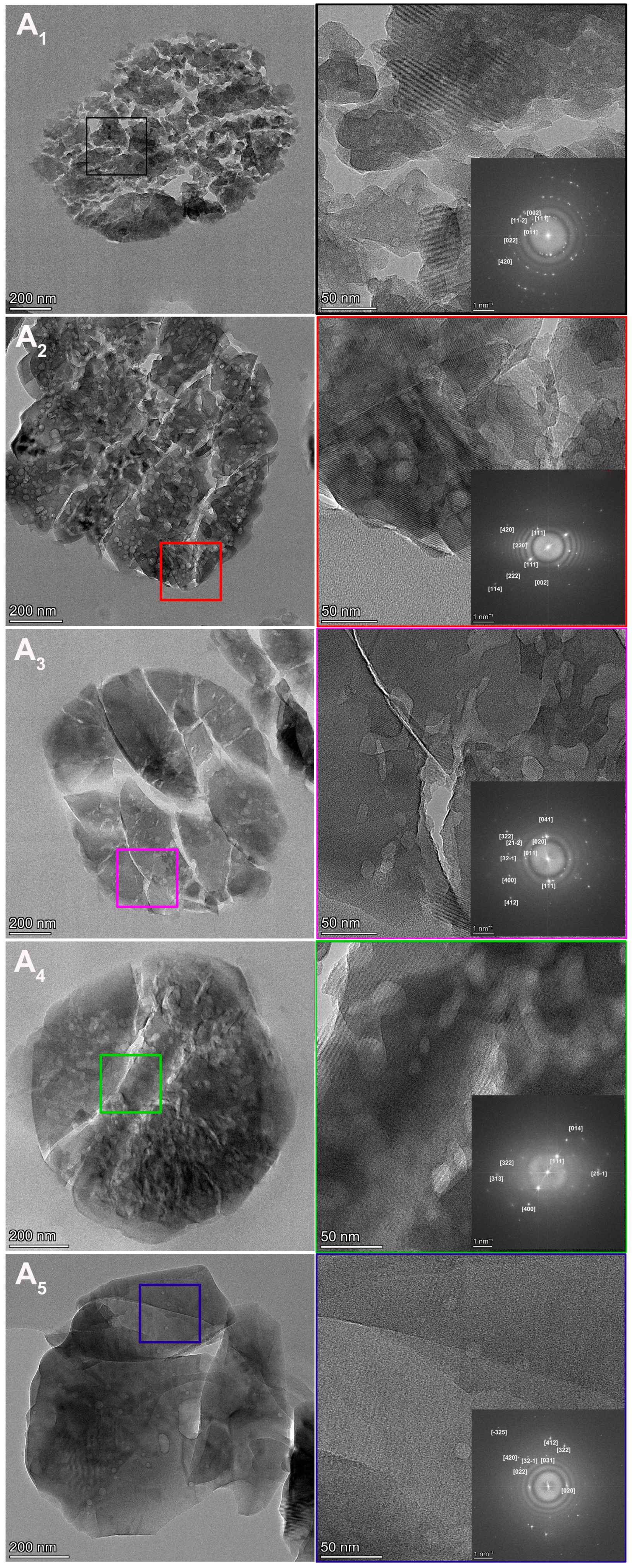
3.1. Characterization Analysis
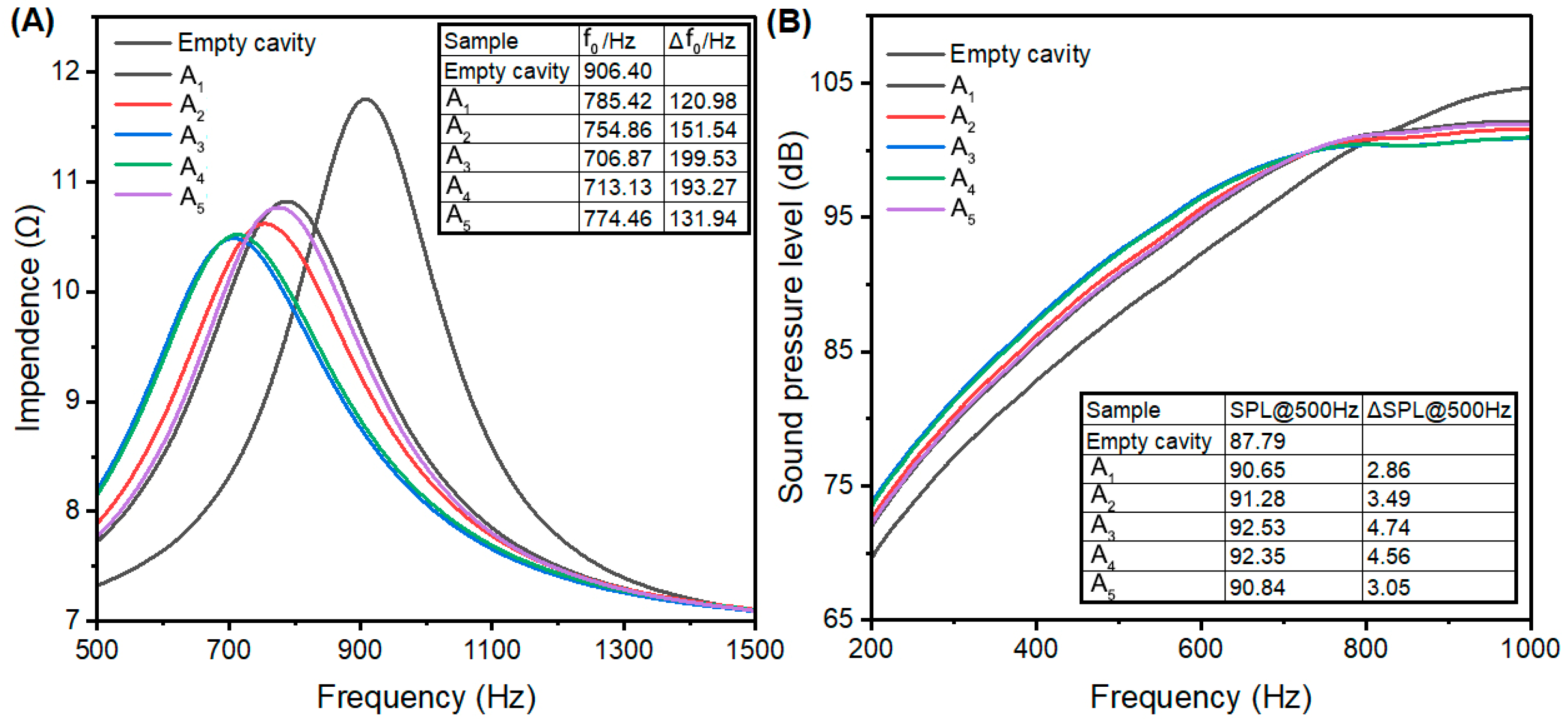
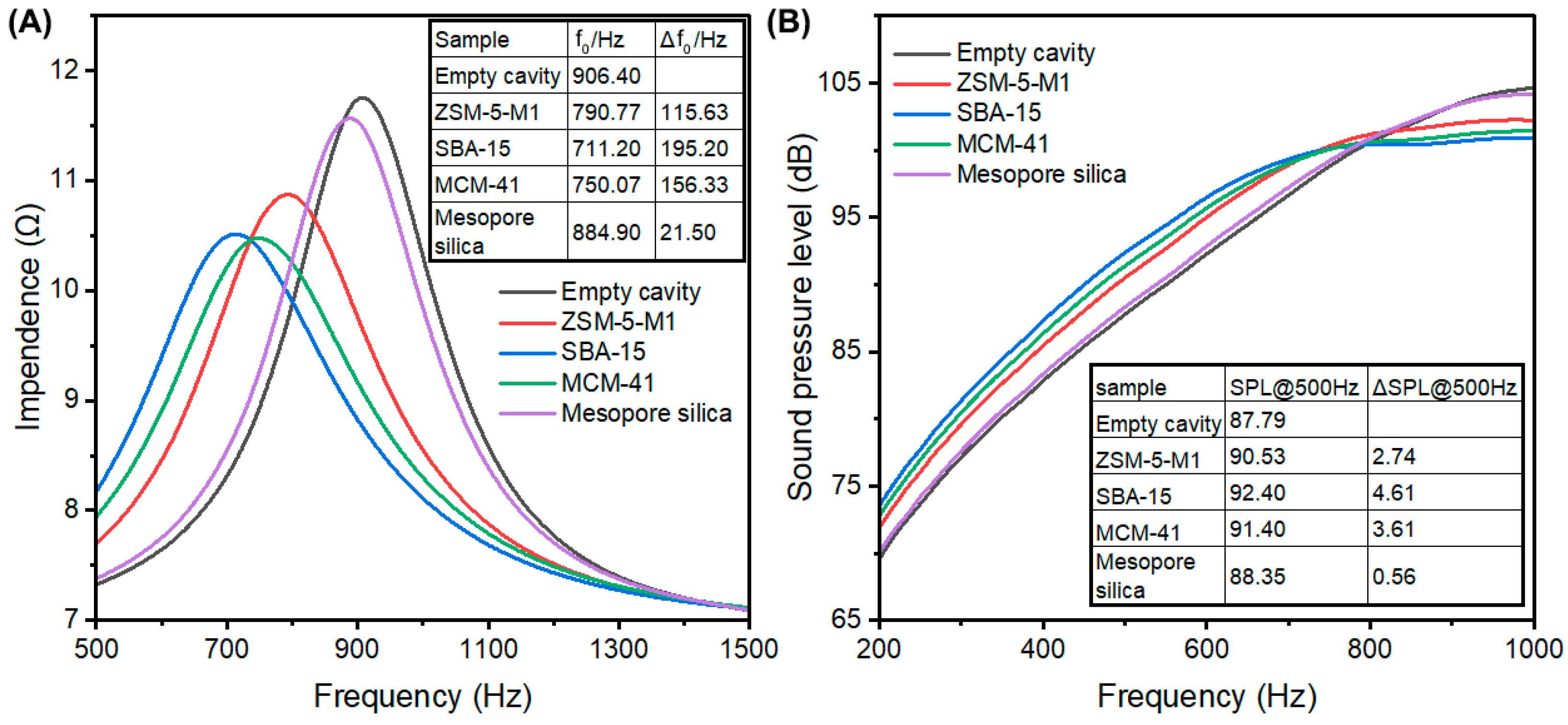
3.2. Acoustic Enhancement Performance
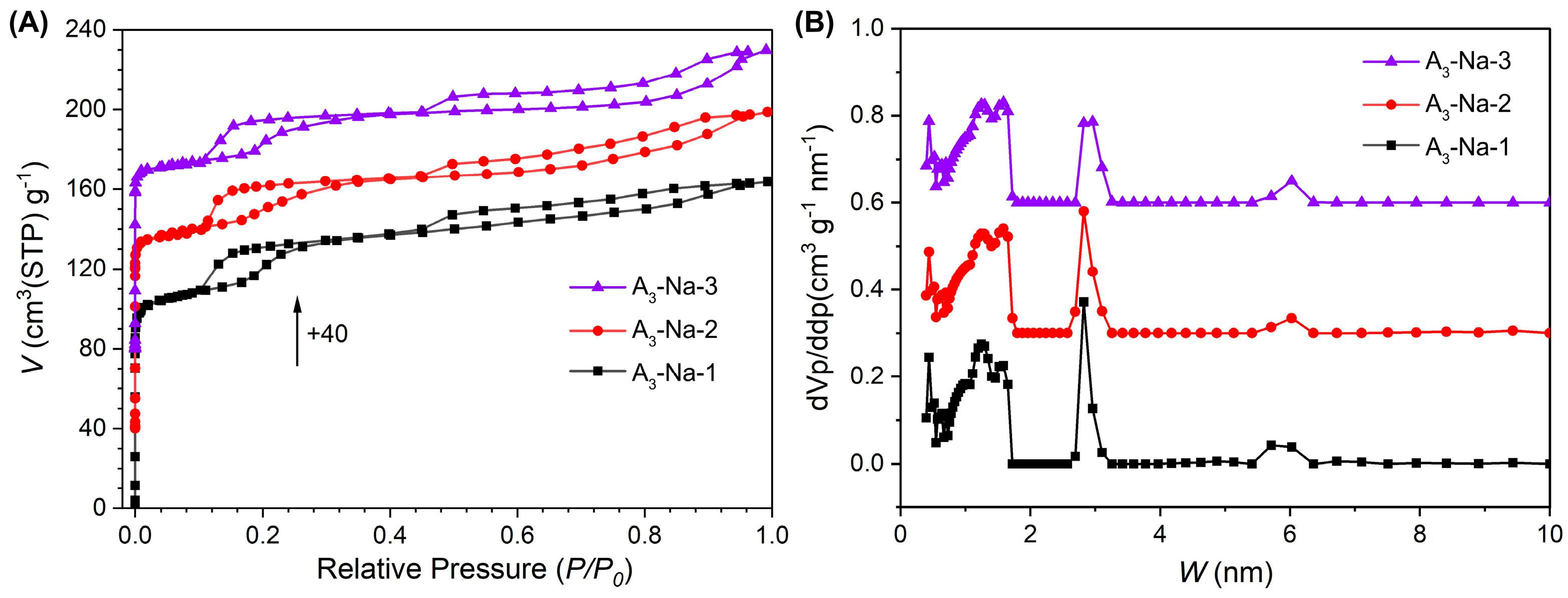
3.3. Impact of Sodium Content
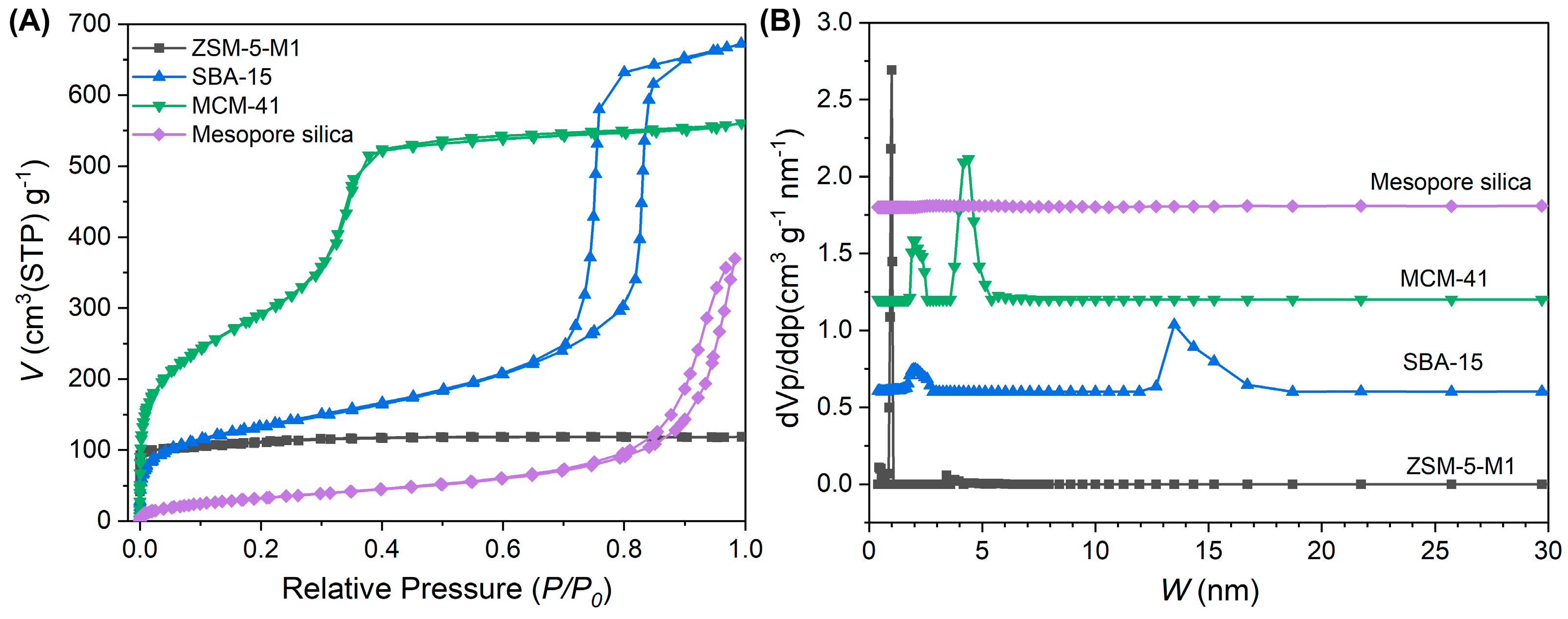
3.4. Impact of Pore Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, M.; Chen, R.L. Analysis and optimal design of miniature loudspeakers. In Proceedings of the 120th AES 6840, Pairs, France, 20–23 May 2006. [Google Scholar]
- Zhang, X.; Che, S. Mesoporous silica microspheres composited with SBA-15s for resonance frequency reduction in a miniature loudspeaker. Chem. Res. Chin. Univ. 2020, 36, 760–767. [Google Scholar] [CrossRef]
- Her, H.-C.; Huang, J.-H. Acoustical analysis for a microspeaker used in cellular phones. Int. Conf. Comput. Exp. Eng. Sci. 2009, 10, 55–56. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Lo, S.-C.; Huang, Z.-R.; Wang, Y.-J.; Wu, M.; Fang, W. On the design of piezoelectric MEMS microspeaker for the sound pressure level enhancement. Sens. Actuators A 2020, 306, 111960. [Google Scholar] [CrossRef]
- Sun, P.; Xu, D.-P.; Hwang, S.-M. Design of microspeaker module considering added stiffness. J. Mech. Sci. Technol. 2014, 28, 1623–1628. [Google Scholar] [CrossRef]
- Lei, T.-H.; Liu, Y.-C. Study on the predictions of acoustic characteristics of microspeakers based on the specific airflow resistance of ventilation materials: Mesh type. Appl. Acoust. 2025, 228, 110304. [Google Scholar] [CrossRef]
- Shiah, Y.C.; Her, H.-C.; Huang, J.H.; Huang, B. Parametric analysis for a miniature loudspeaker used in cellular phones. J. Appl. Phys. 2008, 104, 104905. [Google Scholar] [CrossRef]
- Yang, T.; Hu, L.; Xiong, X.; Petrů, M.; Noman, M.T.; Mishra, R.; Militký, J. Sound Absorption Properties of Natural Fibers: A Review. Sustainability 2020, 12, 8477. [Google Scholar] [CrossRef]
