Silver Nanoparticles and Simvastatin-Loaded PLGA-Coated Hydroxyapatite/Calcium Carbonate Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
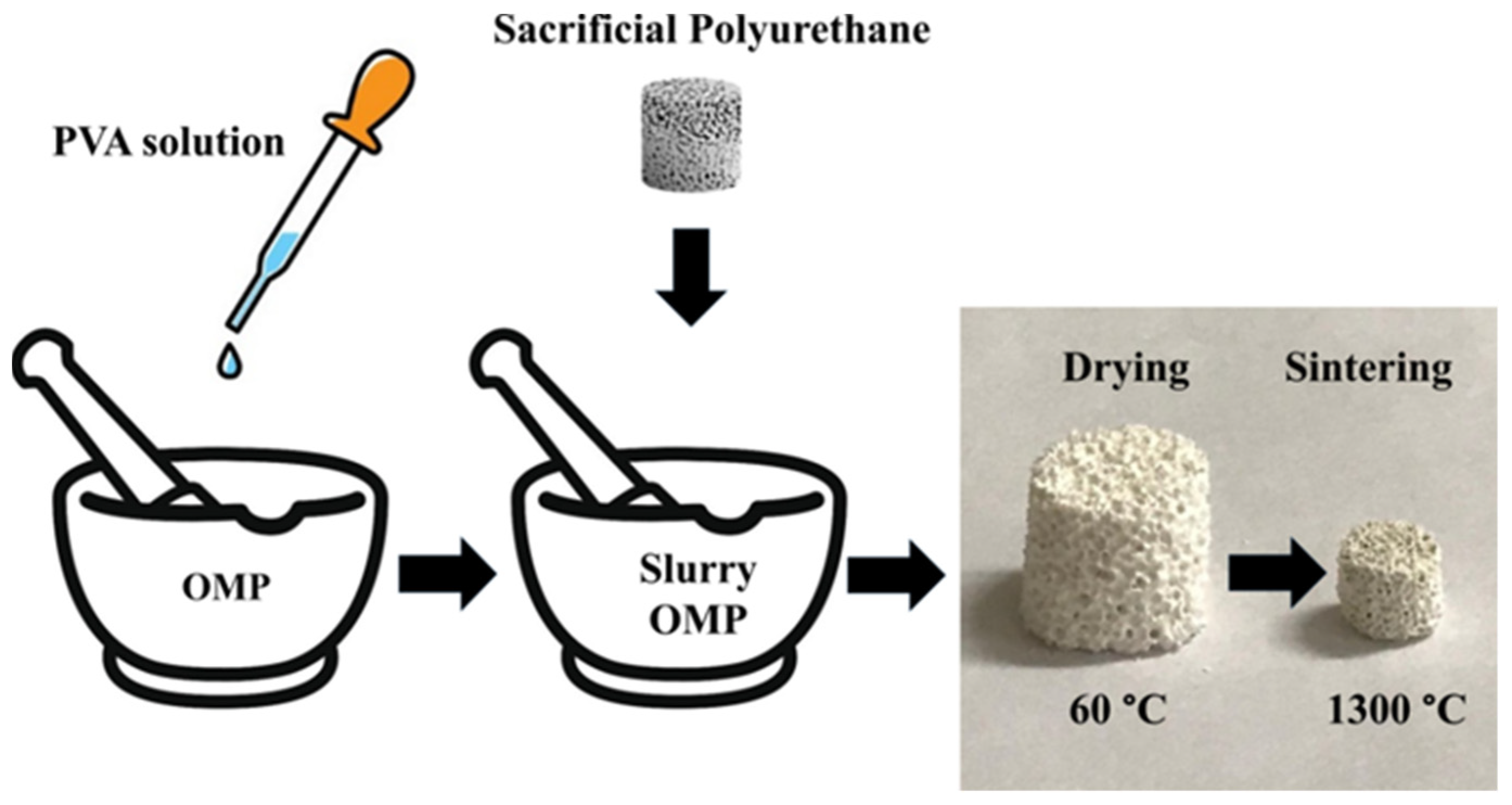
2.2. Preparation of Scaffold Based on OMP
2.3. Preparation of Ag@OMPs
2.4. Coating of OMPs, PMs, Ag@OMPs with PLGA
