Detonation Nanodiamond Soot—A Structurally Tailorable Hybrid Graphite/Nanodiamond Carbon-Based Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Nanocomposites Preparation
3. Results and Discussion
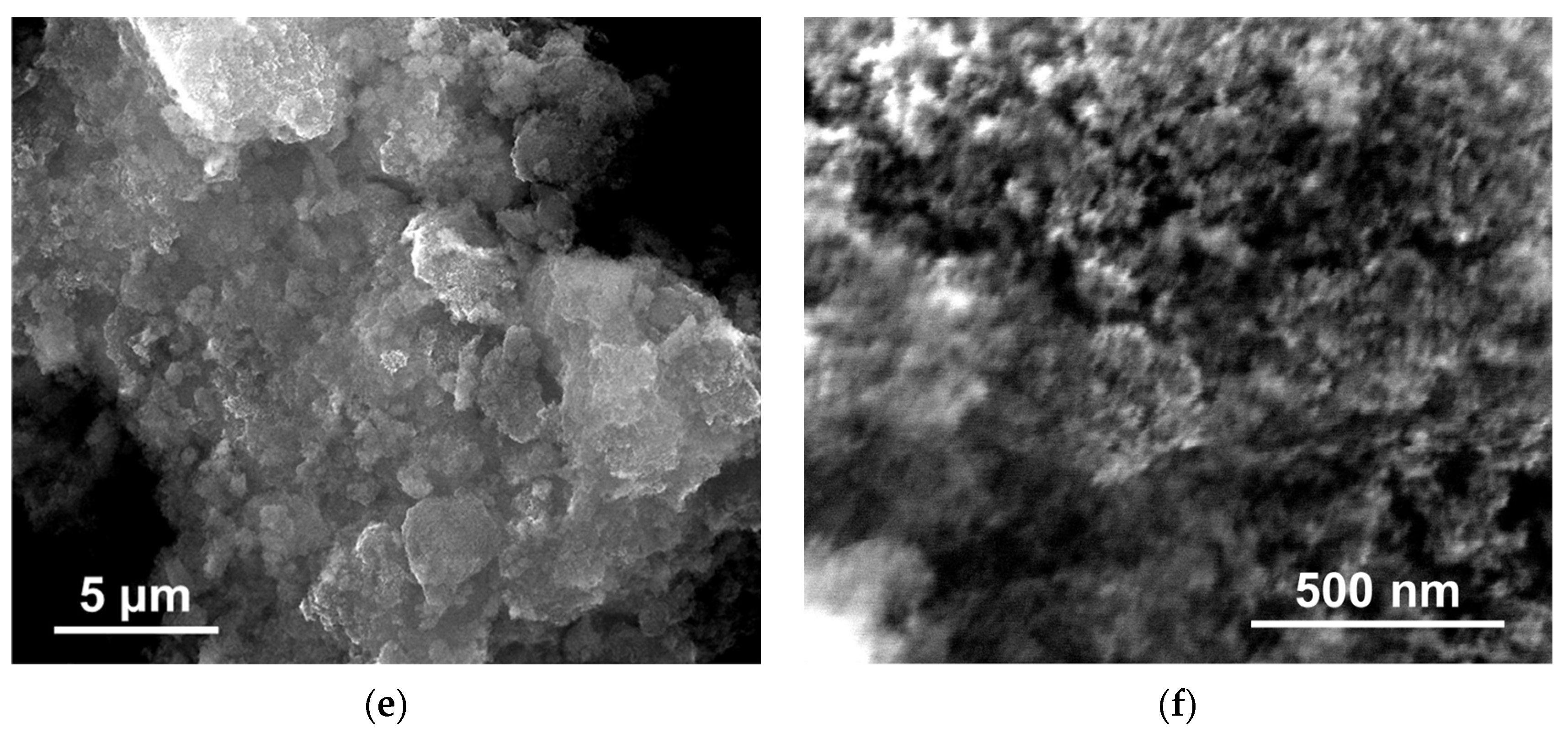
3.1. SEM Results
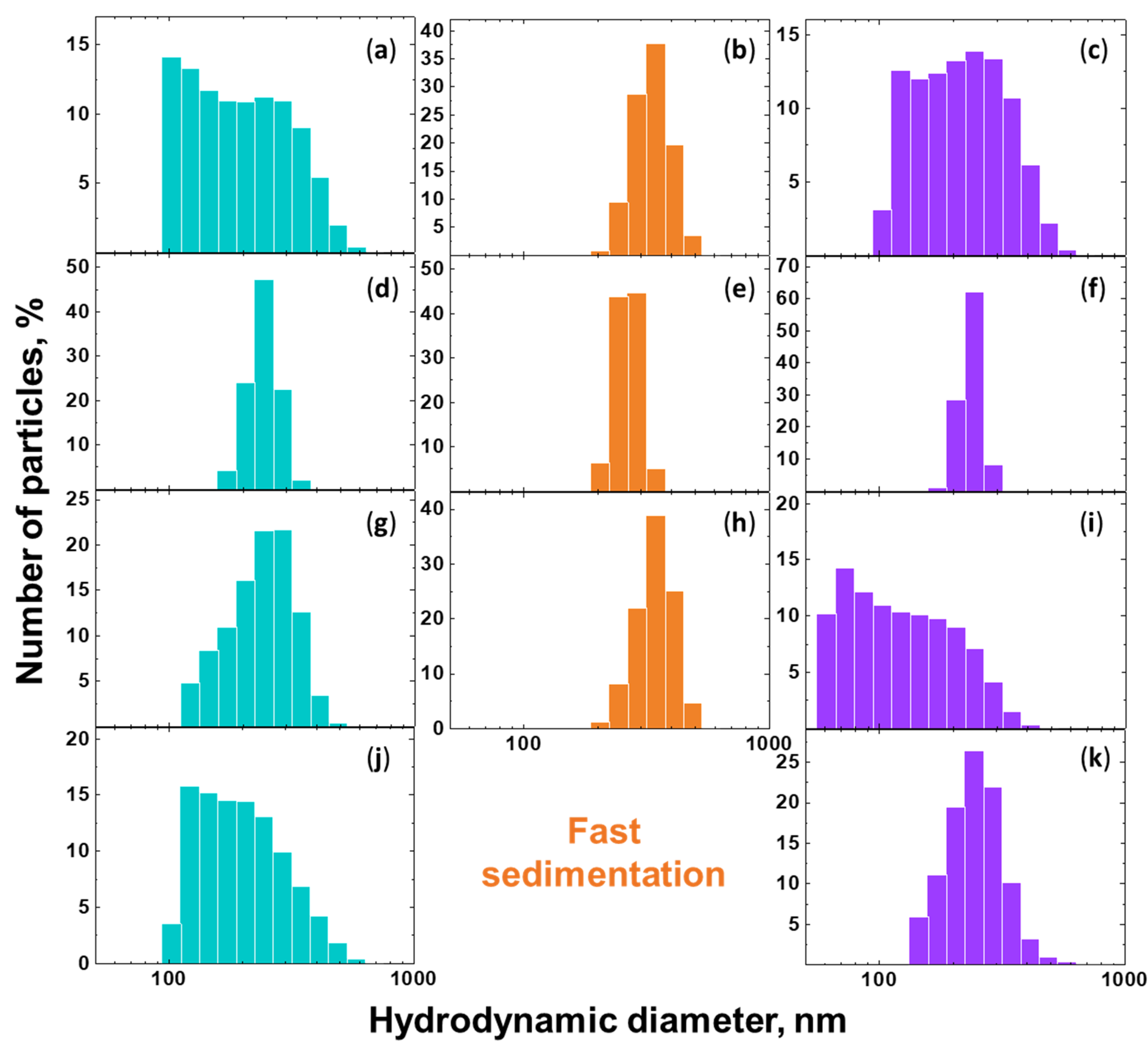
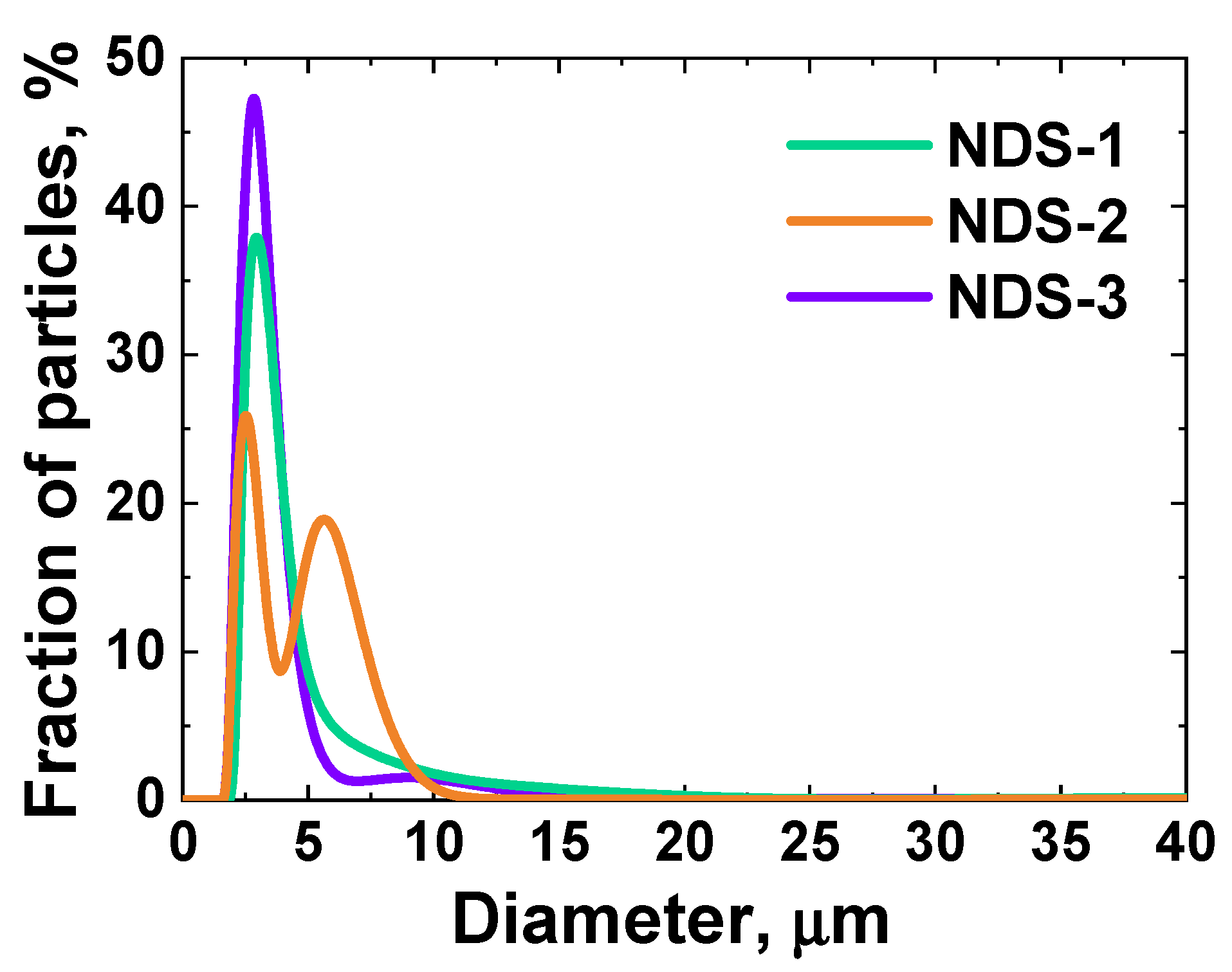
3.2. DLS, Zeta Potential Measurements, and LD Results
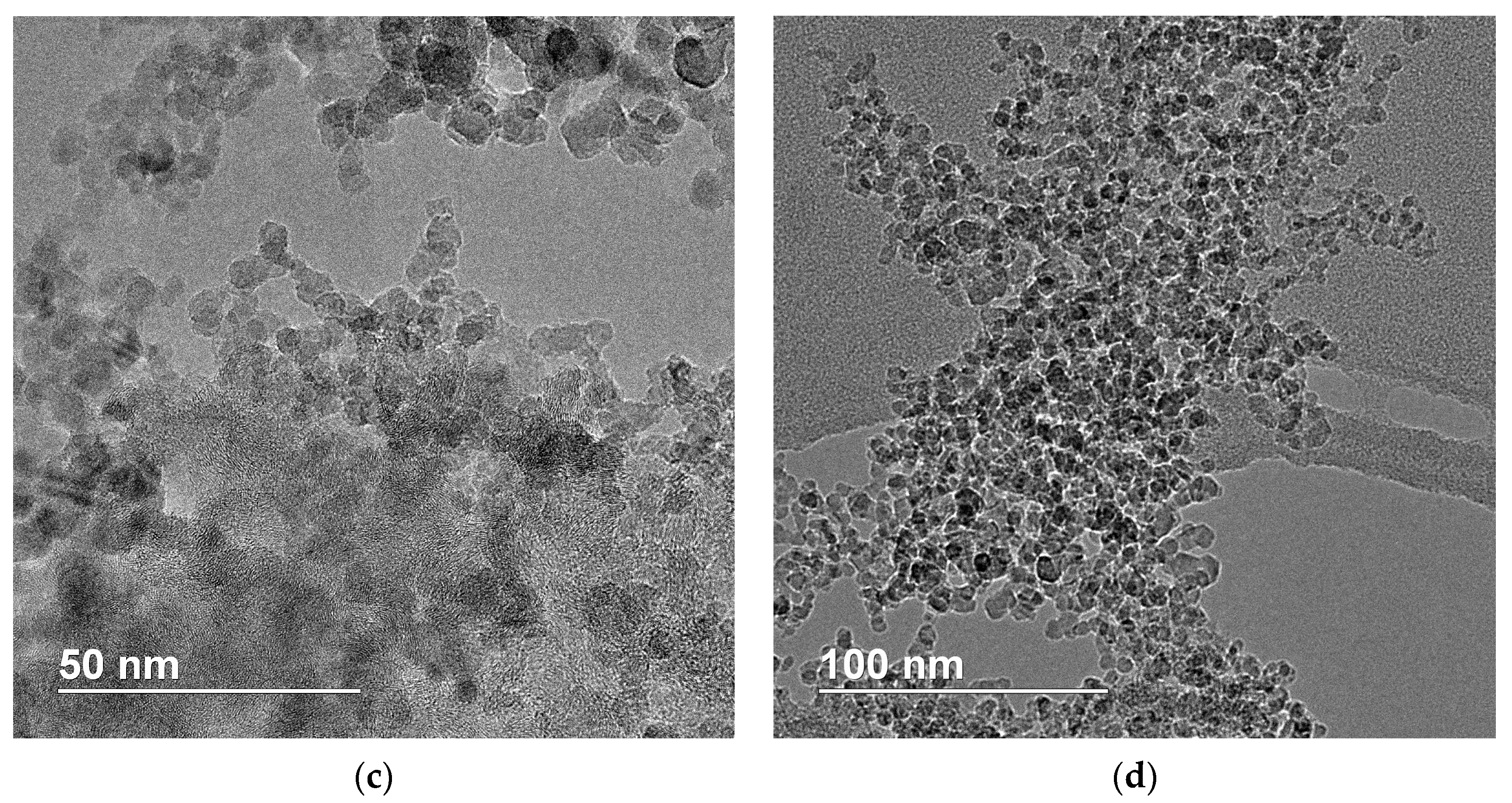
3.3. TEM Results
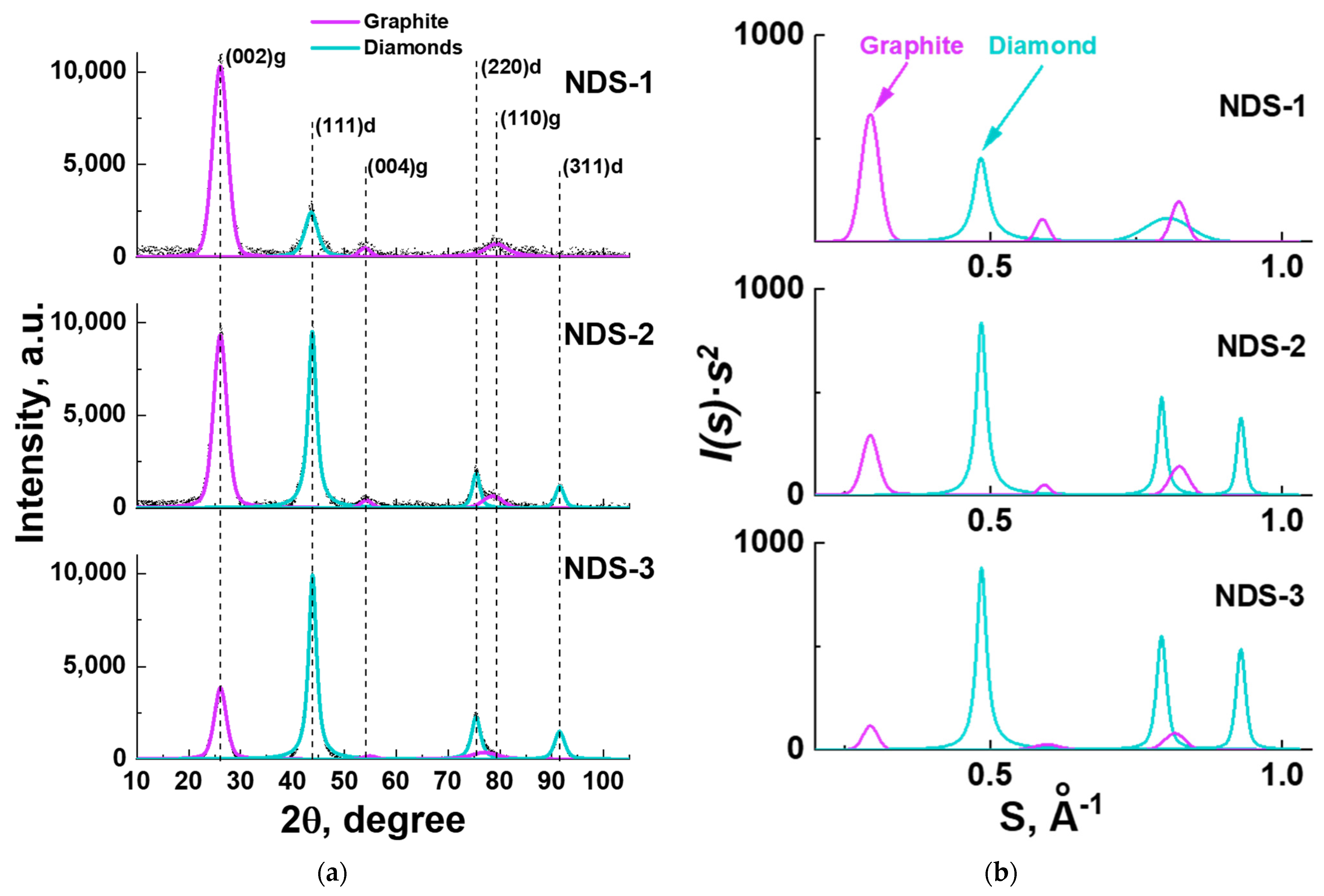
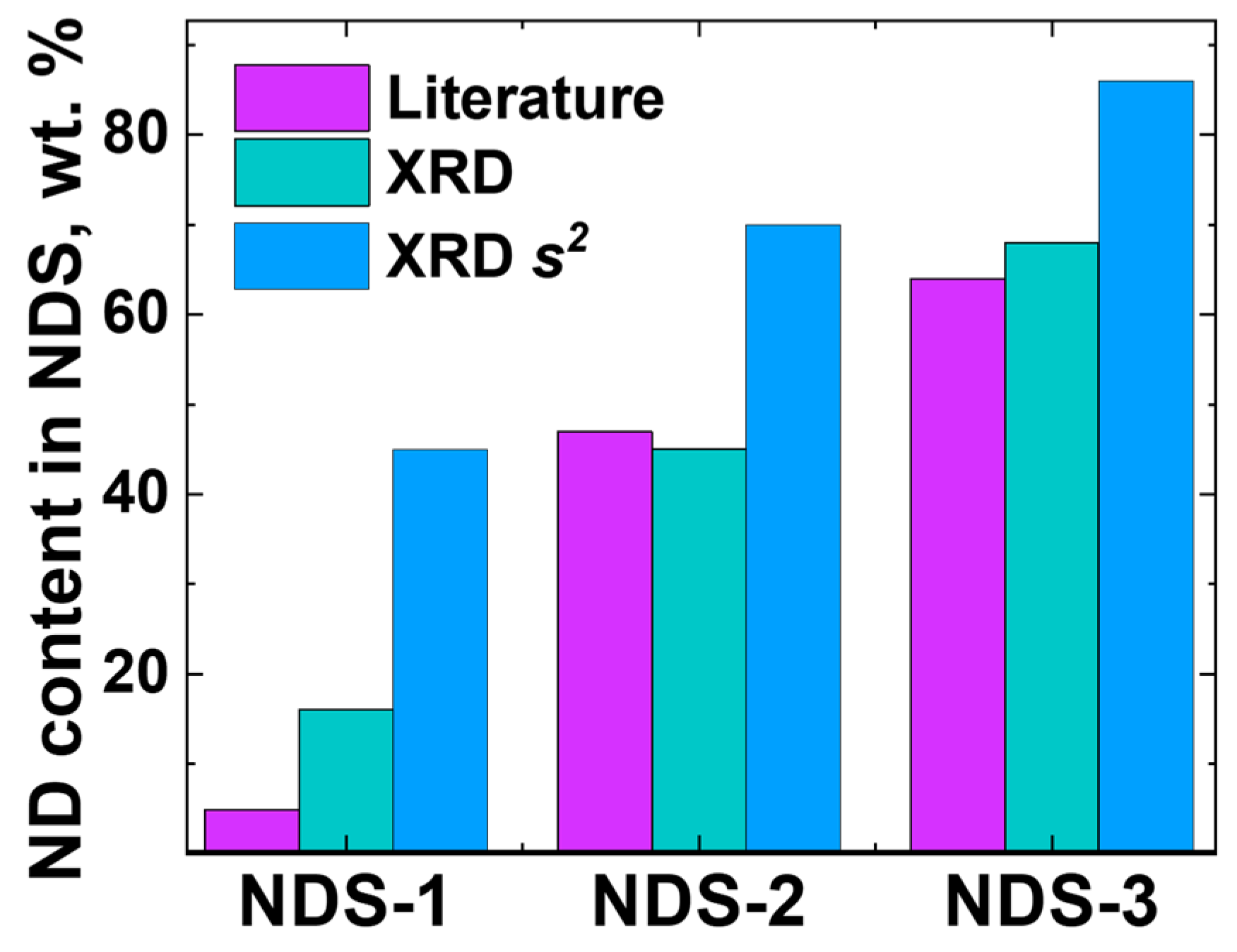
3.4. XRD Analysis Results
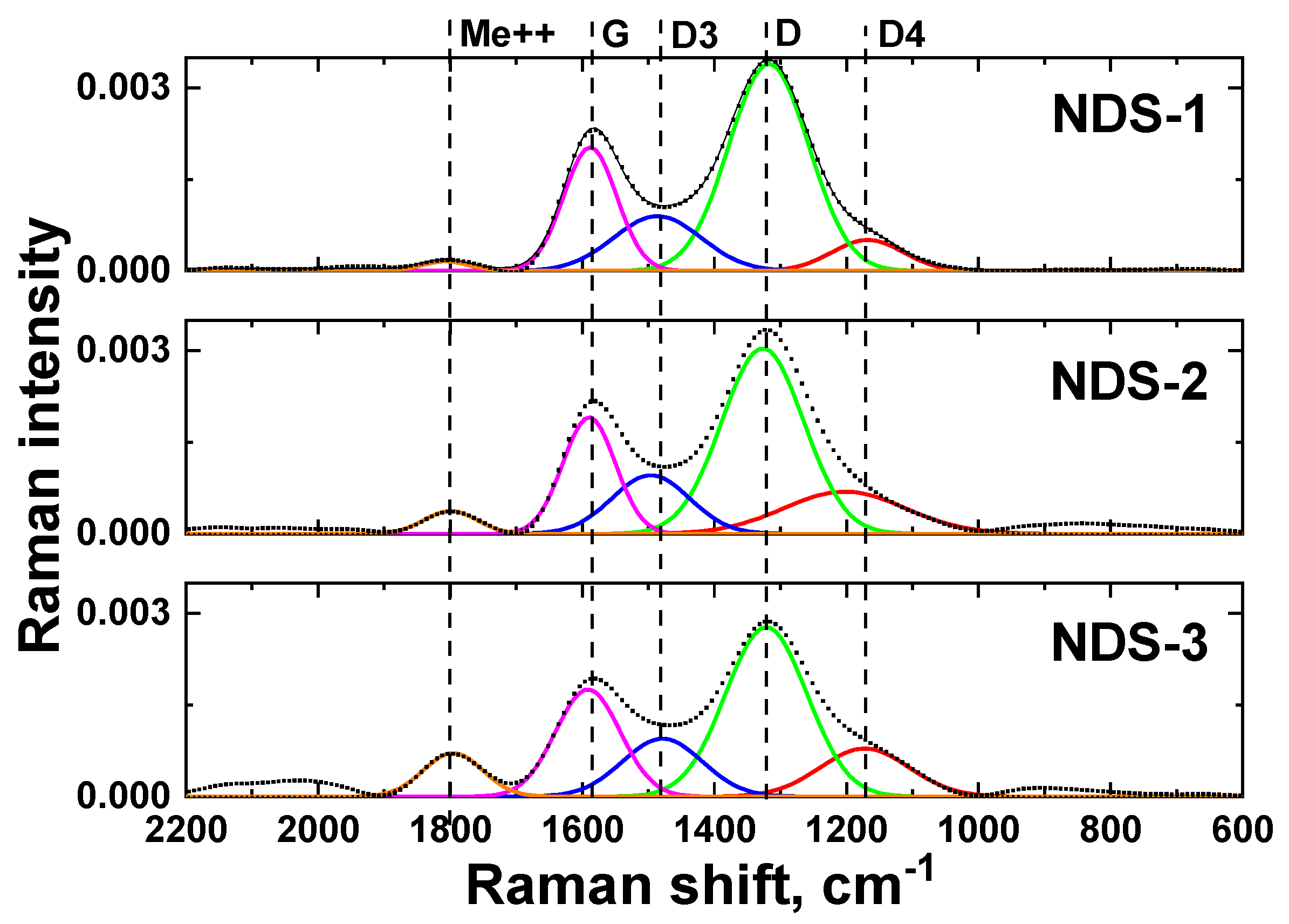
3.5. Raman Spectroscopy Results
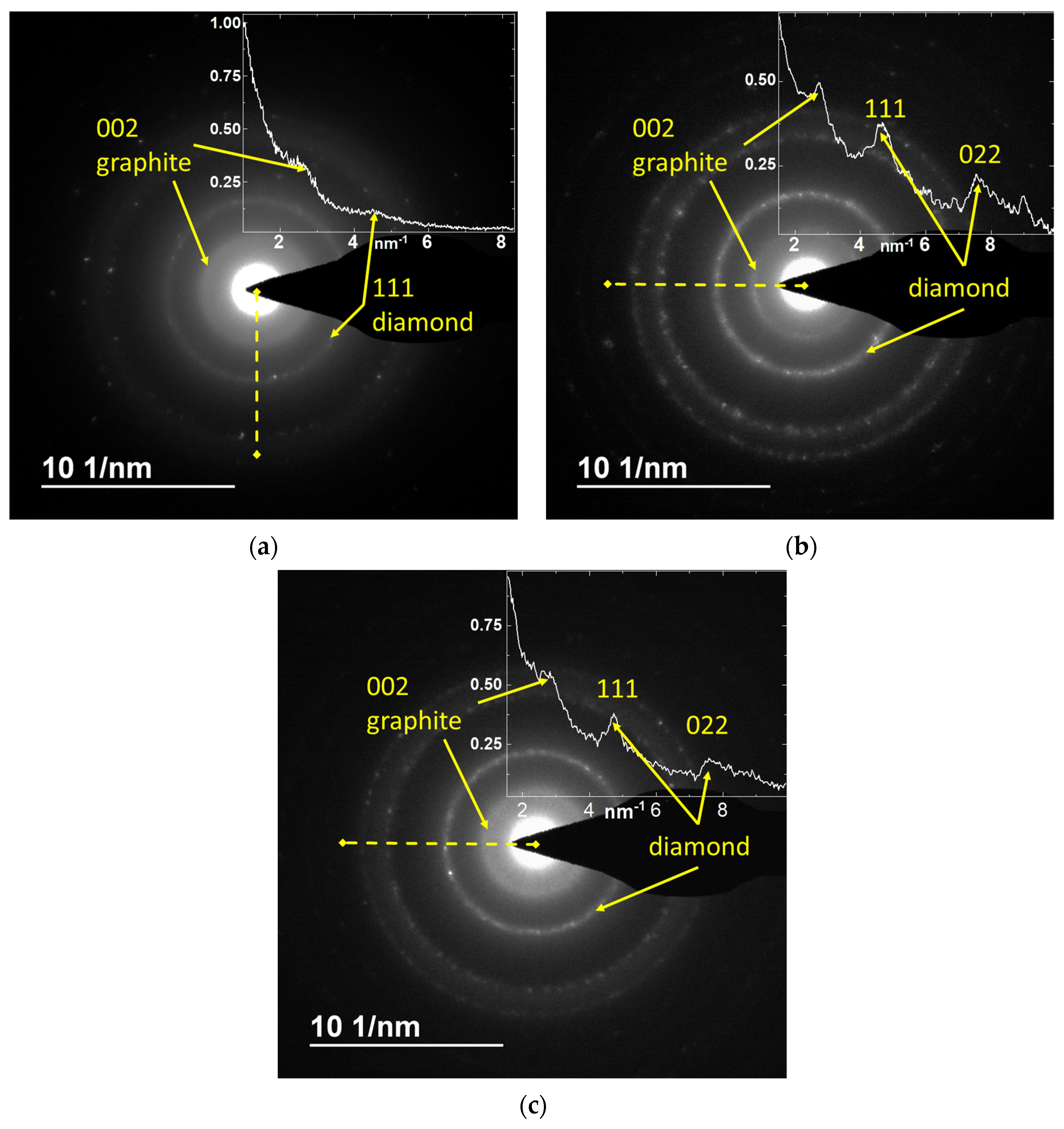
3.6. Electron Diffraction Analysis Results
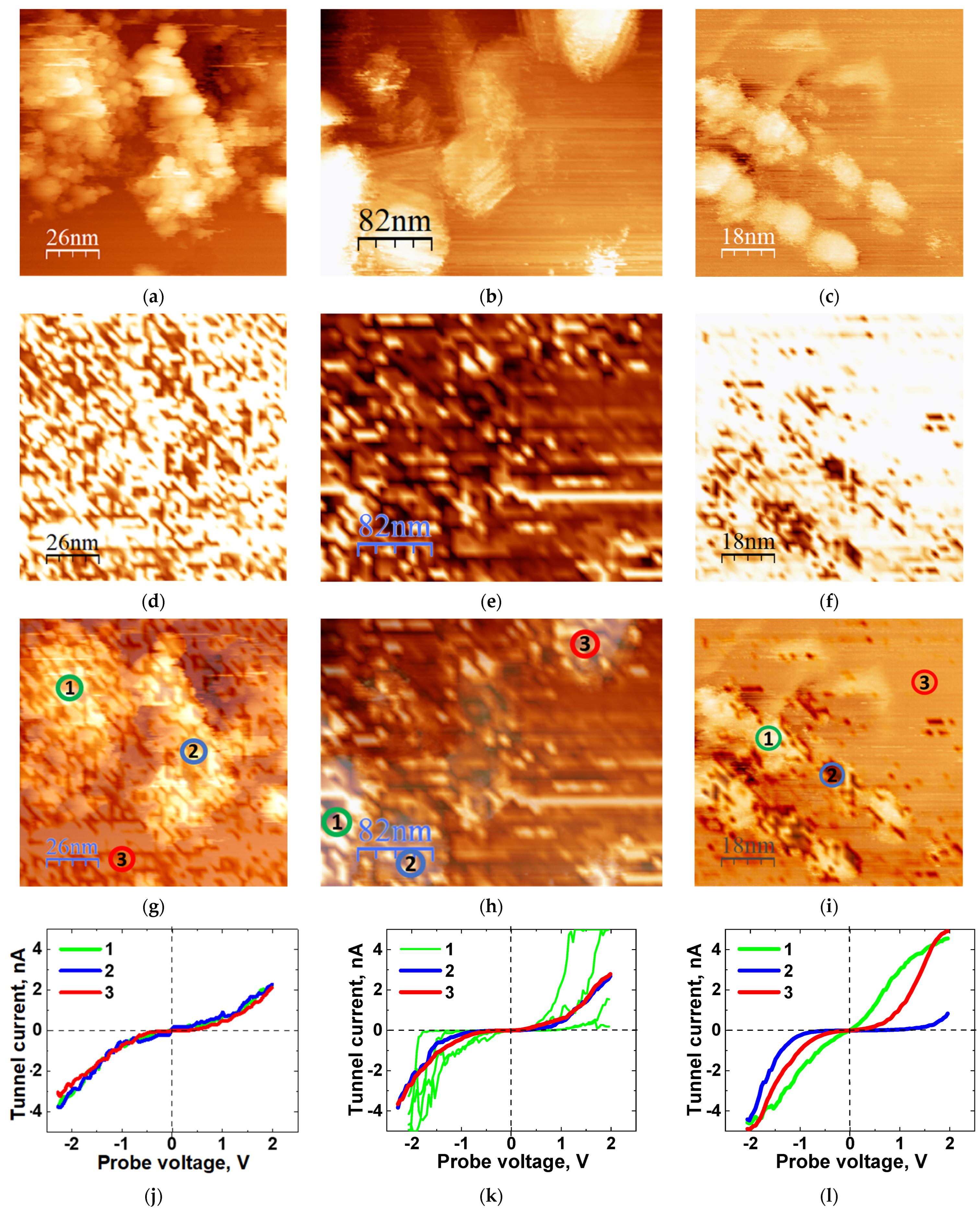
3.7. STM Results
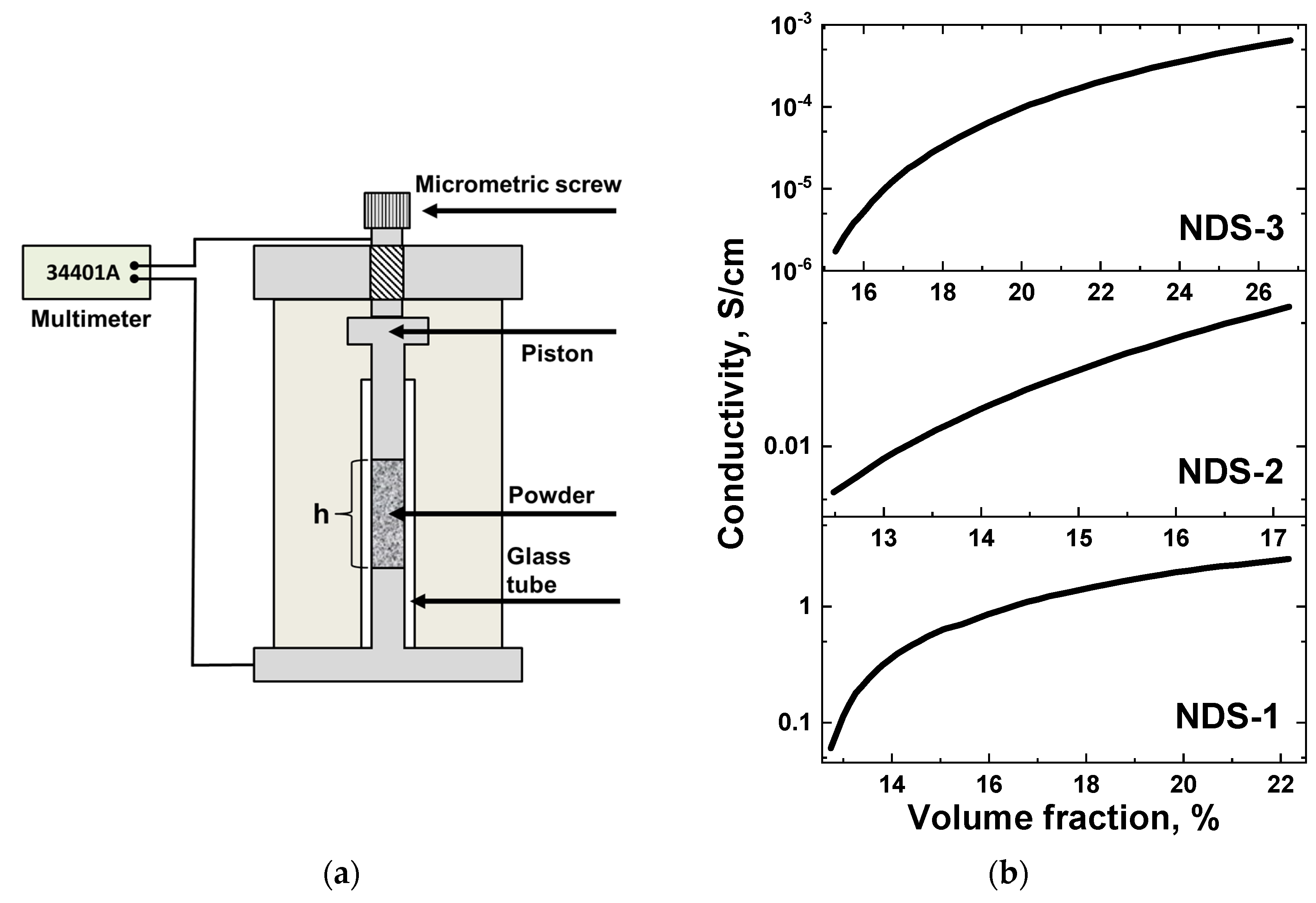
3.8. Electrical Conductivity Measurements
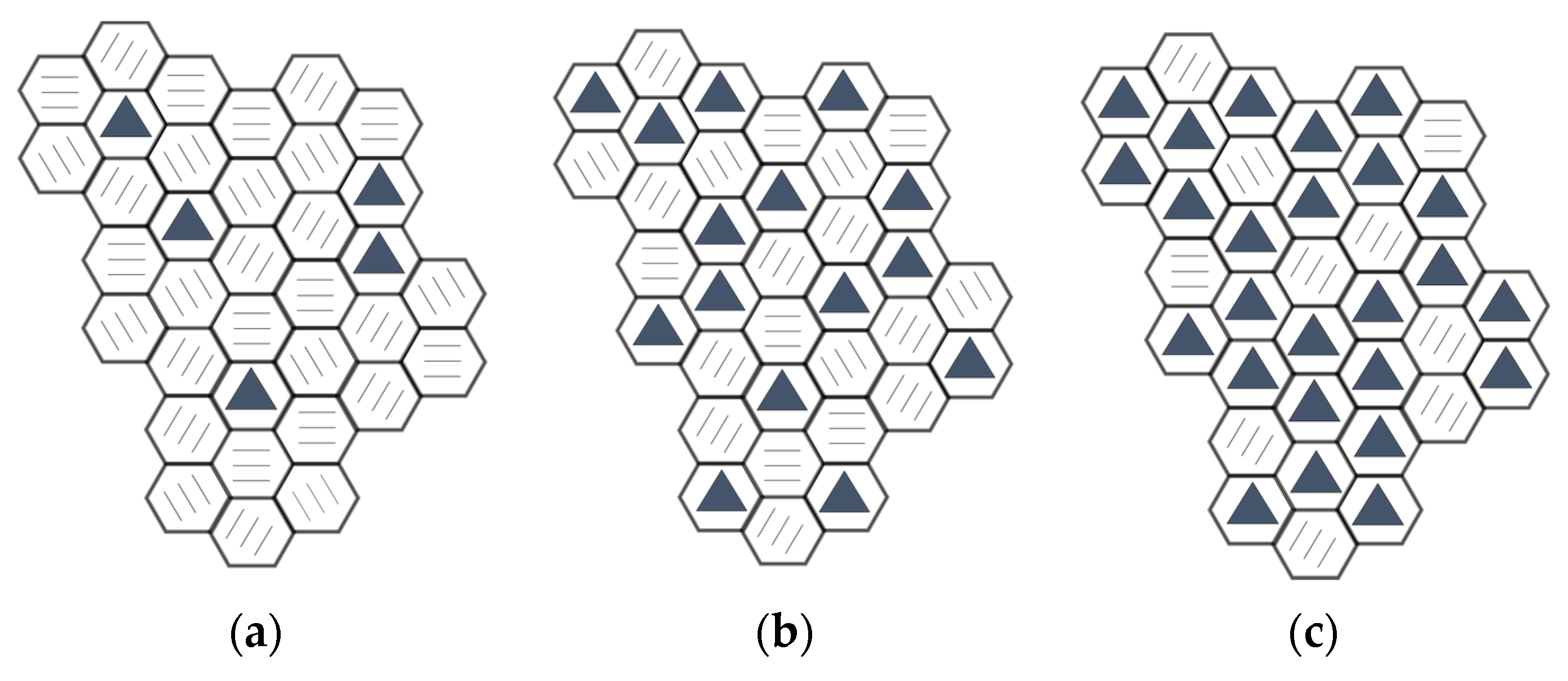
3.9. NDS Nanoparticle Structure Representation
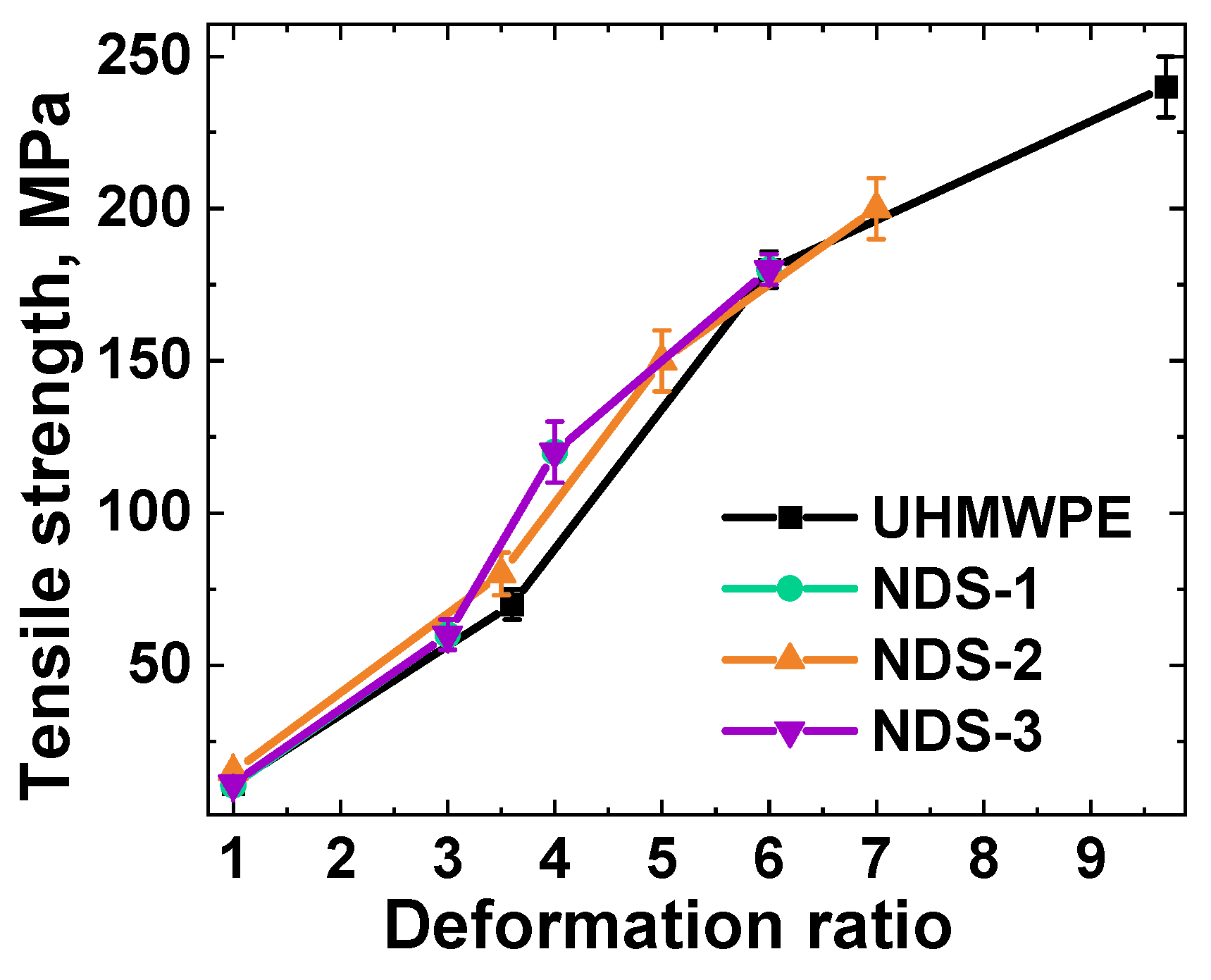
3.10. NDS-Filled Polymer Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurila, T.; Sainio, S.; Caro, M.A. Hybrid carbon based nanomaterials for electrochemical detection of biomolecules. Prog. Mater. Sci. 2017, 88, 499–594. [Google Scholar] [CrossRef]
- Navrotskaya, A.G.; Aleksandrova, D.D.; Krivoshapkina, E.F.; Sillanpää, M.; Krivoshapkin, P. V Hybrid Materials Based on Carbon Nanotubes and Nanofibers for Environmental Applications. Front. Chem. 2020, 8, 546. [Google Scholar] [CrossRef]
- He, Z.; Zhao, Z.; Xiao, S.; Yang, J.; Zhong, M. Preparation of carbon-based hybrid particles and their application in microcellular foaming and flame-retardant materials. RSC Adv. 2018, 8, 26563–26570. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, G.A.; Sevilla, M.; Fuertes, A.B. Free-standing hybrid films based on graphene and porous carbon particles for flexible supercapacitors. Sustain. Energy Fuels 2017, 1, 127–137. [Google Scholar] [CrossRef]
