Synthesis and Characterization of SiO2-Based Graphene Nanoballs Using Copper-Vapor-Assisted APCVD for Thermoelectric Application
Abstract
1. Introduction
| SiO2 Surface | Gas | Flow | Pressure | Temperature | Growth Time | Catalyst | Ref. |
|---|---|---|---|---|---|---|---|
| (sccm) | (Torr) | (°C) | (min) | ||||
| Flat substrate | Ar/H2/CH4 | 65:50:14 | Atmospheric | 1100 | 180 | O2 | [21] 2011 |
| Flat substrate | Ar/H2/CH4 | 230:5:30 | Atmospheric | 1000 | 30 | Cu | [37] 2012 |
| Flat substrate 300 nm | H2/CH4 | 20:30 | 5 | 1000 | 30 | Cu | [38] 2013 |
| Flat substrate Quartz | Ar/H2/CH4 | 100:20:50 | 60–360 | 950–1100 | 5 | Ni | [25] 2014 |
| Nanoparticles 20–30 nm | CH4 | 50 | Atmospheric | 1000 | 60 | - | [26] 2017 |
| Flat substrate Si 500 μm | H2/CH4 | 100:5 | 0.007 | 1100 | 60 | Cu | [39] 2018 |
| Nanoparticles 20–30 nm | Ar/CH4 | 200:25 | Atmospheric | 1000 | 20 | - | [27] 2019 |
| Flat substrate 300 nm | Ar/CH3OH | 0.15 | 1020 | 30 | Cu | [40] 2019 | |
| Flat substrate 300 nm | H2/CH4 | 25:15 | 0.00007 | 950 | 60 | - | [41] 2020 |
| Nanoparticles 20–30 nm | Ar/H2/CH4 | 78:2:50 | Atmospheric | 1000 | 60 | Cu | This work |
2. Experimental Section
2.1. Graphene Growth
2.2. Characterization
3. Results and Discussion
3.1. Optimization of SGB Synthesis Process
3.2. Characterization of the Optimized SGB
3.2.1. Raman Analysis
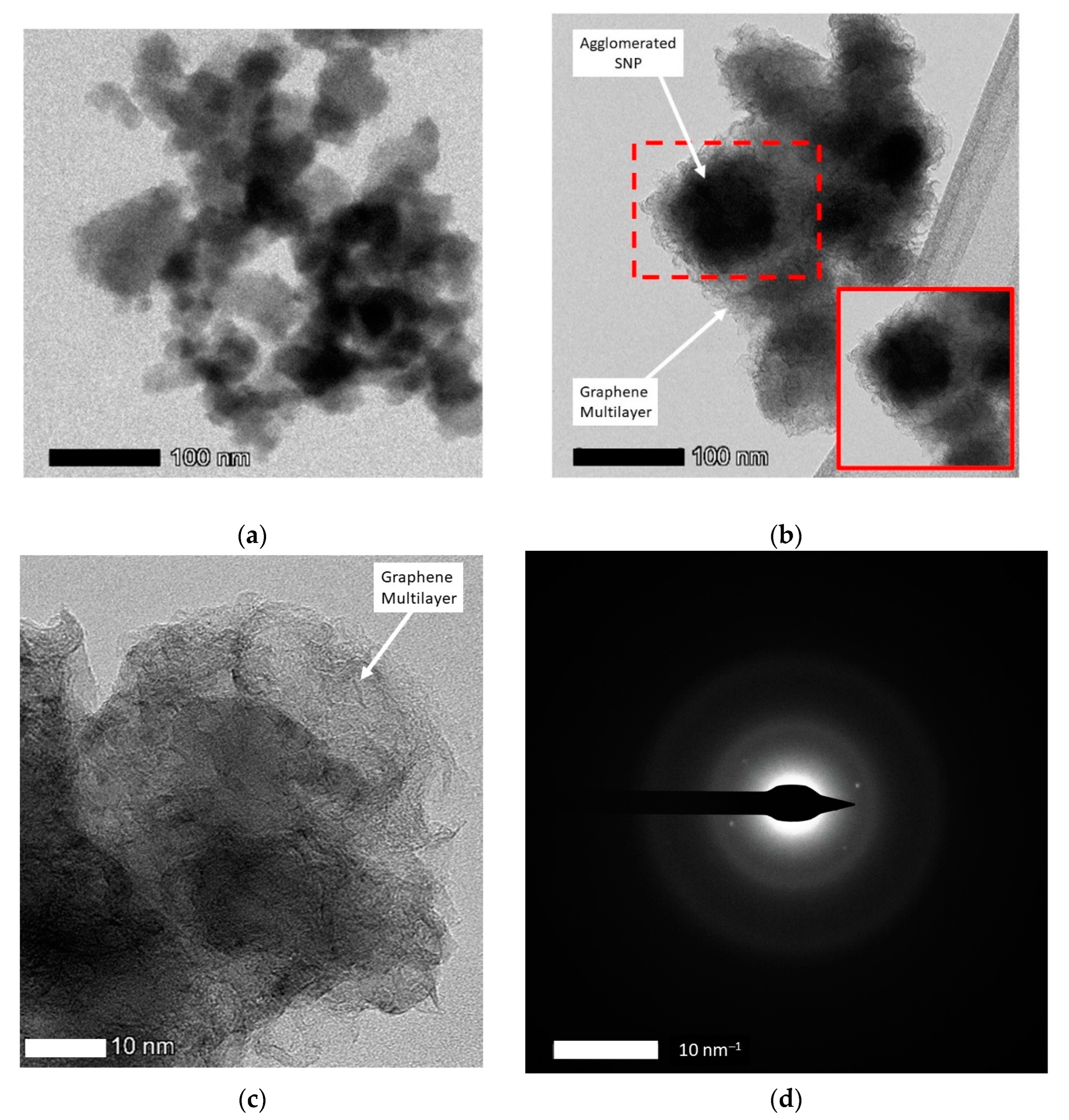
3.2.2. Morphology Analysis
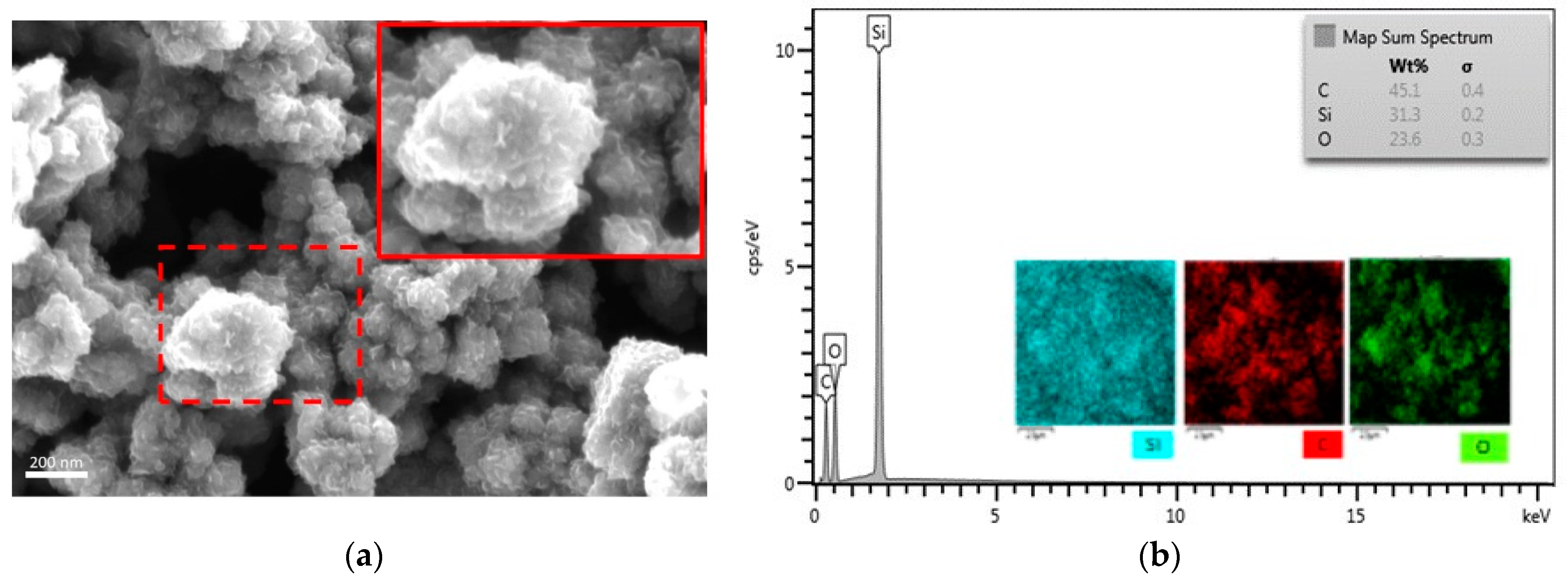
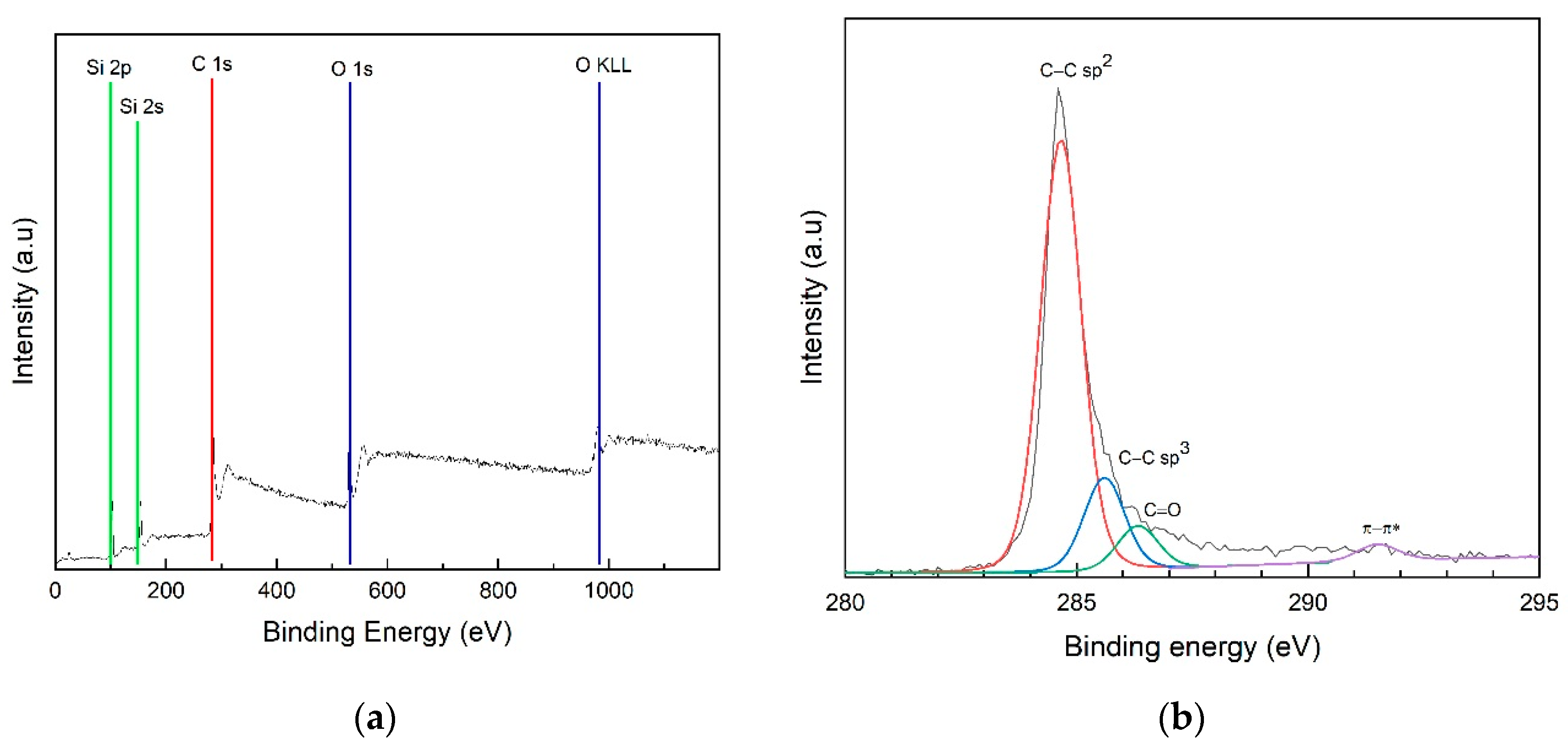
3.2.3. Chemical Composition
3.2.4. Electrical Characterization of SGB
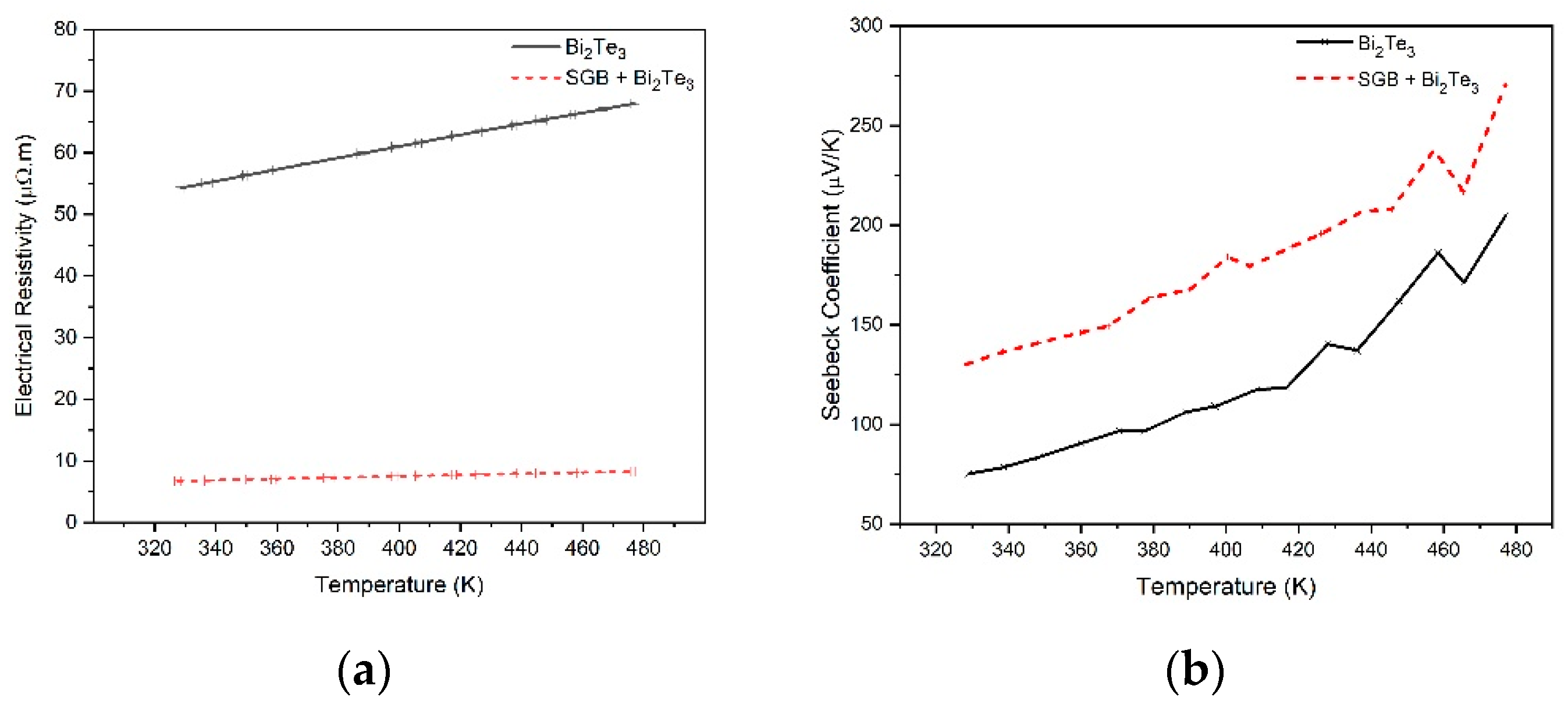
3.3. Material Evaluation for TE Application
