Magnetic Bifunctional Ru-Enzyme Catalyst Allows for Sustainable Conversion of Cellulose Derivative to D-Sorbitol
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cel Biocatalyst Catalytic Activity
3.2. Ru-Containing Nanocatalyst Performance in Hydrogenation
3.3. Bifunctional Catalyst for a One-Pot Cascade Transformation
3.3.1. Nanobiocatalyst Synthesis
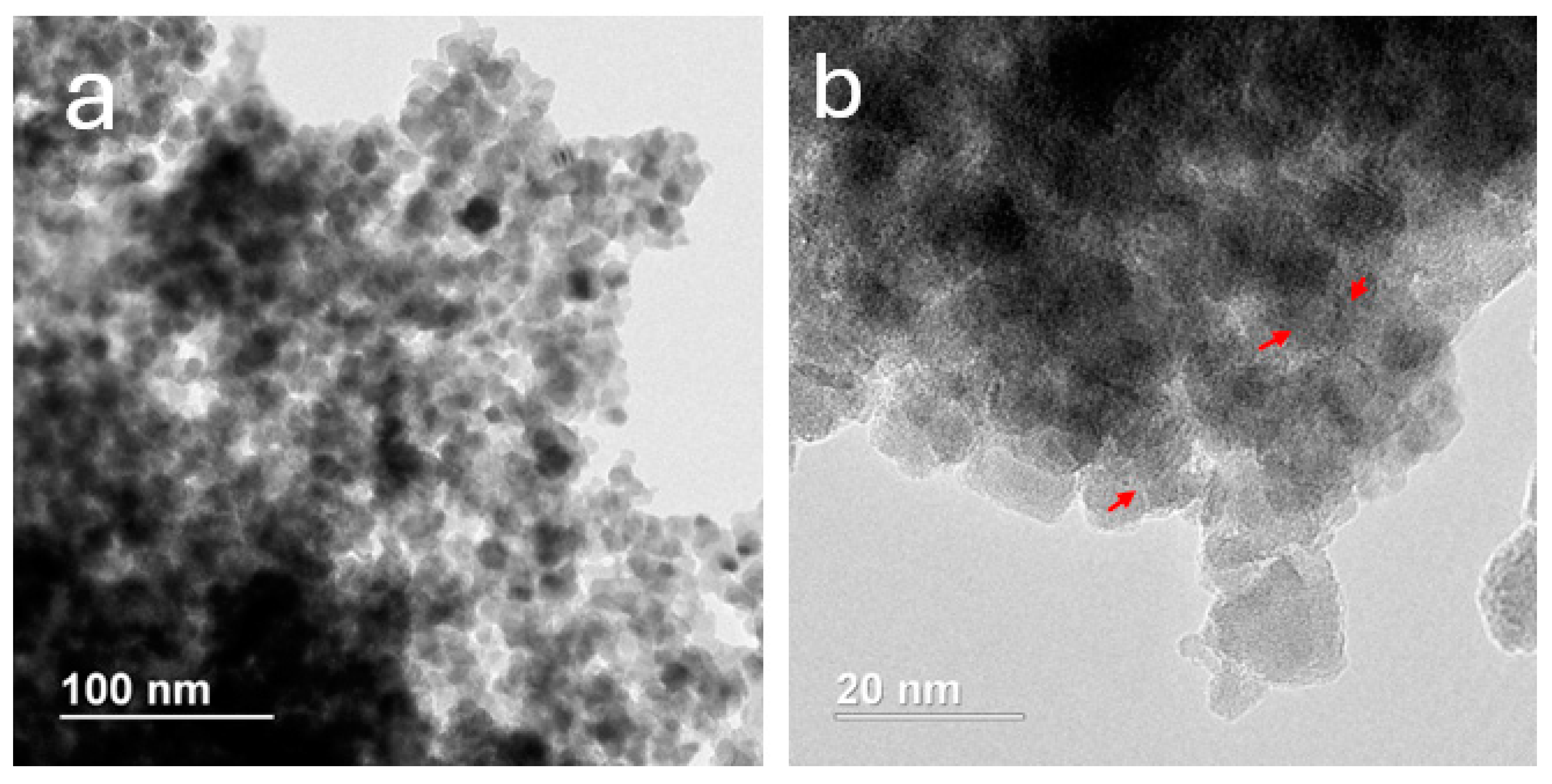
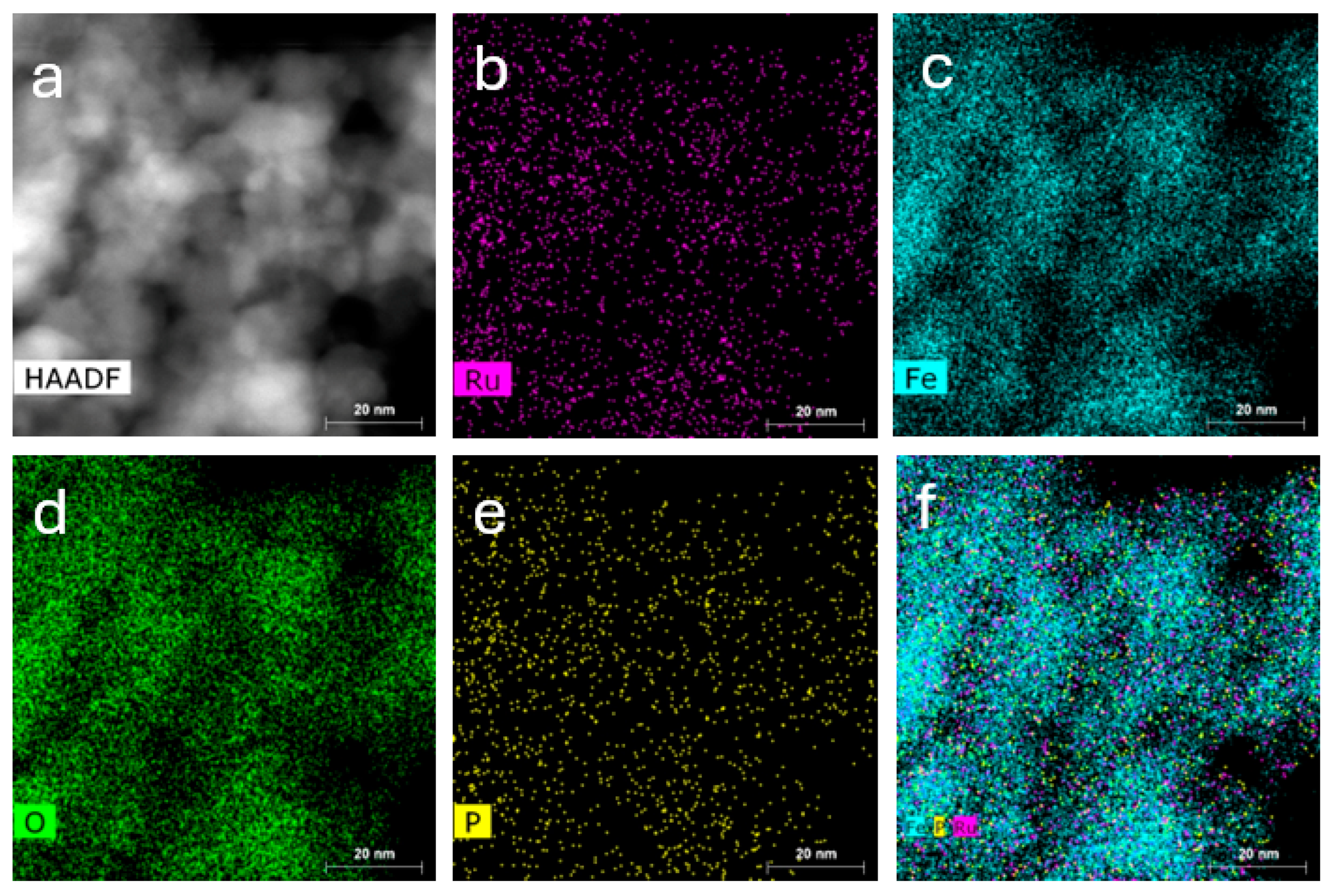
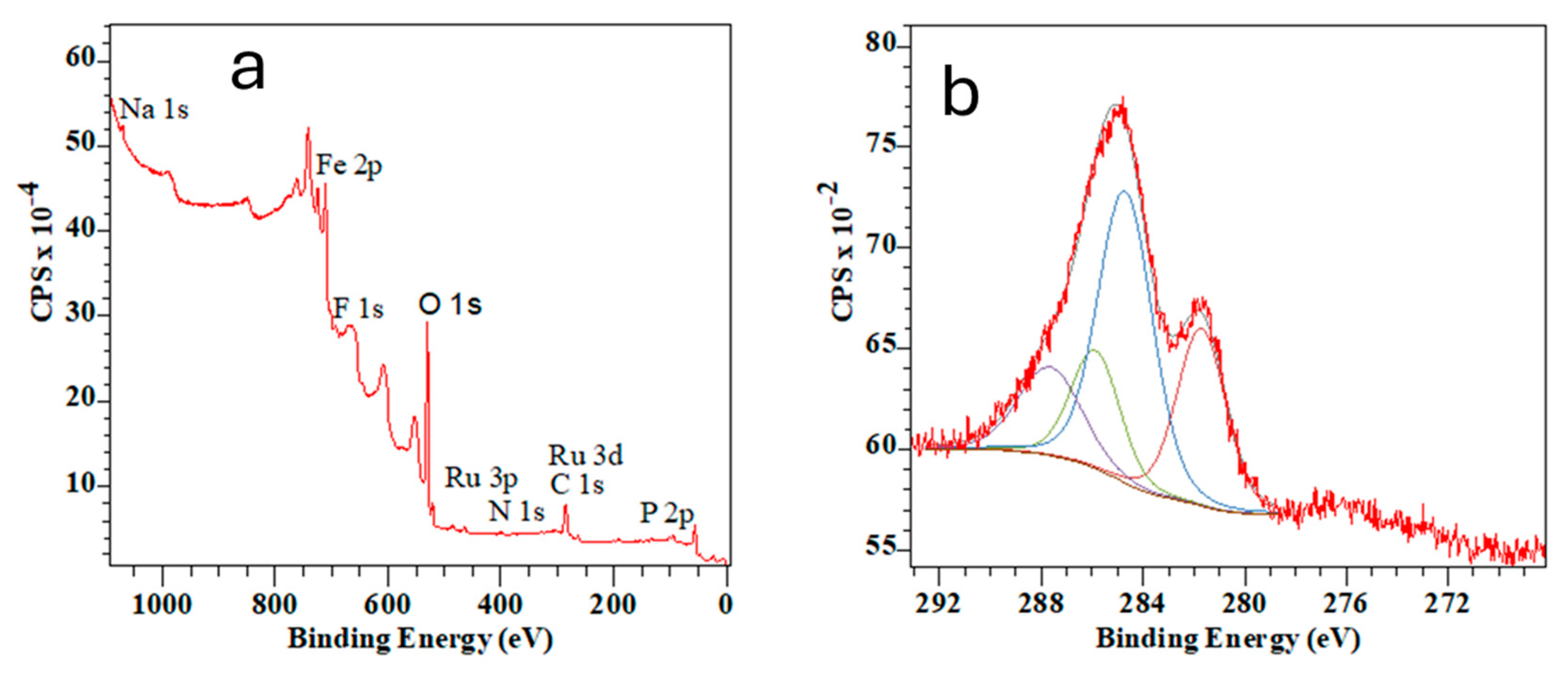
3.3.2. Characterization of MNA-CSP-Ru-Cel
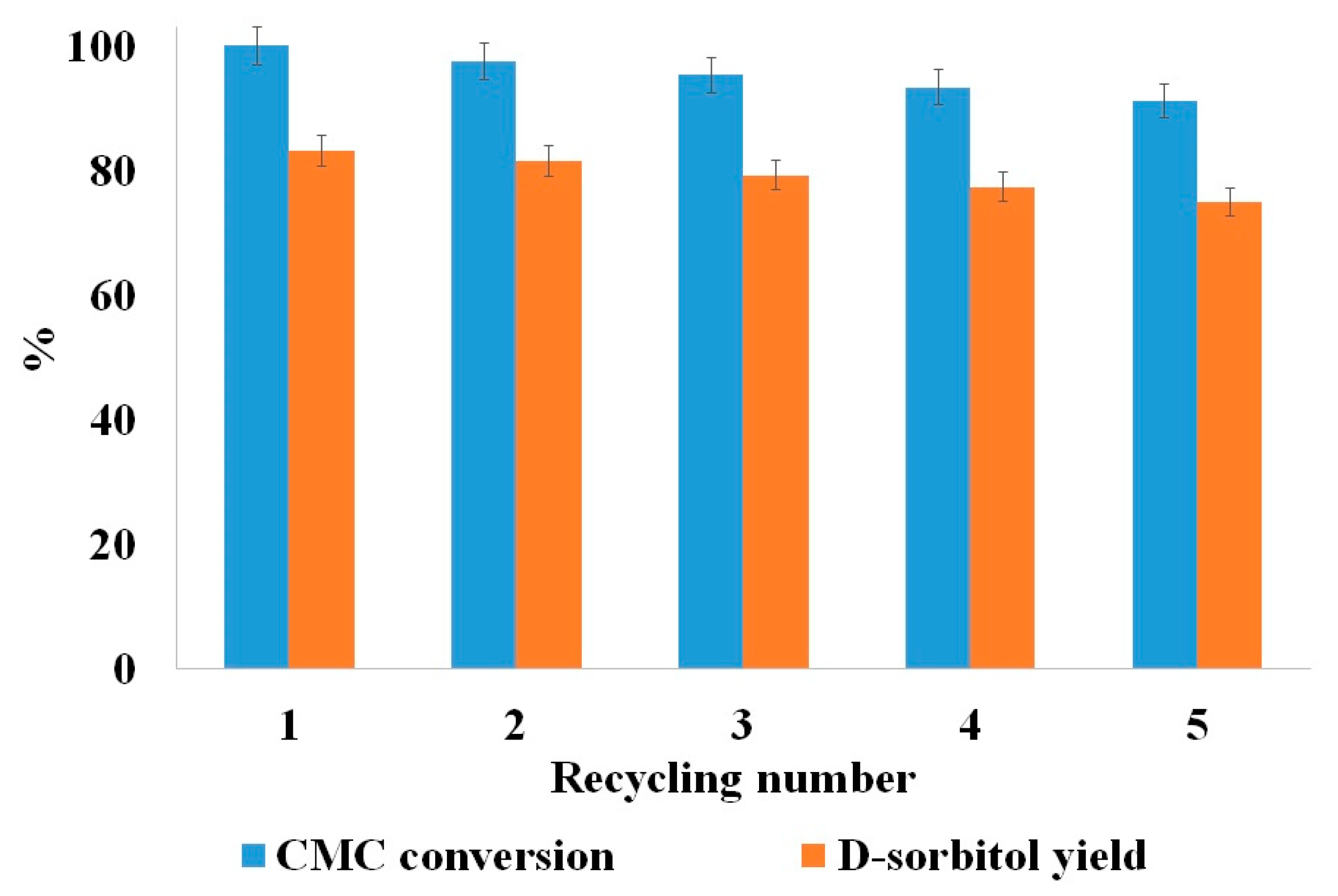
3.3.3. MNA-CSP-Ru-Cel Performance in the Cascade Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.; Duan, X.; Gnanasekarc, P.; Yan, N.; Shi, J. Review: Cascade reactions for conversion of carbohydrates using heteropolyacids as the solid catalysts. Biomass Convers. Biorefin. 2022, 12, 2313–2331. [Google Scholar] [CrossRef]
- Bayu, A.; Abudula, A.; Guan, G. Reaction pathways and selectivity in chemo-catalytic conversion of biomass-derived carbohydrates to high-value chemicals: A review. Fuel Process. Technol. 2019, 196, 106162. [Google Scholar] [CrossRef]
- Xu, H.; Ju, J.; Li, H. Toward efficient heterogeneous catalysts for in-situ hydrodeoxygenation of biomass. Fuel 2022, 320, 123891. [Google Scholar] [CrossRef]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- Arumugam, A.; Malolan, V.V.; Ponnusami, V. Contemporary Pretreatment Strategies for Bioethanol Production from Corncobs: A Comprehensive Review. Waste Biomass Valorization 2021, 12, 577–612. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int. J. Biol. Macromol. 2016, 86, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Cirujano, F.G.; Dhakshinamoorthy, A. Challenges and opportunities for the encapsulation of enzymes over porous solids for Biodiesel production and Cellulose valorization into Glucose. ChemCatChem 2021, 13, 4679–4693. [Google Scholar] [CrossRef]
- Tabah, B.; Pulidindi, I.N.; Chitturi, V.R.; Reddy Arava, L.M.; Varvak, A.; Foran, E.; Gedanken, A. Solar-energy-driven conversion of biomass to bioethanol: A sustainable approach. J. Mater. Chem. A 2017, 5, 15486–15506. [Google Scholar] [CrossRef]
- Zanuso, E.; Gomes, D.G.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Enzyme immobilization as a strategy towards efficient and sustainable lignocellulosic biomass conversion into chemicals and biofuels: Current status and perspectives. Sustain. Energy Fuels 2021, 5, 4233–4247. [Google Scholar] [CrossRef]
- Roy, I.; Sharma, S.; Gupta, M.N. Smart biocatalysts: Design and applications. Adv. Biochem. Eng./Biotechnol. 2004, 86, 159–189. [Google Scholar] [CrossRef]
