Recent Progress in Designing Nanomaterial Biohybrids for Artificial Photosynthesis
Abstract
1. Introduction
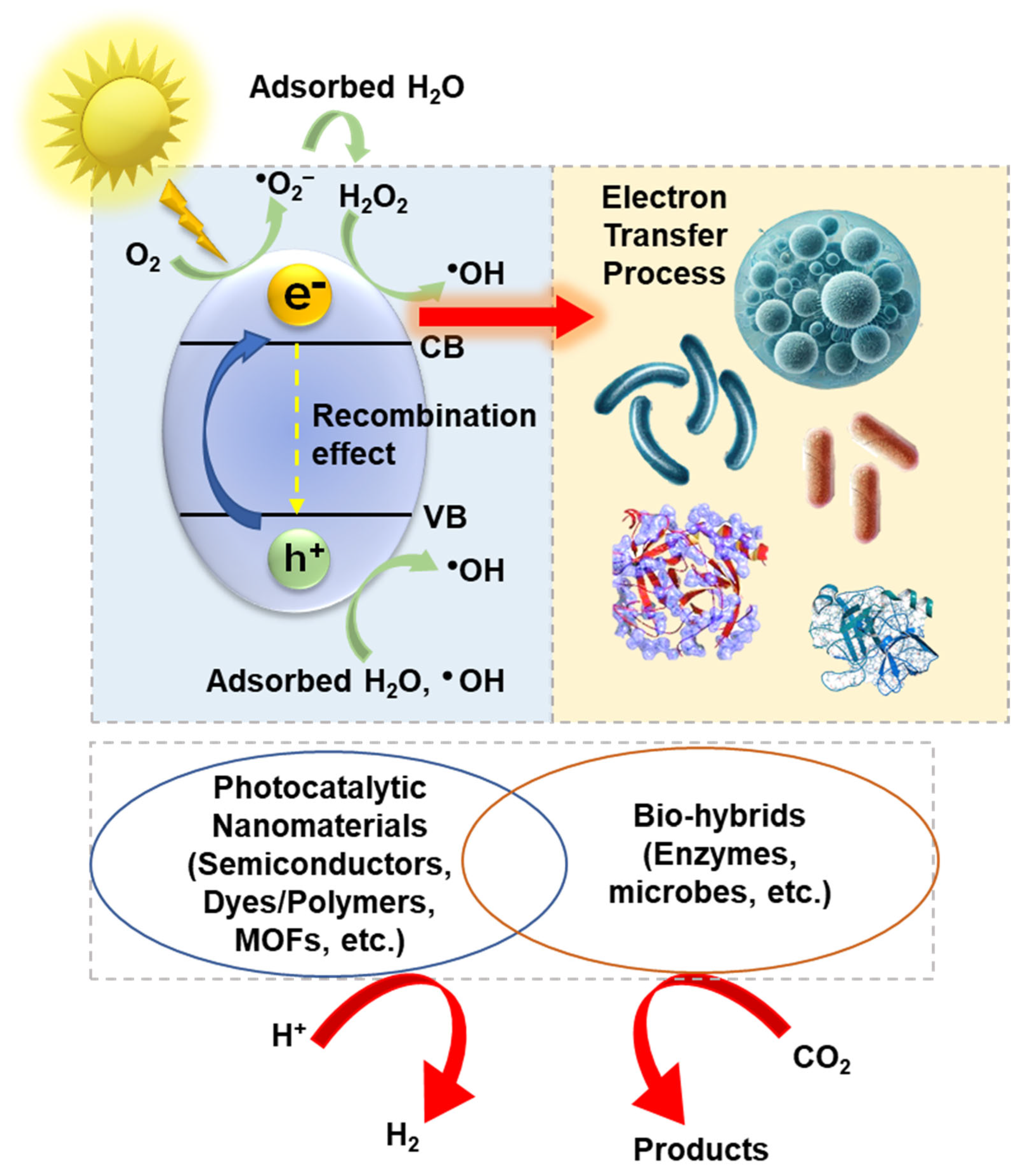
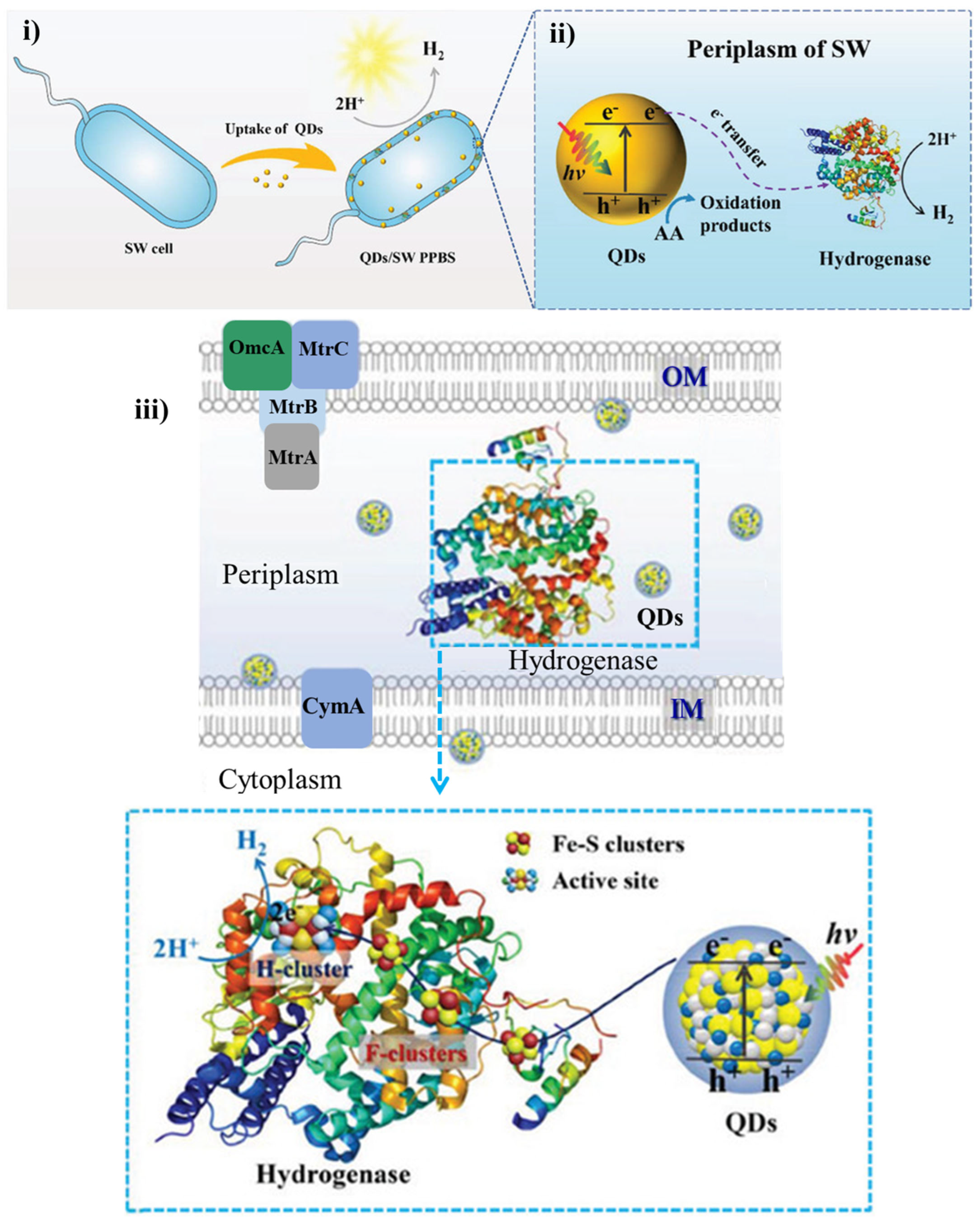
2. Internalization and Compartmentalization of PNMs in Microbial Cells and Mechanisms of Photoelectron Transfer
3. Inorganic Semiconductor–Microorganism-Based NBHs
3.1. Cadmium Sulfide–Microorganism-Based NBHs

3.2. Titanium Dioxide and Silicon Nanowires–Microorganism-Based NBHs
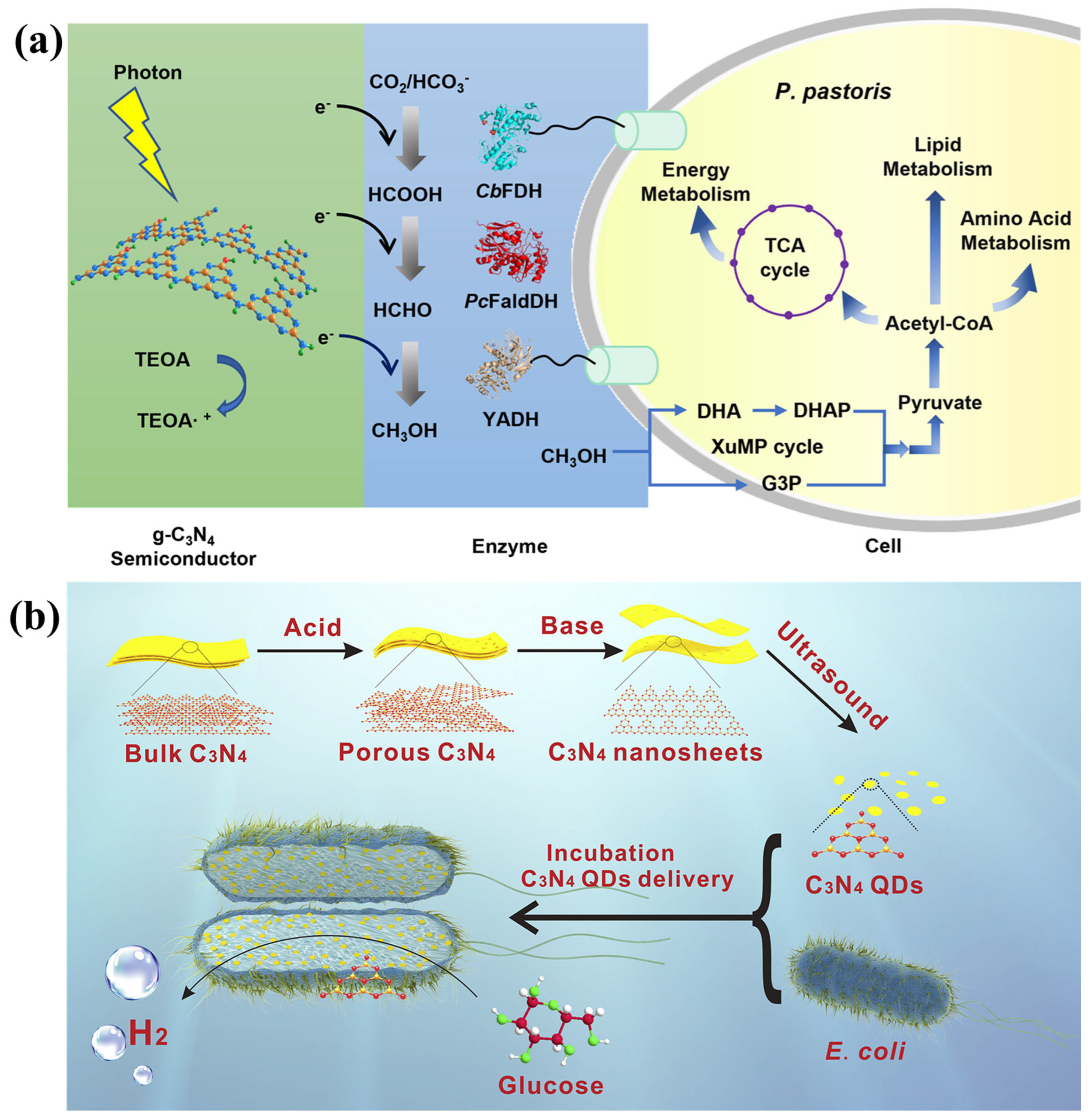
4. Other Inorganic Nanomaterial/Semiconductor–Microorganism Hybrids
5. Carbon-Based Nanomaterials–Microorganism Hybrids
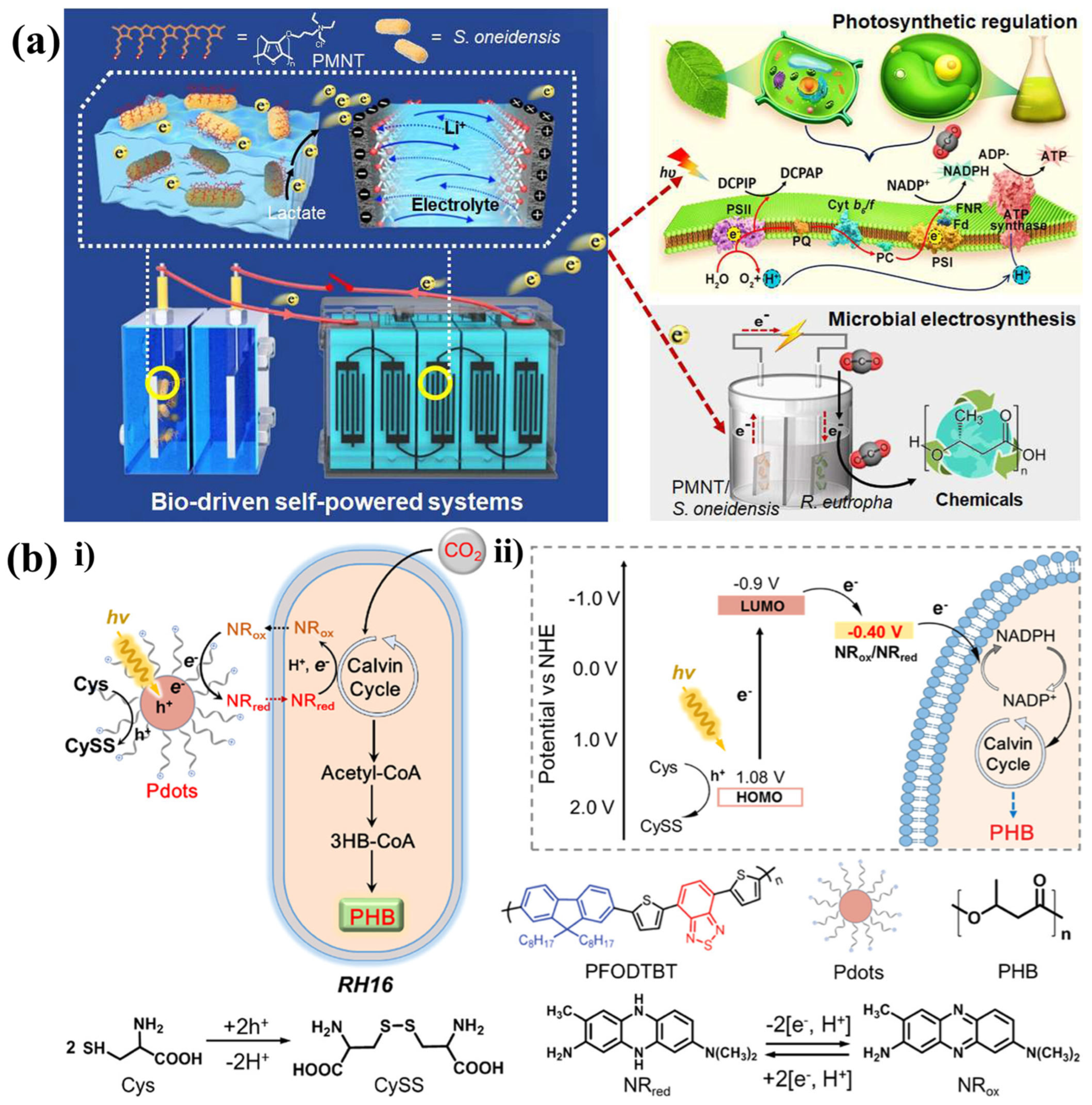
6. Organic Polymer/Semiconductor–Microorganism Hybrids
7. Summary and Future Prospects
- Synergistic Microbial Consortia: Microorganisms thrive as diverse communities. In a shared living environment, different species of microbes exchange metabolic byproducts. Therefore, a synergistic consortium of microbial cells can be combined with PNMs to achieve an effective natural electron transfer pathway and high quantum efficiency.
- Stacking Different PNMs: Stacking different PNMs capable of absorbing various components of solar radiation can enhance light harvesting. Thus, efforts to create NBHs with stacked PNMs need to be increased.
- Nitrogen Fixation: While NBHs have been used for light-driven conversion of CO2 into chemicals and the light-driven splitting of water into H2 and O2, limited attention has been given to nitrogen fixation. This process is critical for the production of carbon-neutral fuels.
- Impact of Positively Charged PNMs: The impact of negatively charged PNMs has been extensively explored, but the effect of positively charged PNMs on the cellular environment remains largely unexplored. Future studies should focus on this aspect.
- Organic Photosensitizers: Recent works have given limited attention to the use of dyes and polymeric photosensitizers. These organic molecules can diffuse inside microbial cells, facilitating the formation of efficient intracellular hybrids.
- Climatic Adaptability: Although NBH-based AP has been successfully explored for chemical and fuel production, the full potential of sunlight-to-chemical conversion has not yet been achieved, with benchmark efficiency. NBHs that function under various climatic conditions must be developed.
- Bio-inspired NBHs: Developing a variety of bio-inspired NBHs that mimic natural photosynthetic structures (e.g., chloroplasts) requires greater insights into light and electron utilization.
