Stearic-Acid-Coated Sand: A Game Changer for Agriculture Water Management
Abstract
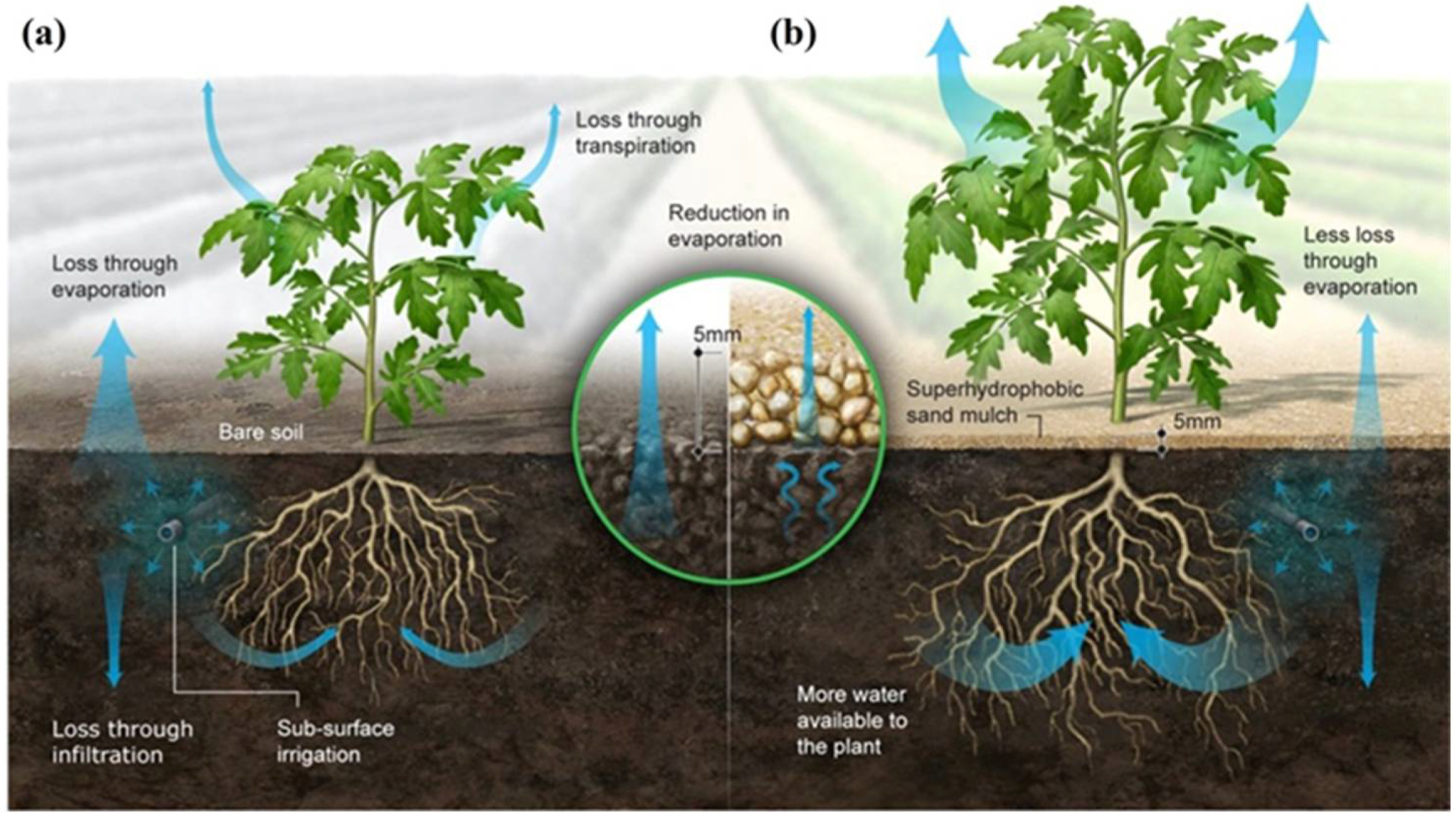
1. Introduction
2. Materials and Methods
2.1. Chemicals
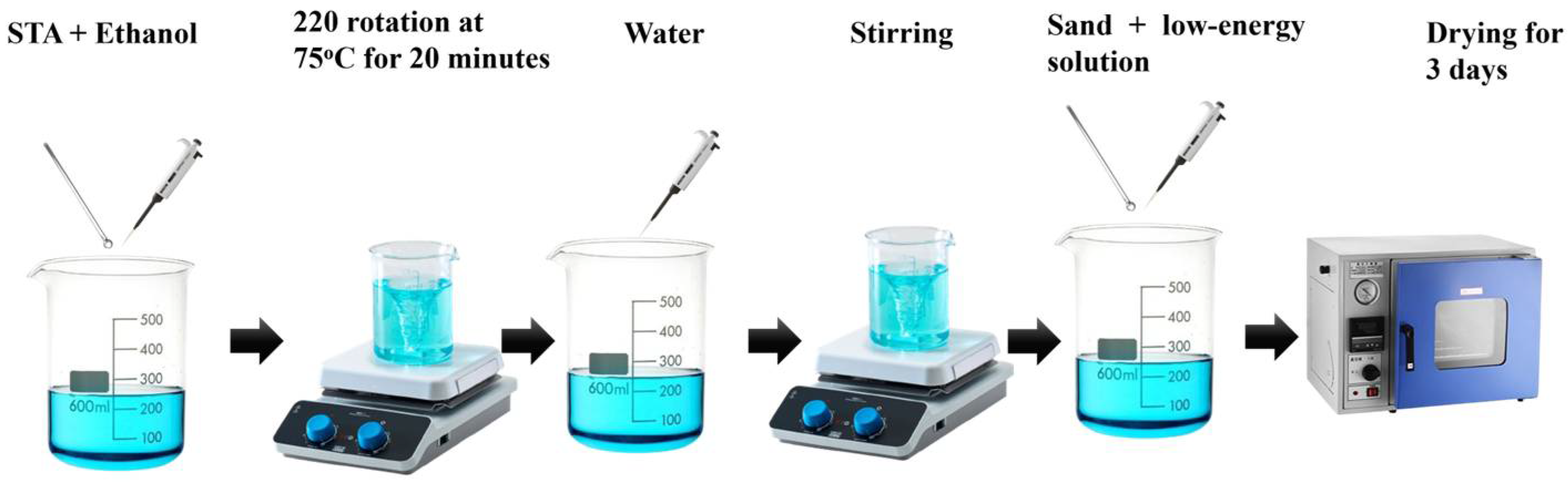
2.2. Preparation of Stearic-Acid-Coated Sand (SACS)
2.3. Reaction Mechanisms
- 1.
