Size-Dependent High-Pressure Behavior of Pure and Eu3+-Doped Y2O3 Nanoparticles: Insights from Experimental and Theoretical Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. Simulation Details
3. Results and Discussion
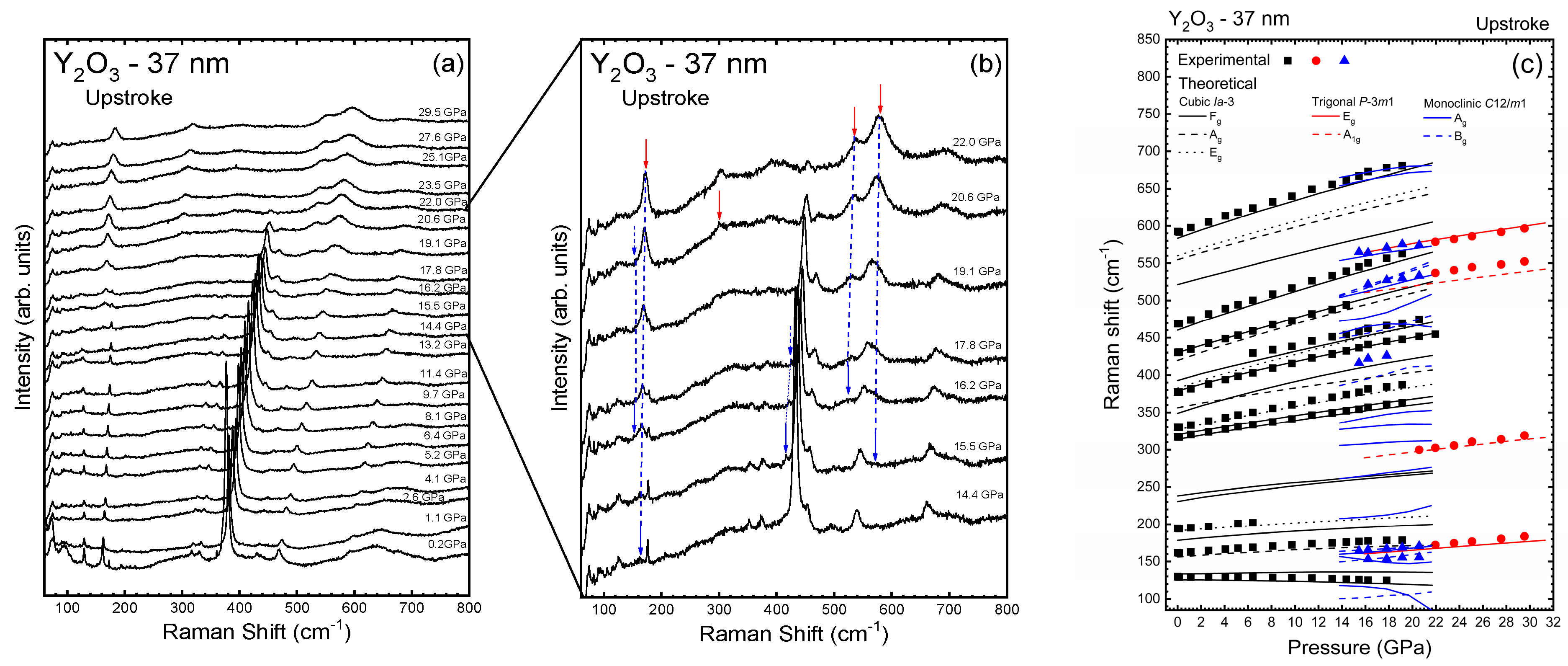
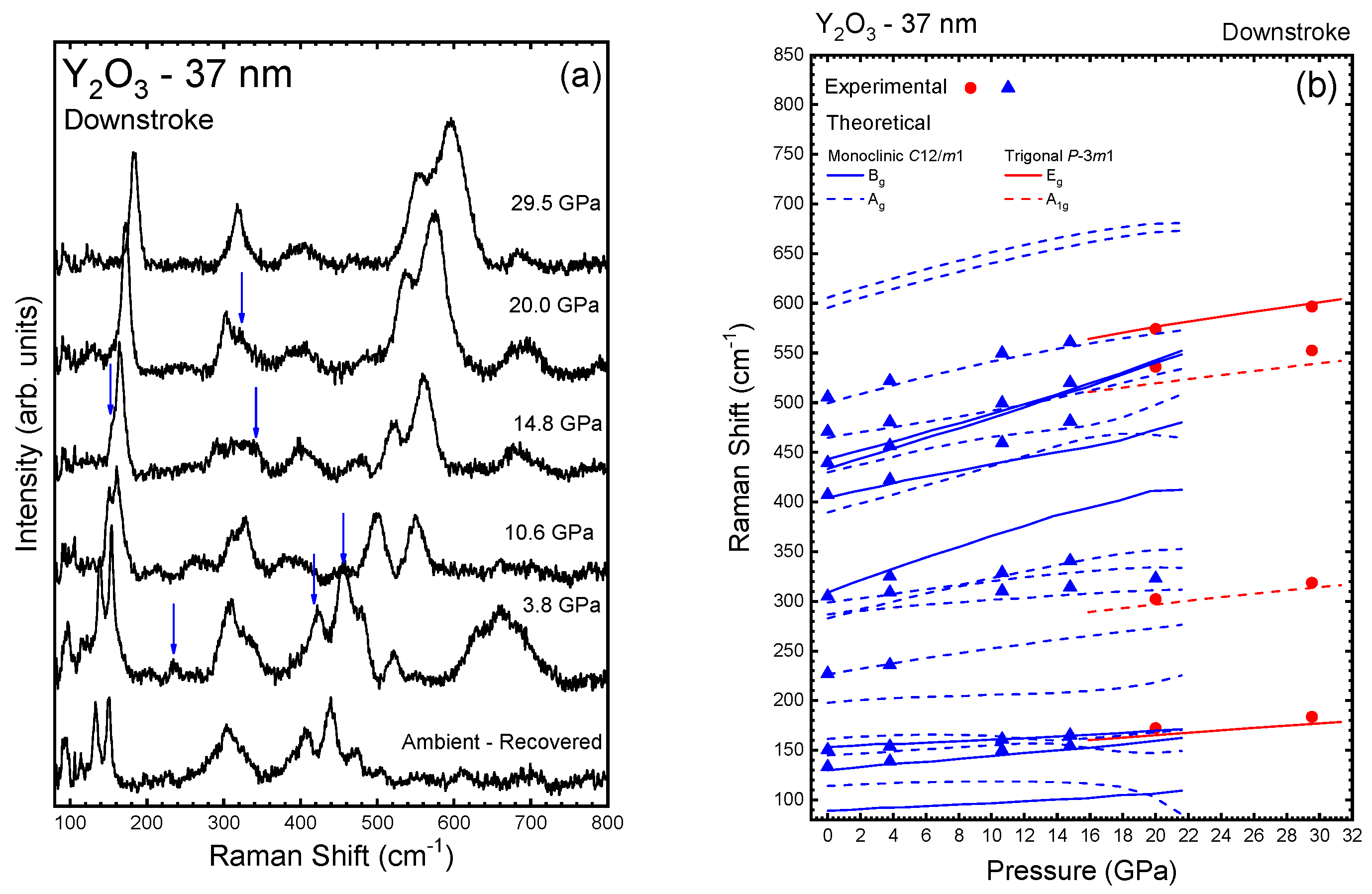
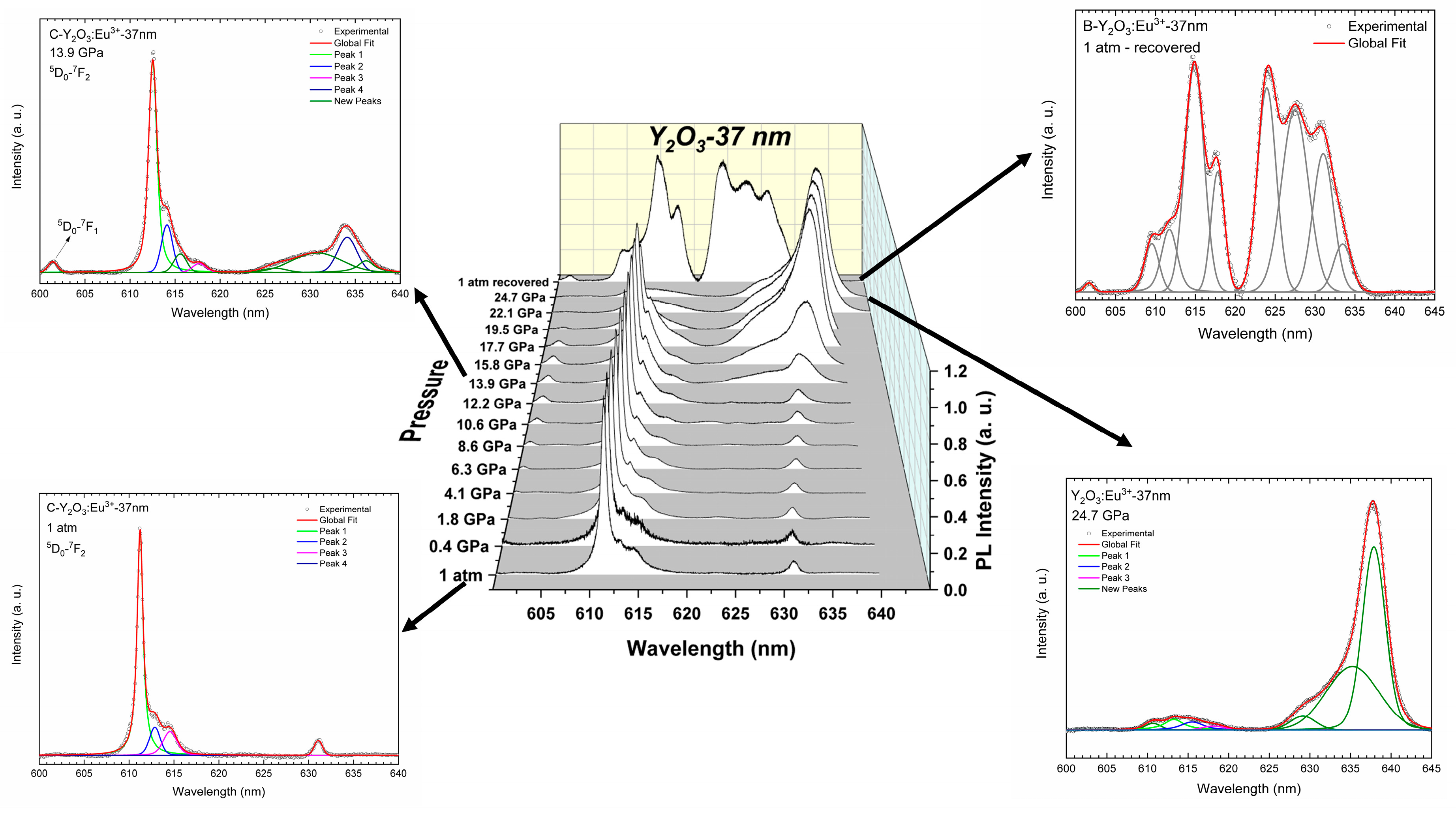
3.1. Y2O3-37 nm and Y2O3:Eu3+-37 nm Nanocrystals
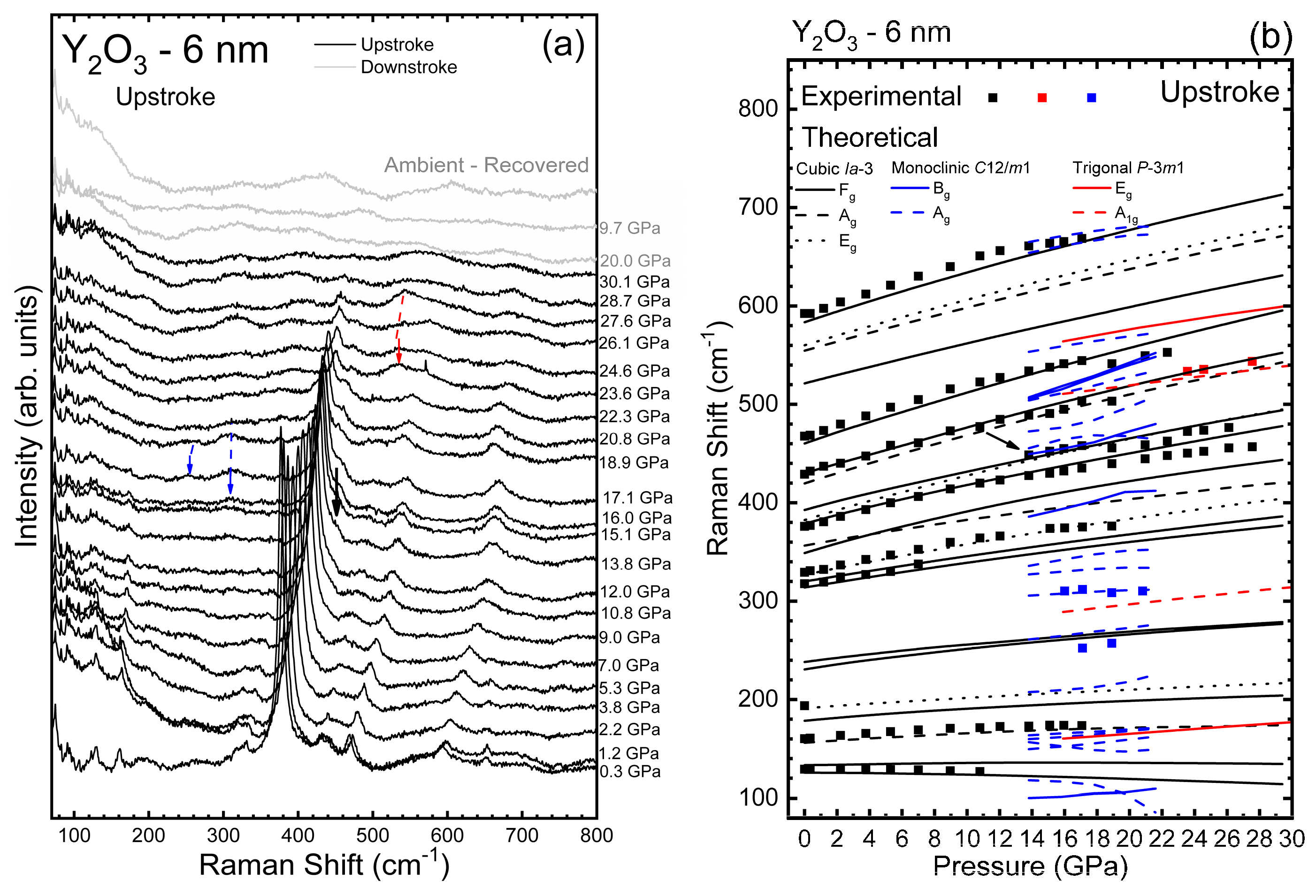
3.2. Y2O3-6 nm and Y2O3:Eu3+-6 nm Nanocrystals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yavetskiy, R.P.; Dobrotvorskaya, M.V.; Doroshenko, A.G.; Tolmachev, V.; Petrusha, I.; Turkevich, V.Z.; Tomala, R.; Hreniak, D.; Strek, W.; Baumer, V.N. Fabrication and luminescent properties of (Y0.99Eu0.01)2O3 transparent nanostructured ceramics. Opt. Mater. 2018, 78, 285–291. [Google Scholar] [CrossRef]
- Singh, O.S.; Wangkhem, R.; Yaiphaba, N.; Singh, N.S. Hydrothermally synthesized rod shaped Y2O3:Eu3+ as red emitter. Phys. B Condens. Matter 2023, 670, 415409. [Google Scholar] [CrossRef]
- Verstegen, J.M.P.J.; Radielović, D.; Vrenken, L.E. A New Generation of “Deluxe” Fluorescent Lamps, Combining an Efficacy of 80 Lumens/W or More with a Color Rendering Index of Approximately 85. J. Electrochem. Soc. 1974, 121, 1627–1631. [Google Scholar] [CrossRef]
- Konrad, A.; Fries, T.; Gahn, A.; Kummer, F.; Herr, U.; Tidecks, R.; Samwer, K. Chemical vapor synthesis and luminescence properties of nanocrystalline cubic Y2O3:Eu. J. Appl. Phys. 1999, 86, 3129–3133. [Google Scholar] [CrossRef]
- Wakefield, G.; Holland, E.; Dobson, P.J.; Hutchison, J.L. Luminescence Properties of Nanocrystalline Y2O3:Eu. Adv. Mater. 2001, 13, 1557–1560. [Google Scholar] [CrossRef]
- Igarashi, T.; Ihara, M.; Kusunoki, T.; Ohno, K.; Isobe, T.; Senna, M. Relationship between optical properties and crystallinity of nanometer Y2O3:Eu phosphor. Appl. Phys. Lett. 2000, 76, 1549–1551. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, C.; Zhang, W.; Zhang, W.; Hu, T. Local structure and luminescence of nanocrystalline Y2O3:Eu. Appl. Phys. Lett. 2002, 81, 2857–2859. [Google Scholar] [CrossRef]
- Boukerika, A.; Guerbous, L. Annealing effects on structural and luminescence properties of red Eu3+-doped Y2O3 nanophosphors prepared by sol-gel method. J. Lumin. 2014, 145, 148–153. [Google Scholar] [CrossRef]
