Proliferation of Human Cervical Cancer Cells Responds to Surface Properties of Bicomponent Polymer Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Surfaces
2.2. Cell Culture
2.3. Atomic Force Microscopy
2.4. Contact Angle Measurements and Surface Energy Determination
2.5. Light Microscopy for Observing Cell Proliferation
2.6. Crystal Violet Staining for Quantifying Cell Growth
2.7. Cell Viability and Cellular Morphology
3. Results
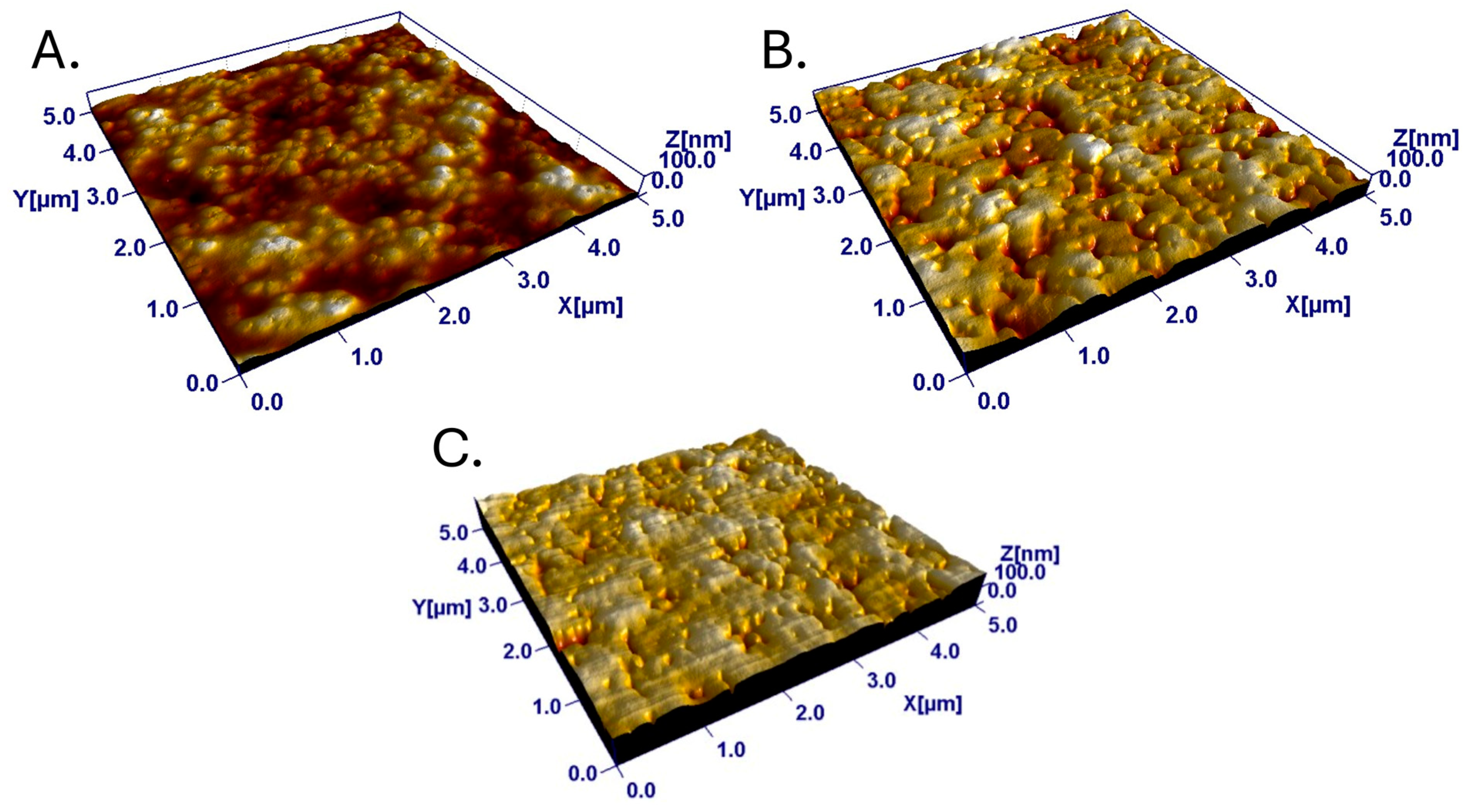
3.1. Properties of the Nanostructured Polymeric Surfaces
3.1.1. Surface Chemistry of the Polymer Coatings
3.1.2. Surface Properties of the Blend Films
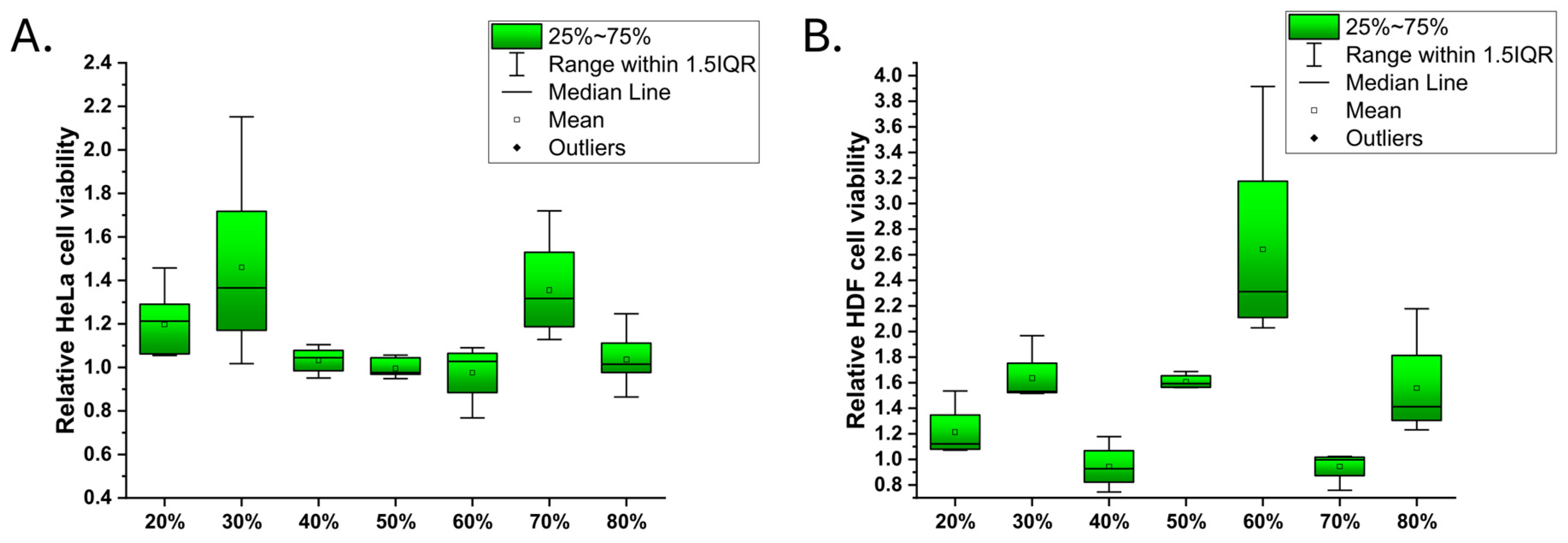
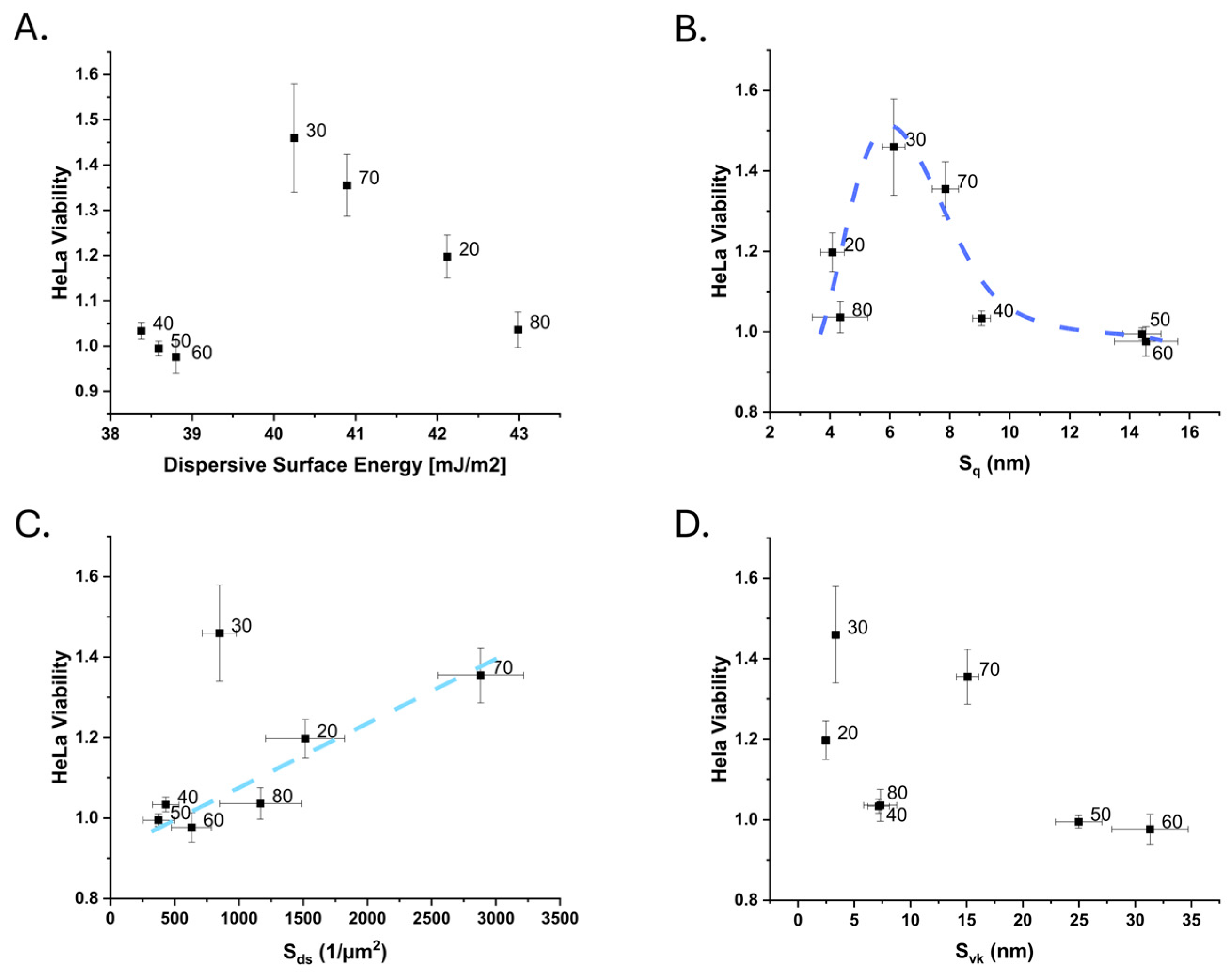
3.2. Cell Growth on the Latex Surfaces and Reference Materials
Cell Morphology and Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile butadiene styrene |
| AFM | Atomic force microscopy |
| CA | Contact angle |
| CAF | Cancer-associated fibroblast |
| CV | Crystal violet |
| DAPI | 4’,6-diamidino-2-phenylindole |
| DIM | Diiodo methane |
| DMEM | Dulbecco’s modified Eagle medium |
| EG | Ethylene glycol |
| HDF | Human dermal fibroblast |
| Hela | Human cervical cancer cells |
| IR | Infrared |
| PS | Polystyrene |
| Sa | Arithmetic average roughness |
| Scl37 | Correlation length to 37% |
| Sdr | Effective surface area |
| SDS | Sodium dodecyl sulphate |
| Sfd | Fractal dimension |
| Sk | Core roughness |
| Sku | Kurtosis |
| Sq | Root mean square height variation roughness |
| Spd | Peak density |
| Spk | Reduced peak height |
| Ssk | Skewness |
| Svk | Reduced valley depth |
| Tg | Glass transition temperature |
References
- Li, Y.; Yin, X.; Changsheng, L. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef] [PubMed]
