Integrated Photonic Biosensors: Enabling Next-Generation Lab-on-a-Chip Platforms
Abstract
1. Introduction
2. Principles of Photonic Biosensing
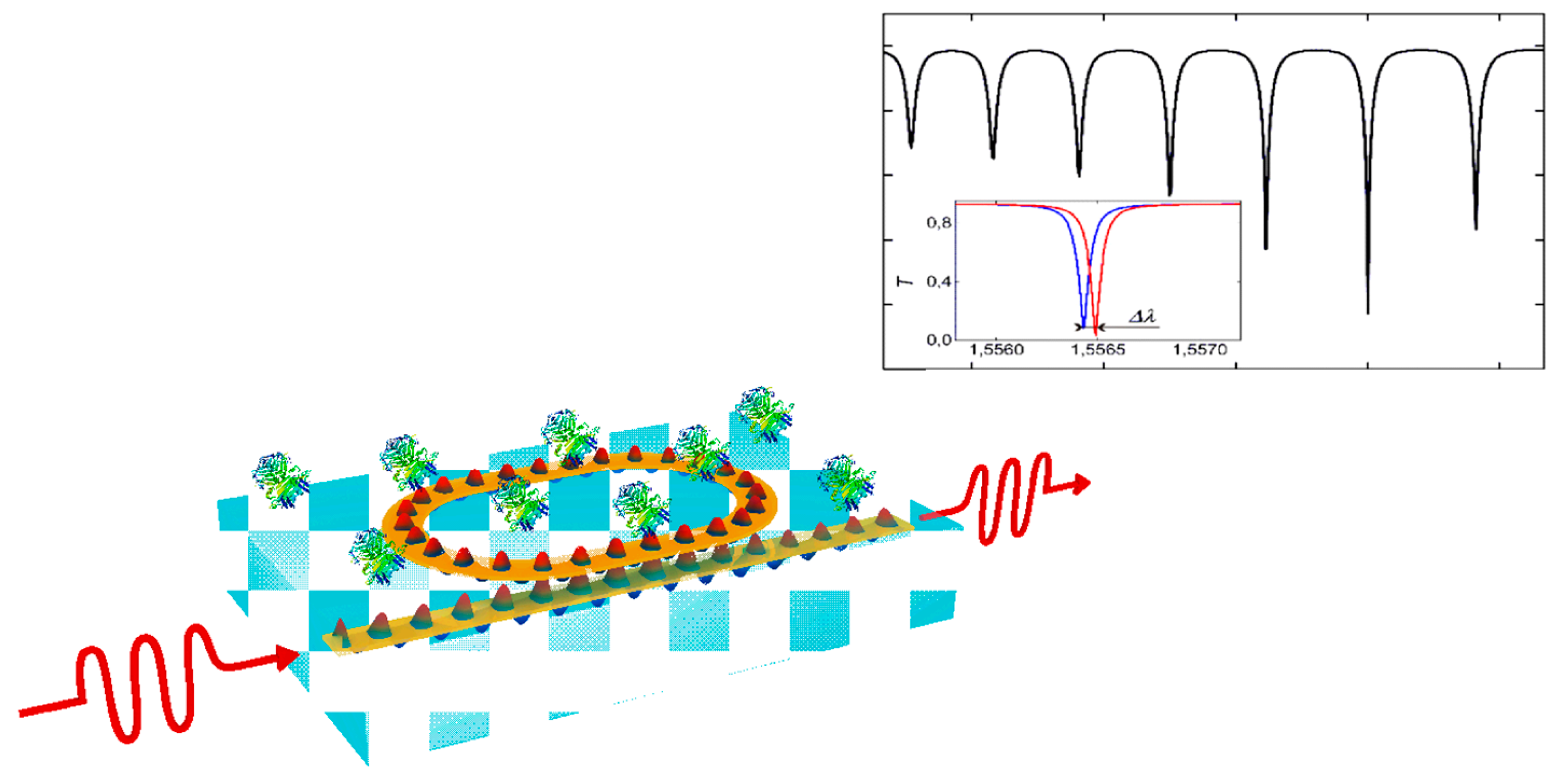
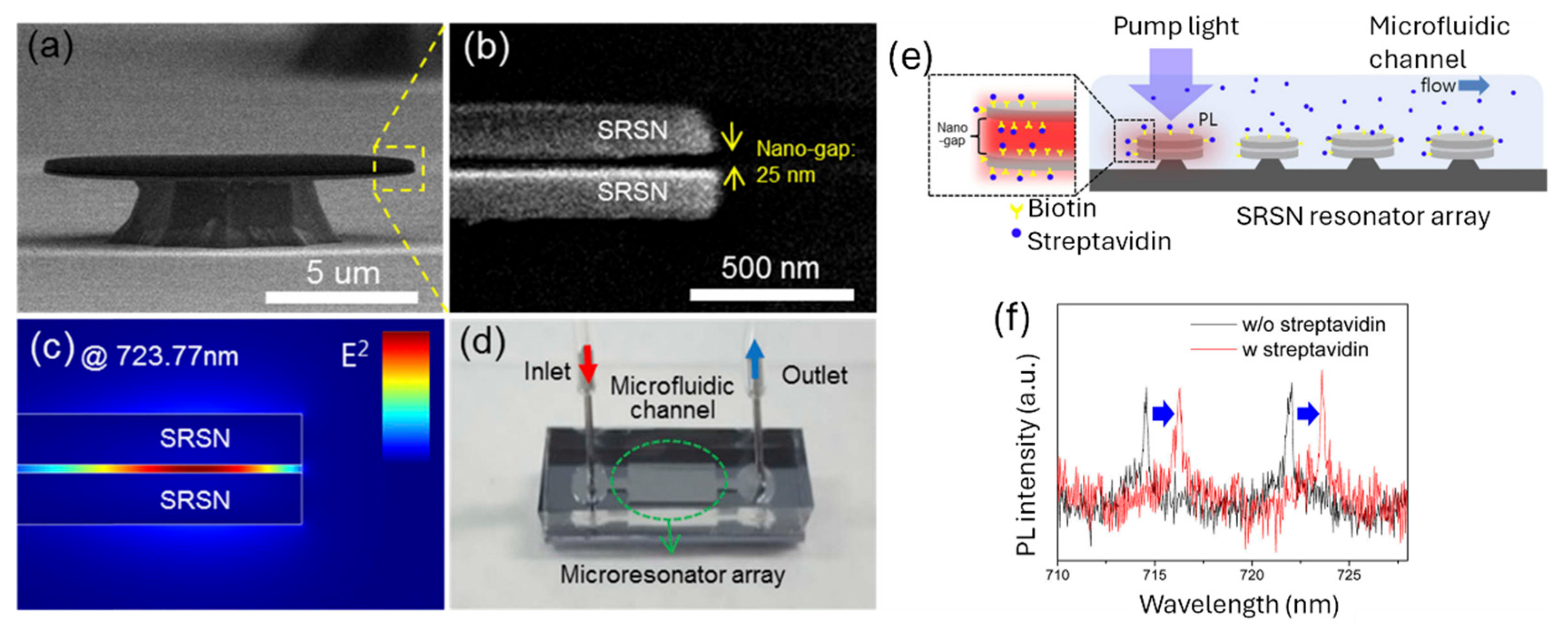
2.1. Resonant Structures
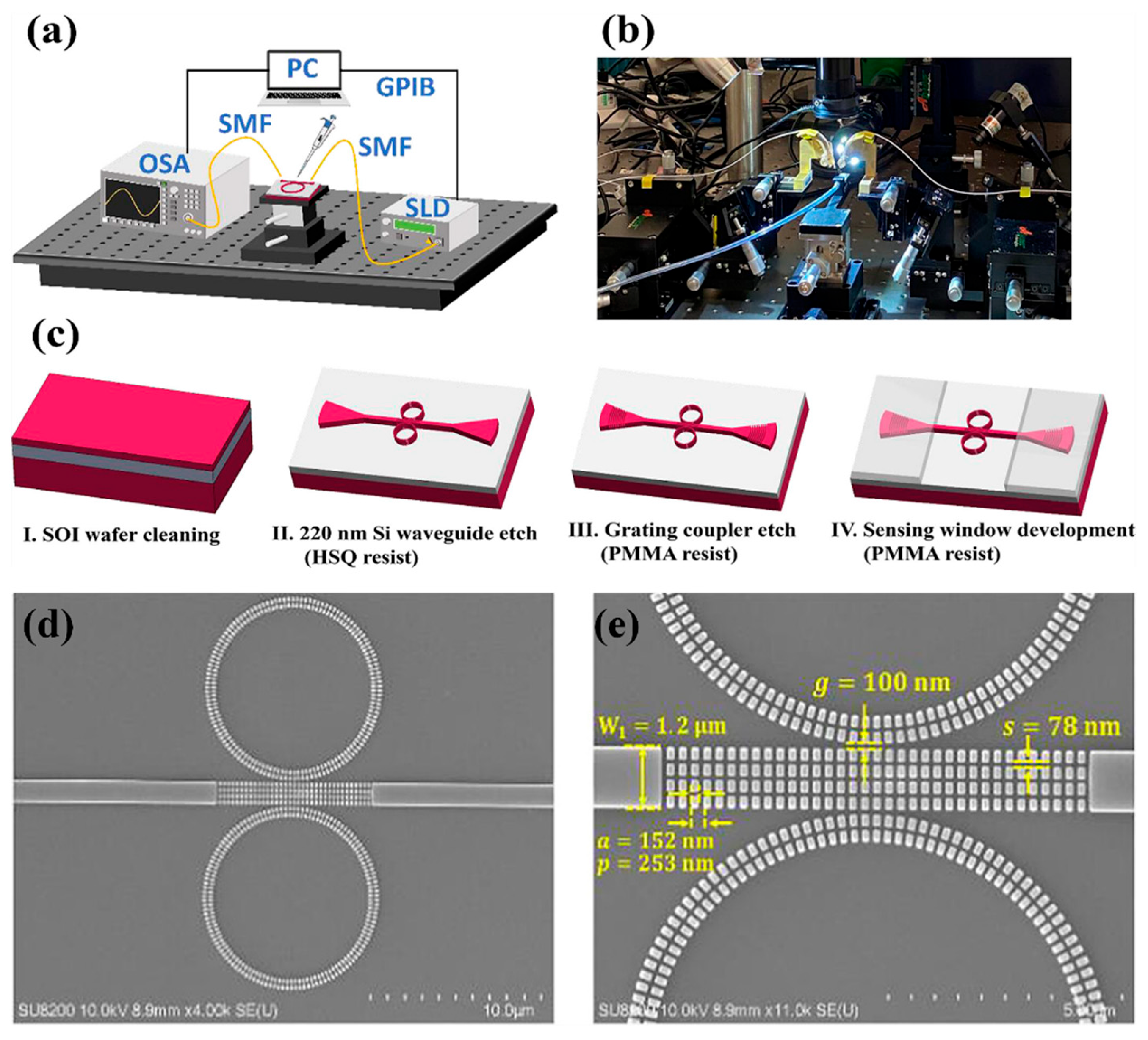
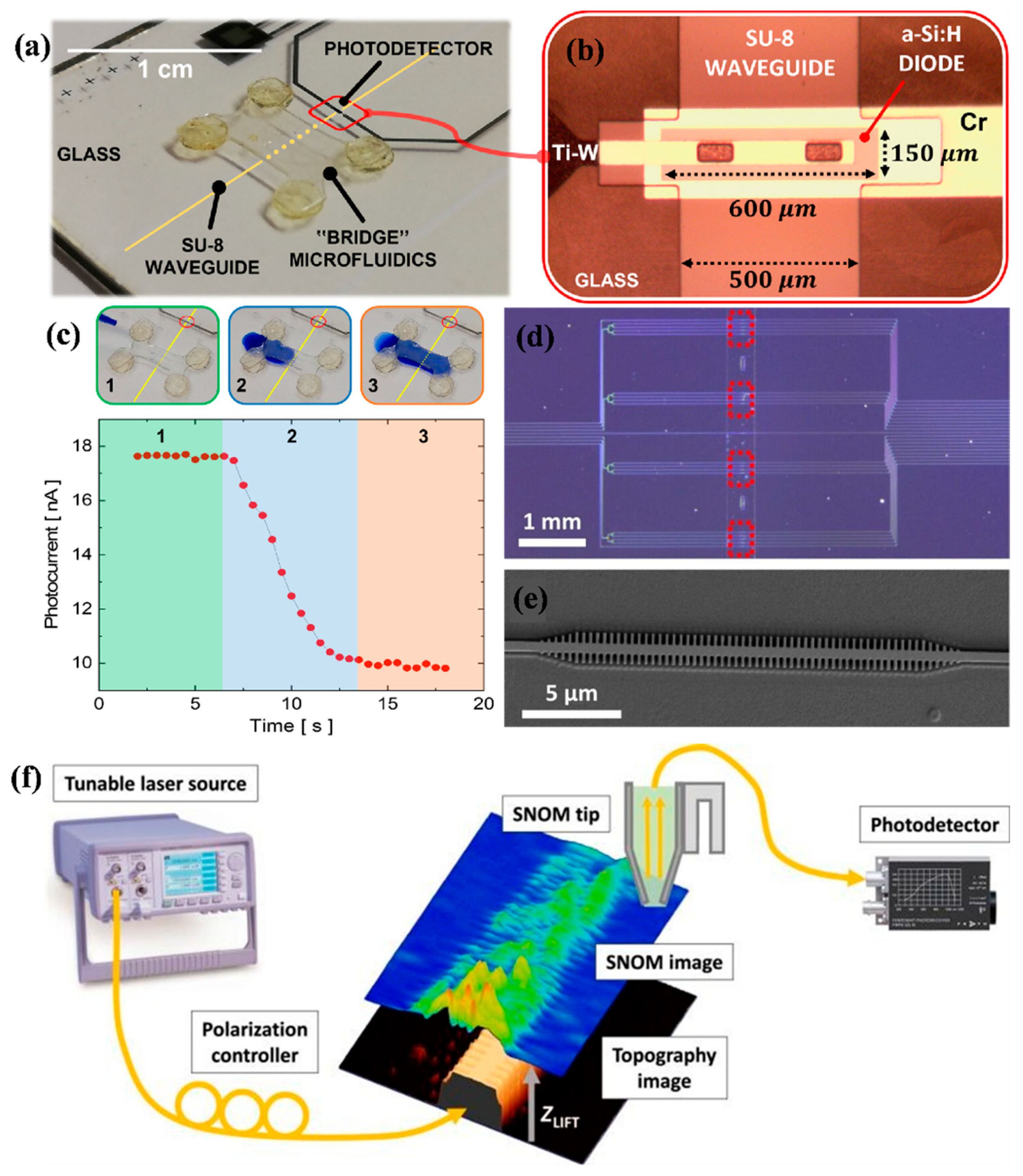
2.2. Interferometric Techniques
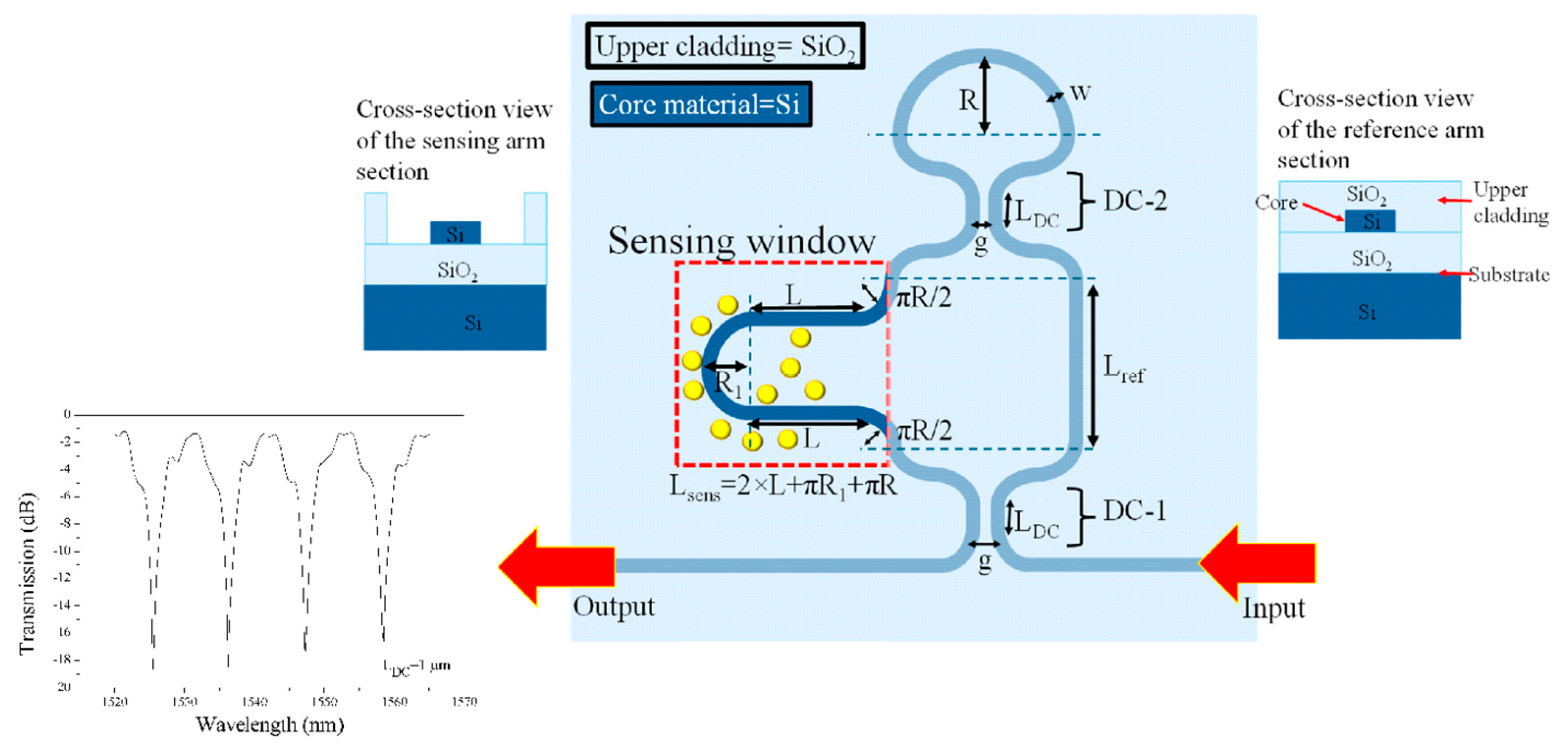
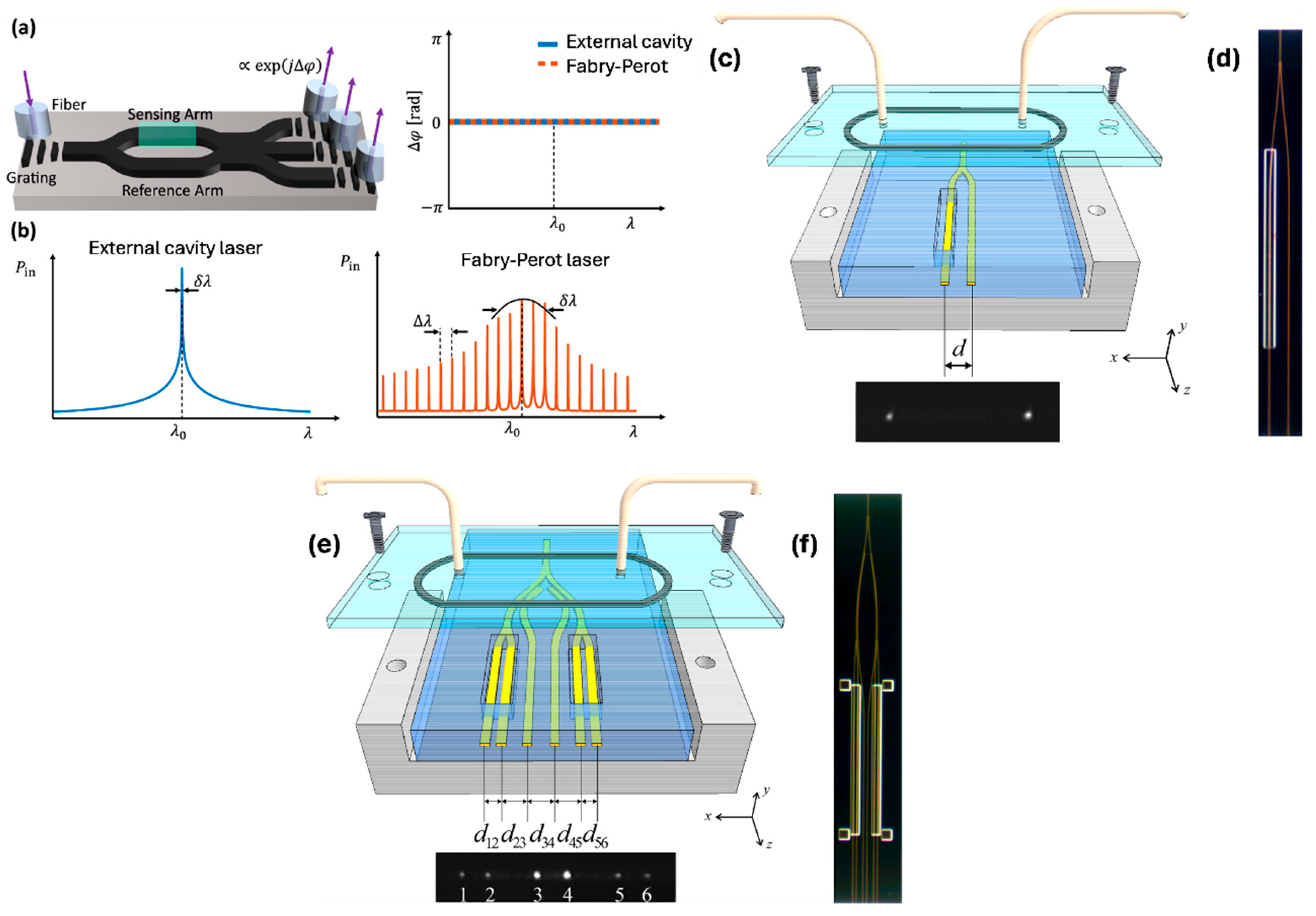
3. Integration with Lab-on-Chip Systems
| Component | Description | Examples/Materials |
|---|---|---|
| Microfluidic Network [91,92] | Engineered microchannels that manipulate and transport nanoliter to microliter volumes of fluids. | Polydimethylsiloxane (PDMS), glass, PMMA; integrated with valves and pumps. |
| Biorecognition Element [93,94] | Molecular entity that provides specificity by selectively interacting with the target analyte. | Antibodies, aptamers, enzymes, nucleic acids (DNA/RNA), and molecularly imprinted polymers. |
| Transduction Unit [95,96] | Converts the biorecognition event into a quantifiable physicochemical signal. | Electrochemical (amperometric, potentiometric), optical (SPR, fluorescence), and piezoelectric. |
| Substrate/Platform Material [33,36,97] | Structural base that supports microfabricated components and defines the chip architecture. | Silicon wafers, glass slides, and thermoplastics (e.g., cyclic olefin copolymer-COC). |
| Signal Processing Module [42] | Amplifies, filters, and digitizes the transduced signal for analysis and interpretation. | Analog front ends, microcontrollers, and signal conditioning circuits. |
| Sample Handling Interface [98] | Facilitates introduction, distribution, and sometimes pre-treatment of biological samples. | Microreservoirs, capillary inlets, filters, micromixers. |
| Data Acquisition and Output Unit [68,99] | Interfaces with user or external devices for data visualization or transmission. | Integrated displays, wireless communication (Bluetooth, NFC), and smartphone integration. |
| Encapsulation and Packaging [100,101] | Protects sensitive components, ensures biocompatibility, and facilitates safe handling. | Biocompatible polymers, epoxy resins, hermetic seals, and micro-packaged enclosures. |
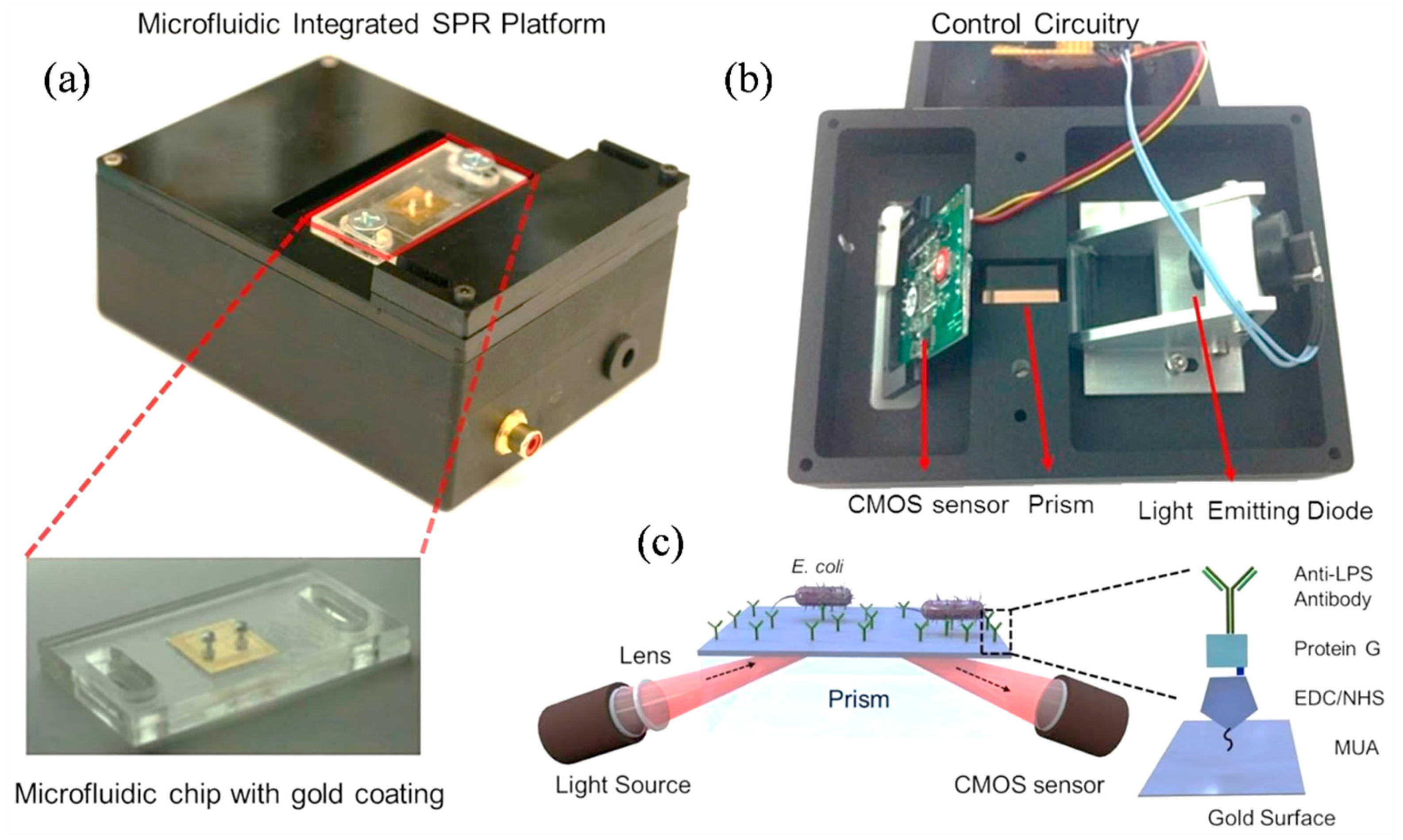
4. Technological Advances
4.1. Silicon Photonics
4.2. Optofluidic Photonic Crystal Cavities
4.3. Integrated Detector Arrays
4.4. Complementary Metal-Oxide-Semiconductor (CMOS) Compatibility
5. Applications
5.1. Medical Diagnostics

5.2. Environmental Monitoring
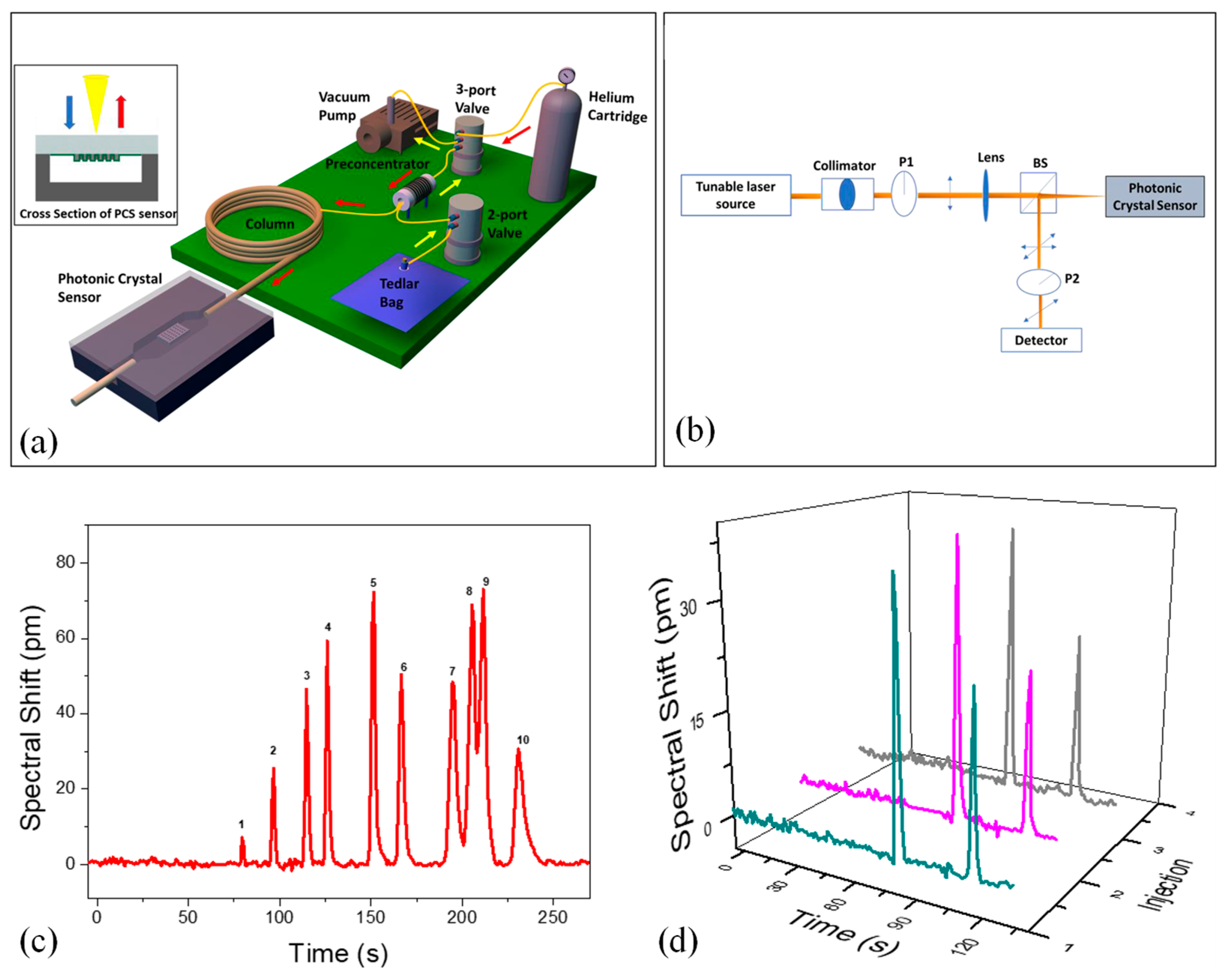
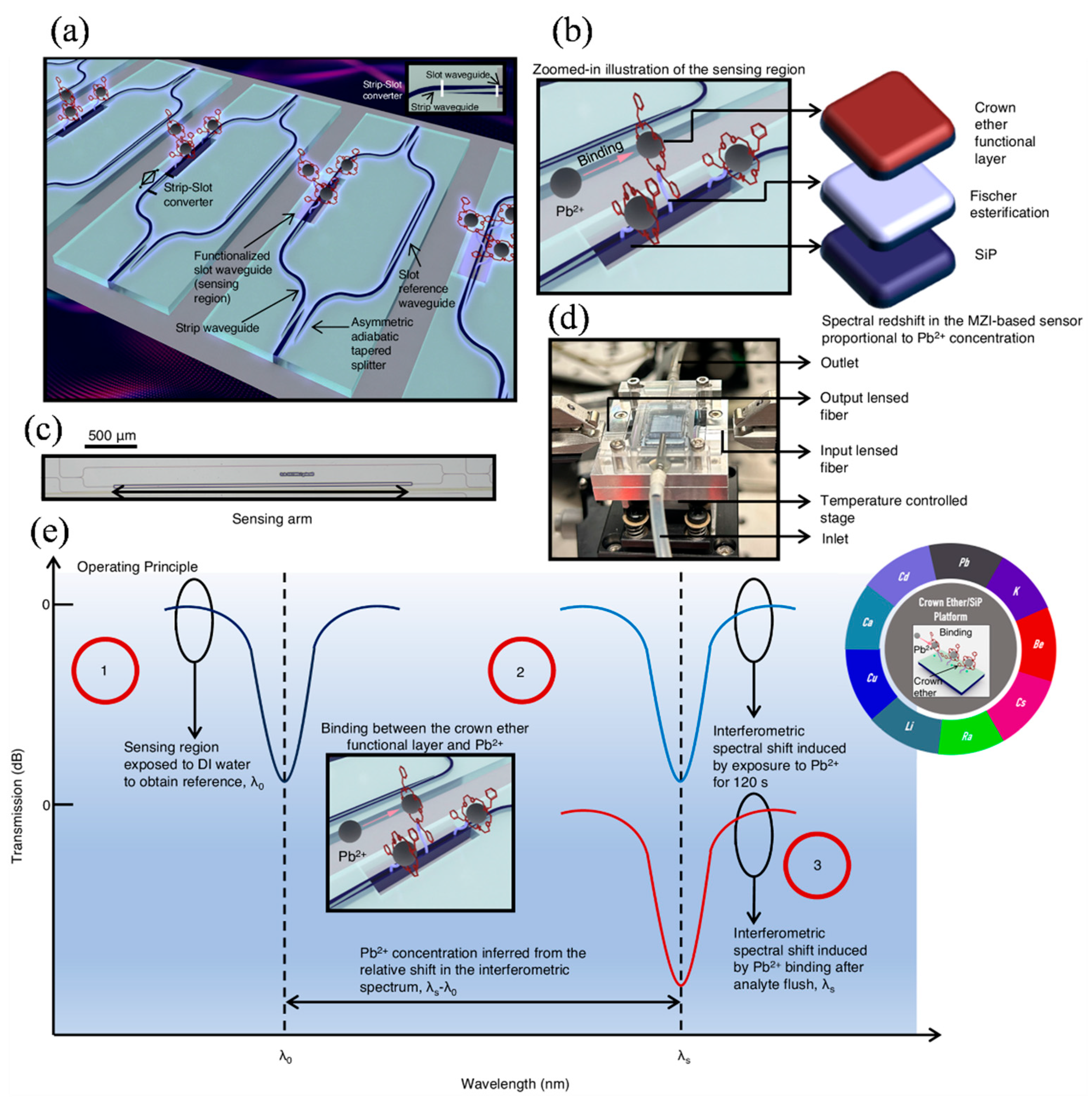
5.3. Food Quality Assurance
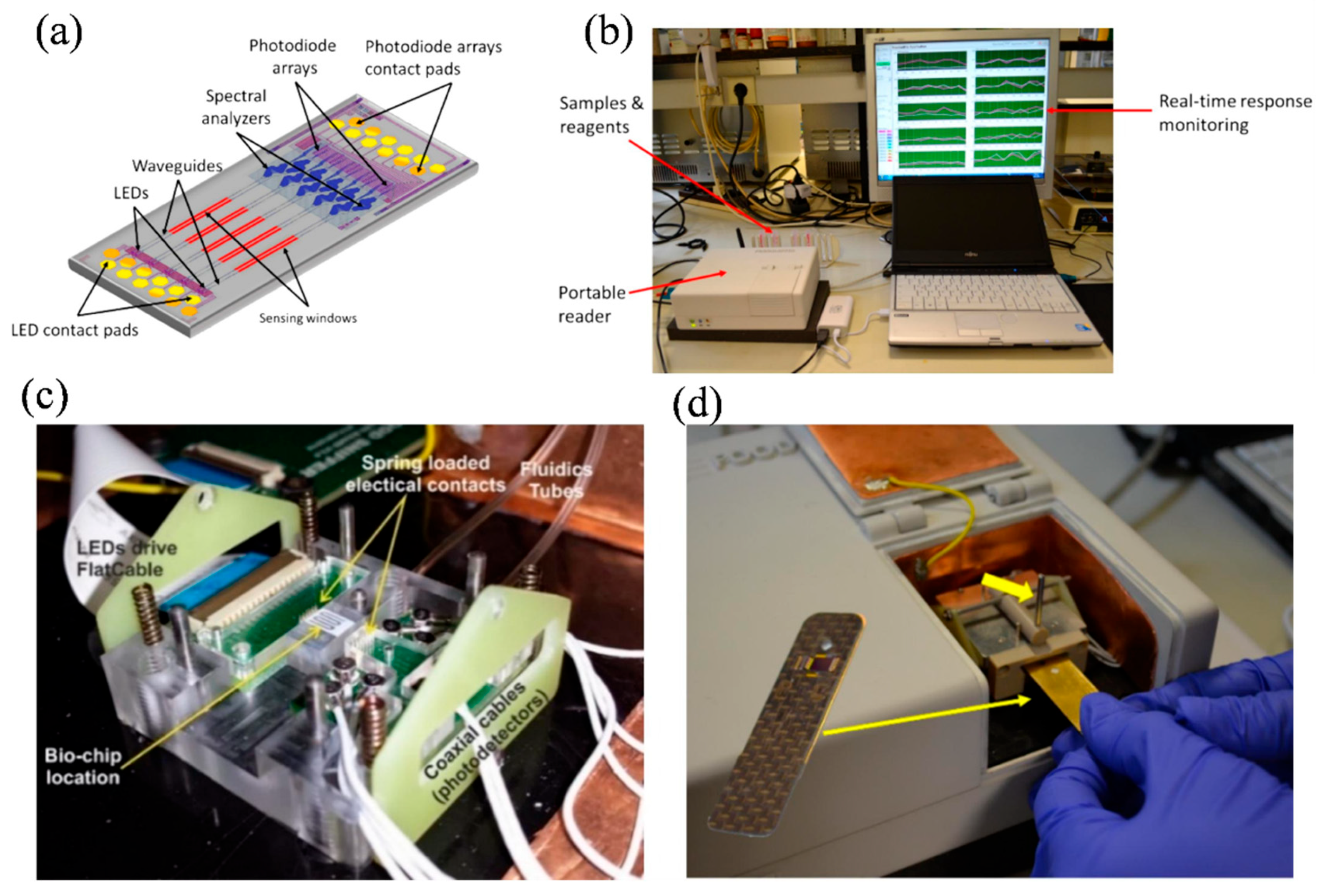
| Application Area | Sensing Principle | Key Metrics | Remarks |
|---|---|---|---|
| Medical Diagnostics [37] | Resonant (Photonic Crystal, MRR) | Sensitivity: 810 nm/RIU, 1430 pm/% NaCl Detection limit: 0.04% NaCl, 2.04 × 10⁻5 RIU | Multiplexed detection using cascaded microring and photonic crystal resonators |
| Medical Diagnostics [17,68] | Interferometric (MZI) | Sensitivity: 510 nm/RIU; CRP LOD: <300 pg/mL with coherent detection | Coherent detection enhances stability and sensitivity; low-cost lasers used |
| Environmental Monitoring [146] | Resonant (Si Photonic + Crown Ethers) | Broad detection range; real-time and selective detection | Ion-selective binding with crown ethers enables selective heavy metal detection |
| Environmental Monitoring [142] | Interferometric (Photothermal) | Detection limit: 870 ppm CO₂ | LiNbO₃ photonic platform using heterodyne phase shift for enhanced gas sensing |
| Food Safety [152] | Interferometric (MZI Array) | Detection limits: 0.01–0.25 μg/mL (allergens) 2–10 ng/mL (mycotoxins) | Multiplexed allergen and toxin detection using integrated BB-MZI array with on-chip photodiodes |
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cinti, S.; Singh, S.; Covone, G.; Tonietti, L.; Ricciardelli, A.; Cordone, A.; Iacono, R.; Mazzoli, A.; Moracci, M.; Rotundi, A.; et al. Reviewing the State of Biosensors and Lab-on-a-Chip Technologies: Opportunities for Extreme Environments and Space Exploration. Front. Microbiol. 2023, 14, 1215529. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.A.; Bañuls, M.J.; González-Pedro, V.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Maquieira, A.; Sohlström, H.; Holgado, M.; Casquel, R. Label-Free Optical Biosensing with Slot-Waveguides. Opt. Lett. 2008, 33, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Amin, R.; Bui, F.M.; Chen, L.; Mohammadd, N.; Al-Zahrani, F.A.; Kumar, S. Design and Analysis of Multi-Analyte Detection Based Biosensor in the Visible to Near-Infrared (VNIR) Region. IEEE Trans. NanoBiosci. 2024, 23, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Skivesen, N.; Têtu, A.; Kristensen, M.; Kjems, J.; Frandsen, L.H.; Borel, P.I. Photonic-Crystal Waveguide Biosensor. Opt. Express 2007, 15, 3169–3176. [Google Scholar] [CrossRef]
- Houssein Jokar, M.; Naraghi, A.; Seifouri, M.; Olyaee, S. Design of Bio-Alcohol Sensor Based on Waveguide-Coupled Photonic Crystal Cavity. Results Opt. 2023, 13, 100563. [Google Scholar] [CrossRef]
- Ho, H.P.; Wong, C.L.; Wu, S.Y.; Yuan, W.; Lin, C.; Kong, S.K. Highly Sensitive Photonic Biosensors Based on Interferometric Detection of Surface Plasmon Waves. In Proceedings of the 2006 International Symposium on Biophotonics, Nanophotonics and Metamaterials, Hangzhou, China, 16–18 October 2006; p. 2. [Google Scholar]