- Yoo, H.S.; Jang, H.S.; Im, W.B.; Kang, J.H.; Jeon, D.Y. Particle size control of a monodisperse spherical Y2O3:Eu3+ phosphor and its photoluminescence properties. J. Mater. Res. 2007, 22, 2017–2024. [Google Scholar] [CrossRef]
- Ogawa, T.; Otani, N.; Yokoi, T.; Fisher, C.A.J.; Kuwabara, A.; Moriwake, H.; Yoshiya, M.; Kitaoka, S.; Takata, M. Density functional study of the phase stability and Raman spectra of Yb2O3, Yb2SiO5 and Yb2Si2O7 under pressure. Phys. Chem. Chem. Phys. 2018, 20, 16518–16527. [Google Scholar] [CrossRef]
- Petit, L.; Svane, A.; Szotek, Z.; Temmerman, W.M. First-principles study of rare-earth oxides. Phys. Rev. B 2005, 72, 205118. [Google Scholar] [CrossRef]
- Gu, J.; Fan, W.; Zhang, Z.; Yao, Q.; Zhan, H. Structure and Optical Property of Pr2O3 Powder Prepared by Reduction. Wuji Cailiao Xuebao/J. Inorg. Mater. 2023, 38, 771–777. [Google Scholar] [CrossRef]
- Schaack, G.; Koningstein, J.A. Phonon and Electronic Raman Spectra of Cubic Rare-Earth Oxides and Isomorphous Yttrium Oxide. J. Opt. Soc. Am. 1970, 60, 1110–1115. [Google Scholar] [CrossRef]
- Sharma, B.D. Crystallographic and spectroscopic symmetry notations. J. Chem. Educ. 1982, 59, 554–557. [Google Scholar] [CrossRef]
- Martel, J.F.; Jandl, S.; Viana, B.; Vivien, D. Crystal-field study of Sm3+ ions in Sm2O3, Sm3+:Gd2O3 and Sm3+:Y2O3. J. Phys. Chem. Solids 2000, 61, 1455–1463. [Google Scholar] [CrossRef]
- Boldish, S.I.; White, W.B. Vibrational spectra of crystals with the A-type rare earth oxide structure-I. La2O3 and Nd2O3. Spectrochim. Acta Part A Mol. Spectrosc. 1979, 35, 1235–1242. [Google Scholar] [CrossRef]
- Dilawar Sharma, N.; Singh, J.; Vijay, A.; Samanta, K.; Dogra, S.; Bandyopadhyay, A.K. Pressure-Induced Structural Transition Trends in Nanocrystalline Rare-Earth Sesquioxides: A Raman Investigation. J. Phys. Chem. C 2016, 120, 11679–11689. [Google Scholar] [CrossRef]
- Ray, S.; León-luis, S.F.; Javier, F.; Alfonso, M.; Gomis, Ó.; Rodríguez-mendoza, U.R.; Agouram, S.; Muñoz, A.; Lavín, V. Broadband, site selective and time resolved photoluminescence spectroscopic studies of fi nely size-modulated Y2O3:Eu3+ phosphors synthesized by a complex based precursor solution method. Curr. Appl. Phys. 2014, 14, 72–81. [Google Scholar] [CrossRef]
- Pereira, A.L.J.; Sans, J.A.; Gomis, O.; Santamaría-Pérez, D.; Ray, S.; Godoy-Jr, A.; da Silva-Sobrinho, A.S.; Rodríguez-Hernández, P.; Muñoz, A.; Popescu, C.; et al. Joint experimental and theoretical study of bulk Y2O3 at high pressure. Results Phys. 2023, 49, 106499. [Google Scholar] [CrossRef]
