Highly Efficient Ru-Based Catalysts for Lactic Acid Conversion to Alanine
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalysts’ Synthesis
2.2. Catalysts’ Characterization
2.3. Catalytic Tests
2.4. Products’ Analysis
3. Results and Discussion
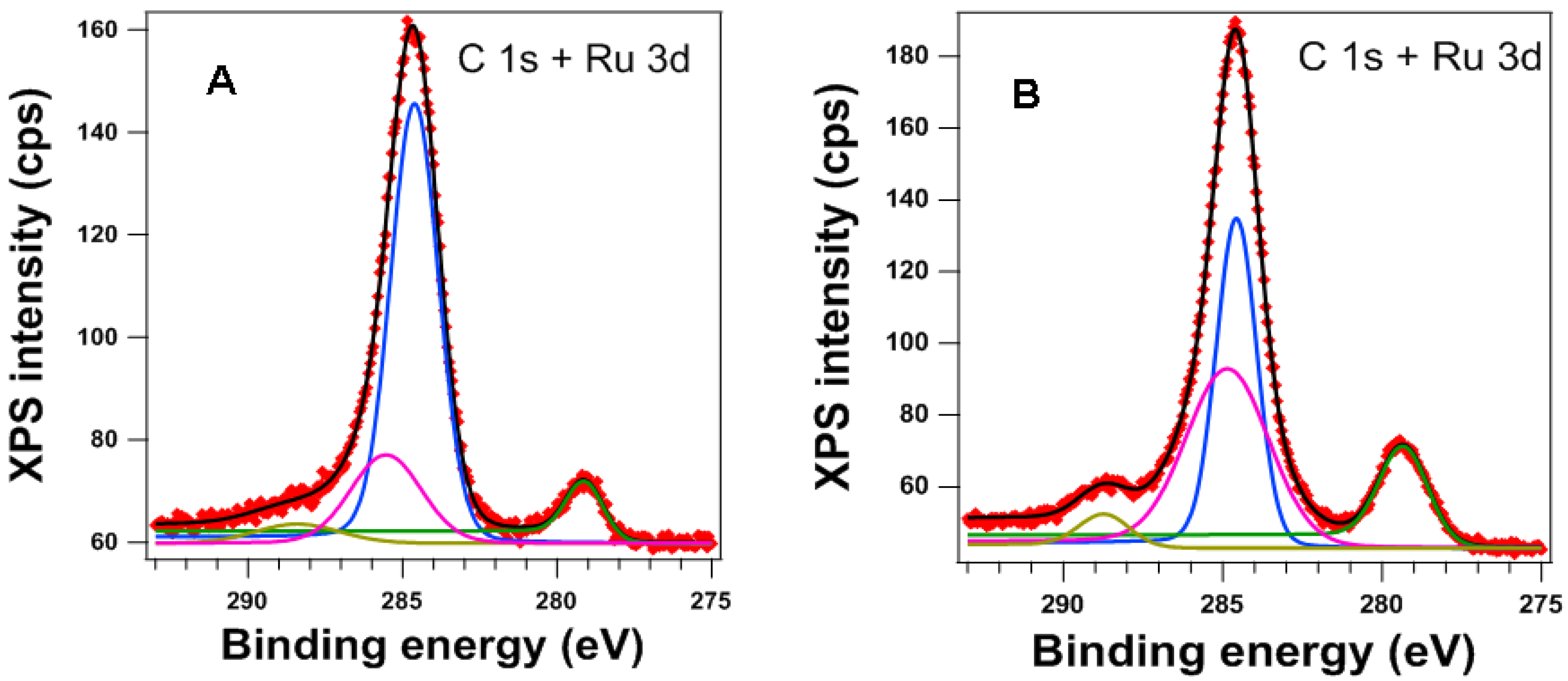
3.1. Catalysts’ Characterization
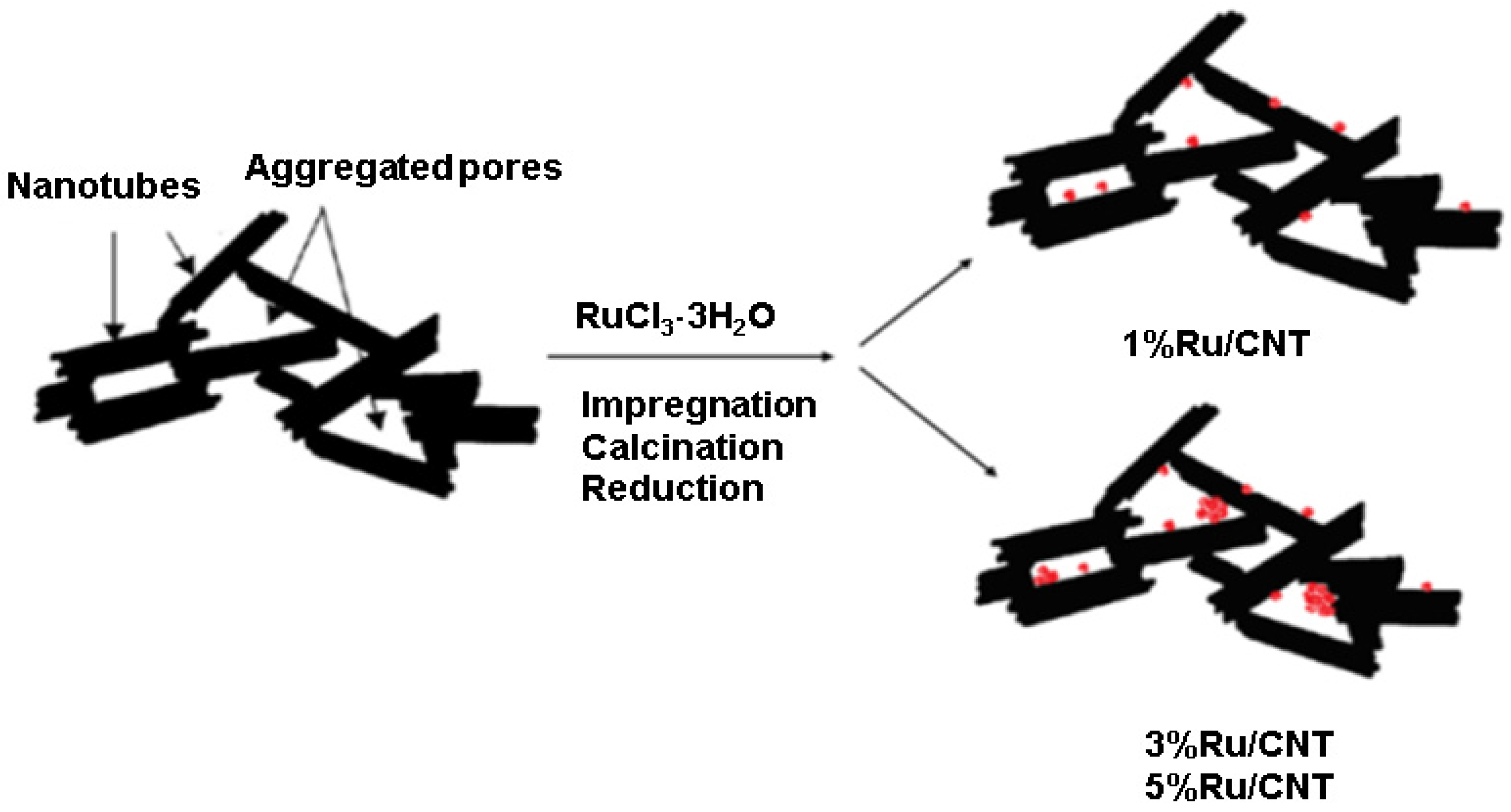
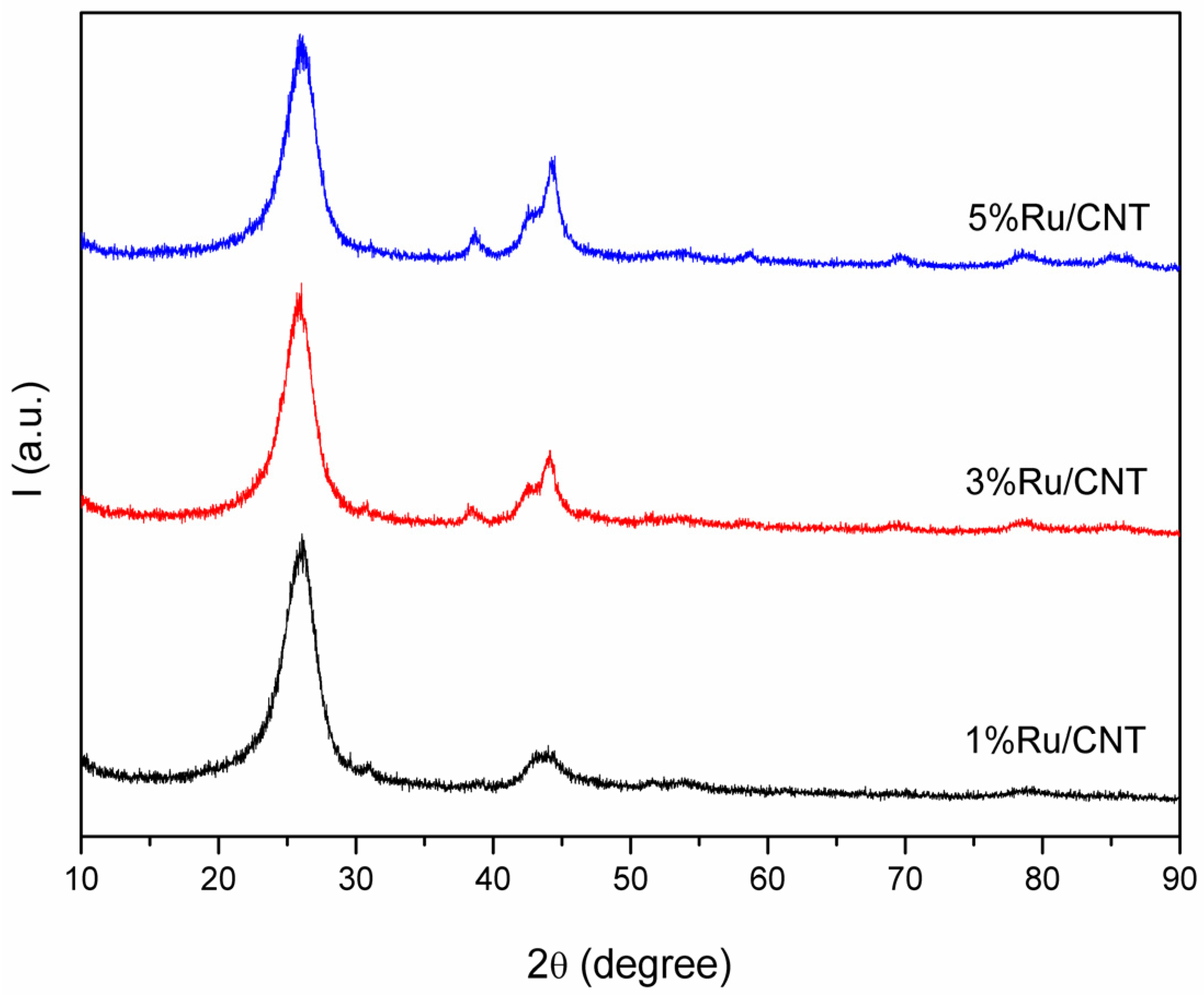
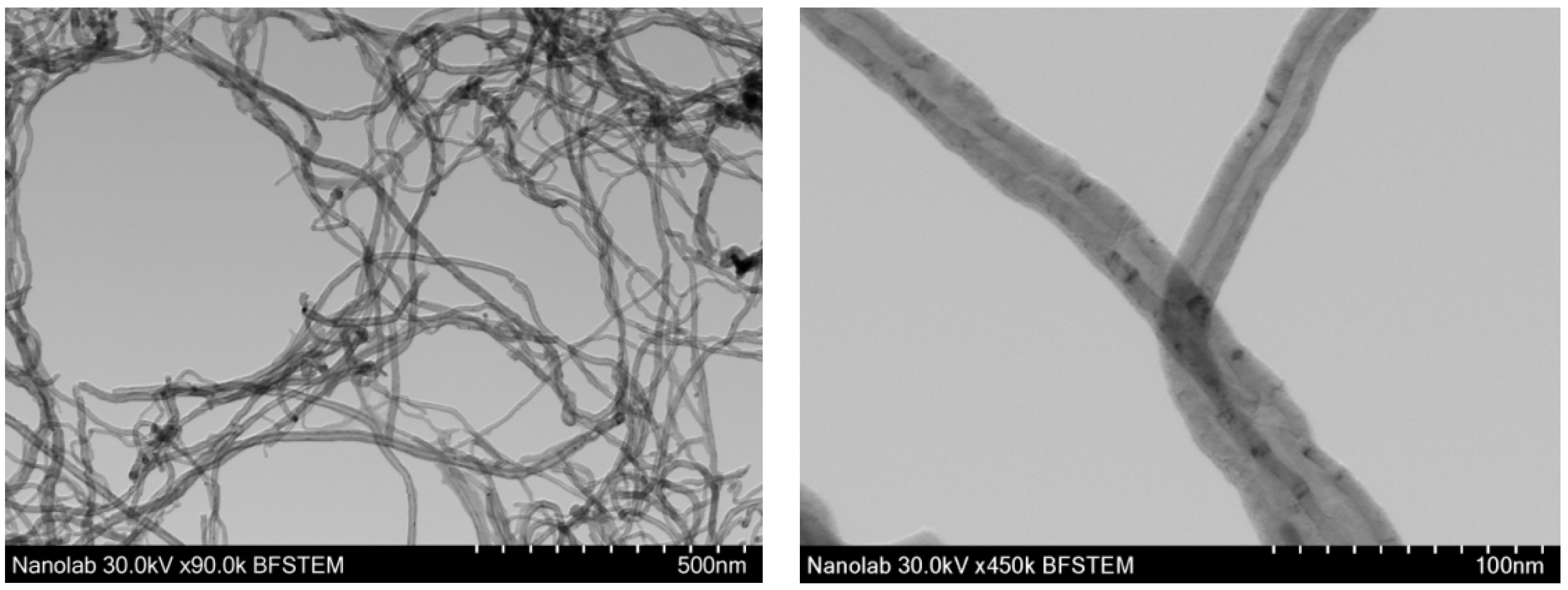
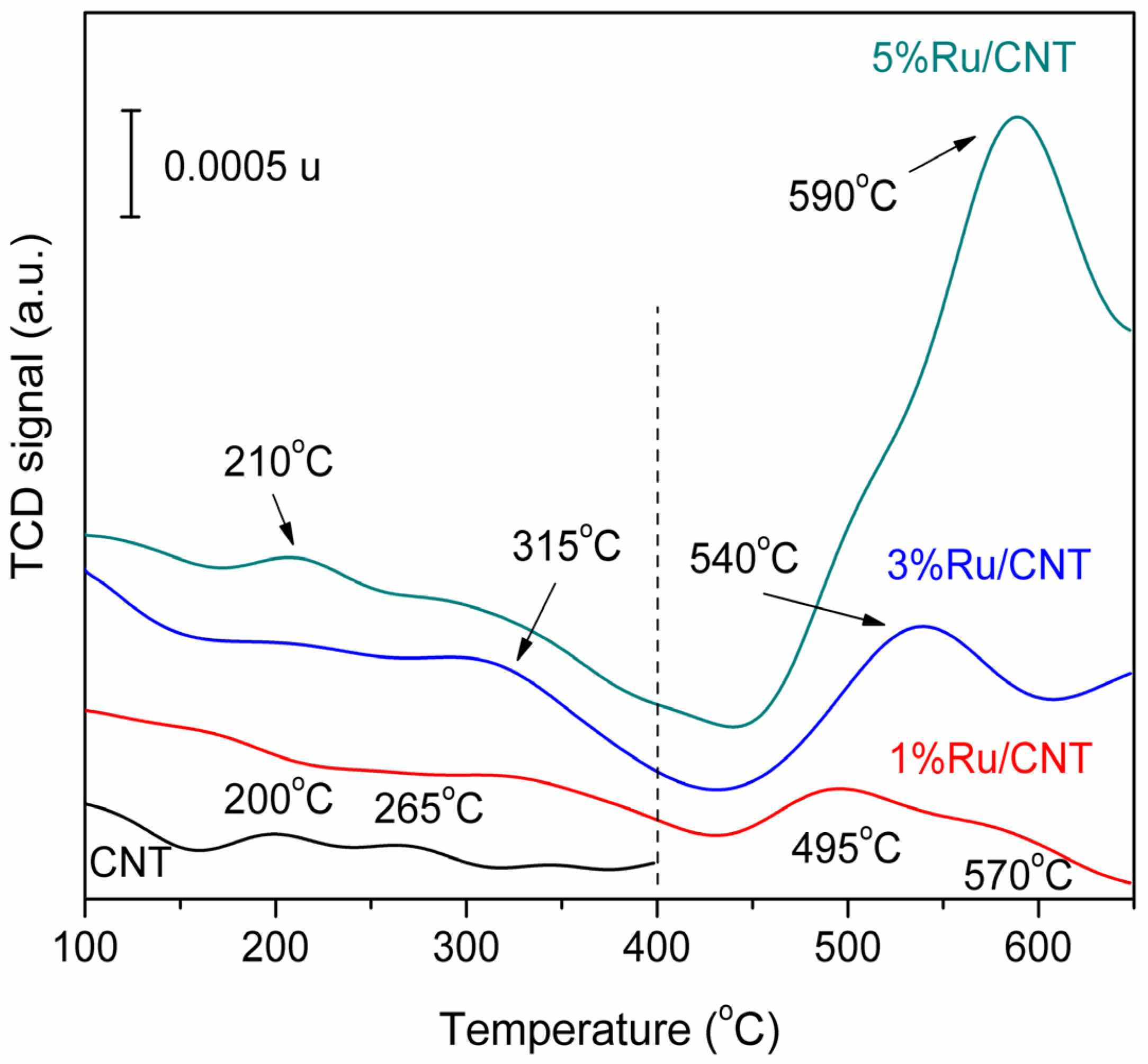
3.1.1. Ruthenium-Based MWCNT Samples
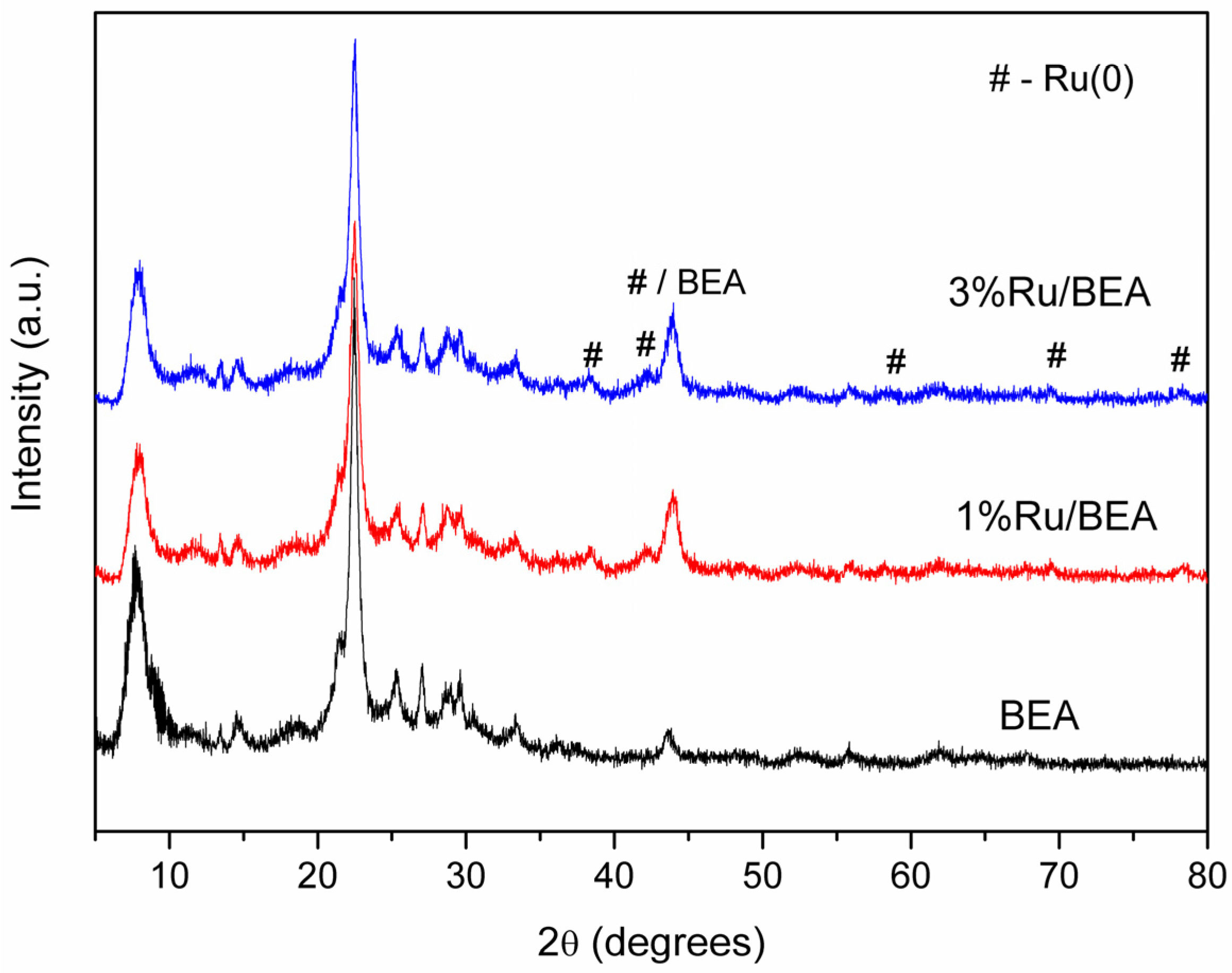
3.1.2. Ruthenium-Based BEA Zeolite Samples
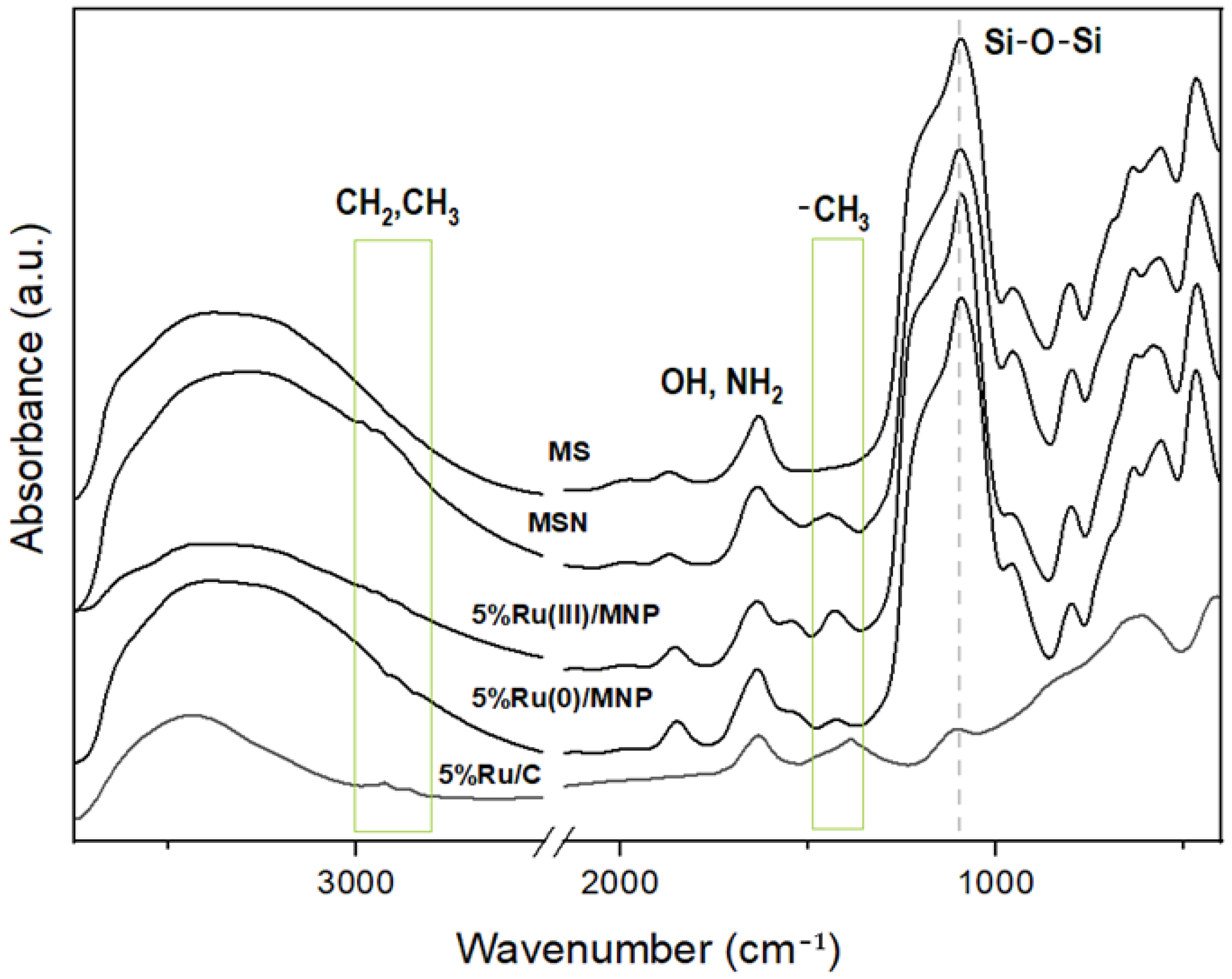
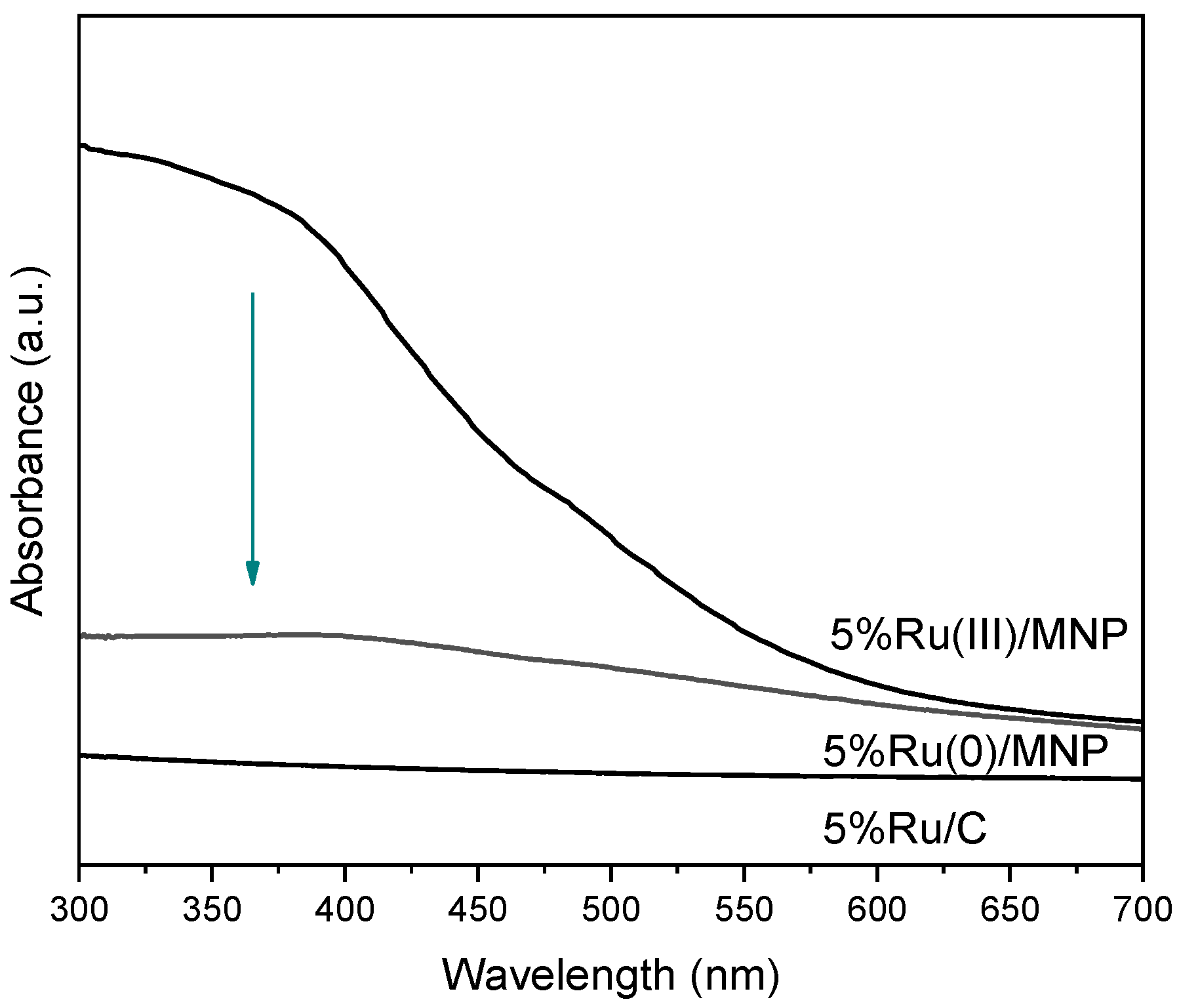
3.1.3. Characterization of Ru/MNP Samples
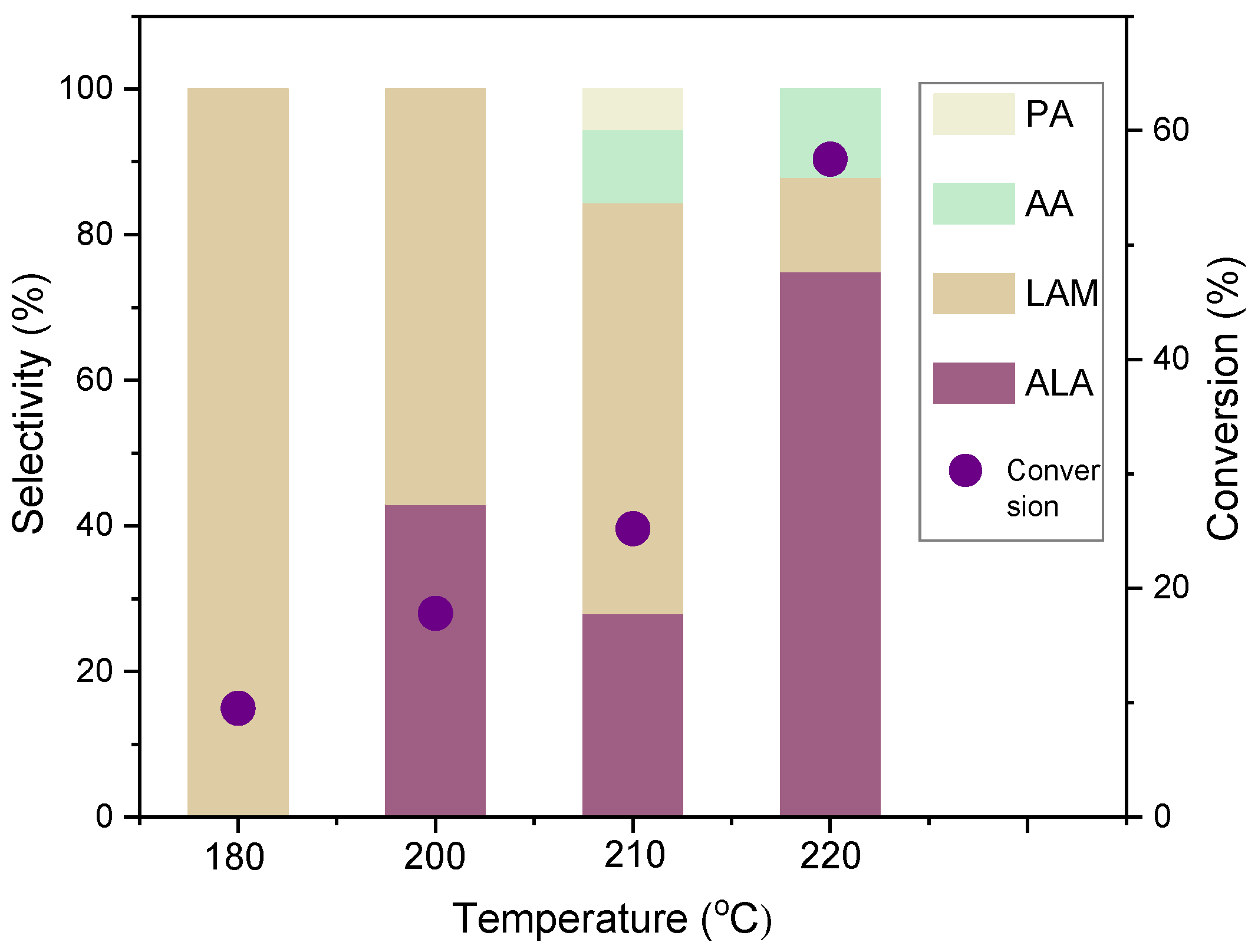
3.2. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scott, E.; Peter, F.; Sanders, J. Biomass in the manufacture of industrial products-the use of proteins and amino acids. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. [Google Scholar] [CrossRef]
- Uneyama, H.; Kobayashi, H.; Tonouchi, N. New functions and potential applications of amino acids. Adv. Biochem. Eng.Biotechnol. 2017, 159, 273–287. [Google Scholar]
- Wendisch, V.F. Metabolic engineering advances and prospects for amino acid production. Metab. Eng. 2020, 58, 17–34. [Google Scholar] [CrossRef] [PubMed]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino acids production focusing on fermentation technologies—A review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Wang, Y.; Zhang, S.; Gupta, K.M.; Hülsey, M.J.; Asakura, H.; Liu, L.; Han, Y.; Karp, E.M.; Beckham, G.T.; et al. Catalytic amino acid production from biomass-derived intermediates. Proc. Natl. Acad. Sci. USA 2018, 115, 5093–5098. [Google Scholar] [CrossRef]