- Butt, M.A.; Kazanskiy, N.L.; Khonina, S.N.; Voronkov, G.S.; Grakhova, E.P.; Kutluyarov, R.V. A Review on Photonic Sensing Technologies: Status and Outlook. Biosensors 2023, 13, 568. [Google Scholar] [CrossRef]
- Butt, M.A.; Kozłowski, Ł.; Słowikowski, M.; Juchniewicz, M.; Drecka, D.A.; Filipiak, M.; Golas, M.; Stonio, B.; Dudek, M.; Piramidowicz, R. Investigation of Modal Characteristics of Silicon Nitride Ridge Waveguides for Enhanced Refractive Index Sensing. Micromachines 2025, 16, 119. [Google Scholar] [CrossRef]
- Altug, H.; Oh, S.-H.; Maier, S.A.; Homola, J. Advances and Applications of Nanophotonic Biosensors. Nat. Nanotechnol. 2022, 17, 5–16. [Google Scholar] [CrossRef]
- Seyyedmasoumian, S.; Attariabad, A.; Pourziad, A.; Bemani, M. Refractive Index Biosensor Using Metamaterial Perfect Absorber Based on Graphene in Near-Infrared for Disease Diagnosis. IEEE Sens. J. 2022, 22, 14870–14877. [Google Scholar] [CrossRef]
- Molina-Fernández, Í.; Leuermann, J.; Ortega-Moñux, A.; Wangüemert-Pérez, J.G.; Halir, R. Fundamental Limit of Detection of Photonic Biosensors with Coherent Phase Read-Out. Opt. Express 2019, 27, 12616–12629. [Google Scholar] [CrossRef]
- Kong, W.; Wan, Y.; Zheng, Z.; Zhao, X.; Liu, Y.; Bian, Y. High-Sensitivity Sensing Based on Intensity-Interrogated Bloch Surface Wave Sensors. In Proceedings of the IEEE Photonics Conference 2012, Burlingame, CA, USA, 23–27 September 2012; pp. 204–205. [Google Scholar]
- Gao, Y.; Yan, X.; Chen, X.; Li, B.; Cheng, T. A Refractive Index Sensor Based on Four-Wave Mixing in D-Shaped Tellurite Photonic Crystal Fiber. Photonic Sens. 2023, 13, 230312. [Google Scholar] [CrossRef]
- Butt, M.A. Racetrack Ring Resonator-Based on Hybrid Plasmonic Waveguide for Refractive Index Sensing. Micromachines 2024, 15, 610. [Google Scholar] [CrossRef] [PubMed]
- Errando-Herranz, C.; Saharil, F.; Romero, A.M.; Sandström, N.; Shafagh, R.Z.; van der Wijngaart, W.; Haraldsson, T.; Gylfason, K.B. Integration of Microfluidics with Grating Coupled Silicon Photonic Sensors by One-Step Combined Photopatterning and Molding of OSTE. Opt. Express 2013, 21, 21293–21298. [Google Scholar] [CrossRef]
- Scullion, M.G.; Di Falco, A.; Krauss, T.F. Slotted Photonic Crystal Cavities with Integrated Microfluidics for Biosensing Applications. Biosens. Bioelectron. 2011, 27, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.A. High Sensitivity Design for Silicon-On-Insulator-Based Asymmetric Loop-Terminated Mach-Zehnder Interferometer. Materials 2025, 18, 798. [Google Scholar] [CrossRef]
- Butt, M.A.; Juchniewicz, M.; Słowikowski, M.; Kozłowski, Ł.; Piramidowicz, R. Mid-Infrared Photonic Sensors: Exploring Fundamentals, Advanced Materials, and Cutting-Edge Applications. Sensors 2025, 25, 1102. [Google Scholar] [CrossRef]
- Butt, M.A.; Janaszek, B.; Piramidowicz, R. Lighting the Way Forward: The Bright Future of Photonic Integrated Circuits. Sens. Int. 2025, 6, 100326. [Google Scholar] [CrossRef]
- Li, K.; Thomson, D.J.; Liu, S.; Zhang, W.; Cao, W.; Littlejohns, C.G.; Yan, X.; Ebert, M.; Banakar, M.; Tran, D.; et al. An Integrated CMOS–Silicon Photonics Transmitter with a 112 Gigabaud Transmission and Picojoule per Bit Energy Efficiency. Nat. Electron. 2023, 6, 910–921. [Google Scholar] [CrossRef]
- Romero-García, S.; Merget, F.; Zhong, F.; Finkelstein, H.; Witzens, J. Silicon Nitride CMOS-Compatible Platform for Integrated Photonics Applications at Visible Wavelengths. Opt. Express 2013, 21, 14036–14046. [Google Scholar] [CrossRef]
- Talamas Simola, E.; Kiyek, V.; Ballabio, A.; Schlykow, V.; Frigerio, J.; Zucchetti, C.; De Iacovo, A.; Colace, L.; Yamamoto, Y.; Capellini, G.; et al. CMOS-Compatible Bias-Tunable Dual-Band Detector Based on GeSn/Ge/Si Coupled Photodiodes. ACS Photonics 2021, 8, 2166–2173. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Chen, X.; Bertrand, M.; Shams-Ansari, A.; Chandrasekhar, S.; Winzer, P.; Lončar, M. Integrated Lithium Niobate Electro-Optic Modulators Operating at CMOS-Compatible Voltages. Nature 2018, 562, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, V.; Kordrostami, Z.; Hosseini, M. Sensitivity and Quality Factor Improvement of Photonic Crystal Sensors by Geometrical Optimization of Waveguides and Micro-Ring Resonators Combination. Sci. Rep. 2024, 14, 2001. [Google Scholar] [CrossRef] [PubMed]
- Barik, P.; Pradhan, M. Selectivity in Trace Gas Sensing: Recent Developments, Challenges, and Future Perspectives. Analyst 2022, 147, 1024–1054. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Roelkens, G.; Baets, R.; Bowers, J.E. Hybrid Integrated Platforms for Silicon Photonics. Materials 2010, 3, 1782–1802. [Google Scholar] [CrossRef]
- Butt, M.A. Surface Plasmon Resonance-Based Biodetection Systems: Principles, Progress and Applications—A Comprehensive Review. Biosensors 2025, 15, 35. [Google Scholar] [CrossRef]
- Elsayed, H.A.; Awasthi, S.K.; Almawgani, A.H.; Mehaney, A.; Ali, Y.A.A.; Alzahrani, A.; Ahmed, A.M. High-Performance Biosensors Based on Angular Plasmonic of a Multilayer Design: New Materials for Enhancing Sensitivity of One-Dimensional Designs. RSC Adv. 2024, 14, 7877–7890. [Google Scholar] [CrossRef]
- Mireles, J., Jr.; Garcia, M.A.; Ambrosio, R.C.; Garcia, E.J.; Calleja, W.; Reyes, C. Optical Fiber Packaging for MEMS Interfacing. In Proceedings of the Micromachining and Microfabrication Process Technology XIV, San Jose, CA, USA, 23 February 2009; SPIE: Bellingham, WA, USA, 2009; Volume 7204, pp. 35–46. [Google Scholar]
