Research on the Microstructure, Mechanical Properties and Strengthening Mechanism of Nanocrystalline Al-Mo Alloy Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Film Deposition
2.2. Films Characterization
3. Results and Discussions
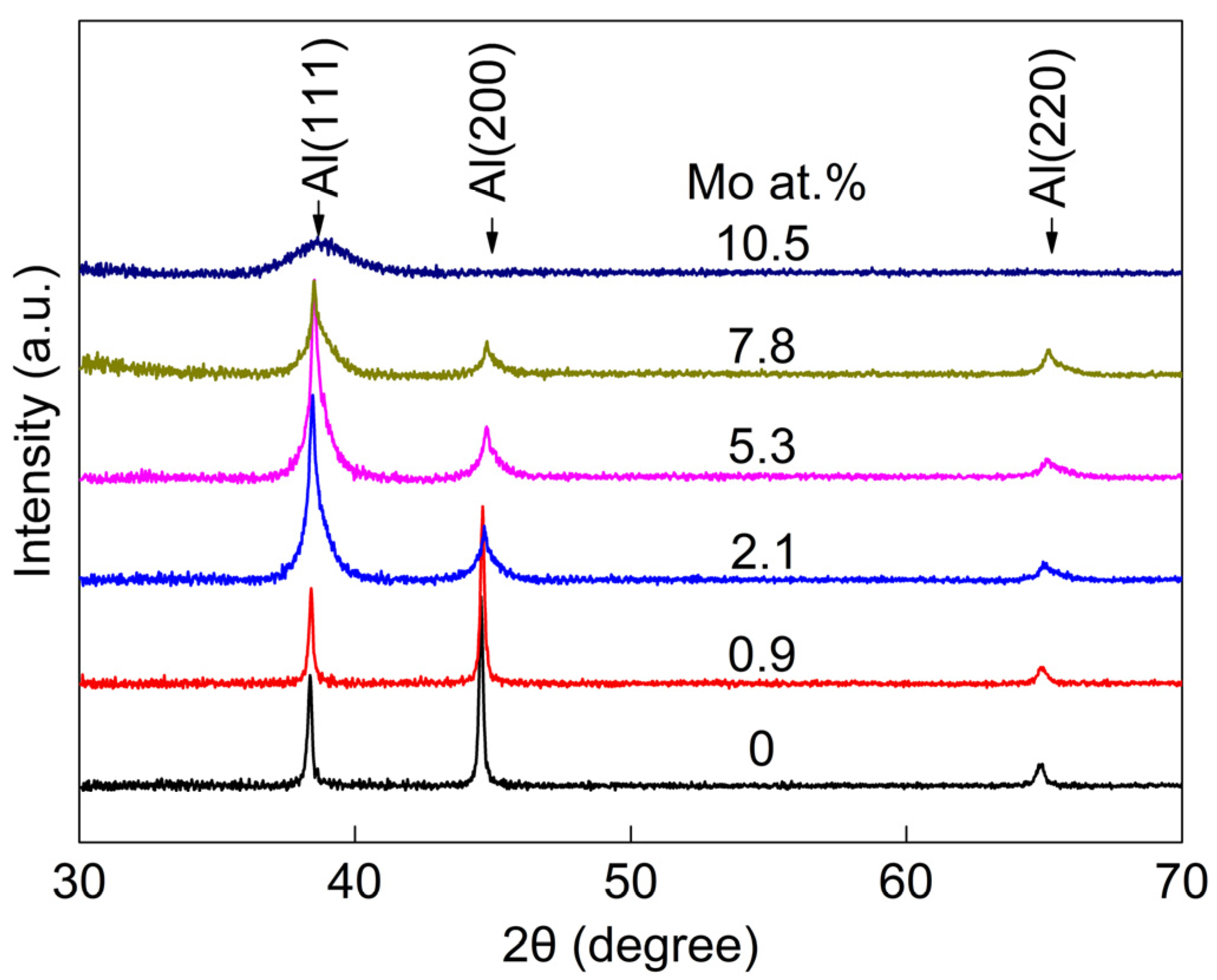