- Muley, A.B.; Mulchandani, K.H.; Singhal, R.S. Immobilization of enzymes on iron oxide magnetic nanoparticles: Synthesis, characterization, kinetics and thermodynamics. Methods Enzymol. 2020, 630, 39–79. [Google Scholar] [CrossRef] [PubMed]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties-Application to oxidoreductase. Molecules 2014, 19, 8995. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Singh, R.; Singhania, R.; Pandey, A.; Larroche, C. Biomass, Biofuels, Biochemicals: Advances in Enzyme Technology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Vasilescu, C.; Marc, S.; Hulka, I.; Paul, C. Enhancement of the Catalytic Performance and Operational Stability of Sol-Gel-Entrapped Cellulase by Tailoring the Matrix Structure and Properties. Gels 2022, 8, 626. [Google Scholar] [CrossRef] [PubMed]
- Guillermo, S.; Swofford, D.R.; Coulter, G.; Vasicek, T.W. Optimization of enzyme activity of cellulases immobilized to magnetic nanoparticles with varying functional group densities. J. Undergrad. Chem. Res. 2022, 21, 96–99. [Google Scholar]
- Hong, K.; Sajjadi, M.; Suh, J.M.; Zhang, K.; Nasrollahzadeh, M.; Jang, H.W.; Varma, R.S.; Shokouhimehr, M. Palladium Nanoparticles on Assorted Nanostructured Supports: Applications for Suzuki, Heck, and Sonogashira Cross-Coupling Reactions. ACS Appl. Nano Mater. 2020, 3, 2070–2103. [Google Scholar] [CrossRef]
- Medina-Castillo, A.L.; Ruzic, L.; Nidetzky, B.; Bolivar, J.M. Hydrophilic Nonwoven Nanofiber Membranes as Nanostructured Supports for Enzyme Immobilization. ACS Appl. Polym. Mater. 2022, 4, 6051–6063. [Google Scholar] [CrossRef]
- Ramirez, N.; Serey, M.; Illanes, A.; Piumetti, M.; Ottone, C. Immobilization strategies of photolyases: Challenges and perspectives for DNA repairing application. J. Photochem. Photobiol. B 2021, 215, 112113. [Google Scholar] [CrossRef]
- Sousa, A.M.L.; Li, T.-D.; Varghese, S.; Halling, P.J.; Aaron Lau, K.H. Highly Active Protein Surfaces Enabled by Plant-Based Polyphenol Coatings. ACS Appl. Mater. Interfaces 2018, 10, 39353–39362. [Google Scholar] [CrossRef]
- Yan, D.; Topsakal, M.; Selcuk, S.; Lyons, J.L.; Zhang, W.; Wu, Q.; Waluyo, I.; Stavitski, E.; Attenkofer, K.; Yoo, S.; et al. Ultrathin amorphous titania on nanowires: Optimization of conformal growth and elucidation of atomic-scale motifs. Nano Lett. 2019, 19, 3457–3463. [Google Scholar] [CrossRef]