- Dissolution: C17H35COOH dissolves in C2H6O through hydrogen bonding between ethanol’s hydroxyl group and the carboxylic acid group of stearic acid.
- 2.
- Micelle Formation: Upon the addition of water, stearic acid becomes amphiphilic and forms micelle-like structures with hydrophilic carboxylic groups associated with water, while the hydrophobic alkyl chains (C17) orient away.
- 3.
- Hydrogen Bonding to Sand: The low-energy solution is poured onto the silica sand surface, where hydrogen bonds form between the -OH groups on the sand and the carboxylic groups of stearic acid.
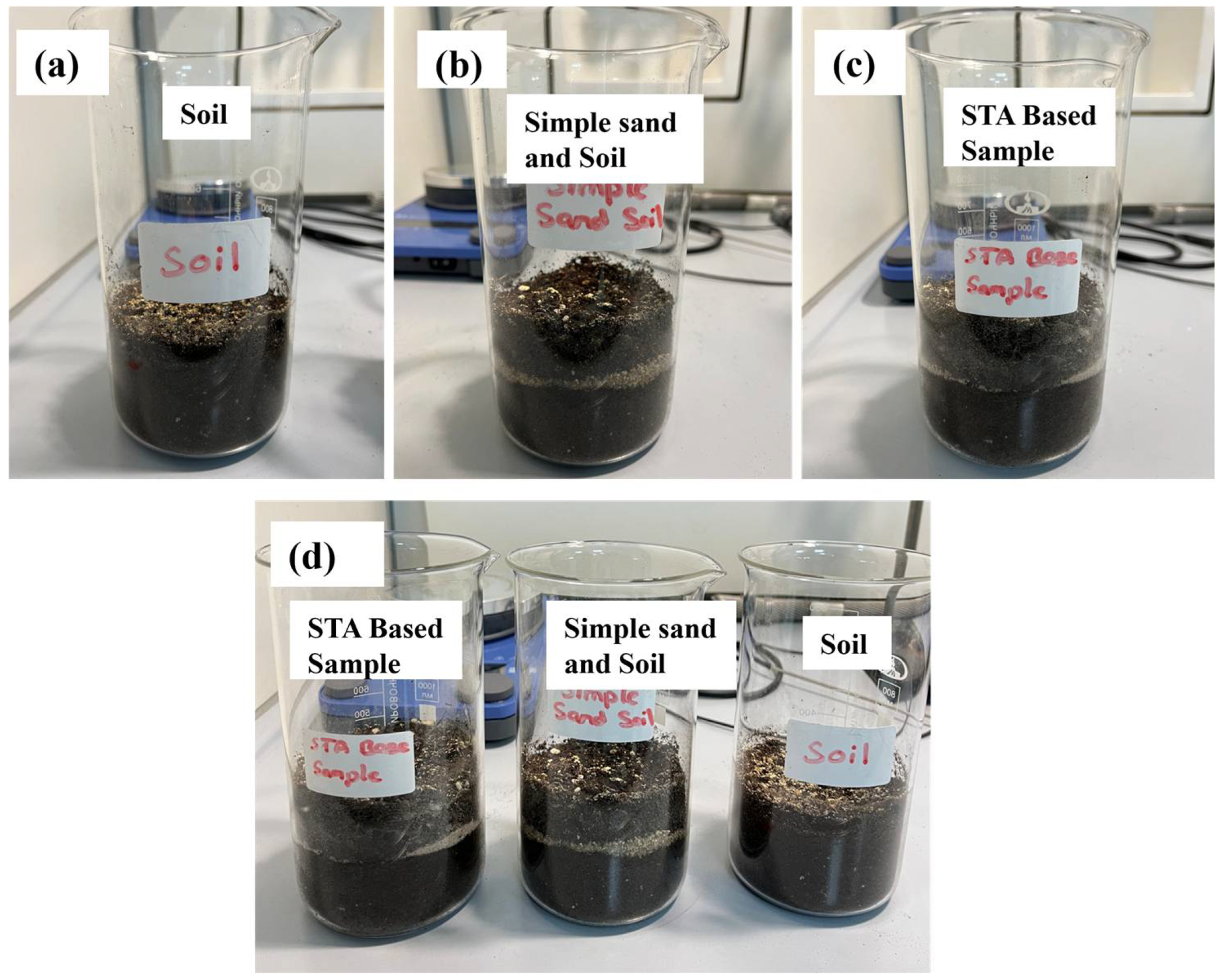
2.4. Experimental Setup
- Soil (Sample A): Universal soil only.
