Nanodots of Transition Metal Sulfides, Carbonates, and Oxides Obtained Through Spontaneous Co-Precipitation with Silica
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Astruc, D. Introduction: Nanoparticles in Catalysis. Chem. Rev. 2020, 120, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Payal; Pandey, P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Pat. Nanotechnol. 2021, 16, 45–66. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy Storage: The Future Enabled by Nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef] [PubMed]
- Sabela, M.; Balme, S.; Bechelany, M.; Janot, J.M.; Bisetty, K. A Review of Gold and Silver Nanoparticle-Based Colorimetric Sensing Assays. Adv. Eng. Mater. 2017, 19, 1700270. [Google Scholar] [CrossRef]
- Jiang, C.; Cao, Y.; Xiao, G.; Zhu, R.; Lu, Y. A Review on the Application of Inorganic Nanoparticles in Chemical Surface Coatings on Metallic Substrates. RSC Adv. 2017, 7, 7531–7539. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Nanocoating Is a New Way for Biofouling Prevention. Front. Nanotechnol. 2021, 3, 771098. [Google Scholar] [CrossRef]
- Khondakar, K.R.; Kaushik, A.K. Nanotechnology in Cancer Management; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128181546. [Google Scholar]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal Oxides Nanoparticles via Sol–Gel Method: A Review on Synthesis, Characterization and Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef]
- Jaji, N.D.; Othman, M.B.H.; Lee, H.L.; Hussin, M.H.; Hui, D. One-Pot Solvothermal Synthesis and Characterization of Highly Stable Nickel Nanoparticles. Nanotechnol. Rev. 2021, 10, 318–329. [Google Scholar] [CrossRef]
- Tsuzuki, T. Mechanochemical Synthesis of Metal Oxide Nanoparticles. Commun. Chem. 2021, 4, 143. [Google Scholar] [CrossRef]