- Butt, M.A.; Mateos, X.; Piramidowicz, R. Photonics Sensors: A Perspective on Current Advancements, Emerging Challenges, and Potential Solutions (Invited). Phys. Lett. A 2024, 516, 129633. [Google Scholar] [CrossRef]
- Butt, M.A.; Mateos, X. Strategic Insights into Integrated Photonics: Core Concepts, Practical Deployments, and Future Outlook. Appl. Sci. 2024, 14, 6365. [Google Scholar] [CrossRef]
- Rafiq, S.M.; Majumder, R.; Joshi, D.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Sidiqi, U.S. Lab-on-a-Chip Device for Food Quality Control and Safety. Food Control 2024, 164, 110596. [Google Scholar] [CrossRef]
- Pol, R.; Céspedes, F.; Gabriel, D.; Baeza, M. Microfluidic Lab-on-a-Chip Platforms for Environmental Monitoring. TrAC Trends Anal. Chem. 2017, 95, 62–68. [Google Scholar] [CrossRef]
- Ramirez, J.C.; Grajales García, D.; Maldonado, J.; Fernández-Gavela, A. Current Trends in Photonic Biosensors: Advances towards Multiplexed Integration. Chemosensors 2022, 10, 398. [Google Scholar] [CrossRef]
- Campbell, D.P. Interferometric Biosensors. In Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A., Eds.; Springer: New York, NY, USA, 2008; pp. 169–211. ISBN 978-0-387-75113-9. [Google Scholar]
- Muhammad, W.; Song, J.; Kim, S.; Ahmed, F.; Cho, E.; Lee, H.; Kim, J. Silicon-Based Biosensors: A Critical Review of Silicon’s Role in Enhancing Biosensing Performance. Biosensors 2025, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ye, S.; Yuan, B.; Marsh, J.H.; Hou, L. Subwavelength Grating Cascaded Microring Resonator Biochemical Sensors with Record-High Sensitivity. ACS Photonics 2024, 11, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chang, M.; Kong, M.; Xu, Y. Two-Peak Envelope Spectrum of a Subwavelength Grating Microring Resonator for Wide-Range and High-Sensitivity Refractive Index Sensing. Photonics Nanostruct.-Fundam. Appl. 2024, 60, 101273. [Google Scholar] [CrossRef]
- Fasching, G.; Tamosiunas, V.; Benz, A.; Andrews, A.M.; Unterrainer, K.; Zobl, R.; Roch, T.; Schrenk, W.; Strasser, G. Subwavelength Microdisk and Microring Terahertz Quantum-Cascade Lasers. IEEE J. Quantum Electron. 2007, 43, 687–697. [Google Scholar] [CrossRef]
- Karnutsch, C.; Smith, C.L.C.; Graham, A.; Tomljenovic-Hanic, S.; McPhedran, R.; Eggleton, B.J.; O’Faolain, L.; Krauss, T.F.; Xiao, S.; Mortensen, N.A. Temperature Stabilization of Optofluidic Photonic Crystal Cavities. Appl. Phys. Lett. 2009, 94, 231114. [Google Scholar] [CrossRef]
- Speijcken, N.W.L.; Dündar, M.A.; Casas Bedoya, A.; Monat, C.; Grillet, C.; Domachuk, P.; Nötzel, R.; Eggleton, B.J.; van der Heijden, R.W. In Situ Optofluidic Control of Reconfigurable Photonic Crystal Cavities. Appl. Phys. Lett. 2012, 100, 261107. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Yang, Y.; Xie, F.; Han, M.; Lin, Z.; Wang, Y.; Zhang, J.; Zhang, S.; Zhang, C.; et al. Photonic Skin for Photonic-Integration-Based Wearable Sensors. Optica 2025, 12, 190–202. [Google Scholar] [CrossRef]
- Wang, Q.; Han, W.; Wang, Y.; Lu, M.; Dong, L. Tape Nanolithography: A Rapid and Simple Method for Fabricating Flexible, Wearable Nanophotonic Devices. Microsyst. Nanoeng. 2018, 4, 31. [Google Scholar] [CrossRef]
- Galli, V.; Sailapu, S.K.; Cuthbert, T.J.; Ahmadizadeh, C.; Hannigan, B.C.; Menon, C. Passive and Wireless All-Textile Wearable Sensor System. Adv. Sci. 2023, 10, 2206665. [Google Scholar] [CrossRef]
- Densmore, A.; Xu, D.-X.; Waldron, P.; Janz, S.; Cheben, P.; Lapointe, J.; Delage, A.; Lamontagne, B.; Schmid, J.H.; Post, E. A Silicon-on-Insulator Photonic Wire Based Evanescent Field Sensor. IEEE Photonics Technol. Lett. 2006, 18, 2520–2522. [Google Scholar] [CrossRef]
- Butt, M.A. Dielectric Waveguide-Based Sensors with Enhanced Evanescent Field: Unveiling the Dynamic Interaction with the Ambient Medium for Biosensing and Gas-Sensing Applications—A Review. Photonics 2024, 11, 198. [Google Scholar] [CrossRef]
- Butt, M.A. Analyzing the Evanescent Field Ratio of Ridge Waveguide Based on Different Material Platforms for Sensing Applications. J. Opt. 2024, 26, 095803. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. Polarization-Insensitive Hybrid Plasmonic Waveguide Design for Evanescent Field Absorption Gas Sensor. Photonic. Sens. 2021, 11, 279–290. [Google Scholar] [CrossRef]
- Acharya, B.; Behera, A.; Behera, S. Optimizing Drug Discovery: Surface Plasmon Resonance Techniques and Their Multifaceted Applications. Chem. Phys. Impact 2024, 8, 100414. [Google Scholar] [CrossRef]
- Buzzin, A.; Asquini, R.; Caputo, D.; de Cesare, G. Evanescent Waveguide Lab-on-Chip for Optical Biosensing in Food Quality Control. Photonics Res. 2022, 10, 1453–1461. [Google Scholar] [CrossRef]
- Sabek, J.; Díaz-Fernández, F.J.; Torrijos-Morán, L.; Díaz-Betancor, Z.; Maquieira, Á.; Bañuls, M.J.; Pinilla-Cienfuegos, E.; García-Rupérez, J. Experimental Study of an Evanescent-Field Biosensor Based on 1D Photonic Bandgap Structures. Beilstein J. Nanotechnol. 2019, 10, 967–974. [Google Scholar] [CrossRef]
- Butt, M.A.; Degtyarev, S.A.; Khonina, S.N.; Kazanskiy, N.L. An Evanescent Field Absorption Gas Sensor at Mid-IR 3.39 Μm Wavelength. J. Mod. Opt. 2017, 64, 1892–1897. [Google Scholar] [CrossRef]
- Butt, M.A.; Piramidowicz, R. Suspended slot membrane waveguide based on germanium-on-silicon-on-insulator at λ = 4.23 µm for CO2 Monitoring. Micromachines 2024, 15, 1434. [Google Scholar] [CrossRef]
