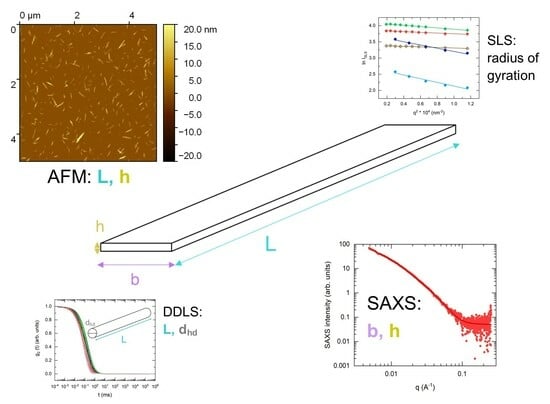
Dimensions of Cellulose Nanocrystals from Cotton and Bacterial Cellulose: Comparison of Microscopy and Scattering Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNC Suspensions
2.3. Experimental Techniques for the Size Characterization of CNC Suspensions
2.4. Data Analysis
- -
- Average length is fixed as obtained from AFM;
- -
- Hydrodynamic cross-section dimension is fixed at either the width or the height as obtained from AFM;
- -
- Length polydispersity σ is fixed at either 0.1, 0.3, 0.5, or 0.8.
3. Results and Discussion
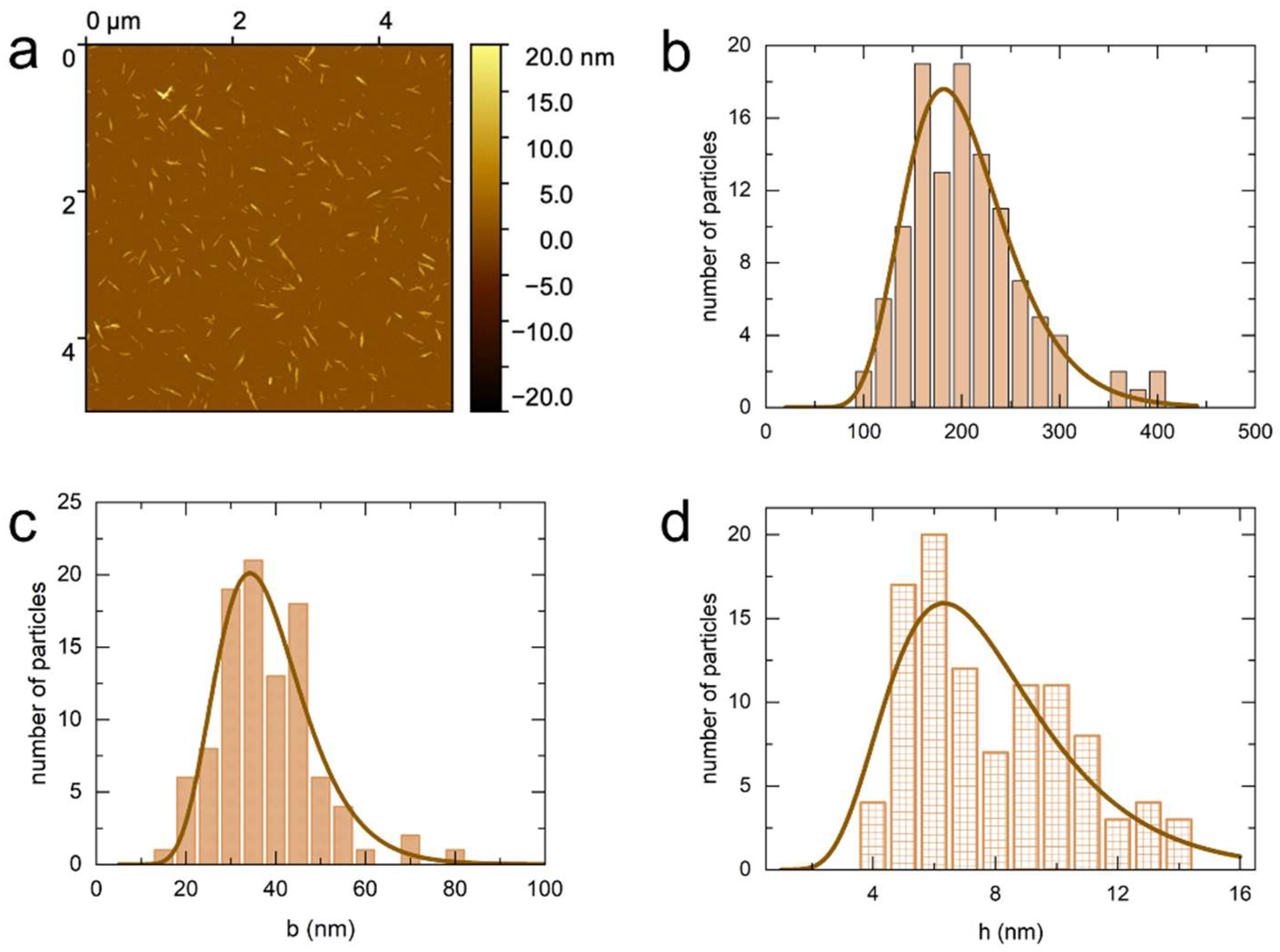
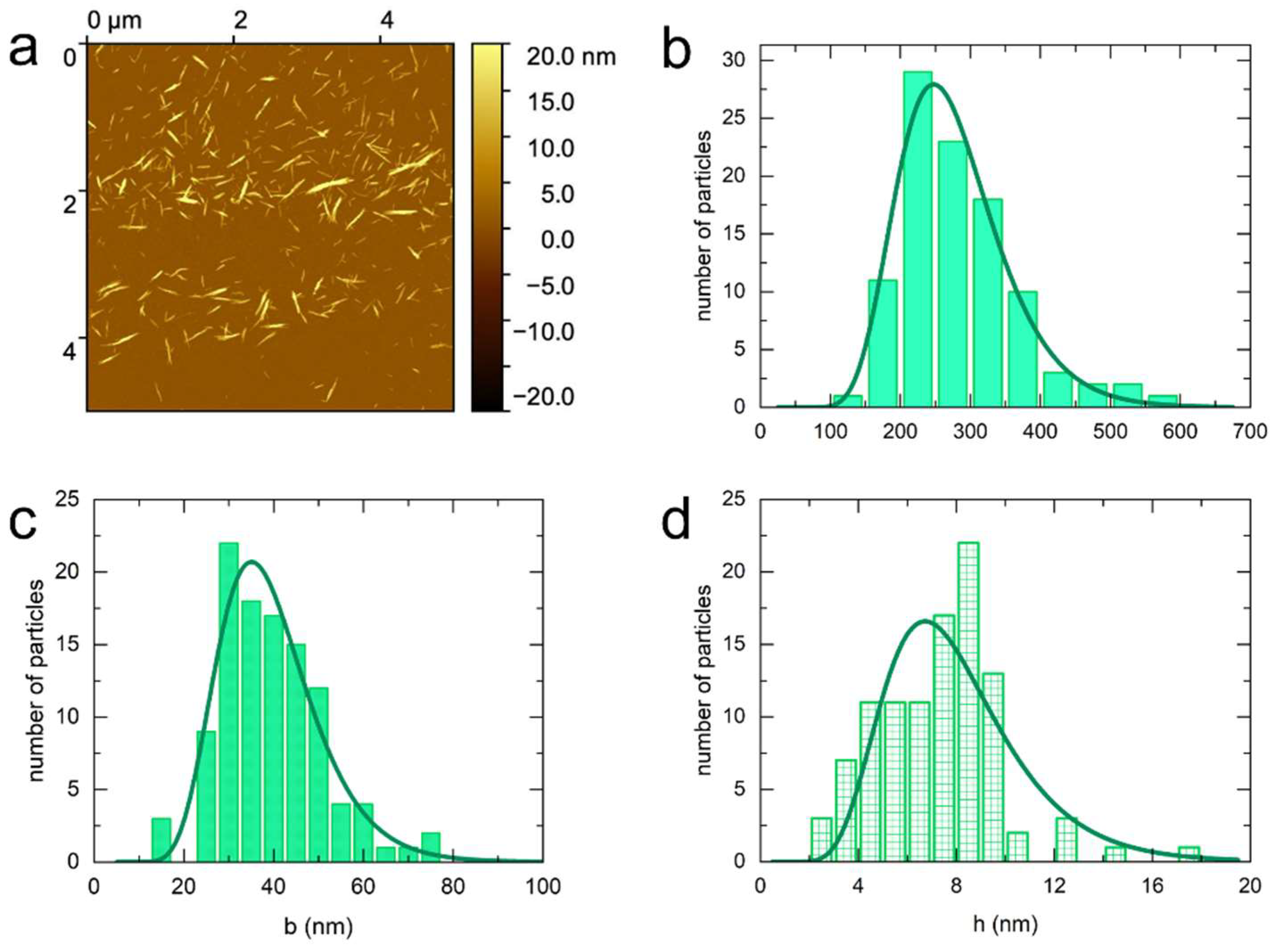
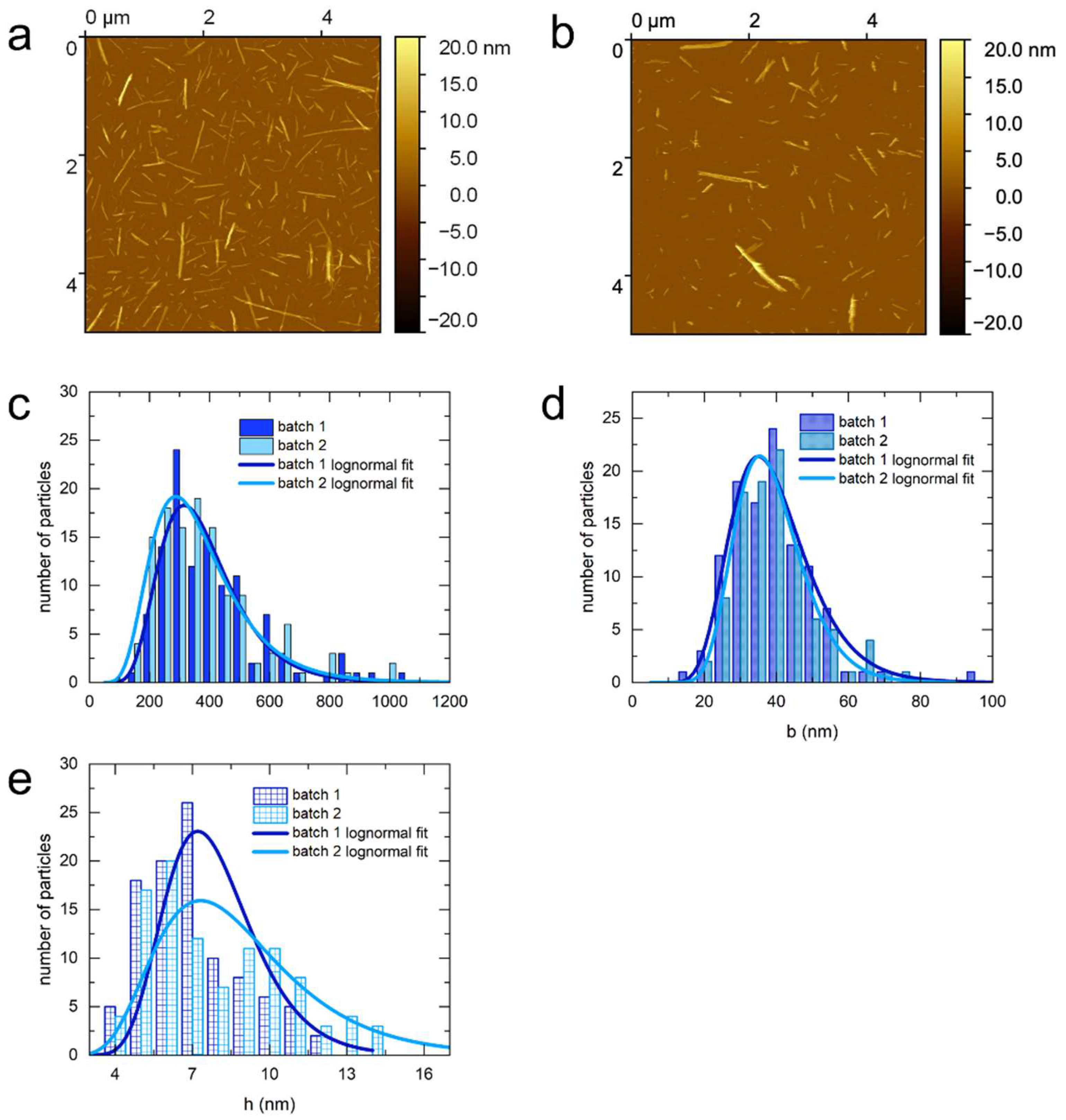
3.1. Atomic Force Microscopy (AFM)
3.2. Small-Angle X-ray Scattering (SAXS)
3.3. Depolarized Dynamic Light Scattering (DDLS)
3.4. Static Light Scattering (SLS)
3.5. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Orts, W.J.; Godbout, L.; Marchessault, R.H.; Revol, J.-F. Enhanced Ordering of Liquid Crystalline Suspensions of Cellulose Microfibrils: A Small Angle Neutron Scattering Study. Macromolecules 1998, 31, 5717–5725. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Influence of surface charge on viscosity behavior of cellulose microcrystal suspension. J. Wood Sci. 1999, 45, 258–261. [Google Scholar] [CrossRef]
- Fagbemi, O.D.; Andrew, J.E.; Sithole, B. Beneficiation of wood sawdust into cellulose nanocrystals for application as a bio-binder in the manufacture of particleboard. Biomass Convers. Biorefinery 2021, 13, 11645–11656. [Google Scholar] [CrossRef]
- Kumar, P.; Miller, K.; Kermanshahi-pour, A.; Brar, S.K.; Beims, R.F.; Xu, C.C. Nanocrystalline cellulose derived from spruce wood: Influence of process parameters. Int. J. Biol. Macromol. 2022, 221, 426–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Q.; Chang, C.; Zhang, L. Phase transition identification of cellulose nanocrystal suspensions derived from various raw materials. J. App. Polym. Sci. 2017, 135, 45702. [Google Scholar] [CrossRef]
- Mondal, K.; Sakurai, S.; Okahisa, Y.; Goud, V.V.; Katiyar, V. Effect of cellulose nanocrystals derived from Dunaliella tertiolecta marine green algae residue on crystallization behaviour of poly(lactic acid). Carbohydr. Polym. 2021, 261, 117881. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Hou, Q.; Liu, R.; Wu, T.; Si, C. Further characterization of cellulose nanocrystal (CNC) preparation from sulfuric acid hydrolysis of cotton fibers. Cellulose 2015, 23, 439–450. [Google Scholar] [CrossRef]
