Gold Nanocages with a Long Surface Plasmon Resonance Peak Wavelength as Contrast Agents for Optical Coherence Tomography Imaging at 1060 nm
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Gold Nanocages with an SPR Wavelength Above 1000 nm
Materials
2.2. Measurement of Optical Properties of Gold Nanocages
2.3. OCT Imaging of Phantoms and In Vivo Mouse Models with Gold Nanocages as Contrast Agent
3. Results
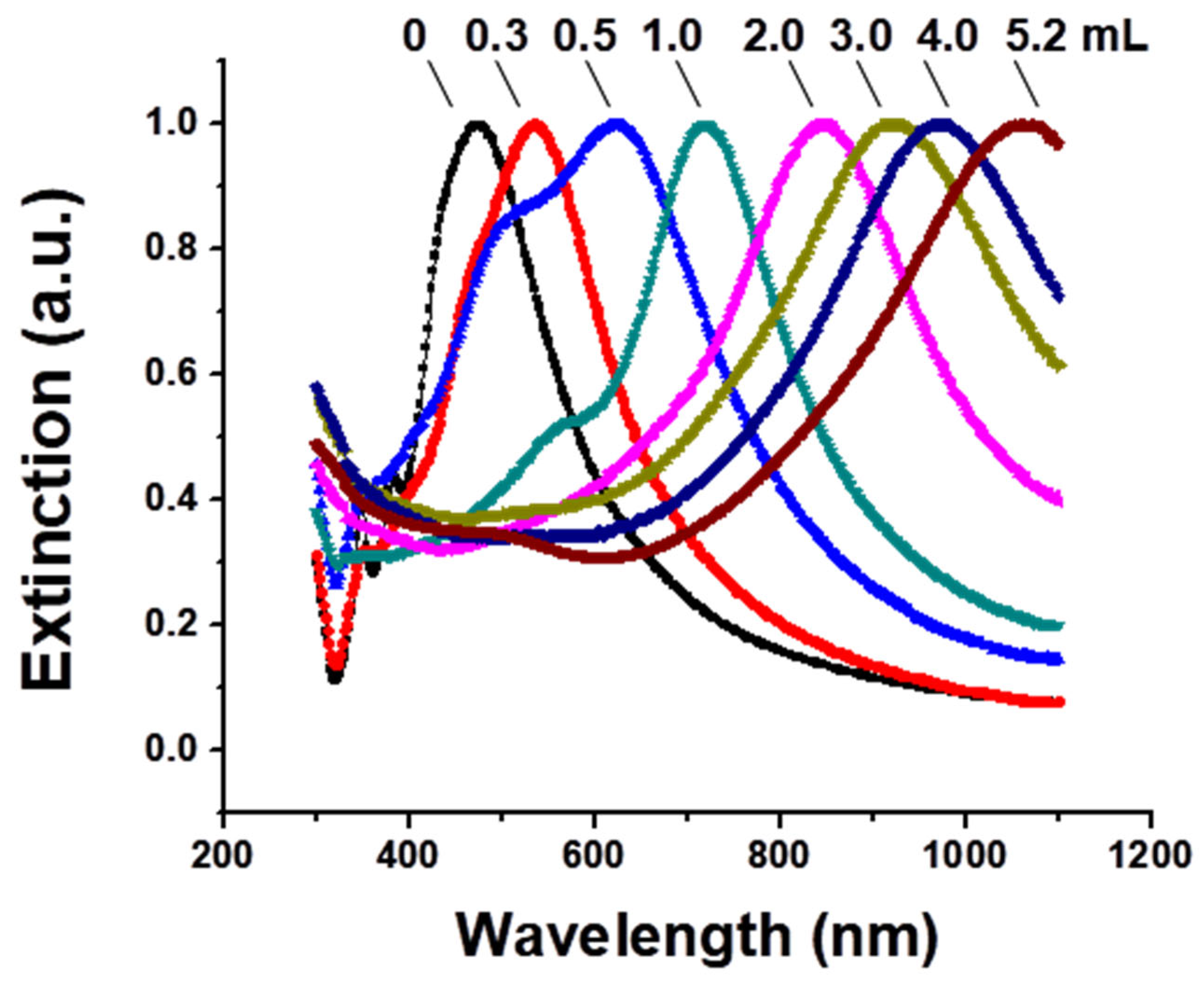
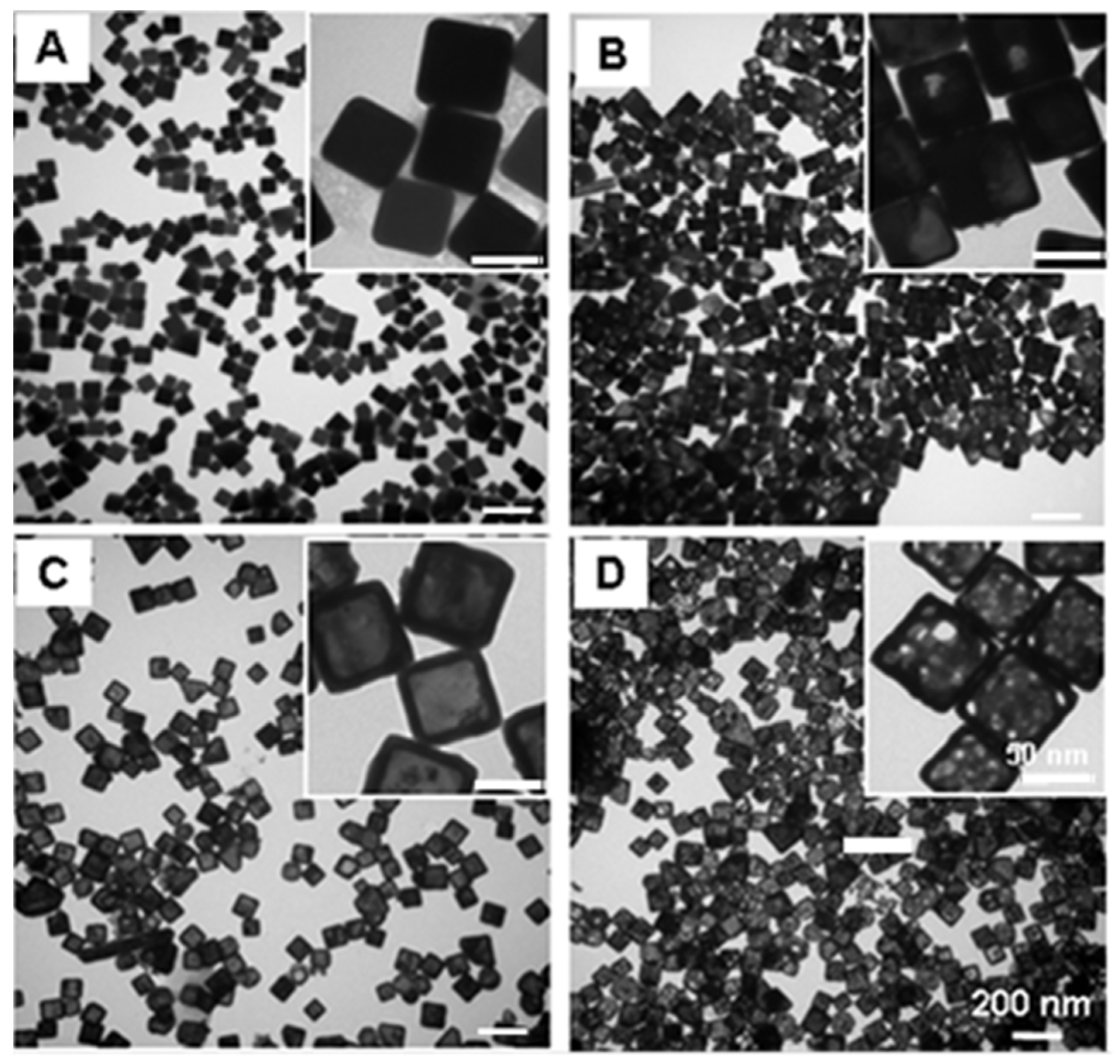
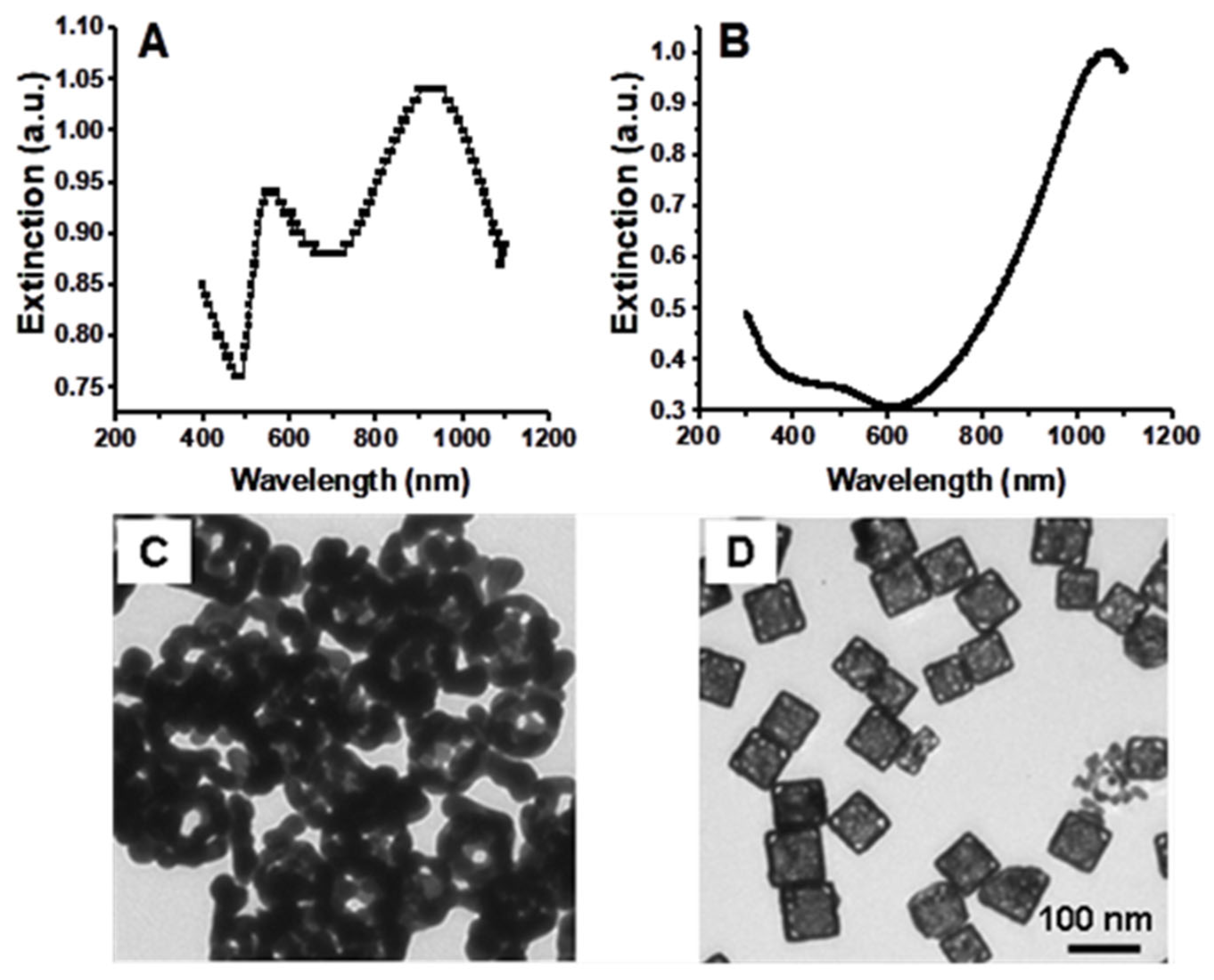
3.1. Gold Nanocage Synthesis, Influence of Synthesis Conditions, and Characterization
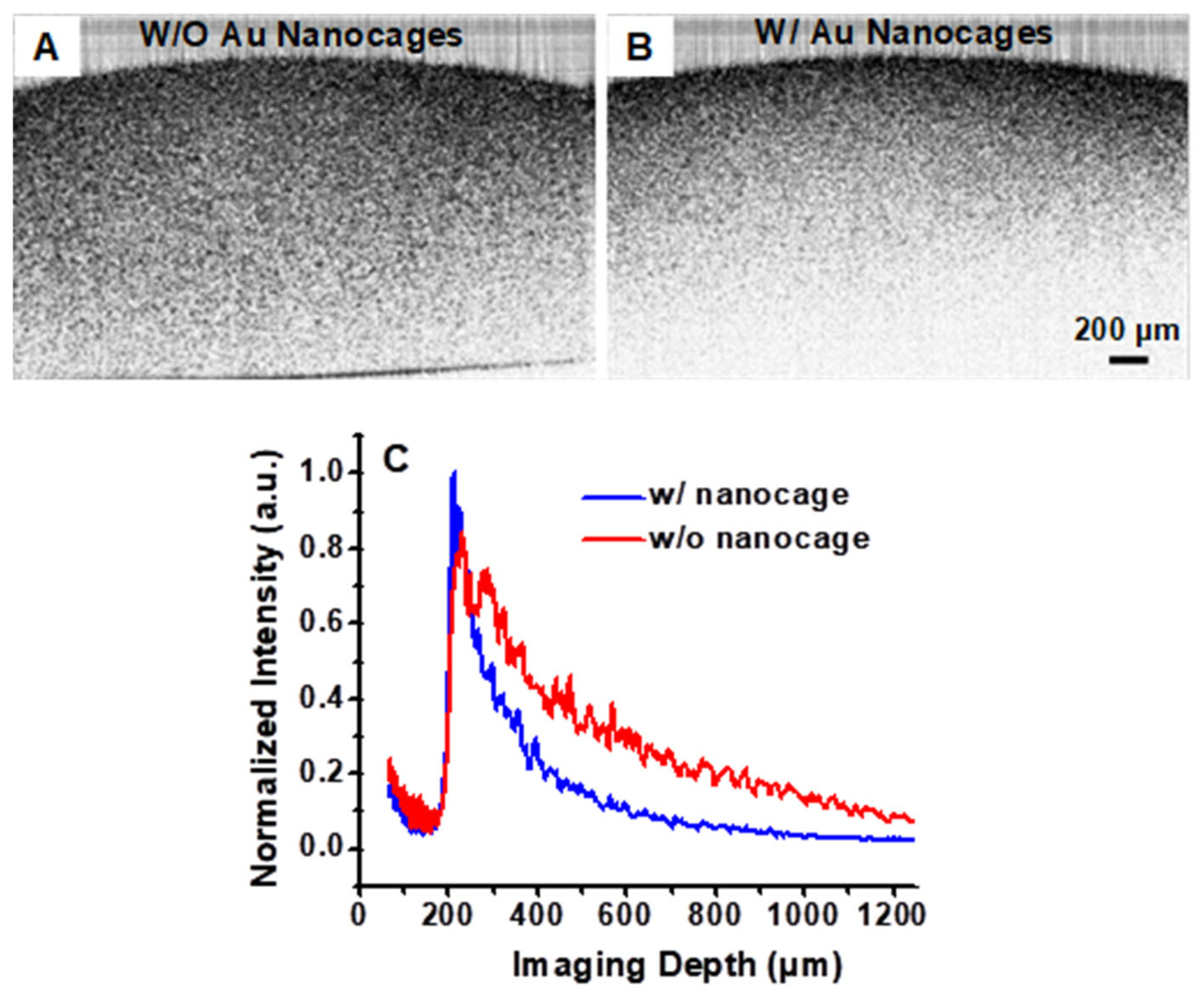
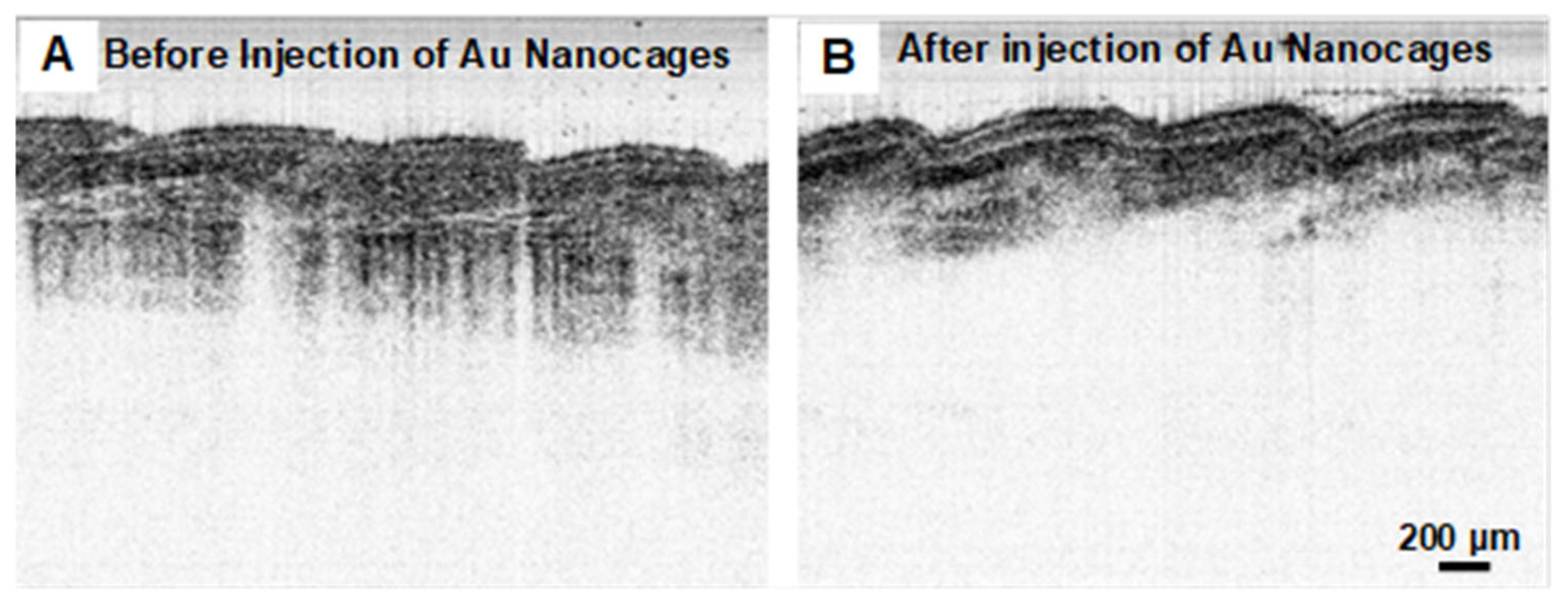
3.2. Gold Nanocages as Contrast Agents for OCT Imaging at 1060 nm
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| OCT | Optical Coherence Tomography |
| SPR | Surface Plasmon Resonance |
| SS-OCT | Swept-Source Optical Coherence Tomography |
| FWHM | Full Width at Half Maximum |
| ICG | Indocyanine Green |
| NIR | Near-Infrared |
| NaYF4:Ho3+/Yb3+ | Sodium yttrium Fluoride doped with Holmium and Ytterbium |
| NaYF4:Ho3+/Yb3+@NaGdF4 | Core-Shell Sodium Yttrium Fluoride doped with Holmium and Ytterbium and Sodium Gadolinium Fluoride |
| DDA | Discrete Dipole Approximation |
| TEM | Transmission Electron Microscopy |
| HauCl4 | Gold(III) Chloride |
| Ag | Silver |
| µa | Absorption Coefficient |
| µs | Scattering Coefficient |
| µs’ | Reduced Scattering Coefficient |