- Ranacher, C.; Consani, C.; Vollert, N.; Tortschanoff, A.; Bergmeister, M.; Grille, T.; Jakoby, B. Characterization of Evanescent Field Gas Sensor Structures Based on Silicon Photonics. IEEE Photonics J. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Stewart, G.; Muhammad, F.A.; Culshaw, B. Sensitivity Improvement for Evanescent-Wave Gas Sensors. Sens. Actuators B: Chem. 1993, 11, 521–524. [Google Scholar] [CrossRef]
- Butt, M.A. Loop-Terminated Mach–Zehnder Interferometer Integrated with Functional Polymer for CO2 Gas Sensing. Appl. Sci. 2024, 14, 4714. [Google Scholar] [CrossRef]
- Butt, M.A.; Shahbaz, M.; Piramidowicz, R. Racetrack Ring Resonator Integrated with Multimode Interferometer Structure Based on Low-Cost Silica–Titania Platform for Refractive Index Sensing Application. Photonics 2023, 10, 978. [Google Scholar] [CrossRef]
- Castelló-Pedrero, L.; Gómez-Gómez, M.I.; García-Rupérez, J.; Griol, A.; Martínez, A. Performance Improvement of a Silicon Nitride Ring Resonator Biosensor Operated in the TM Mode at 1310 Nm. Biomed. Opt. Express 2021, 12, 7244–7260. [Google Scholar] [CrossRef]
- Yoo, K.M.; Fan, K.-C.; Hlaing, M.; Jain, S.; Ning, S.; An, Y.; Chen, R.T. Lab-on-a-Chip Optical Biosensor Platform: A Micro-Ring Resonator Integrated with a near-Infrared Fourier Transform Spectrometer. Opt. Lett. 2023, 48, 5371–5374. [Google Scholar] [CrossRef]
- Álvarez Freile, J.; Choukrani, G.; Zimmermann, K.; Bremer, E.; Dähne, L. Whispering Gallery Modes-Based Biosensors for Real-Time Monitoring and Binding Characterization of Antibody-Based Cancer Immunotherapeutics. Sens. Actuators B Chem. 2021, 346, 130512. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S.; Keng, D. Single Virus Detection from the Reactive Shift of a Whispering-Gallery Mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef]
- Butt, M.A.; Khonina, S.N.; Kazanskiy, N.L. Recent Advances in Photonic Crystal Optical Devices: A Review. Opt. Laser Technol. 2021, 142, 107265. [Google Scholar] [CrossRef]
- Voronin, K.V.; Stebunov, Y.V.; Voronov, A.A.; Arsenin, A.V.; Volkov, V.S. Vertically Coupled Plasmonic Racetrack Ring Resonator for Biosensor Applications. Sensors 2019, 20, 203. [Google Scholar] [CrossRef]
- Foreman, M.R.; Swaim, J.D.; Vollmer, F. Whispering Gallery Mode Sensors. Adv. Opt. Photonics 2015, 7, 168–240. [Google Scholar] [CrossRef]
- Mohammed, N.A.; Khedr, O.E.; El-Rabaie, E.-S.M.; Khalaf, A.A.M. Brain Tumors Biomedical Sensor with High-Quality Factor and Ultra-Compact Size Based on Nanocavity 2D Photonic Crystal. Alex. Eng. J. 2023, 64, 527–540. [Google Scholar] [CrossRef]
- Butt, M.A.; Khonina, S.N.; Kazanskiy, N.L. Highly Sensitive Refractive Index Sensor Based on Hybrid Plasmonic Waveguide Microring Resonator. Waves Random Complex Media 2020, 30, 292–299. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, H. On-Chip Label-Free Biosensing Based on Active Whispering Gallery Mode Resonators Pumped by a Light-Emitting Diode. Opt. Express 2019, 27, 34405–34415. [Google Scholar] [CrossRef] [PubMed]
- Leuermann, J.; Stamenkovic, V.; Ramirez-Priego, P.; Sánchez-Postigo, A.; Fernández-Gavela, A.; Chapman, C.A.; Bailey, R.C.; Lechuga, L.M.; Perez-Inestrosa, E.; Collado, D.; et al. Coherent Silicon Photonic Interferometric Biosensor with an Inexpensive Laser Source for Sensitive Label-Free Immunoassays. Opt. Lett. 2020, 45, 6595–6598. [Google Scholar] [CrossRef]
- Lotfi, F.; Sang-Nourpour, N.; Kheradmand, R. High-Sensitive Plasmonic Sensor Based on Mach-Zehnder Interferometer. Opt. Laser Technol. 2021, 137, 106809. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, M.; Dai, D. Design Rule of Mach-Zehnder Interferometer Sensors for Ultra-High Sensitivity. Sensors 2020, 20, 2640. [Google Scholar] [CrossRef]
- Martens, D.; Bienstman, P. Study on the Limit of Detection in MZI-Based Biosensor Systems. Sci. Rep. 2019, 9, 5767. [Google Scholar] [CrossRef]
- Niu, H.; Yu, P.; Zhu, Y.; Jing, Z.; Li, P.; Wang, B.; Ma, C.; Wang, J.; Wu, J.; Govorov, A.O.; et al. Mach-Zehnder Interferometer Based Integrated-Photonic Acetone Sensor Approaching the Sub-Ppm Level Detection Limit. Opt. Express 2022, 30, 29665–29679. [Google Scholar] [CrossRef]
- Wong, W.R.; Berini, P. Integrated Multichannel Young’s Interferometer Sensor Based on Long-Range Surface Plasmon Waveguides. Opt. Express 2019, 27, 25470–25484. [Google Scholar] [CrossRef]
- Gupta, R.; Labella, E.; Goddard, N.J. An Optofluidic Young Interferometer Sensor for Real-Time Imaging of Refractive Index in μTAS Applications. Sens. Actuators B Chem. 2020, 321, 128491. [Google Scholar] [CrossRef]
- Mulder, H.K.P.; Subramaniam, V.; Kanger, J.S. Background Reduction in a Young Interferometer Biosensor. In Proceedings of the Advanced Photonics (2014), Washington, DC, USA, 27 July 2014; Optica Publishing Group: Washington, DC, USA, 2014; p. SeTh2C.2. [Google Scholar]
- Sahu, S.; Kozadaev, K.V.; Singh, G. Michelson Interferometer Based Refractive Index Biosensor. In Proceedings of the 13th International Conference on Fiber Optics and Photonics (2016), Washington, DC, USA, 4 December 2016; Optica Publishing Group: Washington, DC, USA, 2016; p. Th3A.60.3. [Google Scholar]
- Perera, A.; Shen, J.; Chakravarty, S. Sub-Wavelength Waveguide Michelson Interferometer Sensors. In Proceedings of the Frontiers in Biological Detection: From Nanosensors to Systems XVI, San Francisco, CA, USA, 13 March 2024; SPIE: Bellingham, WA, USA, 2024; Volume 12861, pp. 25–28. [Google Scholar]