- Rostam, H.M.; Singh, S.; Vrana, N.E.; Alexander, M.R.; Ghaemmaghami, A.M. Impact of Surface Chemistry and Topography on the Function of Antigen Presenting Cells. Biomater. Sci. 2015, 3, 424–441. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface Topographical Factors Influencing Bacterial Attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef]
- Webb, H.K.; Truong, V.K.; Hasan, J.; Fluke, C.; Crawford, R.J.; Ivanova, E.P. Roughness Parameters for Standard Description of Surface Nanoarchitecture. Scanning 2012, 34, 257–263. [Google Scholar] [CrossRef]
- Whitehouse, D.J. Handbook of Surface and Nanometrology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-14069-3. [Google Scholar]
- Ferreira, F.V.; Sassi, L.M.; de Souza Camargo, S., Jr. A Computer Simulation Study of Extended DLVO Interactions between Calcite Nanoparticles and Real Rough Surfaces. Surf. Coat. Technol. 2023, 457, 129308. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of Tumor Microenvironment in Tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Morán, M.C.; Ruano, G.; Cirisano, F.; Ferrari, M. Mammalian Cell Viability on Hydrophobic and Superhydrophobic Fabrics. Mater. Sci. Eng. C 2019, 99, 241–247. [Google Scholar] [CrossRef]
- Rahbari, R.; Sheahan, T.; Modes, V.; Collier, P.; Macfarlane, C.; Badge, R.M. A Novel L1 Retrotransposon Marker for HeLa Cell Line Identification. BioTechniques 2009, 46, 277–284. [Google Scholar] [CrossRef]
- Lin, J.; Liu, X.; Ding, D. Evidence for Epithelial-Mesenchymal Transition in Cancer Stem-like Cells Derived from Carcinoma Cell Lines of the Cervix Uteri. Int. J. Clin. Exp. Pathol. 2015, 8, 847–855. [Google Scholar]
- Best, J.P.; Javed, S.; Richardson, J.J.; Cho, K.L.; Kamphuis, M.M.J.; Caruso, F. Stiffness-Mediated Adhesion of Cervical Cancer Cells to Soft Hydrogel Films. Soft Matter 2013, 9, 4580–4584. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth—Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Schneider, A.; Francius, G.; Obeid, R.; Schwinté, P.; Hemmerlé, J.; Frisch, B.; Schaaf, P.; Voegel, J.