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Handayani, M.; Nafi’ah, N.; Nugroho, A.; Rasyida, A.; Prasetyo, A.B.; Febriana, E.; Sulistiyono, E.; Firdiyono, F. The Development of Graphene/Silica Hybrid Composites: A Review for Their Applications and Challenges. Crystals 2021, 11, 1337. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.G.; Jang, H.S.; Moon, J.Y.; Whang, D. Graphene on Group-IV Elementary Semiconductors: The Direct Growth Approach and Its Applications. Adv. Mater. 2019, 31, 1803469. [Google Scholar] [CrossRef]
- Ding, L.P.; McLean, B.; Xu, Z.; Kong, X.; Hedman, D.; Qiu, L.; Page, A.J.; Ding, F. Why Carbon Nanotubes Grow. J. Am. Chem. Soc. 2022, 144, 5606–5613. [Google Scholar] [CrossRef]
- Mowlaei, R.; Lin, J.; Basquiroto de Souza, F.; Fouladi, A.; Habibnejad Korayem, A.; Shamsaei, E.; Duan, W. The Effects of Graphene Oxide-Silica Nanohybrids on the Workability, Hydration, and Mechanical Properties of Portland Cement Paste. Constr. Build. Mater. 2021, 266, 121016. [Google Scholar] [CrossRef]
- Selvarajan, R.S.; Rahim, R.A.; Majlis, B.Y.; Gopinath, S.C.B.; Hamzah, A.A. Ultrasensitive and Highly Selective Graphene-Based Field-Effect Transistor Biosensor for Anti-Diuretic Hormone Detection. Sensors 2020, 20, 2642. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon Nanotube Based Biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Wang, L.; Li, A.; Tang, X.; Choi, J.; Zhang, P.; Jin, M.L.; Joo, S.W. Scalable Fabrication of Carbon Materials Based Silicon Rubber for Highly Stretchable E-Textile Sensor. Nanotechnol. Rev. 2020, 9, 1183–1191. [Google Scholar] [CrossRef]
- Mohammad Haniff, M.A.S.; Muhammad Hafiz, S.; Wahid, K.A.A.; Endut, Z.; Wah Lee, H.; Bien, D.C.S.; Abdul Azid, I.; Abdullah, M.Z.; Ming Huang, N.; Abdul Rahman, S. Piezoresistive Effects in Controllable Defective HFTCVD Graphene-Based Flexible Pressure Sensor. Sci. Rep. 2015, 5, 14751. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, M.; Agusu, L.; Putra, A.A.M.; Maulidiyah, M.; Arham, Z.; Wibowo, D.; Muzakkar, M.Z.; Umar, A.A. Synthesis and Electrochemical Performance of Graphene-TiO2-Carbon Paste Nanocomposites Electrode in Phenol Detection. J. Phys. Chem. Solids 2019, 131, 104–110. [Google Scholar] [CrossRef]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent Advances in Carbon-Based Electrodes for Energy Storage and Conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar] [CrossRef]
