In Situ Polymer-Solution-Processed Graphene–PDMS Nanocomposites for Application in Intracranial Pressure Sensors
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization
3.2. Mechanical Properties
3.3. Electrical Properties
3.4. Pressure Sensing Abilities of Gr-PDMS Nanocomposites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hawryluk, G.W.; Citerio, G.; Hutchinson, P.; Kolias, A.; Meyfroidt, G.; Robba, C.; Stocchetti, N.; Chesnut, R. Intracranial pressure: Current perspectives on physiology and monitoring. Intensive Care Med. 2022, 48, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Nag, D.S.; Sahu, S.; Swain, A.; Kant, S. Intracranial pressure monitoring: Gold standard and recent innovations. World J. Clin. Cases 2019, 7, 1535. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Jia, X.; Pahren, L.; Lee, J.; Foreman, B. Intracranial pressure monitoring signals after traumatic brain injury: A narrative overview and conceptual data science framework. Front. Neurol. 2020, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Murphy, R.K.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Anania, P.; Battaglini, D.; Miller, J.P.; Balestrino, A.; Prior, A.; D’Andrea, A.; Badaloni, F.; Pelosi, P.; Robba, C.; Zona, G.; et al. Escalation therapy in severe traumatic brain injury: How long is intracranial pressure monitoring necessary? Neurosurg. Rev. 2021, 44, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Eide, P.K. Comparison of simultaneous continuous intracranial pressure (ICP) signals from ICP sensors placed within the brain parenchyma and the epidural space. Med. Eng. Phys. 2008, 30, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab A Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Levitt, M.R.; Mandrycky, C.; Abel, A.; Kelly, C.M.; Levy, S.; Chivukula, V.K.; Zheng, Y.; Aliseda, A.; Kim, L.J. Genetic correlates of wall shear stress in a patient-specific 3D-printed cerebral aneurysm model. J. Neurointerv. Surg. 2019, 11, 999–1003. [Google Scholar] [CrossRef]
- de Aguiar, K.M.R.; Nascimento, M.V.; Faccioni, J.L.; Noeske, P.-L.M.; Gaetjen, L.; Rischka, K.; Rodrigues-Filho, U.P. Urethanes PDMS-based: Functional hybrid coatings for metallic dental implants. Appl. Surf. Sci. 2019, 484, 1128–1140. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, K.; Tian, G.; Jiang, B.; Huang, Y. Stretchable Electronics Based on PDMS Substrates. Adv. Mater. 2021, 33, 2003155. [Google Scholar] [CrossRef]
- Victor, A.; Ribeiro, J.; Araújo, F.F. Study of PDMS characterization and its applications in biomedicine: A review. J. Mech. Eng. Biomech. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Z.; Song, H.; Kim, H.S.; Park, J. Wearable Intracranial Pressure Monitoring Sensor for Infants. Biosensors 2021, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Qian, W.; Li, X.; Fei, G.; Luo, G.; Wang, Z.; Xia, H. A novel method to prepare homogeneous biocompatible graphene-based PDMS composites with enhanced mechanical, thermal and antibacterial properties. Polym. Compos. 2019, 40, E1397–E1406. [Google Scholar] [CrossRef]
- Gao, X.; Huang, Y.; He, X.; Fan, X.; Liu, Y.; Xu, H.; Wu, D.; Wan, C. Mechanically Enhanced Electrical Conductivity of Polydimethylsiloxane-Based Composites by a Hot Embossing Process. Polymers 2019, 11, 56. [Google Scholar] [CrossRef]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the Local Chemical Structure of Graphene Oxide and Reduced Graphene Oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef]
- Tamang, S.; Rai, S.; Bhujel, R.; Bhattacharyya, N.K.; Swain, B.P.; Biswas, J. A concise review on GO, rGO and metal oxide/rGO composites: Fabrication and their supercapacitor and catalytic applications. J. Alloys Compd. 2023, 947, 169588. [Google Scholar] [CrossRef]
