Application of Polyvinyl Alcohol–Ethylene Glycol Hydrogel Technology for Removing Animal Glue in Book Restoration Based on Fluorescent Labeling Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVA-EG Hydrogel
2.3. Characterization Techniques
2.4. Fluorescent Animal Glue Removal Experiment
2.4.1. Preparation of Fluorescent Animal Glue
2.4.2. Preparation of Simulated Samples
2.4.3. Animal Glue Removal Experiment on Simulated Samples
2.4.4. Animal Glue Removal Experiment on Book Samples
3. Results and Discussion
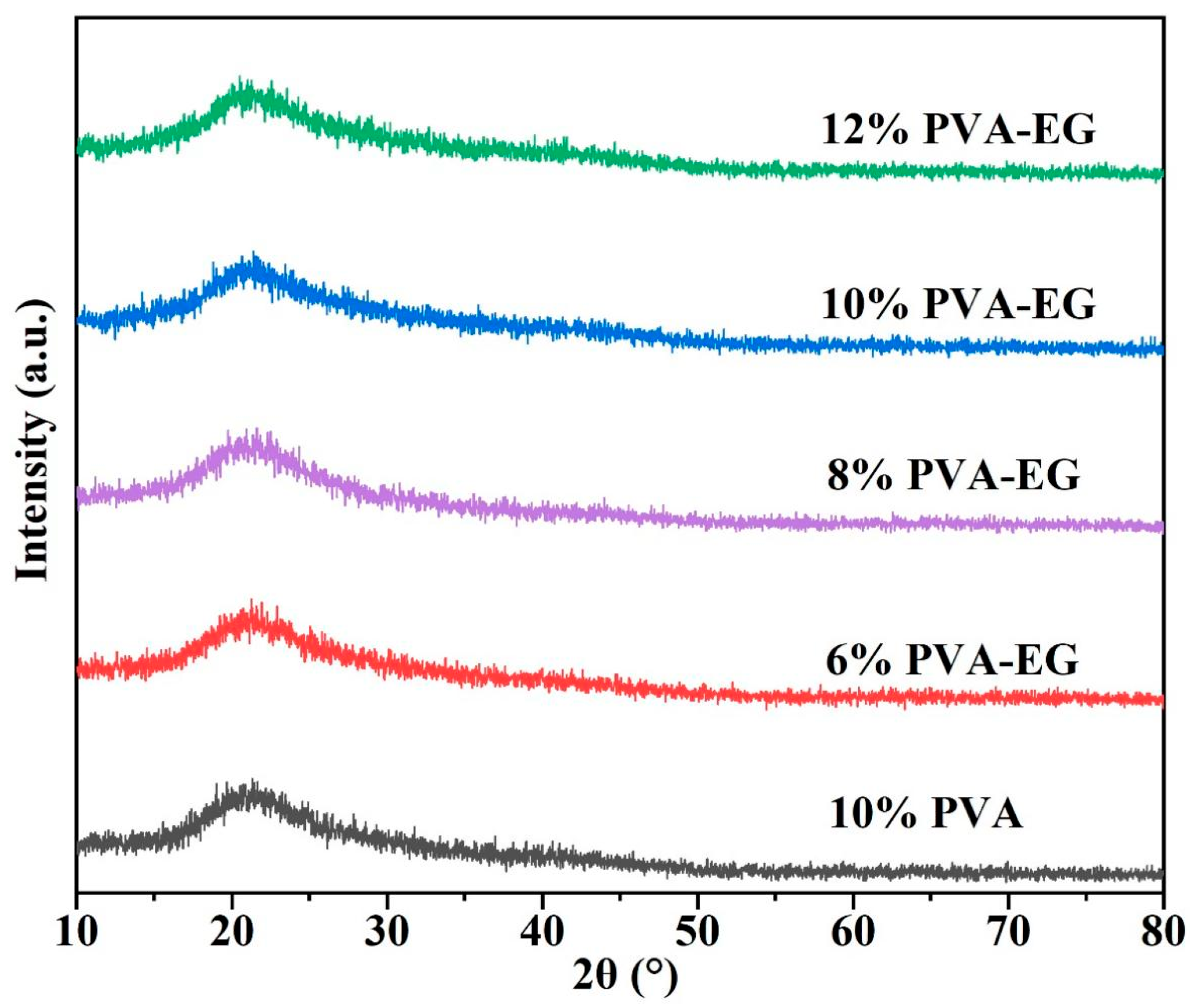
3.1. Crystal Structure Analysis
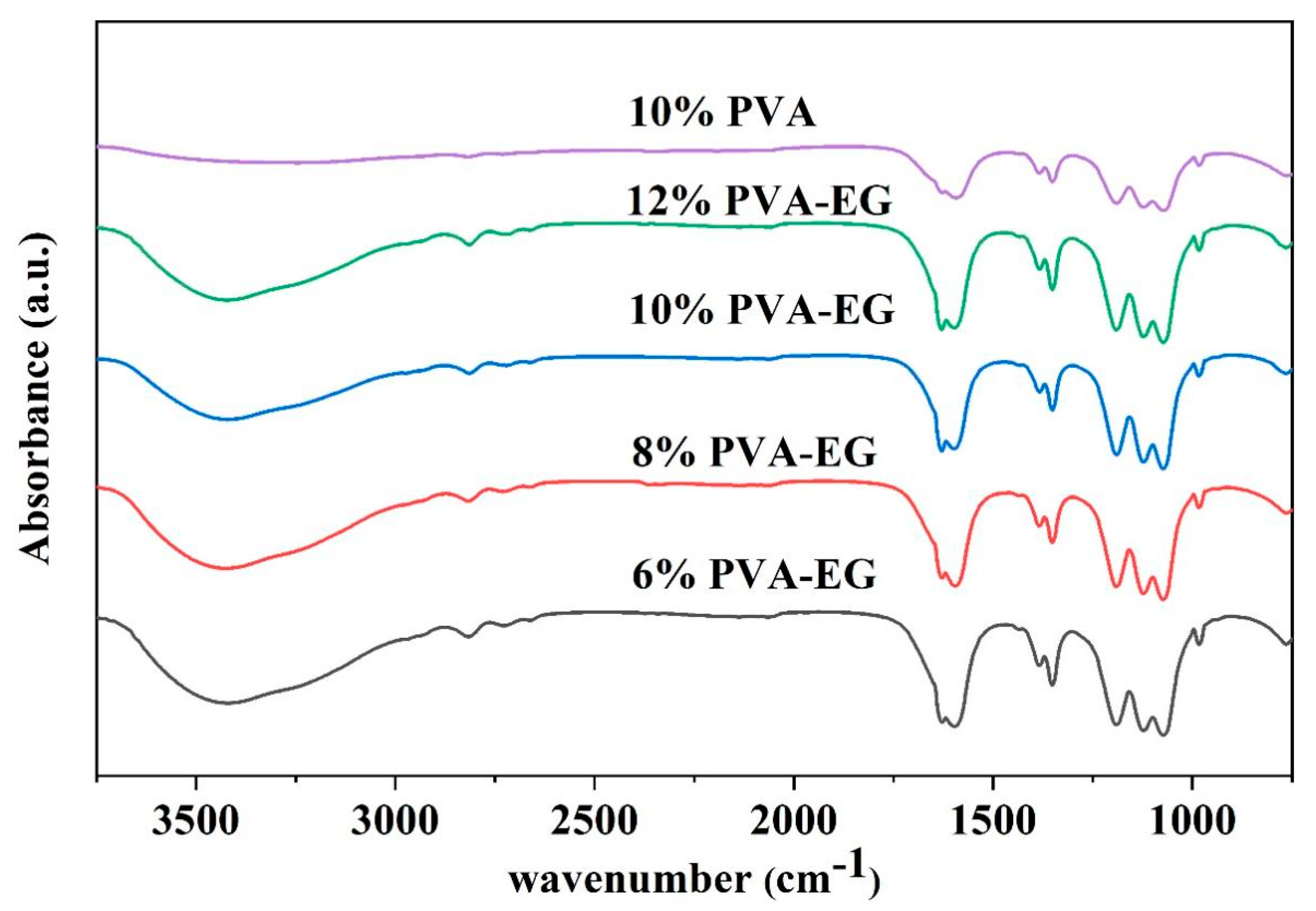
3.2. Chemical Structure Characterization
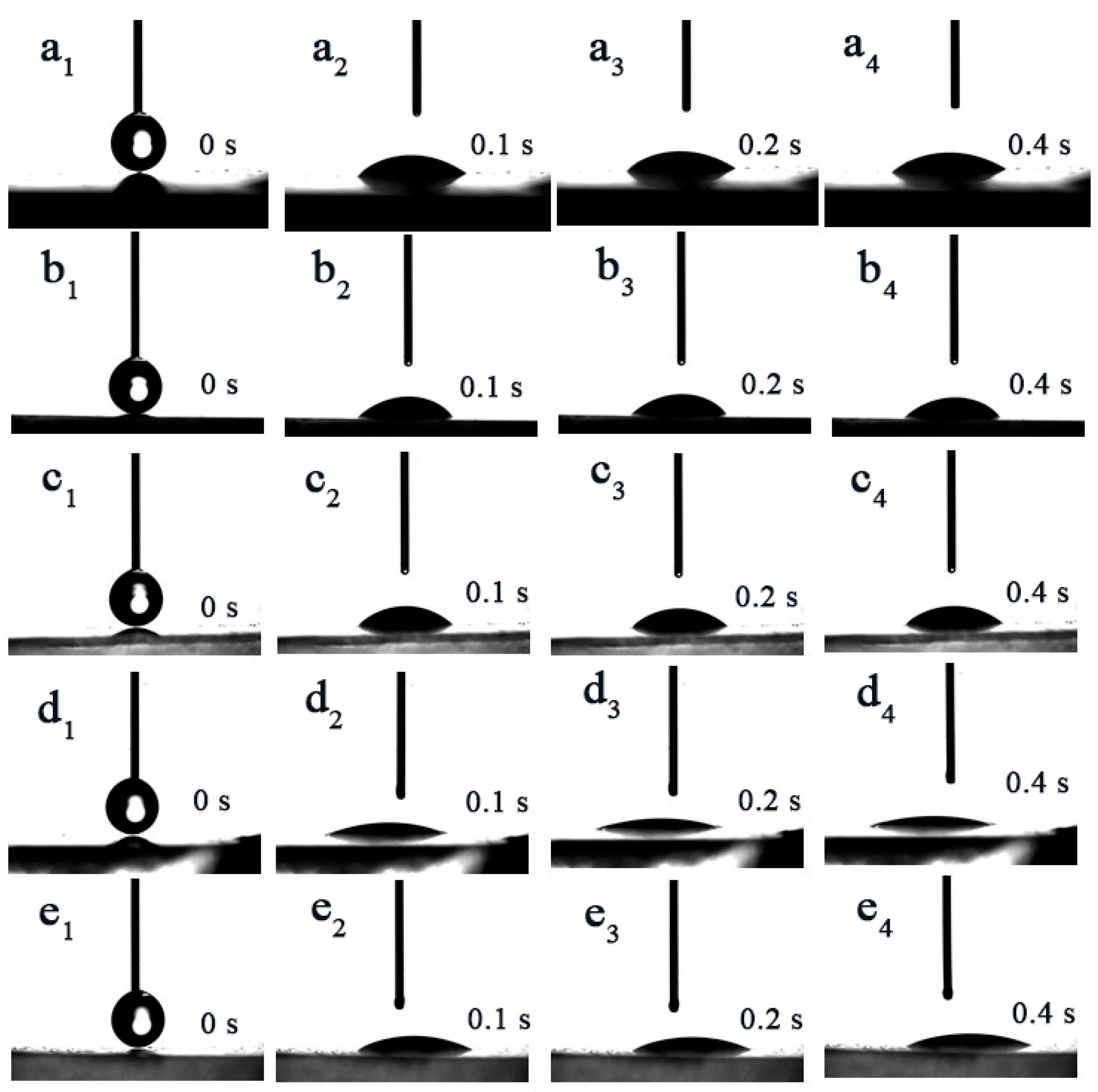
3.3. Surface Wettability Analysis
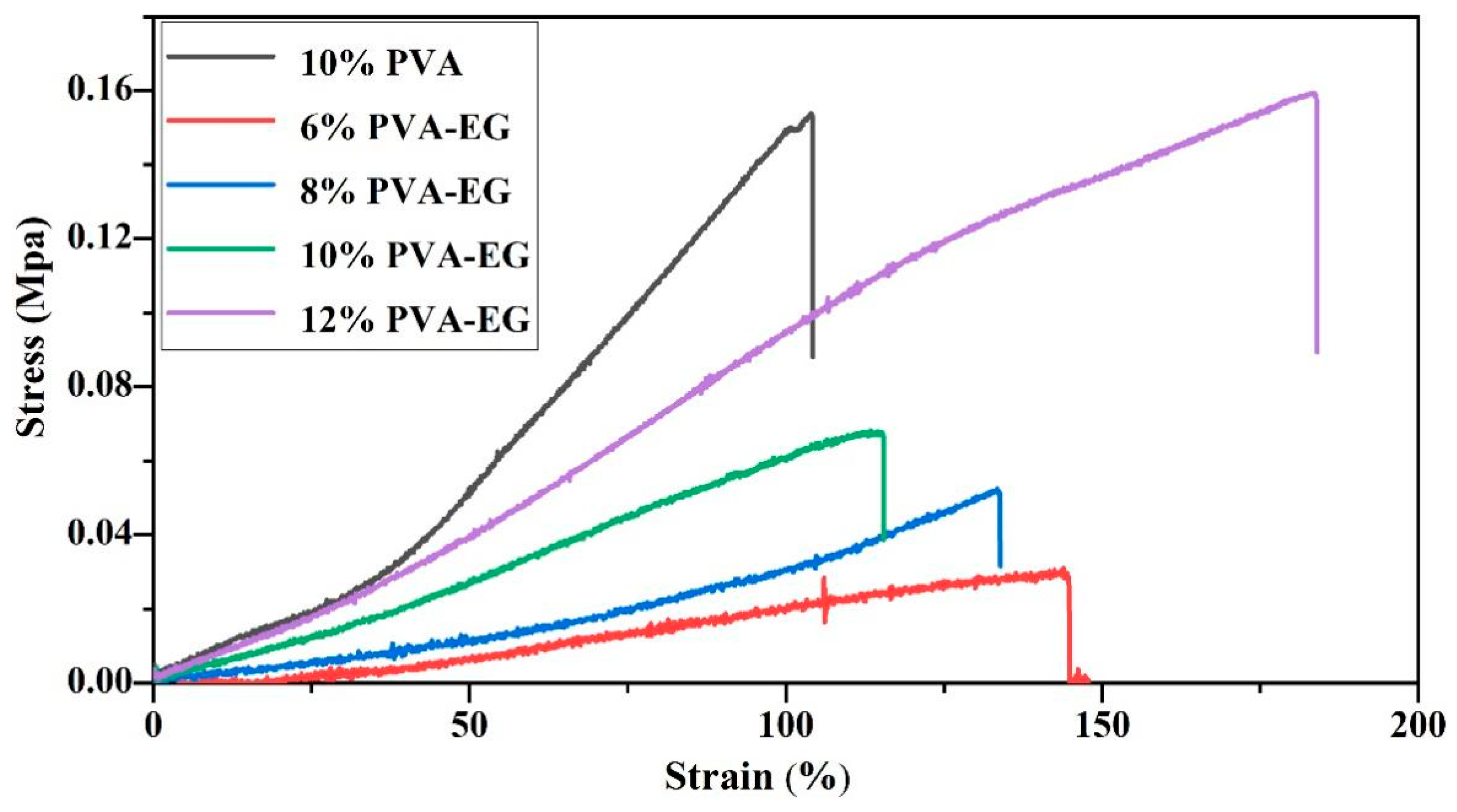
3.4. Mechanical Property Testing
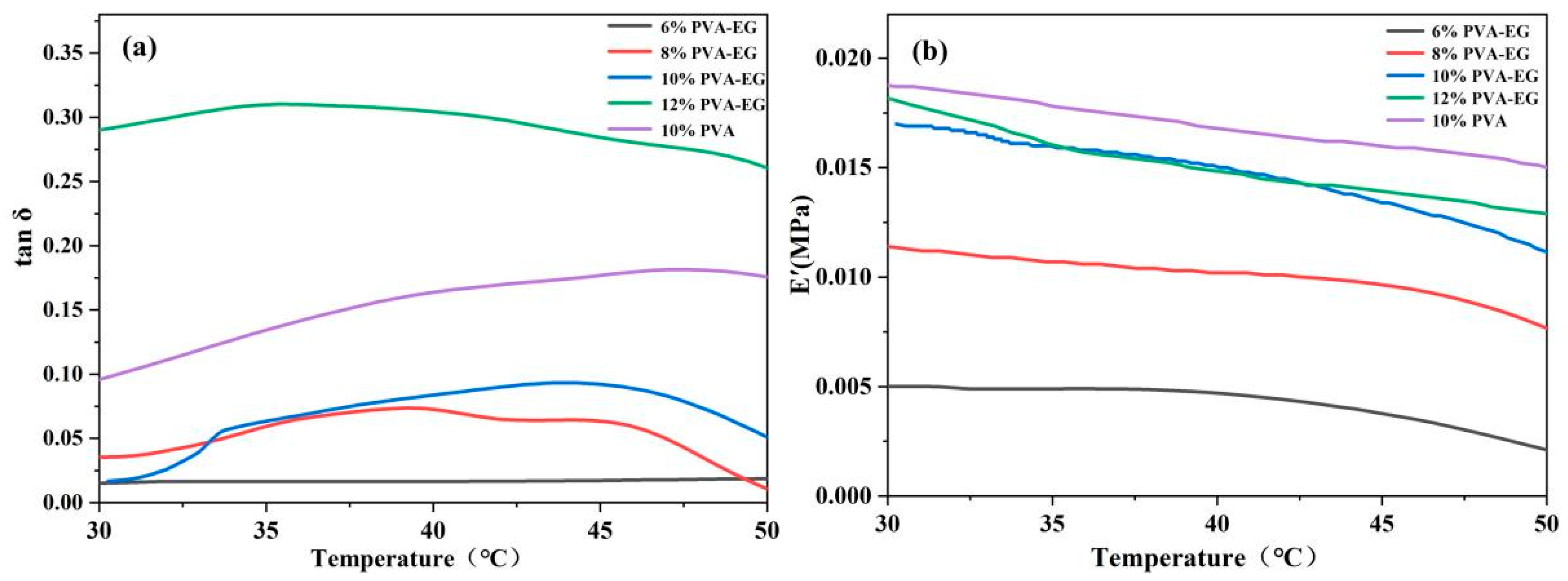
3.5. Dynamic Mechanical Analysis (DMA)
3.6. Analysis of Fluorescent Animal Glue Removaling Results
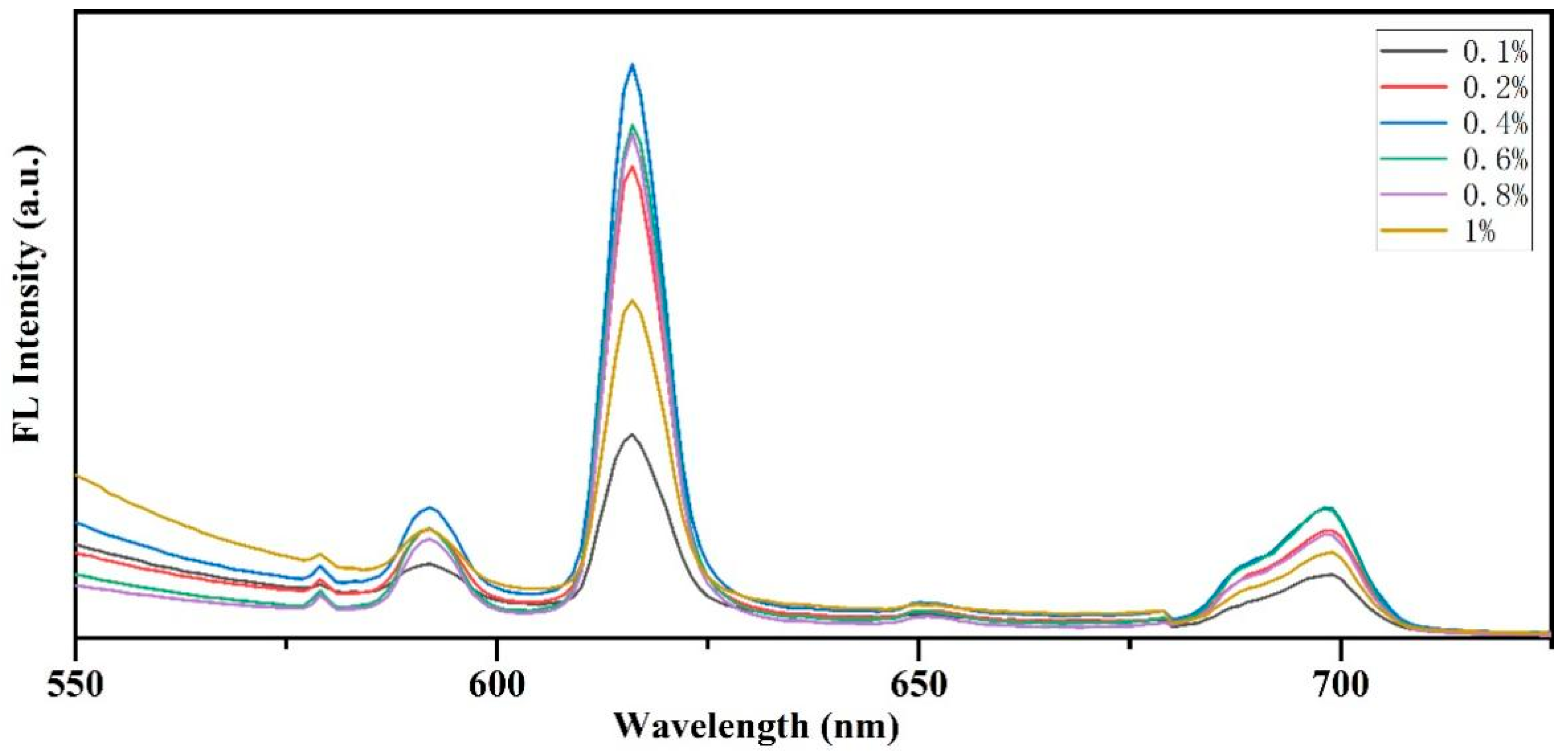
3.6.1. Optimization of Europium Nitrate Concentration and Its Effect on Fluorescent Labeling
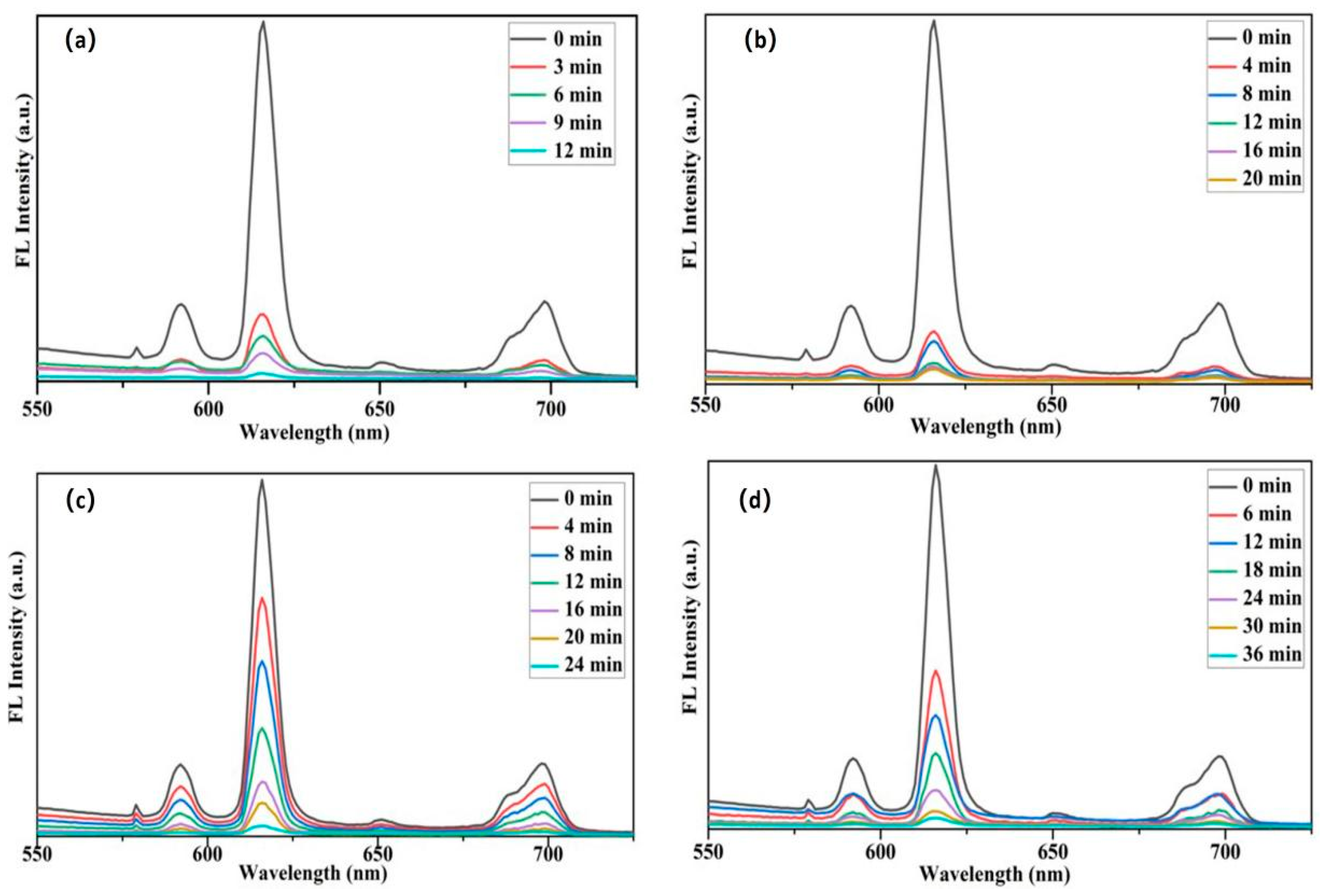
3.6.2. Removal Efficiency of Simulated Animal Glue Layers by PVA-EG Hydrogel
3.6.3. Application of PVA-EG Hydrogel in Removal of Animal Glue from Book Pages
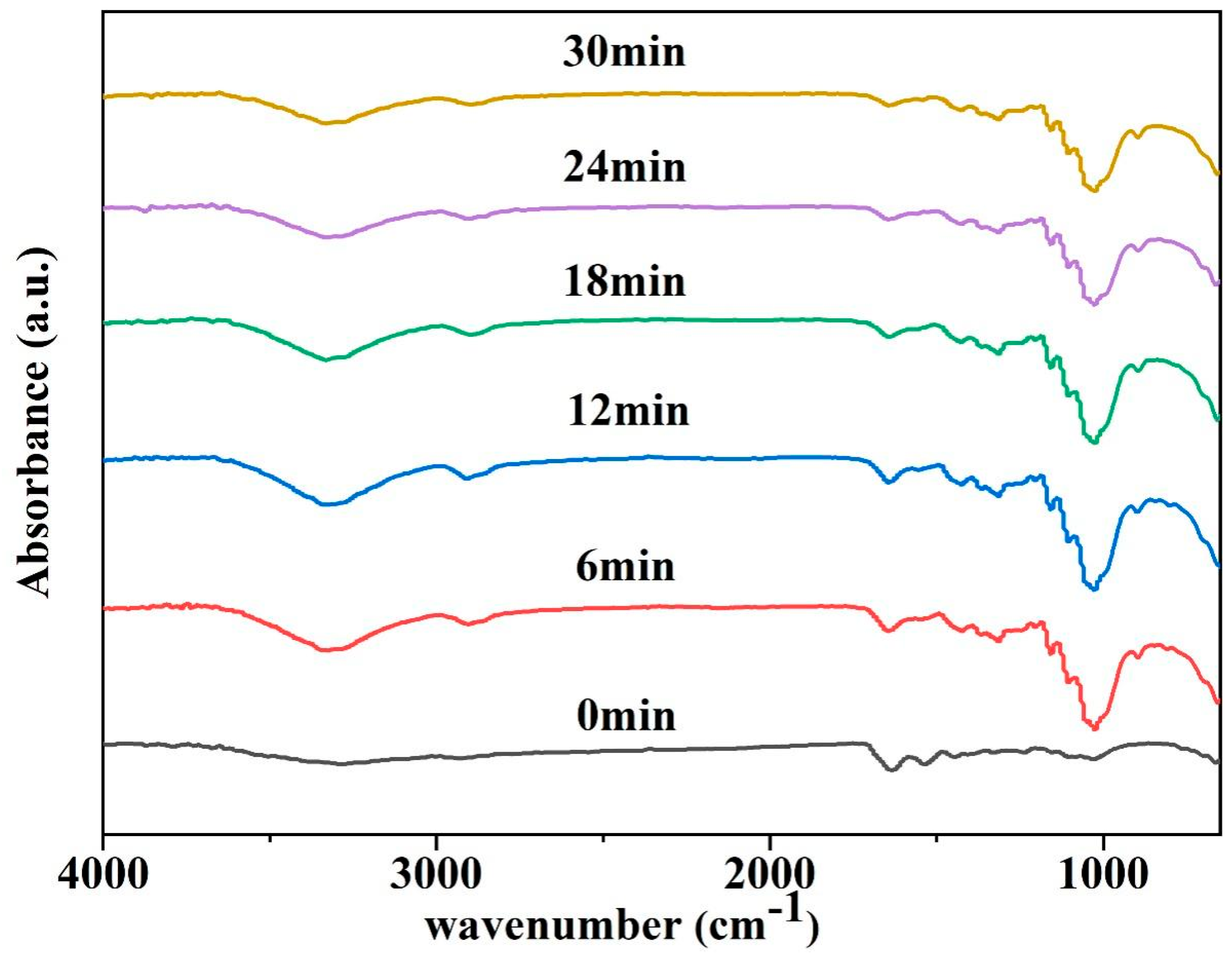
3.6.4. Mechanism of Animal Glue Removal by PVA-EG Hydrogel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Domingues, J.A.