- Kister, T.; Monego, D.; Mulvaney, P.; Widmer-Cooper, A.; Kraus, T. Colloidal Stability of Apolar Nanoparticles: The Role of Particle Size and Ligand Shell Structure. ACS Nano. 2018, 12, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P. Gold Nanoparticle Synthesis, Morphology Control, and Stabilization Facilitated by Functional Polymers. Chem. Eng. Technol. 2011, 34, 15–28. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef] [PubMed]
- Franconetti, A.; Carnerero, J.M.; Prado-Gotor, R.; Cabrera-Escribano, F.; Jaime, C. Chitosan as a Capping Agent: Insights on the Stabilization of Gold Nanoparticles. Carbohydr. Polym. 2019, 207, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Prince, E.; Narayanan, P.; Liu, K.; Nie, Z.; Kumacheva, E. Colloidal Stability of Nanoparticles Stabilized with Mixed Ligands in Solvents with Varying Polarity. Chem. Commun. 2020, 56, 8131–8134. [Google Scholar] [CrossRef] [PubMed]
- Drummer, S.; Madzimbamuto, T.; Chowdhury, M. Green Synthesis of Transition-Metal Nanoparticles and Their Oxides: A Review. Materials 2021, 14, 2700. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M.; Giersig, M.; Mulvaney, P. Synthesis of Nanosized Gold—Silica Core—Shell Particles. Langmuir 1996, 12, 4329–4335. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L.M. Recent Progress on Silica Coating of Nanoparticles and Related Nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, M.Y. Silica-Coated Metal Nanoparticles. Chem. Asian. J. 2010, 5, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.A.; Choi, S.; Jeon, S.M.; Yu, J. Silica Nanoparticle Stability in Biological Media Revisited. Sci. Rep. 2018, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Pham, X.H.; Park, S.M.; Ham, K.M.; Kyeong, S.; Son, B.S.; Kim, J.; Hahm, E.; Kim, Y.H.; Bock, S.; Kim, W.; et al. Synthesis and Application of Silica-Coated Quantum Dots in Biomedicine. Int. J. Mol. Sci. 2021, 22, 10116. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, J.M.; Melero-García, E.; Hyde, S.T. Morphogenesis of Self-Assembled Nanocrystalline Materials of Barium Carbonate and Silica. Science 2009, 323, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Kellermeier, M.; Cölfen, H.; García-Ruiz, J.M. Silica Biomorphs: Complex Biomimetic Hybrid Materials from “Sand and Chalk”. Eur. J. Inorg. Chem. 2012, 2012, 5123–5144. [Google Scholar] [CrossRef]
- Kellermeier, M.; Glaab, F.; Melero-García, E.; García-Ruiz, J.M. Experimental Techniques for the Growth and Characterization of Silica Biomorphs and Silica Gardens. Methods Enzymol. 2013, 532, 225–256. [Google Scholar]
- Kellermeier, M.; Melero-García, E.; Glaab, F.; Klein, R.; Drechsler, M.; Rachel, R.; García-Ruiz, J.M.; Kunz, W. Stabilization of Amorphous Calcium Carbonate in Inorganic Silica-Rich Environments. J. Am. Chem. Soc. 2010, 132, 17859–17866. [Google Scholar] [CrossRef] [PubMed]
- Eiblmeier, J.; Kellermeier, M.; Deng, M.; Kienle, L.; García Ruiz, J.M.; Kunz, W. Bottom-up Self-Assembly of Amorphous Core-Shell-Shell Nanoparticles and Biomimetic Crystal Forms in Inorganic Silica-Carbonate Systems. Chem. Mater. 2013, 25, 1842–1851. [Google Scholar] [CrossRef]
- Kellermeier, M.; Gebauer, D.; Melero-García, E.; Drechsler, M.; Talmon, Y.; Kienle, L.; Cölfen, H.; García-Ruiz, J.M.; Kunz, W. Colloidal Stabilization of Calcium Carbonate Prenucleation Clusters with Silica. Adv. Funct. Mater. 2012, 22, 4301–4311. [Google Scholar] [CrossRef]
- Funkner, D.; Frank, T.; Kohlmann, N.; Zahnweh, D.; Rieder, J.; Kienle, L.; Kunz, W.; Kellermeier, M. Functional Nanoparticles from Chemically Coupled Precipitation Processes. Mater. Today Chem. 2023, 29, 101438. [Google Scholar] [CrossRef]