- Lanone, S.; Andujar, P.; Kermanizadeh, A.; Boczkowski, J. Determinants of carbon nanotube toxicity. Adv. Drug Deliv. Rev. 2013, 65, 2063–2069. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.-M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef]
- Zhang, B.; Ying, Y.; Zhu, Y.; Jiang, Y.; Zhang, Y.; Qiu, Y. Interfacial engineering in PDMS/graphene composites via anchoring polypyrrole nanowires to enhance its electro-photo thermal performance. Carbon 2021, 174, 10–23. [Google Scholar] [CrossRef]
- Monajjemi, M. Liquid-phase exfoliation (LPE) of graphite towards graphene: An ab initio study. J. Mol. Liq. 2017, 230, 461–472. [Google Scholar] [CrossRef]
- Narayan, R.; Kim, S.O. Surfactant mediated liquid phase exfoliation of graphene. Nano Converg. 2015, 2, 20. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Backes, C.; Abdelkader, A.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R. Production and processing of graphene and related materials. 2D Mater. 2020, 7, 022001. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Enhanced mechanical properties of nanocomposites at low graphene content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Tewatia, A.; Hendrix, J.; Dong, Z.; Taghon, M.; Tse, S.; Chiu, G.; Mayo, W.E.; Kear, B.; Nosker, T.; Lynch, J. Characterization of melt-blended graphene–poly (ether ether ketone) nanocomposite. Mater. Sci. Eng. B 2017, 216, 41–49. [Google Scholar] [CrossRef]
- Feng, C.; Zhu, D.; Wang, Y.; Jin, S. Electromechanical behaviors of graphene reinforced polymer composites: A review. Materials 2020, 13, 528. [Google Scholar] [CrossRef]
- Tarhini, A.; Tehrani-Bagha, A.R. Advances in Preparation Methods and Conductivity Properties of Graphene-based Polymer Composites. Appl. Compos. Mater. 2023, 30, 1737–1762. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Hussein, A.H.; Dong, Z.; Lynch-Branzoi, J.; Kear, B.H.; Shan, J.W.; Pelegri, A.A.; Stephen, D.T. Graphene-reinforced polymer matrix composites fabricated by in situ shear exfoliation of graphite in polymer solution: Processing, rheology, microstructure, and properties. Nanotechnology 2021, 32, 175703. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shen, Z.; Yi, M.; Zhang, X.; Ma, S. A green, rapid and size-controlled production of high-quality graphene sheets by hydrodynamic forces. RSC Adv. 2014, 4, 36464–36470. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z. Fluid dynamics: An emerging route for the scalable production of graphene in the last five years. RSC Adv. 2016, 6, 72525–72536. [Google Scholar] [CrossRef]
- Sun, K.; Dong, J.; Wang, Z.; Wang, Z.; Fan, G.; Hou, Q.; An, L.; Dong, M.; Fan, R.; Guo, Z. Tunable Negative Permittivity in Flexible Graphene/PDMS Metacomposites. J. Phys. Chem. C 2019, 123, 23635–23642. [Google Scholar] [CrossRef]
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H.M. Raman, mid-infrared, near-infrared and ultraviolet–visible spectroscopy of PDMS silicone rubber for characterization of polymer optical waveguide materials. J. Mol. Struct. 2010, 976, 274–281. [Google Scholar] [CrossRef]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. Aip Adv. 2014, 4, 027116. [Google Scholar] [CrossRef]
- Hong, H.; Xiong, G.; Dong, Z.; Kear, B.H.; Tse, S.D. Open-atmosphere flame synthesis of monolayer graphene. Carbon 2021, 182, 307–315. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Zhou, K.; Shi, Y.; Jiang, S.; Song, L.; Hu, Y.; Gui, Z. A facile liquid phase exfoliation method to prepare graphene sheets with different sizes expandable graphite. Mater. Res. Bull. 2013, 48, 2985–2992. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Z.; Meng, F.; Zhao, Y.; Wu, Z.; Lei, Y.; Xue, L. Interfacial interaction-induced temperature-dependent mechanical property of graphene-PDMS nanocomposite. J. Mater. Sci. 2020, 55, 1553–1561. [Google Scholar] [CrossRef]
- Wagner, C.D. NIST X-ray Photoelectron Spectroscopy Database, NIST Standarad Reference Database 20; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000.
