Defect-Mediated Energy Transfer Mechanism by Modulating Lattice Occupancy of Alkali Ions for the Optimization of Upconversion Luminescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
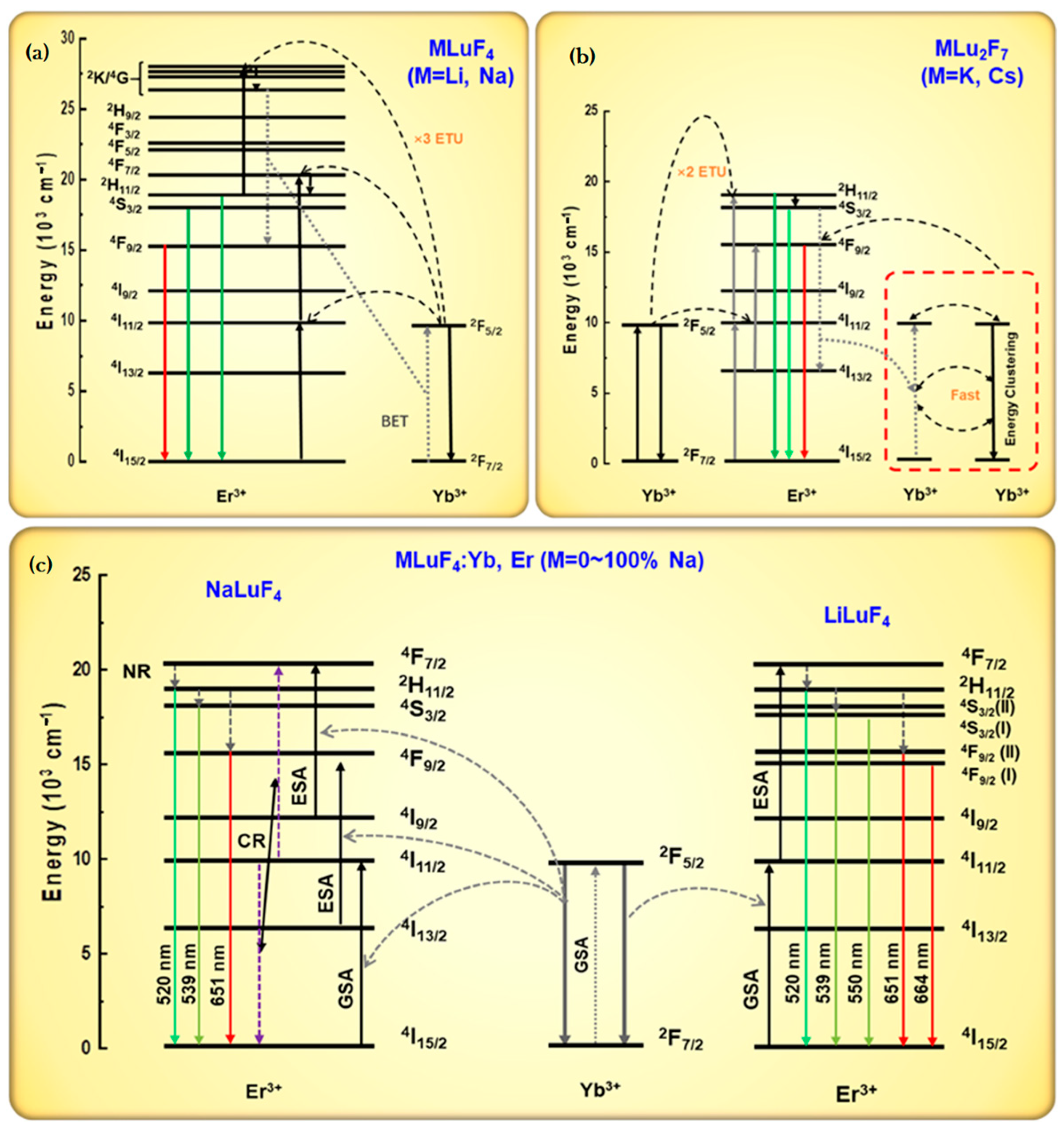
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Auzel, F. Upconversion and Anti-Stokes Processes with f and d Ions in Solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.R.; Kyhm, J.H.; Kang, G.; Jang, H.S. Orthogonal R/G/B Upconversion Luminescence-based Full-Color Tunable Upconversion Nanophosphors for Transparent Displays. Nano Lett. 2021, 21, 4838–4844. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Qin, F.; Chen, R.; Huang, W.; Hong, M.; Liu, X. Temporal full-colour tuning through non-steady-state upconversion highly cited paper. Nat. Nanotechnol. 2015, 10, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Z.; Sun, X.; Ji, J.; Liu, L.; Gu, Z.; Wang, G.F. Graphene quantum dot-rare earth upconversion nanocages with extremely high efficiency of upconversion luminescence, stability and drug loading towards controlled delivery and cancer theranostics. Chem. Eng. J. 2020, 382, 122992. [Google Scholar]
- Zhang, Y.; Zhu, X.; Zhang, Y. Exploring Heterostructured Upconversion Nanoparticles: From Rational Engineering to Diverse Applications. ACS Nano 2021, 15, 3709–3735. [Google Scholar] [CrossRef]
