Exploring Gluconamide-Modified Silica Nanoparticles of Different Sizes as Effective Carriers for Antimicrobial Photodynamic Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silica Nanoparticle Synthesis
2.3. Structural and Chemical Characterization
2.4. Photophysical Characterization
2.5. Antibacterial Activity
2.6. Bacteria Imaging
3. Results and Discussion
3.1. Silica Nanoparticles Characterization
3.2. Silica Nanoparticles Postfunctionalization
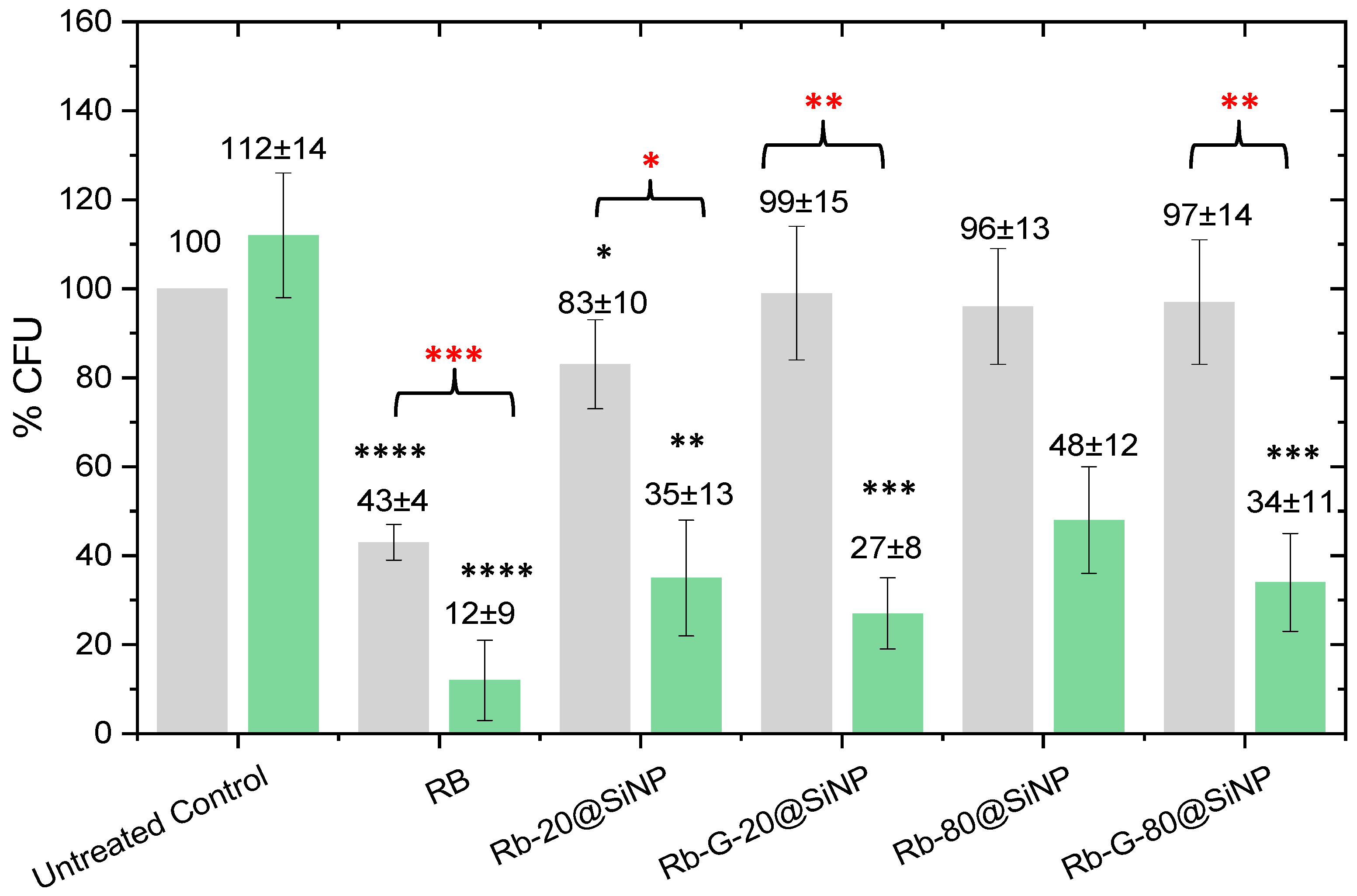
3.3. Antimicrobial Studies with Gram-Negative Bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Songca, S.P.; Adjei, Y. Applications of Antimicrobial Photodynamic Therapy against Bacterial Biofilms. Int. J. Mol. Sci. 2022, 23, 3209. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ran, B.; Wang, Z.; Cai, W.; Ran, L.; Xia, W.; Liu, W.; Peng, X. Organic Photo-antimicrobials: Principles, Molecule Design, and Applications. J. Am. Chem. Soc. 2021, 143, 17891–17909. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Drug-Resistant Infections; World Bank: Washington, DC, USA, 2017.
