Microstructural and Elemental Characterization of Calcium Silicate-Based Sealers
Abstract
1. Introduction
2. Materials and Methods
2.1. Root Canal Sealers
2.2. Sample Preparation
2.3. SEM Imaging
2.4. EDX Analysis
2.5. XRD Investigation
2.6. Statistical Analysis
3. Results
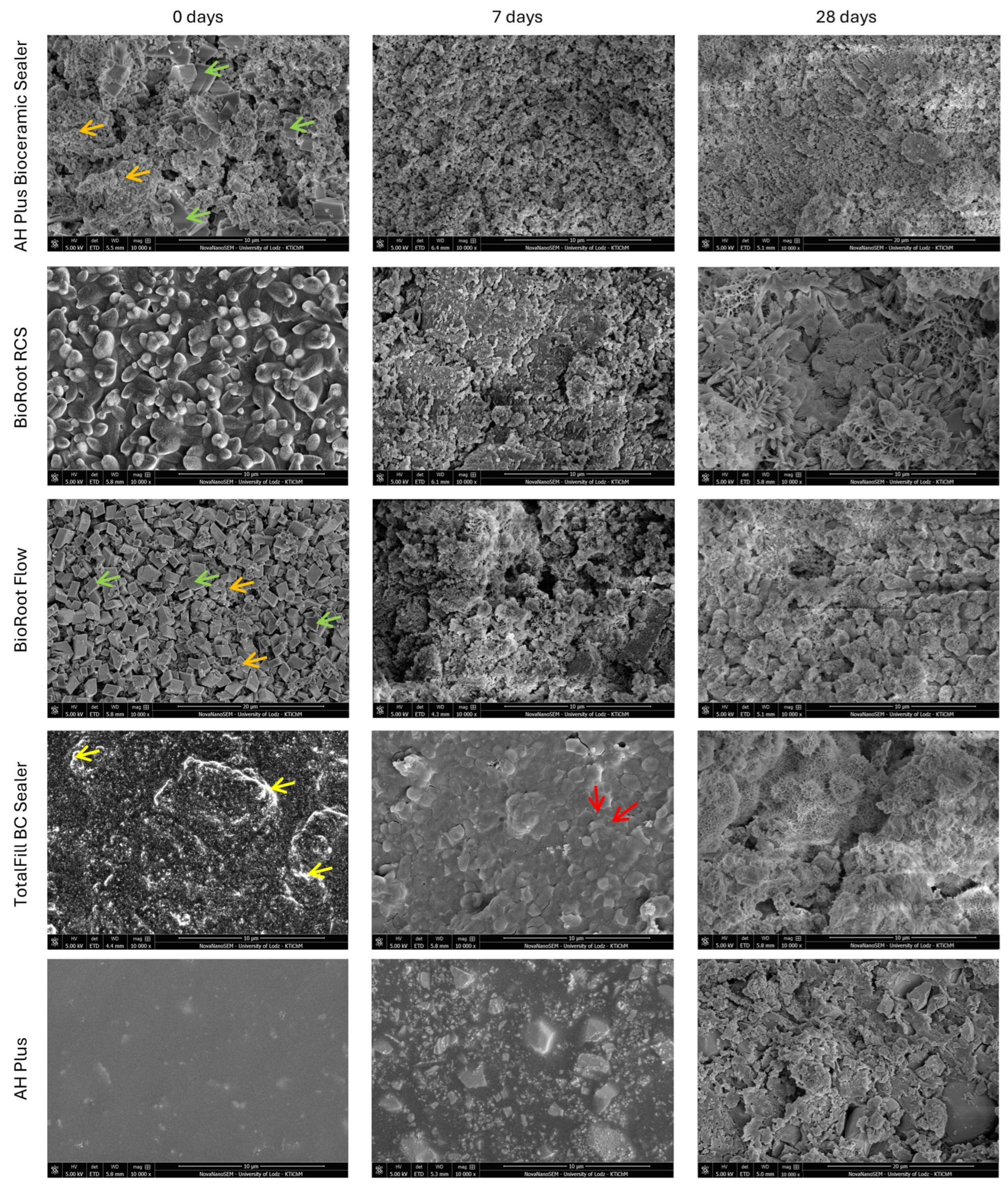
3.1. SEM Imaging