- Tong, N.B.; Van Si, L.; Dung, C.T.M.; Hanh, T.T.K.; Lam, T.T.N.; Thang, P.B.; Binh, D.H.; Uyen, N.T.N.; Van, T.T.T. Intense green upconversion in core-shell structured NaYF4: Er, Yb@SiO2 microparticles for anti-counterfeiting printing. Ceram. Int. 2023, 49, 28484–28491. [Google Scholar] [CrossRef]
- Saidi, K.; Hernández-Álvarez, C.; Runowski, M.; Dammak, M.; Rafael Martín Benenzuela, I. Temperature and Pressure Sensing Using an Optical Platform Based on Upconversion Luminescence in NaSrY(MoO4)3 Codoped with Er3+ and Yb3+ Nanophosphors. ACS Appl. Nano Mater. 2023, 6, 19431–19442. [Google Scholar] [CrossRef]
- Liu, X.; Liu, M.; Chen, J.; Li, Z.; Yuan, Q. Rational design and biomedical applications of DNA-functionalized upconversion nanoparticles. Chin. Chem. Lett. 2018, 29, 1321–1332. [Google Scholar] [CrossRef]
- Chen, J.; Liang, L.; Tan, S.; Xi, S.; Lin, C.H.; Wu, T.; He, Q.; Liu, X. Volumetric Nanocrystal Lattice Reconstruction through Dynamic Metal Complex Docking. Nano Lett. 2023, 23, 7221–7227. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Zhang, X.; Zhang, W.; Yu, Z.; Ling, B.; Yang, X.; Zhang, Y. Artificial Atomic Vacancies Tailor Near-Infrared II Excited Multiplexing Upconversion in Core-Shell Lanthanide Nanoparticles. Nano Lett. 2020, 20, 5236–5242. [Google Scholar] [CrossRef]
- Xu, J.; Yang, D.; Han, W.; Dong, S.; Jia, T.; He, F.; Bi, H.; Gai, S.; Li, L.; Yang, P. A novel strategy for markedly enhancing the red upconversion emission in Er3+/Tm3+ cooperated nanoparticles. J. Mater. Chem. C 2018, 6, 7533–7540. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X. First-principles calculations of strain engineering in NaYF4-based nanocrystals with hydroxyl impurities. Nanoscale 2021, 13, 19561–19567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Q.; Zhao, X.; Luo, X.; Wong, C.P.Y.; Wang, J.; Wan, D.; Venkatesan, T.; Pennycook, S.J.; Loh, K.P.; et al. Photoluminescence Upconversion by Defects in Hexagonal Boron Nitride. Nano Lett. 2018, 18, 6898–6905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Low, J.; Bai, Y.; Bai, Y.; Jiang, Y.; Wang, H.; Liu, W.; Ma, Y.; Chen, Y.; Long, R.; et al. Multilayer core-shell nanostructures for enhanced 808 nm responsive upconversion. Chin. Chem. Lett. 2022, 33, 1087–1090. [Google Scholar] [CrossRef]
- Fan, S.; Gao, G.; Sun, S.; Fan, S.; Sun, H.; Hu, L. Absolute up-conversion quantum efficiency reaching 4% in -NaYF4:Yb3+, Er3+ micro-cylinders achieved by Li+/Na+ ion-exchange. J. Mater. Chem. C 2018, 6, 5453–5461. [Google Scholar] [CrossRef]
- Lai, J.; Zhang, Y.; Pasquale, N.; Lee, K.B. An Upconversion Nanoparticle with Orthogonal Emissions Using Dual NIR Excitations for Controlled Two-Way Photoswitching. Angew. Chem. Int. Ed. 2014, 53, 14419–14423. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Hu, R.; Fang, Z.Q.; Shi, G.; Zhang, S.; Zhang, M. A multifunctional upconversion nanoparticles probe for Cu2+ sensing and pattern recognition of biothiols. Chin. Chem. Lett. 2022, 33, 3782–3786. [Google Scholar] [CrossRef]