- Zhang, S.; Bilal, M.; Zdarta, J.; Cui, J.; Kumar, A.; Franco, M.; Ferreira, L.F.R.; Iqbal, H.M.N. Biopolymers and nanostructured materials to develop pectinases-based immobilized nano-biocatalytic systems for biotechnological applications. Food Res. Int. 2021, 140, 109979. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Valerio, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; de Oliveira, D. Nanomaterials for biocatalyst immobilization—State of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; de A. Falcao, I.R.; da S. Souza, J.E.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; dos Santos, J.C.S. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Ahmed, Z.; Arshad, A.; Bilal, M.; Iqbal, H.M.N.; Ahmed, I. Nano-biocatalytic systems for cellulose de-polymerization: A drive from design to applications. Top. Catal. 2023, 66, 592–605. [Google Scholar] [CrossRef]
- Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. The Synthesis of (Magnetic) Crosslinked Enzyme Aggregates With Laccase, Cellulase, β-Galactosidase and Transglutaminase. Front. Bioeng. Biotechnol. 2022, 10, 813919. [Google Scholar] [CrossRef]
- Jiang, J.; Gong, X.; Li, T.; Huang, J.; Zhou, N.; Jia, X. Immobilized Cellulase on NH2-MIL-88(Fe) and Its Performance as a Biocatalyst. Appl. Biochem. Biotechnol. 2024, 196, 4745–4758. [Google Scholar] [CrossRef]
- Hadadi, M.; Habibi, A. Development of magnetic immobilized cellulase biocatalysts for saccharification of paper waste. Catal. Lett. 2024, 154, 5791–5805. [Google Scholar] [CrossRef]
- Lenertz, M.; Li, Q.; Armstrong, Z.; Scheiwiller, A.; Ni, G.; Wang, J.; Feng, L.; MacRae, A.; Yang, Z. Magnetic Multienzyme@Metal-Organic Material for Sustainable Biodegradation of Insoluble Biomass. ACS Appl. Mater. Interfaces 2024, 16, 11617–11626. [Google Scholar] [CrossRef]
- Marino, M.A.; Moretti, P.; Tasic, L. Immobilized commercial cellulases onto amino-functionalized magnetic beads for biomass hydrolysis: Enhanced stability by non-polar silanization. Biomass Convers. Biorefin. 2023, 13, 9265–9275. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Wu, B.; Gao, Z.; He, B. Immobilization and Characterization of a Processive Endoglucanase EG5C-1 from Bacillus subtilis on Melamine-Glutaraldehyde Dendrimer-Functionalized Magnetic Nanoparticles. Nanomaterials 2024, 14, 340. [Google Scholar] [CrossRef]
- Sun, L.; Xu, C.; Tong, S.; Gu, X. Enhancing cellulose hydrolysis via cellulase immobilization on zeolitic imidazolate frameworks using physical adsorption. Bioprocess Biosyst. Eng. 2024, 47, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Grandio, E.; Demirer, G.S.; Jackson, C.T.; Yang, D.; Ebert, S.; Molawi, K.; Keller, H.; Landry, M.P. Carbon nanotube biocompatibility in plants is determined by their surface chemistry. J. Nanobiotechnol. 2021, 19, 431. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, T.; Karita, H.; Mori-Moriyama, N.; Sato, N.; Yoshimoto, K. Reduced cytotoxicity of polyethyleneimine by covalent modification of antioxidant and its application to microalgal transformation. Sci. Technol. Adv. Mater. 