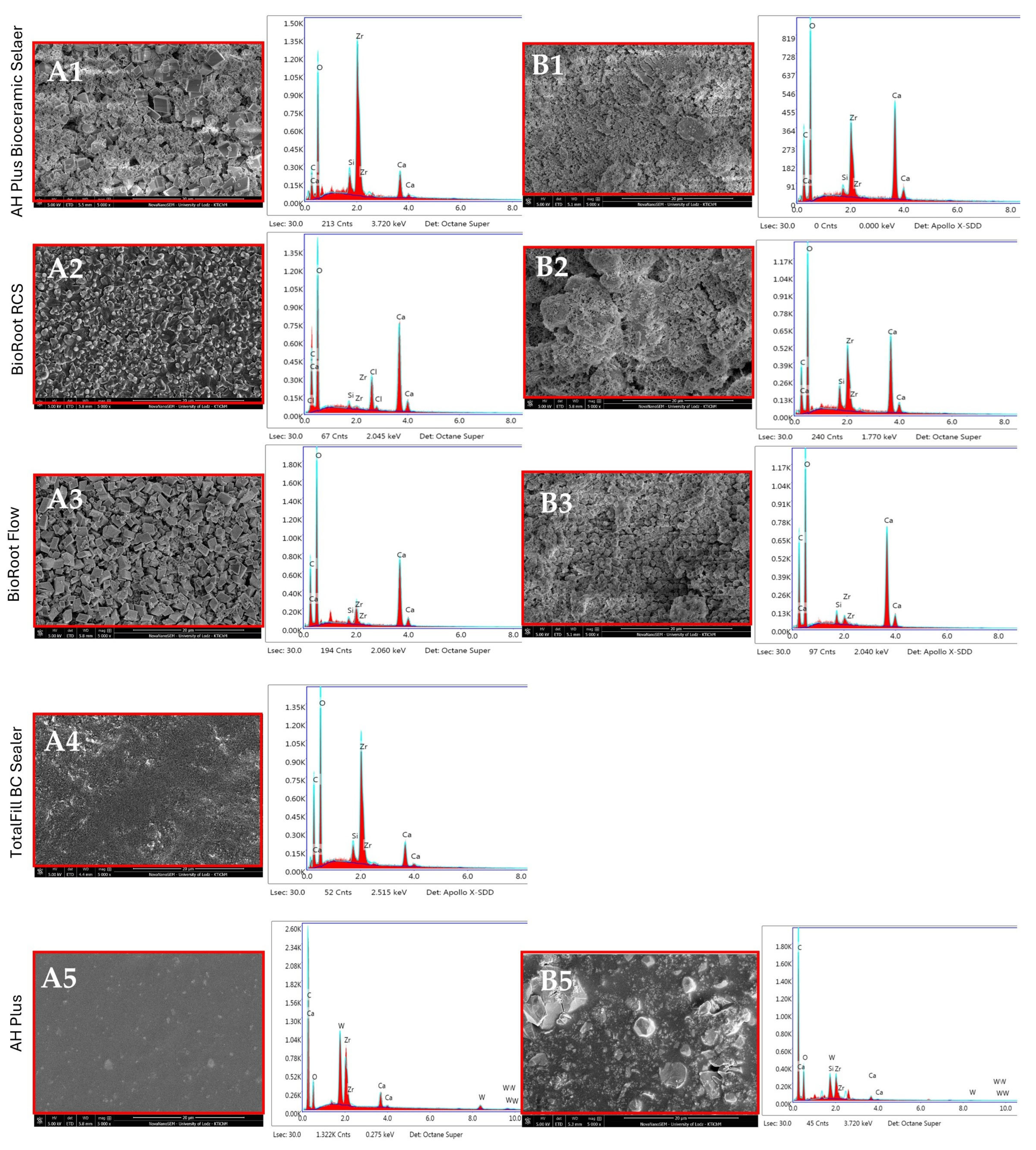
3.2. EDX Analysis
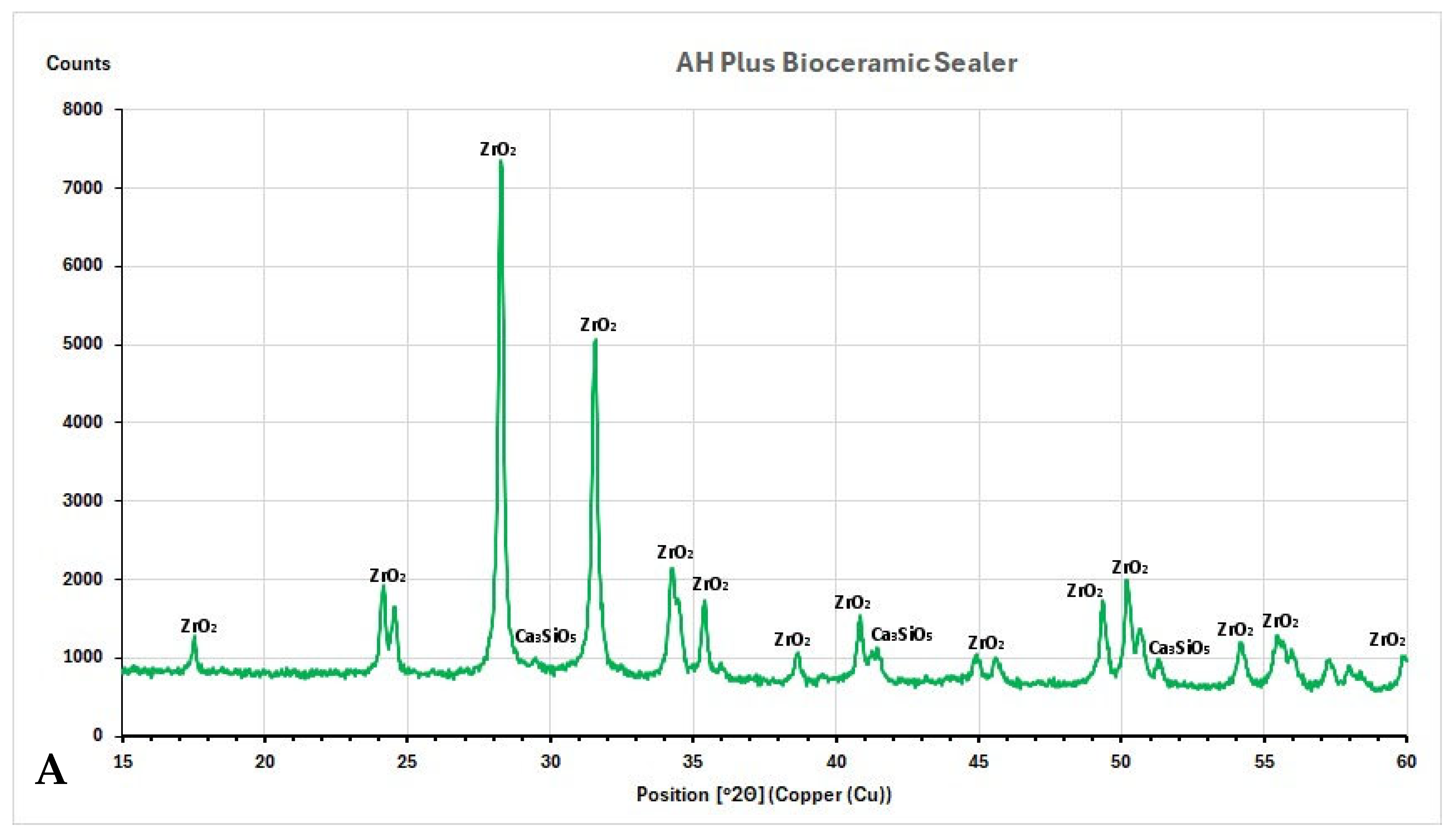
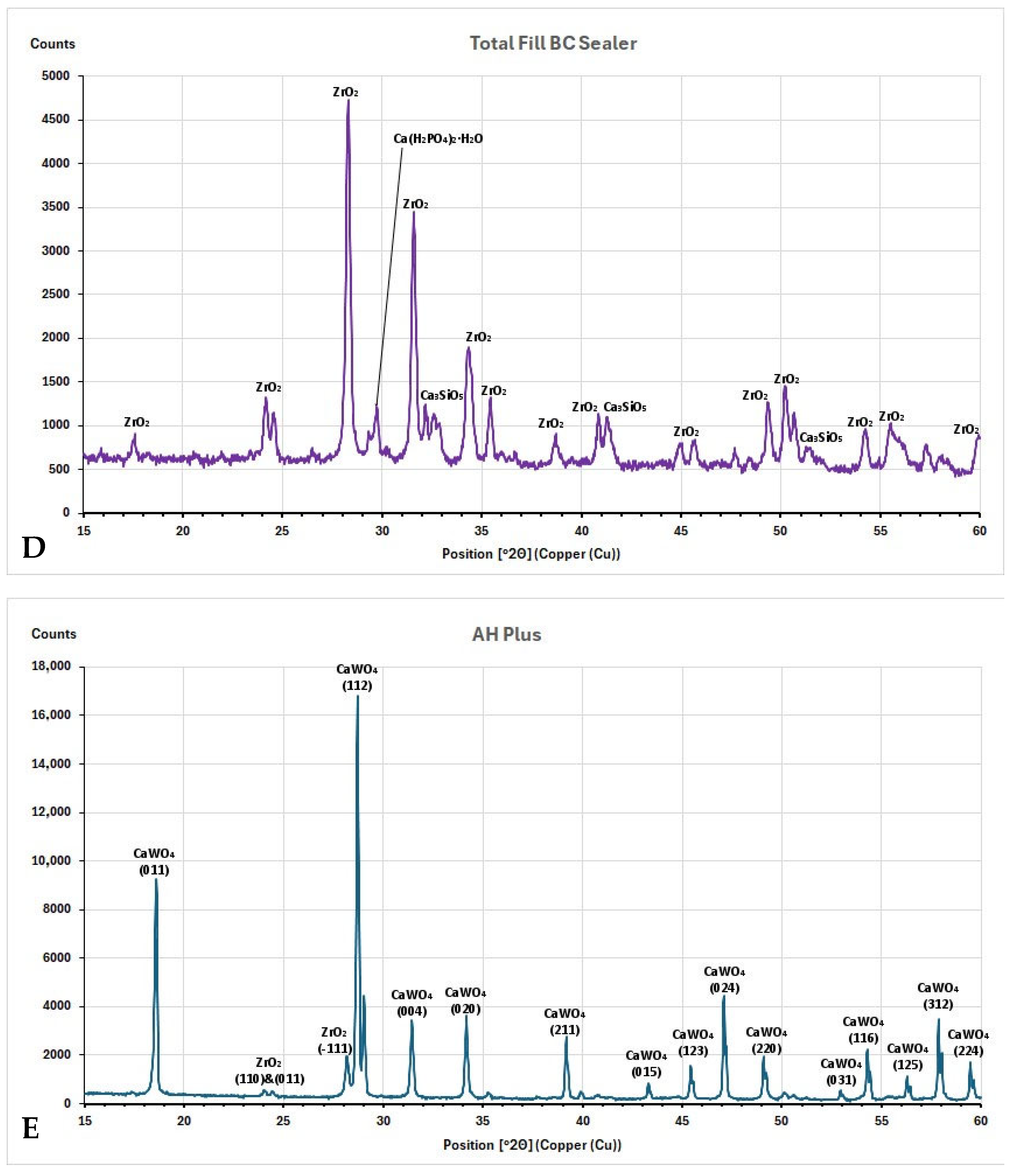
3.3. XRD Investigation
4. Discussion
4.1. Characterization of Root Canal Sealers Through SEM
4.2. Characterization of Root Canal Sealers Through EDX
4.3. Characterization of Root Canal Sealers Through XRD
4.4. Limitations of the Study
4.5. Summary and Future Perspectives
5. Conclusions
- Despite being classified in the same group, calcium-silicate-based sealers showed high heterogeneity in terms of surface characterization both immediately after setting and during the incubation period. The precipitation of crystalline compounds on their surface increased with time.