- Ramasamy, P.; Chandra, P.; Rhee, S.W.; Kim, J. Enhanced upconversion luminescence in NaGdF4: Yb,Er nanocrystals by Fe3+ doping and their application in bioimaging. Nanoscale 2013, 5, 8711–8717. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, J.; Yue, J.; Wei, Y.; Gao, C.; Xie, X.; Huang, L. Recent Development in Sensitizers for Lanthanide-Doped Upconversion Luminescence. Chem. Rev. 2022, 122, 15998–16050. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.D.; Feng, W.; Gu, Y.; Li, F.; Yan, C.H. Versatile Spectral and Lifetime Multiplexing Nanoplatform with Excitation Orthogonalized Upconversion Luminescence. ACS Nano 2017, 11, 3289–3297. [Google Scholar] [CrossRef]
- Wang, Y.F.; Liu, G.Y.; Sun, L.D.; Xiao, J.W.; Zhou, J.C.; Yan, C.H. Nd3+-Sensitized Upconversion Nanophosphors: Efficient In Vivo Bioimaging Probes with Minimized Heating Effect. ACS Nano 2013, 7, 7200–7206. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Zhang, Y. Tuning of the Structure and Emission Spectra of Upconversion Nanocrystals by Alkali Ion Doping. Langmuir 2011, 27, 13236–13241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, J. Recent advances in energy transfer in bulk and nanoscale luminescent materials: From spectroscopy to applications. Chem. Soc. Rev. 2015, 44, 8714–8746. [Google Scholar] [CrossRef]
- Zhou, J.; Chizhik, A.I.; Chu, S.; Jin, D. Single-particle spectroscopy for functional nanomaterials. Nature 2020, 579, 41–50. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Nanoparticle-triggered in situ catalytic chemical reactions for tumour-specific therapy. Chem. Soc. Rev. 2018, 47, 1938–1958. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, X.; Wang, X.; Dong, H.; Sun, L.; Yan, C. Research Progress on Upconversion Emission Modulation of Rare Earth Nanocrystals. Chin. J. Lumin. 2023, 44, 1149–1166. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, T.; Gao, X.; Pan, W.; Li, N.; Tang, B. A highly selective and instantaneous upconversion fluorescent nanoprobe for ascorbic acid detection in biological samples. Chin. Chem. Lett. 2017, 28, 1983–1986. [Google Scholar] [CrossRef]
- Matulionyte, M.; Skripka, A.; Guerra, A.R.; Benayas, A.; Vetrone, F. The Coming of Age of Neodymium: Redefining Its Role in Rare Earth Doped Nanoparticles. Chem. Rev. 2023, 123, 515–554. [Google Scholar] [CrossRef]
- Han, S.; Deng, R.; Xie, X.; Liu, X. Enhancing Luminescence in Lanthanide-Doped Upconversion Nanoparticles highly cited paper. Angew. Chem. Int. Ed. 2014, 53, 11702–11715. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Wang, C.; Liu, Z.; Liu, X. Single-Band Upconversion Emission in Lanthanide-Doped KMnF3 Nanocrystals. Angew. Chem. Int. Ed. 2011, 123, 10553–10556. [Google Scholar] [CrossRef]
- Thakur, H.; Singh, S.; Gathania, A.K.; Singh, S.K.; Singh, R.K. Effect of Li+/K+/Zn2+doping on the optical and temperature sensing properties of Tm3+and Yb3+doped YVO4 phosphor. Ceram. Int. 2023, 49, 25935–25944. [Google Scholar] [CrossRef]