- Wang, L.; Yang, W.; Ding, Y.; Ren, Y.; Xiao, S.; Liu, B.; Sinogeikin, S.V.; Meng, Y.; Gosztola, D.J.; Shen, G.; et al. Size-dependent amorphization of nanoscale Y2O3 at high pressure. Phys. Rev. Lett. 2010, 105, 095701. [Google Scholar] [CrossRef]
- Dai, R.C.; Zhang, Z.M.; Zhang, C.C.; Ding, Z.J. Photoluminescence and Raman Studies of Y2O3:Eu3+ Nanotubes Under High Pressure. J. Nanosci. Nanotechnol. 2010, 10, 7629–7633. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ma, Y.; Yang, H.; Zhu, P.; Du, J.; Ji, C.; Hou, D. Ultrastable structure and luminescence properties of Y2O3 nanotubes. Solid State Commun. 2010, 150, 1208–1212. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Wang, L.; Bai, X.; Song, H.; Zhou, Q.; Wei, T.; An, D.; Liu, B. The pressure induced amorphization and behavior of octahedron in Y2O3/Eu3+ nanotubes. Mater. Res. Express 2014, 1, 025013. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Hou, Y.; Bai, X.; Song, H.; Zhou, Q.; Wei, T.; Li, Y.; Liu, B. Analysis of the upconversion photoluminescence spectra as a probe of local microstructure in Y2O3/Eu3+ nanotubes under high pressure. RSC Adv. 2015, 5, 3130–3134. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Liu, B.; Song, Q.; Zhou, Q.; Song, H.; Liu, B. Investigation of the lattice behavior of cubic Y2O3/Eu3+ nanotubes under high pressure. Phys. Status Solidi Basic Res. 2016, 253, 2204–2208. [Google Scholar] [CrossRef]
- Fauth, F.; Peral, I.; Popescu, C.; Knapp, M. The new Material Science Powder Diffraction beamline at ALBA Synchrotron. Powder Diffr. 2013, 28, S360–S370. [Google Scholar] [CrossRef]
- Merrill, L.; Bassett, W.A. Miniature diamond anvil pressure cell for single crystal X-ray diffraction studies. Rev. Sci. Instrum. 1974, 45, 290–294. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Mezouar, M. Equations of state of six metals above 94 GPa. Phys. Rev. B-Condens. Matter Mater. Phys. 2004, 70, 094112. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Phys. Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Nolze, G.; Kraus, W. PowderCell 2.0 for Windows. Powder Diffr. 2016, 13, 256–259. [Google Scholar]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Errandonea, D.; Muñoz, A.; Gonzalez-Platas, J. Comment on High-pressure x-ray diffraction study of YBO3/Eu3+, GdBO3, and EuBO3: Pressure-induced amorphization in GdBO3. J. Appl. Phys. 2014, 115, 113–116. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B-Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Parlinski, K.; Li, Z.Q.; Kawazoe, Y. First-Principles Determination of the Soft Mode in Cubic ZrO2. Phys. Rev. Lett. 1997, 78, 4063–4066. [Google Scholar] [CrossRef]