- Ye, Z.; Wu, P.; Wang, H.; Jiang, S.; Huang, M.; Lei, D.; Wu, F. Multimode Tunable Terahertz Absorber Based on a Quarter Graphene Disk Structure. Results Phys. 2023, 48, 106420. [Google Scholar] [CrossRef]
- Kuzhir, P.; Celzard, A.; Chen, X. Microwave Absorption by Carbon-Based Materials and Structures. J. Appl. Phys. 2022, 131, 200401. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Hamzah, A.A.; Pang, A.L.; Thi Le, A.; Chiam, S.L.; Ahmad, Z.A.; Rajitha, B.; Pung, S.Y. Photodegradation of Rhodamine B-Dye Pollutant Using CaCu3Ti4O12-Multiwall Carbon Nanotube Nanocomposites. J. Environ. Chem. Eng. 2021, 9, 105185. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Hatta, F.F.; Mohammad Haniff, M.A.S.; Mohamed, M.A. A Review on Applications of Graphene in Triboelectric Nanogenerators. Int. J. Energy Res. 2021, 46, 544–576. [Google Scholar] [CrossRef]
- Tao, T.; Li, G.; He, Y.; Yang, X. 3-D Magnetic Graphene Balls as Sorbents for Cleaning Oil Spills. Nanomater. Nanotechnol. 2019, 9, 184798041985737. [Google Scholar] [CrossRef]
- Solati, E.; Savadkoohi, M.; Dorranian, D. Nonlinear Optical Response of Graphene/Silicon Nanocomposites. Opt. Quantum Electron. 2018, 50, 268. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, L.; Li, C. Facile Fabrication of Silica-Polymer-Graphene Collaborative Nanostructure-Based Hybrid Materials with High Conductivity and Robust Mechanical Performance. RSC Adv. 2015, 5, 25450–25456. [Google Scholar] [CrossRef]
- Ho, S.; Kang, Y.C. Fe3O4-Decorated Hollow Graphene Balls Prepared by Spray Pyrolysis Process for Ultrafast and Long Cycle-Life Lithium Ion Batteries. Carbon N. Y. 2014, 79, 58–66. [Google Scholar] [CrossRef]