2021, 22, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Gracida, J.; Arredondo-Ochoa, T.; Garcia-Almendarez, B.E.; Escamilla-Garcia, M.; Shirai, K.; Regalado, C.; Amaro-Reyes, A. Improved Thermal and Reusability Properties of Xylanase by Genipin Cross-Linking to Magnetic Chitosan Particles. Appl. Biochem. Biotechnol. 2019, 188, 395–409. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Styevko, G.; Madaras, E.; Haktanirlar, G.; Tran, A.T.M.; Bujna, E.; Dam, M.S.; Nguyen, Q.D. Immobilization of β-galactosidase on chitosan-coated magnetic nanoparticles and its application for synthesis of lactulose-based galactooligosaccharides. Process Biochem. 2019, 84, 30–38. [Google Scholar] [CrossRef]
- Nikoshvili, L.Z.; Tikhonov, B.B.; Ivanov, P.E.; Stadolnikova, P.Y.; Sulman, M.G.; Matveeva, V.G. Recent Progress in Chitosan-Containing Composite Materials for Sustainable Approaches to Adsorption and Catalysis. Catalysts 2023, 13, 367. [Google Scholar] [CrossRef]
- Podrepsek, G.H.; Knez, Z.; Leitgeb, M. Development of chitosan functionalized magnetic nanoparticles with bioactive compounds. Nanomaterials 2020, 10, 1913. [Google Scholar] [CrossRef]
- Ashwini John, J.; Samuel, M.S.; Selvarajan, E. Immobilized cellulase on Fe3O4/GO/CS nanocomposite as a magnetically recyclable catalyst for biofuel application. Fuel 2023, 333, 126364. [Google Scholar] [CrossRef]
- Javid, A.; Amiri, H.; Kafrani, A.T.; Rismani-Yazdi, H. Post-hydrolysis of cellulose oligomers by cellulase immobilized on chitosan-grafted magnetic nanoparticles: A key stage of butanol production from waste textile. Int. J. Biol. Macromol. 2022, 207, 324–332. [Google Scholar] [CrossRef]
- Kaur, G.; Taggar, M.S.; Kalia, A.; Kaur, J. Fungal cellulolytic enzyme complex immobilized on chitosan-functionalised magnetic nanoparticles for paddy straw saccharification. Process Saf. Environ. Prot. 2024, 185, 533–544. [Google Scholar] [CrossRef]
- Yi, J.; Qiu, M.; Zhu, Z.; Dong, X.; Andrew Decker, E.; McClements, D.J. Robust and recyclable magnetic nanobiocatalysts for extraction of anthocyanin from black rice. Food Chem. 2021, 364, 130447. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Granda, S.; Escot, L.; Lavandera, I.; Gotor-Fernandez, V. Chemoenzymatic Cascades Combining Biocatalysis and Transition Metal Catalysis for Asymmetric Synthesis. Angew. Chem. Int. Ed. 2023, 62, e202217713. [Google Scholar] [CrossRef]
- Arias, K.S.; Carceller, J.M.; Climent, M.J.; Corma, A.; Iborra, S. Chemoenzymatic Synthesis of 5-Hydroxymethylfurfural (HMF)-Derived Plasticizers by Coupling HMF Reduction with Enzymatic Esterification. ChemSusChem 2020, 13, 1864–1875. [Google Scholar] [CrossRef]