- For AH Plus Bioceramic Selaer, BioRoot RCS, and BioRoot Flow, a significant increase in the calcium content by weight after incubation was observed, while for AH Plus (control), a decrease in this element was found. Consequently, it can be hypothesized that the increase in pH may induce mineralization, apatite formation, and antibacterial potential, promoting healing.
- Apatite nucleation was not observed, suggesting possible increased solubility of the sealer. Consequently, a compromised seal in the apical region could lead to clinical failure.
- The main crystalline phase for all tested CSBS was zirconium oxide, and for ERBS was calcium tungstate.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruddle, C.J. Three-Dimensional Obturation of the Root Canal System. Dent Today 1992, 11, 28–30. [Google Scholar]
- Vishwanath, V.; Rao, H.M. Gutta-Percha in Endodontics—A Comprehensive Review of Material Science. J. Conserv. Dent. Endod. 2019, 22, 216–222. [Google Scholar] [CrossRef]
- Alkahtany, M.F.; Almadi, K.H.; Alahmad, F.A.; Alshehri, A.M.; Alswayyed, A.A.; Alzahran, O.M.; Alhadan, A.; Almustafa, A.S.; Vohra, F.; Abduljabbar, T. Influence of Root Canal Sealers and Obturation Techniques on Vertical Root Fracture Resistance. An in Vitro Experiment. Appl. Sci. 2021, 11, 8022. [Google Scholar] [CrossRef]
- Reszka, P.; Nowicka, A.; Lipski, M.; Dura, W.; Droździk, A.; Woźniak, K. A Comparative Chemical Study of Calcium Silicate-Containing and Epoxy Resin-Based Root Canal Sealers. Biomed Res. Int. 2016, 2016, 9808432. [Google Scholar] [CrossRef]
- Borges, Á.H.; Orçati Dorileo, M.C.G.; Dalla Villa, R.; Borba, A.M.; Semenoff, T.A.D.V.; Guedes, O.A.; Estrela, C.R.A.; Bandeca, M.C. Physicochemical Properties and Surfaces Morphologies Evaluation of MTA FillApex and AH Plus. Sci. World J. 2014, 2014, 589732. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, H.; Yildirim, S.; Cobankara, F.K. Cytotoxicity and Genotoxicity of Salicylate- and Calcium Silicate-Based Root Canal Sealers on Primer Human Periodontal Ligament Fibroblasts. Aust. Endod. J. 2021, 47, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.J.N.L.; Cardoso, M.L.; Rodrigues, J.P.; De-Deus, G.; Fidalgo, T.K.d.S. Solubility of Bioceramic- and Epoxy Resin-Based Root Canal Sealers: A Systematic Review and Meta-Analysis. Aust. Endod. J. 2021, 47, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Eldeniz, A.U.; Ørstavik, D. A Laboratory Assessment of Coronal Bacterial Leakage in Root Canals Filled with New and Conventional Sealers. Int. Endod. J. 2009, 42, 303–312. [Google Scholar] [CrossRef]
- Roggendorf, M.J.; Ebert, J.; Petschelt, A.; Frankenberger, R. Influence of Moisture on the Apical Seal of Root Canal Fillings with Five Different Types of Sealer. J. Endod. 2007, 33, 31–33. [Google Scholar] [CrossRef]
- Najafzadeh, R.; Fazlyab, M.; Esnaashari, E. Comparison of Bioceramic and Epoxy Resin Sealers in Terms of Marginal Adaptation and Tubular Penetration Depth with Different Obturation Techniques in Premolar Teeth: A Scanning Electron Microscope and Confocal Laser Scanning Microscopy Study. J. Fam. Med. Prim. Care 2022, 11, 1794–1797. [Google Scholar] [CrossRef]
- Cardinali, F.; Camilleri, J. A Critical Review of the Material Properties Guiding the Clinician’s Choice of Root Canal Sealers. Clin. Oral Investig. 2023, 27, 4147–4155. [Google Scholar] [CrossRef] [PubMed]
- Donnermeyer, D.; Bürklein, S.; Dammaschke, T.; Schäfer, E. Endodontic Sealers Based on Calcium Silicates: A Systematic Review. Odontology 2019, 107, 421–436. [Google Scholar] [CrossRef]
- Reszka, P.; Kucharski, Ł.; Klimowicz, A.; Lipski, M. Właściwości Alkalizujące Wybranych Krzemianowo-Wapniowych Uszczelniaczy Kanałowych. Badanie in Vitro Alkalizing Properties of Selected Calcium-Silicate Root Canal Sealers. An in Vitro Study. Pomeranian J. Life Sci. 2018, 64, 36–41. [Google Scholar] [CrossRef][Green Version]
- Sfeir, G.; Zogheib, C.; Patel, S.; Giraud, T.; Nagendrababu, V.; Bukiet, F. Calcium Silicate-Based Root Canal Sealers: A Narrative Review and Clinical Perspectives. Materials 2021, 14, 3965. [Google Scholar] [CrossRef] [PubMed]