- Kiany, P.; Goharpey, F.; Karimi, M.; Foudazi, R. Hybrids of Bowl-like and Crumpled Hollow Carbon Particles Synthesized through Encapsulation Templating. Langmuir 2020, 36, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Gooneie, A.; Hufenus, R. Hybrid Carbon Nanoparticles in Polymer Matrix for Efficient Connected Networks: Self-Assembly and Continuous Pathways. Macromolecules 2018, 51, 3547–3562. [Google Scholar] [CrossRef]
- Dolmatov, V.Y.; Rudenko, D.V.; Dorokhov, A.O.; Malygin, A.A.; Kozlov, A.S.; Marchukov, V.A. Obtaining of Detonation Diamonds from Individual Explosives. Combust. Explos. Shock Waves 2021, 57, 232–237. [Google Scholar] [CrossRef]
- Shvidchenko, A.V.; Eidelman, E.D.; Vul’, A.Y.; Kuznetsov, N.M.; Stolyarova, D.Y.; Belousov, S.I.; Chvalun, S.N. Colloids of detonation nanodiamond particles for advanced applications. Adv. Colloid Interface Sci. 2019, 268, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Dolmatov, V.Y. Detonation-synthesis nanodiamonds: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2007, 76, 339–360. [Google Scholar] [CrossRef]
- Satonkina, N.P.; Ershov, A.P.; Kashkarov, A.O.; Rubtsov, I.A. Elongated conductive structures in detonation soot of high explosives. RSC Adv. 2020, 10, 17620–17626. [Google Scholar] [CrossRef] [PubMed]
- Sushchev, V.G.; Dolmatov, V.Y.; Malygin, A.A.; Marchukov, V.A.; Korolev, K.M.; Dorokhov, A.O. Core–Shell Composites Based on Partially Oxidized Blend of Detonation Synthesis Nanodiamonds. Russ. J. Appl. Chem. 2020, 93, 661–671. [Google Scholar] [CrossRef]
- Dorokhov, A.O.; Dolmatov, V.Y.; Malygin, A.A.; Kozlov, A.S.; Marchukov, V.A. Development of the Detonation Nanodiamond Synthesis from Tetryl Based Ternary Mixtures. Russ. J. Appl. Chem. 2020, 93, 1083–1089. [Google Scholar] [CrossRef]