- Zhang, M.; Imm, S.; Bähn, S.; Neumann, H.; Beller, M. Synthesis of α-Amino Acid Amides: Ruthenium-Catalyzed Amination of α-Hydroxy Amides. Angew. Chem. Int. Ed. 2011, 50, 11197–11201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, W.; Wang, B.; Zhang, Q.; Wan, X.; Tang, Z.; Wang, Y.; Zhu, C.; Cao, Z.; Wang, G.; et al. Chemical synthesis of lactic acid from cellulose catalysed by lead(II) ions in water. Nat. Commun. 2013, 4, 2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Furukawa, S.; Song, S.; He, Q.; Asakura, H.; Yan, N. Catalytic Production of Alanine from Waste Glycerol. Angew. Chem. Int. Ed. 2020, 59, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.I.; Kon, K.; Onodera, W.; Yamazaki, H.; Kondo, J.N. Heterogeneous Ni catalyst for direct synthesis of primary amines from alcohols and ammonia. ACS Catal. 2013, 3, 112–117. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Feng, S.; Chen, X. Promoting Effect of Ni on the Catalytic Production of Alanine from Lactic Acid over RuNi/AC Catalyst. Ind. Eng. Chem. Res. 2022, 61, 10285–10293. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, B.; Peng, F.; Liu, M.; Liu, H.; Yang, G.; Han, B. Highly Efficient Synthesis of Amino Acids by Amination of Bio-Derived Hydroxy Acids with Ammonia over Ru Supported on N-Doped Carbon Nanotubes. ChemSusChem 2020, 13, 5683–5689. [Google Scholar] [CrossRef]
- Tian, S.; Jiao, Y.; Gao, Z.; Xu, Y.; Fu, L.; Fu, H.; Zhou, W.; Hu, C.; Liu, G.; Wang, M.; et al. Catalytic Amination of Polylactic Acid to Alanine. J. Am. Chem. Soc. 2021, 143, 16358–16363. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Zhong, R.; Van den Bosch, S.; Coman, S.M.; Parvulescu, V.I.; Sels, B.F. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2018, 47, 8349–8402. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Shah, M.A.; Khalil, I.; Tallarico, S.; Donckels, T.; Eloy, P.; Debecker, D.P.; Oliverio, M.; Dusselier, M. Catalytic amination of lactic acid using Ru–zeolites. Dalton Trans. 2022, 51, 10773–10778. [Google Scholar] [CrossRef]