- Shaheen, W.M.; Selim, M.M. Effect of Thermal Treatment on Physicochemical Properties of Pure and Mixed Manganese Carbonate and Basic Copper Carbonate. Thermochim. Acta. 1998, 322, 117–128. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.; Liu, J. Highly Porous CdO Nanowires: Preparation Based on Hydroxy- and Carbonate-Containing Cadmium Compound Precursor Nanowires, Gas Sensing and Optical Properties. Nanotechnology 2008, 19, 24. [Google Scholar] [CrossRef]
- Cong, H.P.; Yu, S.H. Shape Control of Cobalt Carbonate Particles by a Hydrothermal Process in a Mixed Solvent: An Efficient Precursor to Nanoporous Cobalt Oxide Architectures and Their Sensing Property. Cryst. Growth Des. 2009, 9, 210–217. [Google Scholar] [CrossRef]
- Spinner, N.; Mustain, W.E. Effect of Nickel Oxide Synthesis Conditions on Its Physical Properties and Electrocatalytic Oxidation of Methanol. Electrochim. Acta. 2011, 56, 5656–5666. [Google Scholar] [CrossRef]
- Pourmortazavi, S.M.; Marashianpour, Z.; Karimi, M.S.; Mohammad-Zadeh, M. Electrochemical Synthesis and Characterization of Zinc Carbonate and Zinc Oxide Nanoparticles. J. Mol. Struct. 2015, 1099, 232–238. [Google Scholar] [CrossRef]
- Frisken, B.J. Revisiting the Method of Cumulants for the Analysis of Dynamic Light-Scattering Data. Appl. Opt. 2001, 40, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. A Constrained Regularization Method for Inverting Data Represented by Linear Algebraic or Integral Equations. Comput. Phys. Commun. 1982, 27, 213–227. [Google Scholar] [CrossRef]
- Brus, L.E. Electron-Electron and Electron-Hole Interactions in Small Semiconductor Crystallites: The Size Dependence of the Lowest Excited Electronic State. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef]
- Nemade, K.R.; Waghuley, S.A. Band Gap Engineering of CuS Nanoparticles for Artificial Photosynthesis. Mater. Sci. Semicond. Process. 2015, 39, 781–785. [Google Scholar] [CrossRef]