| G | Avogadro’s Number |
References
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Ye, J.; Marks, D.L.; Boppart, S.A. Near-infrared dyes as contrast-enhancing agents for spectroscopic optical coherence tomography. Opt. Lett. 2004, 29, 1647–1649. [Google Scholar] [CrossRef]
- Lee, T.M.; Oldenburg, A.L.; Sitafalwalla, S.; Marks, D.L.; Luo, W.; Toublan, F.J.J.; Suslick, K.S.; Boppart, S.A. Engineered microsphere contrast agents for optical coherence tomography. Opt. Lett. 2003, 28, 1546–1548. [Google Scholar] [CrossRef]
- Barton, J.K.; Hoying, J.B.; Sullivan, C.J. Use of microbubbles as an optical coherence tomography contrast agent. Acad. Radiol. 2002, 9, S52–S55. [Google Scholar] [CrossRef] [PubMed]
- Calvert, N.D.; Baxter, J.; Torrens, A.A.; Thompson, J.; Kirby, A.; Walia, J.; Ntais, S.; Hemmer, E.; Berini, P.; Hibbert, B.; et al. NIR-II scattering gold superclusters for intravascular optical coherence tomography molecular imaging. Nat. Nanotechnol. 2025, 20, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Troutman, T.S.; Barton, J.K.; Romanowski, M. Optical coherence tomography with plasmon resonant nanorods of gold. Opt. Lett. 2007, 32, 1438–1440. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.H.; Barton, J.; Halas, N.J.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Nguyen, V.-P.; Li, Y.; Henry, J.; Zhang, W.; Aaberg, M.; Jones, S.; Qian, T.; Wang, X.; Paulus, Y.M. Plasmonic gold nanostar-enhanced multimodal photoacoustic microscopy and optical coherence tomography molecular imaging to evaluate choroidal neovascularization. ACS Sens. 2020, 5, 3070–3081. [Google Scholar] [CrossRef]