- Chen, D.; Yi, R.; Chen, S.; Xu, T.; Gordin, M.L.; Wang, D. Facile Synthesis of Graphene—Silicon Nanocomposites with an Advanced Binder for High-Performance Lithium-Ion Battery Anodes. Solid State Ionics 2014, 254, 65–71. [Google Scholar] [CrossRef]
- Chen, J.; Wen, Y.; Guo, Y.; Wu, B.; Huang, L.; Xue, Y.; Geng, D.; Wang, D.; Yu, G.; Liu, Y. Oxygen-Aided Synthesis of Polycrystalline Graphene on Silicon Dioxide Substrates. J. Am. Chem. Soc. 2011, 133, 17548–17551. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Ghoranneviss, M.; Hantehzadeh, M.R. Morphology Control of Graphene by LPCVD. J. Fusion Energy 2015, 34, 532–539. [Google Scholar] [CrossRef]
- Madito, M.J.; Matshoba, K.S.; Mongwaketsi, N.; Mtshali, C.B.; Fabiane, M.; Manyala, N. Nickel-Copper Graphene Foam Prepared by Atmospheric Pressure Chemical Vapour Deposition for Supercapacitor Applications. Surf. Coat. Technol. 2020, 383, 125230. [Google Scholar] [CrossRef]
- Yen, W.C.; Chen, Y.Z.; Yeh, C.H.; He, J.H.; Chiu, P.W.; Chueh, Y.L. Direct Growth of Self-Crystallized Graphene and Graphite Nanoballs with Ni Vapor-Assisted Growth: From Controllable Growth to Material Characterization. Sci. Rep. 2014, 4, 4739. [Google Scholar] [CrossRef]
- Son, I.H.; Park, J.H.; Park, S.; Park, K.; Han, S.; Shin, J.; Doo, S.G.; Hwang, Y.; Chang, H.; Choi, J.W. Graphene Balls for Lithium Rechargeable Batteries with Fast Charging and High Volumetric Energy Densities. Nat. Commun. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, T.; Zhou, X.; Liu, X.; Li, X.; Zhou, K.; Dong, L.; Wei, D. Three-Dimensional Graphene Composite Containing Graphene-SiO2 Nanoballs and Its Potential Application in Stress Sensors. Nanomaterials 2019, 9, 438. [Google Scholar] [CrossRef]