- Lan, Y.-C.; Kamal, S.; Lin, C.-C.; Liu, Y.-H.; Lu, K.-L. Ultra-thin Zr-MOF/PVA/Melamine composites with remarkable sound attenuation effects. Microporous Mesoporous Mater. 2023, 360, 112668. [Google Scholar] [CrossRef]
- Arenas, P.J.; Crocker, M. Recent trends in porous sound-absorbing materials. Sound Vib. 2010, 44, 12–17. [Google Scholar]
- Wright, J.R. The virtual loudspeaker cabinet. J. Audio Eng. Soc. 2003, 51, 244–247. [Google Scholar]
- Venegas, R.; Umnova, O. Influence of sorption on sound propagation in granular activated carbon. J. Acoust. Soc. Am. 2016, 140, 755. [Google Scholar] [CrossRef]
- Zeolite catalysts come into focus. Nat. Mater. 2020, 19, 1037. [CrossRef]
- Yue, B.; Liu, S.; Chai, Y.; Wu, G.; Guan, N.; Li, L. Zeolites for separation: Fundamental and application. J. Energy Chem. 2022, 71, 288–303. [Google Scholar] [CrossRef]
- Townsend, R.P.; Coker, E.N. Chapter 11 Ion exchange in zeolites. In Studies in Surface Science and Catalysis; van Bekkum, H., Flanigen, E.M., Jacobs, P.A., Jansen, J.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 137, pp. 467–524. [Google Scholar]
- Bensafi, B.; Chouat, N.; Djafri, F. The universal zeolite ZSM-5: Structure and synthesis strategies. A review. Coord. Chem. Rev. 2023, 496, 215397. [Google Scholar] [CrossRef]
- Catuzo, G.L.; Santilli, C.V.; Martins, L. Hydrophobic-hydrophilic balance of ZSM-5 zeolites on the two-phase ketalization of glycerol with acetone. Catal. Today 2021, 381, 215–223. [Google Scholar] [CrossRef]
- Yimlamai, I.; Niamlang, S.; Chanthaanont, P.; Kunanuraksapong, R.; Changkhamchom, S.; Sirivat, A. Electrical conductivity response and sensitivity of ZSM-5, Y, and mordenite zeolites towards ethanol vapor. Ionics 2011, 17, 607–615. [Google Scholar] [CrossRef]
- Jiang, Y.-W.; Xu, D.-P.; Kwon, J.-H.; Jiang, Z.-X.; Kim, J.-H.; Hwang, S.-M. Analysis and application of zeolite in microspeaker box. J. Mech. Sci. Technol. 2019, 33, 3679–3683. [Google Scholar] [CrossRef]