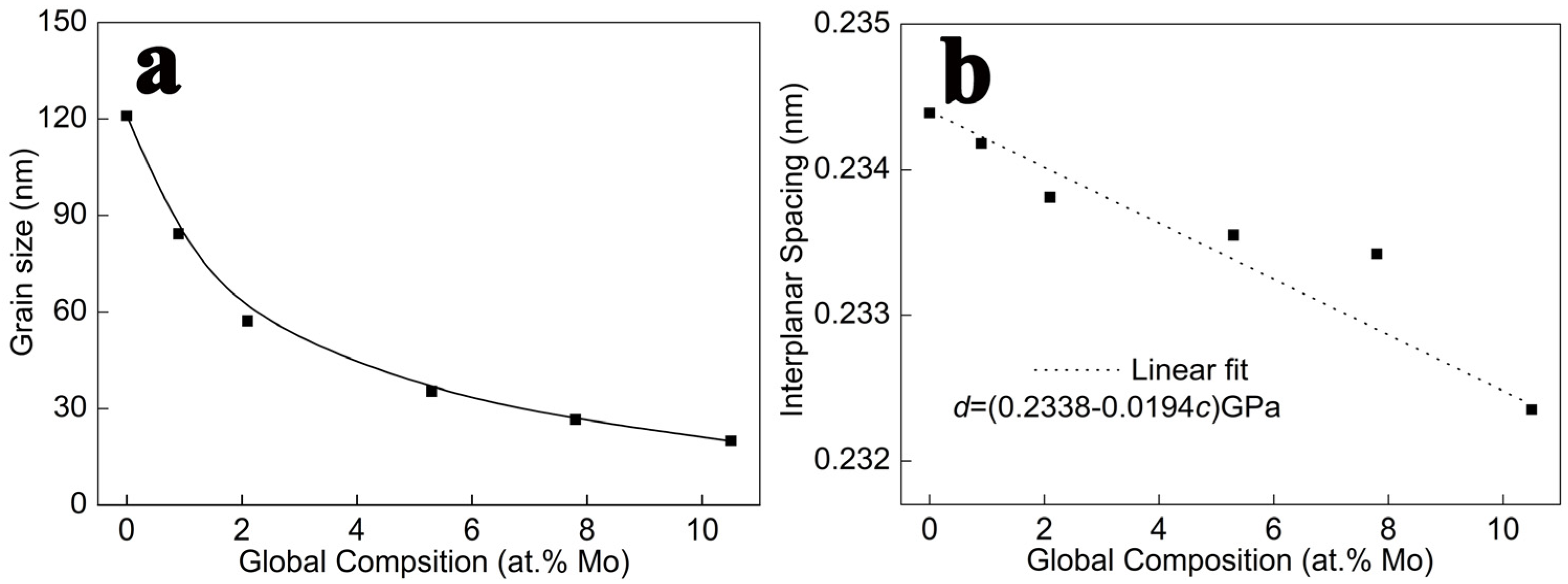
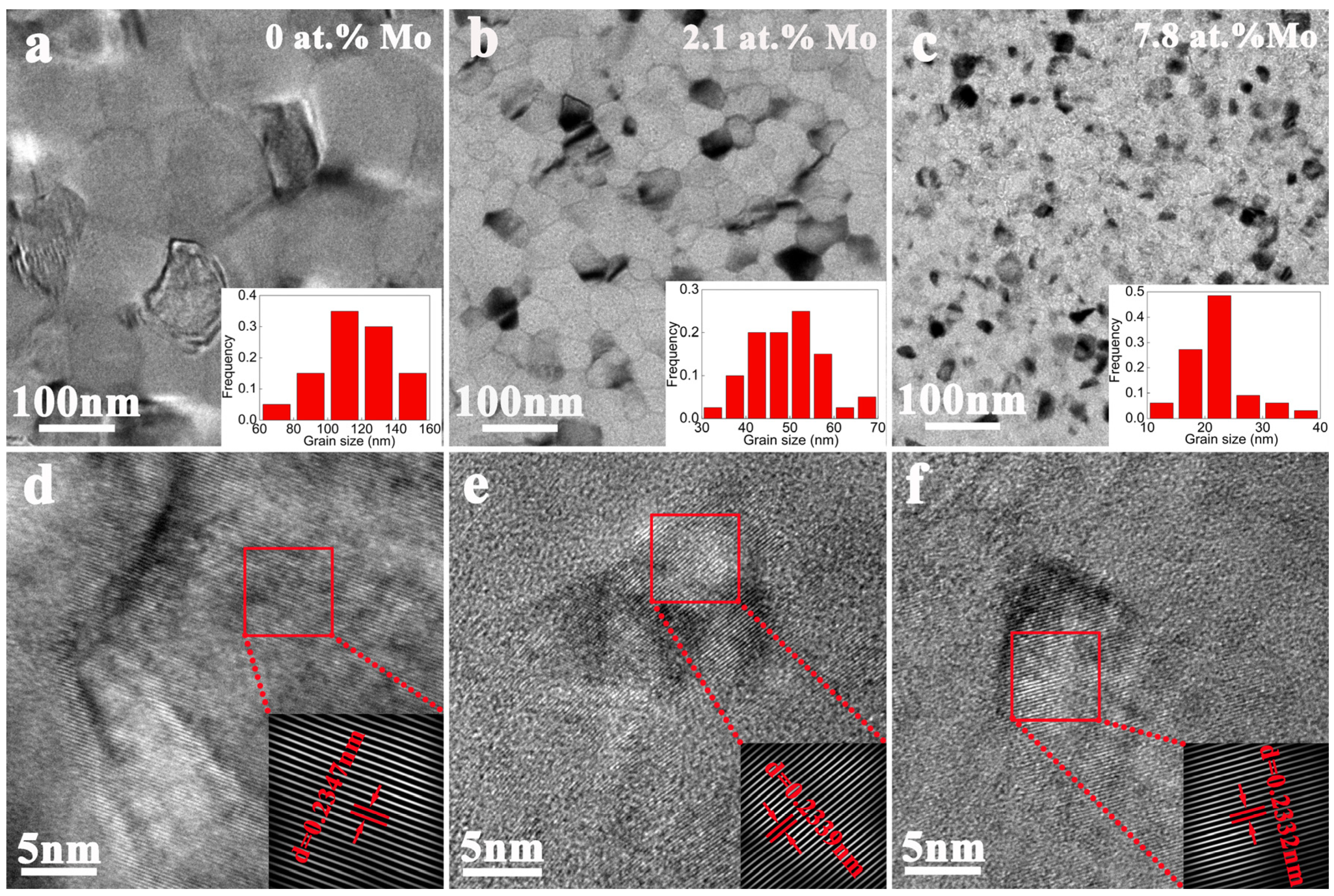
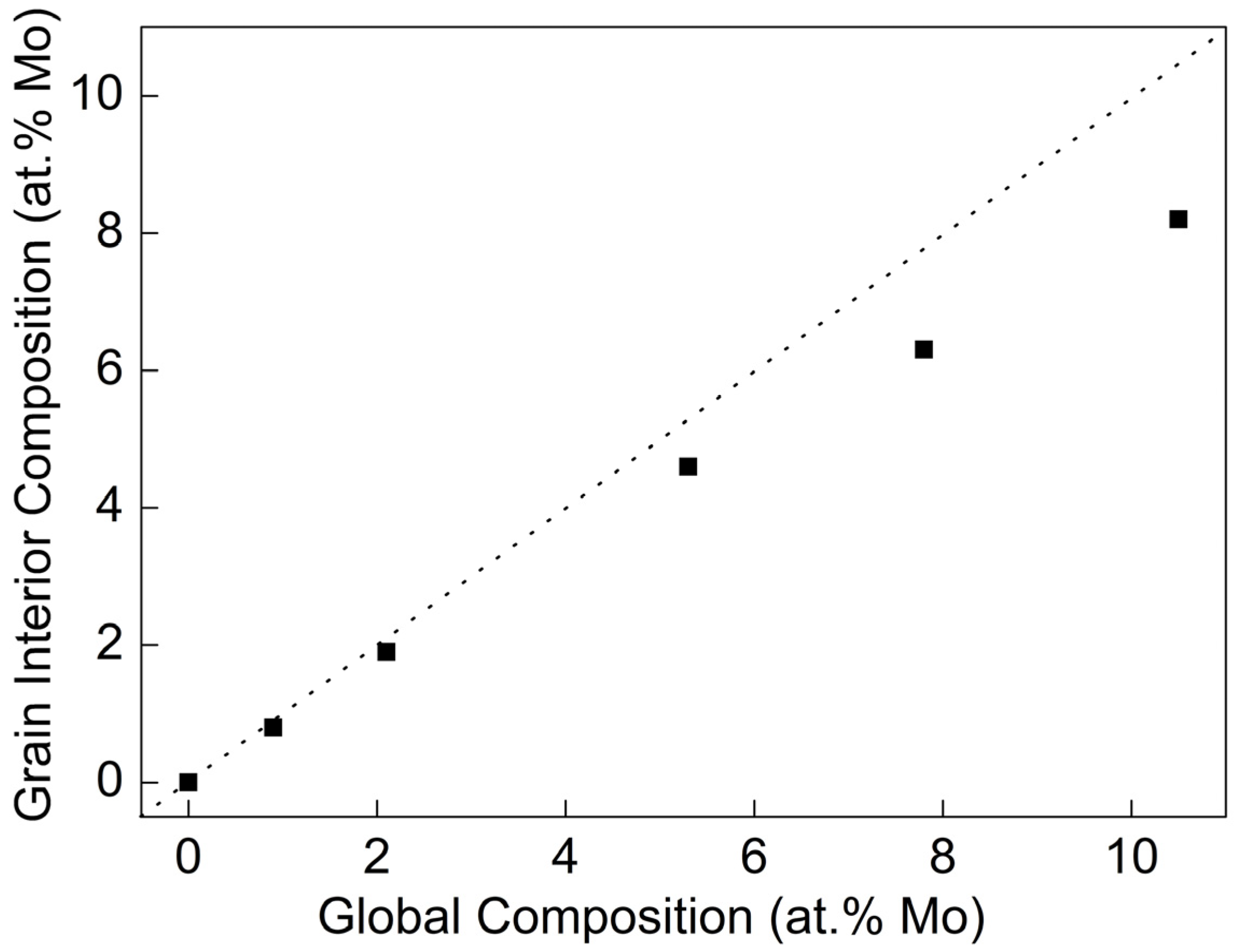
3.1. The Microstructure of Alloy Films
3.2. Mechanical Behaviors of Alloy Films
4. Conclusions
- All the Al-Mo alloy films formed an Al-Mo solid