- Silva, J.G.A.B.; Santos, R.C.; Rodriguez-Castellon, E.; Teixeira, L.S.G.; Pontes, L.A.M. Catalytic conversion of glucose into sorbitol over niobium oxide supported Ru catalysts. Mol. Catal. 2021, 507, 111567. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Hydrogenation of glucose and fructose into hexitols over heterogeneous catalysts: A review. J. Taiwan Inst. Chem. Eng. 2019, 96, 341–352. [Google Scholar] [CrossRef]
- Delgado Arcano, Y.; Valmana Garcia, O.D.; Mandelli, D.; Carvalho, W.A.; Magalhaes Pontes, L.A. Xylitol: A review on the progress and challenges of its production by chemical route. Catal. Today 2020, 344, 2–14. [Google Scholar] [CrossRef]
- Annuar, N.H.R.; Alexzman, Z.A.; Daud, A.R.M.; Alias, A.F.N.; Hairi, H.M.; Setiabudi, H.D. A review on hydrogenolysis of sorbitol over heterogeneous catalysts. J. Environ. Chem. Eng. 2022, 10, 107229. [Google Scholar] [CrossRef]
- Gumina, B.; Mauriello, F.; Pietropaolo, R.; Galvagno, S.; Espro, C. Hydrogenolysis of sorbitol into valuable C3-C2 alcohols at low H2 pressure promoted by the heterogeneous Pd/Fe3O4 catalyst. Mol. Catal. 2018, 446, 152–160. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.; Wang, W.; Liang, J.; Orooji, Y.; Dai, C.; Fu, X.; Yang, Y.; Xu, W.; Zhu, J. Efficient sorbitol producing process through glucose hydrogenation catalyzed by Ru supported amino poly (styrene-co-maleic) polymer (ASMA) encapsulated on γ-Al2O3. Catalysts 2020, 10, 1068. [Google Scholar] [CrossRef]
- Byun, M.Y.; Park, D.-W.; Lee, M.S. Effect of sodium propionate as a stabilizer on the catalytic activity of Pt/C catalysts for D-glucose hydrogenation. Catal. Today 2020, 352, 88–94. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Liu, C.; Wu, S.; Wei, W. Direct conversion of cellulose into sorbitol catalyzed by a bifunctional catalyst. Bioresour. Technol. 2019, 274, 190–197. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, H.; Zhang, Q.; Li, H.; Li, H. Ordered Mesoporous Ni-P Amorphous Alloy Nanowire Arrays: High-Efficiency Catalyst for Production of Polyol from Sugar. ACS Appl. Mater. Interfaces 2020, 12, 26101–26112. [Google Scholar] [CrossRef] [PubMed]
- Gromov, N.V.; Medvedeva, T.B.; Rodikova, Y.A.; Timofeeva, M.N.; Panchenko, V.N.; Taran, O.P.; Kozhevnikov, I.V.; Parmon, V.N. One-pot synthesis of sorbitol via hydrolysis-hydrogenation of cellulose in the presence of Ru-containing composites. Bioresour. Technol. 2021, 319, 124122. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Moreno, J.; Iglesias, J.; Morales, G.; Fierro, J.L.G.; Sanchez-Vazquez, R.; Cubo, A.; Garcia, B. Ru-ZrO2-SBA-15 as efficient and robust catalyst for the aqueous phase hydrogenation of glucose to sorbitol. Mol. Catal. 2020, 484, 110802. [Google Scholar] [CrossRef]