- Xin, H.; Xiu, Z.; Liu, S.; Wang, H.; Wang, C.; Ma, L.; Liu, Q. Efficient conversion of lactic acid to alanine over noble metal supported on Ni@C catalysts. RSC Adv. 2022, 12, 16847–16859. [Google Scholar] [CrossRef] [PubMed]
- Negoi, A.; Trotus, I.T.; Mamula Steiner, O.; Tudorache, M.; Kuncser, V.; Macovei, D.; Parvulescu, V.I.; Coman, S.M. Direct Synthesis of Sorbitol and Glycerol from Cellulose over Ionic Ru/Magnetite Nanoparticles in the Absence of External Hydrogen. ChemSusChem 2013, 6, 2090–2094. [Google Scholar] [CrossRef] [PubMed]
- Podolean, I.; Kuncser, V.; Gheorghe, N.; Macovei, D.; Parvulescu, V.I.; Coman, S.M. Ru-based magnetic nanoparticles (MNP) for succinic acid synthesis from levulinic acid. Green Chem. 2013, 15, 3077–3082. [Google Scholar] [CrossRef]
- Xu, B.; Dai, J.; Du, Z.; Li, F.; Liu, H.; Gu, X.; Wang, X.; Li, N.; Zhao, J. Catalytic conversion of biomass-derived compoUnds to various amino acids: Status and perspectives. Front. Chem. Sci. Eng. 2023, 17, 817–829. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Davis, M.E. Activation of Carbonyl-Containing Molecules with Solid Lewis Acids in Aqueous Media. ACS Catal. 2011, 1, 1566–1580. [Google Scholar] [CrossRef]
- Mardare, D.; Luca, D.; Teodorescu, C.M.; Macovei, D. On the hydrophilicity of nitrogen-doped TiO2 thin films. Surf. Sci. 2007, 601, 4515–4520. [Google Scholar] [CrossRef]
- Teodorescu, C.M.; Esteva, J.M.; Karnatak, R.C.; El Afif, A. An approximation of the Voigt I profile for the fitting of experimental X-ray absorption data. Nucl. Inst. Methods Phys. Res. A 1994, 345, 141–147. [Google Scholar] [CrossRef]
- Sobolevsky, T.G.; Revelsky, I.; Miller, B.; Oriedo, V.; Chernetsova, E.S.; Revelsky, I.A. Comparison of silylation and esterification/ acylation procedures in GC-MS analysis of amino acids. J. Sep. Sci 2003, 26, 1474–1478. [Google Scholar] [CrossRef]