- Zainal Ariffin, N.H.; Mohammad Haniff, M.A.S.; Syono, M.I.; Ambri Mohamed, M.; Hamzah, A.A.; Hashim, A.M. Low-Temperature Nitrogen Doping of Nanocrystalline Graphene Films with Tunable Pyridinic-N and Pyrrolic-N by Cold-Wall Plasma-Assisted Chemical Vapor Deposition. ACS Omega 2021, 6, 23710–23722. [Google Scholar] [CrossRef]
- Fauzi, F.B.; Ismail, E.; Syed Abu Bakar, S.N.; Ismail, A.F.; Mohamed, M.A.; Md Din, M.F.; Illias, S.; Ani, M.H. The Role of Gas-Phase Dynamics in Interfacial Phenomena during Few-Layer Graphene Growth through Atmospheric Pressure Chemical Vapour Deposition. Phys. Chem. Chem. Phys. 2020, 22, 3481–3489. [Google Scholar] [CrossRef]
- Wang, S.; Hibino, H.; Suzuki, S.; Yamamoto, H. Atmospheric Pressure Chemical Vapor Deposition Growth of Millimeter-Scale Single-Crystalline Graphene on the Copper Surface with a Native Oxide Layer. Chem. Mater. 2016, 28, 4893–4900. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Wee, M.F.M.R.; Mohamed, M.A.; Hamzah, A.A.; Menon, P.S. Multi-Response Optimization of Chromium/Gold-Based Nanofilm Kretschmann-Based Surface Plasmon Resonance Glucose Sensor Using Finite-Difference Time-Domain and Taguchi Method. Nanomater. Nanotechnol. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Freddi, A.; Salmon, M. Introduction to the Taguchi Method. In Design Principles and Methodologies; Springer Tracts in Mechanical Engineering; Springer: Cham, Switzerland, 2019; pp. 159–180. ISBN 9783319953427. [Google Scholar]
- Papon, R.; Pierlot, C.; Sharma, S.; Shinde, S.M.; Kalita, G.; Tanemura, M. Optimization of CVD Parameters for Graphene Synthesis through Design of Experiments. Phys. Status Solidi Basic Res. 2017, 254, 1600629. [Google Scholar] [CrossRef]
- Tahir, N.A.M.; Bin Abdollah, M.F.; Tamaldin, N.; Zin, M.R.B.M.; Amiruddin, H. Optimisation of Graphene Grown from Solid Waste Using CVD Method. Int. J. Adv. Manuf. Technol. 2020, 106, 211–218. [Google Scholar] [CrossRef]