- Shi, X.; Yang, Y.; Ye, X.; Zhong, H.; Wang, C.; Jin, F. Enhancing the Synergistic Effect of Bifunctional Pd-Based Catalyst by Phosphonic Acid for Cellobiose Conversion to Sorbitol. ACS Sustain. Chem. Eng. 2024, 12, 5991–6002. [Google Scholar] [CrossRef]
- Tikhonov, B.B.; Lisichkin, D.R.; Sulman, A.M.; Sidorov, A.I.; Bykov, A.V.; Lugovoy, Y.V.; Karpenkov, A.Y.; Bronstein, L.M.; Matveeva, V.G. Magnetic Nanoparticle Support with an Ultra-Thin Chitosan Layer Preserves the Catalytic Activity of the Immobilized Glucose Oxidase. Nanomaterials 2024, 14, 700. [Google Scholar] [CrossRef]
- Martínez-Mera, I.; Espinosa-Pesqueira, M.E.; Pérez-Hernández, R.; Arenas-Alatorre, J. Synthesis of magnetite (Fe3O4) nanoparticles without surfactants at room temperature. Mater. Lett. 2007, 61, 4447–4451. [Google Scholar] [CrossRef]
- Hadadian, Y.; Masoomi, H.; Dinari, A.; Ryu, C.; Hwang, S.; Kim, S.; Cho, B.k.; Lee, J.Y.; Yoon, J. From Low to High Saturation Magnetization in Magnetite Nanoparticles: The Crucial Role of the Molar Ratios Between the Chemicals. ACS Omega 2022, 7, 15996–16012. [Google Scholar] [CrossRef]
- Asar, M.F.; Ahmad, N.; Husain, Q. Chitosan modified Fe3O4/graphene oxide nanocomposite as a support for high yield and stable immobilization of cellulase: Its application in the saccharification of microcrystalline cellulose. Prep. Biochem. Biotechnol. 2020, 50, 460–467. [Google Scholar] [CrossRef]
- Zdarta, J.; Jedrzak, A.; Klapiszewski, L.; Jesionowski, T. Immobilization of cellulase on a functional inorganic-organic hybrid support: Stability and kinetic study. Catalysts 2017, 7, 374. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, X.; Wang, Y.; Yang, Y.; Ma, Y.; Zou, D.; Liu, Y. Optimization of Cellulase Immobilization with Sodium Alginate-Polyethylene for Enhancement of Enzymatic Hydrolysis of Microcrystalline Cellulose Using Response Surface Methodology. Appl. Biochem. Biotechnol. 2021, 193, 2043–2060. [Google Scholar] [CrossRef]
- Yasmin, H.A.N.; Naresh, S.; Kunasundari, B.; Shuit, S.H. Immobilization of cellulase on magnetized multiwall carbon nanotubes (m-MWCNTs) synthesized via eco-friendly (water-based) method. Chem. Pap. 2022, 76, 453–464. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Immobilization of cellulase on functionalized cobalt ferrite nanoparticles. Korean J. Chem. Eng. 2016, 33, 216–222. [Google Scholar] [CrossRef]
- Kaur, P.; Taggar, M.S.; Kalia, A. Characterization of magnetic nanoparticle-immobilized cellulases for enzymatic saccharification of rice straw. Biomass Convers. Biorefin. 2021, 11, 955–969. [Google Scholar] [CrossRef]