- Oliva, A.I.; Solís-Canto, O.; Castro-Rodríguez, R.; Quintana, P. Formation of the Band Gap of CdS Thin Films Growth by Different Techniques. Mod. Phys. Lett. B 2001, 15, 671–674. [Google Scholar] [CrossRef]
- Sheardy, A.T.; Arvapalli, D.M.; Wei, J. Novel Microwave Synthesis of Near-Metallic Copper Sulfide Nanodiscs with Size Control: Experimental and DFT Studies of Charge Carrier Density. Nanoscale Adv. 2020, 2, 1054–1058. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; Wiley: Hoboken, NJ, USA, 1979; ISBN 978-0-471-02404-0. [Google Scholar]
- Lide, D.R. (Ed.) Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9781482208689/1482208687. [Google Scholar]
- Navrotsky, A. Energetic Clues to Pathways to Biomineralization: Precursors, Clusters, and Nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101. [Google Scholar] [CrossRef]
- Seidel, H.; Ehrhardt, H.; Viswanathan, K.; Johannes, W. Darstellung, Struktur Und Eigenschaften von Kupfer(II)—Carbonat. ZAAC—J. Inorg. Gen. Chem. 1974, 410, 138–148. [Google Scholar] [CrossRef]
- Knoll, P.; Steinbock, O. Nanodot-to-Rod Transition and Particle Attachment in Self-Organized Polycrystalline Aggregates. Cryst. Growth Des. 2019, 19, 4218–4223. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef] [PubMed]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic-Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Iyer, A.; Del-Pilar, J.; King’Ondu, C.K.; Kissel, E.; Garces, H.F.; Huang, H.; El-Sawy, A.M.; Dutta, P.K.; Suib, S.L. Water Oxidation Catalysis Using Amorphous Manganese Oxides, Octahedral Molecular Sieves (OMS-2), and Octahedral Layered (OL-1) Manganese Oxide Structures. J. Phys. Chem. C 2012, 116, 6474–6483. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the Rational Design of Non-Precious Transition Metal Oxides for Oxygen Electrocatalysis. Energy Env. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon Nanodots: Synthesis, Properties and Applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Suzuki, N.; Tanaka, H.; Yamanaka, S.; Kanai, M.; Lee, B.K.; Lee, H.Y.; Kawai, T. Epitaxial Nanodot Arrays of Transition—Metal Oxides Fabricated by Dry Deposition Combined with a Nanoimprint—Lithography—Based Molybdenum Lift—Off Technique. Small 2008, 4, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Oaki, Y.; Imai, H. Monolayered Nanodots of Transition Metal Oxides. J. Am. Chem. Soc. 2013, 135, 4501–4508. [Google Scholar] [CrossRef]
- Han, U.B.; Lee, J.S. Bottom-up Synthesis of Ordered Metal/Oxide/Metal Nanodots on Substrates for Nanoscale Resistive Switching Memory. Sci. Rep. 2016, 6, 25537. [Google Scholar] [CrossRef]
- Jana, B.; Reva, Y.; Scharl, T.; Strauss, V.; Cadranel, A.; Guldi, D.M. Carbon Nanodots for All-in-One Photocatalytic Hydrogen Generation. J. Am. Chem. Soc. 2021, 143, 20122–20132. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Fei, Z.; Xie, X.; Lou, J.; Tang, J.; Cui, M.; Qiao, X. CeO2 Nanodots Embedded in a Porous Silica Matrix as an Active yet Durable Catalyst for HCl Oxidation. Catal. Sci. Technol. 2016, 6, 5116–5123. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Vishnu Kirthi, A.; Akksadha, M.; Indu, S.; Dhiviya Dharshini, U.; Pushpamalar, J.; Karthik, L. Recent Advancements in the Applications of Carbon Nanodots: Exploring the Rising Star of Nanotechnology. Nanoscale Adv. 2020, 2, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Koutavarapu, R.; Tamtam, M.R.; Rao, M.C.; Peera, S.G.; Shim, J. Recent Progress in Transition Metal Oxide/Sulfide Quantum Dots-Based Nanocomposites for the Removal of Toxic Organic Pollutants. Chemosphere 2021, 272, 129849. [Google Scholar] [CrossRef]