- Haouache, S.; Jimenez-Saelices, C.; Cousin, F.; Falourd, X.; Pontoire, B.; Cahier, K.; Jérome, F.; Capron, I. Cellulose nanocrystals from native and mercerized cotton. Cellulose 2022, 29, 1567–1581. [Google Scholar] [CrossRef]
- Miriam de Souza Lima, M.; Borsali, R. Static and Dynamic Light Scattering from Polyelectrolyte Microcrystal Cellulose. Langmuir 2002, 18, 992–996. [Google Scholar] [CrossRef]
- Darpentigny, C.; Molina-Boisseau, S.; Nonglaton, G.; Bras, J.; Jean, B. Ice-templated freeze-dried cryogels from tunicate cellulose nanocrystals with high specific surface area and anisotropic morphological and mechanical properties. Cellulose 2020, 27, 233–247. [Google Scholar] [CrossRef]
- Araki, J.; Kuga, S. Effect of Trace Electrolyte on Liquid Crystal Type of Cellulose Microcrystals. Langmuir 2001, 17, 4493–4496. [Google Scholar] [CrossRef]
- Sommer, A.; Staroszczyk, H. Bacterial cellulose vs. bacterial cellulose nanocrystals as stabilizer agents for O/W pickering emulsions. Food Hydrocoll. 2023, 145, 109080. [Google Scholar] [CrossRef]
- Verma, C.; Chhajed, M.; Gupta, P.; Roy, S.; Maji, P.K. Isolation of cellulose nanocrystals from different waste bio-mass collating their liquid crystal ordering with morphological exploration. Int. J. Biol. Macromol. 2021, 175, 242–253. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surf. A Physicochem. Eng. Asp. 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Osorio, D.A.; Cranston, E.D. Optimization of cellulose nanocrystal length and surface charge density through phosphoric acid hydrolysis. Phil. Trans. R. Soc. 2017, 376, 20170041. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W.; Ling, Y.; Liu, B. Facile and rapid one–step extraction of carboxylated cellulose nanocrystals by H2SO4/HNO3 mixed acid hydrolysis. Carbohydr. Polym. 2020, 231, 115701. [Google Scholar] [CrossRef]
- Montanari, S.; Roumani, M.; Heux, L.; Vignon, M.R. Topochemistry of Carboxylated Cellulose Nanocrystals Resulting from TEMPO-Mediated Oxidation. Macromolecules 2005, 38, 1665–1671. [Google Scholar] [CrossRef]
- Fan, W.; Li, J.; Wei, L.; Xu, Y. Carboxylated cellulose nanocrystal films with tunable chiroptical properties. Carbohydr. Polym. 2022, 289, 119442. [Google Scholar] [CrossRef]
- Zhong, L.; Fu, S.; Peng, X.; Zhan, H.; Sun, R. Colloidal stability of negatively charged cellulose nanocrystalline in aqueous systems. Carbohydr. Polym. 2012, 90, 644–649. [Google Scholar] [CrossRef]