- Simple Sand Soil: Untreated sand mixed with universal soil.
- STA Base Sample: Stearic-acid-coated sand mixed with universal soil.
3. Results
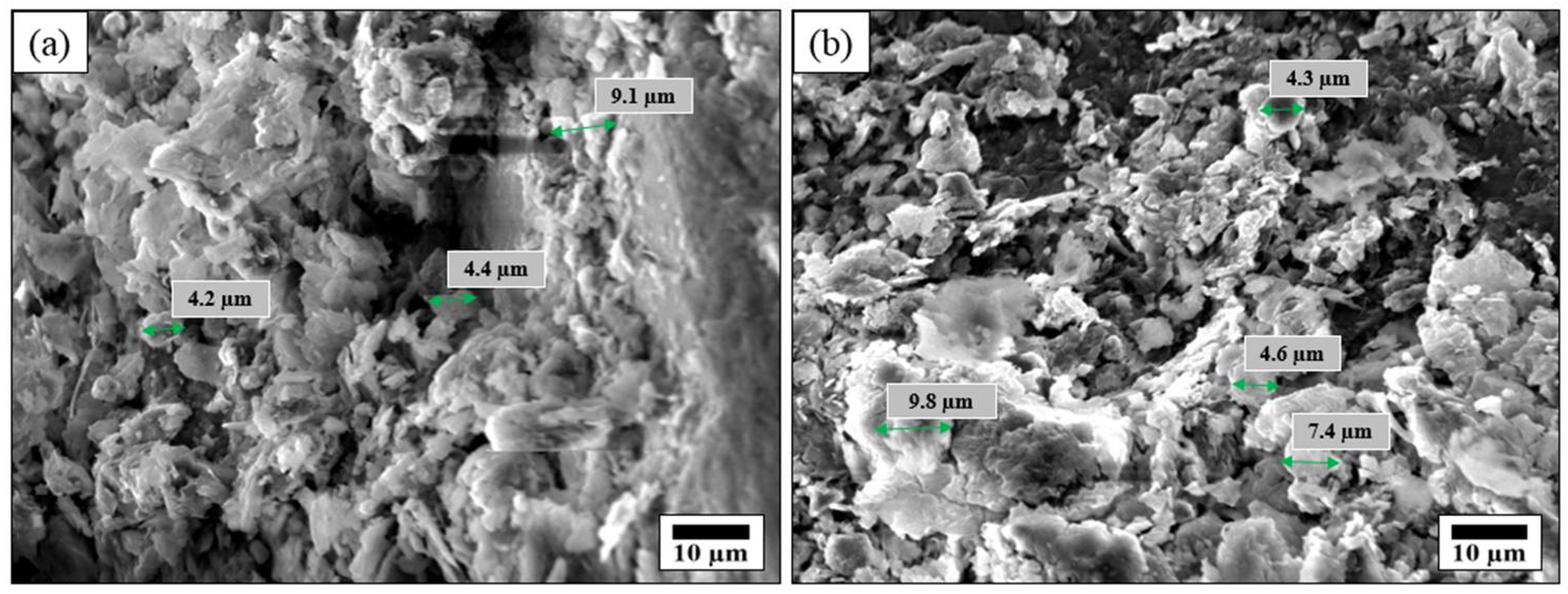
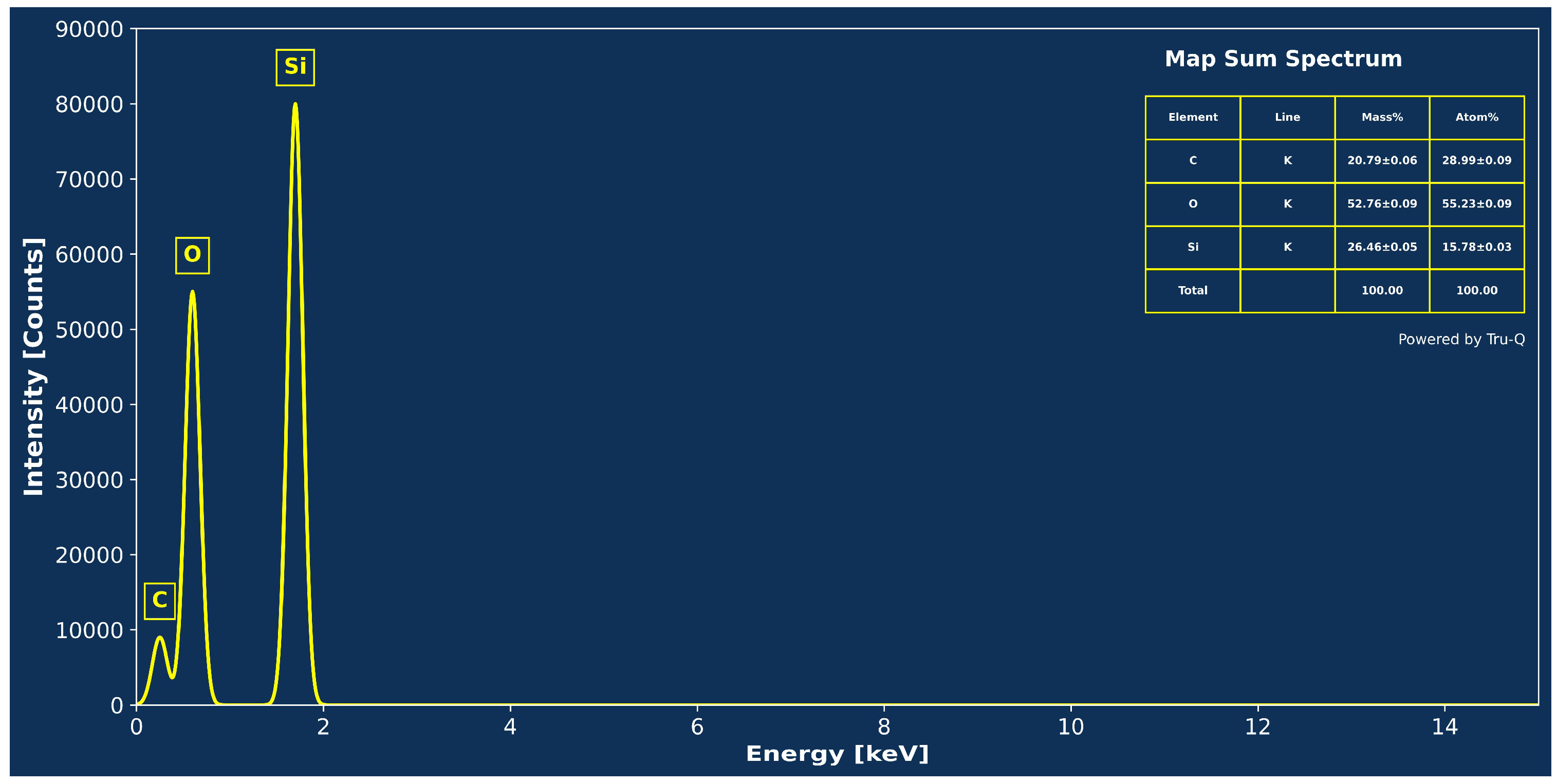
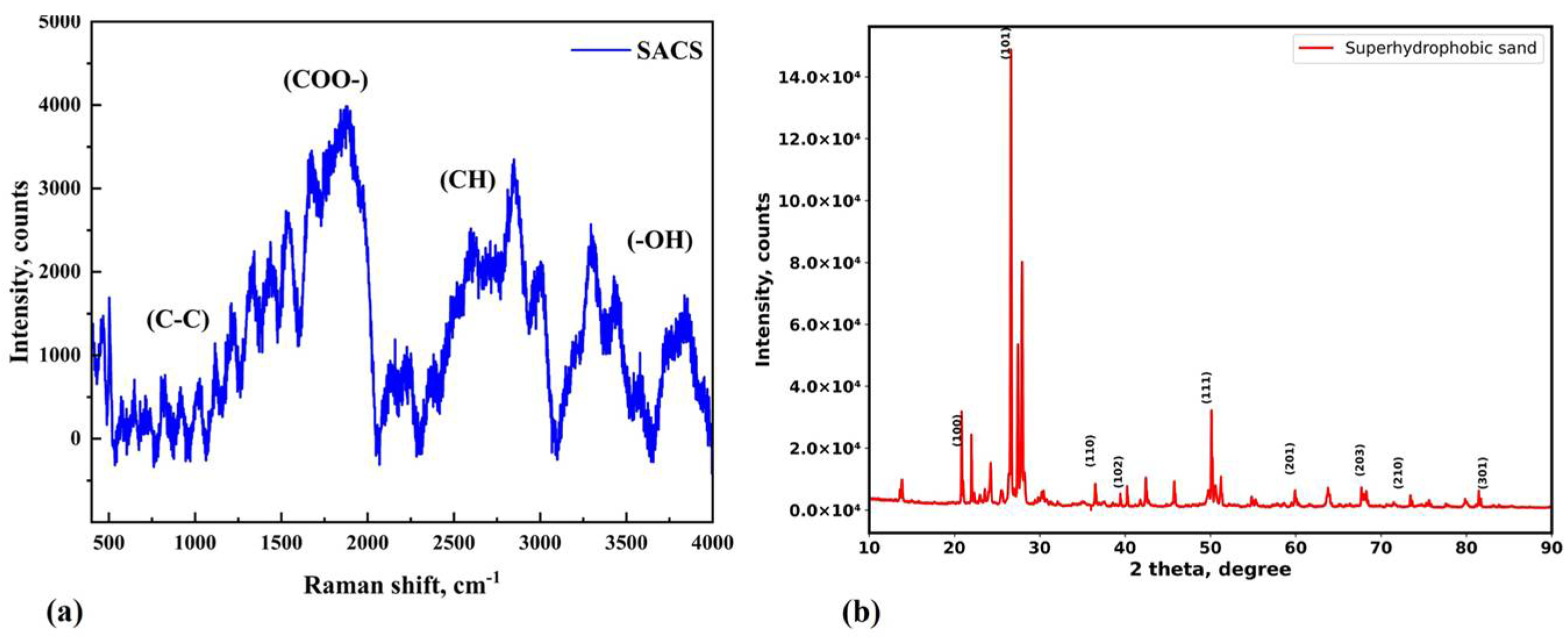
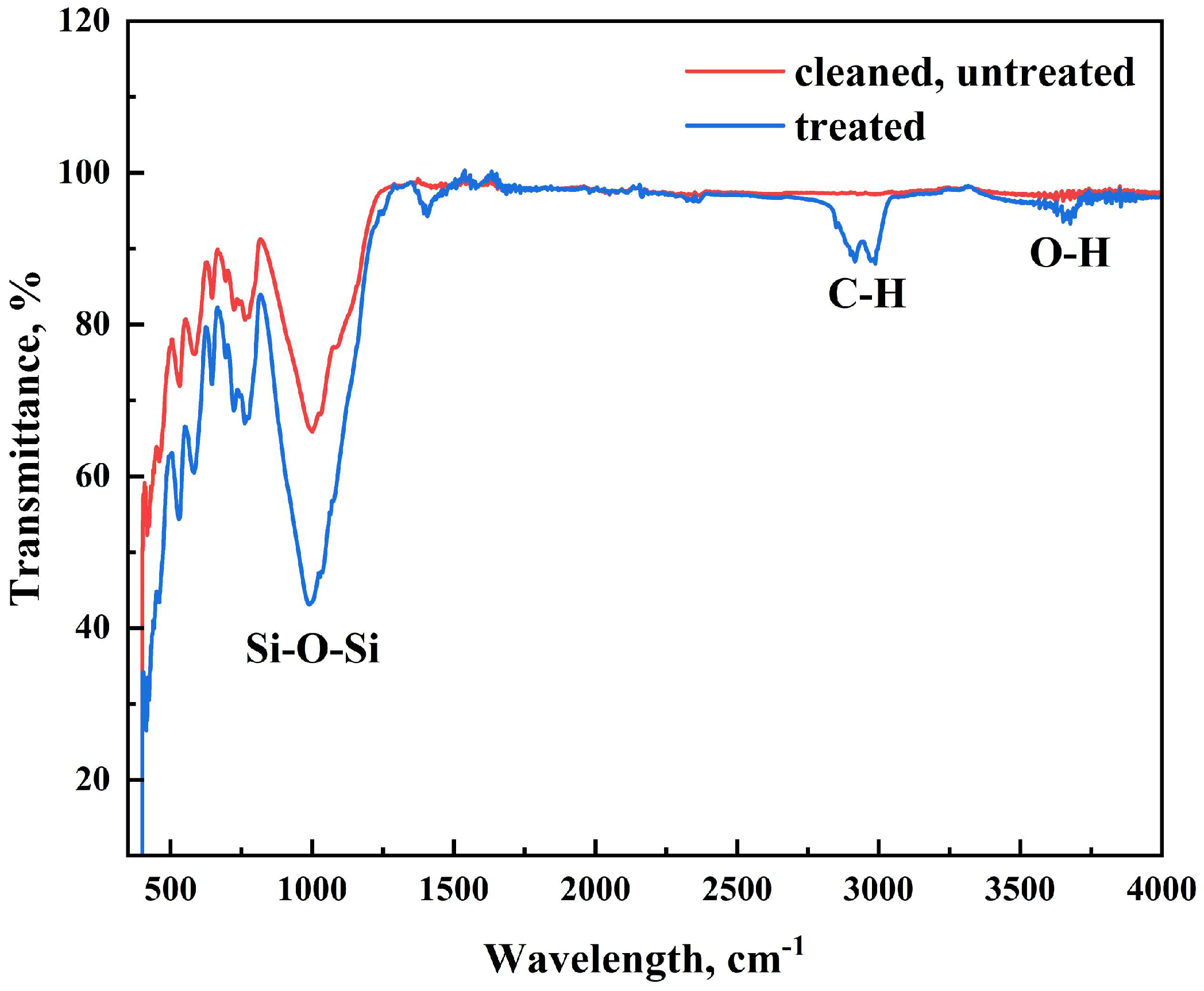
3.1. Characterization
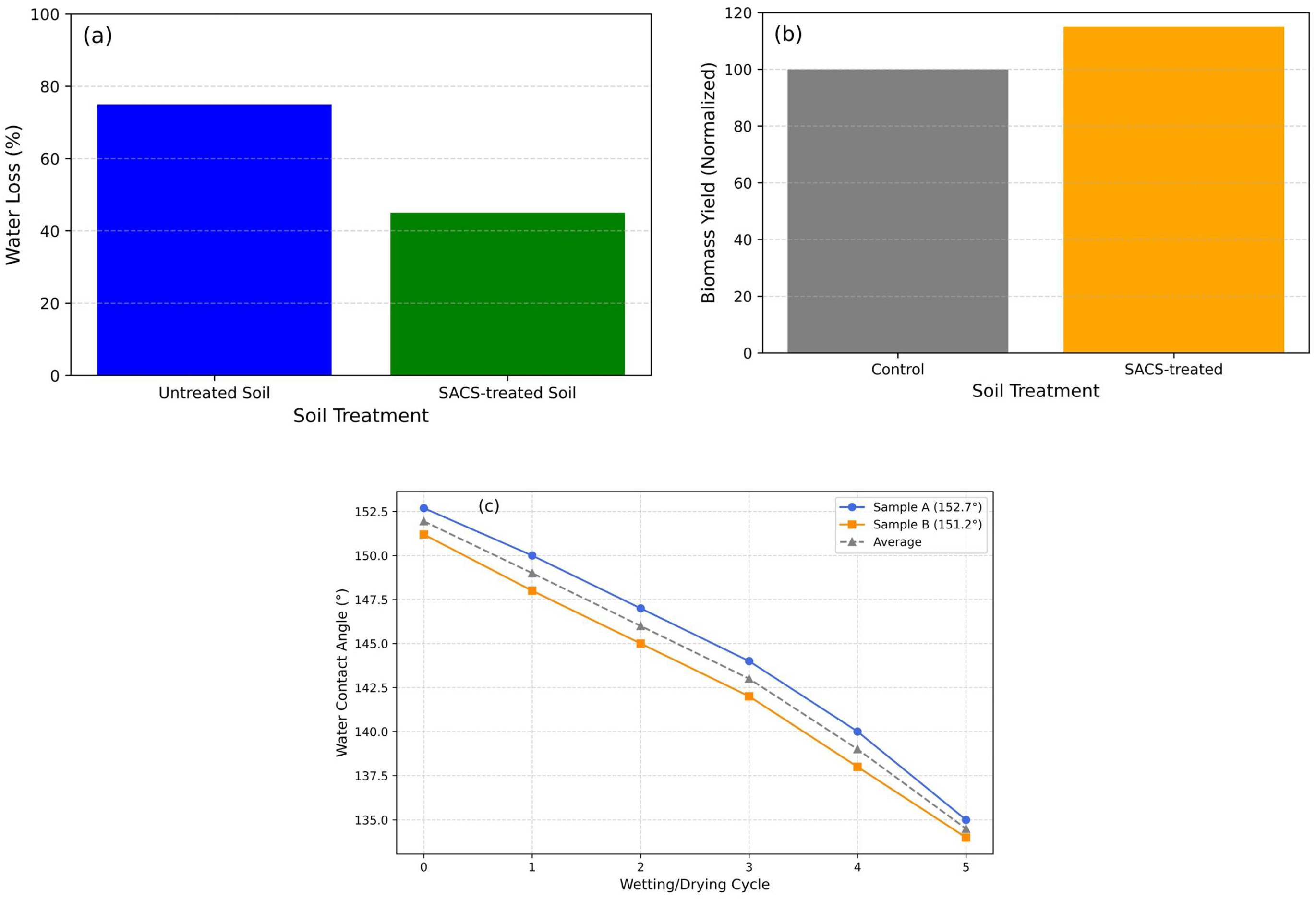
3.2. WCA Measurement
3.3. Validation of Stearic Acid Coating
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biswas, A.; Sarkar, S.; Das, S.; Dutta, S.; Choudhury, M.R.; Giri, A.; Bera, B.; Bag, K.; Mukherjee, B.; Banerjee, K.; et al. Water scarcity: A global hindrance to sustainable development and agricultural production—A critical review of the impacts and adaptation strategies. Camb. Prisms: Water 2025, 3, e4. [Google Scholar] [CrossRef]
- Abdou, D.S.; Othman, A.K.; Moussa, M.N. Examining the impact of water scarcity on agricultural output in the Gulf Cooperation Council (GCC) region. World Water Policy 2024, 10, 1243–1269. [Google Scholar] [CrossRef]
- Toktarbaiuly, O.; Kurbanova, A.; Imekova, G.; Abutalip, M.; Toktarbay, Z. Desert Water Saving and Transportation for Enhanced Oil Recovery: Bridging the Gap for Sustainable Oil Recovery. Eurasian Chem.-Technol. J. 2023, 25, 193–200. [Google Scholar] [CrossRef]
- Jahura, S.; Islam, M.S.; Mostafa, M.G. Impact of water scarcity on rural livelihood in the drought-prone region: A review of global perspectives. Indones. J. Soc. Sci. 2024, 16, 1–13. [Google Scholar] [CrossRef]
- Xie, J.; Qu, S.; Xu, M. Mapping water scarcity risks in global supply chain networks. Int. J. Logist. Res. Appl. 2024, 27, 2541–2555. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Gerbens-Leenes, W. The Water Footprint of Global Food Production. Water 2020, 12, 2696. [Google Scholar] [CrossRef]