- Husson, E.; Proust, C.; Gillet, P.; Itié, J.P. Phase transitions in yttrium oxide at high pressure studied by Raman spectroscopy. Mater. Res. Bull. 1999, 34, 2085–2092. [Google Scholar] [CrossRef]
- White, W.B.; Keramidas, V.G. Vibrational spectra of oxides with the C-type rare earth oxide structure. Spectrochim. Acta Part A Mol. Spectrosc. 1972, 28, 501–509. [Google Scholar] [CrossRef]
- Repelin, Y.; Proust, C.; Husson, E.; Beny, J.M. Vibrational Spectroscopy of the C-Form of Yttrium Sesquioxide. J. Solid State Chem. 1995, 118, 163–169. [Google Scholar] [CrossRef]
- Ubaldini, A.; Carnasciali, M.M. Raman characterisation of powder of cubic RE2O3 (RE=Nd, Gd, Dy, Tm, and Lu), Sc2O3 and Y2O3. J. Alloys Compd. 2008, 454, 374–378. [Google Scholar] [CrossRef]
- Abrashev, M.V.; Todorov, N.D.; Geshev, J. Raman spectra of R2O3 (R—Rare earth) sesquioxides with C-type bixbyite crystal structure: A comparative study. J. Appl. Phys. 2014, 116, 103508. [Google Scholar] [CrossRef]
- Dilawar, N.; Varandani, D.; Mehrotra, S.; Poswal, H.K.; Sharma, S.M.; Bandyopadhyay, A.K. Anomalous high pressure behaviour in nanosized rare earth sesquioxides. Nanotechnology 2008, 19, 115703. [Google Scholar] [CrossRef]
- Wang, J.-H.; Li, Z.-P.; Liu, B.; Liu, B.-B. Local microstructural analysis for Y2O3/Eu3+/Mg2+ nanorods by Raman and photoluminescence spectra under high pressure. Chin. Phys. B 2017, 26, 026101. [Google Scholar] [CrossRef]
- Dilawar, N.; Mehrotra, S.; Varandani, D.; Kumaraswamy, B.V.; Haldar, S.K.; Bandyopadhyay, A.K. A Raman spectroscopic study of C-type rare earth sesquioxides. Mater. Charact. 2008, 59, 462–467. [Google Scholar] [CrossRef]
- Kumar, Y.; Pal, M.; Herrera, M.; Mathew, X. Effect of Eu ion incorporation on the emission behavior of Y2O3 nanophosphors: A detailed study of structural and optical properties. Opt. Mater. 2016, 60, 159–168. [Google Scholar] [CrossRef]
- Allieri, B.; Depero, L.E.; Marino, A.; Sangaletti, L.; Caporaso, L.; Speghini, A.; Bettinelli, M. Growth and microstructural analysis of nanosized Y2O3 doped with rare-earths. Mater. Chem. Phys. 2000, 66, 164–171. [Google Scholar] [CrossRef]