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative hydrogels based on semi-interpenetrating p (HEMA)/PVP networks for the cleaning of water-sensitive cultural heritage artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, N.; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Poly (vinyl alcohol)/poly (vinyl pyrrolidone) hydrogels for the cleaning of art. J. Colloid Interface Sci. 2019, 536, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, R.; Chelazzi, D.; Poggi, G.; Fratini, E.; Buemi, L.P.; Petruzzellis, M.L.; Baglioni, P. Twin-chain polymer hydrogels based on poly (vinyl alcohol) as new advanced tool for the cleaning of modern and contemporary art. Proc. Natl. Acad. Sci. USA 2020, 117, 7011–7020. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiang, N.; Jiang, X.; Hou, L. Preparation of high tough poly (vinyl alcohol) hydrogel by soaking in NaCl aqueous solution. Mater. Lett. 2017, 194, 34–37. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D. How science can contribute to the remedial conservation of cultural heritage. Chem.-A Eur. J. 2021, 27, 10798–10806. [Google Scholar] [CrossRef]
- Rosciardi, V.; Chelazzi, D.; Baglioni, P. “Green” biocomposite poly (vinyl alcohol)/starch cryogels as new advanced tools for the cleaning of artifacts. J. Colloid Interface Sci. 2022, 613, 697–708. [Google Scholar] [CrossRef]
- Chelazzi, D.; Bordes, R.; Giorgi, R.; Holmberg, K.; Baglioni, P. The use of surfactants in the cleaning of works of art. Curr. Opin. Colloid Interface Sci. 2020, 45, 108–123. [Google Scholar] [CrossRef]
- Cardaba, I.; Poggi, G.; Baglioni, M.; Chelazzi, D.; Maguregui, I.; Giorgi, R. Assessment of aqueous cleaning of acrylic paints using innovative cryogels. Microchem. J. 2020, 152, 104311. [Google Scholar] [CrossRef]
