Abstract
Cesium bismuth iodide perovskite material offers good stability toward ambient conditions and has potential optoelectronic characteristics. However, wide bandgap, absorber surface roughness, and poor surface coverage with pinholes are among the key impediments to its adoption as a photovoltaic absorber material. Herein, bandgap modification and the tailoring of surface morphology have been performed through molar ratio variation and antisolvent treatment, whereby type III antisolvent (toluene) based on Hansen space has been utilized. XRD and Raman spectroscopy analyses confirm the formation of a 0D/2D mixed dimensional structure with improved optoelectronic properties when the molar ratio of CsI/BiI3 was adjusted from 1.5:1 to 1:1.5. The absorption results and Tauc plot determination show that the fabricated film has a lower bandgap of 1.80 eV. TRPL analysis reveals that the film possesses a very low charge carrier lifetime of 0.94 ns, suggesting deep defects. Toluene improves the charge carrier lifetime to 1.89 ns. The average grain size also increases from 323.26 nm to 444.3 nm upon toluene addition. Additionally, the inclusion of toluene results in a modest improvement in PCE, from 0.23% to 0.33%.
1. Introduction
Lead-based perovskite thin film solar cell absorbers have demonstrated a significant improvement in power conversion efficiency (PCE) from 3.8% to 26.1%, which is now comparable to that of single-junction crystalline silicon-based solar cells (26.1%) [1,2]. However, silicon-based solar cells account for 95% of the worldwide market, while thin-film perovskite solar cells have yet to enter the commercial sector owing to concerns about lead stability and toxicity [3,4,5,6]. Therefore, improving stability and replacing lead with a less toxic substance are the primary objectives of contemporary perovskite solar cell research. Tin (Sn), bismuth (Bi), antimony (Sb), copper (Cu), and germanium (Ge) have been identified as potential lead substitutes [4,7]. Solar cells using organic–inorganic hybrid lead-free tin-based perovskite absorbers now have a PCE of more than 15%, while all-inorganic tin-based lead-free perovskites have a PCE greater than 10% [6,8,9,10,11,12]. This advancement in PCE paves the way for perovskite thin film material toward overcoming the lead toxicity challenge [13,14,15,16]. Moreover, perovskites made with all-inorganic lead-free absorbers have demonstrated superior stability in the face of humidity, light, and heat [13,17,18,19]. All-inorganic lead-free perovskites solar cells created by chemically combining Cs, Bi, and halide elements are among the lead-free compounds under investigation [20,21,22,23]. When compared to tin-based perovskites which offer the highest efficiency among lead-free substitutes, cesium bismuth iodide provides superior stability under ambient conditions. Sn-based perovskites as well as Ge-based perovskites are very unstable upon air exposure due to self-doping. Sn2+ and Ge2+ normally oxide to Sn4+ and Ge4+ and overcoming their oxidation necessitates the application of several additive engineering techniques, which increases the cost of solar cell production [24,25,26,27]. This is in contrast to Bi-based perovskite films which form a protective layer of Bi2O3 or BiOI upon exposure to air which protects the film from environmental degradation [28,29,30,31].
However, despite its diversity in application, its non-toxicity, and its stability superiority, the research focal point on cesium bismuth iodide perovskite materials is directed toward improving the film’s surface morphology and bandgap adjustment with regard to its modest performance as a photovoltaic solar absorber material. The limited solubility of CsI and BiI3 precursors in organic solvents such as DMF or DMSO, along with the fast crystallization rate, has a negative impact on the quality of the film’s morphology. The surface deteriorates, leaving numerous voids and pinholes, as well as having poor reproducibility. Moreover, low dimensionality causes the materials to have a large bandgap, increased exciton energy, low charge carrier mobility, and modest absorption intensity [20,32]. Nevertheless, uniform surface morphology and good surface coverage are important film formation parameters for the production of perovskite cells with good optoelectronic properties and high reproducibility [6]. Antisolvent engineering techniques have been extensively deployed to tailor the quality of film morphology [33,34,35].
Antisolvents act as heterogeneous catalysts, speeding up the nucleation rate by increasing the rate of instantaneous supersaturation during the spinning process [36]. Supersaturation occurs upon antisolvent addition due to a reduction in solubility of the precursor solvents and the solvent washing away [35]. The type of antisolvent, the ratio between the precursor solvent and the antisolvent, and the antisolvent dripping time and speed are among key parameters to be considered for enhanced efficiency [37]. Even though the interaction between the solvent and antisolvent or between the precursor and antisolvent is governed by the nature of the antisolvent, an ideal antisolvent is required to be insoluble with both the host solvent and perovskite precursors but also miscible with host solvent [38]. When chlorobenzene was utilized in the fabrication of bismuth-based perovskite films, the result was a decrease in grain size and a modest improvement in surface coverage [39]. Ghosh and coworkers performed a comparative study on the use of antisolvents on a lead-free cesium bismuth iodide perovskite for solar cell applications. Type I (isopropanol), II (chlorobenzene), and III (toluene) antisolvents were used. According to Hansen space perspectives, type I antisolvents are alcohol-based and therefore operate on the basis of hydrogen bonding. Type III possesses a low contribution from hydrogen bonding with negligible polar forces and is almost immiscible with the common types of solvents such as DMF and DMSO, while type II receives contributions from both. Upon utilization with cesium bismuth iodide solar cells, type III antisolvents (toluene) offered the highest power conversion efficiency of 0.046%, followed by chlorobenzene (0.04%), and the lowest PCE was achieved with isopropanol (0.02%) [40]. The influence of several kinds of antisolvents, including methanol, toluene, isopropanol, diethyl ether, chlorobenzene, and chloroform, on the crystallization of CsBi3I10 perovskite film was investigated [41]. The produced film was observed to dissolve in diethyl ether, chloroform, and methanol, leading to a low absorption intensity and poor quality of film morphology. It was discovered that the CsBi3I10 film performed better with toluene, chlorobenzene, and isopropanol, with chlorobenzene exhibiting outstanding results in film-quality tailoring. The optimized solar cell device displayed a PCE of 0.63% [41]. Toluene was further used to enhance the crystallinity of bismuth-based perovskite solar cells using a single or double A-site cation; the single A-site cation-based bismuth perovskite achieved a PCE of 0.24% with toluene as the antisolvent, while with the double A-site cation, the PCE was 1.5% [42]. With regard to bandgap adjustment, several techniques have been put in place, such as through compositional engineering as well as molar ratio variation. Adjusting the molar ratio from 1.5:1 to 1:3 resulted in the fabrication of the first cesium bismuth iodide (CsBi3I10) with a bandgap of 1.77 eV and improved its optoelectronic properties. Johanson further performed a comprehensive investigation on molar ratio variation by considering several ratios of CsI, 1:1, 1.5:1, 1:2, 1:3, and 1:9, and BiI3, and discovered that any compound fabricated with a molar ratio of 1.5:1 containing a mixture of CsI and BiI3 possesses improved optoelectronic properties [29,43]. Our group reported an improved morphological quality when cesium bismuth iodide was fabricated with a precursor ratio of 1:1.5 (CsI/BiI3) via solvent vapor annealing. A PCE boost from 0.23% under conventional annealing to 0.98% upon DMF/CH30H solvent vapor annealing was reported [44]. However, this cesium bismuth iodide compound fabricated at a 1:1.5 ratio has not yet been fully explored and most information regarding its electronic properties is unknown.