- Unal, F.; Kaya, F.; Kazmanli, K. Effects of dopant rate and calcination parameters on photoluminescence emission of Y2O3:Eu3+ phosphors: A statistical approach. Ceram. Int. 2019, 45, 17818–17825. [Google Scholar] [CrossRef]
- Bai, X.; Song, H.W.; Liu, B.B.; Hou, Y.Y.; Pan, G.H.; Ren, X.G. Effects of High Pressure on the Luminescent Properties of Nanocrystalline and Bulk Y2O3:Eu3+. J. Nanosci. Nanotechnol. 2008, 8, 1404–1409. [Google Scholar] [CrossRef]
- Sharma, N.D.; Singh, J.; Dogra, S.; Varandani, D.; Poswal, H.K.; Sharma, S.M.; Bandyopadhyay, A.K. Pressure-induced anomalous phase transformation in nano-crystalline dysprosium sesquioxide. J. Raman Spectrosc. 2011, 42, 438–444. [Google Scholar] [CrossRef]
- Pandey, S.D.; Samanta, K.; Singh, J.; Sharma, N.D.; Bandyopadhyay, A.K. Raman scattering of rare earth sesquioxide Ho2O3: A pressure and temperature dependent study. J. Appl. Phys. 2014, 116, 133504. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, J.; Li, X.-D.; Li, Y.-C.; He, S.-M.; Zhang, J.-C. High-Pressure Phase Transitions of Cubic Y2O3 under High Pressures by in-situ Synchrotron X-ray Diffraction. Chinese Phys. Lett. 2019, 36, 046103. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, X.; Qin, S. Pressure-induced phase transition of B-type Y2O3. Chinese Phys. B 2017, 26, 090703. [Google Scholar] [CrossRef]
- Ray, S.; Pramanik, P.; Singha, A.; Roy, A. Optical properties of nanocrystalline Y2O3:Eu3+. J. Appl. Phys. 2005, 97, 094312. [Google Scholar] [CrossRef]
- Ebendorff-Heidepriem, H.; Ehrt, D. Spectroscopic properties of Eu3+ and Tb3+ ions for local structure investigations of fluoride phosphate and phosphate glasses. J. Non. Cryst. Solids 1996, 208, 205–216. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, H.; Zhu, P.; Ma, C.; Wu, X.; Zhu, H.; Ma, Y.; Cui, Q. Photoluminescence studies of Y2O3:Eu3+ under high pressure. J. Appl. Phys. 2014, 115, 3–8. [Google Scholar] [CrossRef]
- Chang, N.C.; Gruber, J.B. Spectra and Energy Levels of Eu3+ in Y2O3. J. Chem. Phys. 1964, 41, 3227–3234. [Google Scholar] [CrossRef]
- Jovanic, B.R.; Dramicanin, M.; Viana, B.; Panic, B.; Radenkovic, B. High-pressure optical studies of Y2O3:Eu3+ nanoparticles. Radiat. Eff. Defects Solids 2008, 163, 925–931. [Google Scholar] [CrossRef]