- Shames, A.I.; Panich, A.M.; Kempiński, W.; Alexenskii, A.E.; Baidakova, M.V.; Dideikin, A.T.; Osipov, V.Y.; Siklitski, V.I.; Osawa, E.; Ozawa, M.; et al. Defects and impurities in nanodiamonds: EPR, NMR and TEM study. J. Phys. Chem. Solids 2002, 63, 1993–2001. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Assessment of applicability of explosive charges for synthesis of detonation nanodiamonds. J. Superhard Mater. 2016, 38, 373–376. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Figueiredo, J.L.; Silva, A.M.T.; Faria, J.L. Photocatalytic activity of functionalized nanodiamond-TiO2 composites towards water pollutants degradation under UV/Vis irradiation. Appl. Surf. Sci. 2018, 458, 839–848. [Google Scholar] [CrossRef]
- Ivanov, M.; Shenderova, O. Nanodiamond-based nanolubricants for motor oils. Curr. Opin. Solid State Mater. Sci. 2017, 21, 17–24. [Google Scholar] [CrossRef]
- Simioni, N.B.; Oliveira, G.G.; Vicentini, F.C.; Lanza, M.R.V.; Janegitz, B.C.; Fatibello-Filho, O. Nanodiamonds stabilized in dihexadecyl phosphate film for electrochemical study and quantification of codeine in biological and pharmaceutical samples. Diam. Relat. Mater. 2017, 74, 191–196. [Google Scholar] [CrossRef]
- Kidalov, S.V.; Shakhov, F.M.; Vul’, A.Y.; Ozerin, A.N. Grain-boundary heat conductance in nanodiamond composites. Diam. Relat. Mater. 2010, 19, 976–980. [Google Scholar] [CrossRef]
- Kurkin, T.S.; Tikunova, E.P.; Yablokova, M.Y.; Kechek’yan, A.S.; Beshenko, M.A.; Dolmatov, V.Y.; Ozerin, A.N. Effect of a detonation-synthesized nanodiamond powder on the adhesive strength of a polymer fiber to an epoxy binder. Dokl. Chem. 2014, 457, 118–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J.J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Dolmatov, V.Y.; Ozerin, A.N.; Kulakova, I.I.; Bochechka, O.O.; Lapchuk, N.M.; Myllymäki, V.; Vehanen, A. Detonation nanodiamonds: New aspects in the theory and practice of synthesis, properties and applications. Russ. Chem. Rev. 2020, 89, 1428–1462. [Google Scholar] [CrossRef]