- Xi, J.F.; Chen, Y.P.; Li, X.D. Characterizing optical properties of nano contrast agents by using cross-referencing OCT imaging. Opt. Lett. Biomed. Opt. Express 2013, 4, 842–851. [Google Scholar] [CrossRef]
- Chen, J.Y.; Saeki, F.; Wiley, B.J.; Cang, H.; Cobb, M.J.; Li, Z.Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X.; et al. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, S.X.; Xu, X.L.; Ren, K.; Dong, M.Z.; Nie, Z.K.; Xu, Y.; Dai, X.H.; Xu, P.; Sun, S.; et al. Recent advances of nanomaterials in imaging liver fibrosis. BMEMat 2024, e12123. [Google Scholar] [CrossRef]
- Xu, Y.L.; Li, C.L.; An, J.S.; Ma, X.; Yang, J.F.; Luo, L.S.; Deng, Y.; Kim, J.S.; Sun, Y. Construction of a 980 nm laser-activated Pt (II) metallacycle nanosystem for efficient and safe photo-induced bacteria sterilization. Sci. China Chem. 2023, 66, 155–163. [Google Scholar] [CrossRef]
- Salesa, B.; Ferrús-Manzano, P.; Tuñón-Molina, A.; Cano-Vicent, A.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Study of biological properties of gold nanoparticles: Low toxicity, no proliferative activity, no ability to induce cell gene expression, and no antiviral activity. Chem. Biol. Interact. 2023, 382, 110646. [Google Scholar] [CrossRef]