- Dynamic Interfacing: Developing stimuli-responsive semiconductors that adjust electron output based on microbial metabolic demand is essential. Integrating biosensors with dynamic interfaces could enable autonomous adjustments. For example, lactate sensors in NBHs could trigger a pH-responsive polymer to release electrons only when product concentrations are low.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Liu, J.; Sha, C.; Yong, Y.-C.; Jiang, Y.; Fang, Z. Nanomaterial-Biological Hybrid Systems: Advancements in Solar-Driven CO2-to-Chemical Conversion. Green Carbon 2024, 2, 322–336. [Google Scholar] [CrossRef]
- Okoro, G.; Husain, S.; Saukani, M.; Mutalik, C.; Yougbaré, S.; Hsiao, Y.C.; Kuo, T.R. Emerging Trends in Nanomaterials for Photosynthetic Biohybrid Systems. ACS Mater. Lett. 2023, 5, 95–115. [Google Scholar] [CrossRef]
- Liang, J.; Xiao, K.; Wang, X.; Hou, T.; Zeng, C.; Gao, X.; Wang, B.; Zhong, C. Revisiting Solar Energy Flow in Nanomaterial-Microorganism Hybrid Systems. Chem. Rev. 2024, 124, 9081–9112. [Google Scholar] [CrossRef]
- Barber, J. Photosynthetic Energy Conversion: Natural and Artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Lewis, N.S. Developing a Scalable Artificial Photosynthesis Technology through Nanomaterials by Design. Nat. Nanotechnol. 2016, 11, 1010–1019. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Sanchez, R.J.; Srinivasan, C.; Munroe, W.H.; Wallace, M.A.; Martins, J.; Kao, T.Y.; Le, K.; Gralla, E.B.; Valentine, J.S. Exogenous Manganous Ion at Millimolar Levels Rescues All Known Dioxygen-Sensitive Phenotypes of Yeast Lacking CuZnSOD. J. Biol. Inorg. Chem. 2005, 10, 913–923. [Google Scholar] [CrossRef]
- Cazelles, R.; Drone, J.; Fajula, F.; Ersen, O.; Moldovan, S.; Galarneau, A. Reduction of CO2 to Methanol by a Polyenzymatic System Encapsulated in Phospholipids-Silica Nanocapsules. New J. Chem. 2013, 37, 3721–3730. [Google Scholar] [CrossRef]
- Kumar, M.; Sahoo, P.C.; Parida, K. Minireview on Microbe-Nanomaterial Synergies: Fundamental, Interface Mechanism, Characterization and CO2 Photo-Fixation. Results Surf. Interfaces 2024, 16, 100272. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Huang, X. Whole-Cell-Based Photosynthetic Biohybrid Systems for Energy and Environmental Applications. Chempluschem 2021, 86, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, Y.; Qiao, L. Photodriven Chemical Synthesis by Whole-Cell-Based Biohybrid Systems: From System Construction to Mechanism Study. ACS Appl. Mater. Interfaces 2023, 15, 6235–6259. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, K.K.; Kornienko, N.; Yang, P. Cyborgian Material Design for Solar Fuel Production: The Emerging Photosynthetic Biohybrid Systems. Acc. Chem. Res. 2017, 50, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, K.K.; Kornienko, N.; Cestellos-Blanco, S.; Lim, J.; Liu, C.; Yang, P. Physical Biology of the Materials-Microorganism Interface. J. Am. Chem. Soc. 2018, 140, 1978–1985. [Google Scholar] [CrossRef]