- Molnar-Perl, I.; Katona, Z.F. GC-MS of amino acids as their trimethylsilyl/t-butyldimethylsilyl derivatives: In model solutions III. Chromatographia 2000, 51, S228–S236. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Yang, Q.-H.; Hou, P.-X.; Bai, S.; Wang, M.-Z.; Cheng, H.-M. Adsorption and capillarity of nitrogen in aggregated multi-walled carbon nanotubes. Chem. Phys. Lett. 2001, 345, 18–24. [Google Scholar] [CrossRef]
- Kang, J.; Deng, W.; Zhang, Q.; Wang, Y. Ru particle size effect in Ru/CNT-catalyzed Fischer-Tropsch synthesis. J. Energy Chem. 2013, 22, 321–328. [Google Scholar] [CrossRef]
- Thoka, S.; Tsai, C.-M.; Tong, Z.; Jena, A.; Wang, F.-M.; Hsu, C.-C.; Chang, H.; Hu, S.-F.; Liu, R.-S. Comparative Study of Li–CO2 and Na–CO2 Batteries with Ru@CNT as a Cathode Catalyst. ACS Appl. Mater. Interfaces 2021, 13, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Lv, L.; Lee, F.Y.; Liu, T.; Cooper, A.I.; Zhao, X.S. Thermally Reduced Ruthenium Nanoparticles as a Highly Active Heterogeneous Catalyst for Hydrogenation of Monoaromatics. J. Am. Chem. Soc. 2007, 129, 14213–14223. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction. Phys. Today 1957, 10, 50. [Google Scholar] [CrossRef]
- Wang, C.; Pan, X.; Bao, X. Direct production of light olefins from syngas over a carbon nanotube confined iron catalyst. Chin. Sci. Bull. 2010, 55, 1117–1119. [Google Scholar] [CrossRef]
- Vidali, G.; Ihm, G.; Kim, H.-Y.; Cole, M.W. Potentials of physical adsorption. Surf. Sci. Rep. 1991, 12, 135–181. [Google Scholar] [CrossRef]
- Masenelli-Varlot, K.; McRae, E.; Dupont-Pavlovsky, N. Comparative adsorption of simple molecules on carbon nanotubes: Dependence of the adsorption properties on the nanotube morphology. Appl. Surf. Sci. 2002, 196, 209–215. [Google Scholar] [CrossRef]