- Sibuyi, N.R.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional gold nanoparticles for improved diagnostic and therapeutic applications: A review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.-H.; Nam, J.-M. Plasmonic photothermal nanoparticles for biomedical applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Au, L.; Li, X.D.; Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, D.L.; Xi, J.F.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.Y.; Zhang, H.; Xia, Y.N.; Li, X.D. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007, 7, 1318–1322. [Google Scholar] [CrossRef]
- Qu, Y.; Lü, X. Aqueous synthesis of gold nanoparticles and their cytotoxicity in human dermal fibroblasts–fetal. Biomed. Mater. 2009, 4, 025007. [Google Scholar] [CrossRef]
- Arnida; Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217. [Google Scholar] [CrossRef]
- Yah, C.S. The toxicity of gold nanoparticles in relation to their physicochemical properties. Biomed. Res. 2013, 24, 3. [Google Scholar]
- Zheng, J.P.; Cheng, X.Z.; Zhang, H.; Bai, X.P.; Ai, R.Q.; Shao, L.; Wang, J.F. Gold nanorods: The most versatile plasmonic nanoparticles. Chem. Rev. 2021, 121, 13342–13353. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Perry, H.L.; Wilton-Ely, J.D. Strategies for the functionalisation of gold nanorods to reduce toxicity and aid clinical translation. Nanotheranostics 2021, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Ngo, N.M.; Tran, H.V.; Lee, T.R. Plasmonic nanostars: Systematic review of their synthesis and applications. ACS Appl. Nano Mater. 2022, 5, 14051–14091. [Google Scholar] [CrossRef]