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.-A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Jørgensen, P.S.; Wernli, D.; Carroll, S.P.; Dunn, R.R.; Harbarth, S.; Levin, S.A.; So, A.D.; Schlüter, M.; Laxminarayan, R. Use antimicrobials wisely. Nature 2016, 537, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.M.; Vaubourgeix, J.; Nathan, C. Biology of antimicrobial resistance and approaches to combat it. Sci. Transl. Med. 2020, 12, eaaz6992. [Google Scholar] [CrossRef]
- Yin, R.; Hamblin, M. Antimicrobial Photosensitizers: Drug Discovery Under the Spotlight. Curr. Med. Chem. 2015, 22, 2159–2185. [Google Scholar] [CrossRef]
- Mula, S.; Koli, M. Helical BODIPY Dyes as Heavy-Atom-Free Triplet Photosensitizers for Photodynamic Therapy of Cancer. ChemMedChem 2024, 19, e202400041. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wan, M.T. Current evidence and applications of photodynamic therapy in dermatology. Clin. Cosmet. Investig. Dermatol. 2014, 7, 145–163. [Google Scholar] [CrossRef]
- Moghissi, K.; Dixon, K.; Gibbins, S. A Surgical View of Photodynamic Therapy in Oncology: A Review. Surg. J. 2015, 1, e1–e15. [Google Scholar]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent advances in photosensitizers as multifunctional theranostic agents for imaging-guided photodynamic therapy of cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef] [PubMed]
- Triesscheijn, M.; Baas, P.; Schellens, J.H.M.; Stewart, F.A. Photodynamic Therapy in Oncology. Oncologist 2006, 11, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- DeRosa, M.; Crutchley, R. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagn. Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Sola-Llano, R.; Agarrabeitia, A.R.; García-Fresnadillo, D.; López-Arbeloa, I.; Villanueva, A.; Ortiz, M.J.; Moya, S.; Martínez-Martínez, V. Exploring BODIPY Derivatives as Singlet Oxygen Photosensitizers for PDT. Photochem. Photobiol. 2020, 96, 458–477. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, M.; Li, C.; Liu, G.; Sun, Q.; Luo, X.; Wu, F. Single molecular-based nanoparticles with aggregation-induced emission characteristics for fluorescence imaging and efficient cancer phototherapy. Dye. Pigment. 2021, 187, 109130–109137. [Google Scholar] [CrossRef]
- Qiao, Y.; Geng, H.; Jiang, N.; Zhu, X.; Li, C.; Cai, Q. Polymyxin B–modified upconversion nanoparticles for selective detection of Gram-negative bacteria such as Escherichia coli. J. Chem. Res. 2020, 44, 756–761. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Medaglia, S.; Candela-Noguera, V.; Tormo-Mas, M.Á.; Marcos, M.D.; Aznar, E.; Deveci, H.; Martínez-Máñez, R. Antibacterial Activity of Linezolid against Gram-Negative Bacteria: Utilization of ε-Poly-l-Lysine Capped Silica Xerogel as an Activating Carrier. Pharmaceutics 2020, 12, 1126. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Kabanov, V.; Press, D.J.; Huynh, R.P.S.; Shimizu, G.K.H.; Heyne, B. Assessment of encapsulated dyes’ distribution in silica nanoparticles and their ability to release useful singlet oxygen. Chem. Commun. 2018, 54, 6320–6323. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Arbeloa, T.; Martínez-Martínez, V. Photosensitizer-Mesoporous Silica Nanoparticles Combination for Enhanced Photodynamic Therapy (PDT). Photochem. Photobiol. 2023, 99, 882–900. [Google Scholar] [CrossRef]
- Abdel Gaber, S.A.; Stepp, H.; Abdel Kader, M.H.; Lindén, M. Mesoporous silica nanoparticles boost aggressive cancer response to hydrophilic chlorin e6-mediated photodynamic therapy. Cancer Nanotechnol. 2023, 14, 67. [Google Scholar] [CrossRef]
- Mangalath, S.; Saneesh Babu, P.S.; Nair, R.R.; Manu, P.M.; Krishna, S.; Nair, S.A.; Joseph, J. Graphene Quantum Dots Decorated with Boron Dipyrromethene Dye Derivatives for Photodynamic Therapy. ACS Appl. Nano Mater. 2021, 4, 4162–4171. [Google Scholar] [CrossRef]
- Simões, J.C.S.; Sarpaki, S.; Papadimitroulas, P.; Therrien, B.; Loudos, G. Conjugated Photosensitizers for Imaging and PDT in Cancer Research. J. Med. Chem. 2020, 63, 14119–14150. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 6, e10246. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, 106818. [Google Scholar] [CrossRef] [PubMed]
- Aflakian, F.; Mirzavi, F.; Aiyelabegan, H.T.; Soleimani, A.; Gholizadeh Navashenaq, J.; Karimi-Sani, I.; Rafati Zomorodi, A.; Vakili-Ghartavol, R. Nanoparticles-based therapeutics for the management of bacterial infections: A special emphasis on FDA approved products and clinical trials. Eur. J. Pharm. Sci. 2023, 188, 106515. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Song, H.; Yu, C. Silica-Based Nanoparticles for Biomedical Applications: From Nanocarriers to Biomodulators. Acc. Chem. Res. 2020, 53, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef]
- Gubala, V.; Giovannini, G.; Kunc, F.; Monopoli, M.P.; Moore, C.J. Dye-doped silica nanoparticles: Synthesis, surface chemistry and bioapplications. Cancer Nanotechnol. 2020, 11, 1. [Google Scholar] [CrossRef]
- Shahabi, S.; Treccani, L.; Rezwan, K. A comparative study of three different synthesis routes for hydrophilic fluorophore-doped silica nanoparticles. J. Nanoparticle Res. 2016, 18, 28. [Google Scholar] [CrossRef]
- Pallavi, P.; Harini, K.; Alshehri, S.; Ghoneim, M.M.; Alshlowi, A.; Gowtham, P.; Girigoswami, K.; Shakeel, F.; Girigoswami, A. From Synthetic Route of Silica Nanoparticles to Theranostic Applications. Processes 2022, 10, 2595. [Google Scholar] [CrossRef]
- Ma, D.; Kell, A.J.; Tan, S.; Jakubek, Z.J.; Simard, B. Photophysical Properties of Dye-Doped Silica Nanoparticles Bearing Different Types of Dye−Silica Interactions. J. Phys. Chem. C 2009, 113, 15974–15981. [Google Scholar] [CrossRef][Green Version]