- Milanovic, I.; Milovanovic, P.; Antonijevic, D.; Dzeletovic, B.; Djuric, M.; Miletic, V. Immediate and Long-Term Porosity of Calcium Silicate-Based Sealers. J. Endod. 2020, 46, 515–523. [Google Scholar] [CrossRef]
- Ersahan, S.; Aydin, C. Solubility and Apical Sealing Characteristics of a New Calcium Silicate-Based Root Canal Sealer in Comparison to Calcium Hydroxide-, Methacrylate Resin- and Epoxy Resin-Based Sealers. Acta Odontol. Scand. 2013, 71, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Maharti, I.D.; Rosseti, R.; Asrianti, D.; Herdianto, N.; Rianti, W. Calcium Silicate-Based Sealers: Apatite Deposition on Root Canal Dentin and PH Variation Analysis. Eur. J. Dent. 2025, 19, 374–381. [Google Scholar] [CrossRef]
- Reyes-Carmona, J.F.; Felippe, M.S.; Felippe, W.T. Biomineralization Ability and Interaction of Mineral Trioxide Aggregate and White Portland Cement with Dentin in a Phosphate-Containing Fluid. J. Endod. 2009, 35, 731–736. [Google Scholar] [CrossRef]
- Madadi, A.; Wei, J. Characterization of Calcium Silicate Hydrate Gels with Different Calcium to Silica Ratios and Polymer Modifications. Gels 2022, 8, 75. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Peng, B. Effects of IRoot SP on Mineralization-Related Genes Expression in MG63 Cells. J. Endod. 2010, 36, 1978–1982. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Peng, B. Ex Vivo Cytotoxicity of a New Calcium Silicate-Based Canal Filling Material. Int. Endod. J. 2010, 43, 769–774. [Google Scholar] [CrossRef]
- Radwanski, M.; Leski, M.; Puszkarz, A.K.; Sokolowski, J.; Hardan, L.; Bourgi, R.; Sauro, S.; Lukomska-Szymanska, M. A Micro-CT Analysis of Initial and Long-Term Pores Volume and Porosity of Bioactive Endodontic Sealers. Biomedicines 2022, 10, 2403. [Google Scholar] [CrossRef] [PubMed]
- Asawaworarit, W.; Pinyosopon, T.; Kijsamanmith, K. Comparison of Apical Sealing Ability of Bioceramic Sealer and Epoxy Resin-Based Sealer Using the Fluid Filtration Technique and Scanning Electron Microscopy. J. Dent. Sci. 2020, 15, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Damion, T.; Cepuritis, R.; Chaunsali, P. Sulfuric Acid and Citric Acid Attack of Calcium Sulfoaluminate-Based Binders. Cem. Concr. Compos. 2022, 130, 104524. [Google Scholar] [CrossRef]
- Allahverdi, A.; Škvára, F. Acidic Corrosion of Hydrated Cement Based Materials. Part 1. Mechanism of the Phenomenon. Ceram.-Silikaty 2000, 44, 114–120. [Google Scholar]
- Kunert, M.; Lukomska-Szymanska, M. Bio-Inductive Materials in Direct and Indirect Pulp Capping—A Review Article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef]
- Pontoriero, D.I.; Ferrari Cagidiaco, E.; Maccagnola, V.; Manfredini, D.; Ferrari, M. Outcomes of Endodontic-Treated Teeth Obturated with Bioceramic Sealers in Combination with Warm Gutta-Percha Obturation Techniques: A Prospective Clinical Study. J. Clin. Med. 2023, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- Zamparini, F.; Lenzi, J.; Duncan, H.F.; Spinelli, A.; Gandolfi, M.G.; Prati, C. The Efficacy of Premixed Bioceramic Sealers versus Standard Sealers on Root Canal Treatment Outcome, Extrusion Rate and Post-Obturation Pain: A Systematic Review and Meta-Analysis. Int. Endod. J. 2024, 57, 1021–1042. [Google Scholar] [CrossRef]
- Sanz, J.L.; López-García, S.; Lozano, A.; Pecci-Lloret, M.P.; Llena, C.; Guerrero-Gironés, J.; Rodríguez-Lozano, F.J.; Forner, L. Microstructural Composition, Ion Release, and Bioactive Potential of New Premixed Calcium Silicate-Based Endodontic Sealers Indicated for Warm Vertical Compaction Technique. Clin. Oral Investig. 2021, 25, 1451–1462. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Galal, M.M.; Ismail, A.G.; Saber, S. Physicochemical Properties of AH plus Bioceramic Sealer, Bio-C Sealer, and ADseal Root Canal Sealer. Head Face Med. 2024, 20, 2. [Google Scholar] [CrossRef]