- Rashid, S.S.; Mustafa, A.H.; Rahim, M.H.A.; Gunes, B. Magnetic nickel nanostructure as cellulase immobilization surface for the hydrolysis of lignocellulosic biomass. Int. J. Biol. Macromol. 2022, 209, 1048–1053. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Zhao, J.; Liang, J.; Zhu, J. Production of Sorbitol via Hydrogenation of Glucose over Ruthenium Coordinated with Amino Styrene-co-maleic Anhydride Polymer Encapsulated on Activated Carbon (Ru/ASMA@AC) Catalyst. Molecules 2023, 28, 4830. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.; Guan, J.; Chen, X.; Qin, Z.; Mu, X.; Xian, M. Selective hydrogenation of D-glucose to D-sorbitol over Ru/ZSM-5 catalysts. Cuihua Xuebao 2014, 35, 733–740. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Mishra, D.K.; Hwang, J.-S. Selective hydrogenation of D-glucose using amine functionalized nanoporous polymer supported Ru nanoparticles based catalyst. Catal. Today 2016, 265, 163–173. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Karakoulia, S.; Delimitis, A.; Coman, S.M.; Parvulescu, V.I.; Triantafyllidis, K.S. D-Glucose hydrogenation/hydrogenolysis reactions on noble metal (Ru, Pt)/activated carbon supported catalysts. Catal. Today 2015, 257, 281–290. [Google Scholar] [CrossRef]
- Manaenkov, O.V.; Mann, J.J.; Kislitza, O.V.; Losovyj, Y.; Stein, B.D.; Morgan, D.G.; Pink, M.; Lependina, O.L.; Shifrina, Z.B.; Matveeva, V.G.; et al. Ru-Containing Magnetically Recoverable Catalysts: A Sustainable Pathway from Cellulose to Ethylene and Propylene Glycols. ACS Appl. Mater. Interfaces 2016, 8, 21285–21293. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, Z.; Xiong, J.; He, Y. Sustainable Chemoenzymatic Cascade Transformation of Corncob to Furfuryl Alcohol with Rice Husk-Based Heterogeneous Catalyst UST-Sn-RH. Catalysts 2023, 13, 37. [Google Scholar] [CrossRef]
- Nzediegwu, E.; Perez-Venegas, M.; Auclair, K.; Dumont, M.-J. Semisynthetic production of hydroxymethylfurfural and furfural: The benefits of an integrated approach. J. Environ. Chem. Eng. 2022, 10, 108515. [Google Scholar] [CrossRef]
- Feng, X.-Q.; Li, Y.-Y.; Ma, C.-L.; Xia, Y.; He, Y.-C. Improved conversion of bamboo shoot shells to furfuryl alcohol and furfurylamine by a sequential catalysis with sulfonated graphite and biocatalysts. RSC Adv. 2020, 10, 40365–40372. [Google Scholar] [CrossRef]
- Musci, J.J.; Chiosso, M.E.; Siri, G.J.; Casella, M.L. Hydrogenation of glucose on a carbon-supported Ru catalyst: Optimization of the reaction conditions. Adv. Chem. Eng. Sci. 2023, 13, 224–240. [Google Scholar] [CrossRef]
- Ahmad, R.; Khare, S.K. Immobilization of Aspergillus niger cellulase on multiwall carbon nanotubes for cellulose hydrolysis. Bioresour. Technol. 2018, 252, 72–75. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248. [Google Scholar] [CrossRef]