- Burnat, D.; Janik, M.; Kwietniewski, N.; Martychowiec, A.; Musolf, P.; Bartnik, K.; Koba, M.; Rygiel, T.P.; Niedziółka-Jönsson, J.; Śmietana, M. Double-Layer Optical Fiber Interferometer with Bio-Layer-Modified Reflector for Label-Free Biosensing of Inflammatory Proteins. Sci. Rep. 2024, 14, 23127. [Google Scholar] [CrossRef] [PubMed]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated Planar Optical Waveguide Interferometer Biosensors: A Comparative Review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Xu, T.; Li, F. Heterodyne Interrogation System for TDM Interferometric Fiber Optic Sensors Array. Opt. Commun. 2015, 341, 74–78. [Google Scholar] [CrossRef]
- Wanser, K.H.; Safar, N.H. Remote Polarization Control for Fiber-Optic Interferometers. Opt. Lett. 1987, 12, 217–219. [Google Scholar] [CrossRef]
- Florjańczyk, M.; Cheben, P.; Janz, S.; Scott, A.; Solheim, B.; Xu, D.-X. Multiaperture Planar Waveguide Spectrometer Formed by Arrayed Mach-Zehnder Interferometers. Opt. Express 2007, 15, 18176–18189. [Google Scholar] [CrossRef]
- Qin, K.; Hu, S.; Retterer, S.T.; Kravchenko, I.I.; Weiss, S.M. Slow Light Mach–Zehnder Interferometer as Label-Free Biosensor with Scalable Sensitivity. Opt. Lett. 2016, 41, 753–756. [Google Scholar] [CrossRef]
- Kuru, C.İ.; Ulucan-Karnak, F.; Akgöl, S. Lab-on-a-Chip Sensors: Recent Trends and Future Applications. In Fundamentals of Sensor Technology; Barhoum, A., Altintas, Z., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2023; pp. 65–98. ISBN 978-0-323-88431-0. [Google Scholar]
- Monajjemi, M.; Mollaamin, F. A Technique of a “Lab-on-a-Chip” for Developing a Novel Biosensor in Viewpoint of Health-Care (PHC) Applications and Biological Regulator Sensors. Sens. Rev. 2024, 44, 353–368. [Google Scholar] [CrossRef]
- Sekhwama, M.; Mpofu, K.; Sudesh, S.; Mthunzi-Kufa, P. Integration of Microfluidic Chips with Biosensors. Discov. Appl. Sci. 2024, 6, 458. [Google Scholar] [CrossRef]
- Luka, G.S.; Nowak, E.; Toyata, Q.R.; Tasnim, N.; Najjaran, H.; Hoorfar, M. Portable On-Chip Colorimetric Biosensing Platform Integrated with a Smartphone for Label/PCR-Free Detection of Cryptosporidium RNA. Sci. Rep. 2021, 11, 23192. [Google Scholar] [CrossRef]
- Kirk, J.T.; Fridley, G.E.; Chamberlain, J.W.; Christensen, E.D.; Hochberg, M.; Ratner, D.M. Multiplexed Inkjet Functionalization of Silicon Photonic Biosensors. Lab Chip 2011, 11, 1372–1377. [Google Scholar] [CrossRef]
- Chen, C.; Ran, B.; Liu, B.; Liu, X.; Zhang, Z.; Li, Y.; Li, H.; Lan, M.; Zhu, Y. Multiplexed Detection of Biomarkers Using a Microfluidic Chip Integrated with Mass-Producible Micropillar Array Electrodes. Anal. Chim. Acta 2023, 1272, 341450. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.W.; Shen, Y.; Palomba, S.; Vigolo, D. Nanofluidic Lab-On-A-Chip Systems for Biosensing in Healthcare. Small 2025, 21, 2407478. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, C.; Gharia, A.; Niknejad, A.; Stojanović, V.; Anwar, M. Microfluidic Packaging Integration with Electronic-Photonic Biosensors Using 3D Printed Transfer Molding. Biosensors 2020, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Kurdadze, T.; Lamadie, F.; Nehme, K.A.; Teychené, S.; Biscans, B.; Rodriguez-Ruiz, I. On-Chip Photonic Detection Techniques for Non-Invasive In Situ Characterizations at the Microfluidic Scale. Sensors 2024, 24, 1529. [Google Scholar] [CrossRef]
- Sande, M.G.; Rodrigues, J.L.; Ferreira, D.; Silva, C.J.; Rodrigues, L.R. Novel Biorecognition Elements against Pathogens in the Design of State-of-the-Art Diagnostics. Biosensors 2021, 11, 418. [Google Scholar] [CrossRef]
- Martinkova, P.; Kostelnik, A.; Valek, T.; Pohanka, M. Main Streams in the Construction of Biosensors and Their Applications. Int. J. Electrochem. Sci. 2017, 12, 7386–7403. [Google Scholar] [CrossRef]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Bilget Güven, E.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Pham, T.N.L.; Nguyen, S.H.; Tran, M.T. A Comprehensive Review of Transduction Methods of Lectin-Based Biosensors in Biomedical Applications. Heliyon 2024, 10, e38371. [Google Scholar] [CrossRef]
- Butt, M.A. Integrated Optics: Platforms and Fabrication Methods. Encyclopedia 2023, 3, 824–838. [Google Scholar] [CrossRef]
- Lakhera, P.; Chaudhary, V.; Bhardwaj, B.; Kumar, P.; Kumar, S. Development and Recent Advancement in Microfluidics for Point of Care Biosensor Applications: A Review. Biosens. Bioelectron. X 2022, 11, 100218. [Google Scholar] [CrossRef]
- Soler, M.; Lechuga, L.M. Label-Free Photonic Biosensors: Key Technologies for Precision Diagnostics. ChemistryEurope 2025, 2400106. [Google Scholar] [CrossRef]
- Gruhl, F.J.; Rapp, B.E.; Rapp, M.; Länge, K. Biosensor Packaging—Adaptation of the Surface Modification Procedure. Procedia Eng. 2010, 5, 363–366. [Google Scholar] [CrossRef]
- Georgas, A.; Nestoras, L.; Kanaris, A.I.; Angelopoulos, S.; Ferraro, A.; Hristoforou, E. Packaging and Optimization of a Capacitive Biosensor and Its Readout Circuit. Sensors 2023, 23, 765. [Google Scholar] [CrossRef] [PubMed]
- Tokel, O.; Yildiz, U.H.; Inci, F.; Durmus, N.G.; Ekiz, O.O.; Turker, B.; Cetin, C.; Rao, S.; Sridhar, K.; Natarajan, N.; et al. Portable Microfluidic Integrated Plasmonic Platform for Pathogen Detection. Sci. Rep. 2015, 5, 9152. [Google Scholar] [CrossRef]
- Luchansky, M.S.; Washburn, A.L.; McClellan, M.S.; Bailey, R.C. Sensitive On-Chip Detection of a Protein Biomarker in Human Serum and Plasma over an Extended Dynamic Range Using Silicon Photonic Microring Resonators and Sub-Micron Beads. Lab Chip 2011, 11, 2042–2044. [Google Scholar] [CrossRef]