- Karami, P.; Salkhi Khasraghi, S.; Hashemi, M.; Rabiei, S.; Shojaei, A. Polymer/nanodiamond composites-a comprehensive review from synthesis and fabrication to properties and applications. Adv. Colloid Interface Sci. 2019, 269, 122–151. [Google Scholar] [CrossRef] [PubMed]
- Kidalov, S.V.; Shakhov, F.M.; Vul’, A.Y. Thermal conductivity of nanocomposites based on diamonds and nanodiamonds. Diam. Relat. Mater. 2007, 16, 2063–2066. [Google Scholar] [CrossRef]
- Houshyar, S.; Sarker, A.; Jadhav, A.; Kumar, G.S.; Bhattacharyya, A.; Nayak, R.; Shanks, R.A.; Saha, T.; Rifai, A.; Padhye, R.; et al. Polypropylene-nanodiamond composite for hernia mesh. Mater. Sci. Eng. C 2020, 111, 110780. [Google Scholar] [CrossRef]
- Neitzel, I.; Mochalin, V.; Bares, J.A.; Carpick, R.W.; Erdemir, A.; Gogotsi, Y. Tribological properties of nanodiamond-epoxy composites. Tribol. Lett. 2012, 47, 195–202. [Google Scholar] [CrossRef]
- Neitzel, I.; Mochalin, V.; Knoke, I.; Palmese, G.R.; Gogotsi, Y. Mechanical properties of epoxy composites with high contents of nanodiamond. Compos. Sci. Technol. 2011, 71, 710–716. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Silva, A.R.; Ribeiro, C.; Carabineiro, S.A.C.; Buijnsters, J.G.; Lanceros-Méndez, S. Nanodiamonds/poly(vinylidene fluoride) composites for tissue engineering applications. Compos. Part B Eng. 2017, 111, 37–44. [Google Scholar] [CrossRef]
- Chen, P.; Huang, F.; Yun, S. Characterization of the condensed carbon in detonation soot. Carbon 2003, 41, 2093–2099. [Google Scholar] [CrossRef]
- Dolmatov, V.Y.; Myllymäki, V.; Vehanen, A.; Dorokhov, A.O.; Kiselev, M.N. Dependence of the Detonation Nanodiamond Yield on the Detonation Process Parameters. J. Superhard Mater. 2019, 41, 355–359. [Google Scholar] [CrossRef]