Herein, we report the morphological, structural, and optical characterization of a cesium bismuth iodide perovskite film fabricated at a molar ratio of (CsI/BiI3) 1:1.5. We further applied antisolvent-assisted crystallization with toluene as the antisolvent to enhance the quality of film morphology through a one-stage approach as shown in Figure 1. XRD and Raman spectroscopy analyses confirmed the formation of a cesium bismuth iodide with a mixed 0D/2D dimensionality structure. The absorption data and Tauc plot determination showed that the fabricated film has a lower bandgap of 1.80 eV than that of Cs3Bi2I9 (2.03–2.30 eV) at a molar ratio of 1.5:1. The average grain size also increased from 323.26 nm to 444.3 nm upon toluene addition. A modest increase in PCE from 0.23% to 0.33% was also observed upon toluene addition.
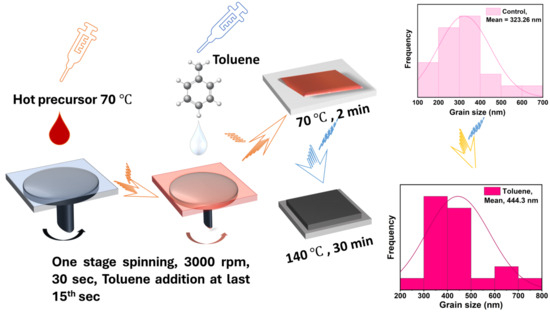
Figure 1.
Fabrication of cesium bismuth iodide with toluene as antisolvent and morphological advancement through grain size enlargement.
2. Materials and Methods
2.1. Materials
Bismuth iodide (BiI3, 99%), 4-tert-butylpyridine (TBP), tris (2-(1H-pyrazol-1-yl)-4-tert-butylpyridine-cobalt (III)Tris(bis(trifluoromethylsulfonyl)imide)) (FK 209), Bis(tri-fluoromethane) sulfonimide lithium salt (99.95%, Li-TFSI, trace metals basis), and chlorobenzene (99.8%, anhydrous) were obtained from Sigma Aldrich (St. Louis, MO, USA). Acetylacetone (C5H8O2, 99.0%) and Tetra-isopropyl orthotitanate (C12H28O4Ti, s.g 0.97) were from TCI (Tokyo, Japan). Acetonitrile, Ti02 paste 30NR-D, and cobalt (III)tris(trifluoromethylsulfonyl)imide) were from Greatcell solar materials (Queanbeyan, NSW, Australia). Cesium iodide (CsI, 99.99%) was obtained from Acros (Geel, Belgium), while ethanol absolute (99.9%) was from Innochem (Pyeongtaek, Republic of Korea). Isopropanol, methanol, dimethyl sulphoxide (DMSO, anhydrous, ≥99.9%), and N, N-dimethylformamide (DMF, anhydrous, 99.8%) were obtained from Alfer Asar (Ward Hill, MA, USA). 2,2,7,7-Tetrakis (N, N-p-dimethoxyphenylamino)-9,9-spirobifluorene (spiro-OMeTAD) Spiro OMeTAD was from Xi’an Polymer Light Technology Corp (Xi’an, China). All chemicals and reagents were used without further purification.
2.2. Perovskite Film and Solar Cell Fabrication Process
The glass substrates coated with fluorine-doped tin oxide (FTO, Pilkington, TEC 15, Lathom, UK) were ultrasonically cleaned with detergent, deionized water, and ethanol for 20 min each prior to ozone treatment. The compact layer was then formed using spray pyrolysis, and the mesoporous electron transport layer was further deposited using the spin coating method. The steps for preparing the solution and for deposition are the same as previously reported [44]. A one-step toluene-assisted crystallization method was used to fabricate the perovskite solar cell absorber. The film was fabricated using CsI and BiI3 at a molar ratio of 1:1.5 dissolved in a mixture of DMF and DMSO at a volume ratio (900/100). The molar ratio of 1:1.5 was adopted instead of 1.5:1 since molar ratio variation has been previously reported to alter the optoelectronic properties of cesium bismuth iodide perovskite absorbers. The antisolvent dripping time was varied between 10 s and 15 s and the volume was adjusted from 150 mL to 200 mL. The optimum dripping time and volume was found to be at the 15th s and 200 mL, respectively. The perovskite precursors were dissolved in 1 mL of the solution mixture of DMF and DMSO at 900:100 (v/v). The hot perovskite precursor solution (70 °C) was spin-coated for 30 s on top of a mesoporous layer at a speed of 3000 rpm. In the last ten to fifteen seconds of the spinning process, about 200 µL of toluene was applied on the antisolvent-treated substrates. The films were then annealed at 70 °C for a few minutes and then at 140 °C for 30 min. After the annealing process, the 2,2,7,7-Tetrakis (N, N-p-dimethoxyphenylamino)-9,9-spirobifluorene (spiro-OMeTAD) was spin-coated at a speed rate of 4000 rpm for 20 s. The spiro-OMeTAD precursor solution was prepared by dissolving 73.5 g of spiro-OMeTAD in 1 mL of chlorobenzene with 29 µL of TBP, 17.0 µL of a Li-TFSI solution, and 8 µL of an FK209–cobalt (III) TFSI solution. The Li-TFSI solution and cobalt (III) solution were prepared by dissolving 520 mg of Li-TFSI salt and 300 mg of FK209–cobalt (III) TFSI salt, respectively, in 1 mL of acetonitrile. We deposited a 70 nm to 80 nm thick gold electrode using the thermal evaporation method at a pressure of 4 × 10−4 MPa. Then, a black mask defined at the 0.0627 cm2 active layer was used for characterization.