- Altobelli, A.; Cennamo, P.; Trojsi, G.; Lumaga, M.R.B.; Carpentieri, A.; Fatigati, G. Experimentation of a PVA-Borax hydrogel for the removal of Paraloid B72® from artifacts of archaeological interest from the National Archaeological Museum in Naples, Italy. Acta IMEKO 2023, 12, 1–8. [Google Scholar] [CrossRef]
- Rafiei-Sarmazdeh, Z.; Sheikh, N. Irradiation-assisted synthesis of smart hydrogels based on nanomagnetic semi-interpenetrating p (HEMA)/PVP networks for the cleaning of cultural heritage artifacts. Discov. Appl. Sci. 2024, 6, 285. [Google Scholar] [CrossRef]
- Mazzuca, C.; Severini, L.; Domenici, F.; Toumia, Y.; Mazzotta, F.; Micheli, L.; Titubante, M.; Di Napoli, B.; Paradossi, G.; Palleschi, A. Polyvinyl alcohol based hydrogels as new tunable materials for application in the cultural heritage field. Colloids Surf. B Biointerfaces 2020, 188, 110777. [Google Scholar] [CrossRef] [PubMed]
- Stagno, V.; Genova, C.; Zoratto, N.; Favero, G.; Capuani, S. Single-sided portable NMR investigation to assess and monitor cleaning action of PVA-borax hydrogel in travertine and Lecce stone. Molecules 2021, 26, 3697. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.T.; Chan, E.P.; Beers, K.L. Quantifying the ‘press and peel’removal of particulates using elastomers and gels. J. Cult. Herit. 2021, 48, 236–243. [Google Scholar] [CrossRef]
- Soto-Bustamante, F.; Bassu, G.; Fratini, E.; Laurati, M. Effect of composition and freeze-thaw on the network structure, porosity and mechanical properties of polyvinyl-alcohol/chitosan hydrogels. Gels 2023, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Al-Emam, E.; Soenen, H.; Caen, J.; Janssens, K. Characterization of polyvinyl alcohol-borax/agarose (PVA-B/AG) double network hydrogel utilized for the cleaning of works of art. Herit. Sci. 2020, 8, 106. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Muhammad, N.; Wang, Z.; Wu, D. Hierarchical networks of anisotropic hydrogels based on cross-linked Poly (vinyl alcohol)/Poly (vinylpyrrolidone). Polymer 2022, 251, 124920. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wu, D. Mapping hierarchical networks of poly (vinyl alcohol)/cellulose nanofiber composite hydrogels via viscoelastic probes. Carbohydr. Polym. 2022, 288, 119372. [Google Scholar] [CrossRef]
- Rosciardi, V. “Green” Poly (Vinyl Alcohol)/Starch Based Cryogels for the Cleaning of Works of Art: Application, Characterization, and Investigation of the Amylose/Amylopectin Structural Role. 2022. Available online: https://flore.unifi.it/handle/2158/1291291 (accessed on 24 November 2022).
- Severini, L.; Tavagnacco, L.; Angelini, R.; Franco, S.; Bertoldo, M.; Calosi, M.; Micheli, L.; Sennato, S.; Chiessi, E.; Ruzicka, B.; et al. Methacrylated gellan gum hydrogel: A smart tool to face complex problems in the cleaning of paper materials. Cellulose 2023, 30, 10469–10485. [Google Scholar] [CrossRef]
- Jiang, C.; Chao, Y.; Xie, W.; Wu, D. Using bacterial cellulose to bridge covalent and physical crosslinks in hydrogels for fabricating multimodal sensors. Int. J. Biol. Macromol. 2024, 263, 130178. [Google Scholar] [CrossRef]
- Warfield, M. Optimal PVA/Starch Complex for Cryogel Development Intended for Artifact Cleaning. 2024. Available online: https://digitalcommons.liberty.edu/research_symp/2024/posters/86/ (accessed on 16 April 2024).