- da Silva Maradini, G.; Oliveira, M.P.; da Silva Guanaes, G.M.; Passamani, G.Z.; Carreira, L.G.; Boschetti, W.T.; Monteiro, S.N.; Pereira, A.C.; de Oliveira, B.F. Characterization of Polyester Nanocomposites Reinforced with Conifer Fiber Cellulose Nanocrystals. Polymers 2020, 12, 2838. [Google Scholar] [CrossRef]
- Feng, K.; Dong, C.; Gao, Y.; Jin, Z. A Green and Iridescent Composite of Cellulose Nanocrystals with Wide Solvent Resistance and Strong Mechanical Properties. ACS Sustain. Chem. Eng. 2021, 9, 6764–6775. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Vaseneva, I.N.; Mikhaylov, V.I.; Martakov, I.S.; Moskalev, A.A.; Koval, L.A.; Zemskaya, N.V.; Paderin, N.M.; Sitnikov, P.A. Pickering emulsions stabilized by partially acetylated cellulose nanocrystals for oral administration: Oils effect and in vivo toxicity. Cellulose 2021, 28, 2365–2385. [Google Scholar] [CrossRef]
- Sun, Z.; Eyley, S.; Guo, Y.; Salminen, R.; Thielemans, W. Synergistic effects of chloride anions and carboxylated cellulose nanocrystals on the assembly of thick three-dimensional high-performance polypyrrole-based electrodes. J. Energy Chem. 2022, 70, 492–501. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Tian, B.; Xiao, H.; Chen, W.; Wu, H.; Jia, J. Immobilization of bismuth oxychloride on cellulose nanocrystal for photocatalytic sulfonylation of arylacetylenic acids with sodium arylsulfinates under visible light. Arab. J. Chem. 2022, 15, 103708. [Google Scholar] [CrossRef]
- Blockx, J.; Verfaillie, A.; Deschaume, O.; Bartic, C.; Muylaert, K.; Thielemans, W. Glycine betaine grafted nanocellulose as an effective and bio-based cationic nanocellulose flocculant for wastewater treatment and microalgal harvesting. Nanoscale Adv. 2021, 3, 4133–4144. [Google Scholar] [CrossRef]
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Hirai, A.; Inui, O.; Horii, F.; Tsuji, M. Phase Separation Behavior in Aqueous Suspensions of Bacterial Cellulose Nanocrystals Prepared by Sulfuric Acid Treatment. Langmuir 2009, 25, 497–502. [Google Scholar] [CrossRef]
- Esmaeili, M.; George, K.; Rezvan, G.; Taheri-Qazvini, N.; Zhang, R.; Sadati, M. Capillary Flow Characterizations of Chiral Nematic Cellulose Nanocrystal Suspensions. Langmuir 2022, 38, 2192–2204. [Google Scholar] [CrossRef]
- Browne, C.; Raghuwanshi, V.S.; Garnier, G.; Batchelor, W. Modulating the chiral nematic structure of cellulose nanocrystal suspensions with electrolytes. J. Colloid Interface Sci. 2023, 650, 1064–1072. [Google Scholar] [CrossRef]