- Shard, A.G. Practical guides for x-ray photoelectron spectroscopy: Quantitative XPS. J. Vac. Sci. Technol. A 2020, 38, 041201. [Google Scholar] [CrossRef]
- Guchhait, P.K.; Bhandari, S.; Singh, S.; Rahaman, M. Study on the effect of nanosilica particles on morphology, thermo-mechanical and electrical properties of liquid polysulfide modified epoxy hybrid nanocomposites. Int. J. Plast. Technol. 2011, 15, 150–162. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced mechanical properties of graphene-based poly (vinyl alcohol) composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, J.H.; Lee, J.-Y.; Capasso, A.; Bonaccorso, F.; Kang, S.-H.; Lee, Y.-K.; Lee, G.-H. Electrically Conducting and Mechanically Strong Graphene–Polylactic Acid Composites for 3D Printing. ACS Appl. Mater. Interfaces 2019, 11, 11841–11848. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, N.; Sun, X.; Lin, X.; Shen, X.; Jia, J.; Zhang, B.; Tang, B.; Chan, M.; Kim, J.K. Highly aligned graphene/polymer nanocomposites with excellent dielectric properties for high-performance electromagnetic interference shielding. Adv. Mater. 2014, 26, 5480–5487. [Google Scholar] [CrossRef]
- Wu, J.; Kong, L. High microwave permittivity of multiwalled carbon nanotube composites. Appl. Phys. Lett. 2004, 84, 4956–4958. [Google Scholar] [CrossRef]