2.3. Characterization
A UV–vis spectrophotometer (UV-2450, Shimadzu corp, A 10834735110, Kyoto, Japan) was used to examine the film’s UV–vis spectra. The XRD patterns were recorded by using an X-ray diffractometer (XRD, Smart Lab, Rigaku, Tokyo, Japan) with Cu-Kα radiation (1.5418). The scanning of diffraction angles ranged from 10° to 60° at a scanning speed of 5° per min. Scanning electron microscopy (SEM) images were obtained using an SU8010 SEM (Hitachi, Matsuda, Tokyo, Japan). The current density vs. voltage (J-V) characterization was examined on a fabricated perovskite solar cell with the configuration of FTO/c-TiO2/m-TiO2/CBI/spiro-OTAD/Au using a Keithley 2400 (Cleveland, OH, USA) source-meter together with a sunlight simulator (XES-300T1, SAN-EI Electric, AM 1.5 G 100 Mw/cm2, Tokyo, Japan). Standard silicon was used as a reference cell. The photoluminescence (PL) and time-resolved photoluminescence (TRPL) spectra on the glass substrates were measured using an FS5 Spectrofluorometer (Edinburgh Instruments, Oxford, UK). The Raman spectra measurements were carried out using a diode laser with a 532 nm light source. X-ray photoelectron spectroscopy (XPS) measurements were carried out using an RBD upgraded PHI-5000C ESCA system (Perkin Elmer, Waltham, MA, USA) with MgKᾳ radiation. hµ = 1486.6 eV and the contact angle were obtained using Dataphysics OCA15EC (Filderstadt, Germany). A multimode atomic force microscope (AFM), namely, an Agilent Technologies 5500 instrument (Santa Clara, CA, USA), was used to assess the surface roughness in tapping mode.
3. Results and Discussion
3.1. XRD Analysis
Cesium bismuth iodide perovskite was fabricated using a mixture of DMF and DMSO (9/1) (v/v) with and without toluene treatment. DMF was selected due to its strong dissolution power, while DMSO was chosen due to its strong coordination ability [45]. The molar ratio of CsI/BiI3 was adjusted from 1.5:1 for Cs3Bi2I9 to 1.15 since adjustments in the precursor molar ratio cause significant improvements in the optoelectronic properties. X-ray diffraction (XRD) measurements were carried out on the fabricated CBI perovskite at diffraction angles ranging from 10° to 60° to evaluate the influence of toluene on the crystallinities of the perovskite films. The XRD patterns of both the control and toluene-treated samples exhibited comparable diffraction peak positions, as shown in Figure 2, implying that the addition of toluene does not lead to structural changes. The highest peaks were found to be at the (101) and (202) planes, with corresponding angles of 12.8 and 25.8, indicating (101) preferred orientation. However, there are diffraction peaks of (113) and (300) planes at 26.97 and 41.56 respectively, in both the control and toluene-treated films. These peaks indicate that BiI3 is present in the crystal lattice. The main characteristic peaks for BiI3 that have been documented in the literature are found in planes (113) and (300) [46,47]. The results validate the existence of hybrid 0D-Cs3Bi2I9 and 2D BiI3 structures. A change in the molar ratio leads to the creation of a mixed-dimensional structure [48]. Moreover, all other diffraction peaks correlate well with the fabricated cesium bismuth iodide perovskite films reported in the literature and there are no peaks that can be assigned to CsI crystals; this signifies the full consumption of cesium iodide [29,44].
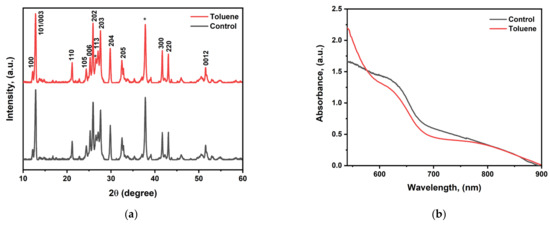
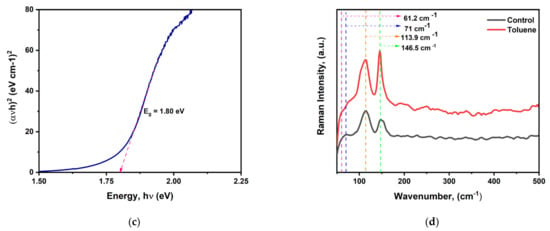
Figure 2.
(a) XRD pattern for cesium bismuth iodide fabricated with and without toluene as antisolvent. (b) UV–visible spectrum for control and toluene-treated samples. (c) Bandgap determination using Tauc plot analysis. (d) Raman spectra for control and toluene-treated perovskite films (* stands for SnO2 from FTO).
3.2. Ultraviolet–Visible Absorption Analysis
The ultraviolet–visible absorption spectrum analysis in Figure 2b shows that the toluene-treated sample does not exhibit a discernible increase in absorption intensity. According to the absorption data in Figure 2c from the Tauc plot analysis, the bandgap of the cesium bismuth iodide photovoltaic absorber fabricated at a ratio of CsI/BiI3 (1:1.5) is determined to be 1.80 eV, which is lower than that of the well-reported Cs3Bi2I9 film fabricated at 1.5:1, which has a bandgap between 2.20 eV and 2.03 eV. Therefore, altering the molar ratio results in a modification of the crystal structure, which in turn enhances the optoelectronic capabilities.