- Onsager, L. The effects of shape on the interaction of colloidal particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Stroobants, A.; Lekkerkerker, H.N.W.; Odijk, T. Effect of Electrostatic Interaction on the Liquid Crystal Phase Transition in Solutions of Rodlike Polyelectrolytes. Macromolecules 1986, 19, 2232–2238. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Morehead, F.F.; Koch, M.J. Some hydrodynamic properties of neutral suspensions of cellulose crystallites as related to size and shape. J. Colloid Sci. 1961, 16, 327–344. [Google Scholar] [CrossRef]
- Terech, P.; Chazeau, L.; Cavaillé, J.Y. A Small-Angle Scattering Study of Cellulose Whiskers in Aqueous Suspensions. Macromolecules 1999, 32, 1872–1875. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Kitchens, C.L. Static light scattering of triaxial nanoparticle suspensions in the RayleighGans-Debye regime: Application to cellulose nanocrystals. RSC Adv. 2012, 2, 1096–1105. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, K.; Zhan, C.; Geng, L.; Chu, B.; Hsiao, B.S. Characterization of Nanocellulose Using Small-Angle Neutron, X-ray, and Dynamic Light Scattering Techniques. J. Phys. Chem. B 2017, 121, 1340–1351. [Google Scholar] [CrossRef]
- Lahiji, R.R.; Xu, X.; Reifenberger, R.; Raman, A.; Rudie, A.; Moon, R.J. Atomic Force Microscopy Characterization of Cellulose Nanocrystals. Langmuir 2010, 26, 4480–4488. [Google Scholar] [CrossRef]
- Bushell, M.; Meija, J.; Chen, M.; Batchelor, W.; Browne, C.; Cho, J.-Y.; Clifford, C.A.; Al-Rekabi, Z.; Vanderfleet, O.M.; Cranston, E.D.; et al. Particle size distributions for cellulose nanocrystals measured by atomic force microscopy: An interlaboratory comparison. Cellulose 2021, 28, 1387–1403. [Google Scholar] [CrossRef]
- Johns, M.A.; Lam, C.; Zakani, B.; Melo, L.; Grant, E.R.; Cranston, E.D. Comparison of cellulose nanocrystal dispersion in aqueous suspension via new and established analytical techniques. Cellulose 2023, 30, 8259–8274. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.-L.; Heux, L.; Dubreuil, F.; Rochas, C. The Shape and Size Distribution of Crystalline Nanoparticles Prepared by Acid Hydrolysis of Native Cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef]
- Meija, J.; Bushell, M.; Couillard, M.; Beck, S.; Bonevich, J.; Cui, K.; Foster, J.; Will, J.; Fox, D.; Cho, W.; et al. Particle Size Distributions for Cellulose Nanocrystals Measured by Transmission Electron Microscopy: An Interlaboratory Comparison. Anal. Chem. 2020, 92, 13434–13442. [Google Scholar] [CrossRef]
- Campano, C.; Lopez-Exposito, P.; Gonzalez-Aguilera, L.; Blanco, Á.; Negro, C. In-depth characterization of the aggregation state of cellulose nanocrystals through analysis of transmission electron microscopy images. Carbohydr. Polym. 2021, 254, 117271. [Google Scholar] [CrossRef]