-C.; Senger, B.; Picart, C.; et al. Polyelectrolyte Multilayers with a Tunable Young’s Modulus: Influence of Film Stiffness on Cell Adhesion. Langmuir ACS J. Surf. Colloids 2006, 22, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Schrade, A.; Mailänder, V.; Ritz, S.; Landfester, K.; Ziener, U. Surface Roughness and Charge Influence the Uptake of Nanoparticles: Fluorescently Labeled Pickering-Type versus Surfactant-Stabilized Nanoparticles. Macromol. Biosci. 2012, 12, 1459–1471. [Google Scholar] [CrossRef]
- Han, J.; Menon, N.V.; Kang, Y.; Tee, S.-Y. An in Vitro Study on the Collective Tumor Cell Migration on Nanoroughened Poly(Dimethylsiloxane) Surfaces. J. Mater. Chem. B 2015, 3, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Haga, H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers 2022, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, E.; Niemelä, E.; Frisk, J.; Öblom, H.; Koppolu, R.; Abdelkader, H.; Soto Véliz, D.; Mennillo, M.; Venu, A.P.; Ihalainen, P.; et al. A Low-Cost Paper-Based Platform for Fast and Reliable Screening of Cellular Interactions with Materials. J. Mater. Chem. B 2020, 8, 1146–1156. [Google Scholar] [CrossRef]
- Rosqvist, E.; Böcker, U.; Gulin-Sarfraz, T.; Afseth, N.K.; Tolvanen, S.; Peltonen, J.; Sarfraz, J. Low-Cost, Mass-Producible Nanostructured Surface on Flexible Substrate with Ultra-Thin Gold or Silver Film for SERS Applications. Nano-Struct. Nano-Objects 2023, 34, 100956. [Google Scholar] [CrossRef]
- Järnström, J.; Ihalainen, P.; Backfolk, K.; Peltonen, J. Roughness of Pigment Coatings and Its Influence on Gloss. Appl. Surf. Sci. 2008, 254, 5741–5749. [Google Scholar] [CrossRef]