- Le, N.T.; Boskovic, T.J.; Allard, M.M.; Nick, K.E.; Kwon, S.R.; Perry, C.C. Gold nanostar characterization by nanoparticle tracking analysis. ACS Omega 2022, 7, 44677–44688. [Google Scholar] [CrossRef]
- Povazay, B.; Bizheva, K.; Hermann, B.; Unterhuber, A.; Sattmann, H.; Fercher, A.F.; Drexler, W.; Schubert, C.; Ahnelt, P.; Mei, M.; et al. Enhanced visualization of choroidal vessels using ultrahigh resolution ophthalmic OCT at 1050 nm. Opt. Express 2003, 11, 1980–1986. [Google Scholar] [CrossRef]
- Wang, Y.M.; Nelson, J.S.; Chen, Z.P.; Reiser, B.J.; Chuck, R.S.; Windeler, R.S. Optimal wavelength for ultrahigh-resolution optical coherence tomography. Opt. Express 2003, 11, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Mishra, T.; Mohan, M.; Chakravarty, M.; Poddar, R. Zinc oxide nanoparticles (ZnONPs) as contrast agent for imaging of animal tissue using swept source optical coherence tomography (SSOCT). Optik 2019, 176, 302–308. [Google Scholar] [CrossRef]
- Shwetabh, K.; Banerjee, A.; Poddar, R.; Kumar, K. NaYF4: Ho3+/Yb3+@ NaGdF4 core@shell upconversion nanoparticles for contrast enhancement in bimodal in-vitro OCT imaging. Microchim. Acta 2024, 191, 261. [Google Scholar] [CrossRef]
- Prahl, S.A. The adding-doubling method. In Optical-Thermal Response of Laser Irradiated Tissue; Welch, A.J., van Gemert, M.J.C., Eds.; Plenum Press: New York, NY, USA, 1995; Chapter 5; pp. 101–129. [Google Scholar]