- Santra, S.; Tapec, R.; Theodoropoulou, N.; Dobson, J.; Hebard, A.; Tan, W. Synthesis and Characterization of Silica-Coated Iron Oxide Nanoparticles in Microemulsion: The Effect of Nonionic Surfactants. Langmuir 2001, 17, 2900–2906. [Google Scholar] [CrossRef]
- Bumb, A.; Brechbiel, M.W.; Choyke, P.L.; Fugger, L.; Eggeman, A.; Prabhakaran, D.; Hutchinson, J.; Dobson, P.J. Synthesis and Characterization of Ultra-Small Superparamagnetic Iron Oxide Nanoparticles Thinly Coated with Silica. Nanotechnology 2008, 19, 33. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, Y.; Hu, H.; Du, X.; Zhang, C.; Shi, X.; Wu, H.; Yang, S. Silica-Coated Manganese Oxide Nanoparticles as a Platform for Targeted Magnetic Resonance and Fluorescence Imaging of Cancer Cells. Adv. Funct. Mater. 2010, 20, 1733–1741. [Google Scholar] [CrossRef]
- Chia, S.L.; Leong, D.T. Reducing ZnO Nanoparticles Toxicity through Silica Coating. Heliyon 2016, 2, e00177. [Google Scholar] [CrossRef]
- Basu, P.; De, K.; Das, S.; Mandal, A.K.; Kumar, A.; Jana, T.K.; Chatterjee, K. Silica-Coated Metal Oxide Nanoparticles: Magnetic and Cytotoxicity Studies. Chem. Sel. 2018, 3, 7346–7353. [Google Scholar] [CrossRef]
- Gui, Z.; Liu, J.; Wang, Z.; Song, L.; Hu, Y.; Fan, W.; Chen, D. From Muticomponent Precursor to Nanoparticle Nanoribbons of ZnO. J. Phys. Chem. B 2005, 109, 1113–1117. [Google Scholar] [CrossRef]
- Liu, J.; Fu, S.; Yuan, B.; Li, Y.; Deng, Z. Toward a Universal “Adhesive Nanosheet” for the Assembly of Multiple Nanoparticles Based on a Protein-Induced Reduction/Decoration of Graphene Oxide. J. Am. Chem. Soc. 2010, 132, 7279–7281. [Google Scholar] [CrossRef] [PubMed]

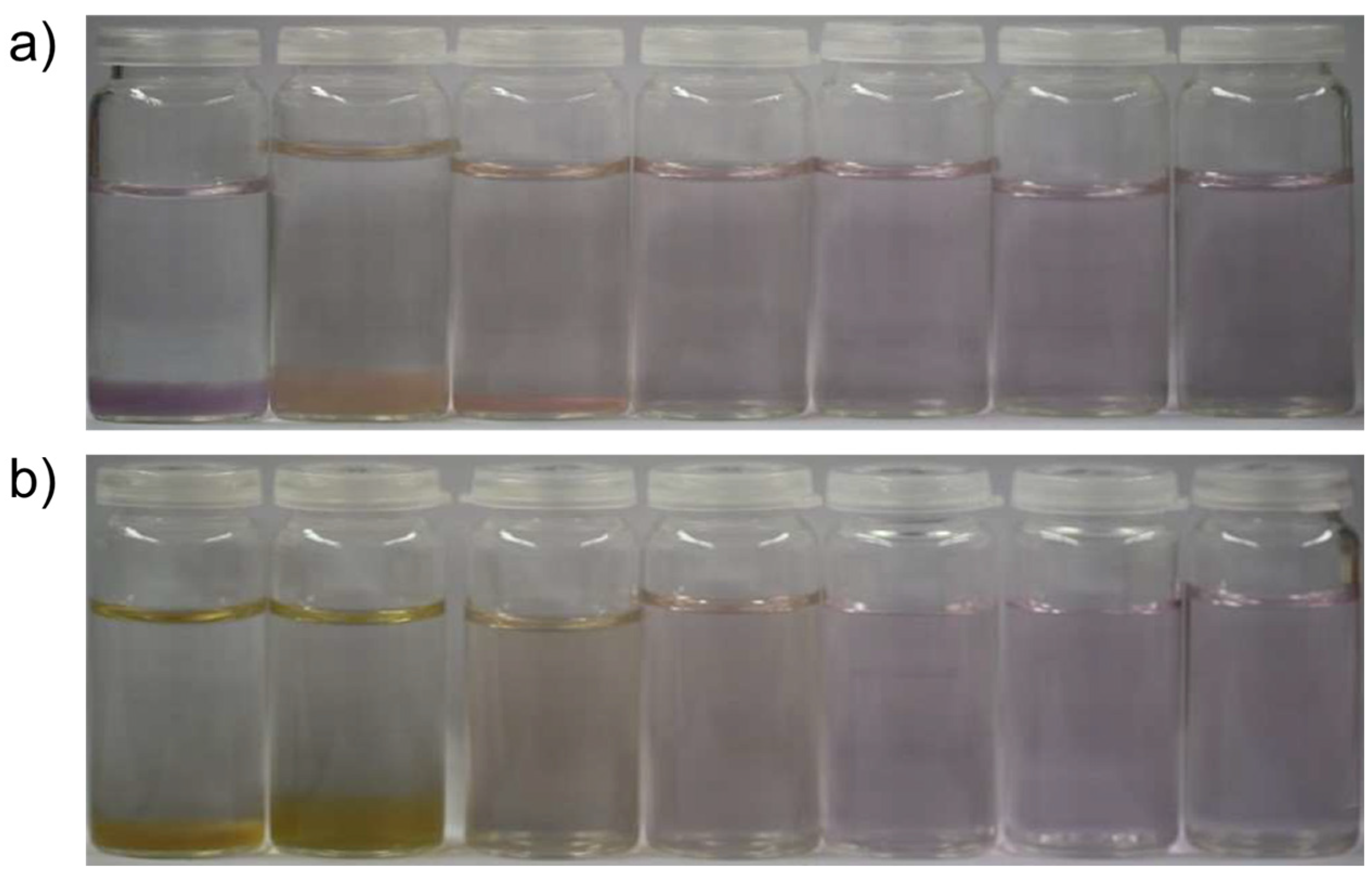
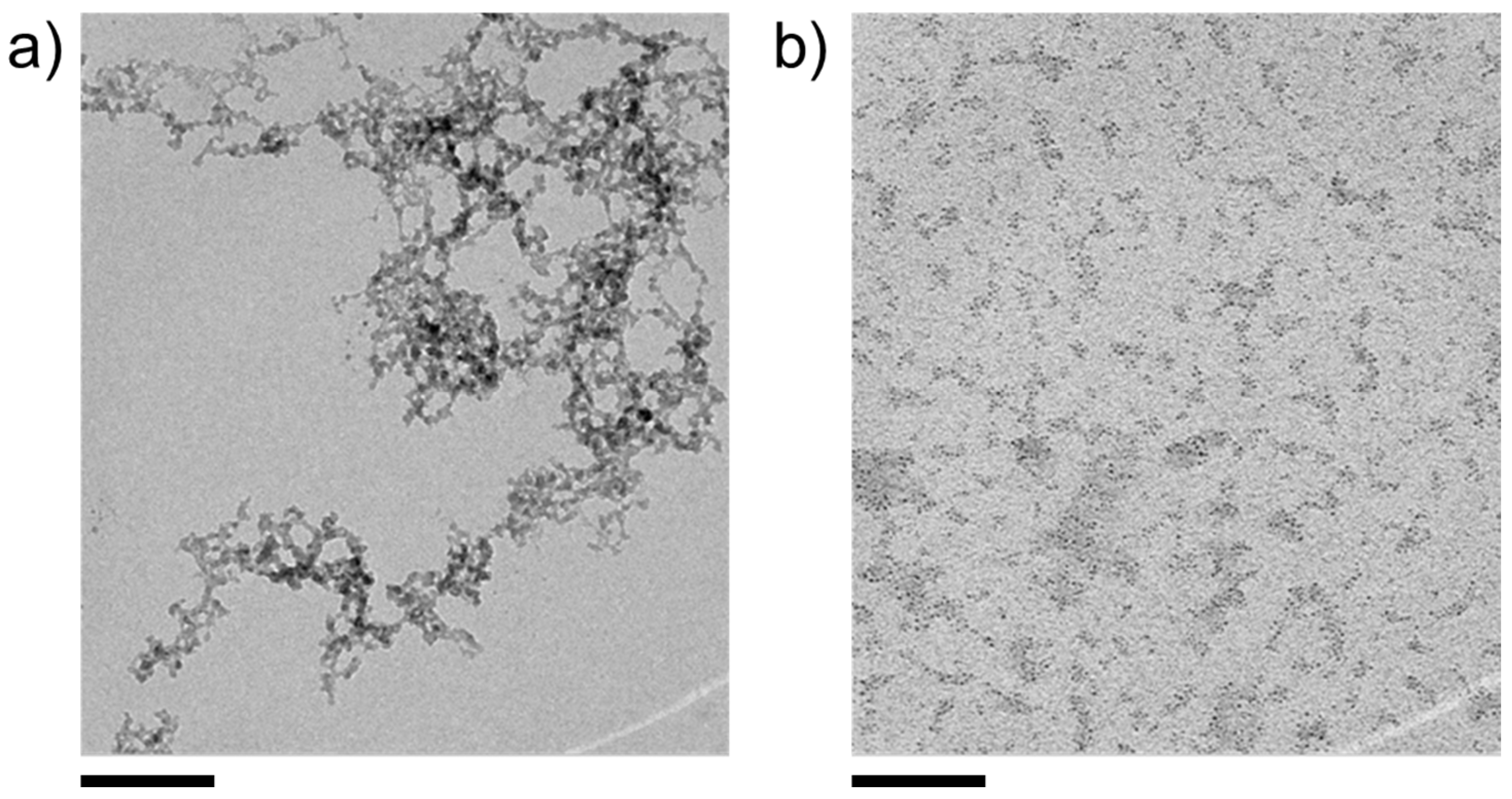
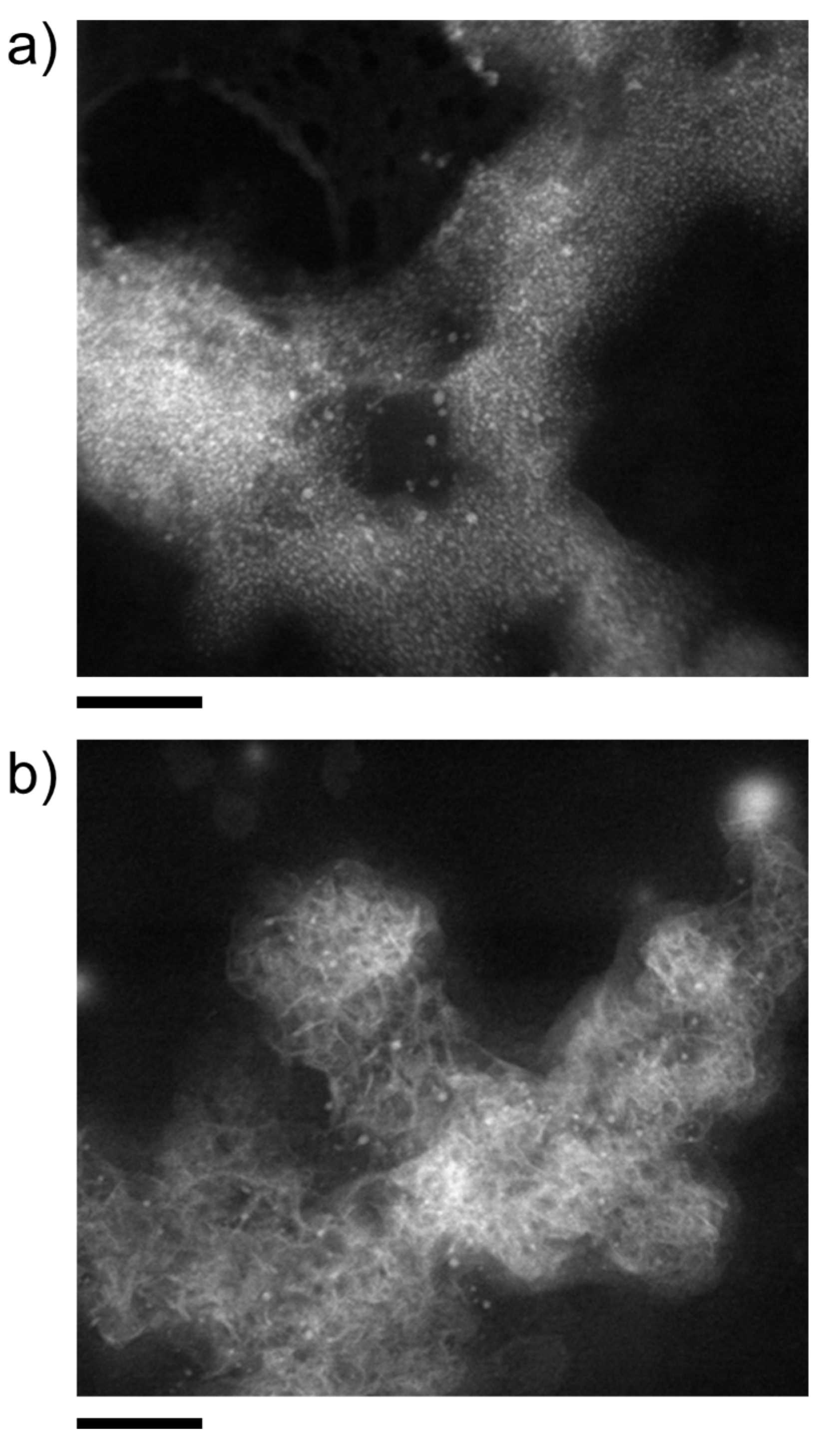
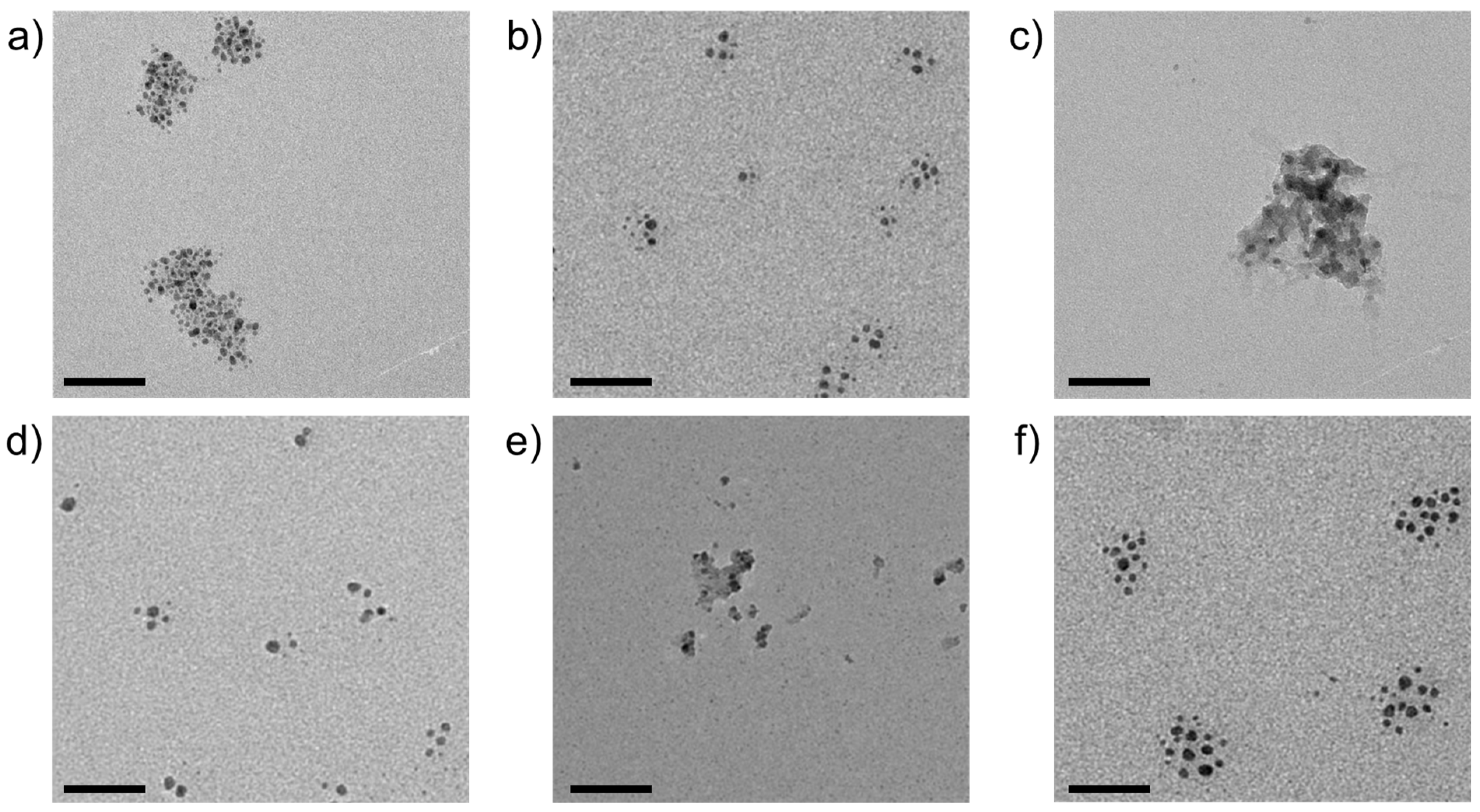
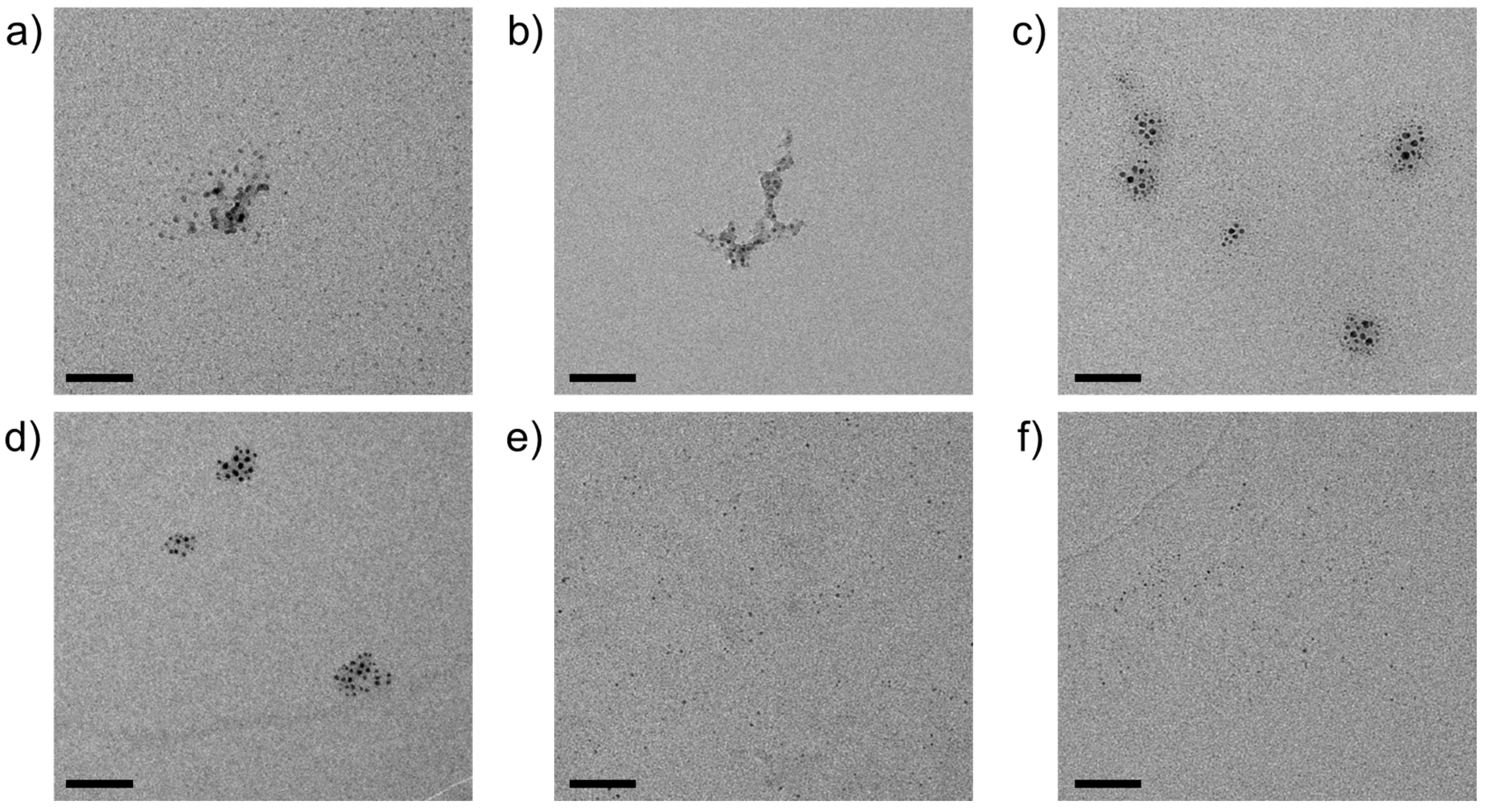