- Rosqvist, E.; Niemelä, E.; Venu, A.P.; Kummala, R.; Ihalainen, P.; Toivakka, M.; Eriksson, J.E.; Peltonen, J. Human Dermal Fibroblast Proliferation Controlled by Surface Roughness of Two-Component Nanostructured Latex Polymer Coatings. Colloids Surf. B Biointerfaces 2019, 174, 136–144. [Google Scholar] [CrossRef]
- San-Martin-Galindo, P.; Rosqvist, E.; Tolvanen, S.; Miettinen, I.; Savijoki, K.; Nyman, T.A.; Fallarero, A.; Peltonen, J. Modulation of Virulence Factors of Staphylococcus aureus by Nanostructured Surfaces. Mater. Des. 2021, 208, 109879. [Google Scholar] [CrossRef]
- Juvonen, H.; Määttänen, A.; Ihalainen, P.; Viitala, T.; Sarfraz, J.; Peltonen, J. Enhanced Protein Adsorption and Patterning on Nanostructured Latex-Coated Paper. Colloids Surf. B Biointerfaces 2014, 118, 261–269. [Google Scholar] [CrossRef]
- Juvonen, H.; Oja, T.; Määttänen, A.; Sarfraz, J.; Rosqvist, E.; Riihimäki, T.A.; Toivakka, M.; Kulomaa, M.; Vuorela, P.; Fallarero, A.; et al. Protein and Bacterial Interactions with Nanostructured Polymer Coatings. Colloids Surf. B Biointerfaces 2015, 136, 527–535. [Google Scholar] [CrossRef]
- Spiro, J.G.; Farinha, J.P.S.; Winnik, M.A. Thermodynamics and Morphology of Latex Blend Films. Macromolecules 2003, 36, 7791–7802. [Google Scholar] [CrossRef]
- Peltonen, J.; Järn, M.; Areva, S.; Linden, M.; Rosenholm, J.B. Topographical Parameters for Specifying a Three-Dimensional Surface. Langmuir 2004, 20, 9428–9431. [Google Scholar] [CrossRef]
- ISO 16610-21:2011; Geometrical product specifications (GPS)—Filtration, Part 21: Linear Profile Filters: Gaussian Filters. International Organization for Standardization: Geneva, Switzerland, 2011.
- Whitehouse, D. 3—Profile and Areal (3D) Parameter Characterization. In Surfaces and Their Measurement; Whitehouse, D., Ed.; Kogan Page Science: Oxford, UK, 2002; pp. 48–95. ISBN 978-1-903996-01-0. [Google Scholar]
- Żak, K.; Grzesik, W. Metrological Aspects of Surface Topographies Produced by Different Machining Operations Regarding Their Potential Functionality. Metrol. Meas. Syst. 2017, 24, 325–335. [Google Scholar] [CrossRef]