- Cestellos-Blanco, S.; Zhang, H.; Kim, J.M.; Shen, Y.; Yang, P. Photosynthetic Semiconductor Biohybrids for Solar-Driven Biocatalysis. Nat. Catal. 2020, 3, 245–255. [Google Scholar] [CrossRef]
- Dogutan, D.K.; Nocera, D.G. Artificial Photosynthesis at Efficiencies Greatly Exceeding That of Natural Photosynthesis. Acc. Chem. Res. 2019, 52, 3143–3148. [Google Scholar] [CrossRef]
- Yang, P. Liquid Sunlight: The Evolution of Photosynthetic Biohybrids. Nano Lett. 2021, 21, 5453–5456. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Y.; Lv, F.; Bai, H.; Wang, S. Organic Semiconductor-Organism Interfaces for Augmenting Natural and Artificial Photosynthesis. Acc. Chem. Res. 2022, 55, 156–170. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, S.; Lv, S.; Tian, R.; Cheng, S.; Chen, Y. Eradicating the Photogenerated Holes in a PhotoAP systems-Microbe Hybrid System: A Review. ACS Appl. Mater. Interfaces 2024, 16, 56545–56554. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Z.; Fu, Q.; Li, Y.; Li, J.; Zhang, L.; Liao, Q.; Zhu, X. Hybrid Microbial Photoelectrochemical System Reduces CO2 to CH4 with 1.28% Solar Energy Conversion Efficiency. Chem. Eng. J. 2020, 390, 124530. [Google Scholar] [CrossRef]
- Lu, X. A Perspective: Photosynthetic Production of Fatty Acid-Based Biofuels in Genetically Engineered Cyanobacteria. Biotechnol. Adv. 2010, 28, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Srirangan, K.; Pyne, M.E.; Perry Chou, C. Biochemical and Genetic Engineering Strategies to Enhance Hydrogen Production in Photosynthetic Algae and Cyanobacteria. Bioresour. Technol. 2011, 102, 8589–8604. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, J.; Feng, W.; Yue, J.; Luo, Y.; Chen, S.; Yang, B.; Jiang, Y.; Hu, H.; Zhou, C.; et al. Periplasmic Biomineralization for Semi-Artificial Photosynthesis. Sci. Adv. 2023, 9, eadg5858. [Google Scholar] [CrossRef]
- Woolerton, T.W.; Sheard, S.; Reisner, E.; Pierce, E.; Ragsdale, S.W.; Armstrong, F.A. Efficient and Clean Photoreduction of CO2 to CO by Enzyme-Modified TiO2 Nanoparticles Using Visible Light. J. Am. Chem. Soc. 2010, 132, 2132–2133. [Google Scholar] [CrossRef]
- Brown, K.A.; Wilker, M.B.; Boehm, M.; Hamby, H.; Dukovic, G.; King, P.W. Photocatalytic Regeneration of Nicotinamide Cofactors by Quantum Dot-Enzyme Biohybrid Complexes. ACS Catal. 2016, 6, 2201–2204. [Google Scholar] [CrossRef]
- Sweeny, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef]
- Kowshik, M.; Deshmukh, N.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial Synthesis of Semiconductor CdS Nanoparticles, Their Characterization, and Their Use in the Fabrication of an Ideal Diode. Biotechnol. Bioeng. 2002, 78, 583–588. [Google Scholar] [CrossRef]