- Chen, J.; Tan, F.; Li, X.; Lu, X.; Zhao, D.; Liu, J.; Zhou, H.; Lu, Q.; Qian, L. Linear enhancement of mechanical compliance by zeolite filling in a compact loudspeaker. J. Phys. D Appl. Phys. 2024, 57, 20LT01. [Google Scholar] [CrossRef]
- Papakyriacou, M.; Kobler, J.; Sauer, J. Loudspeaker System with Improved Sound. U.S. Patent 14/729,882, 23 August 2010. [Google Scholar]
- Papakyriacou, M.; Steinhauster, M.; Jagsich, T. Packaging of Acoustic Volume Increasing Materials for Louderspeaker Devices. U.S. Patent 9,900,675 B2, 2 June 2015. [Google Scholar]
- Papakyriacou, M.; Steinhauster, M.; Jagsich, T. Packaging of Acoustic Volume Increasing Materials for Louderspeaker Devices. U.S. Patent 9,099,073 B2, 5 March 2012. [Google Scholar]
- Alotibi, M.F.; Alshammari, B.A.; Alotaibi, M.H.; Alotaibi, F.M.; Alshihri, S.; Navarro, R.M.; Fierro, J.L.G. ZSM-5 Zeolite Based Additive in FCC Process: A Review on Modifications for Improving Propylene Production. Catal. Surv. Asia 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Aztiria, T.; Rzepka, P.; Verel, R.; Xu, J.; Deng, F.; van Bokhoven, J.A.; Paunović, V. Structural Changes of ZSM-5 Catalysts during Methanol-to-Hydrocarbons Conversion Processes. ACS Catal. 2024, 14, 12410–12424. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, X.; Tong, Y.; Lei, Q.; Li, F.; Zhang, L. The Synthesis of Novel Hierarchical Zeolites and Their Performances in Cracking Large Molecules. Catal. Lett. 2010, 136, 266–270. [Google Scholar] [CrossRef]
- Chen, L.-H.; Li, X.-Y.; Rooke, J.C.; Zhang, Y.-H.; Yang, X.-Y.; Tang, Y.; Xiao, F.-S.; Su, B.-L. Hierarchically structured zeolites: Synthesis, mass transport properties and applications. J. Mater. Chem. 2012, 22, 17381–17403. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannsen, K.; Törnqvist, E.; Schmidt, I.; Topsøe, H.; Christensen, C.H. Mesoporous zeolite single crystal catalysts: Diffusion and catalysis in hierarchical zeolites. Catal. Today 2007, 128, 117–122. [Google Scholar] [CrossRef]