- Nolas, G.S.; Sharp, J.; Goldsmid, J. Thermoelectrics: Basic principles and new materials developments. In Science and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 45, p. 228. ISBN 9781461257080. [Google Scholar]
- Ma, M.; Li, H.; Xiong, Y.; Dong, F. Rational Design, Synthesis, and Application of Silica/Graphene-Based Nanocomposite: A Review. Mater. Des. 2021, 198, 109367. [Google Scholar] [CrossRef]
- Teng, P.Y.; Lu, C.C.; Akiyama-Hasegawa, K.; Lin, Y.C.; Yeh, C.H.; Suenaga, K.; Chiu, P.W. Remote Catalyzation for Direct Formation of Graphene Layers on Oxides. Nano Lett. 2012, 12, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, I.; Park, C.; Son, M.; Hong, M.; Kim, Y.; Kim, J.S.; Shin, H.-J.; Baik, J.; Choi, H.C. Copper-Vapor-Assisted Chemical Vapor Deposition for High-Quality and Metal-Free Single-Layer Graphene on Amorphous SiO2 Substrate. ACS Nano 2013, 7, 6575–6582. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhang, H.; Song, L.; Ye, Q.; Cai, J. Synthesize Monolayer Graphene on SiO2/Si Substrate with Copper-Vapor-Assisted CVD Method. Mater. Res. Express 2018, 5, 125601. [Google Scholar] [CrossRef]
- Zhou, L.; Wei, S.; Ge, C.; Zhao, C.; Guo, B.; Zhang, J.; Zhao, J. Ultrafast Growth of Uniform Multi-Layer Graphene Films Directly on Silicon Dioxide Substrates. Nanomaterials 2019, 9, 964. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Li, Q.; Tian, M.; Li, C.; Sun, L.; Wang, J.; Zhao, X.; Xu, S.; Yu, F. Direct Synthesis of Graphene Dendrites on SiO2/Si Substrates by Chemical Vapor Deposition. Nanoscale Res. Lett. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Ren, S.; Rong, P.; Yu, Q. Preparations, Properties and Applications of Graphene in Functional Devices: A Concise Review. Ceram. Int. 2018, 44, 11940–11955. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman Spectroscopy of Graphene and Graphite: Disorder, Electron–Phonon Coupling, Doping and Nonadiabatic Effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Rushforth, R.J. Review of Design Experiments Using the Taguchi Approach: 16 Steps to Product and Process Improvement; American Society for Testing and Materials: West Conshohocken, PA, USA, 2001; Volume 29, ISBN 978-0-471-36101-5. [Google Scholar]