- Yuan, W.; Kut, C.; Liang, W.; Li, X.D. Robust and fast characterization of OCT-based optical attenuation using a novel frequency-domain algorithm for brain cancer detection. Sci. Rep. 2017, 7, 44909. [Google Scholar] [CrossRef]
- Kut, C.; Chaichana, K.L.; Xi, J.; Raza, S.M.; Ye, X.; McVeigh, E.R.; Rodriguez, F.J.; Quiñones-Hinojosa, A.; Li, X.D. Detection of human brain cancer infiltration ex vivo and in vivo using quantitative optical coherence tomography. Sci. Transl. Med. 2015, 7, 292ra100. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-C.; Li, A.; Guan, H.; Bettegowda, C.; Chaichana, K.; Quiñones-Hinojosa, A.; Li, X.D. Minimizing OCT quantification error via a surface-tracking imaging probe. Biomed. Opt. Express 2021, 12, 3992–4002. [Google Scholar] [CrossRef]
- Park, H.-C.; Li, D.; Liang, R.; Adrales, G.; Li, X.D. Multifunctional ablative gastrointestinal imaging capsule (magic) for esophagus surveillance and interventions. BME Front. 2024, 5, 0041. [Google Scholar] [CrossRef] [PubMed]
- Galvele, J.R. A stress-corrosion cracking mechanism based on surface mobility. Corros. Sci. 1987, 27, 1–33. [Google Scholar] [CrossRef]
- Sieradzki, K.; Newman, R.C. Stress-corrosion cracking. J. Phys. Chem. Solids 1987, 48, 1101–1113. [Google Scholar] [CrossRef]
- Sun, Y.G.; Xia, Y.N. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef]
- Qian, L.H.; Chen, M.W. Ultrafine nanoporous gold by low-temperature dealloying and kinetics of nanopore formation. Appl. Phys. Lett. 2007, 91, 086101. [Google Scholar] [CrossRef]