- Groen, J.C.; Moulijn, J.A.; Pérez-Ramírez, J. Desilication: On the controlled generation of mesoporosity in MFI zeolites. J. Mater. Chem. 2006, 16, 2121–2131. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite Catalysts with Tunable Hierarchy Factor by Pore-Growth Moderators. Adv. Funct. Mater. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Liu, K.; Zeng, Y.; Han, L.; Che, S. Synthesis of a zeolite@mesoporous silica composite to improve the low-frequency acoustic performance of a miniature loudspeaker system. Dalton Trans. 2024, 53, 4764–4771. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Liu, Z.; Xu, S.; Wang, Y.; Xie, Z.; Han, X.; Bao, X. Direct Observation of the Mesopores in ZSM-5 Zeolites with Hierarchical Porous Structures by Laser-Hyperpolarized 129Xe NMR. J. Phys. Chem. C 2008, 112, 15375–15381. [Google Scholar] [CrossRef]
- Tooraji, E.; Ghaemi, A. Nitrogen Adsorption on Molecular Sieve Zeolites: An Experimental and Modeling Study. Iran. J. Oil Gas Sci. Technol. 2020, 9, 47–67. [Google Scholar]
- Nguyen, D.-K.; Dinh, V.-P.; Dang, N.T.; Khan, D.T.; Hung, N.T.; Thi Tran, N.H. Effects of aging and hydrothermal treatment on the crystallization of ZSM-5 zeolite synthesis from bentonite. RSC Adv. 2023, 13, 20565–20574. [Google Scholar] [CrossRef]
- IEC60268-5-2007; Sound System Equipment—Part 5: Loudspeakers. IEC: Geneva, Switzerland, 2007.
- Gao, Y.; Zheng, B.; Wu, G.; Ma, F.; Liu, C. Effect of the Si/Al ratio on the performance of hierarchical ZSM-5 zeolites for methanol aromatization. RSC Adv. 2016, 6, 83581–83588. [Google Scholar] [CrossRef]
- Hu, H.; Lyu, J.; Rui, J.; Cen, J.; Zhang, Q.; Wang, Q.; Han, W.; Li, X. The effect of Si/Al ratio on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol. Catal. Sci. Technol. 2016, 6, 2647–2652. [Google Scholar] [CrossRef]