3.3. Raman Spectroscopy Analysis
We further performed Raman spectroscopy analysis to confirm the formation of cesium bismuth iodide perovskite film and study the exciton–phonon interactions. Raman spectroscopy has been identified as an efficient experimental method to analyze the exciton–phonon interactions [49]. Both the control film and the toluene-treated film exhibited characteristic peaks at 146.5 and 113.9 cm−1. The wavenumber at 146.5 cm−1 corresponds to the terminal Bi-I symmetric stretch, while the characteristic peak at 113.9 cm−1 is not assigned to any stretching or vibrational mode with regard to cesium bismuth iodide but is the strongest characteristic peak for BiI3 crystal which corresponds to the intense zone center C(G) phonon mode of Ag’ symmetry [49,50,51,52]. The findings imply that both the control and toluene-treated films contain a mixture of two phases, 0D-Cs3Bi2I9 and 2D-BiI3. Other minor characteristic peaks were also observed at 71 cm−1 which originated from BiI3 crystal in the control film, which shifted to 61 cm−1 upon toluene addition The wavenumber at 61 cm−1 is the characteristic peak coming from Cs3Bi2I9 crystal. Moreover, the toluene-treated sample was found to have stronger peak intensity compared to the pristine one, as shown in Figure 2d.
3.4. Scanning Electron Microscopy Analysis and Atomic Force Microscope Analysis
Scanning electron microscopy (SEM) analysis was further performed to investigate the effect of toluene addition on the film morphology of CBI perovskite. It can be clearly seen in Figure 3a,b that there is an increase in perovskite crystal size and a reduction in the number of pinholes in the toluene-treated sample. The mean crystal size has increased from 323.3 nm to 444.3 nm upon toluene addition. Both the toluene-treated film and the control film have a mixture of hexagonal and irregular grains. Many pinholes affect the coverage of subsequent layers such as the hole transport layer and metal contact layer, hence limiting the power conversion efficiency. Increased crystal size is advantageous since large grains tend to have a lower density of the grain boundaries and, as a result, a lower number of the corresponding defects located in the grain boundaries. A film containing a high concentration of small grains or grain boundaries is susceptible to Shockley–Read–Hall (SRH) recombination. The grain boundaries serve as non-radiative recombination centers since they are vulnerable to point defect formation, serving as deep traps. An et al. reported that the smallest grain size is linked to a short charge carrier lifetime, while large grains have a longer charge carrier lifetime [53]. The trap density was found to increase linearly with decreasing grain size and it has even been discovered that charge recombination at the grain boundary is significantly more potent than it is at the film surface without surface passivation [53,54,55]. Other film microstructure properties such as the size and density of pinholes, crystallite orientation, and crystallinity quality should also be clearly controlled to achieve a high PCE since nanoscale inhomogeneity and morphological defects are the underlying causes of limited PCE. A decrease in defect density results in an enhancement in charge carrier mobility and a higher diffusion length, which are directly linked with cell power conversion efficiency.
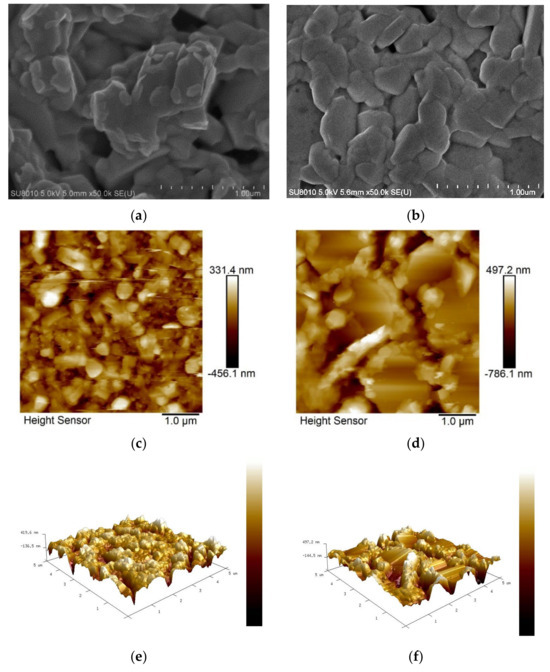
Figure 3.
SEM and AFM analyses for (a,c,e) control and (b,d,f) toluene-treated samples, respectively.
Figure 3c,e show AFM images of the control film with low quality, consisting of irregular, small-sized, randomly distributed grains with voids, confirming the consequence of fast-induced crystallization. When toluene is added, shown in Figure 3d,f, the grains grow in size, revealing a hexagonal lattice crystal with a large number of small-sized irregular grains. The density of pinholes has been reduced, but they can still be visible after adding toluene, indicating that full film coverage has not been attained. There was no improvement in root-mean-square surface roughness seen with the control (77.5 nm) or toluene-treated films (118 nm). The increase in surface roughness may be due to uneven increase in grain size in the target film.
Toluene, as schematically depicted in Figure 4, has a tendency to drive the perovskite into a metastable zone instead of a supersaturated zone. In the metastable zone, there is consistency and uniformity in the nucleation and crystal growth rate, leading to the formation of a perovskite film with large grains and a low grain boundary [56].
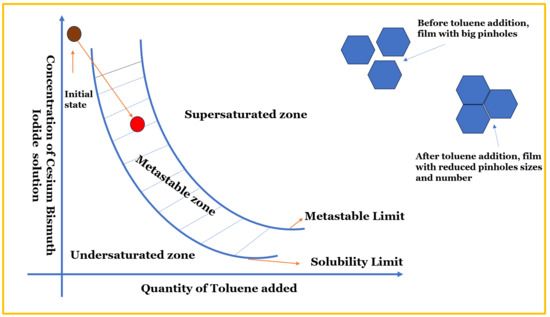
Figure 4.
A schematic diagram showing the toluene tendency to shift the perovskite from the under-supersaturated to metastable zone, hence enhancing film morphology [57].
3.5. X-ray Photoelectron Spectroscopy Analysis
In order to further study the chemical state of Cs, Bi, and I and the interaction between toluene and cesium bismuth iodide perovskite elements, we performed X-ray photoelectron spectroscopy (XPS) measurements. A slight decrease in binding energy from 738.8 eV to 738.6 eV and from 724.9 eV to 724.7 eV can be clearly observed for Cs 3d in Figure 5a upon toluene addition. The shifting of peaks signifies the presence of interaction between toluene and cesium bismuth iodide perovskite. The slight blue shift signifies a modest change in the chemical environment created upon toluene addition. The shift might be caused by the chemical nature of the neighboring atoms on the cesium bismuth iodide surface. The presence of higher valence anionic (Bi2I9)3− octahedron units which are not bound to each other causes the electron density to increase, and consequently, a decrease in binding energy is observed.