- Childres, I.; Jauregui, L.A.; Park, W.; Caoa, H.; Chena, Y.P. Raman Spectroscopy of Graphene and Related Materials. New Dev. Phot. Mater. Res. 2013, 1, 403–418. [Google Scholar]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Vicarelli, L.; Heerema, S.J.; Dekker, C.; Zandbergen, H.W. Controlling Defects in Graphene for Optimizing the Electrical Properties of Graphene Nanodevices. ACS Nano 2015, 9, 3428–3435. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jang, H.; Kim, S.J.; Pang, C.; Chae, H. Copper-Assisted Direct Growth of Vertical Graphene Nanosheets on Glass Substrates by Low-Temperature Plasma-Enhanced Chemical Vapour Deposition Process. Nanoscale Res. Lett. 2015, 10, 308. [Google Scholar] [CrossRef]
- Kristiantoro, T.; Dedi; Fauzia, V. Synthesis and Characterization of Bi2Te3 Thermoelectric Material Using the Cold Pressing and Sintering Method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 553, 012033. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Wu, P.; Guo, J.; Zhou, Y.; Chong, X.; Ge, Z.H.; Feng, J. Achieving a Fine Balance in Mechanical Properties and Thermoelectric Performance in Commercial Bi2Te3 Materials. Ceram. Int. 2020, 46, 14994–15002. [Google Scholar] [CrossRef]
- Ahmad, K.; Wan, C.; Zong, P.A. Thermoelectric Properties of BiSbTe/Graphene Nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 11923–11930. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Krasinski, J.S.; Vashaee, D. N-Type Bismuth Telluride Nanocomposite Materials Optimization for Thermoelectric Generators in Wearable Applications. Materials 2019, 12, 1529. [Google Scholar] [CrossRef] [PubMed]