- Makkonen, L. Faulty Intuitions of Wetting. Int. J. Wettability Sci. Technol. 2018, 1, 13–30. [Google Scholar]
- Makkonen, L. Young’s Equation Revisited. J. Phys. Condens. Matter 2016, 28, 135001. [Google Scholar] [CrossRef]
- Shardt, N.; Elliott, J.A.W. Gibbsian Thermodynamics of Wenzel Wetting (Was Wenzel Wrong? Revisited). Langmuir 2020, 36, 435–446. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van Der Waals and Polar Interactions in Macroscopic Systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Oss, C.J.V.; Good, R.J.; Chaudhury, M.K. The Role of van Der Waals Forces and Hydrogen Bonds in “Hydrophobic Interactions” between Biopolymers and Low Energy Surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Kelpsch, D.J.; Tootle, T.L. Nuclear Actin: From Discovery to Function. Anat. Rec. 2018, 301, 1999–2013. [Google Scholar] [CrossRef]
- Girshovitz, P.; Shaked, N.T. Generalized Cell Morphological Parameters Based on Interferometric Phase Microscopy and Their Application to Cell Life Cycle Characterization. Biomed. Opt. Express 2012, 3, 1757–1773. [Google Scholar] [CrossRef]
- Cadart, C.; Zlotek-Zlotkiewicz, E.; Berre, M.L.; Piel, M.; Matthews, H.K. Exploring the Function of Cell Shape and Size during Mitosis. Dev. Cell 2014, 29, 159–169. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, Y.; Mei, J.; Zhao, J.; Miao, C.; Jiu, Y. Interactive Mechanisms between Caveolin-1 and Actin Filaments or Vimentin Intermediate Filaments Instruct Cell Mechanosensing and Migration. J. Mol. Cell Biol. 2023, 14, mjac066. [Google Scholar] [CrossRef]
- Smoler, M.; Coceano, G.; Testa, I.; Bruno, L.; Levi, V. Apparent Stiffness of Vimentin Intermediate Filaments in Living Cells and Its Relation with Other Cytoskeletal Polymers. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2020, 1867, 118726. [Google Scholar] [CrossRef]