- Muratoglu, A.; Demir, M.S. Understanding spatio-temporal variations in crop water consumption: Applying the WF Methodology Integrated with the SWAT Model. In Proceedings of the European Geosciences Union General Assembly 2024 (EGU24), Vienna, Austria, 14–19 April 2024. [Google Scholar] [CrossRef]
- Monteiro, M.A.; Bahta, Y.T.; Jordaan, H. A Systematic Review on Drivers of Water-Use Behaviour among Agricultural Water Users. Water 2024, 16, 1899. [Google Scholar] [CrossRef]
- Qamar, M.K.; Sharif, A.; Ahmad, M.; Jalil, H.; Bajwa, A. Agriculture and Water. In Global Issues in Water Policy; Springer: Berlin/Heidelberg, Germany, 2023; pp. 269–294. [Google Scholar] [CrossRef]
- Gallo, A.; Odokonyero, K.; Mousa, M.A.A.; Reihmer, J.; Al-Mashharawi, S.; Marasco, R.; Manalastas, E.; Morton, M.J.L.; Daffonchio, D.; McCabe, M.F.; et al. Nature-Inspired Superhydrophobic Sand Mulches Increase Agricultural Productivity and Water-Use Efficiency in Arid Regions. ACS Agric. Sci. Technol. 2022, 2, 276–288. [Google Scholar] [CrossRef]
- Suiindik, Z.; Adotey, E.; Kydyrbay, N.; Zhazitov, M.; Nuraje, N.; Toktarbaiuly, O. Formulating Superhydrophobic Coatings with Silane for Microfiber Applications. Eurasian Chem.-Technol. J. 2024, 26, 53–60. [Google Scholar] [CrossRef]
- Musei, S.K. Sandy soil reclamation technologies to improve crop productivity and soil health: A review. Front. Soil Sci. 2024, 4, 1345895. [Google Scholar] [CrossRef]
- Seralin, A.; Sugurbekova, G.; Kurbanova, A.; Nuraje, N.; Toktarbaiuly, O. Designing Water Repellent Concrete Composites Using Cheap Organic Materials. Eurasian Chem.-Technol. J. 2022, 24, 251–258. [Google Scholar] [CrossRef]
- Liu, W.F.; Huang, Z.; Guo, Z.; López-Vicente, M.; Wang, Z.; Wu, G.L. A nature-based solution to reduce soil water vertical leakage in arid sandy land. Geoderma 2023, 438, 116630. [Google Scholar] [CrossRef]