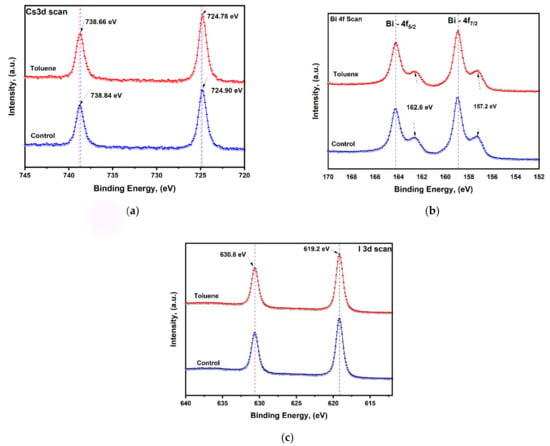
Figure 5.
XPS analysis for control and toluene-treated samples on (a) Cs scan, (b) Bi scan, and (c) iodine scan.
Moreover, for Bi-4f, the characteristic peaks of Bi4f7/2 and Bi4f5/2 are found at 158.9 and 164.2 eV, respectively. Furthermore, on both pristine and toluene-treated films, further peaks have been identified at 162.6 eV after Bi-4f5/2 and 157.2 eV after Bi-4f7/2. These peaks are caused by the existence of metallic Bi0, which is the primary factor causing poor device performance as a result of induced recombination [51,58]. No shifting of peaks has been observed on the Bi-4f scan and I scan, meaning that toluene addition has an effect on cesium only; this indicates that toluene addition does not cause significant changes in the electronic state and hence will have an insignificant contribution to the overall power conversion efficiency.
3.6. FTIR Spectra and Ultraviolet Photoelectron Spectroscopy Analysis
Fourier transform infrared (FTIR) spectra were obtained in order to investigate the effect of toluene on the surface chemistry of cesium bismuth iodide perovskite film, as shown in Figure 6a–c. The peak at wavenumber 1531.7 cm−1 belongs to the functional groups of (S=O) stretching bonds from DMSO, while the peak at 621.4 cm−1 which lies in the fingerprint region is ascribed to the C-S functional group. Upon toluene addition, the peaks have shifted from 1531.7 cm−1 to 1528.05 cm−1 and from 621.4 cm−1 to 619.4 cm−1. The shifting of peaks indicates more robust binding strength between toluene, CsI, and BiI3 complexes.
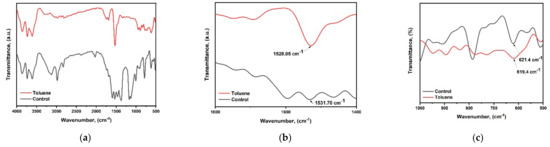
Figure 6.
FTIR analysis (a–c) for control film and toluene-treated film.
3.7. J-V Characterization, and PL and TRPL Analysis
Time-resolved photoluminescence (TRPL) analyses were also carried out to examine the charge recombination behavior of the CBI perovskite films fabricated with and without toluene treatment (Figure 7a). The two exponential decay curve fittings, displayed in Table S1 in the Supporting Information, reveal that the charge carrier lifetime of the pristine film is prolonged from 0.94 ns to 1.53 ns upon toluene addition.
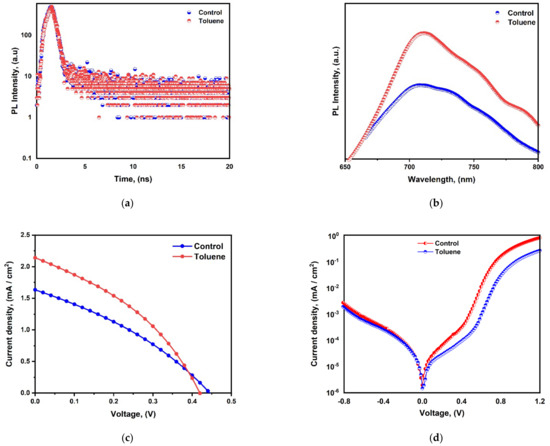
Figure 7.
(a) Time-resolved PL intensity; (b) PL intensity; (c) J-V characteristics; (d) J-V Characteristics under dark current measurements.
This modest improvement in carrier lifetime signifies the ability of toluene to tailor the quality of film morphology, hence reducing trap-assisted surface or bulk recombination and, consequently, non-radiative recombination. Improved crystallinity, which results in a lower defect density, is indicated by an increase in grain size and a notable decrease in density and pinhole size. This is reflected in an improvement in the charge carrier lifetime and PCE. Therefore, a longer charge carrier lifetime denotes better crystallization and less non-radiative recombination activity. An increase in charge carrier concentration, absorption intensity, and absorption wavelength corresponds to a decrease in bandgap and an improvement in electron transport efficiency, which affects current density, FF, and Voc, as well as the device’s overall performance.
However, in general, this charge carrier lifespan is still too short, indicating the possibility of CBI film to still have deep defects that act as recombination sites, resulting in a reduced lifetime. The photoluminescence peak positions for the pristine and toluene-treated films were almost identical and were around 710 nm. However, there was an obvious increase in absorption intensity with toluene-treated sample, as shown in Figure 7b.
The photovoltaic performance of the cesium bismuth iodide perovskite solar cell with a configuration of FTO/c-TiO2/m-TiO2/CBI/Spiro-OMeTAD/Au was further analyzed. The current density–voltage (J-V) characteristics study under standard AM 1.5 G illumination is shown in Figure 7c. The pristine film’s power conversion efficiency increased from 0.24 to 0.33% upon toluene addition. Moreover, the open-circuit voltage, Voc, slightly decreased from 0.45 V to 0.43 V, while the current density, Jsc, and fill factor increased from 1.63 to 2.14 mA/cm2 and from 33.15% to 36.98%, as shown in Table 1. Dark-state J-V measurements were further carried out to estimate the charge accumulation and extraction capabilities at the device interfaces for both pristine and toluene-treated perovskite solar cells. Under forward bias, the toluene-treated perovskite solar cell had a lower dark current and a longer extraction slope than the pristine solar cell (Figure 7d). This indicates that the use of toluene promotes efficient charge extraction capabilities while reducing charge accumulation.

Table 1.
J-V Characteristics Results.