- Piot, L.; Le Floch, S.; Cornier, T.; Daniele, S.; MacHon, D. Amorphization in nanoparticles. J. Phys. Chem. C 2013, 117, 11133–11140. [Google Scholar] [CrossRef]
- Yan, X.; Ren, X.; He, D.; Chen, B.; Yang, W. Mechanical behaviors and phase transition of Ho2O3 nanocrystals under high pressure. J. Appl. Phys. 2014, 116, 033507. [Google Scholar] [CrossRef]
- Jovanic, B.R.; Radenkovic, B.; Zekovic, L.D. The effect of pressure on the position and fluorescence lifetime for the the 5D0 → 7F2 transition in Y1.9Eu0.1O3. J. Phys. Condens. Matter 1996, 8, 4107–4110. [Google Scholar] [CrossRef]
- Machon, D.; Mélinon, P. Size-dependent pressure-induced amorphization: A thermodynamic panorama. Phys. Chem. Chem. Phys. 2015, 17, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Birch, F. Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high pressures and 300°K. J. Geophys. Res. 1978, 83, 1257–1268. [Google Scholar] [CrossRef]
- Cherian, R.; Gerard, C.; Mahadevan, P.; Cuong, N.T.; Maezono, R. Size dependence of the bulk modulus of semiconductor nanocrystals from first-principles calculations. Phys. Rev. B 2010, 82, 235321. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Staun Olsen, J.; Gerward, L.; Mørup, S. Enhanced bulk modulus and reduced transition pressure in γ-Fe2O3 nanocrystals. Europhys. Lett. 1998, 44, 620–626. [Google Scholar] [CrossRef]
- Gu, Q.F.; Krauss, G.; Steurer, W.; Gramm, F.; Cervellino, A. Unexpected high stiffness of Ag and Au nanoparticles. Phys. Rev. Lett. 2008, 100, 045502. [Google Scholar] [CrossRef]
- Abdullah, B.J.; Omar, M.S.; Jiang, Q. Size dependence of the bulk modulus of Si nanocrystals. Sādhanā 2018, 43, 174. [Google Scholar] [CrossRef]
- Clark, S.M.; Prilliman, S.G.; Erdonmez, C.K.; Alivisatos, A.P. Size dependence of the pressure-induced γ to α structural phase transition in iron oxide nanocrystals. Nanotechnology 2005, 16, 2813–2818. [Google Scholar] [CrossRef]
- Beck, C.; Bruch, C.; Ehses, K.-H.; Hempelmann, R. Size dependence of optical phonons in yttria nanoparticles by means of Raman light scattering. J. Phys. IV 2000, 10, Pr7-87–Pr7-90. [Google Scholar] [CrossRef]
- Beck, C.; Ehses, K.; Hempelmann, R.; Bruch, C. Gradients in structure and dynamics of Y2O3 nanoparticles as revealed by X-ray and Raman scattering. Scr. Mater. 2001, 44, 2127–2131. [Google Scholar] [CrossRef]





| V0/Z (Å3) | B0 (GPa) | κa (10−3 GPa−1) | ||
|---|---|---|---|---|
| Experimental | ||||