- Nilahyane, A.; Ghimire, R.; Acharya, B.S.; Schipanski, M.E.; West, C.P.; Obour, A.K. Overcoming agricultural sustainability challenges in water-limited environments through soil health and water conservation: Insights from the Ogallala Aquifer Region, USA. Int. J. Agric. Sustain. 2023, 21, 2211484. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Zhan, H.; Liang, H.; Yang, W.; Zhao, Y.; Li, T. New Comparative Experiments of Different Soil Types for Farmland Water Conservation in Arid Regions. Water 2018, 10, 298. [Google Scholar] [CrossRef]
- Attri, M.; Bharti, V.; Nesar, N.A.; Mehta, S.; Bochalya, R.S.; Bansal, K.K.; Sandhu, R. Improved Irrigation Practices for Higher Agricultural Productivity: A Review. Int. J. Environ. Clim. Change 2022, 12, 51–61. [Google Scholar] [CrossRef]
- Ram, M.; Kamboj, B.R.; Sharma, N. Suitability of Sprinkler and Drip Irrigation in Transplanted and Direct Seeded Rice (Oryza sativa L.) in Haryana, India. Int. J. Plant Soil Sci. 2024, 36, 711–719. [Google Scholar] [CrossRef]
- Esmail, A.A.; Ibrahim, M.A.; Abdallah, S.M.; Radwan, A.E.; Elsayed, M.A.; Elnakeib, N.A.; Dawoud, M.S. Smart irrigation system using IoT and machine learning methods. In Proceedings of the 5th Novel Intelligent and Leading Emerging Sciences Conference, NILES 2023—Proceedings, Giza, Egypt, 21–23 October 2023; pp. 362–367. [Google Scholar] [CrossRef]
- Ehsan, A.F.; Toraman, Ö. Surface coating of micronized calcite (CaCO3) with stearic acid via an attritor mill. Nigde Omer Halisdemir Univ. J. Eng. Sci. 2024, 13, 760–765. [Google Scholar] [CrossRef]
- Sullivan-Porras, J.; Badilla-Sánchez, M.; Rimolo-Donadio, R.; Masís-Meléndez, F. Crushed Glass and Oil Extracted from Palm Oil Sludge as Primary Materials in the Production of Hydrophobic Sand for Capillary Barrier Applications. Sustainability 2022, 14, 12770. [Google Scholar] [CrossRef]
