Doping Characteristics and Band Engineering of InSe for Advanced Photodetectors: A DFT Study
Abstract
1. Introduction
2. Materials and Methods
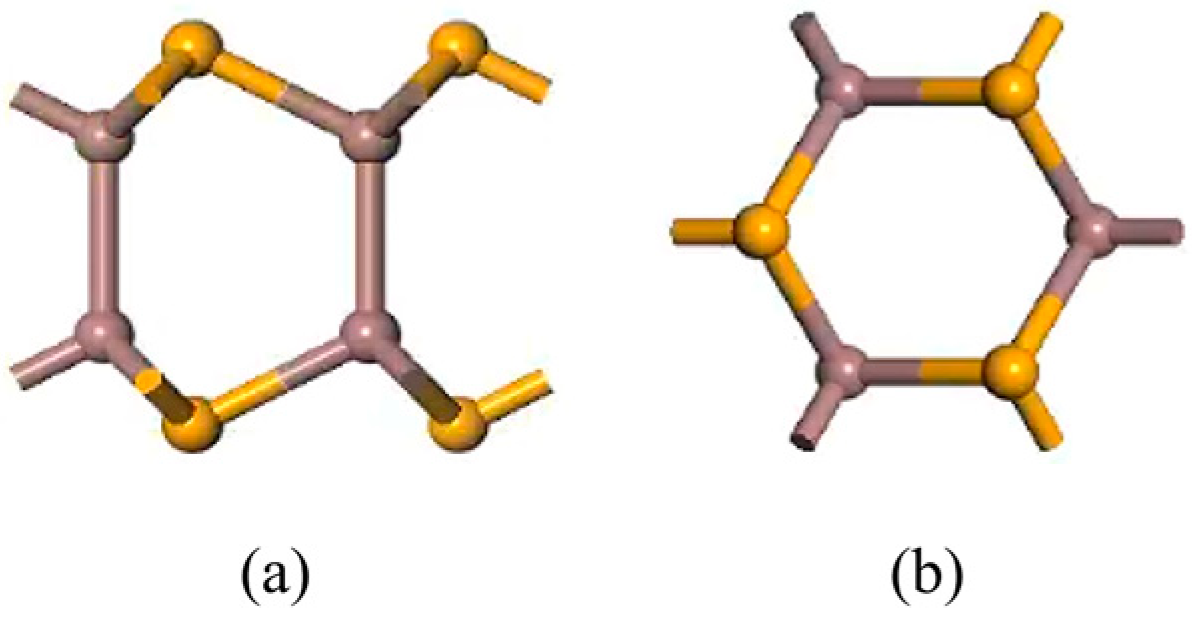
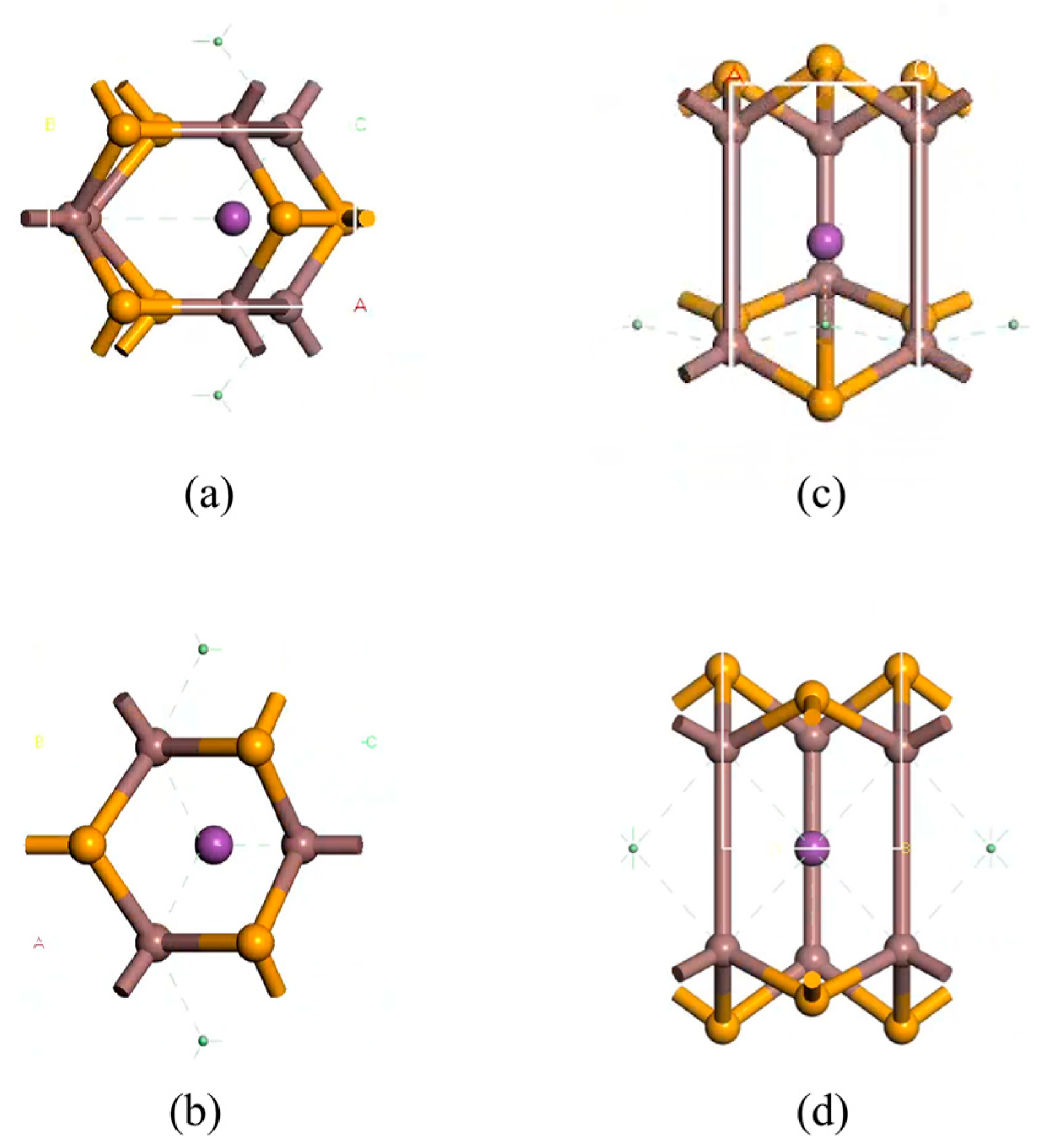
2.1. Structural Modeling and Calculations
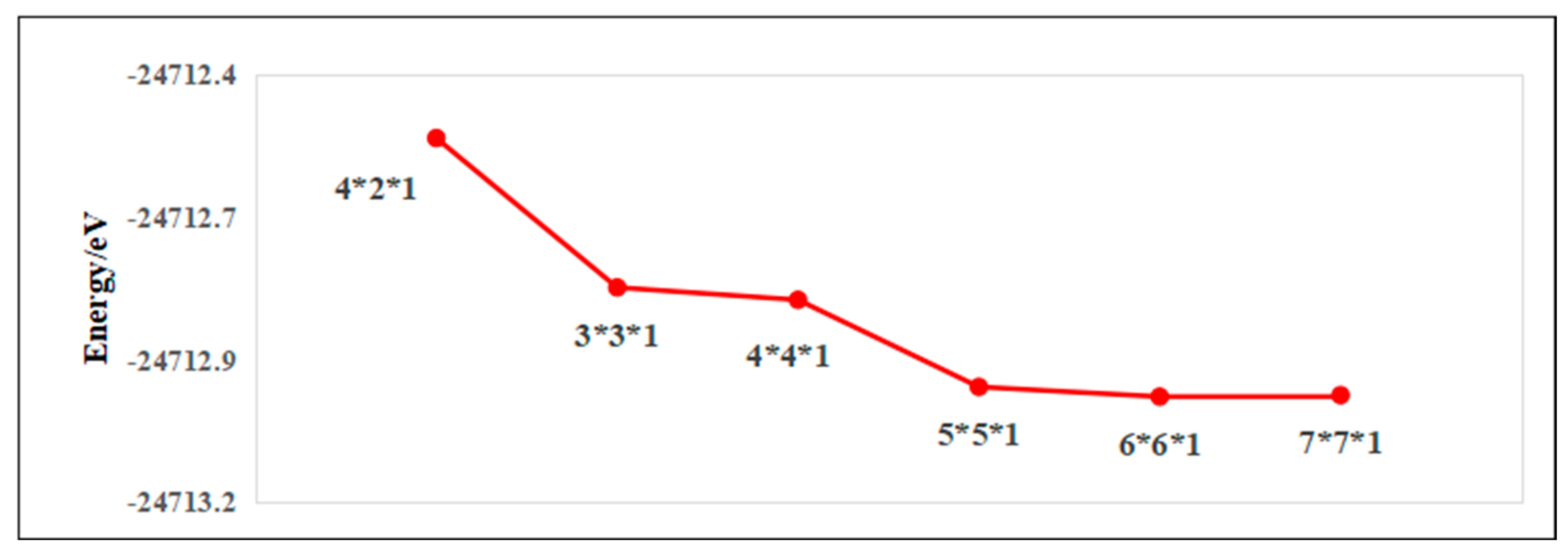
2.2. Parameter Settings and Convergence Tests
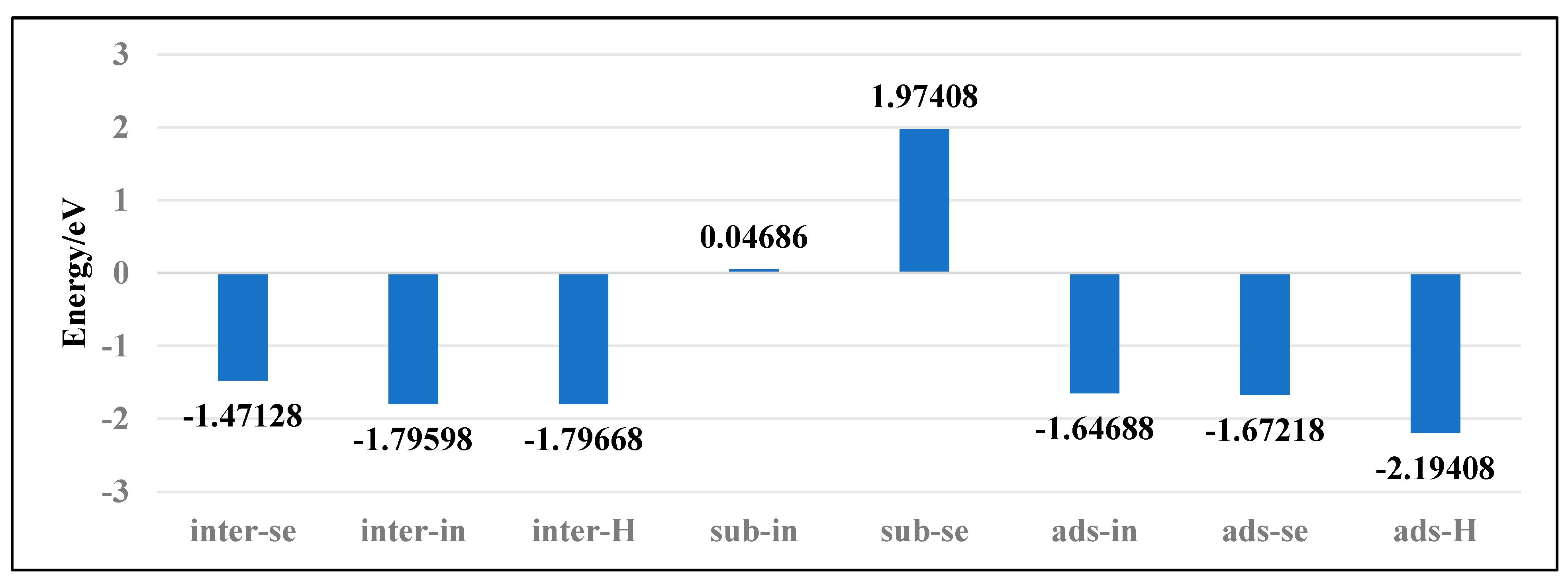
2.3. Binding Energy and Electron Differential Density
3. Results and Discussion
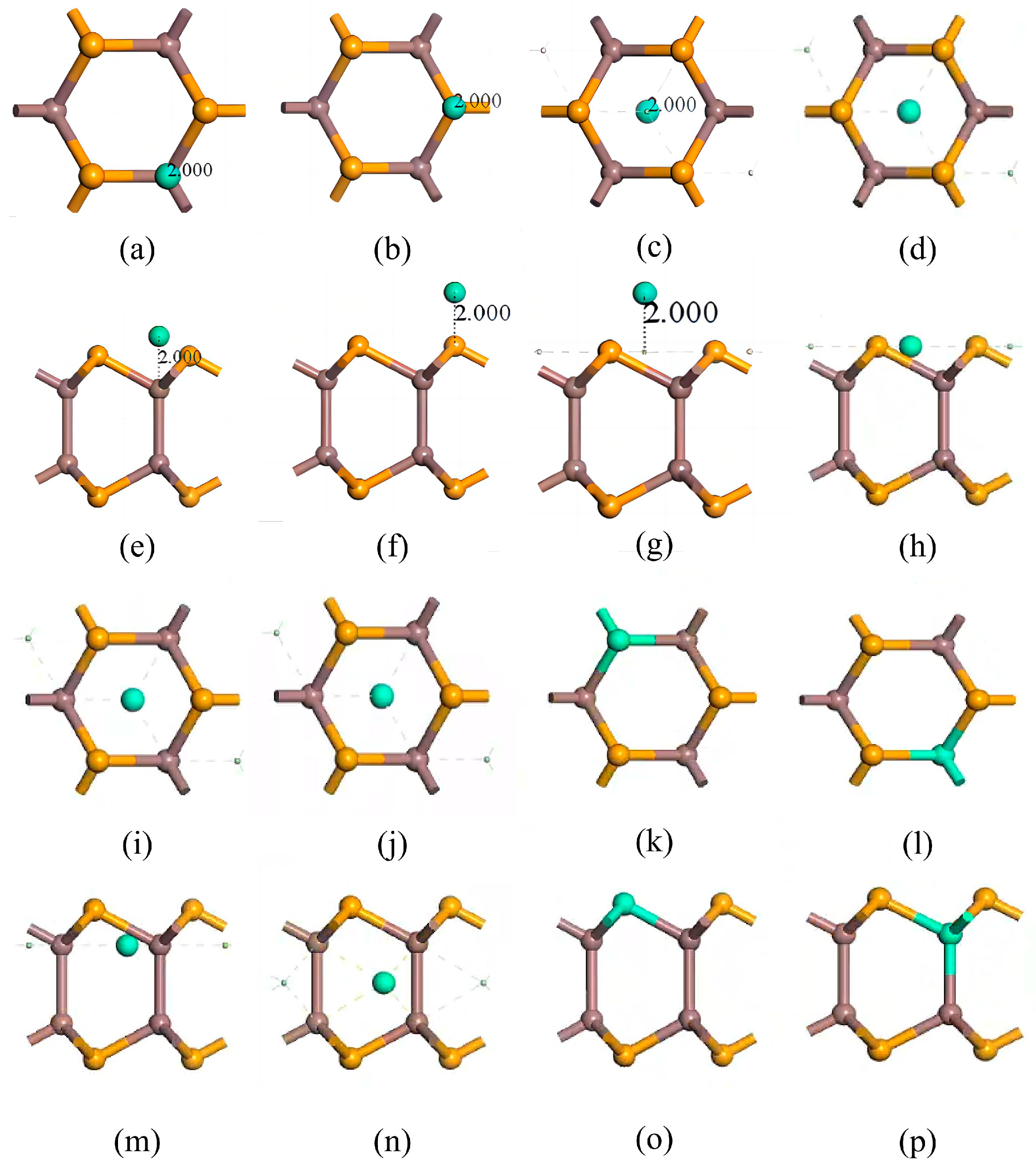
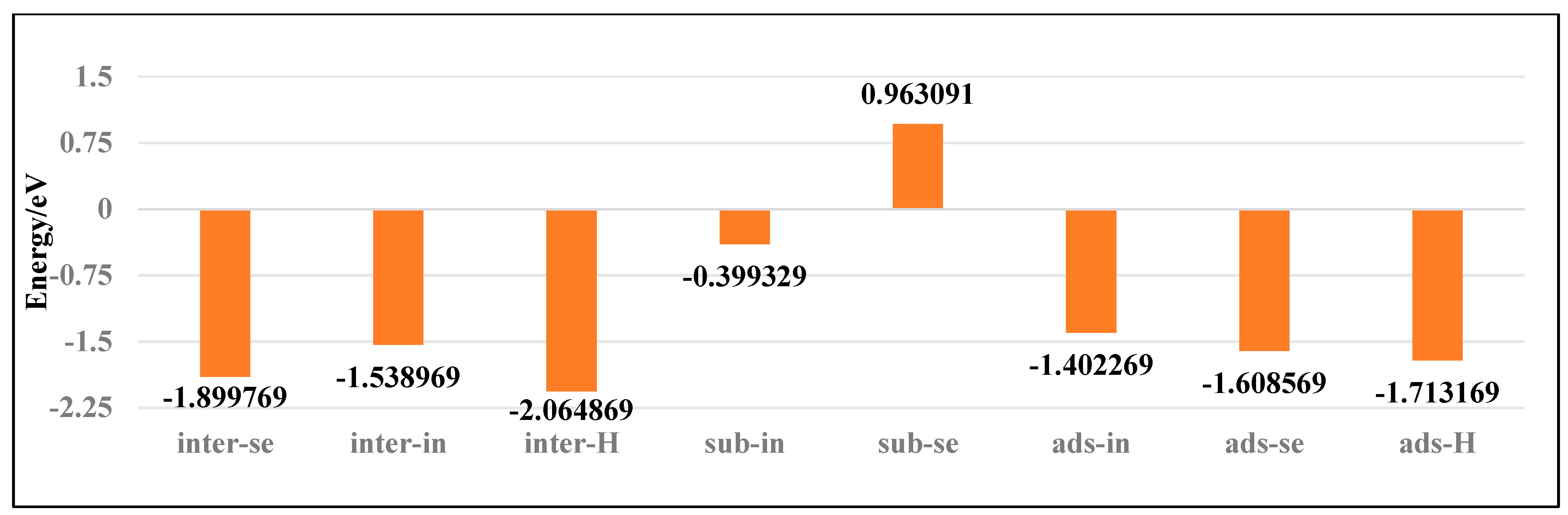
3.1. Doping Structures of InSe
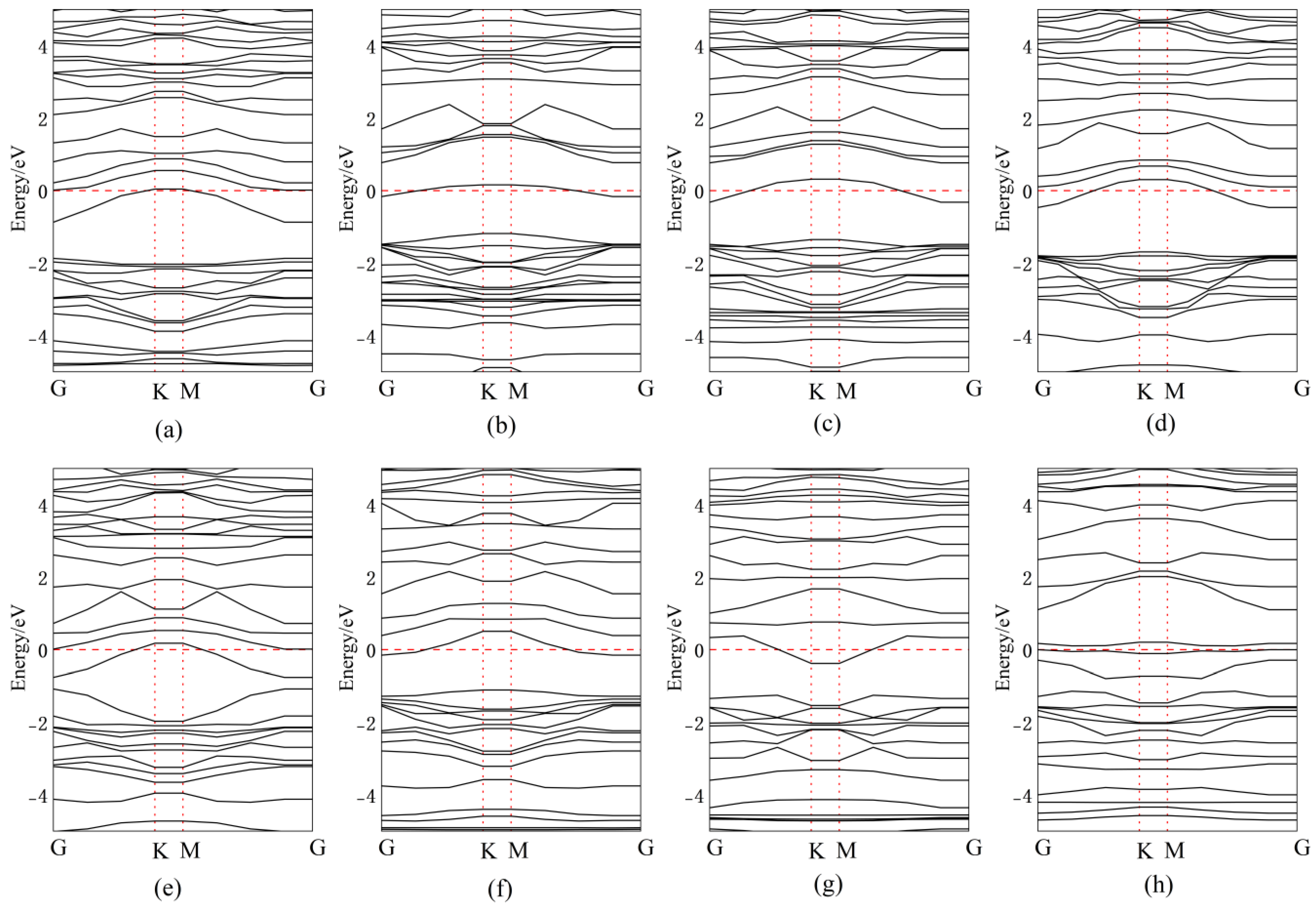
3.2. Band Structure Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, W.; Jiang, J.; Xie, X.; Pal, A.; Chu, J.H.; Kang, J.; Banerjee, K. 2-D Layered Materials for Next-Generation Electronics: Opportunities and Challenges. IEEE Trans. Electron Devices 2018, 65, 4109–4121. [Google Scholar] [CrossRef]
- Akinwande, D.; Huyghebaert, C.; Wang, C.H.; Serna, M.I.; Goossens, S.; Li, L.J.; Wong, H.; Koppens, F. Graphene and two-dimensional materials for silicon technology. Nature 2019, 573, 507–518. [Google Scholar] [CrossRef]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zheng, X.; Han, L.; Sun, Y.; Wang, L.; Li, Z.; Liu, W.; Liu, B.; Han, N.; Khan, S.; et al. High Performance Self-Powered Photodetectors Based on Graphene Nanoribbons/Al2O3/InGaZnO Heterojunctions. IEEE Photonics J. 2024, 16, 6801209. [Google Scholar] [CrossRef]
- Allain, A.; Kang, J.H.; Banerjee, K.; Kis, A. Electrical contacts to two-dimensional semiconductors. Nat. Mater. 2015, 14, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, S.; Gaddemane, G.; Van de Put, M.L.; Fischetti, M.V. Monte Carlo Study of Electronic Transport in Monolayer InSe. Materials 2019, 12, 4210. [Google Scholar] [CrossRef]
- Mudd, G.W.; Svatek, S.A.; Ren, T.; Patanè, A.; Makarovsky, O.; Eaves, L.; Beton, P.H.; Kovalyuk, Z.D.; Lashkarev, G.V.; Kudrynskyi, Z.R.; et al. Tuning the Bandgap of Exfoliated InSe Nanosheets by Quantum Confinement. Adv. Mater. 2013, 25, 5714. [Google Scholar] [CrossRef]
- Brotons-Gisbert, M.; Andres-Peuares, D.; Suh, J.; Hidalgo, F.; Abargues, R.; Rodríguez-Cantó, P.J.; Segura, A.; Cros, A.; Tobias, G.; Canadell, E.; et al. Nanotexturing To Enhance Photoluminescent Response of Atomically Thin Indium Selenide with Highly Tunable Band Gap. Nano Lett. 2016, 16, 3221–3229. [Google Scholar] [CrossRef]