- Wang, J.; Chen, N.; Bian, G.; Mu, X.; Du, N.; Wang, W.; Ma, C.G.; Fu, S.; Huang, B.; Liu, T.; et al. Solar-Driven Overproduction of Biofuels in Microorganisms. Angew. Chem.-Int. Ed. 2022, 61, 7132. [Google Scholar]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based Photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Sakimoto, K.K.; Wong, A.B.; Yang, P. Self-Photosensitization of Nonphotosynthetic Bacteria for Solar-to-Chemical Production. Science 2016, 351, 74–77. [Google Scholar] [CrossRef]
- Kornienko, N.; Sakimoto, K.K.; Herlihy, D.M.; Nguyen, S.C.; Alivisatos, A.P.; Harris, C.B.; Schwartzberg, A.; Yang, P. Spectroscopic Elucidation of Energy Transfer in Hybrid Inorganic-Biological Organisms for Solar-to-Chemical Production. Proc. Natl. Acad. Sci. USA 2016, 113, 11750–11755. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Bertram, J.R.; Eckert, C.; Bommareddy, R.R.; Patel, R.; Conradie, A.; Bryan, S.; Nagpal, P. Nanorg Microbial Factories: Light-Driven Renewable Biochemical Synthesis Using Quantum Dot-Bacteria Nanobiohybrids. J. Am. Chem. Soc. 2019, 141, 10272–10282. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yu, J.; Zhang, Y.; Chen, M.; Liu, X.; Zhou, S.; He, Z. Light-Driven Carbon Dioxide Reduction to Methane by Methanosarcina Barkeri-CdS Biohybrid. Appl. Catal. B 2019, 257, 117916. [Google Scholar] [CrossRef]
- Kumar, M.; Sahoo, P.C.; Srikanth, S.; Bagai, R.; Puri, S.K.; Ramakumar, S.S.V. Photosensitization of Electro-Active Microbes for Solar Assisted Carbon Dioxide Transformation. Bioresour. Technol. 2019, 272, 300–307. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, J.; Sun, Y.; Wu, Y.; Zhang, Y.; Cai, Z.; Chen, Y.; You, C.; Han, P.; Jiang, Z. Artificial Thylakoid for the Coordinated Photoenzymatic Reduction of Carbon Dioxide. ACS Catal. 2019, 9, 3913–3925. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, Z.; Yu, J.C.; Wang, J.; Wong, P.K. Enhanced CO2 Reduction and Valuable C2+ Chemical Production by a CdS-Photosynthetic Hybrid System. Nanoscale 2019, 11, 9296–9301. [Google Scholar] [CrossRef]
- Xu, M.; Tremblay, P.L.; Ding, R.; Xiao, J.; Wang, J.; Kang, Y.; Zhang, T. Photo-Augmented PHB Production from CO2 or Fructose by Cupriavidus Necator and Shape-Optimized CdS Nanorods. Sci. Total. Environ. 2021, 753, 142050. [Google Scholar] [CrossRef]
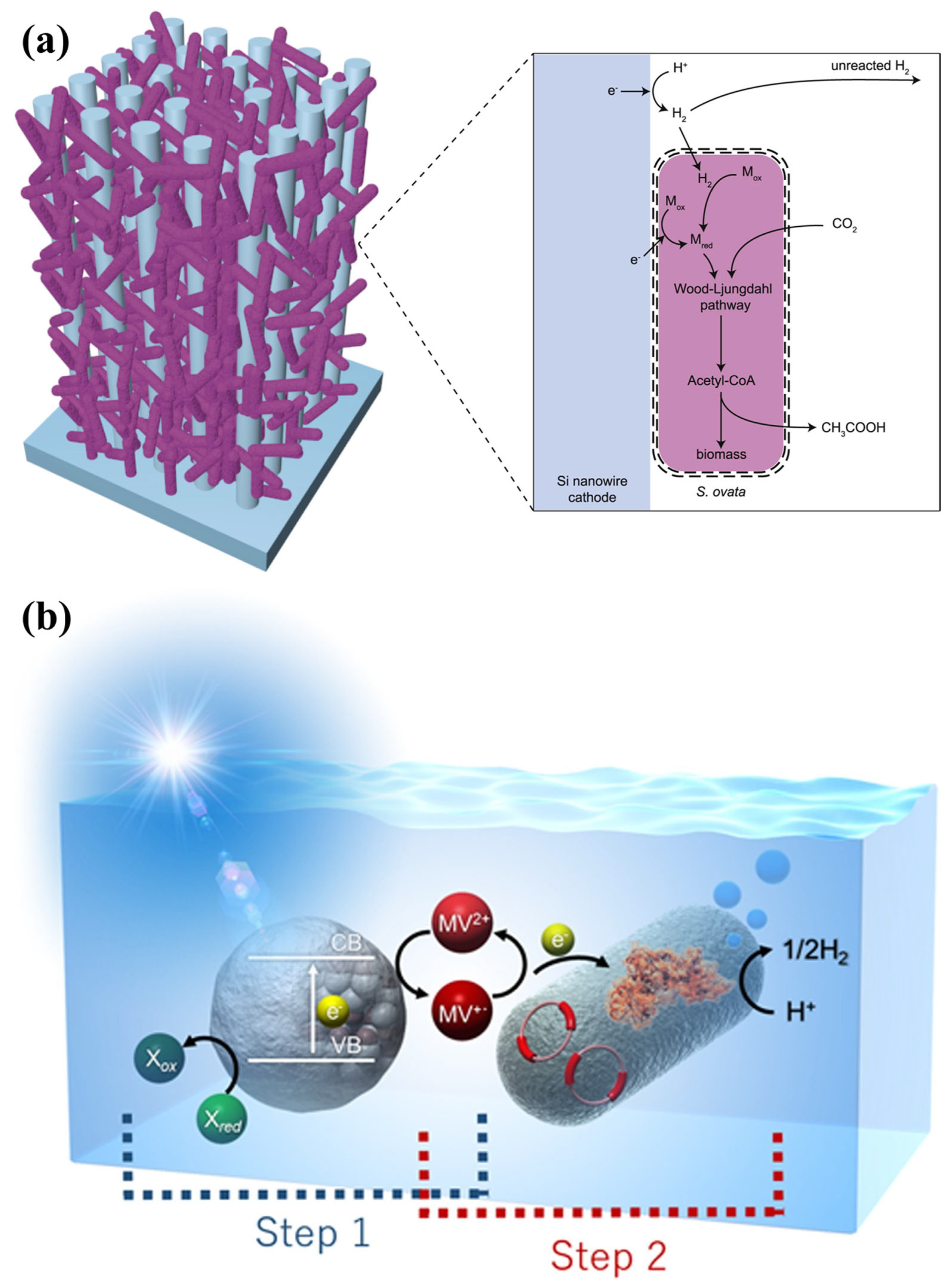
- He, Y.; Wang, S.; Han, X.; Shen, J.; Lu, Y.; Zhao, J.; Shen, C.; Qiao, L. Photosynthesis of Acetate by Sporomusa Ovata-CdS Biohybrid System. ACS Appl. Mater. Interfaces 2022, 14, 23364–23374. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, C.; Chu, K.H.; Wu, D.; Yip, H.Y.; Ye, L.; Wong, P.K. Enhanced Biological Hydrogen Production from Escherichia Coli with Surface Precipitated Cadmium Sulfide Nanoparticles. Adv. Energy Mater. 2017, 7, 1700611. [Google Scholar] [CrossRef]