- Yin, S.F.; Xu, B.Q.; Zhu, W.X.; Ng, C.F.; Zhou, X.P.; Au, C.T. Carbon nanotubes-supported Ru catalyst for the generation of COx-free hydrogen from ammonia. Catal. Today 2004, 93–95, 27–38. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution properties of single-walled carbon nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef]
- Li, W.; Li, F.; Chen, J.; Betancourt, L.E.; Tu, C.; Liao, M.; Ning, X.; Zheng, J.; Li, R. Efficient and Sustainable Hydrogenation of Levulinic Acid to γ-Valerolactone in Aqueous Phase over Ru/MCM-49 Catalysts. Ind. Eng. Chem. Res. 2020, 59, 17338–17347. [Google Scholar] [CrossRef]
- Hu, L.; Wei, X.-Y.; Zong, Z.-M. Ru/Hβ catalyst prepared by the deposition-precipitation method for enhancing hydrodeoxygenation ability of guaiacol and lignin-derived bio-oil to produce hydrocarbons. J. Energy Inst. 2021, 97, 48–57. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Moulijn, J.A.; Pérez-Ramirez, J. On the introduction of intracrystalline mesoporosity in zeolites upon desilication in alkaline medium. Microporous Mesoporous Mater. 2004, 69, 29–34. [Google Scholar] [CrossRef]
- Linares, M.; Vargas, C.; García, A.; Ochoa-Hernández, C.; Čejka, J.; García-Muñoz, R.A.; Serrano, D.P. Effect of hierarchical porosity in Beta zeolites on the Beckmann rearrangement of oximes. Catal. Sci. Technol. 2017, 7, 181–190. [Google Scholar] [CrossRef]
- Balcerzak, J.; Redzynia, W.; Tyczkowski, J. In-situ XPS analysis of oxidized and reduced plasma deposited ruthenium-based thin catalytic films. Appl. Surf. Sci. 2017, 426, 852–855. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Coman, S.; Palade, P.; Macovei, D.; Teodorescu, C.M.; Filoti, G.; Molina, R.; Poncelet, G.; Wagner, F.E. Reducibility of ruthenium in relation with zeolite structure. Appl. Surf. Sci. 1999, 141, 164–176. [Google Scholar] [CrossRef]