- Essam, J.W. Percolation theory. Rep. Prog. Phys. 1980, 43, 833. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ameli, A.; Nofar, M.; Park, C.B.; Pötschke, P.; Rizvi, G. Polypropylene/carbon nanotube nano/microcellular structures with high dielectric permittivity, low dielectric loss, and low percolation threshold. Carbon 2014, 71, 206–217. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Wang, E.; Cakmak, M. Roll-to-Roll continuous manufacturing multifunctional nanocomposites by electric-field-assisted “Z” direction alignment of graphite flakes in poly (dimethylsiloxane). ACS Appl. Mater. Interfaces 2017, 9, 919–929. [Google Scholar] [CrossRef]
- Sohi, N.J.S.; Bhadra, S.; Khastgir, D. The effect of different carbon fillers on the electrical conductivity of ethylene vinyl acetate copolymer-based composites and the applicability of different conductivity models. Carbon 2011, 49, 1349–1361. [Google Scholar] [CrossRef]
- Mohd Radzuan, N.A.; Sulong, A.B.; Sahari, J. A review of electrical conductivity models for conductive polymer composite. Int. J. Hydrogen Energy 2017, 42, 9262–9273. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Qin, G.; Qiu, J. Graphene/polypyrrole nanocomposites with high negative permittivity and low dielectric loss tangent. Ceram. Int. 2019, 45, 5407–5412. [Google Scholar] [CrossRef]