- Bandurin, D.A.; Tyurnina, A.V.; Yu, G.L.; Mishchenko, A.; Zólyomi, V.; Morozov, S.V.; Kumar, R.K.; Gorbachev, R.V.; Kudrynskyi, Z.R.; Pezzini, S.; et al. High electron mobility, quantum Hall effect and anomalous optical response in atomically thin InSe. Nat. Nanotechnol. 2017, 12, 223–227. [Google Scholar] [CrossRef]
- Wei, X.; Liu, F.; Zhu, M.; Wang, L.; Sha, M. Theoretical Study of Halogen Anion Batteries With Ultra-ThinInSe. Int. J. Quantum Chem. 2025, 125, e70012. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Song, N.; Liu, Q.; Wang, S.; Xu, B.; Wang, F. Tailoring the interaction between metal atoms and two-dimensional materials through surface functionalization. AIP Adv. 2025, 15, 025302. [Google Scholar] [CrossRef]
- Felton, J.; Blundo, E.; Ling, S.; Glover, J.; Kudrynskyi, Z.R.; Makarovsky, O.; Kovalyuk, Z.D.; Besley, E.; Walker, G.; Polimeni, A.; et al. The Interaction of Hydrogen with the van der Waals Crystal γ-InSe. Molecules 2020, 25, 2526. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.K.; Wang, H.; Qiu, M.; Cao, R.; Guo, Z.; Zhao, J.; Li, Y.; Xiao, Q.; Fan, D.; Zhang, H. Two Dimensional β-InSe with Layer-Dependent Properties: Band Alignment, Work Function and Optical Properties. Nanomaterials 2019, 9, 82. [Google Scholar] [CrossRef]
- Hao, Q.; Li, P.; Liu, J.; Huang, J.; Zhang, W. Bandgap engineering of high mobility two-dimensional semiconductors toward optoelectronic devices. J. Mater. 2023, 9, 527–540. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Tian, Z.; Li, C.; Braun, K.; Huang, L.; Li, Y.; Luo, X.; Yi, J.; Wu, G.; et al. Sliding Ferroelectricity Induced Ultrafast Switchable Photovoltaic Response in ε-InSe Layers. Adv. Mater. 2024, 36, 9. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Qian, J.; Qiao, R.; Li, X.; Wang, Z.; Zheng, C.; Liu, K.; Cao, T.; Liu, W.T.; et al. Compelling Evidence for the ε-Phase InSe Crystal by Oblique Incident Second Harmonic Generation. Adv. Opt. Mater. 2022, 10, 2201183. [Google Scholar] [CrossRef]
- Segura, A. Layered Indium Selenide under High Pressure: A Review. Crystals 2018, 8, 206. [Google Scholar] [CrossRef]