- Hernando, H.; Moreno, I.; Fermoso, J.; Ochoa-Hernández, C.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. Biomass catalytic fast pyrolysis over hierarchical ZSM-5 and Beta zeolites modified with Mg and Zn oxides. Biomass Convers. Biorefinery 2017, 7, 289–304. [Google Scholar] [CrossRef]
- Yurdakal, S.; Garlisi, C.; Özcan, L.; Bellardita, M.; Palmisano, G. Chapter 4-(Photo)catalyst Characterization Techniques: Adsorption Isotherms and BET, SEM, FTIR, UV–Vis, Photoluminescence, and Electrochemical Characterizations. In Heterogeneous Photocatalysis; Marcì, G., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–152. [Google Scholar]
- Ma, M.; Zhang, Y.; Yu, W.; Shen, H.-y.; Zhang, H.-q.; Gu, N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf. A Physicochem. Eng. Asp. 2003, 212, 219–226. [Google Scholar] [CrossRef]
- Kim, J.; Seidler, P.; Fill, C.; Wan, L.S. Investigations of the effect of curing conditions on the structure and stability of amino-functionalized organic films on silicon substrates by Fourier transform infrared spectroscopy, ellipsometry, and fluorescence microscopy. Surf. Sci. 2008, 602, 3323–3330. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic Processing of Lactic Acid over Pt/Nb2O5. ChemSusChem 2009, 2, 581–586. [Google Scholar] [CrossRef] [PubMed]



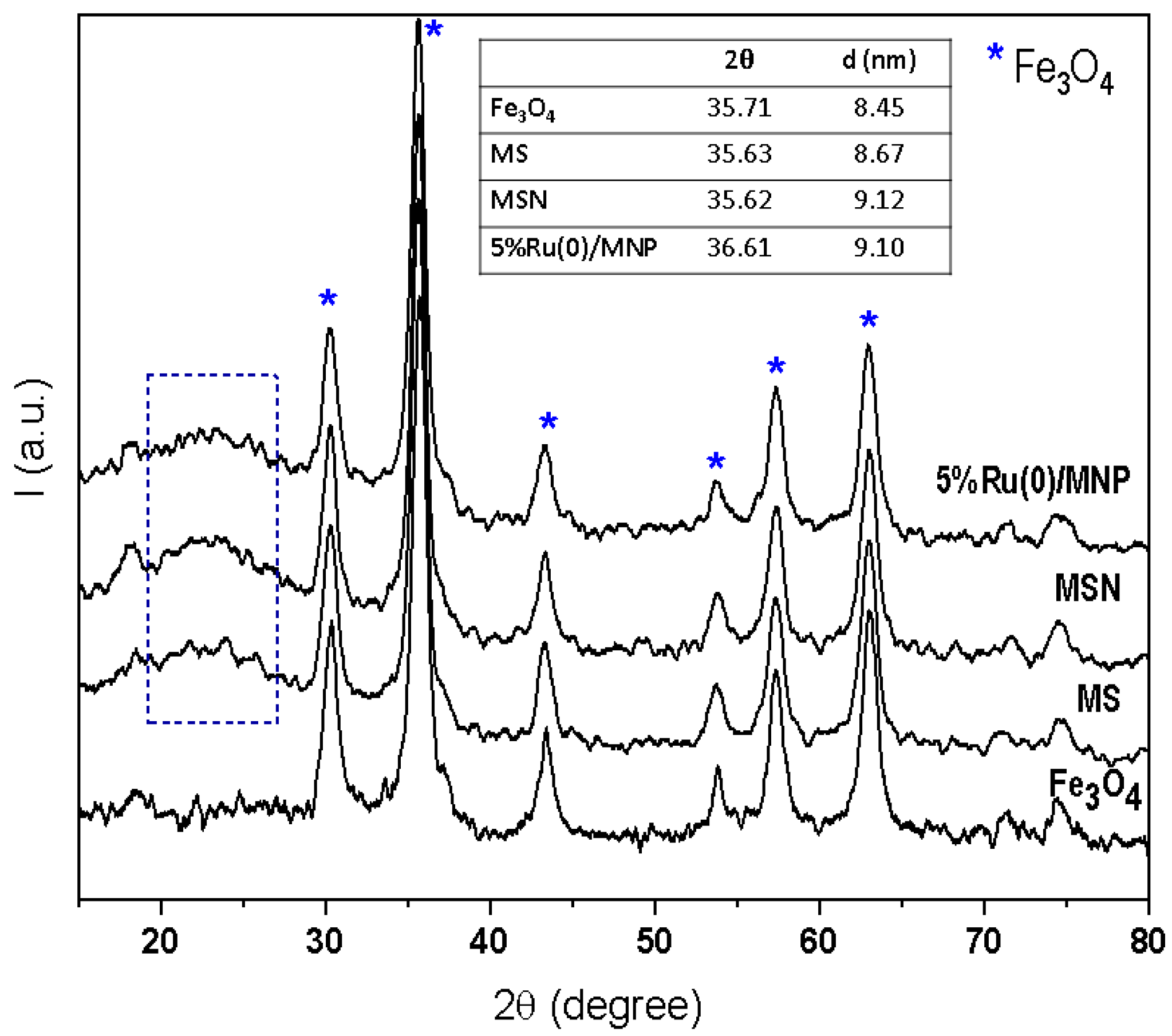

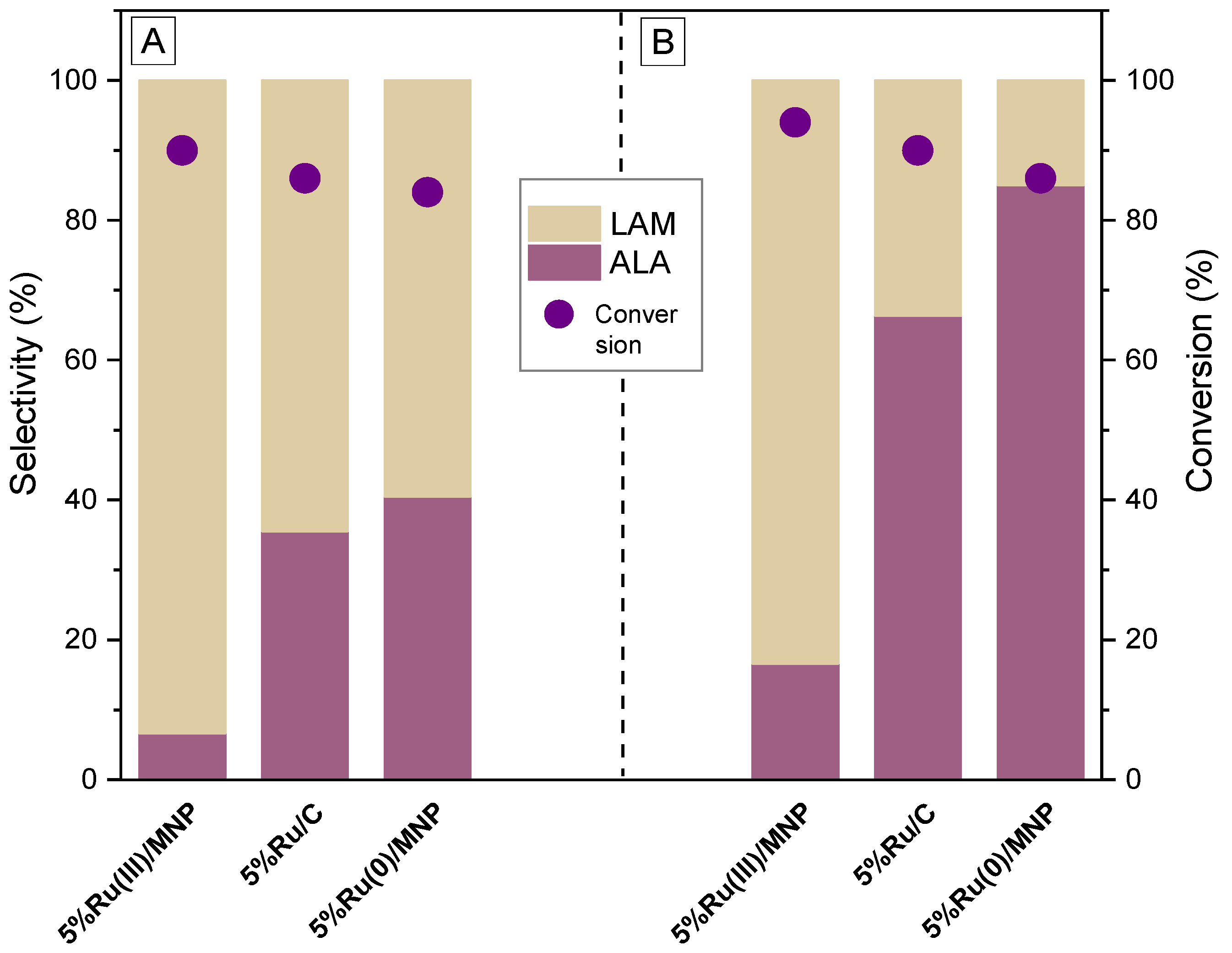
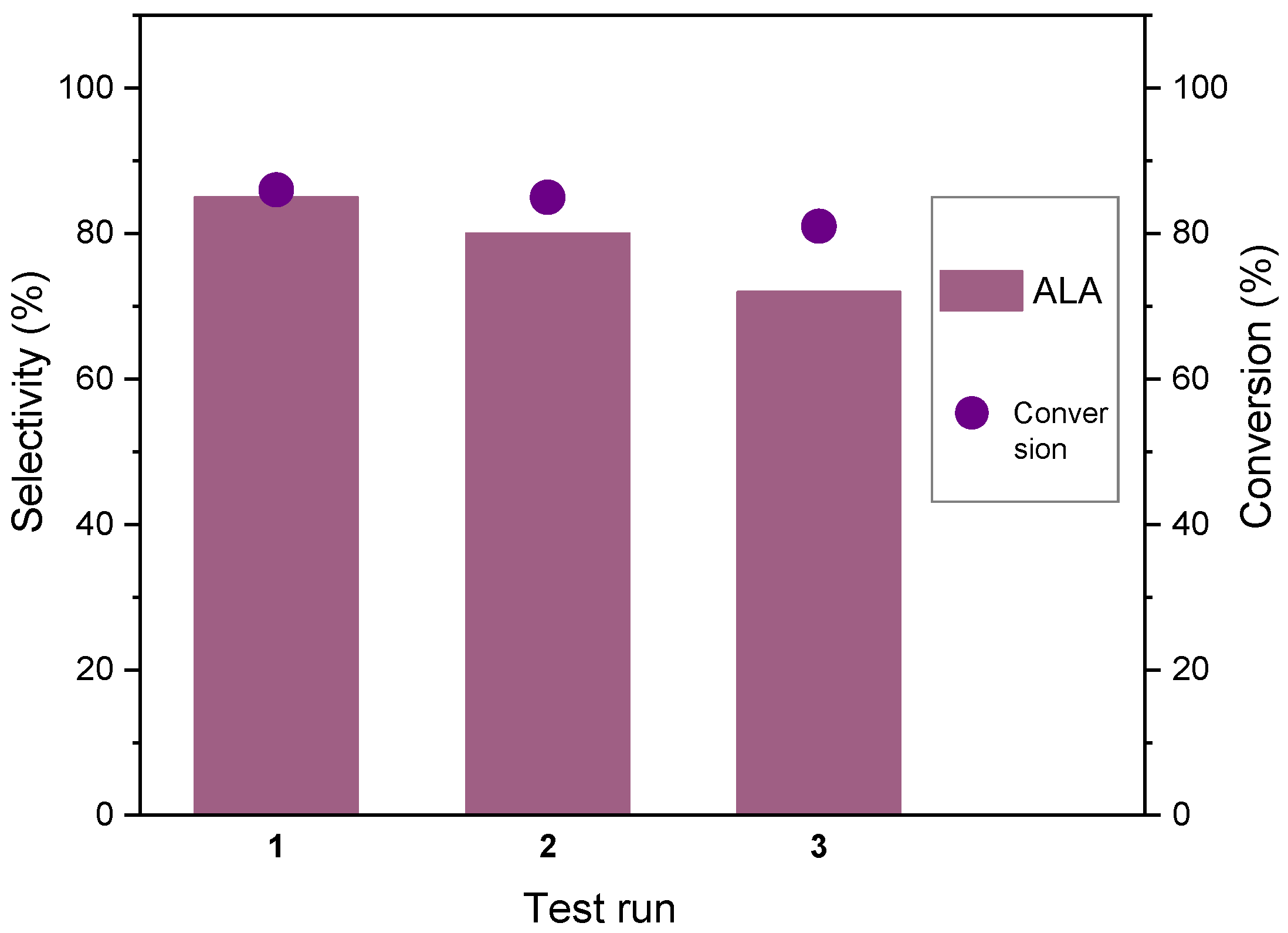

| Entry | Sample | SBET (m2/g) | Micropore Surface Area (m2/g) | External Surface Area (m2/g) | Total pore Volume (cm3/g) | Micropore Volume (cm3/g) | Mezopores Volume (cm3/g) | Average Pore Size (BJH, nm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1% Ru/CNT | 584 | 56 | 527 | 3.82 | 0.02 | 3.80 | 3.3 and 27.7 |
| 2 | 3% Ru/CNT | 315 | 22 | 293 | 1.67 | 0.01 | 1.66 | 3.3 and 31.9 |
| 3 | 5% Ru/CNT | 310 | 20 | 291 | 1.88 | 0.01 | 1.87 | 3.3 and 30.5 |
| Entry | Sample | SBET (m2/g) a | Sext (m2/g) b | S micro (m2/g) b | V total (cm3/g) c | Vmeso (cm3/g) d | Vmicro (cm3/g) e | Vmicro/ Vmeso | Avarage Pore size (nm) f |
