Surface In Situ Growth of Two-Dimensional/Three-Dimensional Heterojunction Perovskite Film for Achieving High-Performance Flexible Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solution Preparation
2.3. Device Fabrication
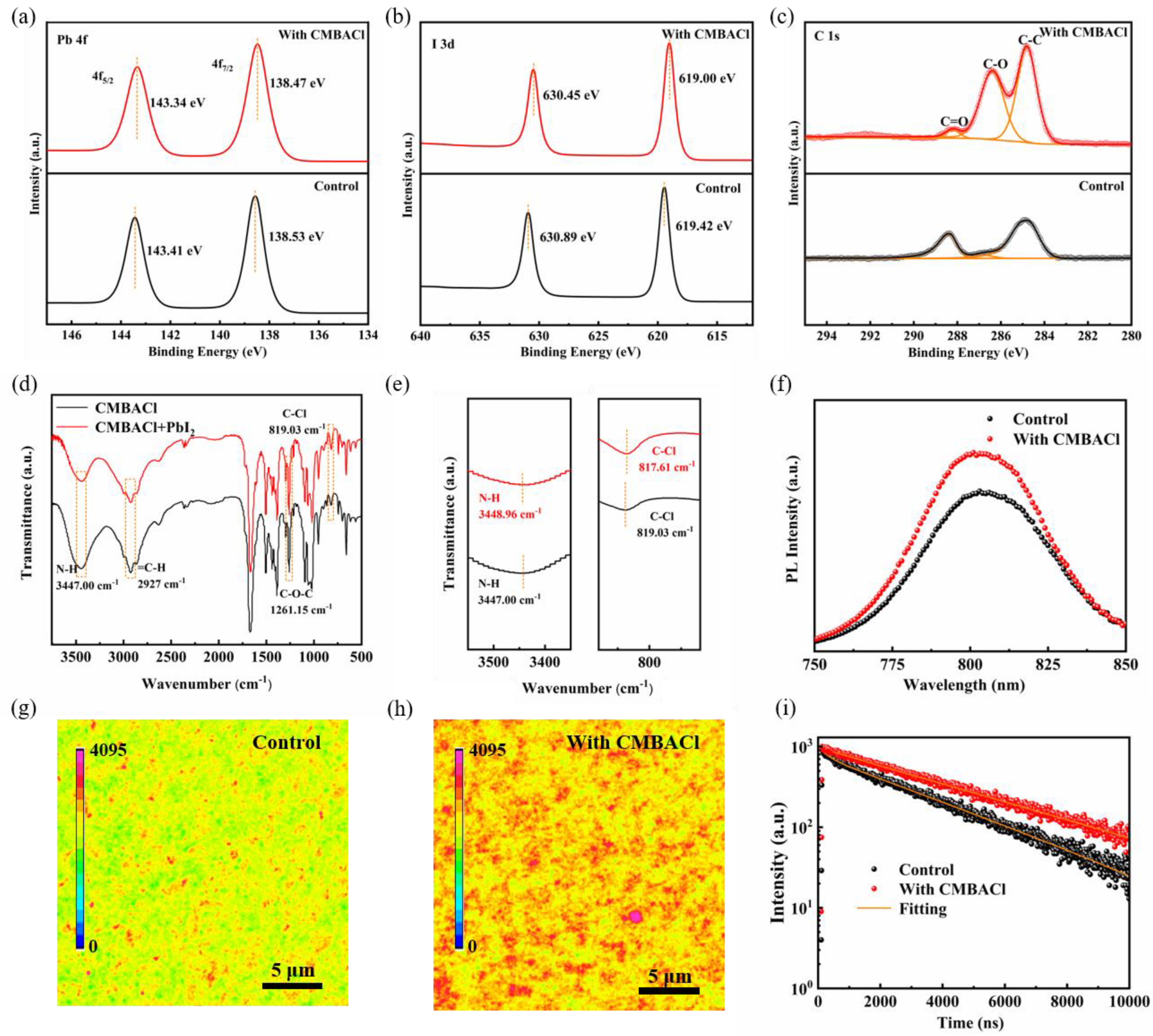
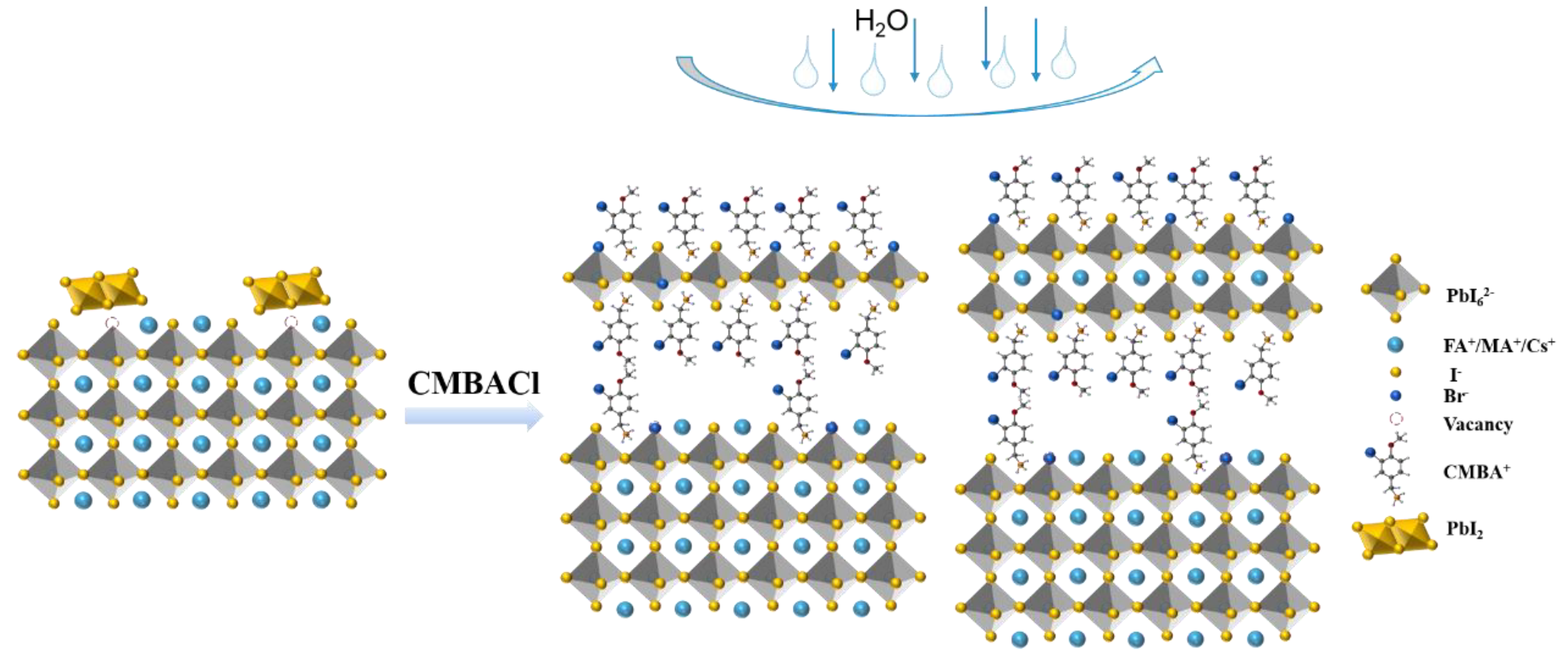
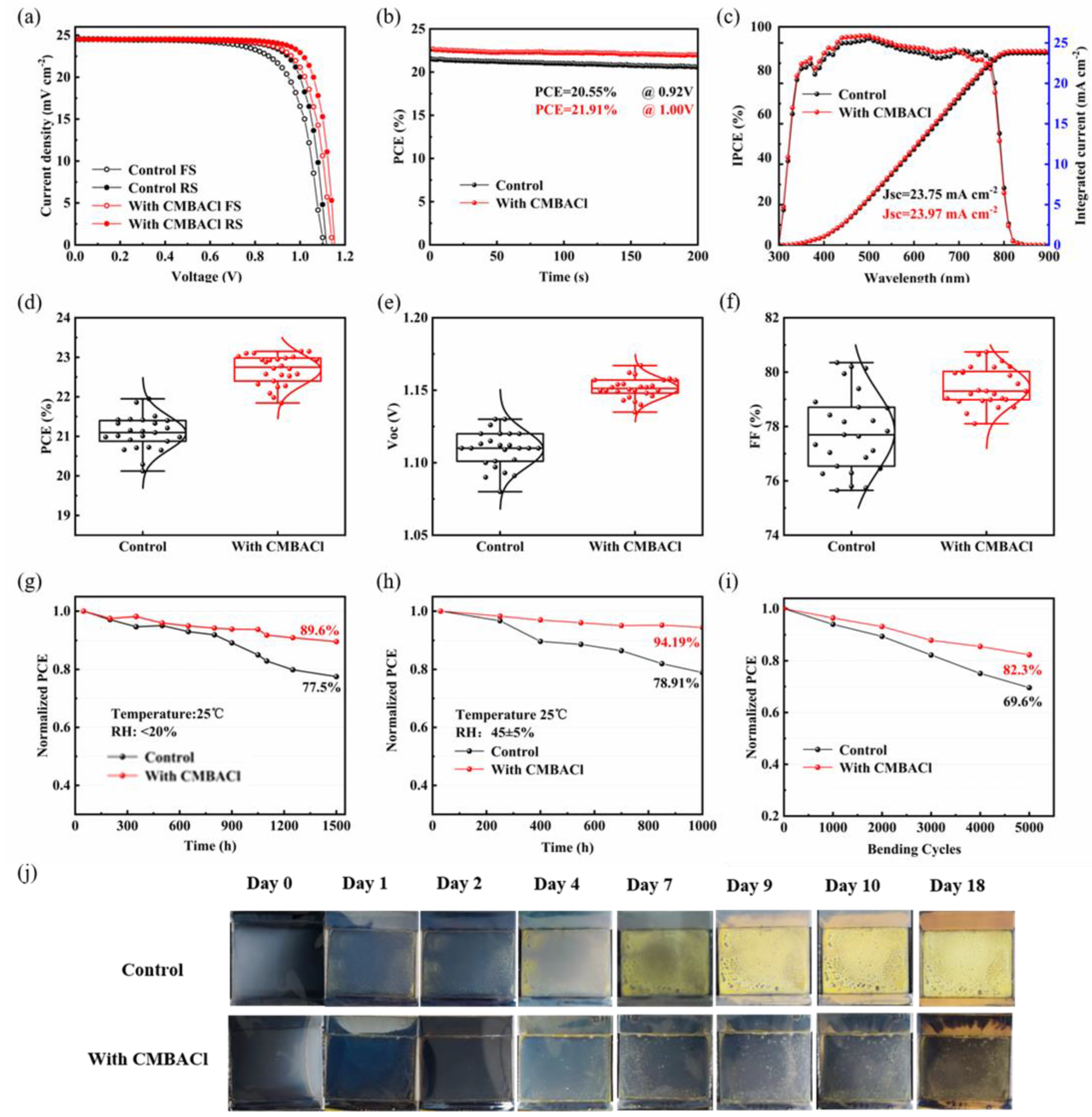
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced Electron Extraction Using SnO2 for High-Efficiency Planar-Structure HC(NH2)2PbI3-Based Perovskite Solar Cells. Nat. Energy 2016, 2, 16177. [Google Scholar] [CrossRef]
- Lu, H.; Krishna, A.; Zakeeruddin, S.M.; Grätzel, M.; Hagfeldt, A. Compositional and Interface Engineering of Organic-Inorganic Lead Halide Perovskite Solar Cells. iScience 2020, 23, 101359. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Guo, X.; Lu, C.; Wei, J.; Fang, J. Constructing Heterojunctions by Surface Sulfidation for Efficient Inverted Perovskite Solar Cells. Science 2022, 375, 434–437. [Google Scholar] [CrossRef]
- Interactive Best Research-Cell Efficiency Chart|Photovoltaic Research|NREL. Available online: https://www.nrel.gov/pv/interactive-cell-efficiency.html (accessed on 25 February 2025).