- Li, X.; Xia, C.; Du, J.; Xiong, W. Magnetism induced by 3d transition metal atom doping in InSe monolayer. J. Mater. Sci. 2018, 53, 3500–3508. [Google Scholar] [CrossRef]
- Boukhvalov, D.; Gürbulak, B.; Duman, S.; Wang, L.; Politano, A.; Caputi, L.; Chiarello, G.; Cupolillo, A. The Advent of Indium Selenide: Synthesis, Electronic Properties, Ambient Stability and Applications. Nanomaterials 2017, 7, 372. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Tian, D.; Fu, Z.; Huang, Y.; Feng, W. Photoelectrochemical-Type Photodetectors Based on Ball Milling InSe for Underwater Optoelectronic Devices. Nanomaterials 2025, 15, 3. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Kudrynskyi, Z.R.; Fay, M.W.; Mudd, G.W.; Svatek, S.A.; Makarovsky, O.; Kovalyuk, Z.D.; Eaves, L.; Beton, P.H.; Patané, A. Room Temperature Electroluminescence from Mechanically Formed van der Waals III-VI Homojunctions and Heterojunctions. Adv. Opt. Mater. 2014, 2, 1064–1069. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.M.; Wu, M.; Cao, T.; Chen, Y.W.; Sankar, R.; Ulaganathan, R.K.; Chou, F.C.; Wetzel, C.; Xu, C.Y.; et al. Ultrasensitive tunability of the direct bandgap of 2D InSe flakes via strain engineering. 2D Mater. 2018, 5, 021002. [Google Scholar] [CrossRef]
- Song, C.; Fan, F.; Xuan, N.; Huang, S.; Zhang, G.; Wang, C.; Sun, Z.; Wu, H.; Yan, H. Largely Tunable Band Structures of Few-Layer InSe by Uniaxial Strain. ACS Appl. Mater. Interfaces 2018, 10, 3994–4000. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, Z.; Dong, Y.; Zhang, Z.; Xia, Y. A simulation optimization method for Verilog-AMS IBIS model under overclocking. Integr. VLSI J. 2025, 102, 102364. [Google Scholar] [CrossRef]
- Kudrynskyi, Z.R.; Mintyanskii, I.V.; Savitskii, P.I.; Kovalyuk, Z.D. Charge Carrier Transport in Van Der Waals Semiconductor InSe Intercalated with RbNO3 Probed by Direct Current Methods. Appl. Sci. 2021, 11, 5181. [Google Scholar] [CrossRef]
- Sorokin, S.V.; Avdienko, P.S.; Sedova, I.V.; Kirilenko, D.A.; Davydov, V.Y.; Komkov, O.S.; Firsov, D.D.; Ivanov, S.V. Molecular Beam Epitaxy of Layered Group III Metal Chalcogenides on GaAs(001) Substrates. Materials 2020, 13, 3447. [Google Scholar] [CrossRef]