- Ouyang, X.; Liu, T.; Zhang, Y.; He, J.; He, Z.; Zhang, A.P.; Tam, H.-Y. Ultrasensitive Optofluidic Enzyme-Linked Immunosorbent Assay by on-Chip Integrated Polymer Whispering-Gallery-Mode Microlaser Sensors. Lab Chip 2020, 20, 2438–2446. [Google Scholar] [CrossRef]
- Han, J.-H.; Kim, H.-J.; Sudheendra, L.; Gee, S.J.; Hammock, B.D.; Kennedy, I.M. Photonic Crystal Lab-On-a-Chip for Detecting Staphylococcal Enterotoxin B at Low Attomolar Concentration. Anal. Chem. 2013, 85, 3104–3109. [Google Scholar] [CrossRef]
- Awasthi, K.; Malviya, N.; Kumar, A. Silicon Subwavelength Grating Slot Waveguide Based Optical Sensor for Label Free Detection of Fluoride Ion in Water. IETE Tech. Rev. 2024, 41, 341–342. [Google Scholar] [CrossRef]
- Billah, M.R.; Blaicher, M.; Hoose, T.; Dietrich, P.-I.; Marin-Palomo, P.; Lindenmann, N.; Nesic, A.; Hofmann, A.; Troppenz, U.; Moehrle, M.; et al. Hybrid Integration of Silicon Photonics Circuits and InP Lasers by Photonic Wire Bonding. Optica 2018, 5, 876–883. [Google Scholar] [CrossRef]
- Chen, X.; Lin, J.; Wang, K. A Review of Silicon-Based Integrated Optical Switches. Laser Photonics Rev. 2023, 17, 2200571. [Google Scholar] [CrossRef]
- Tarik, F.B.; Famili, A.; Lao, Y.; Ryckman, J.D. Scalable and CMOS Compatible Silicon Photonic Physical Unclonable Functions for Supply Chain Assurance. Sci. Rep. 2022, 12, 15653. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.A.; Kozłowski, Ł.; Golas, M.; Slowikowski, M.; Filipiak, M.; Juchniewicz, M.; Bieniek-Kaczorek, A.; Dudek, M.; Piramidowicz, R. Numerical and Experimental Demonstration of a Silicon Nitride-Based Ring Resonator Structure for Refractive Index Sensing. Appl. Sci. 2024, 14, 6082. [Google Scholar] [CrossRef]
- Blumenthal, D.J.; Heideman, R.; Geuzebroek, D.; Leinse, A.; Roeloffzen, C. Silicon Nitride in Silicon Photonics. Proc. IEEE 2018, 106, 2209–2231. [Google Scholar] [CrossRef]
- Vogelbacher, F.; Kothe, T.; Muellner, P.; Melnik, E.; Sagmeister, M.; Kraft, J.; Hainberger, R. Waveguide Mach-Zehnder Biosensor with Laser Diode Pumped Integrated Single-Mode Silicon Nitride Organic Hybrid Solid-State Laser. Biosens. Bioelectron. 2022, 197, 113816. [Google Scholar] [CrossRef]
- Hong, Y.; Ge, H.; Hong, J. Compact Biosensors Based on Thin Film Silicon Nitride Microring Resonators. J. Phys. Conf. Ser. 2021, 2012, 012037. [Google Scholar] [CrossRef]
- Bose, D.; Harrington, M.W.; Isichenko, A.; Liu, K.; Wang, J.; Chauhan, N.; Newman, Z.L.; Blumenthal, D.J. Anneal-Free Ultra-Low Loss Silicon Nitride Integrated Photonics. Light Sci. Appl. 2024, 13, 156. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhou, T.; Wu, Q. Applications and Developments of On-Chip Biochemical Sensors Based on Optofluidic Photonic Crystal Cavities. Lab Chip 2017, 18, 57–74. [Google Scholar] [CrossRef]
- Saldutti, M.; Xiong, M.; Dimopoulos, E.; Yu, Y.; Gioannini, M.; Mørk, J. Modal Properties of Photonic Crystal Cavities and Applications to Lasers. Nanomaterials 2021, 11, 3030. [Google Scholar] [CrossRef]
- Deotare, P.B.; Loncar, M. Photonic Crystal Nanobeam Cavities. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 2060–2069. ISBN 978-90-481-9751-4. [Google Scholar]
- Liu, Y.; Wang, S.; Biswas, P.; Palit, P.; Zhou, W.; Sun, Y. Optofluidic Vapor Sensing with Free-Space Coupled 2D Photonic Crystal Slabs. Sci. Rep. 2019, 9, 4209. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, J.-H.; Lee, S.-K.; Kim, S.-H.; Yang, S.-M.; Lee, Y.-H.; Seassal, C.; Regrency, P.; Viktorovitch, P. Optofluidic Integration of a Photonic Crystal Nanolaser. Opt. Express 2008, 16, 6515–6527. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zeng, G.; Yu, Q.-J.; Shen, L.; Shi, C.-Y.; Lu, H.-L. Photodetectors Integrating Waveguides and Semiconductor Materials. Nanoscale 2024, 16, 5504–5520. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, L.; Zhang, Y.; Hao, J.; Meng, J.; Shi, N. High-Performance Ge Photodetectors on Silicon Photonics Platform for Optical Interconnect. Sens. Actuators A Phys. 2024, 376, 115535. [Google Scholar] [CrossRef]
- Lin, Y.; Yong, Z.; Luo, X.; Azadeh, S.S.; Mikkelsen, J.C.; Sharma, A.; Chen, H.; Mak, J.C.C.; Lo, P.G.-Q.; Sacher, W.D.; et al. Monolithically Integrated, Broadband, High-Efficiency Silicon Nitride-on-Silicon Waveguide Photodetectors in a Visible-Light Integrated Photonics Platform. Nat. Commun. 2022, 13, 6362. [Google Scholar] [CrossRef]
- Yan, R.; Mestas, S.P.; Yuan, G.; Safaisini, R.; Dandy, D.S.; Lear, K.L. Label-Free Silicon Photonic Biosensor System with Integrated Detector Array. Lab Chip 2009, 9, 2163–2168. [Google Scholar] [CrossRef]
- Steglich, P.; Bondarenko, S.; Mai, C.; Paul, M.; Weller, M.G.; Mai, A. CMOS-Compatible Silicon Photonic Sensor for Refractive Index Sensing Using Local Back-Side Release. IEEE Photonics Technol. Lett. 2020, 32, 1241–1244. [Google Scholar] [CrossRef]
- Wang, S.; Dhyani, V.; Mohanraj, S.S.; Shi, X.; Varghese, B.; Chung, W.W.; Huang, D.; Lim, Z.S.; Zeng, Q.; Liu, H.; et al. CMOS-Compatible Photonic Integrated Circuits on Thin-Film ScAlN. APL Photonics 2024, 9, 066109. [Google Scholar] [CrossRef]
- Khumpuang, S.; Koga, K.; Liu, Y.; Kara, S. Process Development for CMOS Fabrication Using Minimal Fab. In Proceedings of the 2017 IEEE Electron Devices Technology and Manufacturing Conference (EDTM), Toyama, Japan, 28 February–2 March 2017; pp. 82–83. [Google Scholar]
- Staff Ayar Labs. CMOS Processing. Available online: https://ayarlabs.com/ (accessed on 15 April 2025).