|---|---|---|---|---|---|---|---|---|---|
| 1 | BEA | 495 | 186 | 309 | 0.72 | 0.58 | 0.14 | 0.24 | 8.5; 31.7 |
| 2 | 1%Ru/BEA | 415 | 168 | 247 | 0.65 | 0.54 | 0.11 | 0.20 | 9.3; 30.0 |
| 3 | 3%Ru/BEA | 402 | 186 | 216 | 0.44 | 0.37 | 0.07 | 0.19 | 9.3; 28.3 |
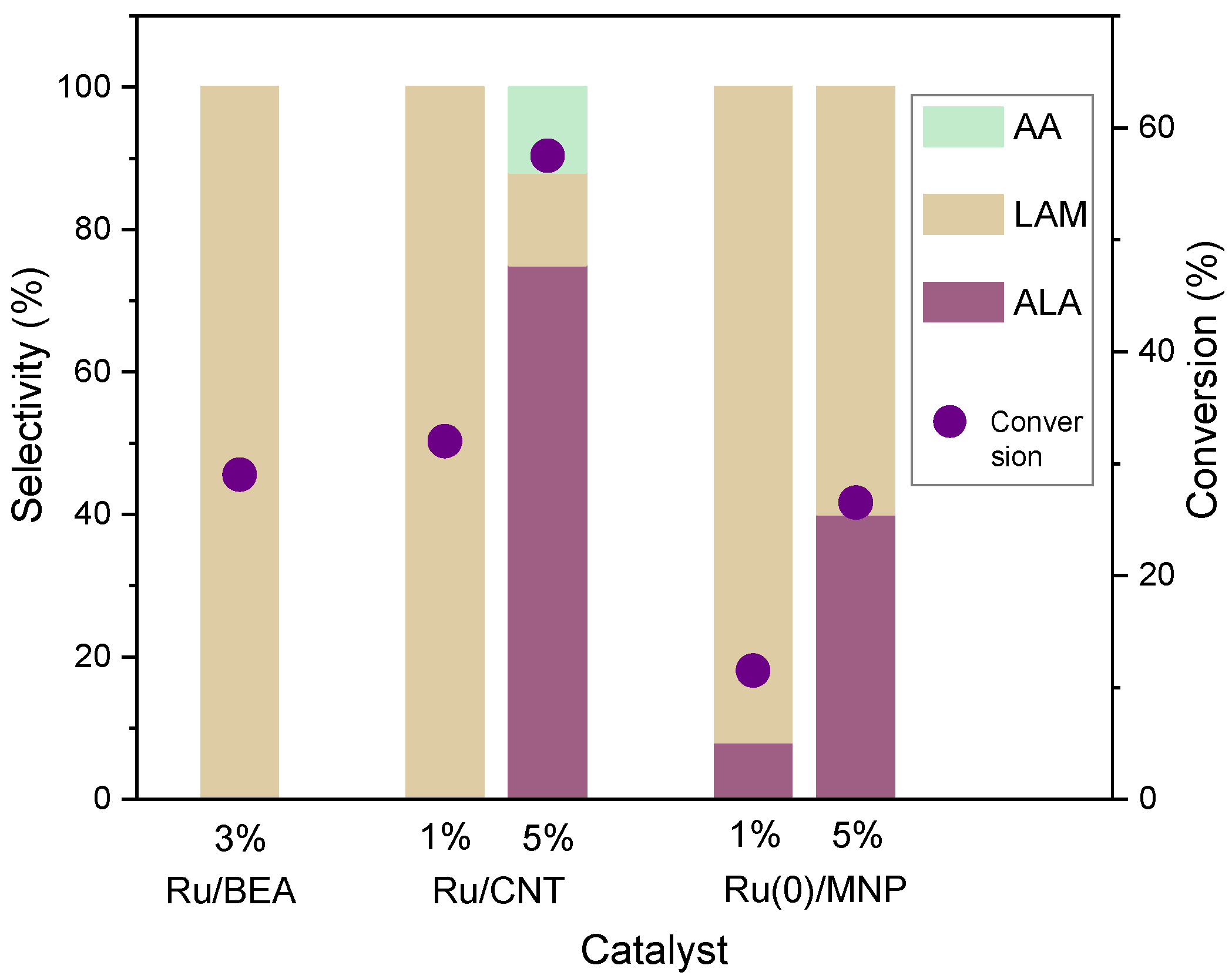
| Entry | Catalyst | LA mmol | C (%) | S(%) | |
|---|---|---|---|---|---|
| ALA | LAM | ||||
| 1 | 5%Ru/CNT | 0.35 | 64.0 | 87.0 | 13.0 |
| 2 | 5%Ru(0)/MNP | 0.35 | 85.0 | 83.0 | 17.0 |
| 3 | 1%Ru/BEA | 0.7 | 4.7 | - | 100 |
| 4 | 3%Ru/BEA | 0.7 | 18.2 | - | 100 |
| 5 | 1%Ru/CNT | 0.7 | 10.3 | - | 100 |
| 6 | 3%Ru/CNT | 0.7 | 8.8 | - | 100 |
| 7 | 5%Ru/CNT | 0.7 | 17.8 | 43.0 | 57.0 |
| 8 | 1%Ru(0)/MNP | 0.7 | 11.0 | 10.0 | 90.0 |
| 9 | 5%Ru(0)/MNP | 0.7 | 40.5 | 11.3 | 88.7 |
| 10 | 1%Ru/BEA | 5.6 | 5.0 | - | 100 |
| 11 | 3%Ru/BEA | 5.6 | 6.3 | - | 100 |
| 12 | 1%Ru/CNT | 5.6 | 13.5 | 5.0 | 95.0 |
| 13 | 5%Ru/CNT | 5.6 | 17.5 | 7.5 | 92.5 |
| 14 | 1%Ru(0)/MNP | 5.6 | 11.6 | 5.3 | 96.7 |
| 15 | 5%Ru(0)/MNP | 5.6 | 31.6 | 17.5 | 82.5 |
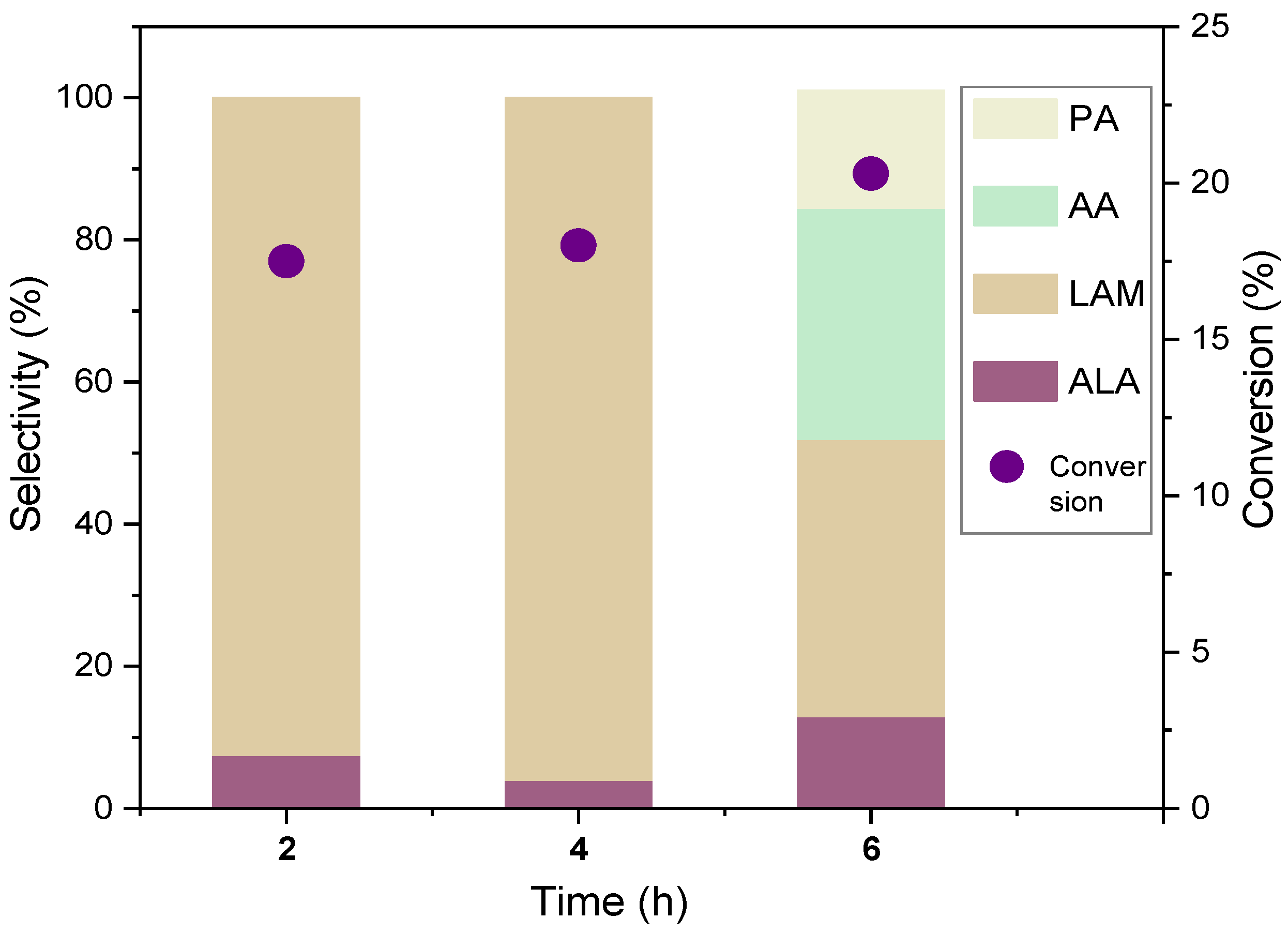
| Entry | Catalyst | Reaction Time (h) | C (%) | S(%) | ||
|---|---|---|---|---|---|---|
| ALA | LAM | AA | ||||
| 1 | 1%Ru/BEA | 4 | 13.5 | 5.1 | 20.9 | 74.0 |
| 2 | 1%Ru/BEA | 8 | 17.0 | 12.0 | 29.0 | 59.0 |
| 3 | 3%Ru/BEA | 4 | 22.2 | 5.5 | 58.5 | 36.0 |
| 4 | 3%Ru/BEA | 8 | 24.5 | 27.0 | 62.0 | 11.0 |
| Entry | Catalyst | LA (mmol) | Time (h) | T (°C) | PH2 (bar) | C% | S% | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Ru/H-β (Si/Al = 12.5) | 2.0 | 4 | 220 | 10 | 55.0 | 80.0 | [15] |
| 2 | Ru/C | 2.0 | 4 | 220 | 10 | 60.0 | 80.0 | [15] |
| 3 | Ru/CNT | 2.0 | 4 | 220 | 10 | 83.0 | 57.0 | [15] |
| 4 | Ru/TiO2 | 50 mg PLA | 72 | 140 | - | >99 | 70.0 | [12] |
| 5 | Ru/TiO2 | 50 mg PLA | 18 | 140 | 10 | >99 | 27.0 | [12] |
| 6 | Ru/Ni@C | 1.11 | 2 | 220 | 15 | 70.5 | 75.0 | [16] |