4. Conclusions
In summary, we have fabricated and characterized a low-bandgap cesium bismuth iodide perovskite solar cell using toluene-assisted crystallization. The fabricated perovskite film was found to have a bandgap of 1.80 eV and an extended absorption wavelength to 710 nm. Toluene addition regulated the crystallization rate, which enabled the advancement in the quality of film morphology. The perovskite average grain size grew from 323.22 nm to 444.3 nm. Perovskite films with large grains are advantageous due to the reduced grain boundary defect density, hence minimizing trap-assisted surface or bulk recombination and, consequently, non-radiative recombination.
As a result, an increase in absorption intensity, phase purity, charge carrier lifetime, and PCE, from 0.24 to 0.33%, have been attained. However, it should be noted that the modest performance may be contributed by other factors such as energy mismatch between electron/hole transport materials. This research study paves the way for future research on electron and hole transport material optimization and other methods for tailoring surface morphology, such as passivation techniques.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano14070626/s1, Figure S1. (a) Ftir at a broder wavenumber from 4000cm−1 and raman spectra for a wavenumber between 70cm−1 and 200cm−1; Figure S2. SEM analyisis for control, (b) for toluene treated film.
Author Contributions
Conceptualization, S.M.M. and J.Y.; methodology, S.M.M., C.Z. and J.L.; validation, J.Y.; formal analysis, S.M.M.; investigation, S.M.M., C.Z. and J.L.; resources, J.Y.; data curation, S.M.M., C.Z. and J.L.; writing—original draft preparation, S.M.M.; writing—review and editing, S.M.M. and J.Y.; visualization, J.Y. and J.X.; supervision, J.Y. and J.X.; project administration, J.Y and J.X.; funding acquisition, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 51772095) and the Hebei Province Key Research and Development Project (No. 20314305D).
Data Availability Statement
Data will be available upon request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar]
- Abate, A. Perovskite Solar Cells Go Lead Free. Joule 2017, 1, 659–664. [Google Scholar] [CrossRef]
- Wadi, M.A.A.; Chowdhury, T.H.; Bedja, I.M.; Lee, J.J.; Amin, N.; Aktharuzzaman, M.; Islam, A. Evolution of Pb-Free and Partially Pb-Substituted Perovskite Absorbers for Efficient Perovskite Solar Cells. Electron. Mater. Lett. 2019, 15, 525–546. [Google Scholar] [CrossRef]
- Miyasaka, T.; Kulkarni, A.; Kim, G.M.; Öz, S.; Jena, A.K. Perovskite Solar Cells: Can We Go Organic-Free, Lead-Free, and Dopant-Free? Adv. Energy Mater. 2020, 10, 1902500. [Google Scholar] [CrossRef]
- Chen, J.; Luo, J.; Hou, E.; Song, P.; Li, Y.; Sun, C.; Feng, W.; Cheng, S.; Zhang, H.; Xie, L.; et al. Efficient tin-based perovskite solar cells with trans-isomeric fulleropyrrolidine additives. Nat. Photonics 2024. [Google Scholar] [CrossRef]
- Pazoki, M.; Edvinsson, T. Metal replacement in perovskite solar cell materials: Chemical bonding effects and optoelectronic properties. Sustain. Energy Fuels 2018, 2, 1430–1445. [Google Scholar] [CrossRef]
- Nishimura, K.; Akmal, M.; Hirotani, D.; Hamada, K.; Shen, Q.; Iikubo, S.; Minemoto, T.; Yoshino, K.; Hayase, S. Nano Energy Lead-free tin-halide perovskite solar cells with 13% efficiency. Nano Energy 2020, 74, 104858. [Google Scholar] [CrossRef]
- Li, B.; Di, H.; Chang, B.; Yin, R.; Fu, L.; Zhang, Y.N.; Yin, L. Efficient Passivation Strategy on Sn Related Defects for High Performance All-Inorganic CsSnI3 Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2007447. [Google Scholar] [CrossRef]
- Ban, H.; Nakajima, T.; Liu, Z.; Yu, H.; Sun, Q.; Dai, L.; Shen, Y.; Zhang, X.-L.; Zhu, J.; Chen, P.; et al. Over 8% efficient CsSnI3-based mesoporous perovskite solar cells enabled by two-step thermal annealing and surface cationic coordination dual treatment. J. Mater. Chem. A 2022, 10, 3642–3649. [Google Scholar] [CrossRef]
- Yu, B.B.; Chen, Z.; Zhu, Y.; Wang, Y.; Han, B.; Chen, G.; Zhang, X.; Du, Z.; He, Z. Heterogeneous 2D/3D Tin-Halides Perovskite Solar Cells with Certified Conversion Efficiency Breaking 14%. Adv. Mater. 2021, 33, 2102055. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Wang, X.; Wang, K.; Ma, S.; Yang, D.; Hou, Y.; Yoon, J.; Wang, K.; Priya, S. Localized Electron Density Engineering for Stabilized B-γCsSnI3-Based Perovskite Solar Cells with Efficiencies > 10%. ACS Energy Lett. 2021, 6, 1480–1489. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Lou, Y.; Zhao, Y. All-inorganic lead-free perovskites for optoelectronic applications. Mater. Chem. Front. 2019, 3, 365–375. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Xiu, J.; Song, H.; Gatti, T.; He, Z. A critical review on bismuth and antimony halide based perovskites and their derivatives for photovoltaic applications: Recent advances and challenges. J. Mater. Chem. A 2020, 8, 16166–16188. [Google Scholar] [CrossRef]