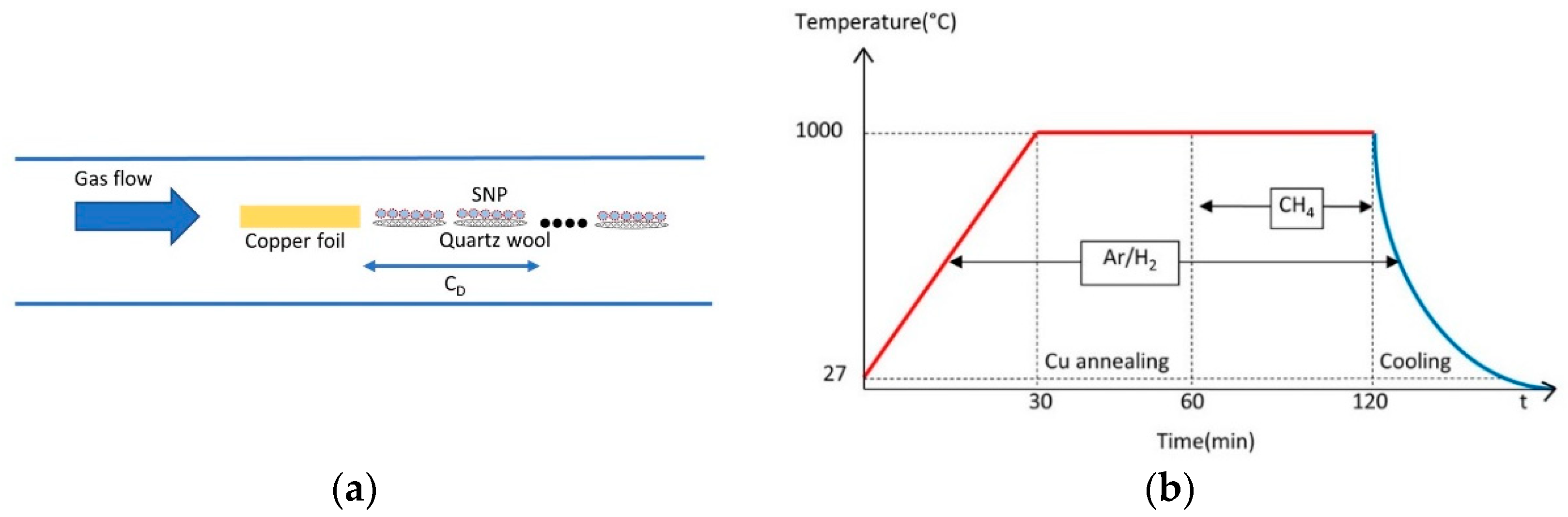
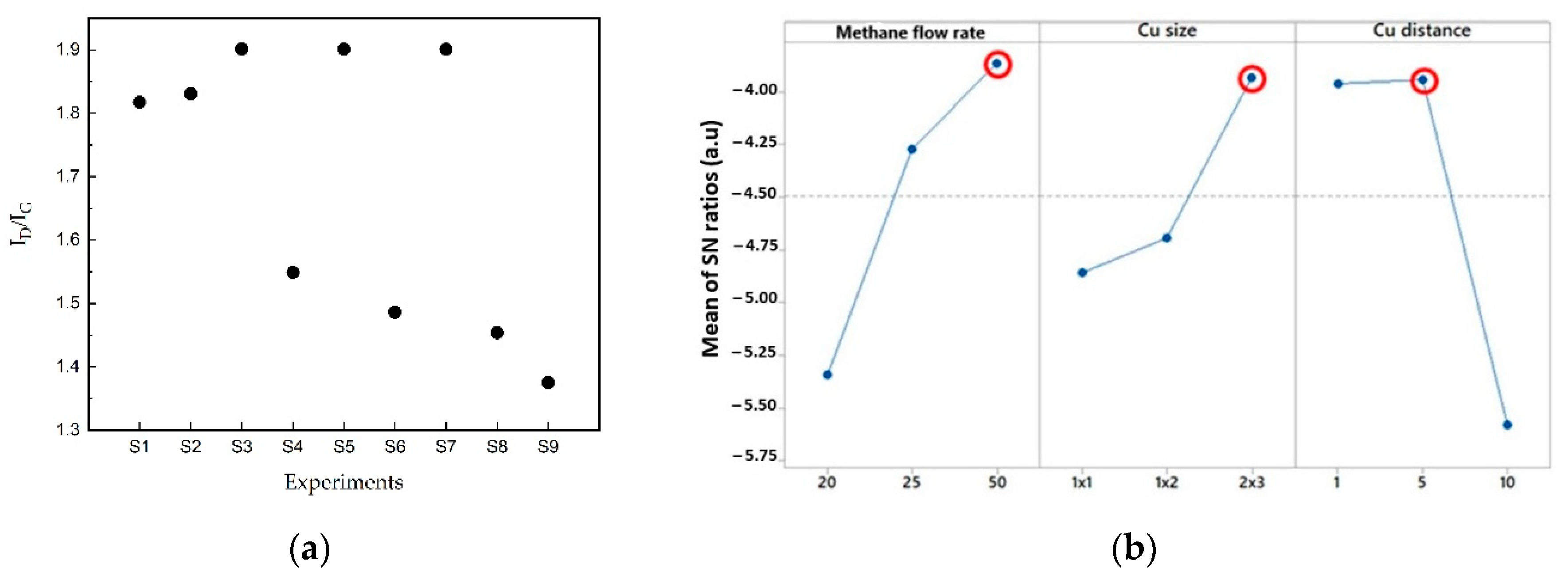

| Experiments | Factors | ||
|---|---|---|---|
| Methane Flow Rate, ΦCH4 (sccm) | Copper Foil Size, CS (cm) | Copper Distance, CD (cm) | |
| S1 | 20 | 1 × 1 | 1 |
| S2 | 20 | 1 × 2 | 5 |
| S3 | 20 | 2 × 3 | 10 |
| S4 | 25 | 1 × 1 | 5 |
| S5 | 25 | 1 × 2 | 10 |
| S6 | 25 | 2 × 3 | 1 |
| S7 | 50 | 1 × 1 | 10 |
| S8 | 50 | 1 × 2 | 1 |
| S9 | 50 | 2 × 3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkepli, N.; Yunas, J.; Mohammad Haniff, M.A.S.; Dedi; Sirat, M.S.; Johari, M.H.; Mohd Maidin, N.N.; Mohd Raub, A.A.; Hamzah, A.A. Synthesis and Characterization of SiO2-Based Graphene Nanoballs Using Copper-Vapor-Assisted APCVD for Thermoelectric Application. Nanomaterials 2024, 14, 618. https://doi.org/10.3390/nano14070618
Zulkepli N, Yunas J, Mohammad Haniff MAS, Dedi, Sirat MS, Johari MH, Mohd Maidin NN, Mohd Raub AA, Hamzah AA. Synthesis and Characterization of SiO2-Based Graphene Nanoballs Using Copper-Vapor-Assisted APCVD for Thermoelectric Application. Nanomaterials. 2024; 14(7):618. https://doi.org/10.3390/nano14070618
Chicago/Turabian StyleZulkepli, Nurkhaizan, Jumril Yunas, Muhammad Aniq Shazni Mohammad Haniff, Dedi, Mohamad Shukri Sirat, Muhammad Hilmi Johari, Nur Nasyifa Mohd Maidin, Aini Ayunni Mohd Raub, and Azrul Azlan Hamzah. 2024. "Synthesis and Characterization of SiO2-Based Graphene Nanoballs Using Copper-Vapor-Assisted APCVD for Thermoelectric Application" Nanomaterials 14, no. 7: 618. https://doi.org/10.3390/nano14070618
APA StyleZulkepli, N., Yunas, J., Mohammad Haniff, M. A. S., Dedi, Sirat, M. S., Johari, M. H., Mohd Maidin, N. N., Mohd Raub, A. A., & Hamzah, A. A. (2024). Synthesis and Characterization of SiO2-Based Graphene Nanoballs Using Copper-Vapor-Assisted APCVD for Thermoelectric Application. Nanomaterials, 14(7), 618. https://doi.org/10.3390/nano14070618