- Hashemi, R.; Weng, G.J. A theoretical treatment of graphene nanocomposites with percolation threshold, tunneling-assisted conductivity and microcapacitor effect in AC and DC electrical settings. Carbon 2016, 96, 474–490. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Zhong, Z.; Weng, G.J. A frequency-dependent theory of electrical conductivity and dielectric permittivity for graphene-polymer nanocomposites. Carbon 2017, 111, 221–230. [Google Scholar] [CrossRef]
- Kou, H.; Zhang, L.; Tan, Q.; Liu, G.; Lv, W.; Lu, F.; Dong, H.; Xiong, J. Wireless flexible pressure sensor based on micro-patterned Graphene/PDMS composite. Sens. Actuators A Phys. 2018, 277, 150–156. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, B.; Duan, J.; Guo, H.; Tang, J. Flexible Pressure Sensor with Ag Wrinkled Electrodes Based on PDMS Substrate. Sensors 2016, 16, 2131. [Google Scholar] [CrossRef] [PubMed]
- Ramalingame, R.; Lakshmanan, A.; Müller, F.; Thomas, U.; Kanoun, O. Highly sensitive capacitive pressure sensors for robotic applications based on carbon nanotubes and PDMS polymer nanocomposite. J. Sens. Sens. Syst. 2019, 8, 87–94. [Google Scholar] [CrossRef]