- Chang, P.; Liu, X.; Liu, F.; Du, G. Remote Phonon Scattering in Two-Dimensional InSe FETs with High-κ Gate Stack. Micromachines 2018, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Lan, X.; Ao, Z.; Cao, J. Enhanced Optoelectronic Performance and Polarized Sensitivity in WSe2 Nanoscrolls Through Quasi-One-Dimensional Structure. Nanomaterials 2024, 14, 1935. [Google Scholar] [CrossRef]
- Sprincean, V.; Leontie, L.; Caraman, I.; Untila, D.; Girtan, M.; Gurlui, S.; Lisnic, P.; Doroftei, C.; Carlescu, A.; Iacomi, F.; et al. Optical and Photosensitive Properties of Flexible n (p)–InSe/In2O3 Heterojunctions. Materials 2022, 15, 3140. [Google Scholar] [CrossRef]
- Huang, X.; Cao, Q.; Wan, M.; Song, H. Electronic and Optical Properties of BP, InSe Monolayer and BP/InSe Heterojunction with Promising Photoelectronic Performance. Materials 2022, 15, 6214. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, L.; Qiu, C.; Peng, L. Ballistic two-dimensional InSe transistors. Nature 2023, 616, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qiu, X.; Huang, S.; Lin, S.; Liu, L.; Xiong, H. Adsorption and Sensing Properties of Ni-Modified InSe Monolayer Towards Toxic Gases: A DFT Study. Chemosensors 2024, 12, 219. [Google Scholar] [CrossRef]
- Wu, W.; Tiong, K.; Tan, S.; Hu, S.; Lee, Y.; Chen, R.; Wu, C. Optical Study on Temperature-Dependent Absorption Edge of γ-InSe-Layered Semiconductor. Appl. Sci. 2024, 14, 6676. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.S.; Lian, W.Y.; Hu, K.; Li, Q.J.; Cui, X.L.; Yao, T.Y.; Shen, L.F. Photo-Energized MoS2/CNT Cathode for High-Performance Li-CO2 Batteries in a Wide-Temperature Range. Nano-Micro Lett. 2025, 17, 5. [Google Scholar] [CrossRef]
- Fu, M.F.; Yang, W.; Du, K.X.; Li, J.B.; Zhang, Y.; Deng, S.K.; Yang, P.Z. Fabrication of Doped Black Phosphorus Crystal by the Chemical Vapor Transport Method. Cryst. Growth Des. 2022, 22, 7168–7175. [Google Scholar] [CrossRef]
- Wu, L.; Shi, J.; Zhou, Z.; Yan, J.; Wang, A.; Bian, C.; Ma, J.; Ma, R.; Liu, H.; Chen, J.; et al. InSe/hBN/graphite heterostructure for high-performance 2D electronics and flexible electronics. Nano Res. 2020, 13, 1127–1132. [Google Scholar] [CrossRef]