- Dolmatov, V.Y.; Dorokhov, A.O.; Kozlov, A.S.; Marchukov, V.A.; Myllymäki, V.; Vehanen, A. Possibility of Predictive Estimation of the Yield of DNDs from Individual Explosives. J. Superhard Mater. 2021, 43, 93–99. [Google Scholar] [CrossRef]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems—A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 521, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.E.; Radwan, R.R. Synthesis, characterization and evaluation of resveratrol-loaded functionalized carbon nanotubes as a novel delivery system in radiation enteropathy. Eur. J. Pharm. Sci. 2021, 167, 106002. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, O.M.; Benelfellah, A.; Papadopoulos, L.; Terzopoulou, Z.; Malletzidou, L.; Vasileiadis, I.G.; Chrissafis, K.; Bikiaris, D.N.; Aït Hocine, N. Influence of montmorillonite/carbon nanotube hybrid nanofillers on the properties of poly(lactic acid). Appl. Clay Sci. 2021, 201, 105925. [Google Scholar] [CrossRef]
- Alaferdov, A.V.; Lebedev, O.V.; Roggero, U.F.S.; Hernandez-Figueroa, H.E.; Nista, S.V.G.; Trindade, G.M.; Danilov, Y.A.; Ozerin, A.N.; Moshkalev, S.A. Highly conductive nanographite/ultra-high-molecular-weight polyethylene composite. Results Mater. 2022, 15, 100298. [Google Scholar] [CrossRef]
- Lebedev, O.V.; Kurkin, T.S.; Golubev, E.K.; Vasiliev, A.L.; Gatin, A.K.; Goncharuk, G.P.; Ozerin, A.N. Detonation Synthesis Nanodiamond Soot as a Promising Filler for Polymer Composites. C 2022, 8, 69. [Google Scholar] [CrossRef]
- Kurkin, T.S.; Ozerin, A.N.; Kechek’yan, A.S.; Gritsenko, O.T.; Ozerina, L.A.; Alkhanishvili, G.G.; Sushchev, V.G.; Dolmatov, V.Y. The structure and properties of polymer composite fibers based on poly(vinyl alcohol) and nanodiamond of detonation synthesis. Nanotechnol. Russ. 2010, 5, 340–351. [Google Scholar] [CrossRef]
- Lebedev, O.V.; Bogdanova, O.I.; Goncharuk, G.P.; Ozerin, A.N. Tribological and percolation properties of polypropylene/nanodiamond soot composites. Polym. Polym. Compos. 2020, 28, 369–377. [Google Scholar] [CrossRef]