- Carski, T.R. An Investigator’s Brochure. In Indocyanine Green: History, Chemistry, Pharmacology, Indications, Adverse Reactions, Investigation, and Prognosis; Becton Dickinson and Company: Hunt Valley, MD, USA, 1994. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xi, J.; Le, V.N.D.; Ramella-Roman, J.; Li, X. Gold Nanocages with a Long Surface Plasmon Resonance Peak Wavelength as Contrast Agents for Optical Coherence Tomography Imaging at 1060 nm. Nanomaterials 2025, 15, 755. https://doi.org/10.3390/nano15100755
Chen Y, Xi J, Le VND, Ramella-Roman J, Li X. Gold Nanocages with a Long Surface Plasmon Resonance Peak Wavelength as Contrast Agents for Optical Coherence Tomography Imaging at 1060 nm. Nanomaterials. 2025; 15(10):755. https://doi.org/10.3390/nano15100755
Chicago/Turabian StyleChen, Yongping, Jiefeng Xi, Vinh Nguyen Du Le, Jessica Ramella-Roman, and Xingde Li. 2025. "Gold Nanocages with a Long Surface Plasmon Resonance Peak Wavelength as Contrast Agents for Optical Coherence Tomography Imaging at 1060 nm" Nanomaterials 15, no. 10: 755. https://doi.org/10.3390/nano15100755
APA StyleChen, Y., Xi, J., Le, V. N. D., Ramella-Roman, J., & Li, X. (2025). Gold Nanocages with a Long Surface Plasmon Resonance Peak Wavelength as Contrast Agents for Optical Coherence Tomography Imaging at 1060 nm. Nanomaterials, 15(10), 755. https://doi.org/10.3390/nano15100755