- Qian, H. Major Factors Influencing the Size Distribution Analysis of Cellulose Nanocrystals Imaged in Transmission Electron Microscopy. Polymers 2021, 13, 3318. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Meinander, K.; Jensen, T.N.; Simonsen, S.B.; Helveg, S.; Lauritsen, J.V. Quantification of tip-broadening in non-contact atomic force microscopy with carbon nanotube tips. Nanotechnology 2012, 23, 405705. [Google Scholar] [CrossRef]
- Bercea, M.; Navard, P. Shear Dynamics of Aqueous Suspensions of Cellulose Whiskers. Macromolecules 2000, 33, 6011–6016. [Google Scholar] [CrossRef]
- Kaushik, M.; Basu, K.; Benoit, C.; Cirtiu, C.M.; Vali, H.; Moores, A. Cellulose Nanocrystals as Chiral Inducers: Enantioselective Catalysis and Transmission Electron Microscopy 3D Characterization. J. Am. Chem. Soc. 2015, 137, 6124–6127. [Google Scholar] [CrossRef]
- Buesch, C.; Smith, S.W.; Eschbach, P.; Conley, J.F., Jr.; Simonsen, J. The Microstructure of Cellulose Nanocrystal Aerogels as Revealed by Transmission Electron Microscope Tomography. Biomacromolecules 2016, 17, 2956–2962. [Google Scholar] [CrossRef]
- Majoinen, J.; Haataja, J.S.; Appelhans, D.; Lederer, A.; Olszewska, A.; Seitsonen, J.; Aseyev, V.; Kontturi, E.; Rosilo, H.; Österberg, M.; et al. Supracolloidal Multivalent Interactions and Wrapping of Dendronized Glycopolymers on Native Cellulose Nanocrystals. J. Am. Chem. Soc. 2014, 136, 866–869. [Google Scholar] [CrossRef]
- Majoinen, J.; Hassinen, J.; Haataja, J.S.; Rekola, H.T.; Kontturi, E.; Kostiainen, M.A.; Ras, R.H.A.; Törmä, P.; Ikkala, O. Chiral Plasmonics Using Twisting along Cellulose Nanocrystals as a Template for Gold Nanoparticles. Adv. Mater. 2016, 28, 5262–5267. [Google Scholar] [CrossRef]
- Bai, L.; Kämäräinen, T.; Ziang, W.; Majoinen, J.; Seitsonen, J.; Grande, R.; Huan, S.; Liu, L.; Fan, Y.; Rojas, O.J. Chirality from Cryo-Electron Tomograms of Nanocrystals Obtained by Lateral Disassembly and Surface Etching of Never-Dried Chitin. ACS Nano 2020, 14, 6921–6930. [Google Scholar] [CrossRef]
- Zhang, F.; Ilavsky, J. Ultra-Small-Angle X-ray Scattering of Polymers. Polym. Rev. 2010, 50, 59–90. [Google Scholar] [CrossRef]
- Schütz, C.; Agthe, M.; Fall, A.B.; Gordeyeva, K.; Guccini, V.; Salajková, M.; Plivelic, T.S.; Lagerwall, J.P.F.; Salazar-Alvarez, G.; Bergström, L. Rod Packing in Chiral Nematic Cellulose Nanocrystal Dispersions Studied by Small-Angle X-ray Scattering and Laser Diffraction. Langmuir 2015, 31, 6507–6513. [Google Scholar] [CrossRef]