- Wu, M.; Chen, X.; Xu, J.; Zhang, H. Freeze-thaw and solvent-exchange strategy to generate physically cross-linked organogels and hydrogels of curdlan with tunable mechanical properties. Carbohydr. Polym. 2022, 278, 119003. [Google Scholar] [CrossRef]
- Mastrangelo, R.; Resta, C.; Carretti, E.; Fratini, E.; Baglioni, P. Sponge-like Cryogels from Liquid-Liquid Phase Separation: Structure, Porosity, and Diffusional Gel Properties. ACS Appl. Mater. Interfaces 2023, 15, 46428–46439. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xing, W.; Wen, J.; Wu, Z.; Zhang, Y.; Zhang, H.; Wu, H.; Yao, H.; Xue, H.; Gao, J. Mixed solvent exchange enabled high-performance polymeric gels. Polymer 2023, 267, 125661. [Google Scholar] [CrossRef]
- Tamburini, G.; Canevali, C.; Ferrario, S.; Bianchi, A.; Sansonetti, A.; Simonutti, R. Optimized semi-interpenetrated p (HEMA)/PVP hydrogels for artistic surface cleaning. Materials 2022, 15, 6739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Wang, J.; Ding, J.; Zhang, P.; Dong, W.; Zhao, X.; Lu, Z.; Li, X. Effectively removing animal glue coated on the surface of ancient mural via dissolution of PVA hydrogel induced by thermal treatment. J. Cult. Herit. 2022, 55, 179–184. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Wang, J.; Ding, J.; Zhao, X.; Dong, W.; Lu, Z.; Li, X. Preparing a microemulsion-loaded hydrogel for cleaning wall paintings and coins. Herit. Sci. 2024, 12, 149. [Google Scholar] [CrossRef]