- Jiang, H.; Jia, H.; Zhang, Y.; Zhang, X.; Feng, Z.; Yuan, Y.; Chen, Z.; Hu, Y.; Peng, F.; Liu, X.; et al. High-performance dual-mode self-calibrating optical thermometry for Er3+, Li+ co-doped oxides. J. Mater. Chem. C 2022, 10, 17917–17924. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Zhang, X.; Yang, K.; Liu, L.; Song, Y. Optical temperature sensor through infrared excited blue upconversion emission in Tm3+/Yb3+ codoped Y2O3. Opt. Commun. 2012, 285, 1925–1928. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, M.; Li, J.; Li, F.; Cui, W.; Jiao, X.; Peng, Y.; Huang, Y.; Chen, L. Upconversion Properties and Temperature-Sensing Behaviors of Alkaline-Earth-Metal Scandate Nanocrystals Doped with Er3+/Yb3+Ions in the Presence of Alkali Ions (Li+, Na+, and K+). Inorg. Chem. 2022, 61, 5309–5317. [Google Scholar] [CrossRef]
- Zeng, J.H.; Su, J.; Li, Z.H.; Yan, R.X.; Li, Y.D. Synthesis and upconversion luminescence of hexagonal-phase NaYF4: Yb, Er3+, phosphors of controlled size and morphology. Adv. Mater. 2005, 17, 2119–2123. [Google Scholar] [CrossRef]
- Anderson, R.B.; Smith, S.J.; May, P.S.; Berry, M.T. Revisiting the NIR-to-Visible Upconversion Mechanism in β-NaYF4:Yb3+, Er3+. J. Phys. Chem. Lett. 2014, 5, 36–42. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.; Lei, P.; Xu, X.; Dong, L.; Guo, X.; Yan, X.; Wang, P.; Song, S.; Feng, J.; et al. Core-shell-shell heterostructures of α-NaLuF4: Yb/Er@NaLuF4: Yb@MF2 (M = Ca, Sr, Ba) with remarkably enhanced upconversion luminescence. Dalton Trans. 2016, 45, 11129–11136. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, J.; Qiu, J.; Song, Z. Comprehensive investigations of near infrared downshift and upconversion luminescence mechanisms in Yb3+ single-doped and Er3+, Yb3+ co-doped SiO2 inverse opals. Phys. Chem. Chem. Phys. 2017, 19, 31997–32006. [Google Scholar] [CrossRef]
- Liang, H.; Chen, G.; Liu, H.; Zhang, Z. Ultraviolet upconversion luminescence enhancement in Yb3+/Er3+-codoped Y2O3 nanocrystals induced by tridoping with Li+ ions. J. Lumin. 2009, 129, 197–202. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, H.; Jiang, Y.; Qi, G.; Chen, G.; Chen, J.; Sun, L. Tunable upconversion emission in Er3+/Yb3+ co-doped oxyfluoride glass ceramics containing NaYF4 nanocrystals by the incorporation of Li+ ions. J. Lumin. 2019, 214, 116524. [Google Scholar] [CrossRef]
- Fujii, M.; Nakano, T.; Imakita, K.; Hayashi, S. Upconversion Luminescence of Er and Yb Codoped NaYF4 Nanoparticles with Metal Shells. J. Phys. Chem. C 2013, 117, 1113–1120. [Google Scholar] [CrossRef]
- Qin, X.; Shen, L.; Liang, L.; Han, S.; Yi, Z.; Liu, X. Suppression of Defect-Induced Quenching via Chemical Potential Tuning: A Theoretical Solution for Enhancing Lanthanide Luminescence. J. Phys. Chem. C 2019, 123, 11151–11161. [Google Scholar] [CrossRef]
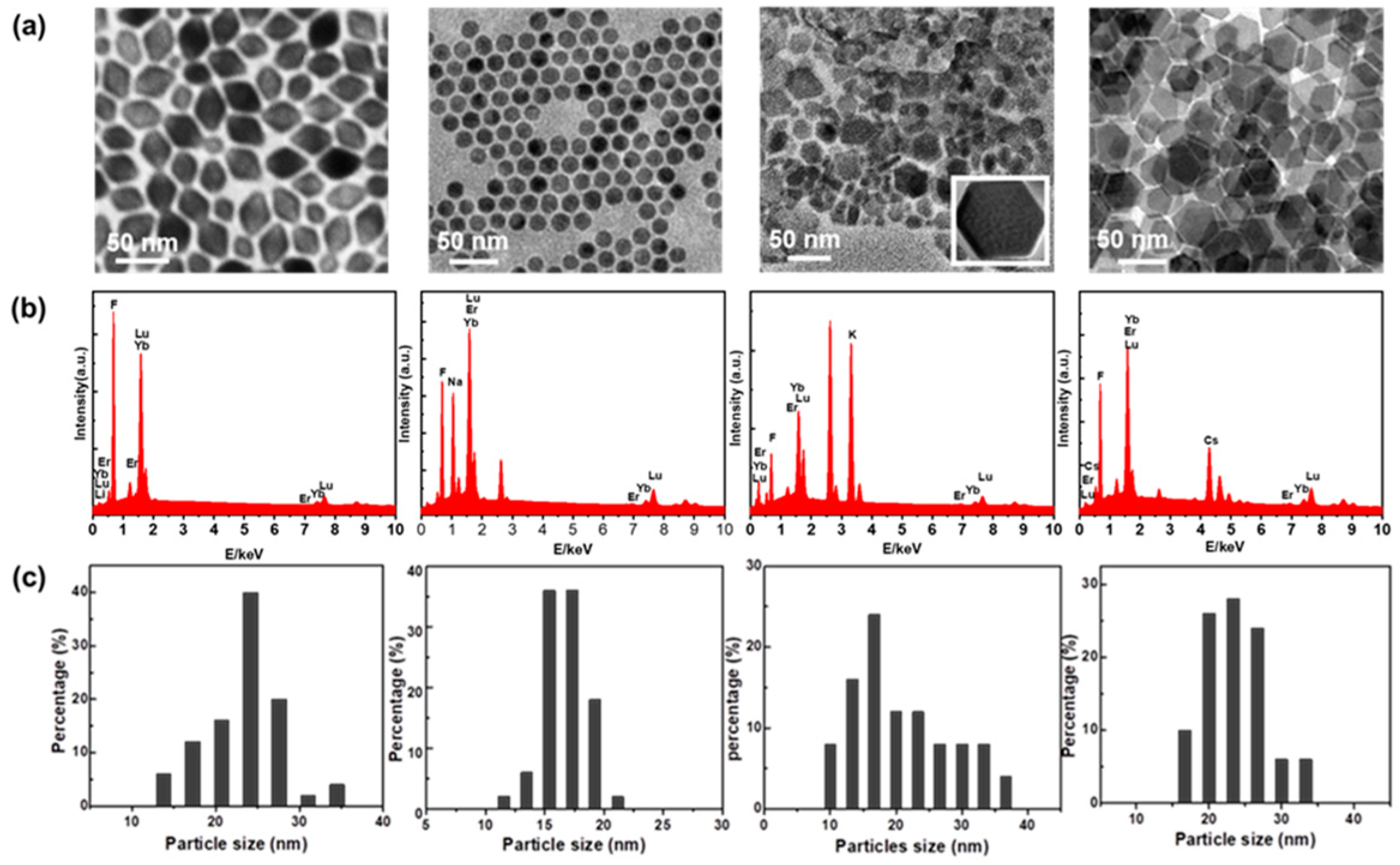
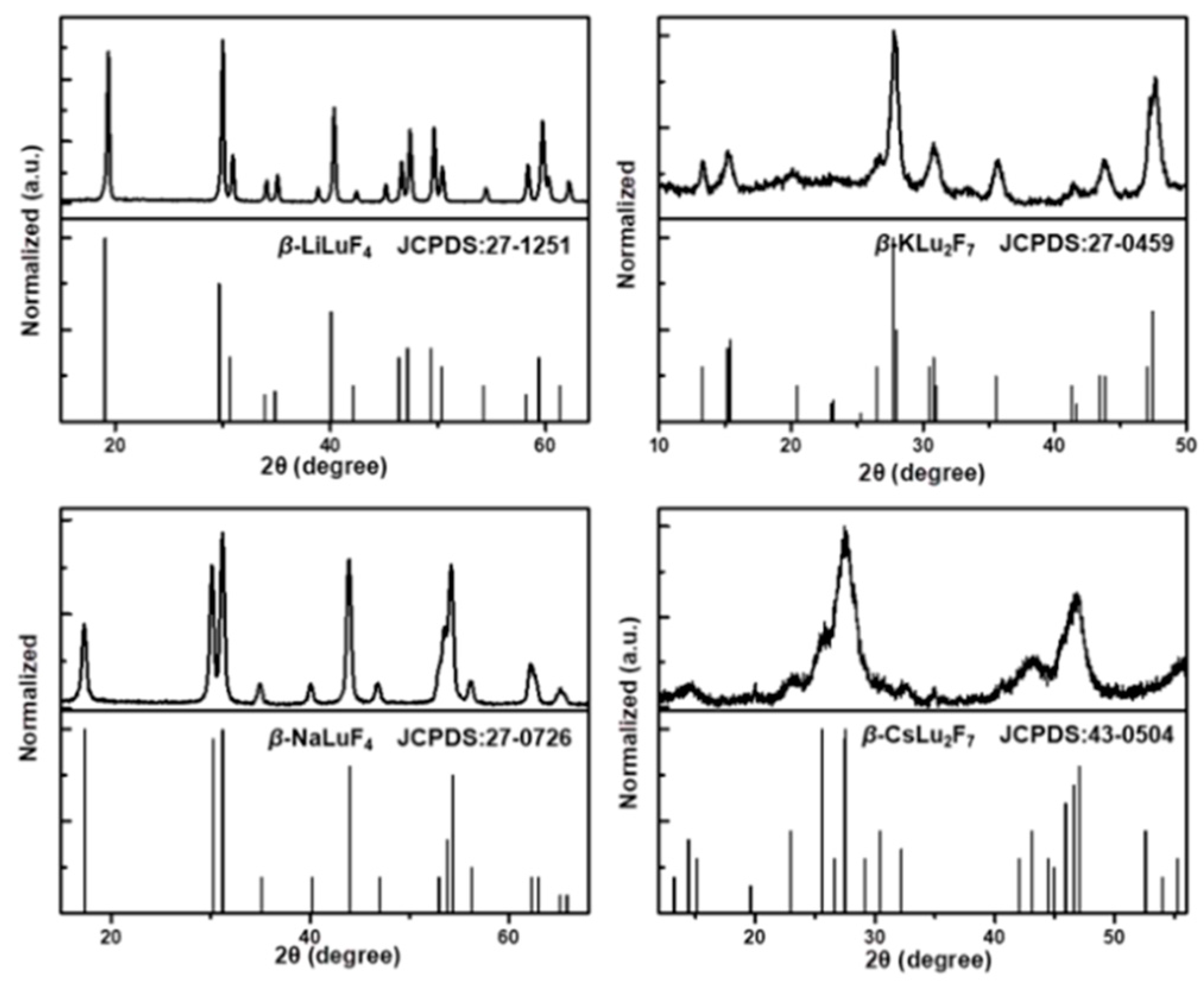
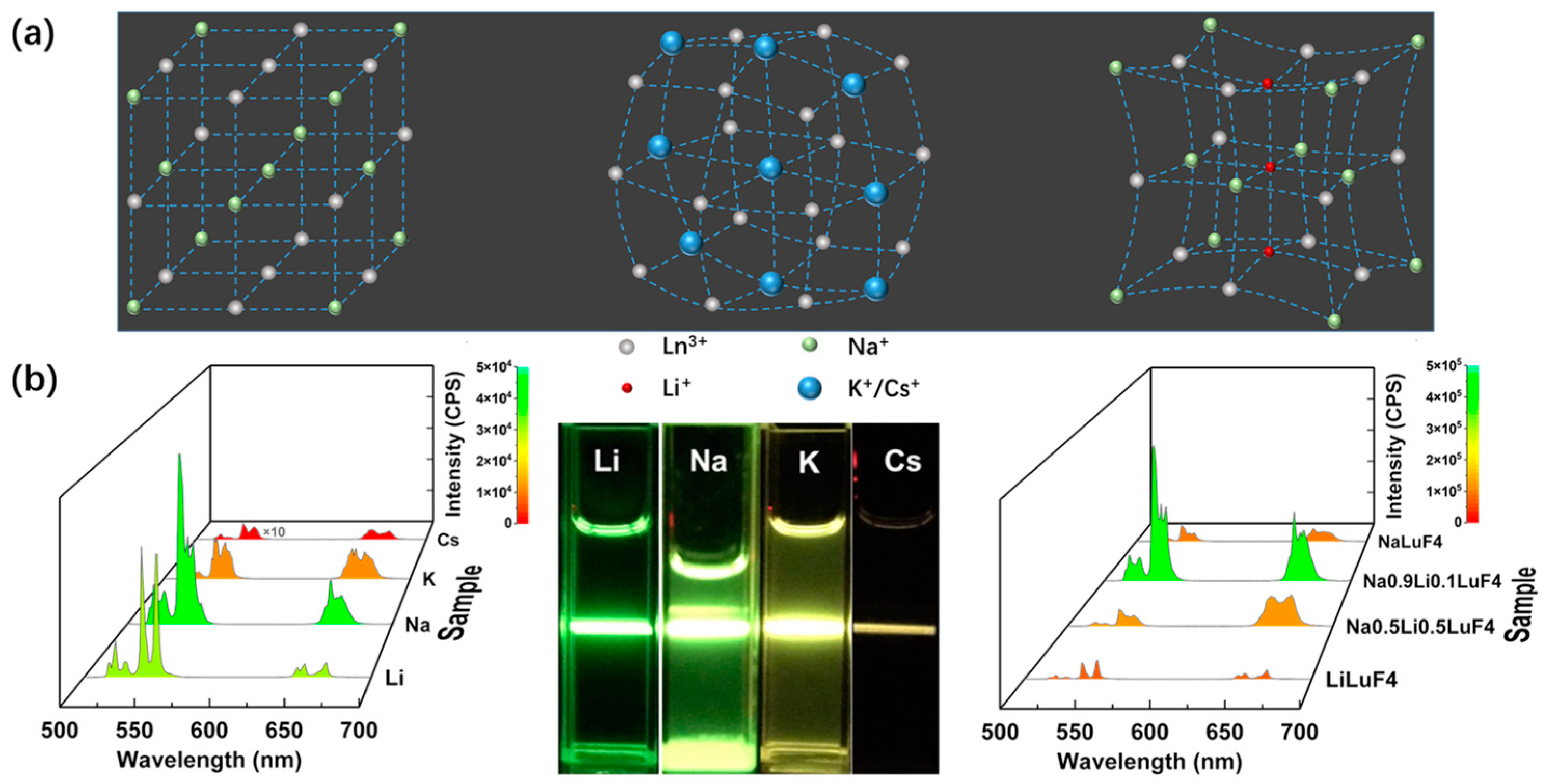
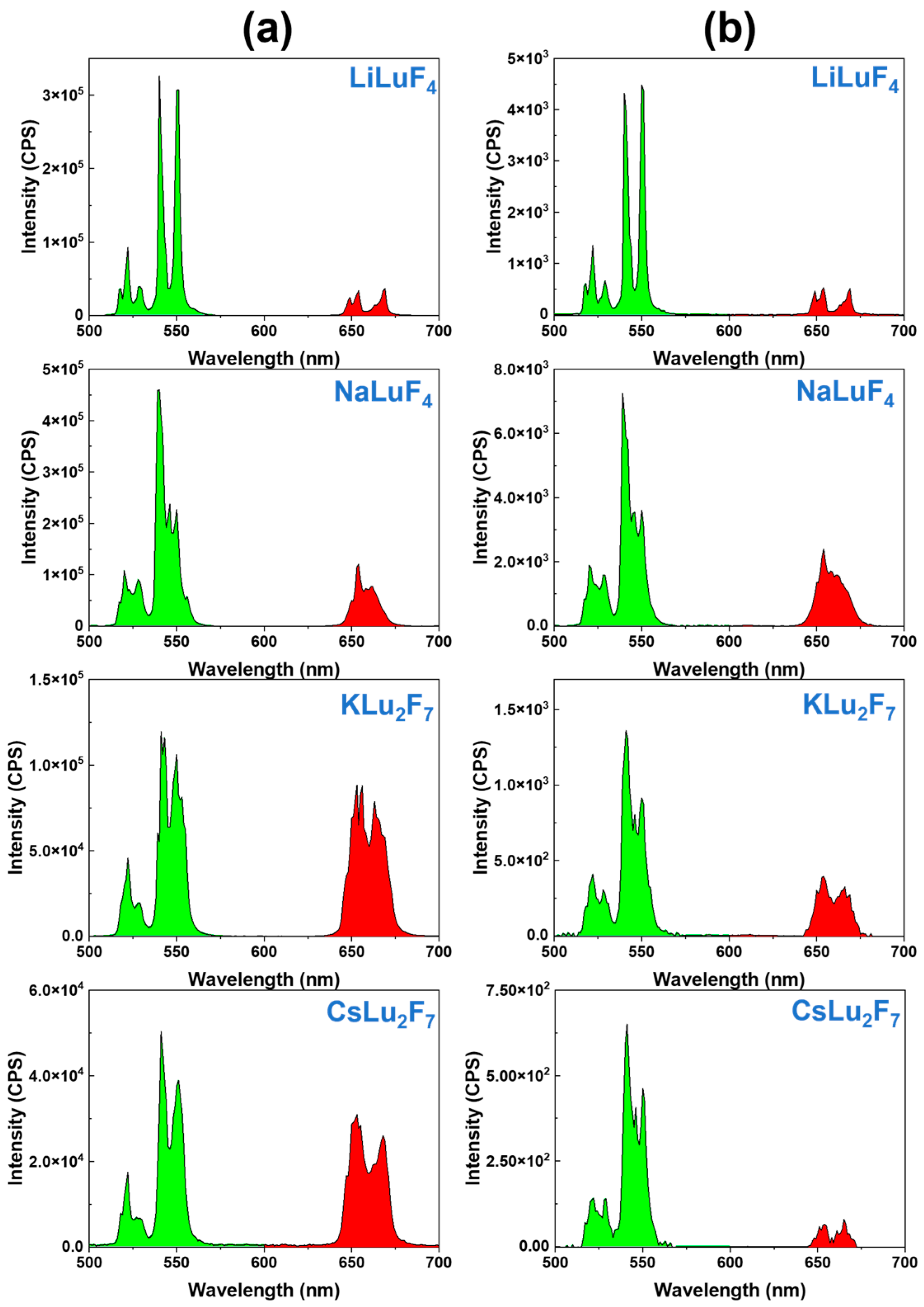
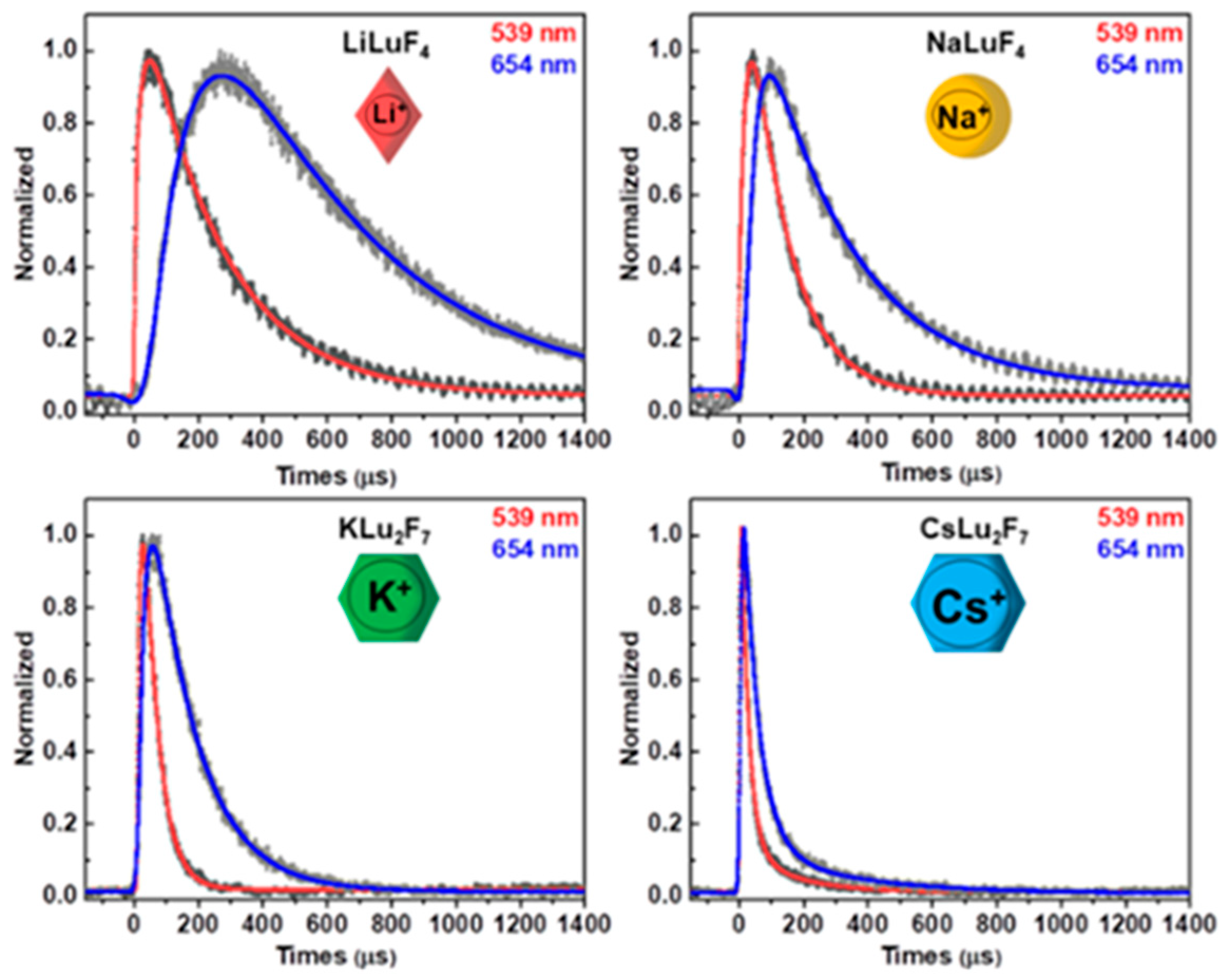
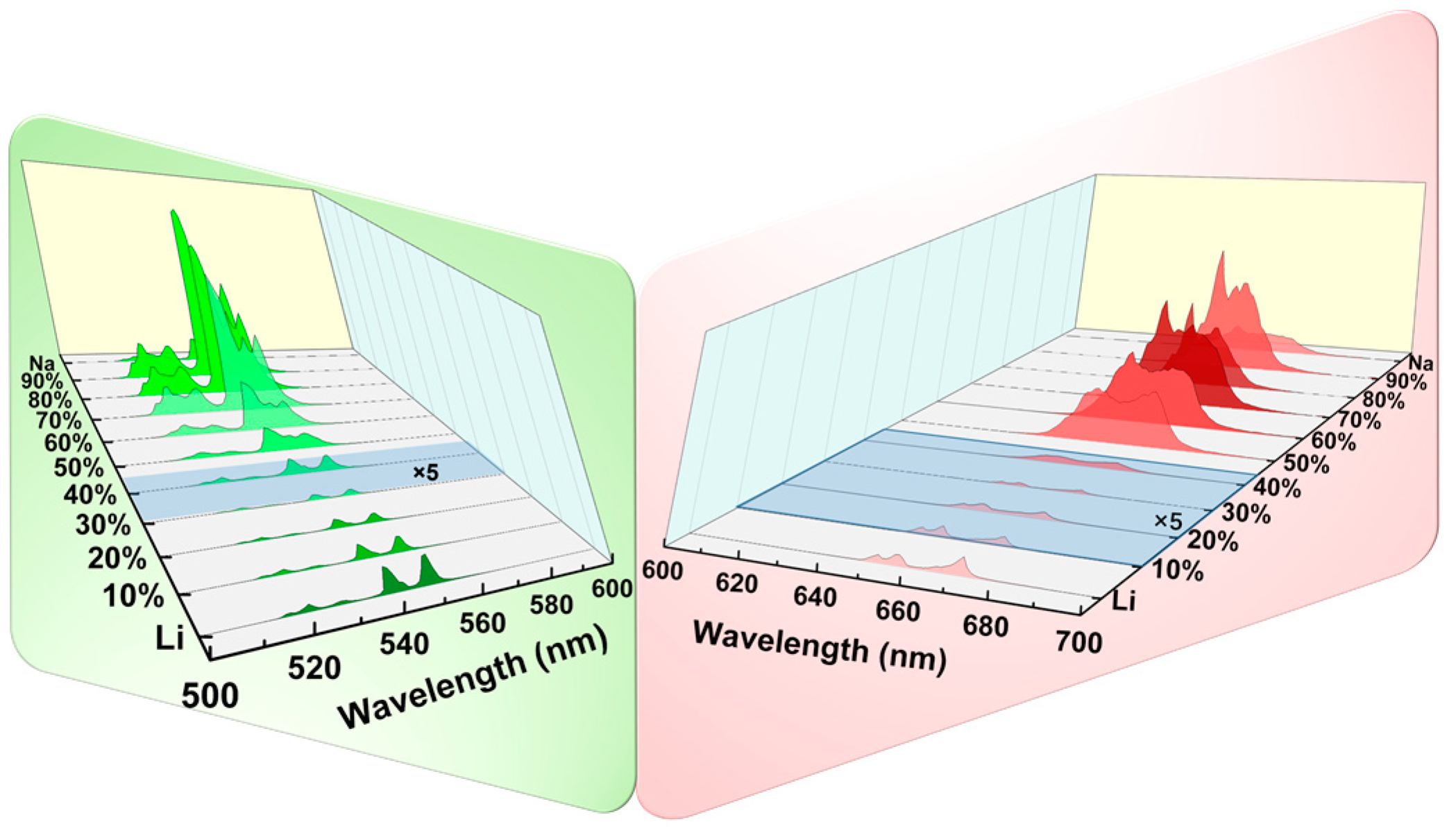
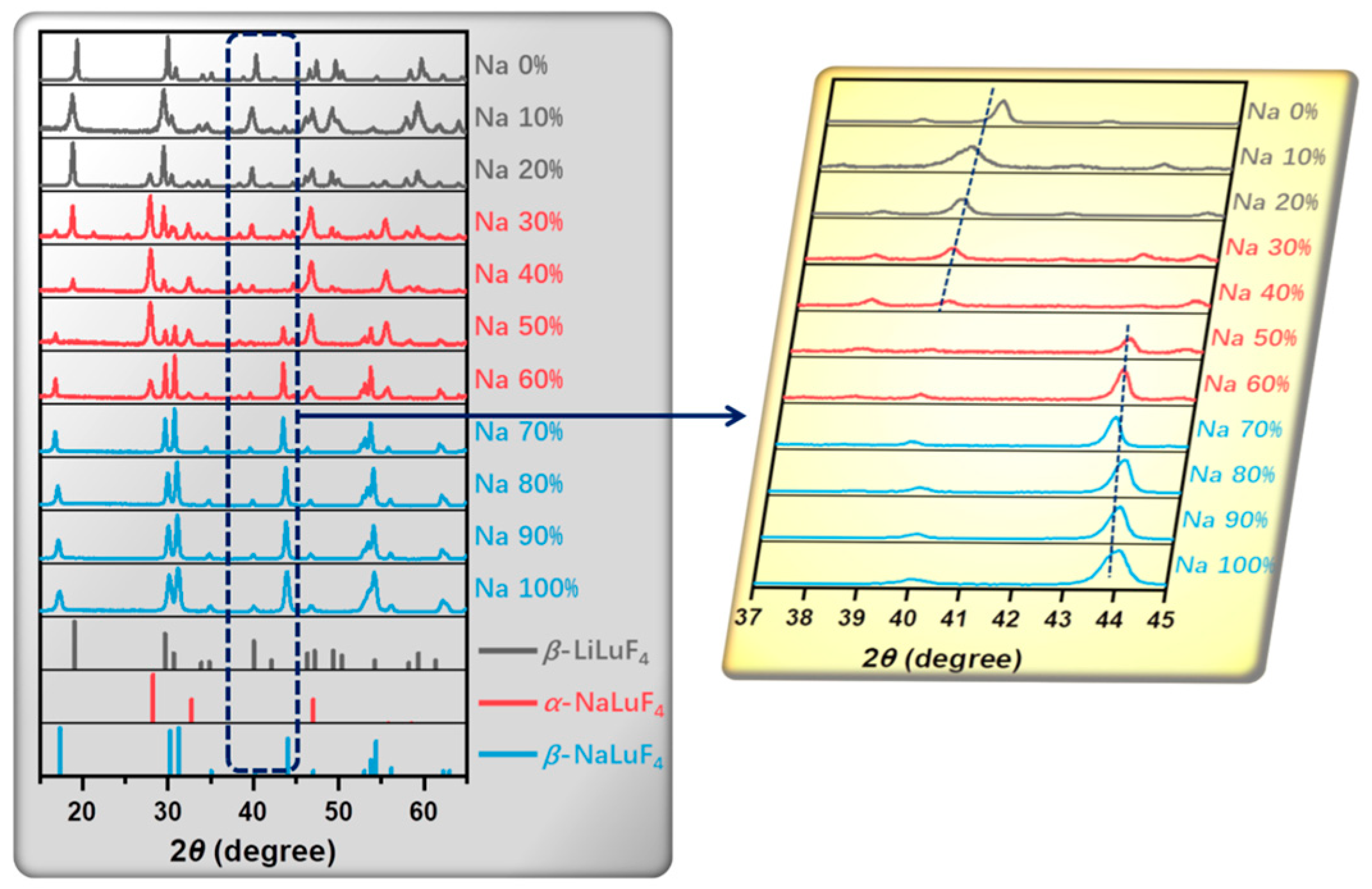