- Stevenson, J.T.M.; Gundlach, A.M. The Application of Photolithography to the Fabrication of Microcircuits. J. Phys. E Sci. Instrum. 1986, 19, 654. [Google Scholar] [CrossRef]
- Balderson, N.R.; Cordova, G.; Yi, K.; Guliani, A.; Manek, S.; Schiavone, G.W.; Harrison, J.; Pearson, R.E. NWELL CMOS Fabrication Process for the Virginia Microelectronics Center. In Proceedings of the Fourteenth Biennial University/Government/Industry Microelectronics Symposium (Cat. No.01CH37197), Richmond, VA, USA, 20 June 2001; pp. 209–212. [Google Scholar]
- Zinoviev, K.; Carrascosa, L.G.; Sánchez del Río, J.; Sepúlveda, B.; Domínguez, C.; Lechuga, L.M. Silicon Photonic Biosensors for Lab-on-a-Chip Applications. Adv. Opt. Technol. 2008, 2008, 383927. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, D.; Widdicombe, B.; Jacob, D.T.; Yang, Y.; Gao, X.; Stewart, A.G.; Unnithan, R.R. Miniaturized multispectral imaging for microfluidic pH sensing. Sensing and Biosensing Research 2025, 47, 100764:. [Google Scholar] [CrossRef]
- Hill, D. Nanophotonic Biosensors within Lab on Chip Optical Systems. In Proceedings of the 2015 International Conference on Photonics, Optics and Laser Technology (PHOTOPTICS), Berlin, Germany, 12–14 March 2015; Volume 2, pp. 60–68. [Google Scholar]
- González-Guerrero, A.B.; Maldonado, J.; Herranz, S.; Lechuga, L.M. Trends in Photonic Lab-on-Chip Interferometric Biosensors for Point-of-Care Diagnostics. Anal. Methods 2016, 8, 8380–8394. [Google Scholar] [CrossRef]
- Høvik, J.; Yadav, M.; Arnfinnsdottir, N.B.; Aksnes, A. Lab-on-a-Chip Photonic Biosensor for Detection of Antigens. In Proceedings of the Biosensing and Nanomedicine XI, 5 September 2018; SPIE: Bellingham, WA, USA, 2018; Volume 10728, pp. 27–33. [Google Scholar]
- Cognetti, J.S.; Moen, M.T.; Brewer, M.G.; Bryan, M.R.; Tice, J.D.; McGrath, J.L.; Miller, B.L. A Photonic Biosensor-Integrated Tissue Chip Platform for Real-Time Sensing of Lung Epithelial Inflammatory Markers. Lab Chip 2023, 23, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kohler, D.; Schindler, G.; Hahn, L.; Milvich, J.; Hofmann, A.; Länge, K.; Freude, W.; Koos, C. Biophotonic Sensors with Integrated Si3N4-Organic Hybrid (SiNOH) Lasers for Point-of-Care Diagnostics. Light Sci. Appl. 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, A. Photonic Sensors for Health and Environmental Monitoring. In Sensors for Environment, Health and Security; Baraton, M.-I., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 191–203. [Google Scholar]
- Longo, D.; Giudice, G.; D’Arrigo, G.; Sciuto, A. Portable Spectroscopy System for Environmental Monitoring: An SO2 Case Study. IEEE Sens. J. 2022, 22, 11135–11143. [Google Scholar] [CrossRef]
- Jang, A.; Zou, Z.; Lee, K.K.; Ahn, C.H.; Bishop, P.L. State-of-the-Art Lab Chip Sensors for Environmental Water Monitoring. Meas. Sci. Technol. 2011, 22, 032001. [Google Scholar] [CrossRef]
- Dhar, B.C.; Lee, N.Y. Lab-on-a-Chip Technology for Environmental Monitoring of Microorganisms. BioChip J. 2018, 12, 173–183. [Google Scholar] [CrossRef]
- Mai, A.; Mai, C.; Steglich, P. From Lab-on-Chip to Lab-in-App: Challenges towards Silicon Photonic Biosensors Product Developments. Results Opt. 2022, 9, 100317. [Google Scholar] [CrossRef]
- Yan, Y.; Feng, H.; Wang, C.; Ren, W. On-Chip Photothermal Gas Sensor Based on a Lithium Niobate Rib Waveguide. Sens. Actuators B Chem. 2024, 405, 135392. [Google Scholar] [CrossRef]
- Min, Y.; Pi, M.; Peng, Z.; Guan, G.; Liang, L.; Song, F.; Wang, Y.; Zhang, Y.; Bai, X.; Zheng, C. On-Chip near-Infrared Multi-Gas Sensing Using Chalcogenide Anti-Resonant Hollow-Core Waveguides. Lab. Chip 2025, 25, 1801–1812. [Google Scholar] [CrossRef]
- Biswas, P.; Zhang, C.; Chen, Y.; Liu, Z.; Vaziri, S.; Zhou, W.; Sun, Y. A Portable Micro-Gas Chromatography with Integrated Photonic Crystal Slab Sensors on Chip. Biosensors 2021, 11, 326. [Google Scholar] [CrossRef]
- Weerasinghe, M.; Jayathilaka, K.; Vithanage, M. Sensors for Detection and Monitoring of Contaminants in Wastewater. Curr. Opin. Environ. Sci. Health 2025, 45, 100609. [Google Scholar] [CrossRef]
- Ranno, L.; Tan, Y.Z.; Ong, C.S.; Guo, X.; Koo, K.N.; Li, X.; Wang, W.; Serna, S.; Liu, C.; Rusli; et al. Crown Ether Decorated Silicon Photonics for Safeguarding against Lead Poisoning. Nat. Commun. 2024, 15, 3820. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-Y.; Kim, B. Lab-on-a-Chip Pathogen Sensors for Food Safety. Sensors 2012, 12, 10713–10741. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Kapoor, A.; Kumar, P.S.; Ponnuchamy, M.; Sivasamy, B.; Vo, D.-V.N. Lab-on-a-Chip Technologies for Food Safety, Processing, and Packaging Applications: A Review. Environ. Chem. Lett. 2022, 20, 901–927. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, H.-Y. Lab-on-a-Chip Devices for Nucleic Acid Analysis in Food Safety. Micromachines 2024, 15, 1524. [Google Scholar] [CrossRef]
- Saini, A.; Yadav, N.; Singh, B.; Rana, J.S. An overview of biosensor advancements for detecting botulinum neurotoxins: Addressing food safety and biowarfare risks. Analytical Biochemistry 2025, 701, 115801:. [Google Scholar] [CrossRef]
- Antolín-Amérigo, D.; Manso, L.; Caminati, M.; de la Hoz Caballer, B.; Cerecedo, I.; Muriel, A.; Rodríguez-Rodríguez, M.; Barbarroja-Escudero, J.; Sánchez-González, M.J.; Huertas-Barbudo, B.; et al. Quality of Life in Patients with Food Allergy. Clin. Mol. Allergy 2016, 14, 4. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Pagkali, V.; Makarona, E.; Misiakos, K.; Raptis, I.; Petrou, P.; Kakabakos, S.; Peters, J.; Jobst, G.; Goustouridis, D.; et al. Multiplexed Detection of Food Contaminants with a Portable Reader Based on All-in-One Monolithic Photonic Chips. Opt. Laser Technol. 2024, 177, 111192. [Google Scholar] [CrossRef]
- Wang, J.; Sanchez, M.M.; Yin, Y.; Herzer, R.; Ma, L.; Schmidt, O.G. Silicon-Based Integrated Label-Free Optofluidic Biosensors: Latest Advances and Roadmap. Adv. Mater. Technol. 2020, 5, 1901138. [Google Scholar] [CrossRef]
- Chauhan, N.; Saxena, K.; Jain, U. Single Molecule Detection; from Microscopy to Sensors. Int. J. Biol. Macromol. 2022, 209, 1389–1401. [Google Scholar] [CrossRef]
- Dey, S.; Dolci, M.; Zijlstra, P. Single-Molecule Optical Biosensing: Recent Advances and Future Challenges. ACS Phys. Chem. Au 2023, 3, 143–156. [Google Scholar] [CrossRef]
- Dashtabi, M.M.; Khoshmehr, M.T.; Nikbakht, H.; Rodriguez, B.L.; Sharma, N.; Zadeh, I.E.; Akca, B.I. Real-Time Measurements of Photonic Microchips with Femtometer-Scale Spectral Precision and Ultrahigh Sensitivity. Laser Photonics Rev. 2024, 18, 2301396. [Google Scholar] [CrossRef]
- Lu, C.; Nikbakht, H.; Karabiyik, M.; Alaydrus, M.; Akca, B.I. A Compound Optical Microresonator Design for Self-Referencing and Multiplexed Refractive Index Sensing. Opt. Express 2021, 29, 42215–42224. [Google Scholar] [CrossRef]
- Charwat, V.; Purtscher, M.; Tedde, S.F.; Hayden, O.; Ertl, P. Standardization of Microfluidic Cell Cultures Using Integrated Organic Photodiodes and Electrode Arrays. Lab Chip 2013, 13, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, G.; Accardo, A.; Angelis, F.D.; Dane, T.; Weinhausen, B.; Burghammer, M.; Riekel, C. A Superhydrophobic Chip Based on SU-8 Photoresist Pillars Suspended on a Silicon Nitride Membrane. Lab Chip 2014, 14, 3705–3709. [Google Scholar] [CrossRef]
- Sobiesierski, A.; Thomas, R.; Buckle, P.; Barrow, D.; Smowton, P.M. A Two-Stage Surface Treatment for the Long-Term Stability of Hydrophilic SU-8. Surf. Interface Anal. 2015, 47, 1174–1179. [Google Scholar] [CrossRef]
- Perdigones, F.; Aracil, C.; Quero, J.M.; Gutiérrez, M.; Jiménez, C.; Giménez, P. Integration Method of Silicon Sensors on SU-8-Based Microfluidic Platforms. Microsyst. Technol. 2015, 21, 155–161. [Google Scholar] [CrossRef]
- Datta-Chaudhuri, T.; Abshire, P.; Smela, E. Packaging Commercial CMOS Chips for Lab on a Chip Integration. Lab Chip 2014, 14, 1753–1766. [Google Scholar] [CrossRef]
- Wang, J.; Maier, S.A.; Tittl, A. Trends in Nanophotonics-Enabled Optofluidic Biosensors. Adv. Opt. Mater. 2022, 10, 2102366. [Google Scholar] [CrossRef]
- Cueff, S.; Poon, J.; Thourhout, D.V.; Vivien, L. Hybrid Photonics: Integration, Design and Devices: Feature Issue Introduction. Opt. Mater. Express 2024, 14, 1456–1458. [Google Scholar] [CrossRef]
- Tan, H.; Du, L.; Yang, F.; Chu, W.; Zhan, Y. Two-Dimensional Materials in Photonic Integrated Circuits: Recent Developments and Future Perspectives [Invited]. Chin. Opt. Lett. 2023, 21, 110007. [Google Scholar]