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Xue, T.; Fan, B.; Jiang, K.-J.; Guo, Q.; Hu, X.; Su, M.; Zhou, E.; Song, Y. Self-Healing Ion-Conducting Elastomer towards Record Efficient Flexible Perovskite Solar Cells with Excellent Recoverable Mechanical Stability. Energy Environ. Sci. 2024, 17, 2621–2630. [Google Scholar] [CrossRef]
- Heo, J.H.; Lee, D.S.; Shin, D.H.; Im, S.H. Recent Advancements in and Perspectives on Flexible Hybrid Perovskite Solar Cells. J. Mater. Chem. A 2019, 7, 888–900. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Tress, W.; Dar, M.I.; Gao, P.; Luo, J.; Renevier, C.; Schenk, K.; Abate, A.; Giordano, F.; Correa Baena, J.-P.; et al. Efficient Luminescent Solar Cells Based on Tailored Mixed-Cation Perovskites. Sci. Adv. 2016, 2, e1501170. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide Management in Formamidinium-Lead-Halide–Based Perovskite Layers for Efficient Solar Cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface Passivation of Perovskite Film for Efficient Solar Cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Y.; Zhang, X.; You, J. Recent Progresses on Defect Passivation toward Efficient Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1902650. [Google Scholar] [CrossRef]
- Zhu, H.; Teale, S.; Lintangpradipto, M.N.; Mahesh, S.; Chen, B.; McGehee, M.D.; Sargent, E.H.; Bakr, O.M. Long-Term Operating Stability in Perovskite Photovoltaics. Nat. Rev. Mater. 2023, 8, 569–586. [Google Scholar] [CrossRef]
- Luo, D.; Su, R.; Zhang, W.; Gong, Q.; Zhu, R. Minimizing Non-Radiative Recombination Losses in Perovskite Solar Cells. Nat. Rev. Mater. 2020, 5, 44–60. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, F.; Gong, J.; Ma, Y.; Gu, J.; Liu, X.; Chen, S.; Liu, M. Pre-Buried Additive for Cross-Layer Modification in Flexible Perovskite Solar Cells with Efficiency Exceeding 22%. Adv. Mater. 2022, 34, 2109879. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Tan, L.; Li, H.; Liu, Y.; Jiang, C.; Ye, Y.; Ding, L.; Tress, W.; Yi, C. Multifunctional Succinate Additive for Flexible Perovskite Solar Cells with More than 23% Power-Conversion Efficiency. Innovation 2022, 3, 100310. [Google Scholar] [CrossRef]
- Huang, K.; Peng, Y.; Gao, Y.; Shi, J.; Li, H.; Mo, X.; Huang, H.; Gao, Y.; Ding, L.; Yang, J. High-Performance Flexible Perovskite Solar Cells via Precise Control of Electron Transport Layer. Adv. Energy Mater. 2019, 9, 1901419. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Guo, J.; Zhang, Z.; Zhang, X.; Zhao, Y. Bio-Inspired Stretchable, Adhesive, and Conductive Structural Color Film for Visually Flexible Electronics. Adv. Funct. Mater. 2020, 30, 200015. [Google Scholar] [CrossRef]
- Bi, C.; Chen, B.; Wei, H.; DeLuca, S.; Huang, J. Efficient Flexible Solar Cell Based on Composition-Tailored Hybrid Perovskite. Adv. Mater. 2017, 29, 1605900. [Google Scholar] [CrossRef]
- Yi, Z.; Xiao, B.; Li, X.; Luo, Y.; Jiang, Q.; Yang, J. Novel Dual-Modification Strategy Using Ce-Containing Compounds toward High-Performance Flexible Perovskite Solar Cells. Nano Energy 2023, 109, 108241. [Google Scholar] [CrossRef]