- Bianca, G.; Zappia, M.I.; Bellani, S.; Ghini, M.; Curreli, N.; Buha, J.; Galli, V.; Prato, M.; Soll, A.; Sofer, Z.; et al. Indium Selenide/Indium Tin Oxide Hybrid Films for Solution-Processed Photoelectrochemical-Type Photodetectors in Aqueous Media. Adv. Mater. Interfaces 2023, 10, 2201635. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Liang, Y.; Cai, X.H.; Du, J.X.; Wang, X.T.; Liu, X.F.; Wang, M.; Wei, Z.M.; Zhang, J.; Zhang, Q. Engineering Near-Infrared Light Emission in Mechanically Exfoliated InSe Platelets through Hydrostatic Pressure for Multicolor Microlasing. Nano Lett. 2022, 22, 3840–3847. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Jiang, Y.J.; Li, C.; Liang, Y.; Wei, Z.M.; Wei, X.D.; Zhang, Q. Probing Anisotropic Deformation and Near-Infrared Emission Tuning in Thin-Layered InSe Crystal under High Pressure. Nano Lett. 2023, 23, 3493–3500. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, Y.; Ning, Y.; Zhang, Z.; Li, H.; Xia, Y. Improved Implementation of Chua’s Circuit on an Active Inductor and Non-Autonomous System. Electronics 2024, 13, 2637. [Google Scholar] [CrossRef]
- Li, M.; Lin, C.Y.; Yang, S.H.; Chang, Y.M.; Chang, J.K.; Yang, F.S.; Zhong, C.; Jian, W.B.; Lien, C.H.; Ho, C.H.; et al. High Mobilities in Layered InSe Transistors with Indium-Encapsulation-Induced Surface Charge Doping. Adv. Mater. 2018, 30, 1803690. [Google Scholar] [CrossRef]
- Curreli, N.; Serri, M.; Spirito, D.; Lago, E.; Petroni, E.; Martín García, B.; Politano, A.; Gürbulak, B.; Duman, S.; Krahne, R.; et al. Liquid Phase Exfoliated Indium Selenide Based Highly Sensitive Photodetectors. Adv. Funct. Mater. 2020, 30, 1908427. [Google Scholar] [CrossRef]
- Liu, H.Q.; Yao, C.B.; Jiang, C.H. Horizontal and vertical stacked Ag/MoS2 nanostructure enabled excellent carrier mobility and optoelectronic properties. Opt. Laser Technol. 2022, 155, 108408. [Google Scholar] [CrossRef]
- Ma, Z.; Li, R.; Xiong, R.; Zhang, Y.; Xu, C.; Wen, C.; Sa, B. InSe/Te van der Waals Heterostructure as a High-Efficiency Solar Cell from Computational Screening. Materials 2021, 14, 3768. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Yang, T.K.; Hsu, M.E.; Hsu, K.Y.; Kondapavuluri, B.; Chyi, J.I. Fabrication of InAs 2D submonolayer nanostructure-based solar cells with InGaAs/GaAsSb double-well structure. Opt. Mater. 2025, 159, 116576. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Gong, Y.; Ke, C.; Guo, N.; Wu, J.; Zeng, X.; Guo, J.; Li, S.; Cheng, Z.; et al. Picometer-Level In Situ Manipulation of Ferroelectric Polarization in Van der Waals layered InSe. Adv. Mater. 2024, 36, 2404628. [Google Scholar] [CrossRef]