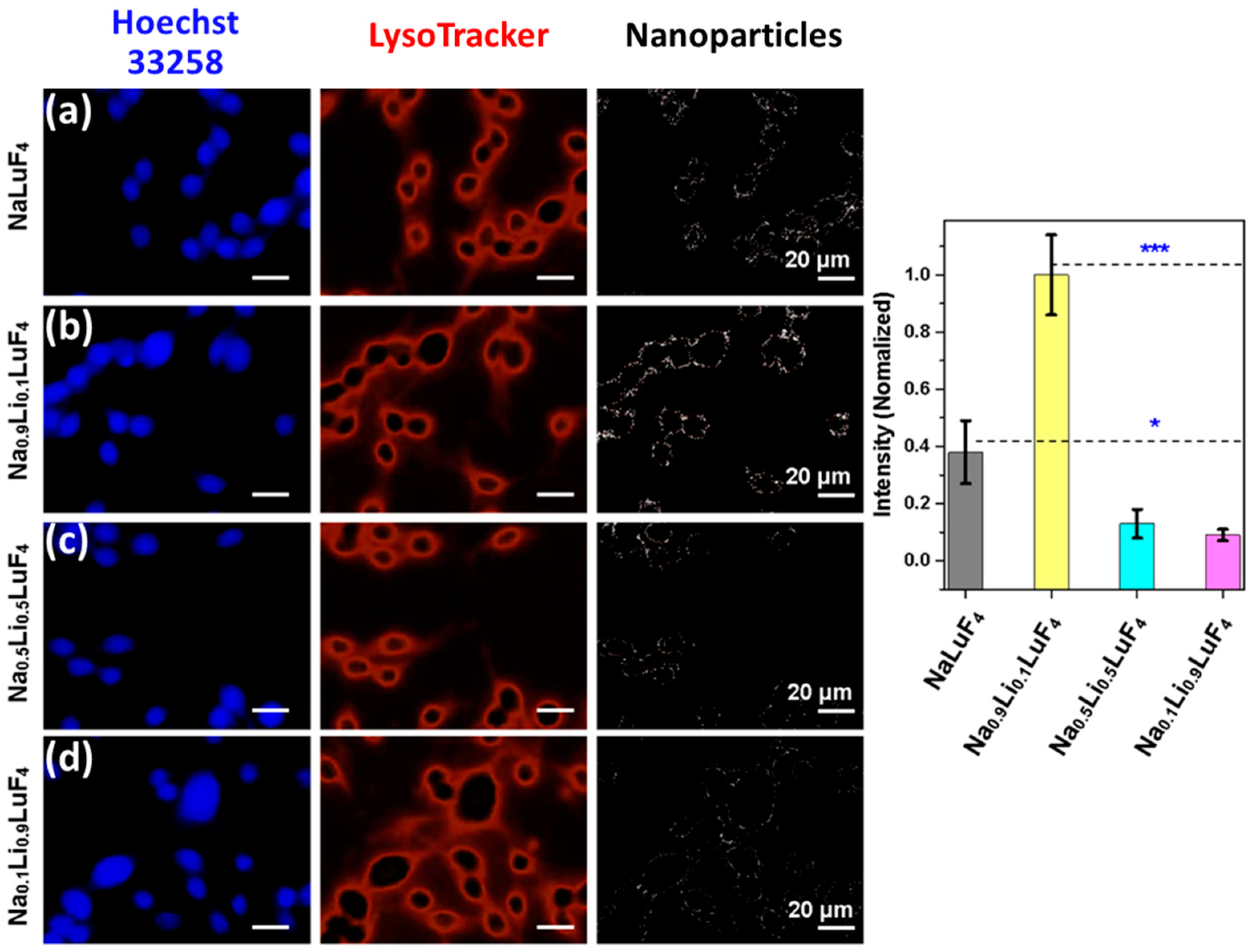
| Matrix | τ (μs) | 2H11/2 | 4S3/2 | 4F9/2 |
|---|---|---|---|---|
| LiLuF4 | τ1 | 17.97 | 22.99 | 121.74 |
| τ2 | 264.59 | 246.95 | 463.43 | |
| NaLuF4 | τ1 | 18.89 | 20.15 | 29.07 |
| τ2 | 125.90 | 121.96 | 283.73 | |
| KLu2F7 | τ1 | 6.21 | 10.57 | 8.59 |
| τ2 | 39.64 | 47.85 | 87.33 | |
| CsLu2F | τ1 | 1.61 | 1.05 | 0.95 |
| τ2 | 27.07 | 45.53 | 61.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Li, Y.; Zhang, Y.; Fu, L.; Li, L. Defect-Mediated Energy Transfer Mechanism by Modulating Lattice Occupancy of Alkali Ions for the Optimization of Upconversion Luminescence. Nanomaterials 2024, 14, 1969. https://doi.org/10.3390/nano14231969
Gao R, Li Y, Zhang Y, Fu L, Li L. Defect-Mediated Energy Transfer Mechanism by Modulating Lattice Occupancy of Alkali Ions for the Optimization of Upconversion Luminescence. Nanomaterials. 2024; 14(23):1969. https://doi.org/10.3390/nano14231969
Chicago/Turabian StyleGao, Rongyao, Yuqian Li, Yuhang Zhang, Limin Fu, and Luoyuan Li. 2024. "Defect-Mediated Energy Transfer Mechanism by Modulating Lattice Occupancy of Alkali Ions for the Optimization of Upconversion Luminescence" Nanomaterials 14, no. 23: 1969. https://doi.org/10.3390/nano14231969
APA StyleGao, R., Li, Y., Zhang, Y., Fu, L., & Li, L. (2024). Defect-Mediated Energy Transfer Mechanism by Modulating Lattice Occupancy of Alkali Ions for the Optimization of Upconversion Luminescence. Nanomaterials, 14(23), 1969. https://doi.org/10.3390/nano14231969