- Chen, M.Y.; Fang, Z.; Xu, L.X.; Zhou, D.; Yang, X.J.; Zhu, H.J.; Yong, Y.C. Enhancement of Photo-Driven Biomethanation under Visible Light by Nano-Engineering of Rhodopseudomonas Palustris. Bioresour. Bioprocess. 2021, 8, 30. [Google Scholar] [CrossRef]
- Liang, G.; Xu, X.; Chen, X.; Wu, J.; Song, W.; Wei, W.; Liu, J.; Li, X.; Liu, L.; Gao, C. Designing a Periplasmic Photosynthetic Biohybrid System for Succinate and Electric Energy Production. Chem. Eng. J. 2023, 477, 147152. [Google Scholar] [CrossRef]
- Wei, W.; Sun, P.; Li, Z.; Song, K.; Su, W.; Wang, B.; Liu, Y.; Zhao, J. A Surface-Display Biohybrid Approach to Light-Driven Hydrogen Production in Air. Sci. Adv. 2018, 4, eaap9253. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, J.; Xia, Q.; Liu, J.; Zhu, J.J.; Zhang, J.R.; Chen, X.; Zhu, W.; Chen, Z. Operando Spectroscopic Elucidation of the Bubble Sunshade Effect in Inorganic-Biological Hybrids for Photosynthetic Hydrogen Production. ACS Nano 2024, 18, 14546–14557. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Yu, Y.H.; Van Der Linden, B.; Wu, J.C.S.; Mul, G. Artificial Photosynthesis over Crystalline TiO2-Based Catalysts: Fact or Fiction? J. Am. Chem. Soc. 2010, 132, 8398–8406. [Google Scholar] [CrossRef]
- Yuan, L.; Han, C.; Pagliaro, M.; Xu, Y.J. Origin of Enhancing the Photocatalytic Performance of TiO2 for Artificial Photoreduction of CO2 through a SiO2 Coating Strategy. J. Phys. Chem. C 2016, 120, 265–273. [Google Scholar] [CrossRef]
- Ruan, X.; Li, S.; Huang, C.; Zheng, W.; Cui, X.; Ravi, S.K. Catalyzing Artificial Photosynthesis with TiO2 Heterostructures and Hybrids: Emerging Trends in a Classical yet Contemporary Photocatalyst. Adv. Mater. 2024, 36, 2305285. [Google Scholar] [CrossRef]
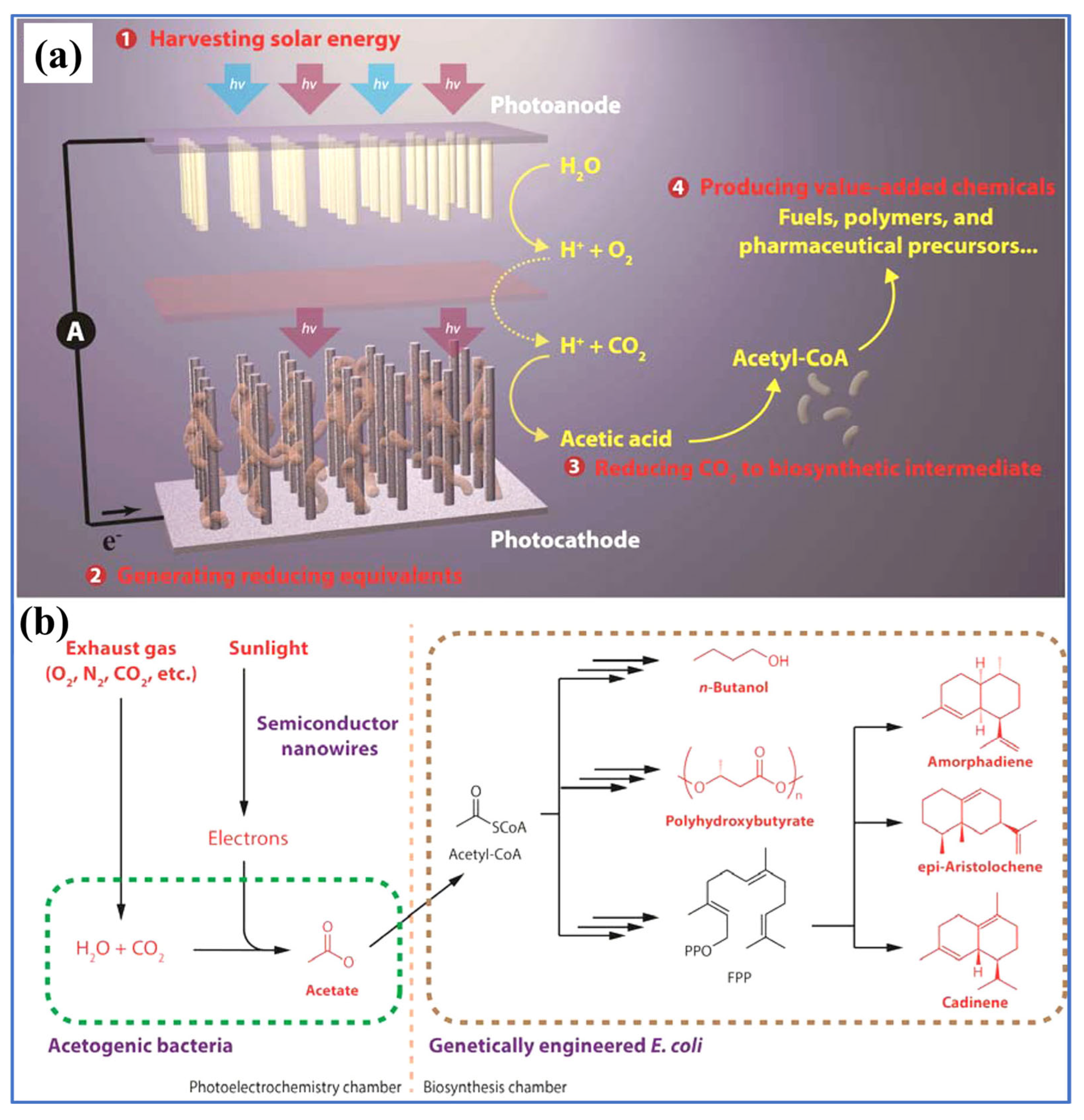
- Liu, C.; Gallagher, J.J.; Sakimoto, K.K.; Nichols, E.M.; Chang, C.J.; Chang, M.C.Y.; Yang, P. Nanowire-Bacteria Hybrids for Unassisted Solar Carbon Dioxide Fixation to Value-Added Chemicals. Nano Lett. 2015, 15, 3634–3639. [Google Scholar] [CrossRef]
- Su, Y.; Cestellos-Blanco, S.; Kim, J.M.; Shen, Y.; Kong, Q.; Lu, D.; Liu, C.; Zhang, H.; Cao, Y.; Yang, P. Close-Packed Nanowire-Bacteria Hybrids for Efficient Solar-Driven CO2 Fixation. Joule 2020, 4, 800–811. [Google Scholar] [CrossRef]
- Honda, Y.; Watanabe, M.; Hagiwara, H.; Ida, S.; Ishihara, T. Inorganic/Whole-Cell Biohybrid Photocatalyst for Highly Efficient Hydrogen Production from Water. Appl. Catal. B 2017, 210, 400–406. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Guo, P.; Zhang, J.; Tira, G.; Kim, Y.J.; Wu, Y.A.; Liu, Y.; Wen, J.; Rajh, T.; et al. Semi-Artificial Photosynthetic CO2 Reduction through Purple Membrane Re-Engineering with Semiconductor. J. Am. Chem. Soc. 2019, 141, 11811–11815. [Google Scholar] [CrossRef]