- Stepanov, R.S.; Marland, P.I.; Kolobov, A.V. Compositional and Structural Disorder in Two-Dimensional AIIIBVI Materials. Crystals 2023, 13, 1209. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, Y.; Jiang, Y.; Sui, L.; Chen, A.; Jin, M. Strain-induced variations in the Raman and infrared spectra of monolayer InSe: A first-principles study. J. Appl. Phys. 2024, 136, 065101. [Google Scholar] [CrossRef]
- Yan, S.; Li, H.; Liu, C.; Xiang, S.; Long, M. Strain regulation of the photoelectric performance of 2D InSe–AlAs vdW heterojunction: A DFT study. J. Phys. D Appl. Phys. 2025, 58, 75002. [Google Scholar] [CrossRef]
- Engel, E.; Höck, A.; Schmid, R.N.; Dreizler, R.M.; Chetty, N. Role of the core-valence interaction for pseudopotential calculations with exact exchange. Phys. Rev. B 2001, 64, 125111. [Google Scholar] [CrossRef]
- Voss, D.; Krüger, P.; Mazur, A.; Pollman, J. Atomic and electronic structure of WSe2 from ab initio theory: Bulk crystal and thin film systems. Phys. Rev. B 1999, 60, 14311–14317. [Google Scholar] [CrossRef]
- Hamann, D.R.; Schluter, M.; Chiang, C. Norm-Conserving Pseudopotentials. Phys. Rev. Lett. 1979, 43, 1494–1497. [Google Scholar] [CrossRef]
- Hu, H.W.; Sun, Y.L.; Chai, M.S.; Xie, D.; Ma, J.; Zhu, H.W. Room-temperature out-of-plane and in-plane ferroelectricity of two-dimensional β-InSe nanoflakes. Appl. Phys. Lett. 2019, 114, 252903. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, H.; Wang, W.; Huang, H. Polarization-Resolved and Helicity-Resolved Raman Intensity of Monolayer and Bilayer β-InSe. J. Phys. Chem. C 2022, 126, 11219–11228. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Avazli, N.; Durgun, E.; Cahangirov, S. Structural and electronic properties of monolayer group III monochalcogenides. Phys. Rev. B 2017, 95, 115409. [Google Scholar] [CrossRef]