- Leelamanie, D.A.L.; Karube, J.; Yoshida, A. Relative humidity effects on contact angle and water drop penetration time of hydrophobized fine sand. Soil Sci. Plant Nutr. 2008, 54, 695–700. [Google Scholar] [CrossRef]
- Subedi, S.; Kawamoto, K.; Jayarathna, L.; Vithanage, M.; Moldrup, P.; de Jonge, L.W.; Komatsu, T. Characterizing Time-Dependent Contact Angles for Sands Hydrophobized with Oleic and Stearic Acids. Vadose Zone J. 2012, 11, vzj2011-0055. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; He, Y. Facile preparation of superhydrophilic and superoleophobic sand for efficient oil-water separation. J. Water Process Eng. 2024, 61, 105355. [Google Scholar] [CrossRef]
- Smith, M.D.; Sikka, A.; Dirwai, T.L.; Mabhaudhi, T. Research and innovation in agricultural water management for a water-secure world. Irrig. Drain. 2023, 72, 1245–1259. [Google Scholar] [CrossRef]
- Kydyrbay, N.; Adotey, E.; Zhazitov, M.; Suiindik, Z.; Toktarbay, Z.; Nuraje, N.; Toktarbaiuly, O. Enhancing road durability and Safety: A study on Silica-Based Superhydrophobic coating for cement surfaces in road construction. Eng. Sci. 2024, 30, 1221. [Google Scholar] [CrossRef]
- Zilberman, D.; Huang, A.; Goldberg, L.; Reardon, T. The evolution of symbiotic innovation, water, and agricultural supply chains. Appl. Econ. Perspect. Policy 2023, 45, 1592–1603. [Google Scholar] [CrossRef]
- Pronti, A.; Auci, S.; Berbel, J. Water conservation and saving technologies for irrigation. A structured literature review of econometric studies on the determinants of adoption. Agric. Water Manag. 2024, 299, 108838. [Google Scholar] [CrossRef]
- Paschke, R.F.; Nordgren, R.; Wheeler, D.H. Conditioning of agricultural soils with fatty acid still pitch. J. Am. Oil Chem. Soc. 1957, 34, 149–151. [Google Scholar] [CrossRef]
- Śpitalniak, M.; Bogacz, A.; Zięba, Z. The assessment of water retention efficiency of different soil amendments in comparison to water absorbing geocomposite. Materials 2021, 14, 6658. [Google Scholar] [CrossRef]