- Kim, J.; Cestellos-Blanco, S.; Shen, Y.X.; Cai, R.; Yang, P. Enhancing Biohybrid CO2 to Multicarbon Reduction via Adapted Whole-Cell Catalysts. Nano Lett. 2022, 22, 5503–5509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, Q.; Li, Y.; Feng, L.; Fu, S.; Mazarji, M.; Pan, J. The Role of Semi-Artificial Photosynthetic Systems in Energy and Environmental Solutions: A Critical Review. Biofuel Res. J. 2024, 11, 2082–2098. [Google Scholar] [CrossRef]
- Surana, K.; Singh, P.K.; Rhee, H.W.; Bhattacharya, B. Synthesis, Characterization and Application of CdSe Quantum Dots. J. Ind. Eng. Chem. 2014, 20, 4188–4193. [Google Scholar] [CrossRef]
- Li, X.B.; Tung, C.H.; Wu, L.Z. Semiconducting Quantum Dots for Artificial Photosynthesis. Nat. Rev. Chem. 2018, 2, 160–173. [Google Scholar] [CrossRef]
- Tsuneda, T.; Ten-No, S.L. Water-Oxidation Mechanism of Cobalt Phosphate Co-Catalyst in Artificial Photosynthesis: A Theoretical Study. Phys. Chem. Chem. Phys. 2022, 24, 4674–4682. [Google Scholar] [CrossRef]
- Gautam, A.; Gore, P.M.; Kandasubramanian, B. Nanocluster Materials in Photosynthetic Machines. Chem. Eng. J. 2020, 385, 123951. [Google Scholar] [CrossRef]
- Rahman, A.; Parwaiz, S.; Sohn, Y.; Mansoob Khan, M. Advances in Artificial Photosynthesis: The Role of Chalcogenides and Chalcogenide-Based Heterostructures. ChemPhotoChem 2024, 9, e202400234. [Google Scholar] [CrossRef]
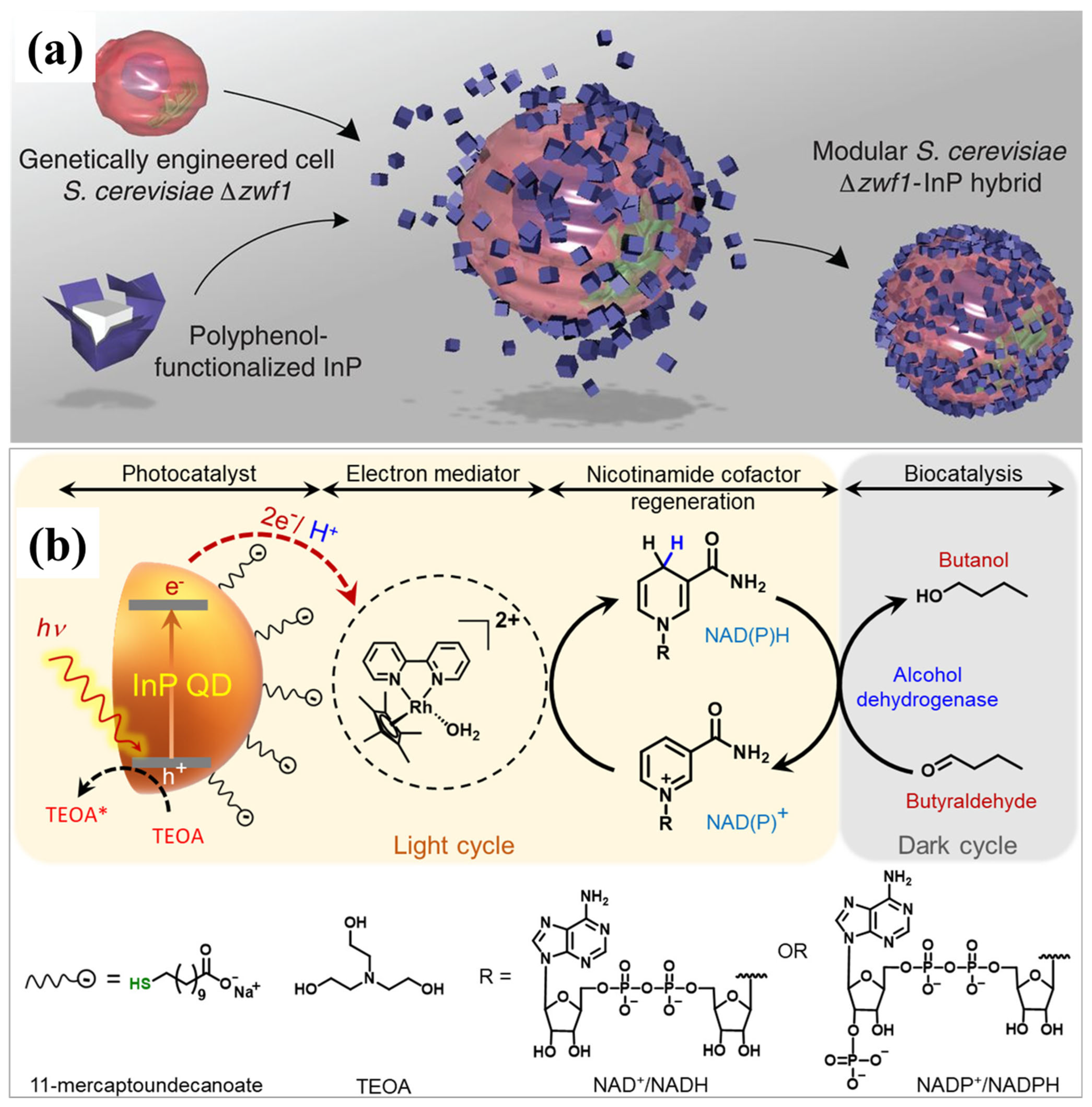
- Guo, J.; Suástegui, M.; Sakimoto, K.K.; Moody, V.M.; Xiao, G.; Nocera, D.G.; Joshi, N.S. Light-Driven Fine Chemical Production in Yeast Biohybrids. Science 2018, 362, 813–816. [Google Scholar] [CrossRef]
- Chakraborty, I.N.; Jain, V.; Roy, P.; Kumar, P.; Vinod, C.P.; Pillai, P.P. Photocatalytic Regeneration of Reactive Cofactors with InP Quantum Dots for the Continuous Chemical Synthesis. ACS Catal. 2024, 14, 6740–6748. [Google Scholar] [CrossRef]
- Liu, C.; Colón, B.C.; Ziesack, M.; Silver, P.A.; Nocera, D.G. Water Splitting–Biosynthetic System with CO2 Reduction Efficiencies Exceeding Photosynthesis. Science 2016, 352, 1210–1213. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Tian, Z.; Lu, D.; Yu, Y.; Cestellos-Blanco, S.; Sakimoto, K.K.; Yang, P. Bacteria Photosensitized by Intracellular Gold Nanoclusters for Solar Fuel Production. Nat. Nanotechnol. 2018, 13, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, B.; Yu, J.C.; Wang, J.; An, T.; Zhao, H.; Li, H.; Yuan, S.; Wong, P.K. AglnS2/In2S3 Heterostructure Sensitization of Escherichia Coli for Sustainable Hydrogen Production. Nano Energy 2018, 46, 234–240. [Google Scholar] [CrossRef]