- Rosén, T.; Wang, R.; Zhan, C.; He, H.; Chodankar, S.; Hsiao, B.S. Cellulose nanofibrils and nanocrystals in confined flow: Single-particle dynamics to collective alignment revealed through scanning small-angle X-ray scattering and numerical simulations. Phys. Rev. E 2020, 101, 032610. [Google Scholar] [CrossRef]
- Cherhal, F.; Cousin, F.; Capron, I. Influence of Charge Density and Ionic Strength on the Aggregation Process of Cellulose Nanocrystals in Aqueous Suspension, as Revealed by Small-Angle Neutron Scattering. Langmuir 2015, 31, 5596–5602. [Google Scholar] [CrossRef]
- Cherhal, F.; Cousin, F.; Capron, I. Structural Description of the Interface of Pickering Emulsions Stabilized by Cellulose Nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef]
- Uhlig, M.; Fall, A.; Weller, S.; Lehmann, M.; Prévost, S.; Wågberg, L.; von Klitzing, R.; Nyström, G. Two-Dimensional Aggregation and Semidilute Ordering in Cellulose Nanocrystals. Langmuir 2016, 32, 442–450. [Google Scholar] [CrossRef]
- Azzam, F.; Frka-Petesic, B.; Semeraro, E.F.; Cousin, F.; Jean, B. Small-Angle Neutron Scattering Reveals the Structural Details of Thermosensitive Polymer-Grafted Cellulose Nanocrystal Suspensions. Langmuir 2020, 36, 8511–8519. [Google Scholar] [CrossRef]
- Delepierre, G.; Eyley, S.; Thielemans, W.; Weder, C.; Cranston, E.D.; Zoppe, J.O. Patience is a Virtue: Self-Assembly and Physico-Chemical Properties of Cellulose Nanocrystal Allomorphs. Nanoscale 2020, 12, 17480–17493. [Google Scholar] [CrossRef]
- Jakubek, Z.J.; Chen, M.; Couillard, M.; Leng, T.; Liu, L.; Zou, S.; Baxa, U.; Clogston, J.D.; Hamad, W.Y.; Johnston, L.J. Characterization challenges for a cellulose nanocrystal reference material: Dispersion and particle size distributions. J. Nanopart. Res. 2018, 20, 98. [Google Scholar] [CrossRef]
- Miriam de Souza Lima, M.; Wong, J.T.; Paillet, M.; Borsali, R.; Pecora, R. Translational and Rotational Dynamics of Rodlike Cellulose Whiskers. Langmuir 2003, 19, 24–29. [Google Scholar] [CrossRef]
- Khouri, S.; Shams, M.; Tam, K.C. Determination and prediction of physical properties of cellulose nanocrystals from dynamic light scattering measurements. J. Nanopart. Res. 2014, 16, 2499. [Google Scholar] [CrossRef]
- Van Rie, J.; Schütz, C.; Gençer, A.; Lombardo, S.; Gasser, U.; Kumar, S.; Salazar-Alvarez, G.; Kang, K.; Thielemans, W. Anisotropic Diffusion and Phase Behavior of Cellulose Nanocrystal Suspensions. Langmuir 2019, 35, 2289–2302. [Google Scholar] [CrossRef]