- Adotey, E.; Kurbanova, A.; Ospanova, A.; Ardakkyzy, A.; Toktarbay, Z.; Kydyrbay, N.; Zhazitov, M.; Nuraje, N.; Toktarbaiuly, O. Development of Superhydrophobic Reduced Graphene Oxide (rGO) for Potential Applications in Advanced Materials. Nanomaterials 2025, 15, 363. [Google Scholar] [CrossRef]
- Zulkharnay, R.; Ualibek, O.; Toktarbaiuly, O.; May, P.W. Hydrophobic behaviour of reduced graphene oxide thin filmfabricated via electrostatic spray deposition. Bull. Mater. Sci. 2021, 44, 112. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Krishnan, M.R. Method of Making Multilayer Soil with Property for Extended Release Water for Extended Release for Desert Agriculture. U.S. Patent 10,772,265, 15 September 2020. [Google Scholar]
- Umar, W.; Balogh, J.; Hameed, M.K.; Ayub, M.A.; Anwaar, M.H.; Czinkota, I.; Gulyás, M. Reduction of nitrous oxide emission by using stearic acid combined zinc coated urea in silty clay and sandy loam soils under bare and planted conditions. Heliyon 2023, 9, e22578. [Google Scholar] [CrossRef]
- Thorsøe, M.H.; Keesstra, S.; De Boever, M.; Buchová, K.; Bøe, F.; Castanheira, N.L.; Chenu, C.; Cornu, S.; Don, A.; Fohrafellner, J.; et al. Sustainable soil management: Soil knowledge use and gaps in Europe. Eur. J. Soil Sci. 2023, 74, e13439. [Google Scholar] [CrossRef]
- Mamgain, H.P.; Pandey, J.K.; Brajpuriya, R. Aqua Barrier: Polypropylene/Stearic Acid-Infused Zn-ZnO-Cu Multilayer Superhydrophobic Coatings on Steel for Enhancing Anti-Microbial and Anti-Corrosion Efficiency. ECS J. Solid State Sci. Technol. 2024, 13, 123003. [Google Scholar] [CrossRef]
- Mofidabadi, A.H.J.; Bahlakeh, G.; Ramezanzadeh, B. Fabrication of a novel hydrophobic anti-corrosion film based on Eu2O3/stearic acid on steel surface; Experimental and detailed computer modeling studies. J. Taiwan Inst. Chem. Eng. 2020, 114, 228–240. [Google Scholar] [CrossRef]