| Y2O3—Bulk a (Up to 12 GPa) | 74.517 (8) | 147 (1) | 3.2 (3) | 2.3 (5) |
| Y2O3-37 nm (Up to 12 GPa) | 74.82 (5) | 144 (7) | 3 (1) | 2.5 (5) |
| Y2O3—6 nm (Up to 10 GPa) | 74.88 (7) | 127 (2) | 4 (Fixed) | 3.3 (2) |
| Theoretical | ||||
| C-type Bulk Y2O3 (Up to 12 GPa) a | 74.24 (1) | 154 (1) | 3.5 (2) | 2.18 (8) |
| Symmetry | Experimental | Theoretical | Sharma et al. | |||||
|---|---|---|---|---|---|---|---|---|
| 37 nm | 6 nm | |||||||
| ω0 | dω/dP | ω0 | dω/dP | ω0 | dω/dP | ω0 | dω/dP | |
| Fg1 | 129.7 | −0.27 | 129.3 | −0.19 | 125.7 | −0.34 | ||
| Fg2 | 133.4 | 0.11 | ||||||
| Ag1 | 161.8 | 0.93 | 160.2 | 0.80 | 156.1 | 0.74 | ||
| Fg3 | 178.5 | 0.97 | ||||||
| Eg1 | 194.4 | 1.26 | 193.7 | - | 191.2 | 0.92 | ||
| Fg4 | 230.6 | 1.73 | ||||||
| Fg5 | 238.2 | 1.50 | ||||||
| Fg6 | 316.9 | 2.44 | 317.8 | 2.74 | 313.9 | 2.29 | 316 | |
| Fg7 | 320.1 | 2.37 | ||||||
| Eg2 | 329.8 | 3.06 | 329.3 | 3.18 | 326.8 | 2.81 | 331 | |
| Fg8 | 348.9 | 3.57 | ||||||
| Ag2 | 356.4 | 2.31 | ||||||
| Fg9 | 377.2 | 3.65 | 376.1 | 2.87 | 378.8 | 3.51 | 381.3 | 3.79 |
| Eg3 | 382.4 | 4.10 | ||||||
| Fg10 | 408.0 * | 3.25 | 392.7 | 3.58 | ||||
| Ag3 | 419.7 | 4.47 | ||||||
| Fg11 | 430.4 | 4.44 | 429.1 | 4.01 | 430.1 | 4.42 | ||
| Fg12 | 468.9 | 4.95 | 467.7 | 3.98 | 460.3 | 4.80 | 472.0 | 5.02 |
| Fg13 | 521.4 | 3.86 | ||||||
| Ag4 | 554.6 | 4.11 | ||||||
| Eg4 | 560.0 | 4.30 | ||||||
| Fg14 | 592.4 | 4.78 | 592.4 | 3.71 | 583.4 | 4.63 | 598.1 | 4.58 |
| Symmetry | Experimental | Theoretical | ||
|---|---|---|---|---|
| ω22GPa | dω/dP | ω22GPa | dω/dP | |
| Eg1 | 184.7 | 1.35 | 168.4 | 1.16 |
| A1g1 | 319.4 | 2.10 | 300.5 | 1.71 |
| A1g2 | 553.1 | 2.27 | 523.7 | 2.00 |
| Eg2 | 596.7 | 2.27 | 581.6 | 2.42 |
| Symmetry | Experimental | Theoretical | ||
|---|---|---|---|---|
| ω0 | dω/dP | ω0 | dω/dP | |
| Bg1 | 89.2 | 0.77 | ||
| Ag1 | 114.2 | 0.37 | ||
| Bg2 | 133.3 | 1.45 | 130.0 | 1.40 |
| Ag2 | 145.0 | 1.00 | ||
| Bg3 | 150.3 | 1.01 | 153.2 | 0.72 |
| Ag3 | 161.5 | 0.08 | ||
| Ag4 | 197.7 | 0.71 | ||
| Ag5 | 226.3 | 2.54 | ||
| Ag6 | 282.8 | 1.58 | ||
| Ag7 | 286.7 | 3.33 | ||
| Ag8 | 298.8 | 2.53 | ||
| Bg4 | 308.7 | 5.58 | ||
| Ag9 | 389.5 | 4.69 | ||
| Bg5 | 407.0 | 3.99 | 404.3 | 3.30 |
| Ag10 | 429.7 | 3.39 | ||
| Bg6 | 433.3 | 5.12 | ||
| Bg7 | 439.5 | 2.40 | 443.0 | 4.60 |
| Ag11 | 470.7 | 3.24 | 464.6 | 2.73 |
| Ag12 | 505.6 | 3.79 | 499.4 | 4.06 |
| Ag13 | 595.5 | 4.36 | ||