- Lebedev, O.V.; Yablokov, M.Y.; Mukhortov, L.A.; Goncharuk, G.P.; Ozerin, A.N. Migration of Carbon Nanoparticles to the Surface of a Polymer Composite Melt. Dokl. Chem. 2019, 489, 283–287. [Google Scholar] [CrossRef]
- Yablokov, M.Y.; Shchegolikhin, A.N.; Lebedev, O.V.; Goncharuk, G.P.; Ozerin, A.N. Gradient structure of polypropylene composites filled with carbon black. Russ. Chem. Bull. 2021, 70, 1816–1821. [Google Scholar] [CrossRef]
- Lebedev, O.V.; Ozerin, A.N.; Kechek’yan, A.S.; Shevchenko, V.G.; Kurkin, T.S.; Golubev, E.K.; Karpushkin, E.A.; Sergeyev, V.G.; Kechek’yan, A.S.; Shevchenko, V.G.; et al. A study of oriented conductive composites with segregated network structure obtained via solid-state processing of UHMWPE reactor powder and carbon nanofillers. Polym. Compos. 2019, 40, E146–E155. [Google Scholar] [CrossRef]
- Ivanchev, S.S.; Ruppel’, E.I.; Ozerin, A.N. Optimization of the conditions of ethylene polymerization into reactor powders of ultra-high-molecular-weight polyethylene suitable for solid-phase formation into oriented ultra-high-strength and ultra-high-modulus film yarns. Dokl. Phys. Chem. 2016, 468, 89–92. [Google Scholar] [CrossRef]
- Golubev, E.K.; Kurkin, T.S.; Ozerin, A.N. High-strength tapes prepared by the solid-state processing of nascent reactor powders of ultrahigh molecular weight polyethylene. Russ. Chem. Bull. 2023, 72, 749–763. [Google Scholar] [CrossRef]
- Lebedev, O.V.; Tikunova, E.P.; Golubev, E.K.; Kurkin, T.S.; Shevchenko, V.G.; Ozerin, A.N. Solid-State Processing of Nascent Disentangled UHMWPE Reactor Powders Mixed with Carbon Nanoparticles. Macromol. Symp. 2024, 413, 2400008. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Justice, R.S. How Nano Are Nanocomposites? Macromolecules 2007, 40, 8501–8517. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Empirical Parameters of Solvent Polarity. In Solvents and Solvent Effects in Organic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 425–508. ISBN 9783527632220. [Google Scholar]