- Mazloumi, M.; Johnston, L.J.; Jakubek, Z.J. Dispersion, stability and size measurements for cellulose nanocrystals by static multiple light scattering. Cellulose 2018, 25, 5751–5768. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; Pignon, F.; El Kissi, N.; Dufresne, A. Surface adsorption of triblock copolymer (PEO–PPO–PEO) on cellulose nanocrystals and their melt extrusion with polyethylene. RSC Adv. 2016, 6, 66224–66232. [Google Scholar] [CrossRef]
- Gicquel, E.; Bras, J.; Rey, C.; Putaux, J.-L.; Pignon, F.; Jean, B.; Martin, C. Impact of sonication on the rheological and colloidal properties of highly concentrated cellulose nanocrystal suspensions. Cellulose 2019, 26, 7619–7634. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Andrade Feitosa, J.P.; Portela da Gama, F.M.; Saraiva Morais, J.P.; Andrade, F.K.; Moreira de Souza Filho, M.S.; de Freitas Rosa, M. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Nayuk, R.; Huber, K. Formfactors of Hollow and Massive Rectangular Parallelepipeds at Variable Degree of Anisometry. Z. Phys. Chem. 2012, 226, 837–854. [Google Scholar] [CrossRef]
- Boluk, Y.; Danumah, C. Analysis of cellulose nanocrystal rod lengths by dynamic light scattering and electron microscopy. J. Nanopart. Res. 2014, 16, 2174. [Google Scholar] [CrossRef]
- Tirado, M.M.; García de la Torre, J. Rotational dynamics of rigid, symmetric top macromolecules. Application to circular cylinders. J. Chem. Phys. 1980, 73, 1986–1993. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]

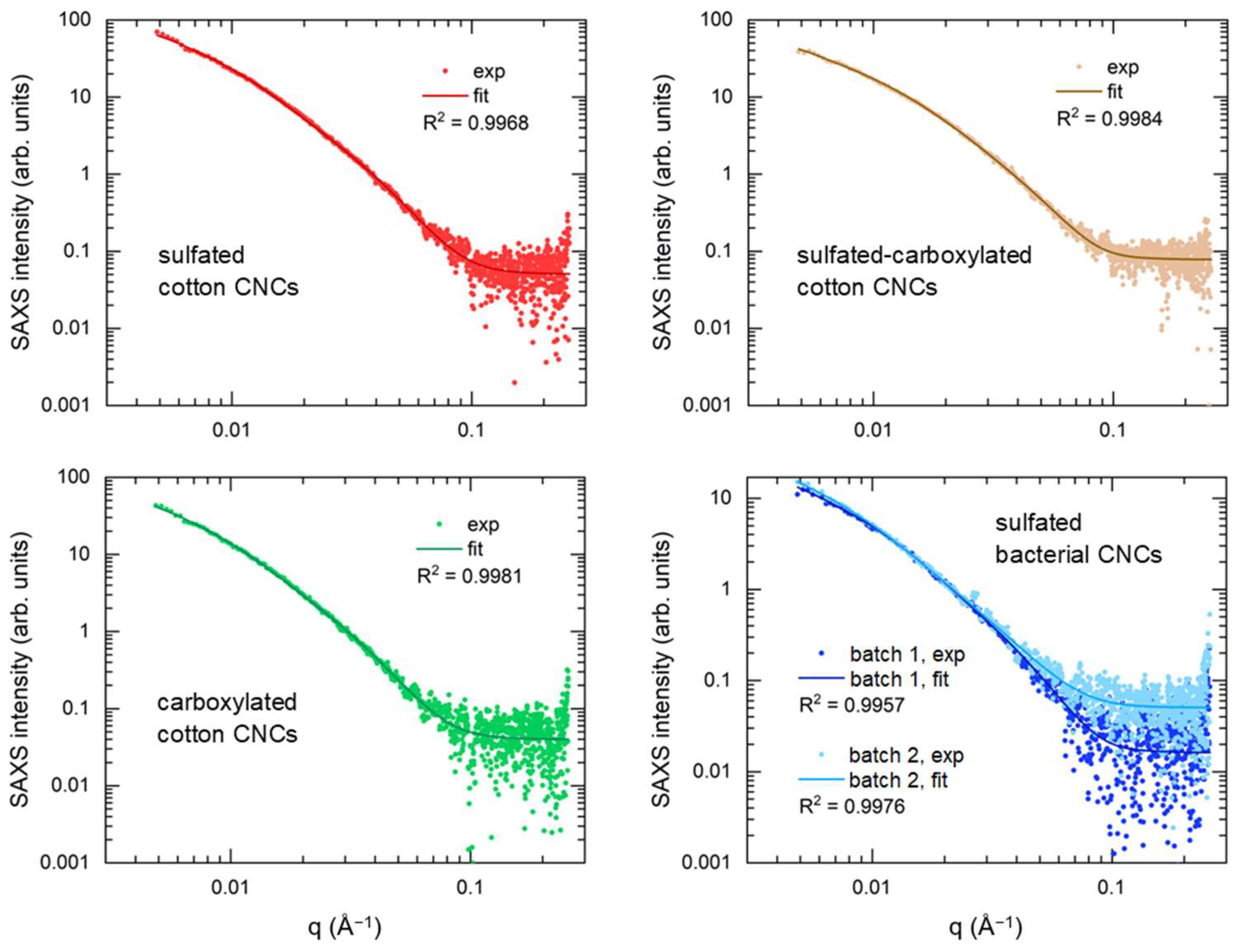

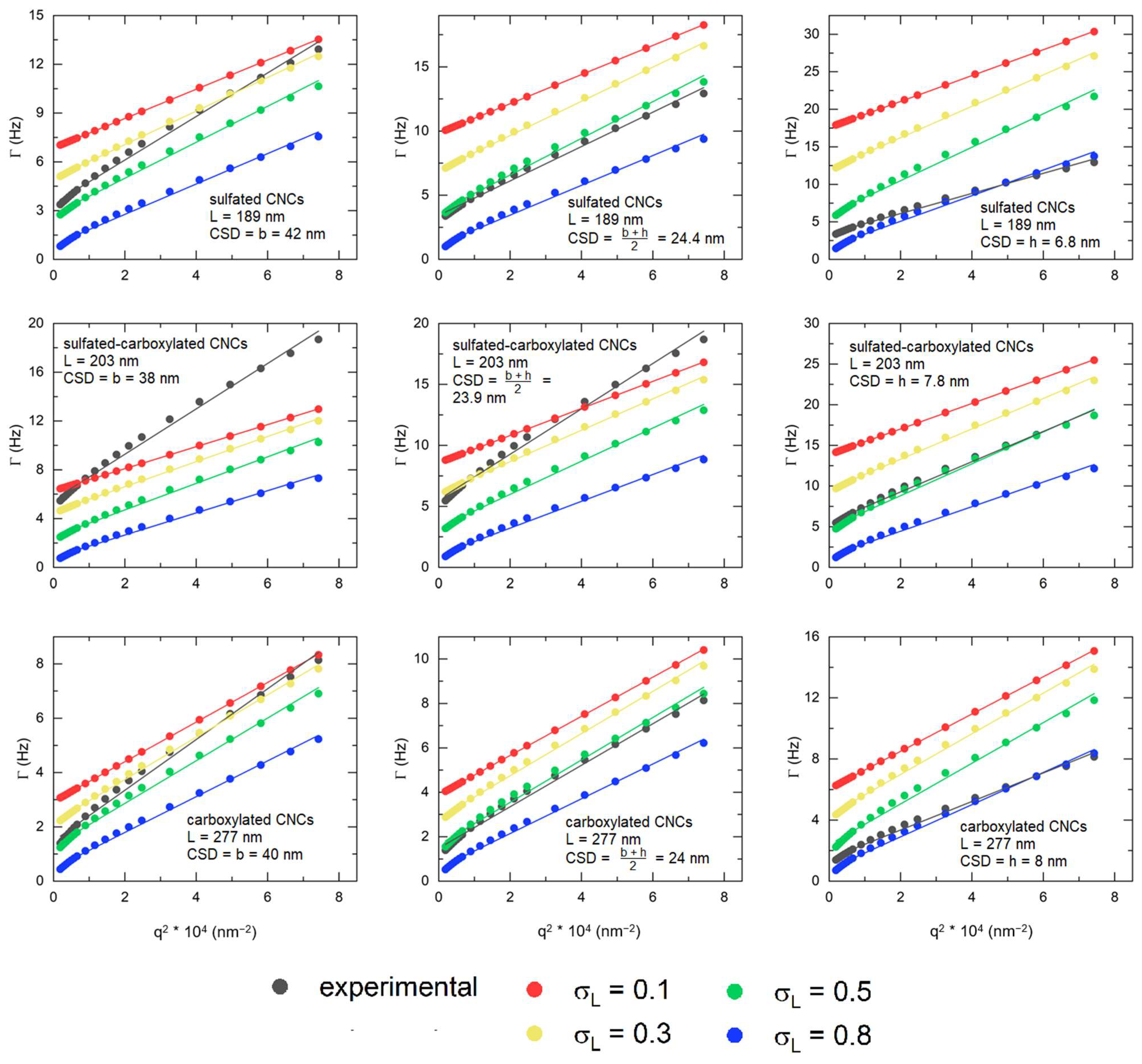

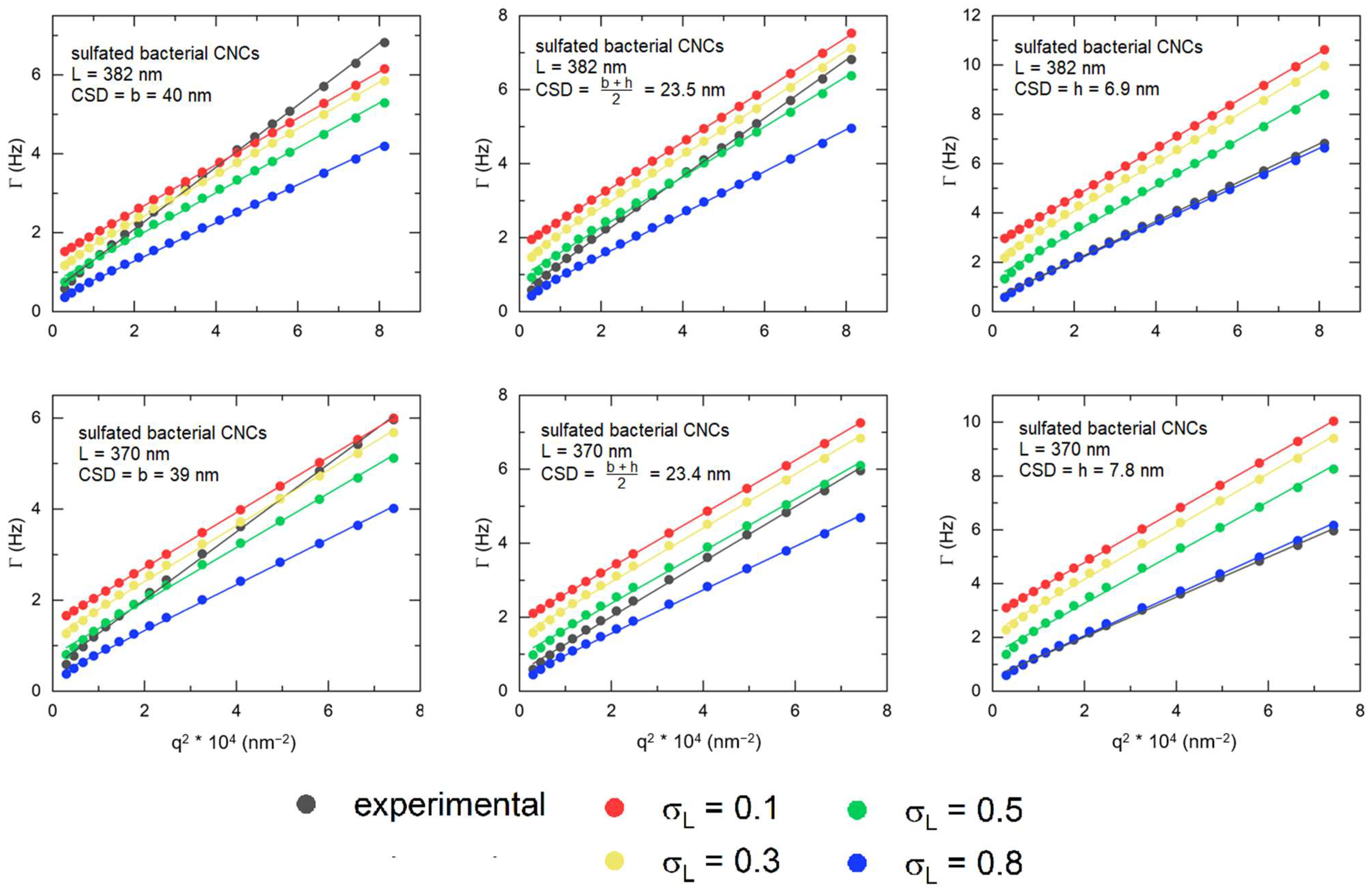
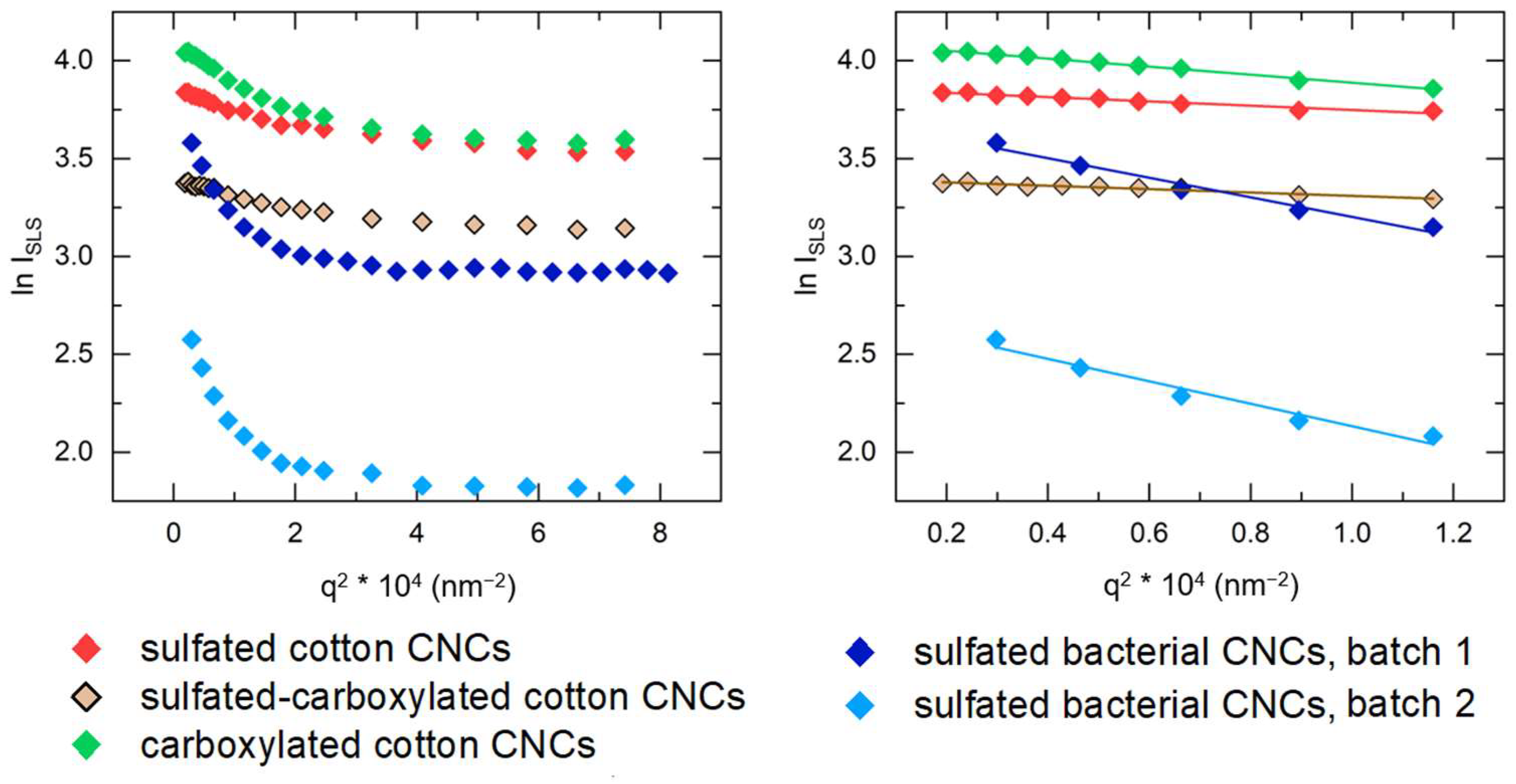
| Sample | c*, vol% | cSAXS, vol% | cDDLS, vol% |
|---|---|---|---|
| sulfated cotton CNCs | 0.25 | 0.10 | 0.05 |
| sulfated-carboxylated cotton CNCs | 0.28 | 0.10 | 0.05 |
| carboxylated cotton CNCs | 0.16 | 0.05 | 0.05 |
| sulfated bacterial CNCs, 1st batch | 0.06 | 0.02 | 0.01 |
| sulfated bacterial CNCs, 2nd batch | 0.09 | 0.02 | 0.01 |
| Parameter | Sulfated Cotton CNCs | Sulfated-Carboxylated Cotton CNCs | Carboxylated Cotton CNCs | Sulfated Bacterial CNCs, 1st Batch | Sulfated Bacterial CNCs, 2nd Batch |
|---|---|---|---|---|---|
| , nm | 189 | 203 | 277 | 382 | 370 |
| σL | 0.31 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.01 | 0.36 ± 0.03 | 0.41 ± 0.03 |
| , nm | 42 | 38 | 40 | 40 | 39 |
| σb | 0.27 ± 0.05 | 0.28 ± 0.02 | 0.28 ± 0.02 | 0.29 ± 0.02 | 0.25 ± 0.01 |
| , nm | 6.8 | 7.8 | 8.0 | 6.9 | 7.8 |