- Miller, G.L. Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426. [Google Scholar] [CrossRef]
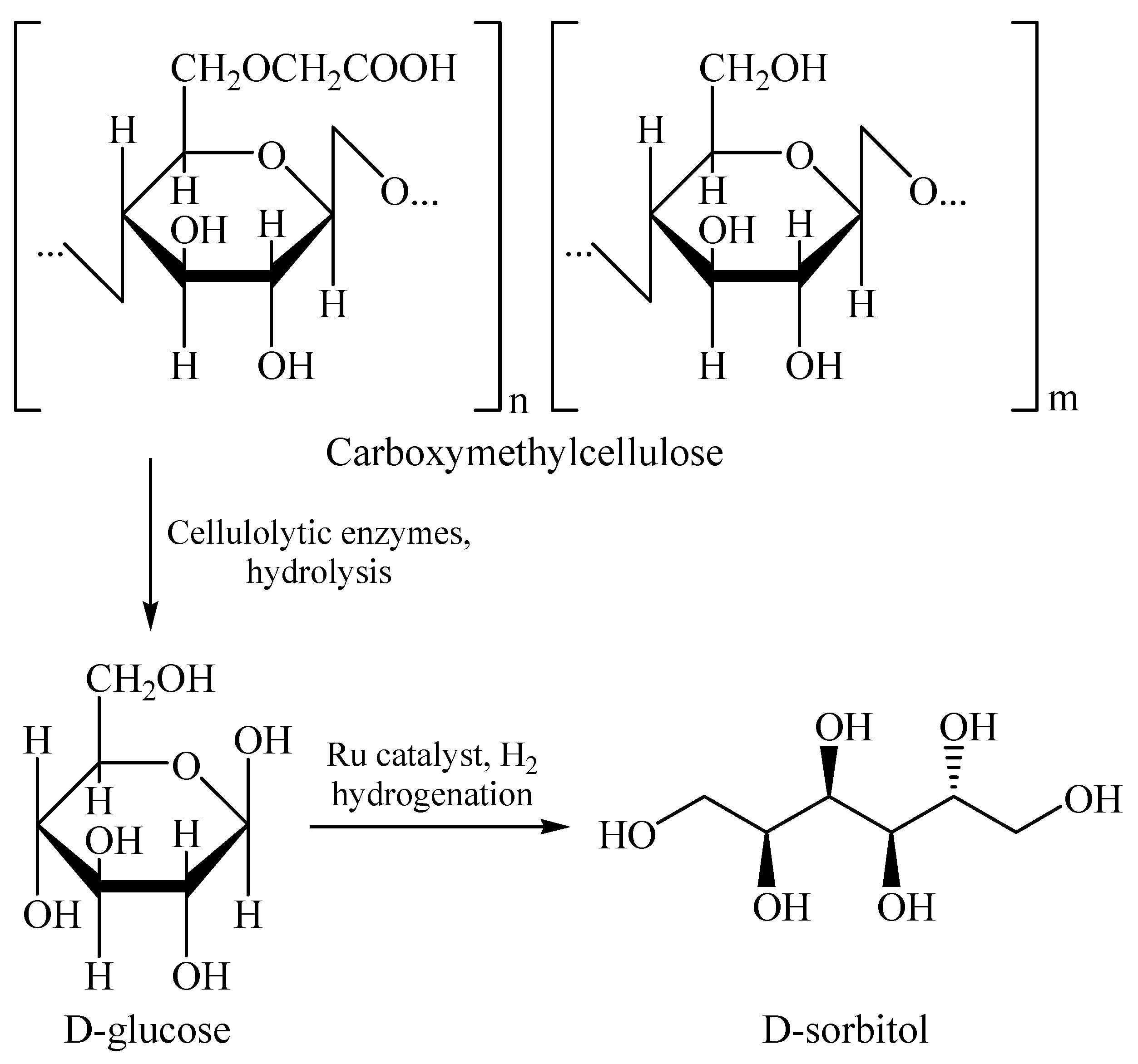
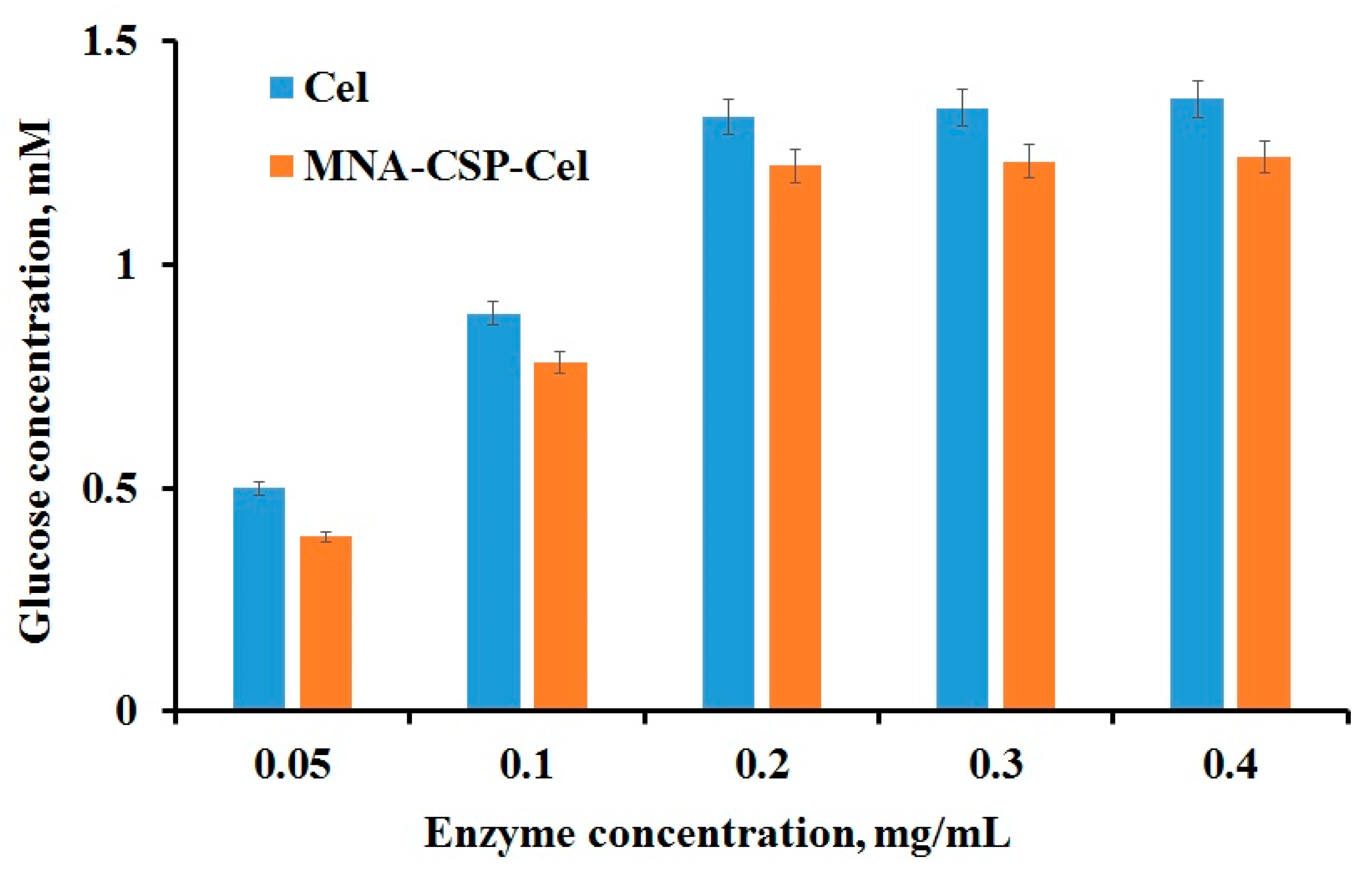
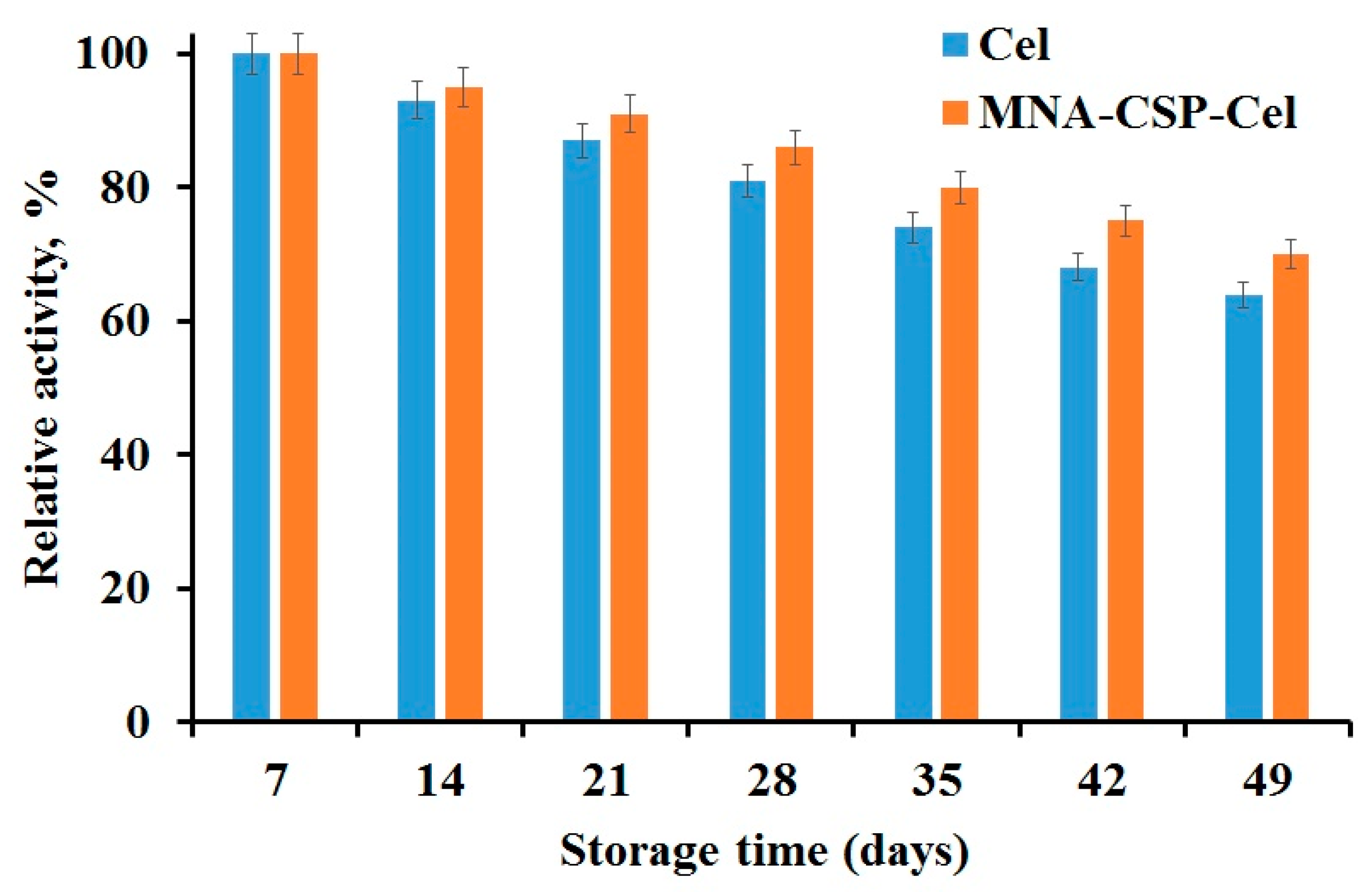
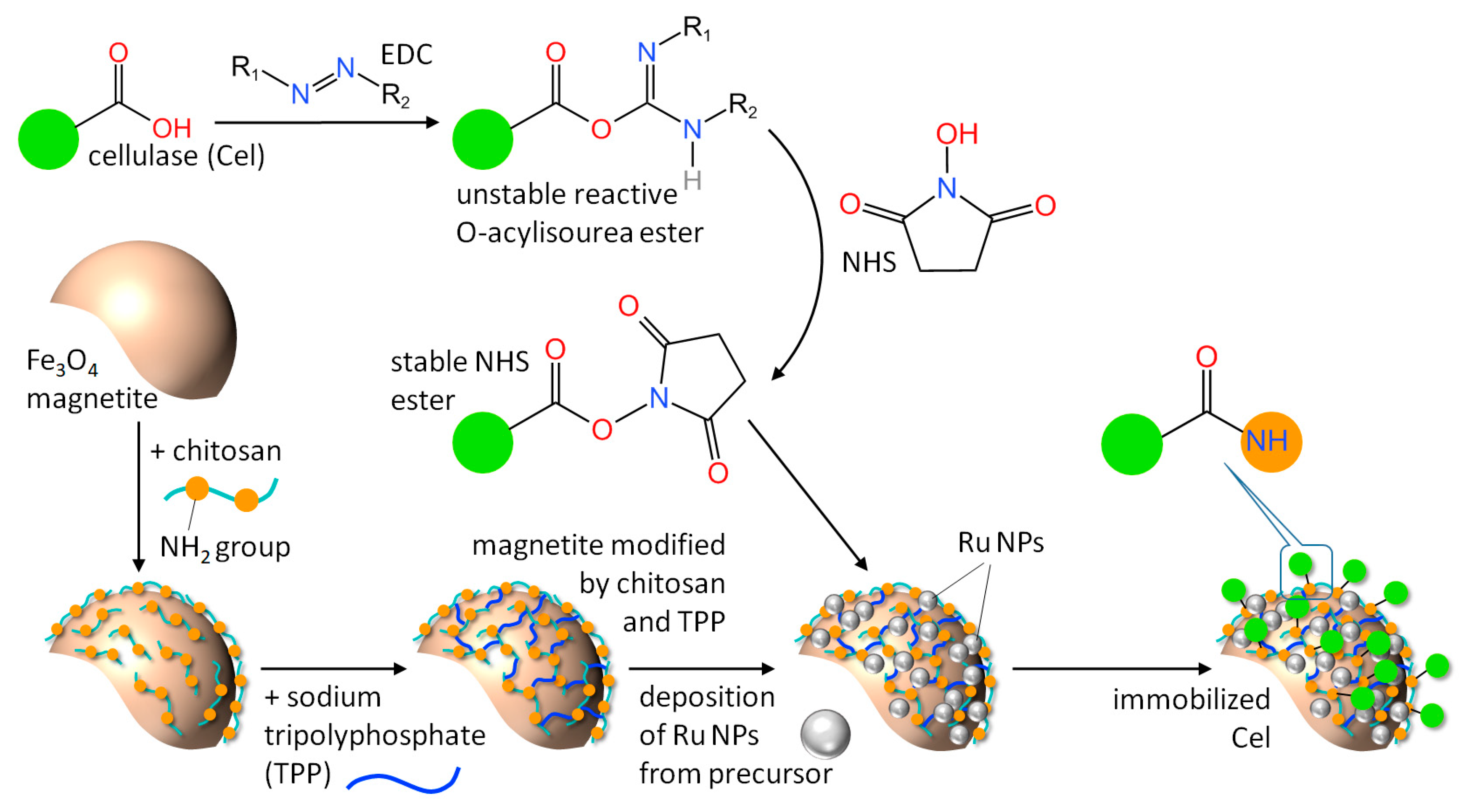

| Kinetic Constants | Native Cel | MNA-CSP-Cel |
|---|---|---|
| Km, mM | 69.5 | 143.4 |
| Vmax, μM/s | 7.7 | 7.1 |
| Ru wt.% by Loading | Ru Concentration ×104, mol/L | D-Glucose Conversion, % | Selectivity, % |
|---|---|---|---|
| 1 | 1.98 | 34.0 | 99.2 |
| 2 | 3.96 | 65.0 | 99.3 |
| 3 | 5.94 | 99.9 | 99.3 |
| 5 | 9.90 | 99.9 | 90.8 |
| Catalyst | Ru Content, wt.% | T, °C | P(H2), MPa | Time, h | Conversion, % * | Selectivity, % * | Ref. |
|---|---|---|---|---|---|---|---|
| MNA-CSP-Ru | 3 | 100 | 4 | 2 | 99.9 | 99.3 | This work |
| Ru/ASMA@AC | 2.5 | 130 | 4 | 3 | 99.7 | 93.0 | [67] |
| 5%Ru/-Al2O3@ASMA | 5 | 120 | 5 | 3 | 100 | 95.2 | [50] |
| Ru/ZSM-5 | 5 | 200 | 4 | 99.6 | 99.2 | [68] | |
| Ru/polymer catalyst | 5 | 100 | 5.5 | 1 | 67.5 | 98 | [69] |
| Ru/AC | 3 | 180 | 1.6 | 3 | 97 | 90 | [70] |
| Time, h | Reaction Mixture Composition, % * | |||
|---|---|---|---|---|
| CMC | D-Glucose | D-Sorbitol | D-Mannitol | |
| 5.0 | 6.7 | 43.1 | 49.7 | 0.5 |
| 7.5 | - | 29.1 | 70.0 | 0.9 |
| 10.0 | - | - | 83.2 | 16.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonov, B.B.; Lisichkin, D.R.; Sulman, A.M.; Sidorov, A.I.; Bykov, A.V.; Lugovoy, Y.V.; Karpenkov, A.Y.; Bronstein, L.M.; Matveeva, V.G. Magnetic Bifunctional Ru-Enzyme Catalyst Allows for Sustainable Conversion of Cellulose Derivative to D-Sorbitol. Nanomaterials 2025, 15, 740. https://doi.org/10.3390/nano15100740
Tikhonov BB, Lisichkin DR, Sulman AM, Sidorov AI, Bykov AV, Lugovoy YV, Karpenkov AY, Bronstein LM, Matveeva VG. Magnetic Bifunctional Ru-Enzyme Catalyst Allows for Sustainable Conversion of Cellulose Derivative to D-Sorbitol. Nanomaterials. 2025; 15(10):740. https://doi.org/10.3390/nano15100740
Chicago/Turabian StyleTikhonov, Boris B., Daniil R. Lisichkin, Alexandrina M. Sulman, Alexander I. Sidorov, Alexey V. Bykov, Yury V. Lugovoy, Alexey Y. Karpenkov, Lyudmila M. Bronstein, and Valentina G. Matveeva. 2025. "Magnetic Bifunctional Ru-Enzyme Catalyst Allows for Sustainable Conversion of Cellulose Derivative to D-Sorbitol" Nanomaterials 15, no. 10: 740. https://doi.org/10.3390/nano15100740
APA StyleTikhonov, B. B., Lisichkin, D. R., Sulman, A. M., Sidorov, A. I., Bykov, A. V., Lugovoy, Y. V., Karpenkov, A. Y., Bronstein, L. M., & Matveeva, V. G. (2025). Magnetic Bifunctional Ru-Enzyme Catalyst Allows for Sustainable Conversion of Cellulose Derivative to D-Sorbitol. Nanomaterials, 15(10), 740. https://doi.org/10.3390/nano15100740