- Abdullah; Afzal, U.; Choi, M.; Shah, N.A.; Chung, J.D. Analysis of various system designs for adsorption cycle in building heating applications. J. Build. Eng. 2024, 97, 110849. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Li, B.; Liu, S.P.; Liu, X.; Jiang, Q. Determining the adsorption energies of small molecules with the intrinsic properties of adsorbates and substrates. Nat. Commun. 2020, 11, 1196. [Google Scholar] [CrossRef]
- Mei, D.; Deskins, N.A.; Dupuis, M.; Ge, Q. Methanol adsorption on the clean CeO2(111) surface: A density functional theory study. J. Phys. Chem. C 2007, 111, 10514–10522. [Google Scholar] [CrossRef]
- Sun, Z.N.; Song, Y.X.; Lv, W.D.; Zeng, X.Y.; Fu, Z.T. Photocatalytic Mechanism and Charge Transfer of PtS2/WSe2 Heterostructures: First-principles Study. Bull. Chem. React. Eng. Catal. 2025, 20, 44–52. [Google Scholar] [CrossRef]
- Hoffmannostenhof, M.; Hoffmannostenhof, T. Schrodinger Inequalities and Asymptotic-Behavior of Electron-Density of Atoms and Molecules. Phys. Rev. A 1977, 16, 1782–1785. [Google Scholar] [CrossRef]
- Guillory, J.K. CRC Handbook of Chemistry and Physics, 2009–2010, 90th ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-9084-0. [Google Scholar]
- Al-Malah, K.I. Aqueous solubility of a diatomic molecule as a function of its size & electronegativity difference. J. Mol. Model. 2011, 17, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Petrini, N.; Asaithambi, A.; Rebecchi, L.; Curreli, N. Bismuth telluride iodide monolayer flakes with nonlinear optical response obtained via gold-assisted mechanical exfoliation. Opt. Mater. X 2023, 19, 100255. [Google Scholar] [CrossRef]
- He, T.H.; Zeng, X.S.; Rong, S.P. The controllable synthesis of substitutional and interstitial nitrogen-doped manganese dioxide: The effects of doping sites on enhancing the catalytic activity. J. Mater. Chem. A 2020, 8, 8383–8396. [Google Scholar] [CrossRef]
- Lu, Y.; Warner, J.H. Synthesis and Applications of Wide Bandgap 2D Layered Semiconductors Reaching the Green and Blue Wavelengths. ACS Appl. Electron. Mater. 2020, 2, 1777–1814. [Google Scholar] [CrossRef]
- Wei, X.; Dong, C.F.; Xu, A.N.; Li, X.G. First-principles study of the surface reparation of ultrathin InSe with Se-atom vacancies by thiol chemistry. Appl. Surf. Sci. 2019, 475, 487–493. [Google Scholar] [CrossRef]