- Liu, C.; Huang, K.; Hu, B.; Li, Y.; Zhang, L.; Zhou, X.; Liu, Y.; Liu, Z.; Sheng, Y.; Chen, S.; et al. Concurrent Top and Buried Surface Optimization for Flexible Perovskite Solar Cells with High Efficiency and Stability. Adv. Funct. Mater. 2023, 33, 2212698. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, X.; Yang, Z.; Zhang, X.; Niu, J.; Wang, Z.; Zuo, S.; Priya, S.; Liu, S.; Yang, D. Record Efficiency Stable Flexible Perovskite Solar Cell Using Effective Additive Assistant Strategy. Adv. Mater. 2018, 30, 1801418. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, G.; Xi, J.; Shen, Y.; Wu, X.; Tang, X.; Ding, J.; Yang, H.; Cheng, Q.; Chen, Z.; et al. In Situ Crosslinking-Assisted Perovskite Grain Growth for Mechanically Robust Flexible Perovskite Solar Cells with 23.4% Efficiency. Joule 2023, 7, 398–415. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, W.; Shang, Y.; Zhou, W.; Zhang, W.; Yang, S. Surface Passivation of Perovskite Film by Sodium Toluenesulfonate for Highly Efficient Solar Cells. Solar RRL 2020, 4, 2000113. [Google Scholar] [CrossRef]
- Wu, G.; Liang, R.; Ge, M.; Sun, G.; Zhang, Y.; Xing, G. Surface Passivation Using 2D Perovskites toward Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2022, 34, 2105635. [Google Scholar] [CrossRef]
- Krishna, A.; Gottis, S.; Nazeeruddin, M.K.; Sauvage, F. Mixed Dimensional 2D/3D Hybrid Perovskite Absorbers: The Future of Perovskite Solar Cells? Adv. Funct. Mater. 2019, 29, 1806482. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.-U.; Lee, D.Y.; Paik, M.J.; Na, H.; Lee, J.; Seok, S.I. Optimal Interfacial Engineering with Different Length of Alkylammonium Halide for Efficient and Stable Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1902740. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Wang, S.; Lyu, M.; Yun, J.-H.; Wang, L. In Situ Growth of 2D Perovskite Capping Layer for Stable and Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1706923. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, T.; Loo, Y.-L. Advancing 2D Perovskites for Efficient and Stable Solar Cells: Challenges and Opportunities. Adv. Mater. 2022, 34, 210584. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Ye, J.; Xu, S.; Zhang, L.; Xu, H.; Liang, Z.; Chen, X.; Pan, X. Mixed-Phase Low-Dimensional Perovskite-Assisted Interfacial Lead Directional Management for Stable Perovskite Solar Cells with Efficiency over 24%. ACS Energy Lett. 2021, 6, 4395–4404. [Google Scholar] [CrossRef]
- Du, D.; Liu, G.; Zhang, L.; Tao, Y.; Zhang, H.; Guo, T.; Xu, H.; Xu, S.; Ye, J.; Zheng, H.; et al. Conjugate Cyclic Cations Engineering for High-Performance 2D/3D Heterojunction Perovskite Solar Cells. Chem. Eng. J. 2023, 467, 143392. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, M.; Liu, Y.; Eickemeyer, F.T.; Zhao, W.; Dai, Z.; Yin, Y.; Jiang, C.; Feng, J.; Jin, S.; et al. Flexible Perovskite Solar Cells with Simultaneously Improved Efficiency, Operational Stability, and Mechanical Reliability. Joule 2021, 5, 1587–1601. [Google Scholar] [CrossRef]