solution. No compounds or second phases appeared. With the increase in Mo, the grain size rapidly refines. The additional Mo atoms exhibited the phenomenon of grain boundary segregation. The higher the Mo content, the more pronounced the trend of grain boundary segregation.
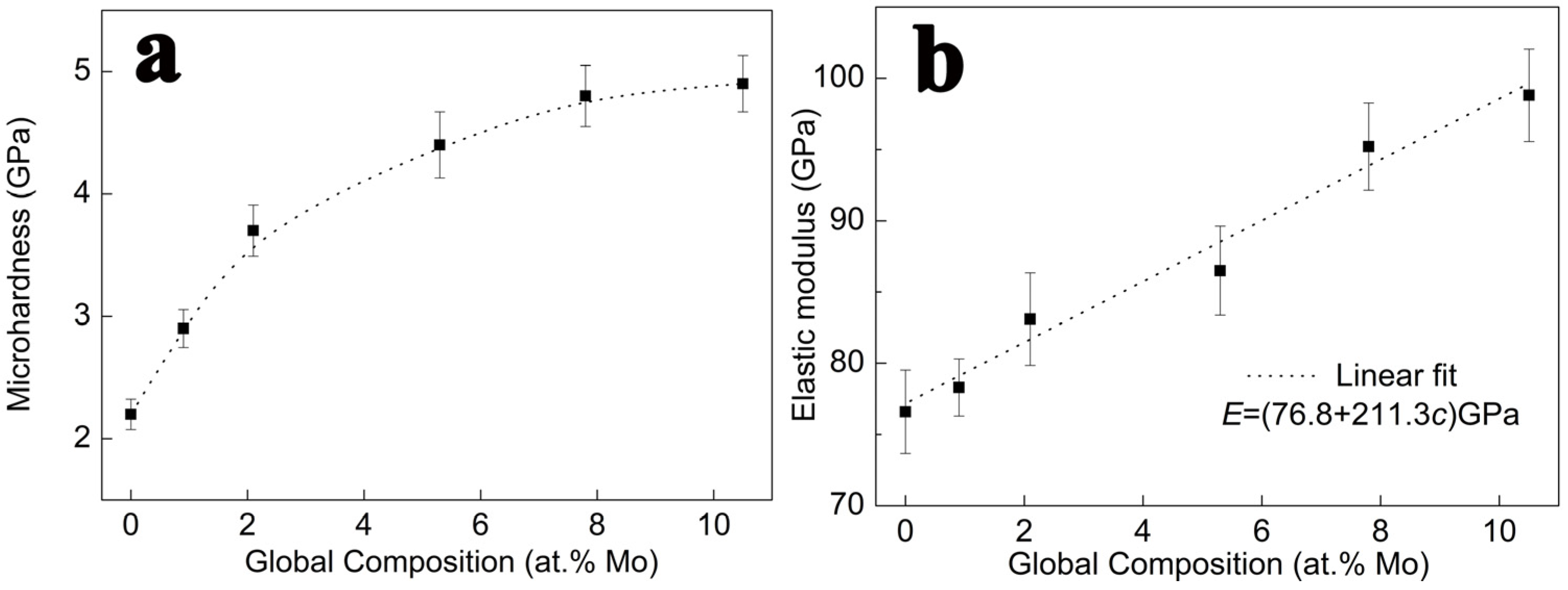
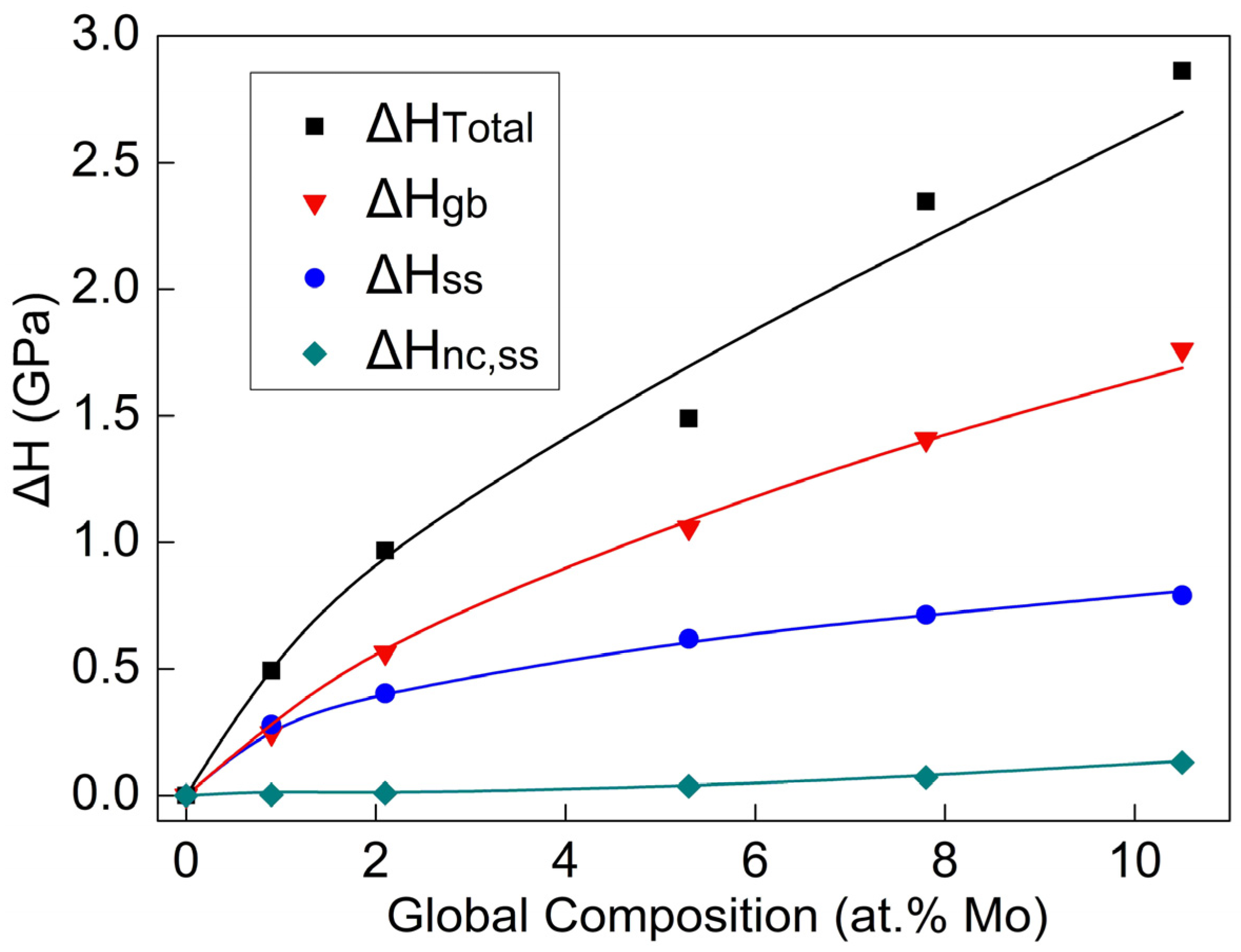
- The hardness and elastic modulus of the Al-Mo nanocrystalline alloy films gradually increased with the increase in Mo content. The fine grain (), solid solution (), and nanocrystalline solute pinning () are the three main reasons for the increase in the hardness of alloy thin films. Among them, the contribution of is the largest, accounting for over 60% of the total response, while the accounts for about 30%, ranking second. The rest is due to the contribution of .