- Andalibi Miandoab, S.; Talebzadeh, R. Ultra-Sensitive and Selective 2D Hybrid Highly Doped Semiconductor-Graphene Biosensor Based on SPR and SEIRA Effects in the Wide Range of Infrared Spectral. Opt. Mater. 2022, 129, 112572. [Google Scholar] [CrossRef]
- Guo, B.; Xiao, Q.; Wang, S.; Zhang, H. 2D Layered Materials: Synthesis, Nonlinear Optical Properties, and Device Applications. Laser Photonics Rev. 2019, 13, 1800327. [Google Scholar] [CrossRef]
- Abbas, K.; Ji, P.; Ullah, N.; Shafique, S.; Zhang, Z.; Ameer, M.F.; Qin, S.; Yang, S. Graphene Photodetectors Integrated with Silicon and Perovskite Quantum Dots. Microsyst. Nanoeng. 2024, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.-H.; bin Mohd Yusoff, A.R.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite Light-Emitting Diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Khan, S.; F Didar, T. Bio-Functionalization of Microfluidic Platforms Made of Thermoplastic Materials: A Review. Anal. Chim. Acta 2022, 1209, 339283. [Google Scholar] [CrossRef]
- Damiati, L.A.; El-Yaagoubi, M.; Damiati, S.A.; Kodzius, R.; Sefat, F.; Damiati, S. Role of Polymers in Microfluidic Devices. Polymers 2022, 14, 5132. [Google Scholar] [CrossRef]
- Juang, Y.-J.; Chiu, Y.-J. Fabrication of Polymer Microfluidics: An Overview. Polymers 2022, 14, 2028. [Google Scholar] [CrossRef]
- Yang, J.; Tang, M.; Chen, S.; Liu, H. From Past to Future: On-Chip Laser Sources for Photonic Integrated Circuits. Light Sci. Appl. 2023, 12, 16. [Google Scholar] [CrossRef]
- Shang, C.; Hughes, E.; Wan, Y.; Dumont, M.; Koscica, R.; Selvidge, J.; Herrick, R.; Gossard, A.C.; Mukherjee, K.; Bowers, J.E. High-Temperature Reliable Quantum-Dot Lasers on Si with Misfit and Threading Dislocation Filters. Optica 2021, 8, 749–754. [Google Scholar] [CrossRef]
- Chen, S.; Li, W.; Wu, J.; Jiang, Q.; Tang, M.; Shutts, S.; Elliott, S.N.; Sobiesierski, A.; Seeds, A.J.; Ross, I.; et al. Electrically Pumped Continuous-Wave III–V Quantum Dot Lasers on Silicon. Nat. Photonic 2016, 10, 307–311. [Google Scholar] [CrossRef]
- Norman, J.C.; Jung, D.; Wan, Y.; Bowers, J.E. Perspective: The Future of Quantum Dot Photonic Integrated Circuits. APL Photonics 2018, 3, 030901. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, J.; Guo, J.; Chang, L.; Wang, R.N.; Weng, W.; Peters, J.; Xie, W.; Zhang, Z.; Riemensberger, J.; et al. Laser Soliton Microcombs Heterogeneously Integrated on Silicon. Science 2021, 373, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Elshaari, A.W.; Pernice, W.; Srinivasan, K.; Benson, O.; Zwiller, V. Hybrid Integrated Quantum Photonic Circuits. Nat. Photonics 2020, 14, 285–298. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.W.; Jeon, H.-J. Machine Learning-Driven Innovations in Microfluidics. Biosensors 2024, 14, 613. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, T.J.; El Shamy, R.; Swillam, M.A.; Li, X. Enhanced Performance of On-Chip Integrated Biosensor Using Deep Learning. Opt. Quant. Electron. 2023, 55, 967. [Google Scholar] [CrossRef]
- Han, G.-R.; Goncharov, A.; Eryilmaz, M.; Ye, S.; Palanisamy, B.; Ghosh, R.; Lisi, F.; Rogers, E.; Guzman, D.; Yigci, D.; et al. Machine Learning in Point-of-Care Testing: Innovations, Challenges, and Opportunities. Nat. Commun. 2025, 16, 3165. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Panigrahi, D.; Rewatkar, P.; Haick, H. Role of Machine Learning Assisted Biosensors in Point-of-Care-Testing For Clinical Decisions. ACS Sens. 2024, 9, 4495–4519. [Google Scholar] [CrossRef]
- Mencattini, A.; Rizzuto, V.; Antonelli, G.; Di Giuseppe, D.; D’Orazio, M.; Filippi, J.; Comes, M.C.; Casti, P.; Vives Corrons, J.L.; Garcia-Bravo, M.; et al. Machine Learning Microfluidic Based Platform: Integration of Lab-on-Chip Devices and Data Analysis Algorithms for Red Blood Cell Plasticity Evaluation in Pyruvate Kinase Disease Monitoring. Sens. Actuators A Phys. 2023, 351, 114187. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-End Design of Wearable Sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Althuwayb, A.A.; Alibakhshikenari, M.; Virdee, B.S.; Rashid, N.; Kaaniche, K.; Atitallah, A.B.; Armghan, A.; Elhamrawy, O.I.; See, C.H.; Falcone, F. Metasurface-Inspired Flexible Wearable MIMO Antenna Array for Wireless Body Area Network Applications and Biomedical Telemetry Devices. IEEE Access 2023, 11, 1039–1056. [Google Scholar] [CrossRef]
- Childs, A.; Mayol, B.; Lasalde-Ramírez, J.A.; Song, Y.; Sempionatto, J.R.; Gao, W. Diving into Sweat: Advances, Challenges, and Future Directions in Wearable Sweat Sensing. ACS Nano 2024, 18, 24605–24616. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Huang, F.; Zhang, X.; Song, P.; Zheng, H.; Chevali, V.; Wang, H.; Xu, Z. Flexible Pressure Sensors via Engineering Microstructures for Wearable Human-Machine Interaction and Health Monitoring Applications. iScience 2022, 25, 104148. [Google Scholar] [CrossRef] [PubMed]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. A Review on Flexible Wearables—Recent Developments in Non-Invasive Continuous Health Monitoring. Sens. Actuators A Phys. 2024, 366, 114993. [Google Scholar] [CrossRef]
- Butt, M.A. A Perspective on Smart Contact Lenses: Pioneering Non-Intrusive Eye Health Monitoring. Sens. Actuators A Phys. 2025, 387, 116399. [Google Scholar] [CrossRef]
- Jacobo-Martín, A.; Jost, N.; Hernández, J.J.; Domínguez, C.; Vallerotto, G.; Askins, S.; Antón, I.; Rodríguez, I. Roll-to-Roll Nanoimprint Lithography of High Efficiency Fresnel Lenses for Micro-Concentrator Photovoltaics. Opt. Express 2021, 29, 34135–34149. [Google Scholar] [CrossRef]
- Chamanzar, M.; Xia, Z.; Yegnanarayanan, S.; Adibi, A. Hybrid Integrated Plasmonic-Photonic Waveguides for on-Chip Localized Surface Plasmon Resonance (LSPR) Sensing and Spectroscopy. Opt. Express 2013, 21, 32086–32098. [Google Scholar] [CrossRef]
- De Angelis, F.; Patrini, M.; Das, G.; Maksymov, I.; Galli, M.; Businaro, L.; Andreani, L.C.; Di Fabrizio, E. A Hybrid Plasmonic–Photonic Nanodevice for Label-Free Detection of a Few Molecules. Nano Lett. 2008, 8, 2321–2327. [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; Wang, X.; Zhao, W.; Liu, H.; Li, M.; Zhao, Y.; Zhang, L.; Huang, C. Plasmonic-Photonic Hybrid Configuration on Optical Fiber Tip: Toward Low-Cost and Miniaturized Biosensing Probe. Sens. Actuators B Chem. 2022, 367, 132059. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Quantum Dots for Biosensing: Classification and Applications. Biosens. Bioelectron. 2025, 273, 117180. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Xiao, S.; Song, Q. Recent Advances in Perovskite Micro- and Nanolasers. Adv. Opt. Mater. 2018, 6, 1800278. [Google Scholar] [CrossRef]
- Gubbi, V.; Ivanov, T.; Ved, K.; Lenk, C.; Ziegler, M. Bio-Inspired Acoustic MEMS Sensor with Tunable Resonance Frequency. Sens. Actuators A Phys. 2025, 387, 116369. [Google Scholar] [CrossRef]
- Edinger, P.; Jo, G.; Nguyen, C.P.V.; Takabayashi, A.Y.; Errando-Herranz, C.; Antony, C.; Talli, G.; Verheyen, P.; Khan, U.; Bleiker, S.J.; et al. Vacuum-Sealed Silicon Photonic MEMS Tunable Ring Resonator with an Independent Control over Coupling and Phase. Opt. Express 2023, 31, 6540–6551. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, X.; Lu, G. Recent Developments of Flexible and Transparent SERS Substrates. J. Mater. Chem. C 2020, 8, 3956–3969. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Chang, J.; Lyu, Y.; Zhao, J.; Qiu, S. Programmable Mechanical Metamaterials: Basic Concepts, Types, Construction Strategies—A Review. Front. Mater. 2024, 11, 1361408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butt, M.A.; Imran Akca, B.; Mateos, X. Integrated Photonic Biosensors: Enabling Next-Generation Lab-on-a-Chip Platforms. Nanomaterials 2025, 15, 731. https://doi.org/10.3390/nano15100731
Butt MA, Imran Akca B, Mateos X. Integrated Photonic Biosensors: Enabling Next-Generation Lab-on-a-Chip Platforms. Nanomaterials. 2025; 15(10):731. https://doi.org/10.3390/nano15100731
Chicago/Turabian StyleButt, Muhammad A., B. Imran Akca, and Xavier Mateos. 2025. "Integrated Photonic Biosensors: Enabling Next-Generation Lab-on-a-Chip Platforms" Nanomaterials 15, no. 10: 731. https://doi.org/10.3390/nano15100731
APA StyleButt, M. A., Imran Akca, B., & Mateos, X. (2025). Integrated Photonic Biosensors: Enabling Next-Generation Lab-on-a-Chip Platforms. Nanomaterials, 15(10), 731. https://doi.org/10.3390/nano15100731