- Han, B.; Wang, Y.; Liu, C.; Sun, K.; Yang, M.; Xie, L.; Yang, S.; Meng, Y.; Lin, S.; Xu, P.; et al. Rational Design of Ferroelectric 2D Perovskite for Improving the Efficiency of Flexible Perovskite Solar Cells Over 23 %. Angew. Chem. Int. Ed. 2023, 62, e202217526. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Tsai, C.-C.; Liao, M.-Y.; Su, Y.-A.; Lin, S.-T.; Chueh, C.-C. Stable, Color-Tunable 2D SCN-Based Perovskites: Revealing the Critical Influence of an Asymmetric Pseudo-Halide on Constituent Ions. Nanoscale 2019, 11, 2608–2616. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Song, T.-B.; Luo, S.; Hong, Z.; Duan, H.-S.; Dou, L.; Liu, Y.; Yang, Y. Controllable Self-Induced Passivation of Hybrid Lead Iodide Perovskites toward High Performance Solar Cells. Nano Lett. 2014, 14, 4158–4163. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, T.; Wang, B.; Li, J.; Li, D.; Wang, G.; Lian, Q.; Amini, A.; Chen, S.; Cheng, C.; et al. Antisolvent Engineering to Optimize Grain Crystallinity and Hole-Blocking Capability of Perovskite Films for High-Performance Photovoltaics. Adv. Mater. 2021, 33, 2102816. [Google Scholar] [CrossRef]
- Li, N.; Niu, X.; Pei, F.; Liu, H.; Cao, Y.; Liu, Y.; Xie, H.; Gao, Y.; Chen, Q.; Mo, F.; et al. Energy-Level Modulation in Diboron-Modified SnO2 for High-Efficiency Perovskite Solar Cells. Solar RRL 2020, 4, 1900217. [Google Scholar] [CrossRef]
- SunLi, Z.; Liu, Y.; Li, S.; Ren, J.; Wu, Y.; Sun, Q.; Cui, Y.; Chen, M.; Hao, Y. 2D Perovsktie Substrate-Assisted CsPbI3 Film Growth for High-Efficiency Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 7417–7742. [Google Scholar] [CrossRef]
- Xu, D.; Li, T.; Han, Y.; He, X.; Yang, S.; Che, Y.; Xu, J.; Zou, H.; Guo, X.; Wang, J.; et al. Fluorine Functionalized MXene QDs for Near-Record-Efficiency CsPbI3 Solar Cell with High Open-Circuit Voltage. Adv. Funct. Mater. 2022, 32, 2203704. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, X.; Yang, R.; Yang, Z.; Yu, W.; Wang, X.; Li, C.; Liu, S.; Chang, R.P.H. Surface Optimization to Eliminate Hysteresis for Record Efficiency Planar Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 3071–3078. [Google Scholar] [CrossRef]
- Gao, C.; Jia, S.; Yin, X.; Li, Z.; Yang, G.; Chen, J.; Li, Z.; An, X. Enhancing Open-Circuit Voltage in FAPbI3 Perovskite Solar Cells via Self-Formation of Coherent Buried Interface FAPbIxCl3−x. Chem. Commun. 2025, 61, 2758–2761. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Li, Y.; Gao, C.; Liu, G.; Ren, Y.; He, C.; An, X.-T. Realization of P-Type MA-Based Perovskite Solar Cells Based on Exposure of the (002) Facet. Appl. Phys. Lett. 2025, 126, 023908. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Chen, H.; Spanopoulos, I.; Bati, A.S.R.; Gilley, I.W.; Chen, J.; Maxwell, A.; Vishal, B.; Reynolds, R.P.; et al. Two-Dimensional Perovskitoids Enhance Stability in Perovskite Solar Cells. Nature 2024, 633, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Park, S.Y.; Yao, C.; Lu, H.; Dunfield, S.P.; Xiao, C.; Uličná, S.; Zhao, X.; Du Hill, L.; Chen, X.; et al. Metastable Dion-Jacobson 2D Structure Enables Efficient and Stable Perovskite Solar Cells. Science 2022, 375, 71–76. [Google Scholar] [CrossRef]