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.Y.; Jin, Z.H.; Zhou, X.; Lu, K. Constrained minimal-interface structures in polycrystalline copper with extremely fine grains. Science 2020, 370, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feng, Z.; Zhu, L.; Xu, J.; Miyagi, L.; Dong, H.; Sheng, H.; Wang, Y.; Li, Q.; Ma, Y.; et al. High-pressure strengthening in ultrafine-grained metals. Nature 2020, 579, 67–72. [Google Scholar] [CrossRef]
- Ke, X.; Ye, J.; Pan, Z.; Geng, J.; Besser, M.F.; Qu, D.; Caro, A.; Marian, J.; Ott, R.T.; Wang, Y.M.; et al. Ideal maximum strengths and defect-induced softening in nanocrystalline-nanotwinned metals. Nat. Mater. 2019, 18, 1207–1214. [Google Scholar] [CrossRef]
- Sotniczuk, A.; Kuczyńska-Zemła, D.; Królikowski, A.; Garbacz, H. Enhancement of the corrosion resistance and mechanical properties of nanocrystalline titanium by low-temperature annealing. Corros. Sci. 2019, 147, 342–349. [Google Scholar] [CrossRef]
- Simões, S.; Calinas, R.; Vieira, M.F.; Ferreira, P.J. In situ TEM study of grain growth in nanocrystalline copper thin films. Nanotechnology 2010, 21, 145701. [Google Scholar] [CrossRef]
- Klement, U.; Erb, U.; El-Sherik, A.M.; Aust, K.T. Thermal stability of nanocrystalline Ni. Mater. Sci. Eng. A 1995, 203, 177–186. [Google Scholar] [CrossRef]
- Gianola, D.; Van Petegem, S.; Legros, M.; Brandstetter, S.; Van Swygenhoven, H.; Hemker, K. Stress-assisted discontinuous grain growth and its effect on the deformation behavior of nanocrystalline aluminum thin films. Acta Mater. 2006, 54, 2253–2263. [Google Scholar] [CrossRef]
- Malow, T.; Koch, C.C. Thermal Stability of Nanocrystalline Materials. Mater. Sci. Forum 1996, 225–227, 595–604. [Google Scholar] [CrossRef]
- Peng, H.; Huo, W.; Zhang, W.; Tang, Y.; Zhang, S.; Huang, L.; Hou, H.; Ding, Z.; Liu, F. Correlation between stabilizing and strengthening effects due to grain boundary segregation in iron-based alloys: Theoretical models and first-principles calculations. Acta Mater. 2023, 251, 118899. [Google Scholar] [CrossRef]
- Liang, N.; Zhao, Y. A review on thermal stability of nanostructured materials. J. Alloys Compd. 2023, 938, 168528. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.Y.; Lu, K. Enhanced thermal stability of nanograined metals below a critical grain size. Science 2018, 360, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, A.; Wang, W.; Vemuri, R.; Kovarik, L.; Jiang, X.; Bowden, M.; Trelewicz, J.; Mathaudhu, S.; Rohatgi, A. Grain boundary segregation and intermetallic precipitation in coarsening resistant nanocrystalline aluminum alloys. Acta Mater. 2019, 165, 698–708. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Darling, K.; Turnage, S.; Koju, R.; Hornbuckle, B.; Mishin, Y.; Solanki, K. Microstructural evolution in a nanocrystalline Cu-Ta alloy: A combined in-situ TEM and atomistic study. Mater. Des. 2017, 113, 178–185. [Google Scholar] [CrossRef]
- Atwater, M.A.; Scattergood, R.O.; Koch, C.C. The stabilization of nanocrystalline copper by zirconium. Mater. Sci. Eng. A 2012, 559, 250–256. [Google Scholar] [CrossRef]