| σh | 0.18 ± 0.01 | 0.38 ± 0.05 | 0.34 ± 0.05 | 0.27 ± 0.03 | 0.38 ± 0.05 |
| : ratio | 4.5 | 5.3 | 6.9 | 9.6 | 9.5 |
| : ratio | 6.2 | 4.9 | 5.0 | 5.8 | 5.0 |
| Parameter | Sulfated Cotton CNCs | Sulfated-Carboxylated Cotton CNCs | Carboxylated Cotton CNCs | Sulfated Bacterial CNCs, 1st Batch | Sulfated Bacterial CNCs, 2nd Batch |
|---|---|---|---|---|---|
| , nm | 28 | 20 | 31 | 26 | 29 |
| σb | 0.28 ± 0.02 | 0.34 ± 0.03 | 0.29 ± 0.03 | 0.29 ± 0.05 | 0.35 ± 0.06 |
| , nm | 4.5 | 5.0 | 5.1 | 5.0 | 3.9 |
| σh | 0.42 ± 0.02 | 0.29 ± 0.01 | 0.42 ± 0.01 | 0.29 ± 0.08 | 0.44 ± 0.02 |
| : ratio | 6.2 | 4.0 | 6.1 | 5.2 | 7.4 |
| Parameter | Sulfated Cotton CNCs | Sulfated-Carboxylated Cotton CNCs | Carboxylated Cotton CNCs | Sulfated Bacterial CNCs, 1st Batch | Sulfated Bacterial CNCs, 2nd Batch |
|---|---|---|---|---|---|
| , nm | 28.4 | 9.6 | 25.0 | 5.7 | 10.0 |
| (AFM), nm | 42 | 38 | 40 | 40 | 39 |
| (AFM), nm | 6.8 | 7.8 | 8.0 | 6.9 | 7.8 |
| σL (DDLS) | 0.50 | 0.45 | 0.53 | 0.81 | 0.78 |
| σL (AFM) | 0.31 | 0.27 | 0.27 | 0.36 | 0.41 |
| Method | Sulfated Cotton CNCs | Sulfated-Carboxylated Cotton CNCs | Carboxylated Cotton CNCs | Sulfated Bacterial CNCs, 1st Batch | Sulfated Bacterial CNCs, 2nd Batch |
|---|---|---|---|---|---|
| AFM | 56 ± 18 | 60 ± 17 | 81 ± 23 | 111 ± 41 | 107 ± 46 |
| SLS | 57 ± 2 | 51 ± 2 | 78 ± 1 | 122 ± 6 | 131 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grachev, V.; Deschaume, O.; Lang, P.R.; Lettinga, M.P.; Bartic, C.; Thielemans, W. Dimensions of Cellulose Nanocrystals from Cotton and Bacterial Cellulose: Comparison of Microscopy and Scattering Techniques. Nanomaterials 2024, 14, 455. https://doi.org/10.3390/nano14050455
Grachev V, Deschaume O, Lang PR, Lettinga MP, Bartic C, Thielemans W. Dimensions of Cellulose Nanocrystals from Cotton and Bacterial Cellulose: Comparison of Microscopy and Scattering Techniques. Nanomaterials. 2024; 14(5):455. https://doi.org/10.3390/nano14050455
Chicago/Turabian StyleGrachev, Vladimir, Olivier Deschaume, Peter R. Lang, Minne Paul Lettinga, Carmen Bartic, and Wim Thielemans. 2024. "Dimensions of Cellulose Nanocrystals from Cotton and Bacterial Cellulose: Comparison of Microscopy and Scattering Techniques" Nanomaterials 14, no. 5: 455. https://doi.org/10.3390/nano14050455
APA StyleGrachev, V., Deschaume, O., Lang, P. R., Lettinga, M. P., Bartic, C., & Thielemans, W. (2024). Dimensions of Cellulose Nanocrystals from Cotton and Bacterial Cellulose: Comparison of Microscopy and Scattering Techniques. Nanomaterials, 14(5), 455. https://doi.org/10.3390/nano14050455