- Jensen, M.H.; Morris, E.J.; Goldman, R.D.; Weitz, D.A. Emergent Properties of Composite Semiflexible Biopolymer Networks. BioArchitecture 2014, 4, 138–143. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin Structure and Function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Kloc, M.; Chanana, P.; Vaughn, N.; Uosef, A.; Kubiak, J.Z.; Ghobrial, R.M. New Insights into Cellular Functions of Nuclear Actin. Biology 2021, 10, 304. [Google Scholar] [CrossRef]
- Kumari, R.; Ven, K.; Chastney, M.; Kokate, S.B.; Peränen, J.; Aaron, J.; Kogan, K.; Almeida-Souza, L.; Kremneva, E.; Poincloux, R.; et al. Focal Adhesions Contain Three Specialized Actin Nanoscale Layers. Nat. Commun. 2024, 15, 2547. [Google Scholar] [CrossRef]
- Tsuruta, D.; Jones, J.C.R. The Vimentin Cytoskeleton Regulates Focal Contact Size and Adhesion of Endothelial Cells Subjected to Shear Stress. J. Cell Sci. 2003, 116, 4977–4984. [Google Scholar] [CrossRef]
- Lou, H.-Y.; Zhao, W.; Li, X.; Duan, L.; Powers, A.; Akamatsu, M.; Santoro, F.; McGuire, A.F.; Cui, Y.; Drubin, D.G.; et al. Membrane Curvature Underlies Actin Reorganization in Response to Nanoscale Surface Topography. Proc. Natl. Acad. Sci. USA 2019, 116, 23143–23151. [Google Scholar] [CrossRef]
- Record, J.; Saeed, M.B.; Venit, T.; Percipalle, P.; Westerberg, L.S. Journey to the Center of the Cell: Cytoplasmic and Nuclear Actin in Immune Cell Functions. Front. Cell Dev. Biol. 2021, 9, 682294. [Google Scholar] [CrossRef]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-Actin Cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent Advance in Surface Modification for Regulating Cell Adhesion and Behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Mechanical Strength of Glass. In Glass; John Wiley & Sons, Ltd.: London, UK, 2007; pp. 135–159. ISBN 978-3-527-61702-9.