- Zhang, Q.; Hao, F.; Li, J.; Zhou, Y.; Wei, Y.; Lin, H. Perovskite solar cells: Must lead be replaced—And can it be done? Sci. Technol. Adv. Mater. 2018, 19, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Ahmad, W.; Liu, W.; Yang, J.; Zhang, R.; Sun, Y.; Yang, J.; Li, X. Lead-Free Halide Double Perovskite Materials: A New Superstar toward Green and Stable Optoelectronic Applications. Nano Micro Lett. 2019, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Kumar, P.; Mobin, S.M. Inorganic Pb-Free Perovskite Light Absorbers for Efficient Perovskite Solar Cells with Enhanced Performance. Chemistry 2020, 15, 2859–2863. [Google Scholar] [CrossRef] [PubMed]
- Eun, D.; Soo, L.; Kim, Y.; Won, H. Lead-free all-inorganic halide perovskite quantum dots: Review and outlook. J. Korean Ceram. Soc. 2020, 57, 455–479. [Google Scholar]
- Masawa, S.M.; Bakari, R.; Xu, J.; Yao, J. Progress and challenges in the fabrication of lead-free all-inorganic perovskites solar cells using solvent and compositional engineering Techniques—A review. J. Solid State Chem. 2023, 317, 123608. [Google Scholar] [CrossRef]
- Li, S.; He, J.; Ran, R.; Zhou, W.; Wang, W.; Shao, Z. Lead-Free All-Inorganic Cesium Bismuth Iodide-Based Perovskite Solar Cells: Recent Advances, Current Limitations, and Future Prospects. Solar RRL 2024, 8, 2300984. [Google Scholar] [CrossRef]
- Ji, F.; Zhang, B.; Chen, W.M.; Buyanova, I.A.; Wang, F. Amine Gas-Induced Reversible Optical Bleaching of Bismuth-Based Lead-Free Perovskite Thin Films. Adv. Sci. 2024, 11, 2306391. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Kumar, P.; Quasim Khan, M.; Alsulmi, A.; Kim, H. Improved Stability of CsBi3I10 Based Pb-Free Perovskite Solar Cells. ChemistrySelect 2023, 8, e202300520. [Google Scholar] [CrossRef]
- Mariyappan, P.; Pandian, M.G.M.; Chowdhury, T.H.; Babu, S.M.; Subashchandran, S. Investigations on the stability of the ambient processed bismuth based lead-free A3Bi2I9 (A = MA; Cs) perovskite thin-films for optoelectronic applications. Mater. Sci. Eng. B 2023, 297, 116706. [Google Scholar] [CrossRef]
- Song, T.B.; Yokoyama, T.; Stoumpos, C.C.; Logsdon, J.; Cao, D.H.; Wasielewski, M.R.; Aramaki, S.; Kanatzidis, M.G. Importance of reducing vapor atmosphere in the fabrication of Tin-based perovskite solar cells. J. Am. Chem. Soc. 2017, 139, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.F.; Igbari, F.; Lou, Y.H.; Wang, Z.K.; Liao, L.S. Tin Halide Perovskites: Progress and Challenges. Adv. Energy Mater. 2020, 10, 1902584. [Google Scholar] [CrossRef]
- Nasti, G.; Abate, A. Tin Halide Perovskite (ASnX3) Solar Cells: A Comprehensive Guide toward the Highest Power Conversion Efficiency. Adv. Energy Mater. 2020, 10, 1902467. [Google Scholar] [CrossRef]
- Yao, H.; Zhou, F.; Li, Z.; Ci, Z.; Ding, L.; Jin, Z. Strategies for Improving the Stability of Tin-Based Perovskite (ASnX3) Solar Cells. Adv. Sci. 2020, 7, 1903540. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Philippe, B.; Zhang, X.; Rensmo, H.; Boschloo, G.; Johansson, E.M.J. Bismuth Based Hybrid Perovskites A3Bi2I9 (A: Methylammonium or Cesium) for Solar Cell Application. Adv. Mater. 2015, 9, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.B.; Philippe, B.; Banerjee, A.; Phuyal, D.; Mukherjee, S.; Chakraborty, S.; Cameau, M.; Zhu, H.; Ahuja, R.; Boschloo, G.; et al. Cesium Bismuth Iodide Solar Cells from Systematic Molar Ratio Variation of CsI and BiI3. Inorg. Chem. 2019, 58, 12040–12052. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Chen, X.; Fan, P.; Liang, G. Inorganic and lead-free CsBi3I10 thin-film solar cell prepared by single-source thermal evaporation. J. Mater. Sci. Mater. Electron. 2021, 32, 11183–11192. [Google Scholar] [CrossRef]
- Sanders, S.; Stümmler, D.; Pfeiffer, P.; Ackermann, N.; Simkus, G.; Heuken, M.; Baumann, P.K.; Vescan, A.; Kalisch, H. Chemical Vapor Deposition of Organic-Inorganic Bismuth-Based Perovskite Films for Solar Cell Application. Sci. Rep. 2019, 9, 9774. [Google Scholar] [CrossRef] [PubMed]
- Hamukwaya, S.L.; Hao, H.; Mashingaidze, M.M.; Zhong, T.; Tang, S.; Dong, G.; Xing, J.; Liu, H.; Zhao, Z. Blended bismuth-based Cs3 Bi2 I9/Ag2 BiI5 perovskite films incorporated potassium iodide for high-efficiency carbon electrode solar cells. Res. Sq. 2022, 1–23. [Google Scholar] [CrossRef]
- Sun, M.; Zheng, Y.; Shi, Y.; Zhang, G.; Li, Q.; Shao, Y. Superfast crystalline powder synthetic strategy toward scale-up of perovskite solar cells. Mater. Today Energy 2022, 27, 101049. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, J.H.; Ramming, P.; Zhong, Y.; Schötz, K.; Kwon, S.J.; Huettner, S.; Panzer, F.; Park, N.G. How antisolvent miscibility affects perovskite film wrinkling and photovoltaic properties. Nat. Commun. 2021, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mishra, S.; Singh, T. Antisolvents in Perovskite Solar Cells: Importance, Issues, and Alternatives. Adv. Mater. Interfaces 2020, 7, 2000950. [Google Scholar] [CrossRef]
- Sun, J.; Li, F.; Yuan, J.; Ma, W. Advances in Metal Halide Perovskite Film Preparation: The Role of Anti-Solvent Treatment. Small Methods 2021, 5, 2100046. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.D.; Sun, Q.; Goetz, K.P.; An, Q.; Schramm, T.; Hofstetter, Y.; Litterst, M.; Paulus, F.; Vaynzof, Y. A general approach to high-efficiency perovskite solar cells by any antisolvent. Nat. Commun. 2021, 12, 1878. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Quijano, J.R.; Telschow, O.; Paulus, F.; Vaynzof, Y. Solvent-antisolvent interactions in metal halide perovskites. Chem. Commun. 2023, 59, 10588–10603. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Correa Baena, J.P.; Kurchin, R.C.; Polizzotti, A.; Yoo, J.J.; Wieghold, S.; Bawendi, M.G.; Buonassisi, T. Solvent-Engineering Method to Deposit Compact Bismuth-Based Thin Films: Mechanism and Application to Photovoltaics. Chem. Mater. 2018, 30, 336–343. [Google Scholar] [CrossRef]