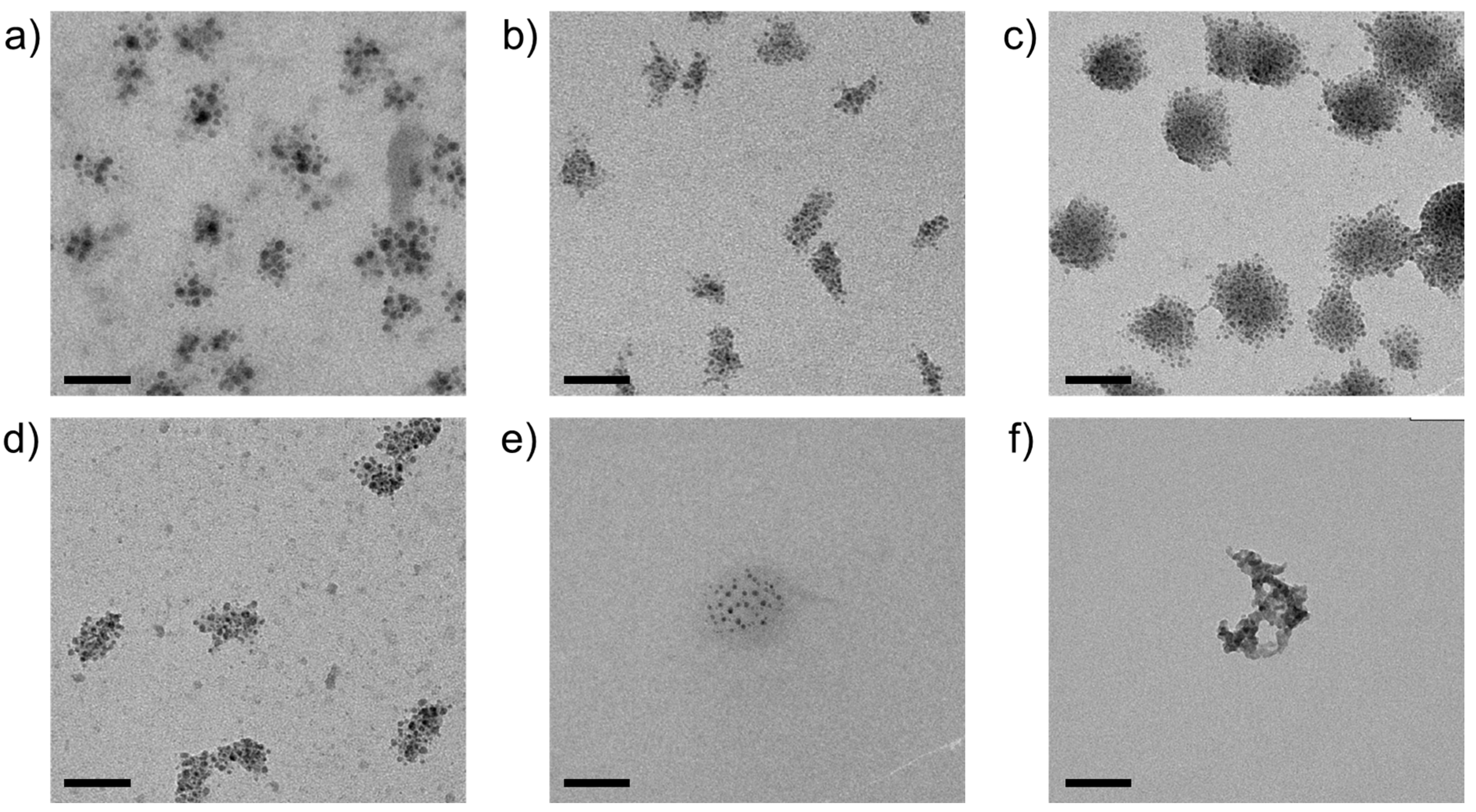
| Metal Cation | dCarbonate/nm | dOxide/nm | dSulfide/nm |
|---|---|---|---|
| Cd2+ | 4.8 ± 1.1 (n = 90) | 3.5 ± 0.9 (n = 55) | 5.3 ± 2.2 (n = 68) |
| Co2+ | 4.1 ± 1.2 (n = 140) | 2.8 ± 0.7 (n = 70) | 4.4 ± 1.5 (n = 47) |
| Cu2+ | 4.8 ± 1.1 (n = 10) | 4.3 ± 1.4 (n = 86) | 5.5 ± 1.3 (n = 29) |
| Mn2+ | 5.7 ± 1.7 (n = 70) | 3.7 ± 1.0 (n = 230) | 4.5 ± 1.7 (n = 40) |
| Ni2+ | 4.4 ± 1.1 (n = 21) | 2.9 ± 0.7 (n = 107) | 4.7 ± 1.0 (n = 45) |
| Zn2+ | 4.7 ± 1.4 (n = 90) | 2.5 ± 0.5 (n = 133) | 3.3 ± 0.7 (n = 38) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rödig, B.; Funkner, D.; Frank, T.; Schürmann, U.; Rieder, J.; Kienle, L.; Kunz, W.; Kellermeier, M. Nanodots of Transition Metal Sulfides, Carbonates, and Oxides Obtained Through Spontaneous Co-Precipitation with Silica. Nanomaterials 2024, 14, 2054. https://doi.org/10.3390/nano14242054
Rödig B, Funkner D, Frank T, Schürmann U, Rieder J, Kienle L, Kunz W, Kellermeier M. Nanodots of Transition Metal Sulfides, Carbonates, and Oxides Obtained Through Spontaneous Co-Precipitation with Silica. Nanomaterials. 2024; 14(24):2054. https://doi.org/10.3390/nano14242054
Chicago/Turabian StyleRödig, Bastian, Diana Funkner, Thomas Frank, Ulrich Schürmann, Julian Rieder, Lorenz Kienle, Werner Kunz, and Matthias Kellermeier. 2024. "Nanodots of Transition Metal Sulfides, Carbonates, and Oxides Obtained Through Spontaneous Co-Precipitation with Silica" Nanomaterials 14, no. 24: 2054. https://doi.org/10.3390/nano14242054
APA StyleRödig, B., Funkner, D., Frank, T., Schürmann, U., Rieder, J., Kienle, L., Kunz, W., & Kellermeier, M. (2024). Nanodots of Transition Metal Sulfides, Carbonates, and Oxides Obtained Through Spontaneous Co-Precipitation with Silica. Nanomaterials, 14(24), 2054. https://doi.org/10.3390/nano14242054