- Zuliani, A.; Chen, S.; Giorgi, R. Re-usable cross-linked poly (ethyl methacrylate) gels for cleaning purposes of artworks. Appl. Mater. Today 2023, 30, 101716. [Google Scholar] [CrossRef]
- Meng, Q.; Li, X.; Geng, J.; Liu, C.; Ben, S. A biological cleaning agent for removing mold stains from paper artifacts. Herit. Sci. 2023, 11, 243. [Google Scholar] [CrossRef]
- Li, Q.; Wu, C.; Peng, Y.; Zhang, B. Toward a non-invasive cleaning of the wall painting using polyelectrolyte hydrogel. Sci. China Technol. Sci. 2023, 66, 2213–2224. [Google Scholar] [CrossRef]
- Casini, A.; Chelazzi, D.; Baglioni, P. Advanced methodologies for the cleaning of works of art. Sci. China Technol. Sci. 2023, 66, 2162–2182. [Google Scholar] [CrossRef]
- Chelazzi, D.; Baglioni, P. From nanoparticles to gels: A breakthrough in art conservation science. Langmuir 2023, 39, 10744–10755. [Google Scholar] [CrossRef]
- Poggi, G.; Santan, H.D.; Smets, J.; Chelazzi, D.; Noferini, D.; Petruzzellis, M.L.; Buemi, L.P.; Fratini, E.; Baglioni, P. Nanostructured bio-based castor oil organogels for the cleaning of artworks. J. Colloid Interface Sci. 2023, 638, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Casagli, M.; Poggi, G.; Chelazzi, D.; Baglioni, P. Tuning Local Order in Starch Nanoparticles Exploiting Nonsolvency with “Green” Solvents. ACS Appl. Mater. Interfaces 2024, 16, 21185–21196. [Google Scholar] [CrossRef] [PubMed]
- Pensabene Buemi, L.; Petruzzellis, M.L.; Chelazzi, D.; Baglioni, M.; Mastrangelo, R.; Giorgi, R.; Baglioni, P. Twin-chain polymer networks loaded with nanostructured fluids for the selective removal of a non-original varnish from Picasso’s “L’Atelier” at the Peggy Guggenheim Collection, Venice. Herit. Sci. 2020, 8, 77. [Google Scholar] [CrossRef]