- Mehmood, A.; Mubarak, N.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E. Graphene/PVA buckypaper for strain sensing application. Sci. Rep. 2020, 10, 20106. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Uhrich, K.E. Biodegradable and biocompatible polymers for electronic applications: A review. J. Bioact. Compat. Polym. 2018, 34, 3–15. [Google Scholar] [CrossRef]
- Kim, S.; Choi, G.Y.; Nezaj, J.; Ulman, A.; Fleischer, C. Studies of adhesion to molecularly engineered surfaces using contact mechanics methods. In Macromolecular Symposia; Wiley-VCH GmbH: Weinheim, Germany, 1998; Volume 126, pp. 1–6. [Google Scholar]
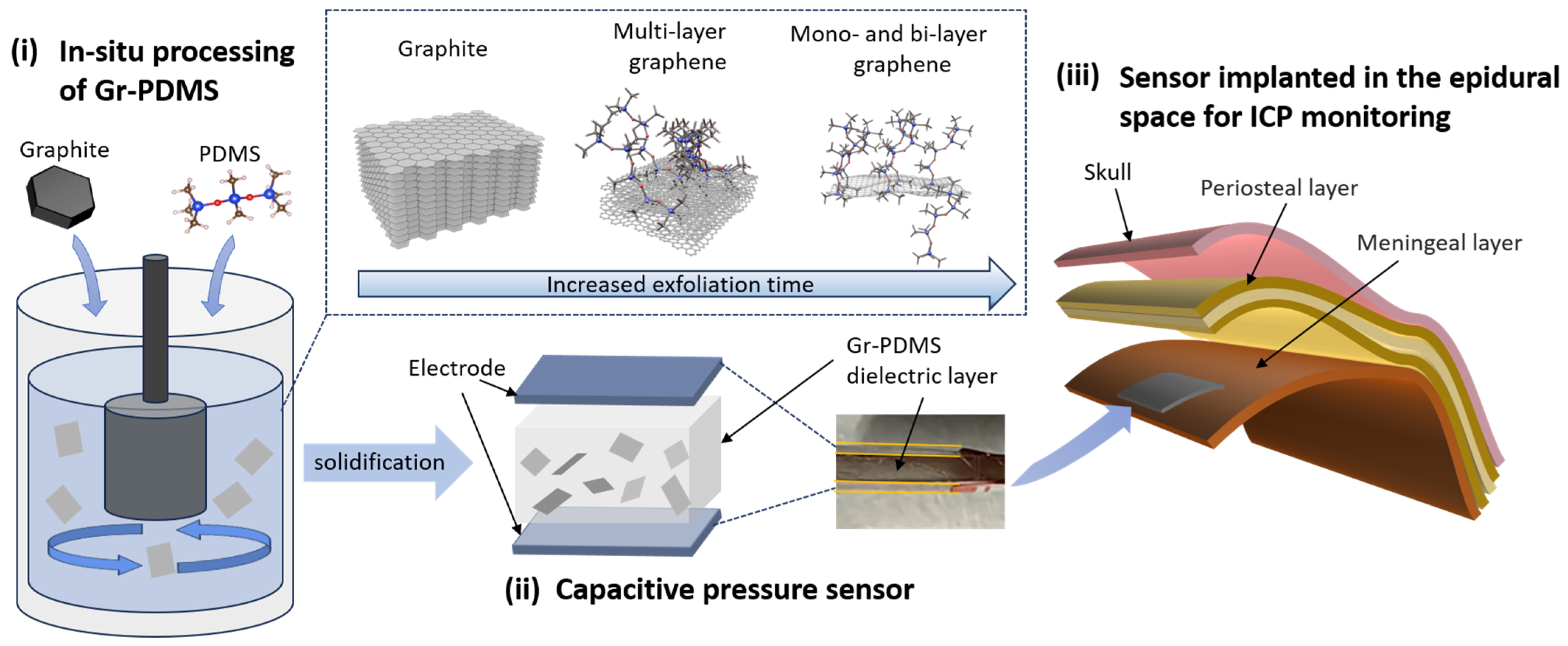
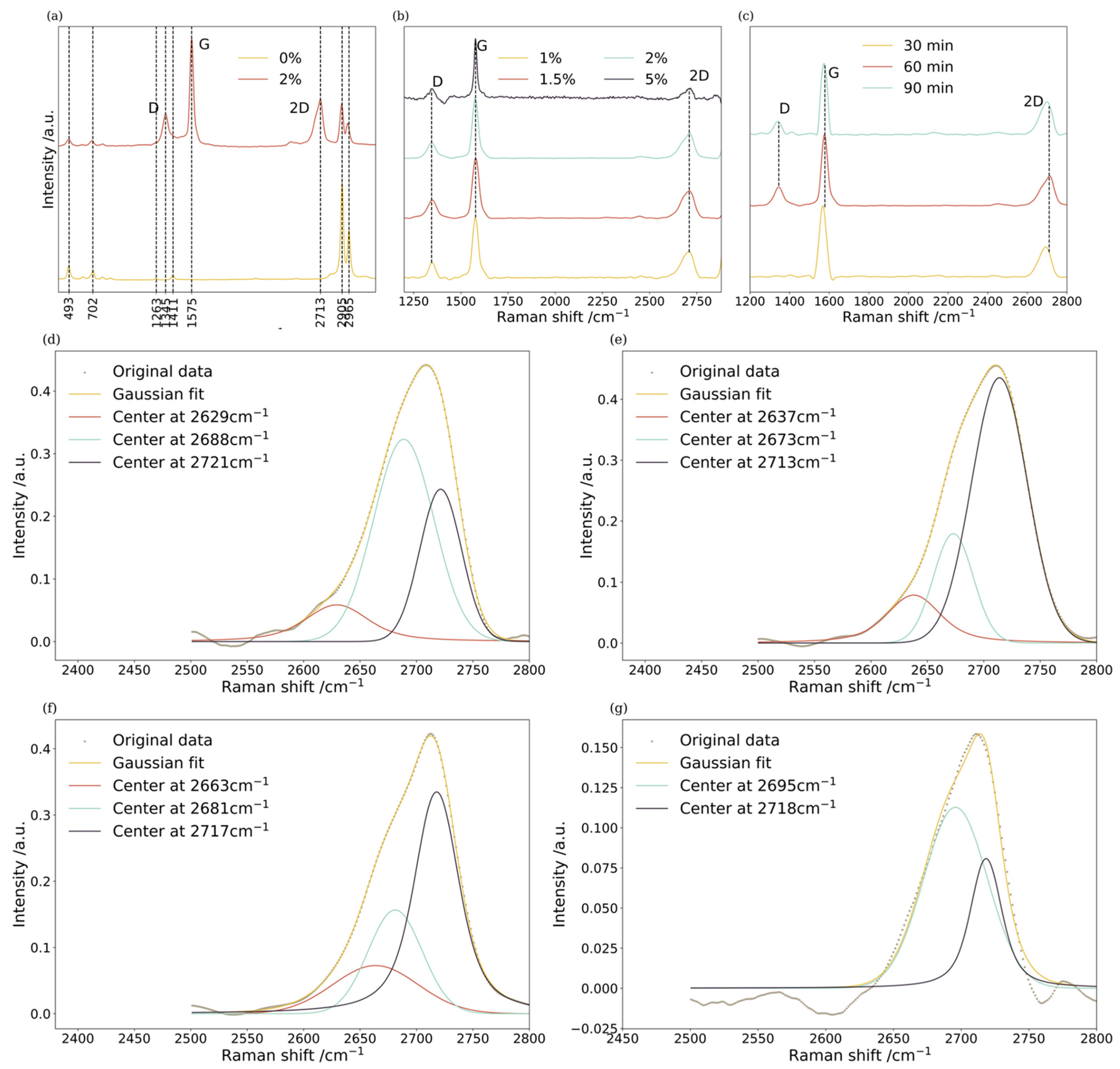


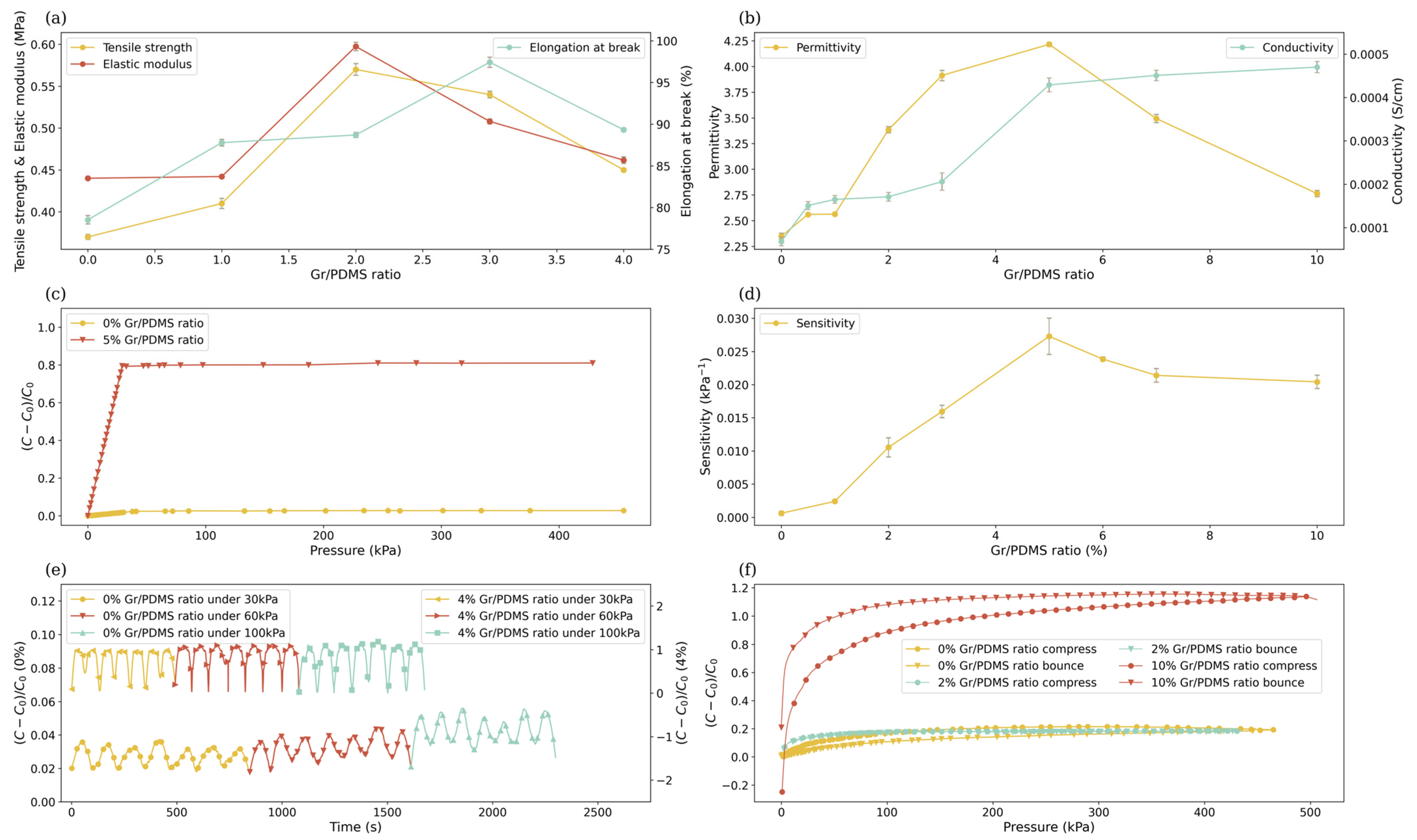
| Gr/PDMS (%) | Graphite Particles (g) | PDMS (g) |
|---|---|---|
| 0.0 | 0 | 30 |
| 0.5 | 0.15 | 30 |
| 1.0 | 0.3 | 30 |
| 1.5 | 0.45 | 30 |
| 2.0 | 0.6 | 30 |
| 3.0 | 0.9 | 30 |
| 4.0 | 1.2 | 30 |
| 5.0 | 1.5 | 30 |
| 6.0 | 1.8 | 30 |
| 7.0 | 2.1 | 30 |
| 10.0 | 3.0 | 30 |
| Gr/PDMS (%) | (%) | (%) | C–Si (%) | C–O (%) |
|---|---|---|---|---|
| 0.0 | 0 | 0 | 85.26 | 13.87 |
| 2.0 | 33.45 | 16.12 | 43.78 | 6.65 |
| Gr/PDMS (%) | O–Si–CH3 | O–Si–O | Si–C | Si–O |
|---|---|---|---|---|
| 0.0 | 53.73 | 32.87 | 0 | 13.40 |