- Yang, T.; Ma, C.; Cai, W.; Wang, S.; Wu, Y.; Feng, J.; Wu, N.; Li, H.; Huang, W.; Ding, Z.; et al. Amidino-Based Dion-Jacobson 2D Perovskite for Efficient and Stable 2D/3D Heterostructure Perovskite Solar Cells. Joule 2023, 7, 574–586. [Google Scholar] [CrossRef]
- Wang, H.; Su, S.; Chen, Y.; Ren, M.; Wang, S.; Wang, Y.; Zhu, C.; Miao, Y.; Ouyang, C.; Zhao, Y. Impurity-Healing Interface Engineering for Efficient Perovskite Submodules. Nature 2024, 634, 1091–1095. [Google Scholar] [CrossRef]
- Yao, K.; Wang, X.; Xu, Y.; Li, F. A General Fabrication Procedure for Efficient and Stable Planar Perovskite Solar Cells: Morphological and Interfacial Control by in-Situ-Generated Layered Perovskite. Nano Energy 2015, 18, 165–175. [Google Scholar] [CrossRef]
- Sidhik, S.; Metcalf, I.; Li, W.; Kodalle, T.; Dolan, C.J.; Khalili, M.; Hou, J.; Mandani, F.; Torma, A.; Zhang, H.; et al. Two-Dimensional Perovskite Templates for Durable, Efficient Formamidinium Perovskite Solar Cells. Science 2024, 384, 1227–1235. [Google Scholar] [CrossRef]
- Gong, C.; Chen, X.; Zeng, J.; Wang, H.; Li, H.; Qian, Q.; Zhang, C.; Zhuang, Q.; Yu, X.; Gong, S.; et al. Functional-Group-Induced Single Quantum Well Dion–Jacobson 2D Perovskite for Efficient and Stable Inverted Perovskite Solar Cells. Adv. Mater. 2024, 36, 2307422. [Google Scholar] [CrossRef]
- Azmi, R.; Ugur, E.; Seitkhan, A.; Aljamaan, F.; Subbiah, A.S.; Liu, J.; Harrison, G.T.; Nugraha, M.I.; Eswaran, M.K.; Babics, M.; et al. Damp Heat–Stable Perovskite Solar Cells with Tailored-Dimensionality 2D/3D Heterojunctions. Science 2022, 376, 73–77. [Google Scholar] [CrossRef]
- Sidhik, S.; Wang, Y.; De Siena, M.; Asadpour, R.; Torma, A.J.; Terlier, T.; Ho, K.; Li, W.; Puthirath, A.B.; Shuai, X.; et al. Deterministic Fabrication of 3D/2D Perovskite Bilayer Stacks for Durable and Efficient Solar Cells. Science 2022, 377, 1425–1430. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liu, H.; Liu, J.; Xu, J.; Tan, Z.; Yao, J. Surface In Situ Growth of Two-Dimensional/Three-Dimensional Heterojunction Perovskite Film for Achieving High-Performance Flexible Perovskite Solar Cells. Nanomaterials 2025, 15, 798. https://doi.org/10.3390/nano15110798
Zhang Z, Liu H, Liu J, Xu J, Tan Z, Yao J. Surface In Situ Growth of Two-Dimensional/Three-Dimensional Heterojunction Perovskite Film for Achieving High-Performance Flexible Perovskite Solar Cells. Nanomaterials. 2025; 15(11):798. https://doi.org/10.3390/nano15110798
Chicago/Turabian StyleZhang, Zhiyu, Huijing Liu, Jing Liu, Jia Xu, Zhan’ao Tan, and Jianxi Yao. 2025. "Surface In Situ Growth of Two-Dimensional/Three-Dimensional Heterojunction Perovskite Film for Achieving High-Performance Flexible Perovskite Solar Cells" Nanomaterials 15, no. 11: 798. https://doi.org/10.3390/nano15110798
APA StyleZhang, Z., Liu, H., Liu, J., Xu, J., Tan, Z., & Yao, J. (2025). Surface In Situ Growth of Two-Dimensional/Three-Dimensional Heterojunction Perovskite Film for Achieving High-Performance Flexible Perovskite Solar Cells. Nanomaterials, 15(11), 798. https://doi.org/10.3390/nano15110798