| Ag14 | 605.7 | 4.44 | ||
| Peak | Y2O3:Eu3+-37 nm | Y2O3:Eu3+-6 nm | Zhang et al. | Bai et al. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Bulk | λ0 (nm) | Bulk | 20 nm Nanoparticle | ||||||
| λ0 (nm) | dλ/dP (nm/GPa) | λ0 (nm) | dλ/dP (nm/GPa) | λ0 (nm) | dλ/dP (nm/GPa) | dλ/dP (nm/GPa) | dλ/dP (nm/GPa) | ||
| 5D0–7F1 | 600.6 | 0.11 (1) | 600.8 | 0.08 (1) | |||||
| 0 | - | - | 610.1 | 0.06 (1) | |||||
| 1 | 611.2 | 0.09 (1) | 611.0 | 0.08 (1) | 611.9 | 0.098 | 611.4 | 0.101 | 0.102 |
| 2 | 612.9 | 0.10 (1) | 612.9 | 0.08 (1) | |||||
| 3 | 614.3 | 0.15 (2) | 614.5 | 0.14 (2) | |||||
| 4 | 631.1a | 0.20 (1) a | 631.1 | 0.17 (1) | 631.4 | 0.198 | 0.184 | ||
| 5 | - | - | 623.6 | 0.14 (2) | |||||
| 6 | - | - | 627.7 | 0.10 (1) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.L.d.J.; Sans, J.Á.; Gomis, Ó.; Santamaría-Pérez, D.; Ray, S.; Godoy, A., Jr.; da Silva-Sobrinho, A.S.; Rodríguez-Hernández, P.; Muñoz, A.; Popescu, C.; et al. Size-Dependent High-Pressure Behavior of Pure and Eu3+-Doped Y2O3 Nanoparticles: Insights from Experimental and Theoretical Investigations. Nanomaterials 2024, 14, 721. https://doi.org/10.3390/nano14080721
Pereira ALdJ, Sans JÁ, Gomis Ó, Santamaría-Pérez D, Ray S, Godoy A Jr., da Silva-Sobrinho AS, Rodríguez-Hernández P, Muñoz A, Popescu C, et al. Size-Dependent High-Pressure Behavior of Pure and Eu3+-Doped Y2O3 Nanoparticles: Insights from Experimental and Theoretical Investigations. Nanomaterials. 2024; 14(8):721. https://doi.org/10.3390/nano14080721
Chicago/Turabian StylePereira, André Luis de Jesus, Juan Ángel Sans, Óscar Gomis, David Santamaría-Pérez, Sudeshna Ray, Armstrong Godoy, Jr., Argemiro Soares da Silva-Sobrinho, Plácida Rodríguez-Hernández, Alfonso Muñoz, Catalin Popescu, and et al. 2024. "Size-Dependent High-Pressure Behavior of Pure and Eu3+-Doped Y2O3 Nanoparticles: Insights from Experimental and Theoretical Investigations" Nanomaterials 14, no. 8: 721. https://doi.org/10.3390/nano14080721
APA StylePereira, A. L. d. J., Sans, J. Á., Gomis, Ó., Santamaría-Pérez, D., Ray, S., Godoy, A., Jr., da Silva-Sobrinho, A. S., Rodríguez-Hernández, P., Muñoz, A., Popescu, C., & Manjón, F. J. (2024). Size-Dependent High-Pressure Behavior of Pure and Eu3+-Doped Y2O3 Nanoparticles: Insights from Experimental and Theoretical Investigations. Nanomaterials, 14(8), 721. https://doi.org/10.3390/nano14080721