- Luo, B.; Wang, Y.Z.; Li, D.; Shen, H.; Xu, L.X.; Fang, Z.; Xia, Z.; Ren, J.; Shi, W.; Yong, Y.C. A Periplasmic Photosensitized Biohybrid System for Solar Hydrogen Production. Adv. Energy Mater. 2021, 11, 2100256. [Google Scholar] [CrossRef]
- Edwards, E.H.; Jelušić, J.; Kosko, R.M.; McClelland, K.P.; Ngarnim, S.S.; Chiang, W.; Lampa-Pastirk, S.; Krauss, T.; Bren, K.L. Shewanella Oneidensis MR-1 Respires CdSe Quantum Dots for Photocatalytic Hydrogen Evolution. Proc. Natl. Acad. Sci. USA 2023, 120, e2206975120. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wang, S.; Xiao, G.; Jin, Y.; Wang, Z.; Su, H. Visible-Light-Driven Enhanced Biohydrogen Production by Photo-Biohybrid System Based on Photoelectron Transfer between Intracellular Photosensitizer Gold Nanoparticles and Clostridium Butyricum. ACS Sustain. Chem. Eng. 2023, 11, 300–311. [Google Scholar] [CrossRef]
- Lu, L.; Lv, Z.; Si, Y.; Liu, M.; Zhang, S. Recent Progress on Band and Surface Engineering of Graphitic Carbon Nitride for Artificial Photosynthesis. Appl. Surf. Sci. 2018, 462, 693–712. [Google Scholar] [CrossRef]
- Cruz, D.; Żółtowska, S.; Savateev, O.; Antonietti, M.; Giusto, P. Carbon Nitride Caught in the Act of Artificial Photosynthesis. Nat. Commun. 2025, 16, 374. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Y.; Zong, Y.; Li, J.; Yang, N.; Zhang, M.; Guo, Z.; Song, H. Construction of Functionally Compartmental Inorganic Photocatalyst-Enzyme System via Imitating Chloroplast for Efficient Photoreduction of CO2 to Formic Acid. ACS Appl. Mater. Interfaces 2020, 12, 34795–34805. [Google Scholar] [CrossRef]
- Tremblay, P.L.; Xu, M.; Chen, Y.; Zhang, T. Nonmetallic Abiotic-Biological Hybrid Photocatalyst for Visible Water Splitting and Carbon Dioxide Reduction. iScience 2020, 23, 100784. [Google Scholar] [CrossRef]
- Sheng, Y.; Guo, F.; Guo, B.; Wang, N.; Sun, Y.; Liu, H.; Feng, X.; Han, Q.; Yu, Y.; Li, C. Light-Driven CO2 Reduction with a Surface-Displayed Enzyme Cascade-C3N4 Hybrid. ACS Synth. Biol. 2023, 12, 2715–2724. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Fu, B.; Zhang, Z. Living Intracellular Inorganic-Microorganism Biohybrid System for Efficient Solar Hydrogen Generation. Joule 2022, 6, 2293–2303. [Google Scholar] [CrossRef]
- Hu, A.; Ye, J.; Ren, G.; Qi, Y.; Chen, Y.; Zhou, S. Metal-Free Semiconductor-Based Bio-Nano Hybrids for Sustainable CO2-to-CH4 Conversion with High Quantum Yield. Angew. Chem.-Int. Ed. 2022, 61, e202206508. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hu, J.; Li, L.; Hong, M.; Yang, C.; Ren, G.; Ye, J.; Zhou, S. Natural AIEgens as Ultraviolet Sunscreens and Photosynergists for Solar Fuel Production. Environ. Sci. Technol. 2024, 58, 20434–20443. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, H.; Yu, W.; Huang, Y.; Lv, F.; Bai, H.; Wang, S. Organic Semiconducting Polymers for Augmenting Biosynthesis and Bioconversion. JACS Au 2024, 4, 3–19. [Google Scholar] [CrossRef]