- Ginés, L.; Mandal, S.; Cheng, C.-L.; Sow, M.; Williams, O.A. Positive zeta potential of nanodiamonds. Nanoscale 2017, 9, 12549–12555. [Google Scholar] [CrossRef]
- Napper, D.H. Polymeric Stabilization of Colloidal Dispersions; Colloid Science: A Series of Monographs. 2; Academic Press: Cambridge, MA, USA, 1983; ISBN 9780125139809. [Google Scholar]
- Riddick, T.M. Control of Colloid Stability Through Zeta Potential; Livingston: Hongkong, China, 1968. [Google Scholar]
- Mogilevskaya, E.L.; Akopova, T.A.; Zelenetskii, A.N.; Ozerin, A.N. The crystal structure of chitin and chitosan. Polym. Sci. Ser. A 2006, 48, 116–123. [Google Scholar] [CrossRef]
- Iwashita, N.; Park, C.R.; Fujimoto, H.; Shiraishi, M.; Inagaki, M. Specification for a standard procedure of X-ray diffraction measurements on carbon materials. Carbon 2004, 42, 701–714. [Google Scholar] [CrossRef]
- Calderon, H.A.; Okonkwo, A.; Estrada-Guel, I.; Hadjiev, V.G.; Alvarez-Ramírez, F.; Robles Hernández, F.C. HRTEM low dose: The unfold of the morphed graphene, from amorphous carbon to morphed graphenes. Adv. Struct. Chem. Imaging 2016, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Mermoux, M.; Chang, S.; Girard, H.A.; Arnault, J.-C. Raman spectroscopy study of detonation nanodiamond. Diam. Relat. Mater. 2018, 87, 248–260. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Zou, Q.; Li, Y.G.; Zou, L.H.; Wang, M.Z. Characterization of structures and surface states of the nanodiamond synthesized by detonation. Mater. Charact. 2009, 60, 1257–1262. [Google Scholar] [CrossRef]
- Lebedev, O.V.; Goncharuk, G.P.; Ozerin, A.N. Changes in Electrical Conductance of Polymer Composites Melts Due to Carbon Nanofiller Particles Migration. Polymers 2021, 13, 1030. [Google Scholar] [CrossRef] [PubMed]