- Walcott, S.; Sun, S.X. A Mechanical Model of Actin Stress Fiber Formation and Substrate Elasticity Sensing in Adherent Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 7757–7762. [Google Scholar] [CrossRef]
- Zhou, K.; Li, Y.; Zhang, L.; Jin, L.; Yuan, F.; Tan, J.; Yuan, G.; Pei, J. Nano-Micrometer Surface Roughness Gradients Reveal Topographical Influences on Differentiating Responses of Vascular Cells on Biodegradable Magnesium. Bioact. Mater. 2021, 6, 262–272. [Google Scholar] [CrossRef]
- Gentile, F.; Tirinato, L.; Battista, E.; Causa, F.; Liberale, C.; Fabrizio, E.M.d.; Decuzzi, P. Cells Preferentially Grow on Rough Substrates. Biomaterials 2010, 31, 7205–7212. [Google Scholar] [CrossRef]
- Bhushan, B. Surface Roughness Analysis and Measurement Techniques. In Modern Tribology Handbook; CRC Press: Boca Raton, FL, USA, 2000; pp. 49–119. [Google Scholar]
- Hansson, K.N.; Hansson, S. Skewness and Kurtosis: Important Parameters in the Characterization of Dental Implant Surface Roughness—A Computer Simulation. ISRN Mater. Sci. 2011, 2011, 305312. [Google Scholar] [CrossRef]
- Ong, S.-E.; Zhang, S.; Du, H.; Wang, Y.; Ma, L.-L. In-Vitro Cellular Behavior on Amorphous Carbon Containing Silicon. Thin Solid Films 2008, 516, 5152–5156. [Google Scholar] [CrossRef]
- Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface Free Energy Predominates in Cell Adhesion to Hydroxyapatite through Wettability. Mater. Sci. Eng. C 2016, 62, 283–292. [Google Scholar] [CrossRef]
- Kubies, D.; Himmlová, L.; Riedel, T.; Mázl Chánová, E.; Balík, K.; Douděrová, M.; Bártová, J.; Pešáková, V. The Interaction of Osteoblasts With Bone-Implant Materials: 1. The Effect of Physicochemical Surface Properties of Implant Materials. Physiol. Res. Acad. Sci. Bohemoslov. 2010, 60, 95–111. [Google Scholar] [CrossRef]
- Satriano, C.; Carnazza, S.; Guglielmino, S.; Marletta, G. Surface Free Energy and Cell Attachment onto Ion-Beam Irradiated Polymer Surfaces. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 287–293. [Google Scholar] [CrossRef]
- Boulange-Petermann, L.; Baroux, B.; Bellon-Fontaine, M.-N. The Influence of Metallic Surface Wettability on Bacterial Adhesion. J. Adhes. Sci. Technol. 1993, 7, 221–230. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q. The CQ Ratio of Surface Energy Components Influences Adhesion and Removal of Fouling Bacteria. Biofouling 2011, 27, 275–285. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q. Influence of Surface-Energy Components of Ni–P–TiO2–PTFE Nanocomposite Coatings on Bacterial Adhesion. Langmuir 2011, 27, 9512–9519. [Google Scholar] [CrossRef]
- Whitehouse, D.J. Fractal or Fiction. Wear 2001, 249, 345–353. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosqvist, E.; Niemelä, E.; Liang, S.; Eriksson, J.E.; Wang, X.; Peltonen, J. Proliferation of Human Cervical Cancer Cells Responds to Surface Properties of Bicomponent Polymer Coatings. Nanomaterials 2025, 15, 716. https://doi.org/10.3390/nano15100716
Rosqvist E, Niemelä E, Liang S, Eriksson JE, Wang X, Peltonen J. Proliferation of Human Cervical Cancer Cells Responds to Surface Properties of Bicomponent Polymer Coatings. Nanomaterials. 2025; 15(10):716. https://doi.org/10.3390/nano15100716
Chicago/Turabian StyleRosqvist, Emil, Erik Niemelä, Shujun Liang, John E. Eriksson, Xiaoju Wang, and Jouko Peltonen. 2025. "Proliferation of Human Cervical Cancer Cells Responds to Surface Properties of Bicomponent Polymer Coatings" Nanomaterials 15, no. 10: 716. https://doi.org/10.3390/nano15100716
APA StyleRosqvist, E., Niemelä, E., Liang, S., Eriksson, J. E., Wang, X., & Peltonen, J. (2025). Proliferation of Human Cervical Cancer Cells Responds to Surface Properties of Bicomponent Polymer Coatings. Nanomaterials, 15(10), 716. https://doi.org/10.3390/nano15100716