- Baglioni, M.; Poggi, G.; Giorgi, R.; Rivella, P.; Ogura, T.; Baglioni, P. Selective removal of over-paintings from “Street Art” using an environmentally friendly nanostructured fluid loaded in highly retentive hydrogels. J. Colloid Interface Sci. 2021, 595, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Volpi, F.; Fiocco, G.; Weththimuni, M.L.; Licchelli, M.; Malagodi, M. Preliminary cleaning approach with alginate and konjac glucomannan polysaccharide gel for the surfaces of east Asian and western string musical instruments. Materials 2022, 15, 1100. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, F.; Bruno, L.; Casieri, C.; Ranaldi, R.; Rugnini, L.; Spreti, N. Application and monitoring of oxidative alginate–biocide hydrogels for two case studies in “The Sassi and the Park of the Rupestrian Churches of Matera”. Coatings 2022, 12, 462. [Google Scholar] [CrossRef]
- Ortega Saez, N.; Arno, R.; Marchetti, A.; Cauberghs, S.; Janssens, K.; Van der Snickt, G.; Al-Emam, E. Towards a novel strategy for soot removal from water-soluble materials: The synergetic effect of hydrogels and cyclomethicone on gelatine emulsion-based photographs. Herit. Sci. 2023, 11, 78. [Google Scholar] [CrossRef]
- Richard, F.; Hermans, J.J.; Angelova, L. Rigid Solvent-Gels in Paper Conservation: A New Approach to Sticky Problems. J. Pap. Conserv. 2024, 25, 86–106. [Google Scholar] [CrossRef]