- Kim, S.D.; Noh, S.H.; Park, J.W.; Kim, W.J. Organic-free synthesis of ZSM-5 with narrow crystal size distribution using two-step temperature process. Microporous Mesoporous Mater. 2006, 92, 181–188. [Google Scholar] [CrossRef]
- Kim, S.D.; Noh, S.H.; Seong, K.H.; Kim, W.J. Compositional and kinetic study on the rapid crystallization of ZSM-5 in the absence of organic template under stirring. Microporous Mesoporous Mater. 2004, 72, 185–192. [Google Scholar] [CrossRef]
- Xu, R.R.; Pang, W.Q.; Yu, J.J.; Huo, Q.S.; Chen, J.S. Chemistry of Zeolite and Related Porous Materials; Science Press: Beijing, China, 2004; pp. 1–4. [Google Scholar]
- Zhang, Q.; Chen, G.; Wang, Y.; Chen, M.; Guo, G.; Shi, J.; Luo, J.; Yu, J. High-Quality Single-Crystalline MFI-Type Nanozeolites: A Facile Synthetic Strategy and MTP Catalytic Studies. Chem. Mater. 2018, 30, 2750–2758. [Google Scholar] [CrossRef]
- van Koningsveld, H.; Jansen, J.C.; van Bekkum, H. The monoclinic framework structure of zeolite H-ZSM-5. Comparison with the orthorhombic framework of as-synthesized ZSM-5. Zeolites 1990, 10, 235–242. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.Y.; Zhang, H.K.; Lv, J.E.; Ren, P.J.; Ren, J. Synthesis and characterization of an unusual snowflake-shaped ZSM-5 zeolite with high catalytic performance in the methanol to olefin reaction. Chin. J. Catal. 2016, 37, 308–315. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Feng, W.; Lin, Y.; Wang, S. Effect of triethylamine treatment of titanium silicalite-1 on propylene epoxidation. Front. Chem. Sci. Eng. 2014, 8, 478–487. [Google Scholar] [CrossRef]
- Song, G.; Chen, W.; Dang, P.; Yang, S.; Zhang, Y.; Wang, Y.; Xiao, R.; Ma, R.; Li, F. Synthesis and Characterization of Hierarchical ZSM-5 Zeolites with Outstanding Mesoporosity and Excellent Catalytic Properties. Nanoscale Res. Lett. 2018, 13, 364. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Yao, Z.; Guo, Y.; Gao, Z.; Zheng, J.; Li, W.; Fan, B.; Wang, Y.; Chen, S.; et al. Synthesis of loosely aggregating polycrystalline ZSM-5 with luxuriant mesopore structure and its hierarchically cracking for bulky reactants. Mater. Chem. Phys. 2020, 243, 122610. [Google Scholar] [CrossRef]
- Hou, Y.; Li, X.; Sun, M.; Li, C.; Bakhtiar, S.U.H.; Lei, K.; Yu, S.; Wang, Z.; Hu, Z.; Chen, L.; et al. The effect of hierarchical single-crystal ZSM-5 zeolites with different Si/Al ratios on its pore structure and catalytic performance. Front. Chem. Sci. Eng. 2021, 15, 269–278. [Google Scholar] [CrossRef]
- Narayanan, S.; Vijaya, J.J.; Sivasanker, S.; Ragupathi, C.; Sankaranarayanan, T.M.; Kennedy, L.J. Hierarchical ZSM-5 catalytic performance evaluated in the selective oxidation of styrene to benzaldehyde using TBHP. J. Porous Mater. 2016, 23, 741–752. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, Y.; Mokaya, R. Zeolite ZSM-5 with Unique Supermicropores Synthesized Using Mesoporous Carbon as a Template. Adv. Mater. 2004, 16, 727–732. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, L.; Song, Y.; Shang, Y.; Zhai, Y.; Gong, Y. A comparative synthesis of ZSM-5 with ethanol or TPABr template: Distinction of Brønsted/Lewis acidity ratio and its impact on n-hexane cracking. Catal. Sci. Technol. 2018, 8, 1923–1935. [Google Scholar] [CrossRef]
- Lutsko, J.F.; Basios, V.; Nicolis, G.; Kozak, J.J.; Sleutel, M.; Maes, D. Kinetic rougheninglike transition with finite nucleation barrier. J. Chem. Phys. 2010, 132, 035102. [Google Scholar] [CrossRef]
- Chawla, A.; Li, R.; Jain, R.; Clark, R.J.; Sutjianto, J.G.; Palmer, J.C.; Rimer, J.D. Cooperative effects of inorganic and organic structure-directing agents in ZSM-5 crystallization. Mol. Syst. Des. Eng. 2018, 3, 159–170. [Google Scholar] [CrossRef]
- Tao, Y.; Ren, M.; Zhang, H.; Peijs, T. Recent progress in acoustic materials and noise control strategies—A review. Appl. Mater. Today 2021, 24, 101141. [Google Scholar] [CrossRef]