- Bouramtane, S.; Bretin, L.; Pinon, A.; Leger, D.; Liagre, B.; Richard, L.; Brégier, F.; Sol, V.; Chaleix, V. Porphyrin-xylan-coated silica nanoparticles for anticancer photodynamic therapy. Carbohydr. Polym. 2019, 213, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hong, X.; Zong, S.; Tang, C.; Cui, Y.; Zheng, Q. BODIPY-doped silica nanoparticles with reduced dye leakage and enhanced singlet oxygen generation. Sci. Rep. 2015, 5, 12602. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Watanabe, R.; Yokoi, T.; Kobayashi, E.; Otsuka, Y.; Shimojima, A.; Okubo, T.; Tatsumi, T. Extension of size of monodisperse silica nanospheres and their well-ordered assembly. J. Colloid Interface Sci. 2011, 360, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Capeletti, L.B.; de Oliveira, J.F.A.; Loiola, L.M.D.; Galdino, F.E.; da Silva Santos, D.E.; Soares, T.A.; de Oliveira Freitas, R.; Cardoso, M.B. Gram-Negative Bacteria Targeting Mediated by Carbohydrate–Carbohydrate Interactions Induced by Surface-Modified Nanoparticles. Adv. Funct. Mater. 2019, 29, 1904216. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Landry, M.P.; Moore, A.; Coreas, R. The protein corona from nanomedicine to environmental science. Nat. Rev. Mater. 2023, 8, 422–438. [Google Scholar] [CrossRef]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Åberg, C.; Dawson, K.A.; Salvati, A. Effects of the Presence or Absence of a Protein Corona on Silica Nanoparticle Uptake and Impact on Cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar] [CrossRef]
- Bañuelos Prieto, J.; López Arbeloa, F.; Martínez Martínez, V.; Arbeloa López, T.; López Arbeloa, I. Photophysical Properties of the Pyrromethene 597 Dye: Solvent Effect. J. Phys. Chem. A 2004, 108, 5503–5508. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Cajaraville, M.P.; Agarrabeitia, A.R.; Ortiz, M.J.; Martínez-Martínez, V. Functionalization of Photosensitized Silica Nanoparticles for Advanced Photodynamic Therapy of Cancer. Int. J. Mol. Sci. 2021, 22, 6618. [Google Scholar] [CrossRef]
- Prieto-Montero, R.; Tejón, M.; Albaya, A.; Arbeloa, T.; Chiara, J.L.; Fanarraga, M.L.; Martínez-Martínez, V. Targeted photodynamic therapy: Gluconamide-modified cellulose nanocrystals as efficient photosensitizer delivery platforms against Gram-negative bacteria. Carbohydr. Polym. 2024, 3, 122784. [Google Scholar] [CrossRef]
- Arulprakasajothi, M.; Elangovan, K.; Chandrasekhar, U.; Suresh, S. Performance study of conical strip inserts in tube heat exchanger using water based titanium oxide nanofluid. Therm. Sci. 2018, 22, 477–485. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.T.; Faria, M.; Crampin, E.J. Understanding Nano-Engineered Particle–Cell Interactions: Biological Insights from Mathematical Models. Nanoscale Adv. 2021, 3, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Atrash, M.; Hovor, I.; Gurianov, Y.; Barel, M.; Semenova, O.; Brider, T.; Nisnevitch, M.; Nakonechny, F. Antibacterial Properties of Rose Bengal Conjugated to Hyaluronic Acid. Int. J. Mol. Sci. 2024, 25, 3330. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, M.; Mitachi, K.; Yang, J.; Pershing, E.V.; Horowitz, B.D.; Wachter, E.A.; Lacey, J.W.; Ji, Y.; Rodrigues, D.J. Antibacterial Activity of Pharmaceutical-Grade Rose Bengal: An Application of a Synthetic Dye in Antibacterial Therapies. Molecules 2022, 27, 322. [Google Scholar] [CrossRef]
- Cui, Z.J.; Kanno, T. Photodynamic Triggering of Calcium Oscillation in the Isolated Rat Pancreatic Acini. J. Physiol. 1997, 504, 47–55. [Google Scholar] [CrossRef]



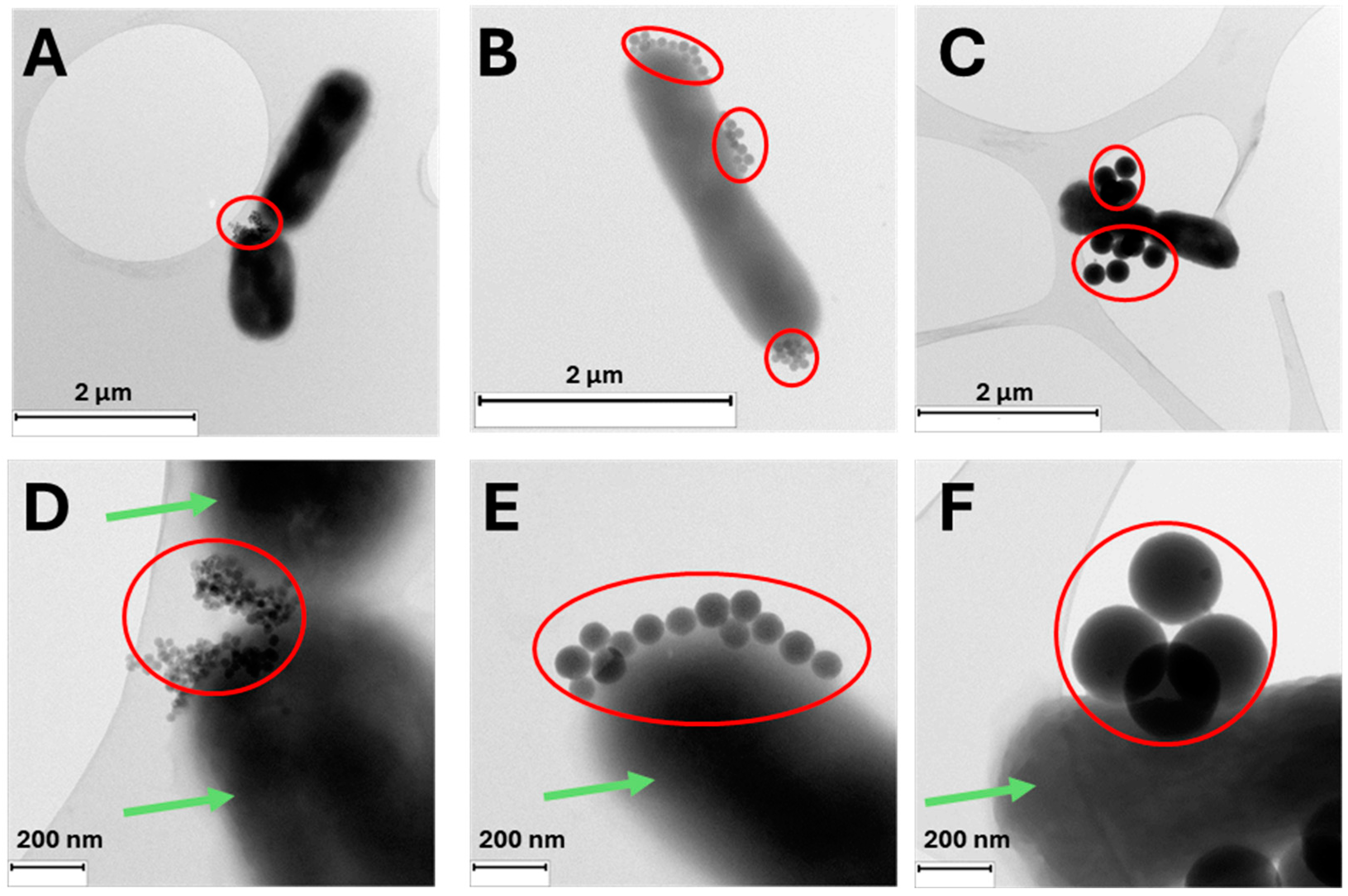
| Samples | TEM (nm) | DLS (nm) | PDI Index | ζ (mV) | XPS | ||
|---|---|---|---|---|---|---|---|
| %C | %O | %Si | |||||
| 20@SiNP | 21 ± 2 | 50 | 0.44 | −31 | 2.8 | 68.8 | 28.3 |
| 80@SiNP | 80 ± 5 | 100 | 0.21 | −33 | 3.1 | 63.0 | 33.9 |
| 250@SiNP | 260 ± 27 | 250 | 0.20 | −53 | 2.0 | 70.1 | 27.9 |
| Samples | DLS (nm) | PDI Index | ζ (mV) | (RB) μmol/g |
|---|---|---|---|---|
| RB-20@SiNP | 41 | 0.37 | −22 | 7.0 |
| RB-G-20@SiNP | 45 | 0.44 | −29 | 7.0 |
| RB-80@SiNP | 81 | 0.14 | −23 | 2.1 |
| RB-G-80@SiNP | 83 | 0.25 | −40 | 2.1 |
| RB-250@SiNP | 261 | 0.17 | −44 | 0.8 |
| RB-G-250@SiNP | 280 | 0.25 | −46 | 0.8 |
| Samples | λab (nm) | λfl (nm) | Φfl | Φ∆ |
|---|---|---|---|---|
| RB | 556 | 578 | 0.10 | 0.86 |
| RB-G-20@SiNP | 557 | 572 | 0.07 | 0.84 |
| RB-G-80@SiNP | 557 | 573 | 0.05 | 0.76 |
| RB-G-250@SiNP | 557 | 576 | 0.02 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto-Montero, R.; Herrera, L.; Tejón, M.; Albaya, A.; Chiara, J.L.; Fanarraga, M.L.; Martínez-Martínez, V. Exploring Gluconamide-Modified Silica Nanoparticles of Different Sizes as Effective Carriers for Antimicrobial Photodynamic Therapy. Nanomaterials 2024, 14, 1982. https://doi.org/10.3390/nano14241982
Prieto-Montero R, Herrera L, Tejón M, Albaya A, Chiara JL, Fanarraga ML, Martínez-Martínez V. Exploring Gluconamide-Modified Silica Nanoparticles of Different Sizes as Effective Carriers for Antimicrobial Photodynamic Therapy. Nanomaterials. 2024; 14(24):1982. https://doi.org/10.3390/nano14241982
Chicago/Turabian StylePrieto-Montero, Ruth, Lucia Herrera, Maite Tejón, Andrea Albaya, Jose Luis Chiara, Mónica L. Fanarraga, and Virginia Martínez-Martínez. 2024. "Exploring Gluconamide-Modified Silica Nanoparticles of Different Sizes as Effective Carriers for Antimicrobial Photodynamic Therapy" Nanomaterials 14, no. 24: 1982. https://doi.org/10.3390/nano14241982
APA StylePrieto-Montero, R., Herrera, L., Tejón, M., Albaya, A., Chiara, J. L., Fanarraga, M. L., & Martínez-Martínez, V. (2024). Exploring Gluconamide-Modified Silica Nanoparticles of Different Sizes as Effective Carriers for Antimicrobial Photodynamic Therapy. Nanomaterials, 14(24), 1982. https://doi.org/10.3390/nano14241982