| 7 | Ru/CS | 1.11 | 2 | 220 | 15 | 63.4 | 77.5 | [16] |
| 8 | Ru/CNT | 0.5 | 2 | 220 | 10 | 75.0 | 49.0 | [5] |
| 9 | Ru1Ni7MgO | 1.11 (glycerol) | 1 | 220 | 10 | 15.0 | 0.08 | [8] |
| 10 | Ru/N-CNTs | 0.5 | 2 | 180 | 10 | 99.9 | 70.0 | [11] |
| 11 | Ru/ZSM-5 | 0.5 | 2 | 180 | 10 | 69.0 | 0 | [11] |
| 12 | Ru/CNTs | 0.5 | 2 | 180 | 10 | 94.5 | 53.9 | [11] |
| 13 | Ru/CNT | 0.35 | 2 | 200 | 10 | 64.0 | 87.0 | This work |
| 14 | Ru/CNT | 0.7 | 2 | 220 | 10 | 60.0 | 75.0 | This work |
| 14 | Ru/BEA | 5.6 | 8 | 200 | 10 | 24.5 | 27.0 | This work |
| 15 | Ru/MNP | 0.35 | 2 | 200 | 10 | 87.0 | 85.0 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podolean, I.; Dogaru, M.; Guzo, N.C.; Petcuta, O.A.; Jacobsen, E.E.; Nicolaev, A.; Cojocaru, B.; Tudorache, M.; Parvulescu, V.I.; Coman, S.M. Highly Efficient Ru-Based Catalysts for Lactic Acid Conversion to Alanine. Nanomaterials 2024, 14, 277. https://doi.org/10.3390/nano14030277
Podolean I, Dogaru M, Guzo NC, Petcuta OA, Jacobsen EE, Nicolaev A, Cojocaru B, Tudorache M, Parvulescu VI, Coman SM. Highly Efficient Ru-Based Catalysts for Lactic Acid Conversion to Alanine. Nanomaterials. 2024; 14(3):277. https://doi.org/10.3390/nano14030277
Chicago/Turabian StylePodolean, Iunia, Mara Dogaru, Nicolae Cristian Guzo, Oana Adriana Petcuta, Elisabeth E. Jacobsen, Adela Nicolaev, Bogdan Cojocaru, Madalina Tudorache, Vasile I. Parvulescu, and Simona M. Coman. 2024. "Highly Efficient Ru-Based Catalysts for Lactic Acid Conversion to Alanine" Nanomaterials 14, no. 3: 277. https://doi.org/10.3390/nano14030277
APA StylePodolean, I., Dogaru, M., Guzo, N. C., Petcuta, O. A., Jacobsen, E. E., Nicolaev, A., Cojocaru, B., Tudorache, M., Parvulescu, V. I., & Coman, S. M. (2024). Highly Efficient Ru-Based Catalysts for Lactic Acid Conversion to Alanine. Nanomaterials, 14(3), 277. https://doi.org/10.3390/nano14030277