- Xu, D.-P.; Sun, P.; Kwon, J.-H.; Hwang, S.-M. An integrated design of microspeaker module with smaller volume. J. Appl. Phys. 2014, 115, 17A339. [Google Scholar] [CrossRef]
- Foust Iii, H.C. Equations of State. In Thermodynamics, Gas Dynamics, and Combustion; Foust Iii, H.C., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–23. [Google Scholar]
- Beranek, L.L.; Mellow, T.J. Chapter 1—Introduction and terminology. In Acoustics: Sound Fields and Transducers; Beranek, L.L., Mellow, T.J., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 1–19. [Google Scholar]
- Wang, Y. A study of kinetic air separation at low temperatures: Oxygen, nitrogen, and argon in carbon molecular sieve. Sep. Purif. Technol. 2023, 312, 123372. [Google Scholar] [CrossRef]
- Feng, C.; Jiaqiang, E.; Han, W.; Deng, Y.; Zhang, B.; Zhao, X.; Han, D. Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 2021, 144, 110954. [Google Scholar] [CrossRef]
- Cai, W.; He, H.; Zhu, S.; Wang, N. Biological Effect of Audible Sound Control on Mung Bean (Vigna radiate) Sprout. BioMed Res. Int. 2014, 2014, 931740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, S.; Chen, J.; Wei, Y.; Li, J.; Fan, D.; Yu, Z.; Qi, Y.; He, Y.; Xu, S.; et al. Synthesis of mesoporous ZSM-5 catalysts using different mesogenous templates and their application in methanol conversion for enhanced catalyst lifespan. RSC Adv. 2014, 4, 21479–21491. [Google Scholar] [CrossRef]


| Sample | Na2O (wt%) | Al2O3 (wt%) | SiO2 (wt%) | Actual SAR | Relative Crystallinity (%) |
|---|---|---|---|---|---|
| A1 | 1.53 | 1.88 | 96.29 | 87 | 76 |
| A2 | 0.64 | 0.97 | 98.10 | 172 | 97 |
| A3 | 0.22 | 0.28 | 99.31 | 614 | 100 |
| A4 | 0.20 | 0.14 | 99.35 | 1233 | 73 |
| A5 | 0.17 | 0.03 | 99.59 | 6625 | 30 |
| Sample | Stotal (m2·g−1) | Vtotal (cm3·g−1) | Sext (m2·g−1) | Vmicro (cm3·g−1) | Vmeso (cm3·g−1) |