- Gai, P.; Yu, W.; Zhao, H.; Qi, R.; Li, F.; Liu, L.; Lv, F.; Wang, S. Solar-Powered Organic Semiconductor–Bacteria Biohybrids for CO2 Reduction into Acetic Acid. Angew. Chem.-Int. Ed. 2020, 59, 7224–7229. [Google Scholar] [CrossRef]
- Chen, W.; Yu, W.; Wang, Z.; Gao, Z.; Zhang, M.; Zhu, C.; Lv, F.; Huang, Y.; Bai, H.; Wang, S. Self-Powered Biohybrid Systems Based on Organic Materials for Sustainable Biosynthesis. ACS Appl. Mater. Interfaces 2023, 16, 19914–19925. [Google Scholar] [CrossRef]
- Yu, W.; Pavliuk, M.V.; Liu, A.; Zeng, Y.; Xia, S.; Huang, Y.; Bai, H.; Lv, F.; Tian, H.; Wang, S. Photosynthetic Polymer Dots-Bacteria Biohybrid System Based on Transmembrane Electron Transport for Fixing CO2 into Poly-3-Hydroxybutyrate. ACS Appl. Mater. Interfaces 2023, 15, 2183–2191. [Google Scholar] [CrossRef]
- Utschig, L.M.; Dimitrijevic, N.M.; Poluektov, O.G.; Chemerisov, S.D.; Mulfort, K.L.; Tiede, D.M. Photocatalytic Hydrogen Production from Noncovalent Biohybrid Photosystem I/Pt Nanoparticle Complexes. J. Phys. Chem. Lett. 2011, 2, 236–241. [Google Scholar] [CrossRef]
- Holá, K.; Pavliuk, M.V.; Németh, B.; Huang, P.; Zdražil, L.; Land, H.; Berggren, G.; Tian, H. Carbon Dots and [FeFe] Hydrogenase Biohybrid Assemblies for Efficient Light-Driven Hydrogen Evolution. ACS Catal. 2020, 10, 9943–9952. [Google Scholar] [CrossRef]
- Yang, Y.; Zwijnenburg, M.A.; Gardner, A.M.; Adamczyk, S.; Yang, J.; Sun, Y.; Jiang, Q.; Cowan, A.J.; Sprick, R.S.; Liu, L.N.; et al. Conjugated Polymer/Recombinant Escherichia Coli Biohybrid Systems for Photobiocatalytic Hydrogen Production. ACS Nano 2024, 18, 13484–13495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeevanandham, S.; Ramasundaram, S.; Vijay, N.; Oh, T.H.; Selvan, S.T. Recent Progress in Designing Nanomaterial Biohybrids for Artificial Photosynthesis. Nanomaterials 2025, 15, 730. https://doi.org/10.3390/nano15100730
Jeevanandham S, Ramasundaram S, Vijay N, Oh TH, Selvan ST. Recent Progress in Designing Nanomaterial Biohybrids for Artificial Photosynthesis. Nanomaterials. 2025; 15(10):730. https://doi.org/10.3390/nano15100730
Chicago/Turabian StyleJeevanandham, Sampathkumar, Subramaniyan Ramasundaram, Natarajan Vijay, Tae Hwan Oh, and Subramanian Tamil Selvan. 2025. "Recent Progress in Designing Nanomaterial Biohybrids for Artificial Photosynthesis" Nanomaterials 15, no. 10: 730. https://doi.org/10.3390/nano15100730
APA StyleJeevanandham, S., Ramasundaram, S., Vijay, N., Oh, T. H., & Selvan, S. T. (2025). Recent Progress in Designing Nanomaterial Biohybrids for Artificial Photosynthesis. Nanomaterials, 15(10), 730. https://doi.org/10.3390/nano15100730