- Voznyakovskii, A.P.; Smirnov, A.V.; Fedorov, B.A.; Khoreva, A.K.; Shumilov, F.A. Geometrical characteristics of detonation diamond particles by the data of small-angle X-ray scattering. J. Superhard Mater. 2015, 37, 357–362. [Google Scholar] [CrossRef]




| Pin Material | AISI 420 (45HRc) Steel |
|---|---|
| Pin diameter, mm | 5 |
| Track radius, mm | 25 |
| Rotation speed, m/s | 0.8 |
| Test duration, h | 8 |
| Normal load, N | 10 |
| NDS Type | ND Fraction, wt.% | Nanographite Fraction, wt.% | Nanographite Domain Size (002 Reflection), nm | ||
|---|---|---|---|---|---|
| I(s) − s | I(s)∙s2 − s | I(s) − s | I(s)∙s2 − s | ||
| NDS-1 | 16 | 45 | 84 | 55 | 2.64 |
| NDS-2 | 45 | 70 | 55 | 30 | 2.96 |
| NDS-3 | 68 | 86 | 32 | 14 | 3.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurkin, T.S.; Lebedev, O.V.; Golubev, E.K.; Gatin, A.K.; Nepomnyashchikh, V.V.; Dolmatov, V.Y.; Ozerin, A.N. Detonation Nanodiamond Soot—A Structurally Tailorable Hybrid Graphite/Nanodiamond Carbon-Based Material. Nanomaterials 2025, 15, 56. https://doi.org/10.3390/nano15010056
Kurkin TS, Lebedev OV, Golubev EK, Gatin AK, Nepomnyashchikh VV, Dolmatov VY, Ozerin AN. Detonation Nanodiamond Soot—A Structurally Tailorable Hybrid Graphite/Nanodiamond Carbon-Based Material. Nanomaterials. 2025; 15(1):56. https://doi.org/10.3390/nano15010056
Chicago/Turabian StyleKurkin, Tikhon S., Oleg V. Lebedev, Evgeny K. Golubev, Andrey K. Gatin, Victoria V. Nepomnyashchikh, Valery Yu. Dolmatov, and Alexander N. Ozerin. 2025. "Detonation Nanodiamond Soot—A Structurally Tailorable Hybrid Graphite/Nanodiamond Carbon-Based Material" Nanomaterials 15, no. 1: 56. https://doi.org/10.3390/nano15010056
APA StyleKurkin, T. S., Lebedev, O. V., Golubev, E. K., Gatin, A. K., Nepomnyashchikh, V. V., Dolmatov, V. Y., & Ozerin, A. N. (2025). Detonation Nanodiamond Soot—A Structurally Tailorable Hybrid Graphite/Nanodiamond Carbon-Based Material. Nanomaterials, 15(1), 56. https://doi.org/10.3390/nano15010056