| 2.0 | 17.16 | 12.20 | 36.81 | 33.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, H.; Zhang, J.; Zhu, Y.; Tse, S.D.; Guo, H.; Lai, Y.; Xi, Y.; He, L.; Zhu, Z.; Yin, K.; et al. In Situ Polymer-Solution-Processed Graphene–PDMS Nanocomposites for Application in Intracranial Pressure Sensors. Nanomaterials 2024, 14, 399. https://doi.org/10.3390/nano14050399
Hong H, Zhang J, Zhu Y, Tse SD, Guo H, Lai Y, Xi Y, He L, Zhu Z, Yin K, et al. In Situ Polymer-Solution-Processed Graphene–PDMS Nanocomposites for Application in Intracranial Pressure Sensors. Nanomaterials. 2024; 14(5):399. https://doi.org/10.3390/nano14050399
Chicago/Turabian StyleHong, Hua, Junjie Zhang, Yuchen Zhu, Stephen D. Tse, Hongxuan Guo, Yilin Lai, Yubo Xi, Longbing He, Zhen Zhu, Kuibo Yin, and et al. 2024. "In Situ Polymer-Solution-Processed Graphene–PDMS Nanocomposites for Application in Intracranial Pressure Sensors" Nanomaterials 14, no. 5: 399. https://doi.org/10.3390/nano14050399
APA StyleHong, H., Zhang, J., Zhu, Y., Tse, S. D., Guo, H., Lai, Y., Xi, Y., He, L., Zhu, Z., Yin, K., & Sun, L. (2024). In Situ Polymer-Solution-Processed Graphene–PDMS Nanocomposites for Application in Intracranial Pressure Sensors. Nanomaterials, 14(5), 399. https://doi.org/10.3390/nano14050399