- Radwanski, M.; Rozpedek-Kaminska, W.; Galita, G.; Siwecka, N.; Sokolowski, J.; Majsterek, I.; Özcan, M.; Lukomska-Szymanska, M. Cytotoxicity and Genotoxicity of Bioceramic Root Canal Sealers Compared to Conventional Resin-Based Sealer. Sci. Rep. 2024, 14, 4124. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F., Jr.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Camilleri, J.; et al. PRILE 2021 Guidelines for Reporting Laboratory Studies in Endodontology: A Consensus-Based Development. Int. Endod. J. 2021, 54, 1482–1490. [Google Scholar] [CrossRef]
- Nagendrababu, V.; Murray, P.E.; Ordinola-Zapata, R.; Peters, O.A.; Rôças, I.N.; Siqueira, J.F., Jr.; Priya, E.; Jayaraman, J.; Pulikkotil, S.J.; Suresh, N.; et al. PRILE 2021 Guidelines for Reporting Laboratory Studies in Endodontology: Explanation and Elaboration. Int. Endod. J. 2021, 54, 1491–1515. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.L.; López-García, S.; Rodríguez-Lozano, F.J.; Melo, M.; Lozano, A.; Llena, C.; Forner, L. Cytocompatibility and Bioactive Potential of AH Plus Bioceramic Sealer: An in Vitro Study. Int. Endod. J. 2022, 55, 1066–1080. [Google Scholar] [CrossRef]
- Anthrayose, P.; Aggarwal, A.; Yadav, S.; Nawal, R.R.; Talwar, S. Microscopic and Elemental Characterization of Hydrated Dental Pulp Capping Agents. J. Conserv. Dent. 2021, 24, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Hydration Mechanisms of Mineral Trioxide Aggregate. Int. Endod. J. 2007, 40, 462–470. [Google Scholar] [CrossRef]
- Zamparini, F.; Prati, C.; Taddei, P.; Spinelli, A.; Di Foggia, M.; Gandolfi, M.G. Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int. J. Mol. Sci. 2022, 23, 13914. [Google Scholar] [CrossRef]
- Raman, V.; Camilleri, J. Characterization and Assessment of Physical Properties of 3 Single Syringe Hydraulic Cement–Based Sealers. J. Endod. 2024, 50, 381–388. [Google Scholar] [CrossRef]
- Assiry, A.A.; Karobari, M.I.; Lin, G.S.S.; Batul, R.; Snigdha, N.T.; Luke, A.M.; Shetty, K.P.; Scardina, G.A.; Noorani, T.Y. Microstructural and Elemental Characterization of Root Canal Sealers Using FTIR, SEM, and EDS Analysis. Appl. Sci. 2023, 13, 4517. [Google Scholar] [CrossRef]
- Bilvinaitė, G.; Drukteinis, S.; Šakirzanovas, S.; Dummer, P.M. A Laboratory Study to Assess the Physico-Chemical Properties of BioRoot RCS and BioRoot Flow Exposed to Citric Acid and EDTA Irrigating Solutions. Clin. Oral Investig. 2024, 28, 662. [Google Scholar] [CrossRef]
- Touchefeu, Y.; Franken, P.; Harrington, K.J. Radiovirotherapy: Principles and Prospects in Oncology. Curr. Pharm. Des. 2012, 18, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Kandemir Demirci, G.; Çöven, F.O.; Güneri, P.; Karavana, S.Y.; Nalbantsoy, A.; Köse, T.; Kaval, M.E. The Solubility, PH Value, Chemical Structure, Radiopacity, and Cytotoxicity of Four Different Root Canal Sealers: An in Vitro Study. Clin. Oral Investig. 2023, 27, 5413–5425. [Google Scholar] [CrossRef]
- Reszka, P.; Nowicka, A.; Dura, W.; Marek, E.; Lipski, M. SEM and EDS Study of Totalfill BC Sealer and Guttaflow Bioseal Root Canal Sealers. Dent. Med. Probl. 2019, 56, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Juntha, S.; Tungsawat, P.; Wongwatanasanti, N.; Suksaphar, W.; Lertnantapanya, S. Evaluation of Setting Time, Flowability, Film Thickness, and Radiopacity of Experimental Monocalcium Silicate-Based Root Canal Sealers. Int. J. Dent. 2024, 2024, 8541653. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Evaluation of the Physical Properties of an Endodontic Portland Cement Incorporating Alternative Radiopacifiers Used as Root-End Filling Material. Int. Endod. J. 2010, 43, 231–240. [Google Scholar] [CrossRef]
- Lin, G.S.S.; Sim, D.H.H.; Luddin, N.; Lai, J.C.H.; Ghani, H.A.; Noorani, T.Y. Fabrication and Characterisation of Novel Algin Incorporated Bioactive-Glass 58S Calcium-Silicate-Based Root Canal Sealer. J. Dent. Sci. 2023, 18, 604–612. [Google Scholar] [CrossRef]
- Lim, M.; Jung, C.; Shin, D.-H.; Cho, Y.-B.; Song, M. Calcium Silicate-Based Root Canal Sealers: A Literature Review. Restor. Dent. Endod. 2020, 45, e35. [Google Scholar] [CrossRef]