- Sun, X.; Lu, C.; Liu, Y.; Zhang, W.; Zhang, X. Melt-processed poly (vinyl alcohol) composites filled with microcrystalline cellulose from waste cotton fabrics. Carbohydr. Polym. 2014, 101, 642–649. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Zagorskaya, S.A. Determination of the degree of crystallinity of poly (vinyl alcohol) by FTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Fei, W.; Wu, Z.; Cheng, H.; Xiong, Y.; Chen, W.; Meng, L. Molecular mobility and morphology change of poly (vinyl alcohol)(PVA) film as induced by plasticizer glycerol. J. Polym. Sci. 2023, 61, 1959–1970. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, Y.; Tian, C.; Yu, Y.; Zou, C. Application of Polyvinyl Alcohol–Ethylene Glycol Hydrogel Technology for Removing Animal Glue in Book Restoration Based on Fluorescent Labeling Evaluation. Nanomaterials 2024, 14, 1878. https://doi.org/10.3390/nano14231878
Wang J, Xu Y, Tian C, Yu Y, Zou C. Application of Polyvinyl Alcohol–Ethylene Glycol Hydrogel Technology for Removing Animal Glue in Book Restoration Based on Fluorescent Labeling Evaluation. Nanomaterials. 2024; 14(23):1878. https://doi.org/10.3390/nano14231878
Chicago/Turabian StyleWang, Jia, Yuting Xu, Canxin Tian, Yunjiang Yu, and Changwei Zou. 2024. "Application of Polyvinyl Alcohol–Ethylene Glycol Hydrogel Technology for Removing Animal Glue in Book Restoration Based on Fluorescent Labeling Evaluation" Nanomaterials 14, no. 23: 1878. https://doi.org/10.3390/nano14231878
APA StyleWang, J., Xu, Y., Tian, C., Yu, Y., & Zou, C. (2024). Application of Polyvinyl Alcohol–Ethylene Glycol Hydrogel Technology for Removing Animal Glue in Book Restoration Based on Fluorescent Labeling Evaluation. Nanomaterials, 14(23), 1878. https://doi.org/10.3390/nano14231878