- Mokobia, K.; Jonathan, E.M.; Oyiborhoro, G.; Maliki, M.; Ifijen, I.H. Environmental Degradation of Polymer-Based Composite Materials: Challenges and Mitigation Strategies. In The Minerals, Metals & Materials Series; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1218–1236. [Google Scholar] [CrossRef]
- Lisco, F.; Bukhari, F.; Uličná, S.; Isbilir, K.; Barth, K.L.; Taylor, A.; Walls, J.M. Degradation of Hydrophobic, Anti-Soiling Coatings for Solar Module Cover Glass. Energies 2020, 13, 3811. [Google Scholar] [CrossRef]
- Ferraro, J.R.; Nakamoto, K. Introductory Raman Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Bansal, N.P.; Doremus, R.H. Handbook of Glass Properties; Academic Press: Cambridge, MA, USA, 1986. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, M.; Zhazitov, M.; Kydyrbay, N.; Duisebayev, T.; Tezekbay, Y.; Toktarbaiuly, O. Stearic-Acid-Coated Sand: A Game Changer for Agriculture Water Management. Nanomaterials 2025, 15, 721. https://doi.org/10.3390/nano15100721
Abdullah M, Zhazitov M, Kydyrbay N, Duisebayev T, Tezekbay Y, Toktarbaiuly O. Stearic-Acid-Coated Sand: A Game Changer for Agriculture Water Management. Nanomaterials. 2025; 15(10):721. https://doi.org/10.3390/nano15100721
Chicago/Turabian StyleAbdullah, Muhammad, Mergen Zhazitov, Nazerke Kydyrbay, Tolagay Duisebayev, Yerbolat Tezekbay, and Olzat Toktarbaiuly. 2025. "Stearic-Acid-Coated Sand: A Game Changer for Agriculture Water Management" Nanomaterials 15, no. 10: 721. https://doi.org/10.3390/nano15100721
APA StyleAbdullah, M., Zhazitov, M., Kydyrbay, N., Duisebayev, T., Tezekbay, Y., & Toktarbaiuly, O. (2025). Stearic-Acid-Coated Sand: A Game Changer for Agriculture Water Management. Nanomaterials, 15(10), 721. https://doi.org/10.3390/nano15100721