|---|---|---|---|---|---|
| A1 | 394 | 0.24 | 31 | 0.19 | 0.05 |
| A2 | 384 | 0.23 | 47 | 0.14 | 0.09 |
| A3 | 384 | 0.24 | 60 | 0.12 | 0.12 |
| A4 | 389 | 0.25 | 68 | 0.13 | 0.12 |
| A5 | 382 | 0.23 | 50 | 0.13 | 0.10 |
| Sample | Na (wt%) | Stotal (m2·g−1) | Vtotal (cm3·g−1) | Sext (m2·g−1) | Vmicro (cm3·g−1) | Vmeso (cm3·g−1) |
|---|---|---|---|---|---|---|
| A3-Na-1 | 1.04 | 430 | 0.25 | 27 | 0.21 | 0.04 |
| A3-Na-2 | 0.68 | 400 | 0.25 | 47 | 0.17 | 0.08 |
| A3-Na-3 | 0.53 | 378 | 0.23 | 57 | 0.13 | 0.10 |
| Sample | Stotal (m2·g−1) | Vtotal (cm3·g−1) | Sext (m2·g−1) | Vmicro (cm3·g−1) | Vmeso (cm3·g−1) |
|---|---|---|---|---|---|
| ZSM-5-M1 | 418 | 0.18 | 0 | 0.18 | 0 |
| SBA-15 | 466 | 1.04 | 70 | 0.91 | 0.13 |
| MCM-41 | 245 | 0.87 | 19 | 0.83 | 0.04 |
| Mesoporous silica | 125 | 0.57 | 125 | 0 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Wang, Y.; Zhang, L.; Lu, J.; Gong, C.; Zhang, W.; Fang, Y.; Zhu, X.; Che, S. Acoustic Enhancement Performance of Hierarchical ZSM-5 Zeolites with Different Si/Al Ratios. Nanomaterials 2025, 15, 797. https://doi.org/10.3390/nano15110797
Guo M, Wang Y, Zhang L, Lu J, Gong C, Zhang W, Fang Y, Zhu X, Che S. Acoustic Enhancement Performance of Hierarchical ZSM-5 Zeolites with Different Si/Al Ratios. Nanomaterials. 2025; 15(11):797. https://doi.org/10.3390/nano15110797
Chicago/Turabian StyleGuo, Mingbo, Yijun Wang, Lei Zhang, Junran Lu, Chang Gong, Wanning Zhang, Yuxi Fang, Xinyuan Zhu, and Shunai Che. 2025. "Acoustic Enhancement Performance of Hierarchical ZSM-5 Zeolites with Different Si/Al Ratios" Nanomaterials 15, no. 11: 797. https://doi.org/10.3390/nano15110797
APA StyleGuo, M., Wang, Y., Zhang, L., Lu, J., Gong, C., Zhang, W., Fang, Y., Zhu, X., & Che, S. (2025). Acoustic Enhancement Performance of Hierarchical ZSM-5 Zeolites with Different Si/Al Ratios. Nanomaterials, 15(11), 797. https://doi.org/10.3390/nano15110797