- Ghosh, B.; Wu, B.; Mulmudi, H.K.; Guet, C.; Weber, K.; Sum, T.C.; Mhaisalkar, S.; Mathews, N. Limitations of Cs3Bi2I9 as Lead-Free Photovoltaic Absorber Materials. ACS Appl. Mater. Interfaces 2018, 10, 35000–35007. [Google Scholar] [CrossRef] [PubMed]
- Mariyappan, P.; Chowdhury, T.H.; Subashchandran, S.; Bedja, I.; Ghaithan, H.M.; Islam, A. Fabrication of lead-free CsBi3I10 based compact perovskite thin films by employing solvent engineering and anti-solvent treatment techniques: An efficient photo-conversion efficiency up to 740 nm. Sustain. Energy Fuels 2020, 4, 5042–5049. [Google Scholar] [CrossRef]
- Ünlü, F.; Kulkarni, A.; Lê, K.; Bohr, C.; Bliesener, A.; Öz, S.D.; Jena, A.K.; Ando, Y.; Miyasaka, T.; Kirchartz, T.; et al. Single- or double A-site cations in A3Bi2I9 bismuth perovskites: What is the suitable choice? J. Mater. Res. 2021, 36, 1794–1804. [Google Scholar] [CrossRef]
- Johansson, M.B.; Zhu, H.; Johansson, E.M.J. Extended Photo-Conversion Spectrum in Low-Toxic Bismuth Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 3467–3471. [Google Scholar] [CrossRef] [PubMed]
- Masawa, S.M.; Li, J.; Zhao, C.; Liu, X.; Yao, J. 0D/2D Mixed Dimensional Lead-Free Caesium Bismuth Iodide Perovskite for Solar Cell Application. Materials 2022, 15, 2180. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Hao, L.; Liu, Z.; Su, G.; He, X.; Zeng, Q.; Wei, J. All green solvent engineering of organic–inorganic hybrid perovskite layer for high-performance solar cells. Chem. Eng. J. 2022, 437, 135458. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.; Jung, S.; Kim, C.S.; Park, J.; Song, A.; Chung, K.B.; Jin, S.H.; Lee, J.H.; Song, M. Enhanced efficiency in lead-free bismuth iodide with post treatment based on a hole-conductor-free perovskite solar cell. Nano Res. 2018, 11, 6283–6293. [Google Scholar] [CrossRef]
- Mcrae, J.C.; Mcrae, J. Enhancing Bismuth Iodide Solar Cells Controlling Crystal Orientation of 2D Bismuth; Worcester Polytechnic Institute: Worcester, MA, USA, 2019. [Google Scholar]
- Hamdeh, U.H.; Nelson, R.D.; Ryan, B.J.; Bhattacharjee, U.; Petrich, J.W.; Panthani, M.G. Solution-processed BiI3 thin films for photovoltaic applications: Improved carrier collection via solvent annealing. Chem. Mater. 2016, 28, 6567–6574. [Google Scholar] [CrossRef]
- Baibarac, M.; Matea, A.; Mitran, R.; Baltog, I.; Nillă, A.; Bismuth, I. Exciton-phonon interactions in the Cs3Bi2I9 crystal structure revealed by Raman spectroscopic studies. Phys. Status Solidi B 2017, 254, 1552805. [Google Scholar]
- Mccall, K.M.; Stoumpos, C.C.; Kostina, S.S.; Kanatzidis, M.G.; Wessels, B.W. Strong Electron-Phonon Coupling and Self-Trapped Excitons in the Defect Halide Perovskites A3M2I9 (A = Cs, Rb; M = Bi, Sb). Chem. Mater. 2017, 29, 4129–4145. [Google Scholar] [CrossRef]
- Waykar, R.; Bhorde, A.; Nair, S.; Pandharkar, S.; Gabhale, B. Environmentally stable lead-free cesium bismuth iodide (Cs3Bi2I9) perovskite: Synthesis to solar cell application. J. Phys. Chem. Solids 2020, 146, 109608. [Google Scholar] [CrossRef]
- Lenzer, T. Pronounced exciton and coherent phonon dynamics in BiI3. Phys. Chem. Chem. Phys. 2017, 20, 10677–10685. [Google Scholar]
- An, Q.; Paulus, F.; Becker-Koch, D.; Cho, C.; Sun, Q.; Weu, A.; Bitton, S.; Tessler, N.; Vaynzof, Y. Small grains as recombination hot spots in perovskite solar cells. Matter 2021, 4, 1683–1701. [Google Scholar] [CrossRef]
- Gedamu, D.; Asuo, I.M.; Benetti, D.; Basti, M.; Ka, I.; Cloutier, S.G.; Rosei, F.; Nechache, R. Solvent-Antisolvent Ambient Processed Large Grain Size Perovskite Thin Films for High-Performance Solar Cells. Sci. Rep. 2018, 8, 12885. [Google Scholar] [CrossRef] [PubMed]
- Adhyaksa, G.W.P.; Brittman, S.; Āboliņš, H.; Lof, A.; Li, X.; Keelor, J.D.; Luo, Y.; Duevski, T.; Heeren, R.M.A.; Ellis, S.R.; et al. Understanding Detrimental and Beneficial Grain Boundary Effects in Halide Perovskites. Adv. Mater. 2018, 30, 1804792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, H.; Qi, J.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. Antisolvent-Derived Intermediate Phases for Low-Temperature Flexible Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 6477–6486. [Google Scholar] [CrossRef]
- Lee, K.; Lin, C.; Liou, B.; Yu, S.; Hsu, C. Solar Energy Materials and Solar Cells Selection of anti-solvent and optimization of dropping volume for the preparation of large area sub-module perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 172, 368–375. [Google Scholar] [CrossRef]
- Kang, J.; Liu, J.; Allen, O.; Al-Mamun, M.; Liu, P.; Yin, H.; Wang, Y.; Chen, S.; Zhao, H. Fabrication of High-Quality CsBi3I10 Films via a Gas-Assisted Approach for Efficient Lead-Free Perovskite Solar Cells. Energy Technol. 2022, 10, 2200318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).