- Wang, X.Y.; Jiang, Z.W.; Wang, P.; Chen, Z.; Sheng, T.; Wu, Z.C.; Xiong, Y.J. Ag+-Doped InSe Nanosheets for Membrane Electrode Assembly Electrolyzer toward Large-Current Electroreduction of CO2 to Ethanol. Angew. Chem. Int. Edit. 2023, 62, e202313646. [Google Scholar] [CrossRef]


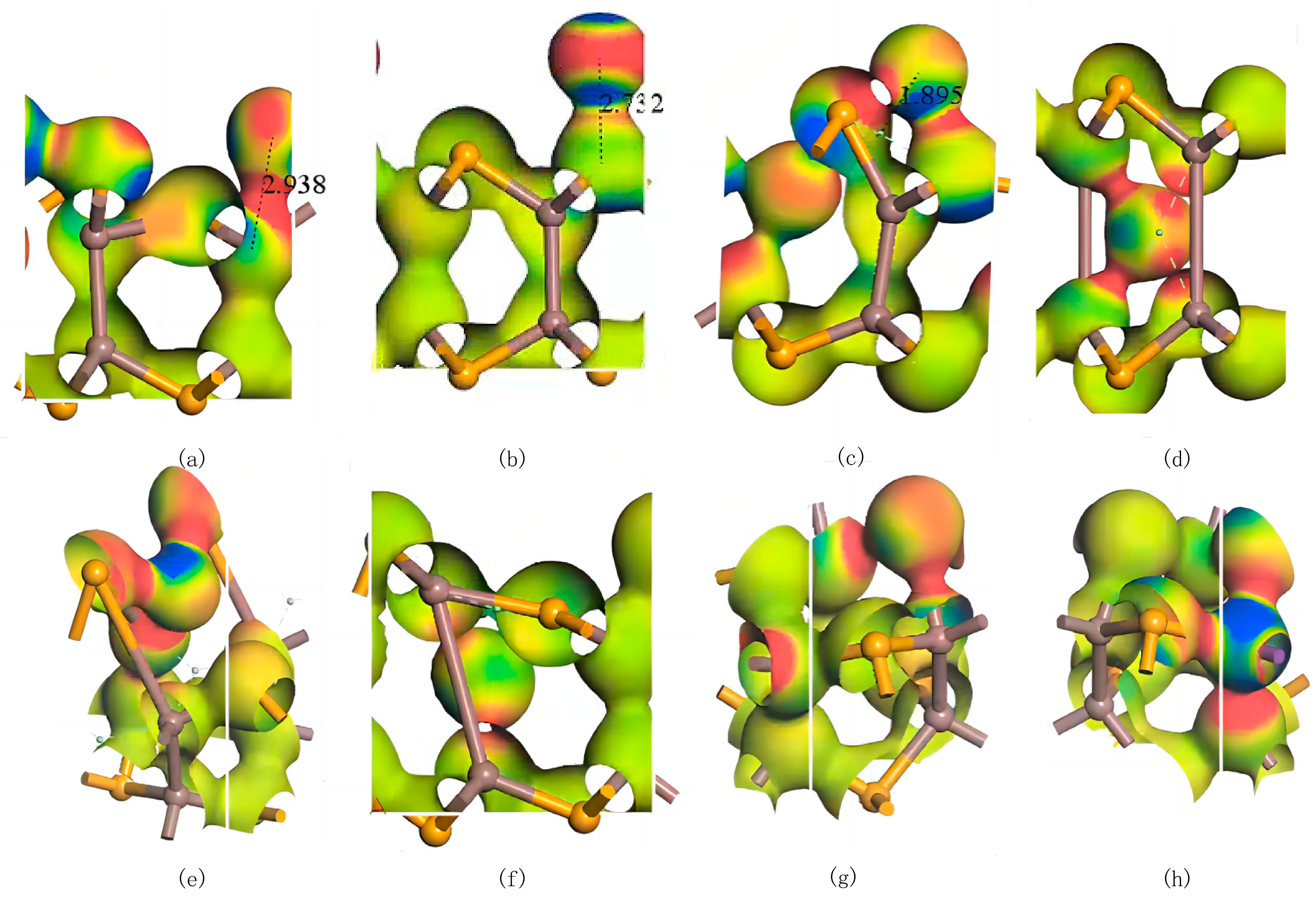

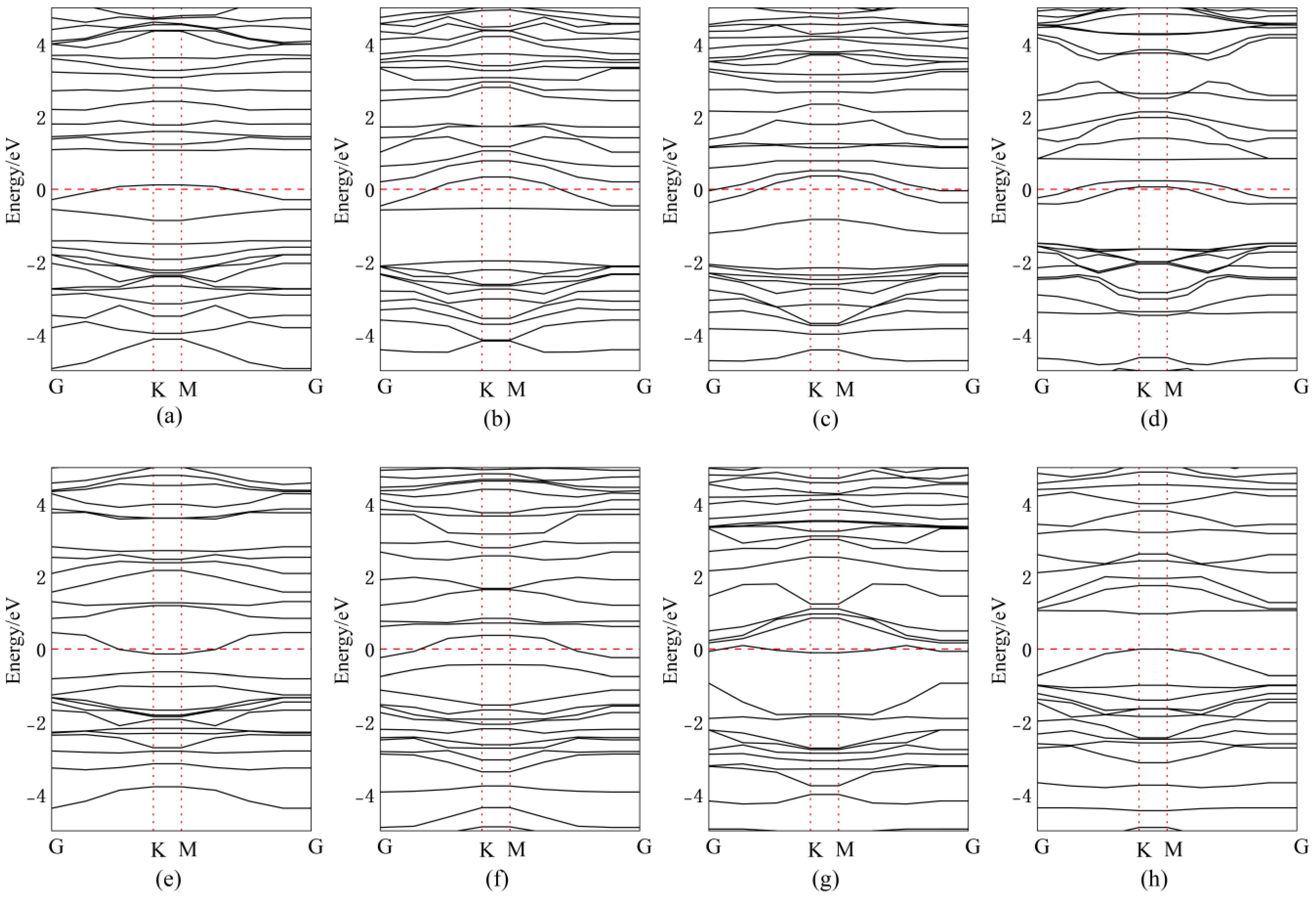
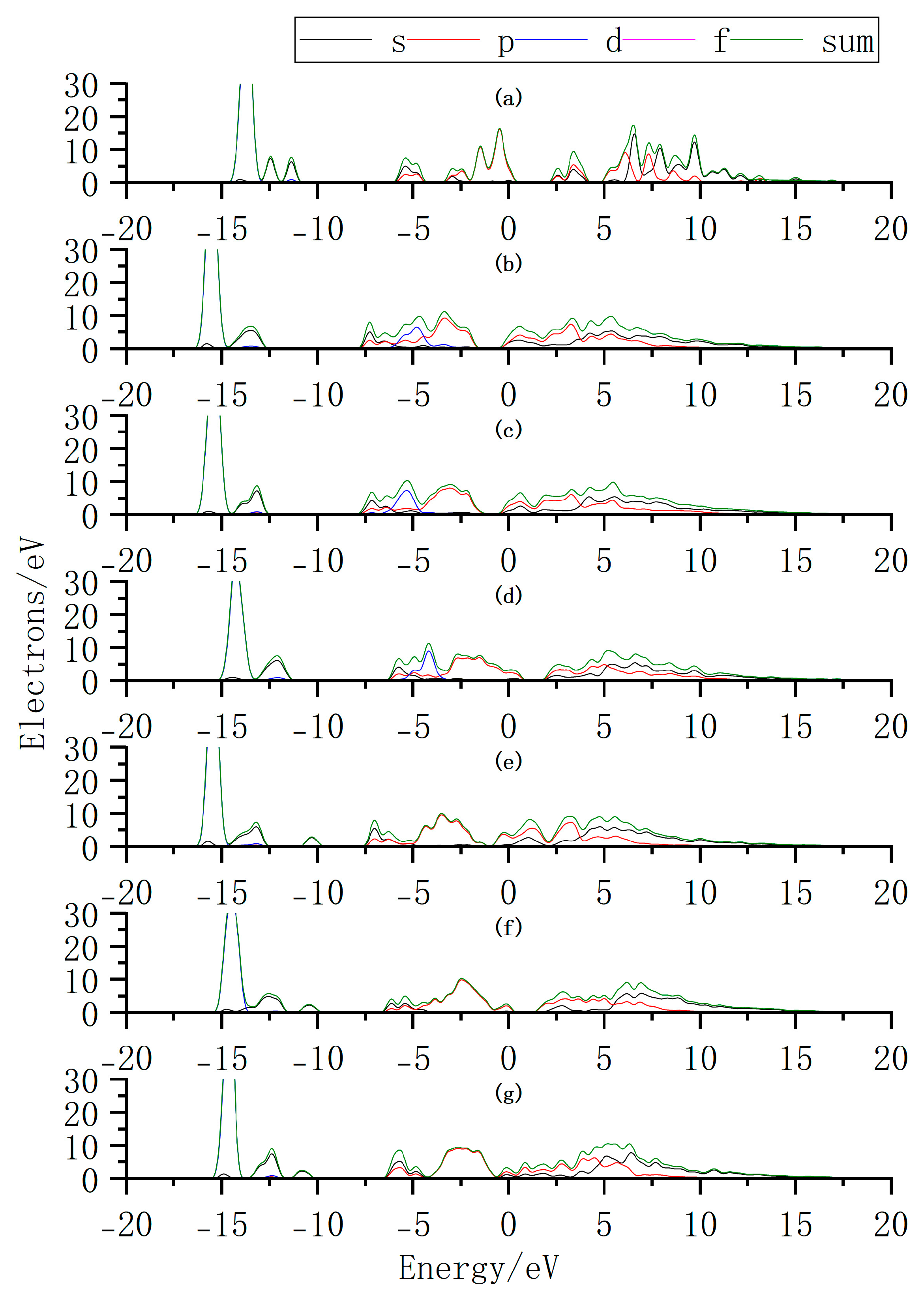
| Doping Position | Eg | △E | Bandgap Type |
|---|---|---|---|
| ads-In | 1.01 eV | −0.50 eV | Direct bandgap |
| ads-Se | 0.98 eV | −0.52 eV | Indirect bandgap |
| ads-H | 0.96 eV | −0.54 eV | Indirect bandgap |
| inter-In | 0.86 eV | −0.65 eV | Indirect bandgap |
| inter-Se | 0.29 eV | −1.22 eV | Direct bandgap |
| inter-H | 1.16 eV | −0.35 eV | Indirect bandgap |
| sub-In | 0.83 eV | −0.68 eV | Indirect bandgap |
| sub-Se | 0.84 eV | −0.67 eV | Indirect bandgap |
| Doping Position | Eg | △E | Bandgap Type |
|---|---|---|---|
| ads-In | 0.92 eV | −0.59 eV | Direct bandgap |
| ads-Se | 1.49 eV | −0.02 eV | Direct bandgap |
| ads-H | 0.73 eV | −0.78 eV | Direct bandgap |
| inter-In | 0.19 eV | −1.32 eV | Direct bandgap |
| inter-Se | 0.49 eV | −1.02 eV | Direct bandgap |
| inter-H | 1.05 eV | −0.46 eV | Indirect bandgap |
| sub-In | 0.98 eV | −0.53 eV | Indirect bandgap |
| sub-Se | 0.85 eV | −0.66 eV | Direct bandgap |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ning, Y.; Li, H.; Xu, C.; Wang, Y.; Xia, Y. Doping Characteristics and Band Engineering of InSe for Advanced Photodetectors: A DFT Study. Nanomaterials 2025, 15, 720. https://doi.org/10.3390/nano15100720
Zhang W, Ning Y, Li H, Xu C, Wang Y, Xia Y. Doping Characteristics and Band Engineering of InSe for Advanced Photodetectors: A DFT Study. Nanomaterials. 2025; 15(10):720. https://doi.org/10.3390/nano15100720
Chicago/Turabian StyleZhang, Wenkai, Yafei Ning, Hu Li, Chaoqian Xu, Yong Wang, and Yuhan Xia. 2025. "Doping Characteristics and Band Engineering of InSe for Advanced Photodetectors: A DFT Study" Nanomaterials 15, no. 10: 720. https://doi.org/10.3390/nano15100720
APA StyleZhang, W., Ning, Y., Li, H., Xu, C., Wang, Y., & Xia, Y. (2025). Doping Characteristics and Band Engineering of InSe for Advanced Photodetectors: A DFT Study. Nanomaterials, 15(10), 720. https://doi.org/10.3390/nano15100720