- Kotan, H.; Darling, K.A.; Saber, M.; Koch, C.C.; Scattergood, R.O. Effect of zirconium on grain growth and mechanical properties of a ball-milled nanocrystalline FeNi alloy. J. Alloys Compd. 2012, 551, 621–629. [Google Scholar] [CrossRef]
- Detor, A.J.; Schuh, C.A. Grain boundary segregation, chemical ordering and stability of nanocrystalline alloys: Atomistic computer simulations in the Ni–W system. Acta Mater. 2007, 55, 4221–4232. [Google Scholar] [CrossRef]
- Chookajorn, T.; Murdoch, H.A.; Schuh, C.A. Design of Stable Nanocrystalline Alloys. Science 2012, 337, 951–954. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2013, 62, 141–155. [Google Scholar] [CrossRef]
- Scattergood, R.O.; Koch, C.C.; Murty, K.L.; Brenner, D. Strengthening mechanisms in nanocrystalline alloys. Mater. Sci. Eng. A 2008, 493, 3–11. [Google Scholar] [CrossRef]
- Wen, H.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J. Strengthening mechanisms in a high-strength bulk nanostructured Cu–Zn–Al alloy processed via cryomilling and spark plasma sintering. Acta Mater. 2013, 61, 2769–2782. [Google Scholar] [CrossRef]
- Rupert, T.J.; Trenkle, J.C.; Schuh, C.A. Enhanced solid solution effects on the strength of nanocrystalline alloys. Acta Mater. 2011, 59, 1619–1631. [Google Scholar] [CrossRef]
- Shanmugasundaram, T.; Heilmaier, M.; Murty, B.S.; Sarma, V.S. On the Hall–Petch relationship in a nanostructured Al–Cu alloy. Mater. Sci. Eng. A 2010, 527, 7821–7825. [Google Scholar] [CrossRef]
- Darling, K.; Roberts, A.; Armstrong, L.; Kapoor, D.; Tschopp, M.; Kecskes, L.; Mathaudhu, S. Influence of Mn solute content on grain size reduction and improved strength in mechanically alloyed Al–Mn alloys. Mater. Sci. Eng. A 2013, 589, 57–65. [Google Scholar] [CrossRef]
- Dong, Y.J.; Yang, D.; Shen, J.; Li, G.Y. Improved soluble salt substrate method for thin film TEM sample preparation. Mater. Res. Innov. 2012, 16, 158–160. [Google Scholar] [CrossRef]
- Pharr, G.; Oliver, W. Measurement of Thin Film Mechanical Properties Using Nanoindentation. MRS Bull. 1992, 17, 28–33. [Google Scholar] [CrossRef]
- Yu, R.H. The empirical electron theory of solids and molecules. Chin. Sci. Bull. 1978, 23, 217–224. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, H.; Chen, Y.; Qi, X.; Zhong, N. Research on the Microstructure, Mechanical Properties and Strengthening Mechanism of Nanocrystalline Al-Mo Alloy Films. Nanomaterials 2024, 14, 1990. https://doi.org/10.3390/nano14241990
Wang Y, Xu H, Chen Y, Qi X, Zhong N. Research on the Microstructure, Mechanical Properties and Strengthening Mechanism of Nanocrystalline Al-Mo Alloy Films. Nanomaterials. 2024; 14(24):1990. https://doi.org/10.3390/nano14241990
Chicago/Turabian StyleWang, Ying, Huanqing Xu, Yulan Chen, Xiaoben Qi, and Ning Zhong. 2024. "Research on the Microstructure, Mechanical Properties and Strengthening Mechanism of Nanocrystalline Al-Mo Alloy Films" Nanomaterials 14, no. 24: 1990. https://doi.org/10.3390/nano14241990
APA StyleWang, Y., Xu, H., Chen, Y., Qi, X., & Zhong, N. (2024). Research on the Microstructure, Mechanical Properties and Strengthening Mechanism of Nanocrystalline Al-Mo Alloy Films. Nanomaterials, 14(24), 1990. https://doi.org/10.3390/nano14241990