- Siboni, F.; Taddei, P.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Properties of BioRoot RCS, a Tricalcium Silicate Endodontic Sealer Modified with Povidone and Polycarboxylate. Int. Endod. J. 2017, 50, e120–e136. [Google Scholar] [CrossRef]
- Antunes, T.B.M.; Janini, A.C.P.; Pelepenko, L.E.; Abuna, G.F.; Paiva, E.M.; Sinhoreti, M.A.C.; Raimundo, I.M.J.; Gomes, B.P.F.A.; de-Jesus-Soares, A.; Marciano, M.A. Heating Stability, Physical and Chemical Analysis of Calcium Silicate-Based Endodontic Sealers. Int. Endod. J. 2021, 54, 1175–1188. [Google Scholar] [CrossRef]
- Kim, H.I.; Jang, Y.E.; Kim, Y.; Kim, B.S. Physicochemical Changes in Root-Canal Sealers under Thermal Challenge: A Comparative Analysis of Calcium Silicate- and Epoxy-Resin-Based Sealers. Materials 2024, 17, 1932. [Google Scholar] [CrossRef]
- Kebudi Benezra, M.; Schembri Wismayer, P.; Camilleri, J. Influence of Environment on Testing of Hydraulic Sealers. Sci. Rep. 2017, 7, 17927. [Google Scholar] [CrossRef] [PubMed]
- Janini, A.C.P.; Pelepenko, L.E.; Gomes, B.P.F.A.; Marciano, M.A. Physico-Chemical Properties of Calcium Silicate-Based Sealers in Powder/Liquid and Ready-to-Use Forms. Braz. Dent. J. 2022, 33, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Badawy, R.E.S.; Mohamed, D.A. Evaluation of New Bioceramic Endodontic Sealers: An in Vitro Study. Dent. Med. Probl. 2022, 59, 85–92. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Le Goff, F.; Pochard, I.; Dauzères, A. Effect of Magnesium on Calcium Silicate Hydrate (C-S-H). Cem. Concr. Res. 2017, 97, 61–72. [Google Scholar] [CrossRef]
- Kunert, M.; Piwonski, I.; Hardan, L.; Bourgi, R.; Sauro, S.; Inchingolo, F.; Lukomska-Szymanska, M. Dentine Remineralisation Induced by “Bioactive” Materials through Mineral Deposition: An In Vitro Study. Nanomaterials 2024, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L.; Monticelli, F.; Brackett, W.W.; Loushine, R.J.; Rockman, R.A.; Ferrari, M.; Pashley, D.H.; Tay, F.R. Sealing Properties of Mineral Trioxide Aggregate Orthograde Apical Plugs and Root Fillings in an in Vitro Apexification Model. J. Endod. 2007, 33, 272–275. [Google Scholar] [CrossRef]
- Newbury, D.E. Mistakes Encountered during Automatic Peak Identification of Minor and Trace Constituents in Electron-Excited Energy Dispersive X-Ray Microanalysis. Scanning 2009, 31, 91–101. [Google Scholar] [CrossRef]
- Inada, R.N.H.; Silva, E.C.A.; Lopes, C.S.; Queiroz, M.B.; Torres, F.F.E.; da Silva, G.F.; Cerri, P.S.; Guerreiro–Tanomaru, J.M.; Tanomaru-Filho, M. Biocompatibility, Bioactivity, Porosity, and Sealer/Dentin Interface of Bioceramic Ready-to-Use Sealers Using a Dentin-Tube Model. Sci. Rep. 2024, 14, 16768. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Van Landuyt, K.; Taddei, P.; Modena, E.; Van Meerbeek, B.; Prati, C. Environmental Scanning Electron Microscopy Connected with Energy Dispersive X-Ray Analysis and Raman Techniques to Study ProRoot Mineral Trioxide Aggregate and Calcium Silicate Cements in Wet Conditions and in Real Time. J. Endod. 2010, 36, 851–857. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Siboni, F.; Modena, E.; Ciapetti, G.; Prati, C. Development of the Foremost Light-Curable Calcium-Silicate MTA Cement as Root-End in Oral Surgery. Chemical-Physical Properties, Bioactivity and Biological Behavior. Dent. Mater. 2011, 27, e134–e157. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Prati, C. Properties of a Novel Polysiloxane-Guttapercha Calcium Silicate-Bioglass-Containing Root Canal Sealer. Dent. Mater. 2016, 32, e113–e126. [Google Scholar] [CrossRef]
- Forni, M.; Bernardini, C.; Zamparini, F.; Zannoni, A.; Salaroli, R.; Ventrella, D.; Parchi, G.; Degli Esposti, M.; Polimeni, A.; Fabbri, P.; et al. Vascular Wall-Mesenchymal Stem Cells Differentiation on 3D Biodegradable Highly Porous CaSi-DCPD Doped Poly (α-Hydroxy) Acids Scaffolds for Bone Regeneration. Nanomaterials 2020, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Parrilli, A.P.; Fini, M.; Prati, C.; Dummer, P.M.H. 3D Micro-CT Analysis of the Interface Voids Associated with Thermafil Root Fillings Used with AH Plus or a Flowable MTA Sealer. Int. Endod. J. 2013, 46, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zeid, S.T.; Saif, R.E.; Mostafa, H.A.; Edrees, H.Y. Characterization and Crystallinity of Two Bioactive Sealers: Qualitative and Quantitative Analysis. Appl. Sci. 2024, 14, 1285. [Google Scholar] [CrossRef]
- Edanami, N.; Takenaka, S.; Ibn Belal, R.S.; Yoshiba, K.; Takahara, S.; Yoshiba, N.; Ohkura, N.; Noiri, Y. In Vivo Assessment of the Apatite-Forming Ability of New-Generation Hydraulic Calcium Silicate Cements Using a Rat Subcutaneous Implantation Model. J. Funct. Biomater. 2023, 14, 213. [Google Scholar] [CrossRef]
- Okamoto, M.; Matsumoto, S.; Moriyama, K.; Huang, H.; Watanabe, M.; Miura, J.; Sugiyama, K.; Hirose, Y.; Mizuhira, M.; Kuriki, N.; et al. Biological Evaluation of the Effect of Root Canal Sealers Using a Rat Model. Pharmaceutics 2022, 14, 2038. [Google Scholar] [CrossRef]


| Root Canal Sealer | Manufacturer | Composition | LOT |
|---|---|---|---|
| AH Plus Bioceramic Sealer | Manufactured by Maruchi Distributed by Denstply DeTrey GmbH Konstanz, Germany | Zirconium dioxide (50–75%), tricalcium silicate (5–15%), dimethyl sulfoxide (10–30%), lithium carbonate (<0.5%), thickening agent. | KI211103 |
| BioRoot RCS | Septodont, Saint Maur Des Fosses, France | Powder: Tricalcium silicate, zirconium oxide, and povidone. Liquid: Aqueous solution of calcium chloride and polycarboxylate. | B30546 |
| BioRoot Flow | Septodont, Saint Maur Des Fosses, France | Tricalcium silicate, propylene glycol, povidone, calcium carbonate, aerosil (silica), zirconium oxide, acrylamide/sodium acryloyldimethyltaurate copolymer, isohexadecane and polysorbate. | B33990AAB |
| Total Fill BC Sealer | FKG Dentaire, La Chaux-de-Fonds, Switzerland | Calcium silicates, calcium phosphate monobasic, zirconium oxide, tantalum oxide, and thickening agents. | 24003SP |
| AH Plus | Denstply DeTrey GmbH, Konstanz, Germany | Paste A: bisphenol-A epoxy resin, bisphenol-F epoxy resin, calcium tungstate, zirconium oxide, iron oxide, pigments. Paste B: dibenyldiamine, aminoadamantane, tricyclodecane-diamine, calcium tungstate, zirconium oxide, silica, silicone oil. | 2207000296 |
| Element | AH Plus Bioceramic Sealer | BioRoot RCS | BioRoot Flow | TotalFill BC Sealer | AH Plus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 d | 28 d | 0 d | 28 d | 0 d | 28 d | 0d | 28 d | 0 d | 28 d | |
| C | 8.56 | 12.18 ↑ | 7.99 | 7.94 | 10.71 | 14.16 ↑ | 22.28 | ND | 70.52 | 71.47 |
| O | 30.74 | 38.95 ↑ | 40.74 | 37.06 ↓ | 43.74 | 44.15 | 32.99 | ND | 21.64 | 24.94 ↑ |
| Si | 2.57 | 0.77 ↓ | 0.80 | 2.78 ↑ | 0.76 | 1.22 ↑ | 1.92 | ND | ND | 0.66 |
| Zr | 45.48 | 16.24 ↓ | 10.91 | 17.84 ↑ | 8.04 | 2.06 ↓ | 32.86 | ND | 4.37 | 2.49 ↓ |
| Cl | ND | ND | 7.56 | ND | ND | ND | ND | ND | ND | ND |
| Ca | 12.65 | 31.86 ↑ | 32.00 | 34.38 ↑ | 36.75 | 38.41 ↑ | 9.94 | ND | 1.09 | 0.26 ↓ |
| W | ND | ND | ND | ND | ND | ND | ND | ND | 2.38 | 0.17 ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwanski, M.; Piwonski, I.; Szmechtyk, T.; Sauro, S.; Lukomska-Szymanska, M. Microstructural and Elemental Characterization of Calcium Silicate-Based Sealers. Nanomaterials 2025, 15, 756. https://doi.org/10.3390/nano15100756
Radwanski M, Piwonski I, Szmechtyk T, Sauro S, Lukomska-Szymanska M. Microstructural and Elemental Characterization of Calcium Silicate-Based Sealers. Nanomaterials. 2025; 15(10):756. https://doi.org/10.3390/nano15100756
Chicago/Turabian StyleRadwanski, Mateusz, Ireneusz Piwonski, Tomasz Szmechtyk, Salvatore Sauro, and Monika Lukomska-Szymanska. 2025. "Microstructural and Elemental Characterization of Calcium Silicate-Based Sealers" Nanomaterials 15, no. 10: 756. https://doi.org/10.3390/nano15100756
APA StyleRadwanski, M., Piwonski, I., Szmechtyk, T., Sauro, S., & Lukomska-Szymanska, M. (2025). Microstructural and Elemental Characterization of Calcium Silicate-Based Sealers. Nanomaterials, 15(10), 756. https://doi.org/10.3390/nano15100756