2.5. Coating of OMPs and Ag@OMPs with PLGA and SIMV
2.6. Bioactivity Tests in Simulated Body Fluid
2.7. In Vitro Silver and SIMV Release from PLGA/SIMV/Ag@OMPs
2.8. Microbial Strains
2.9. Antimicrobial Susceptibility Testing
2.10. Characterization
3. Results and Discussion
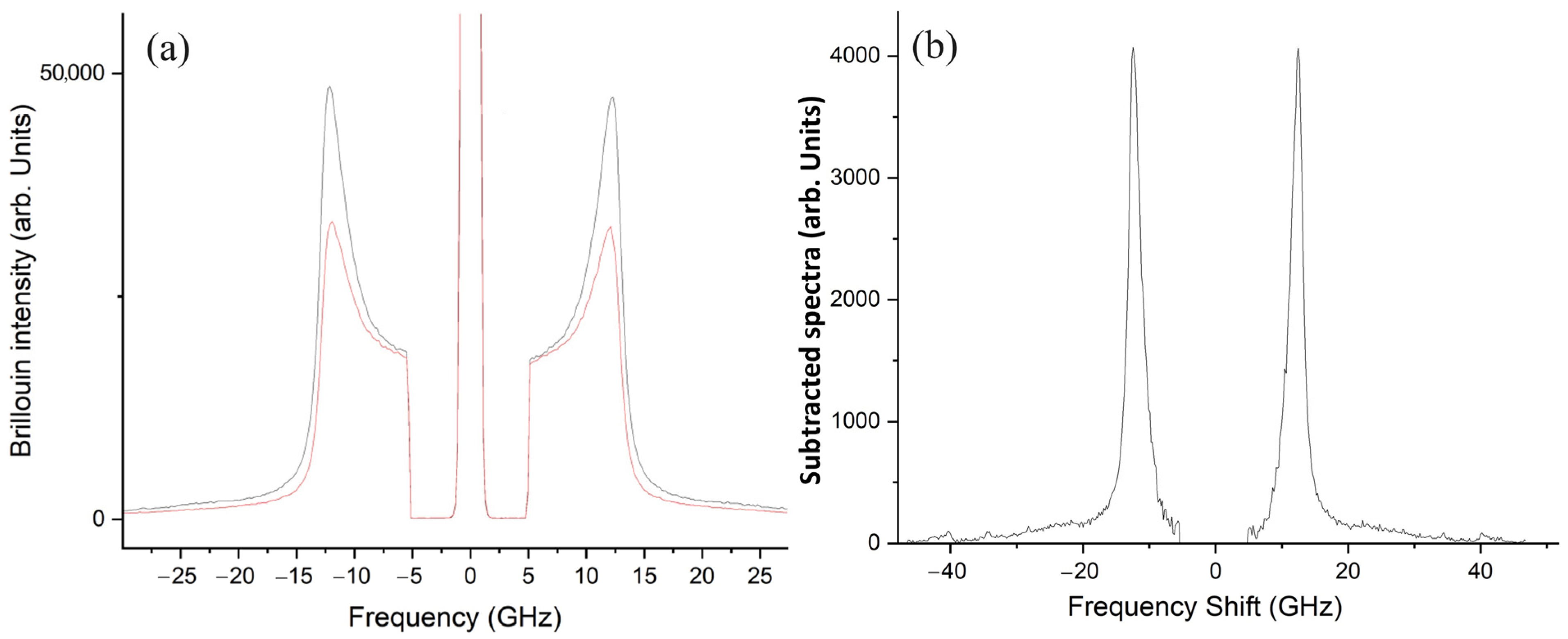
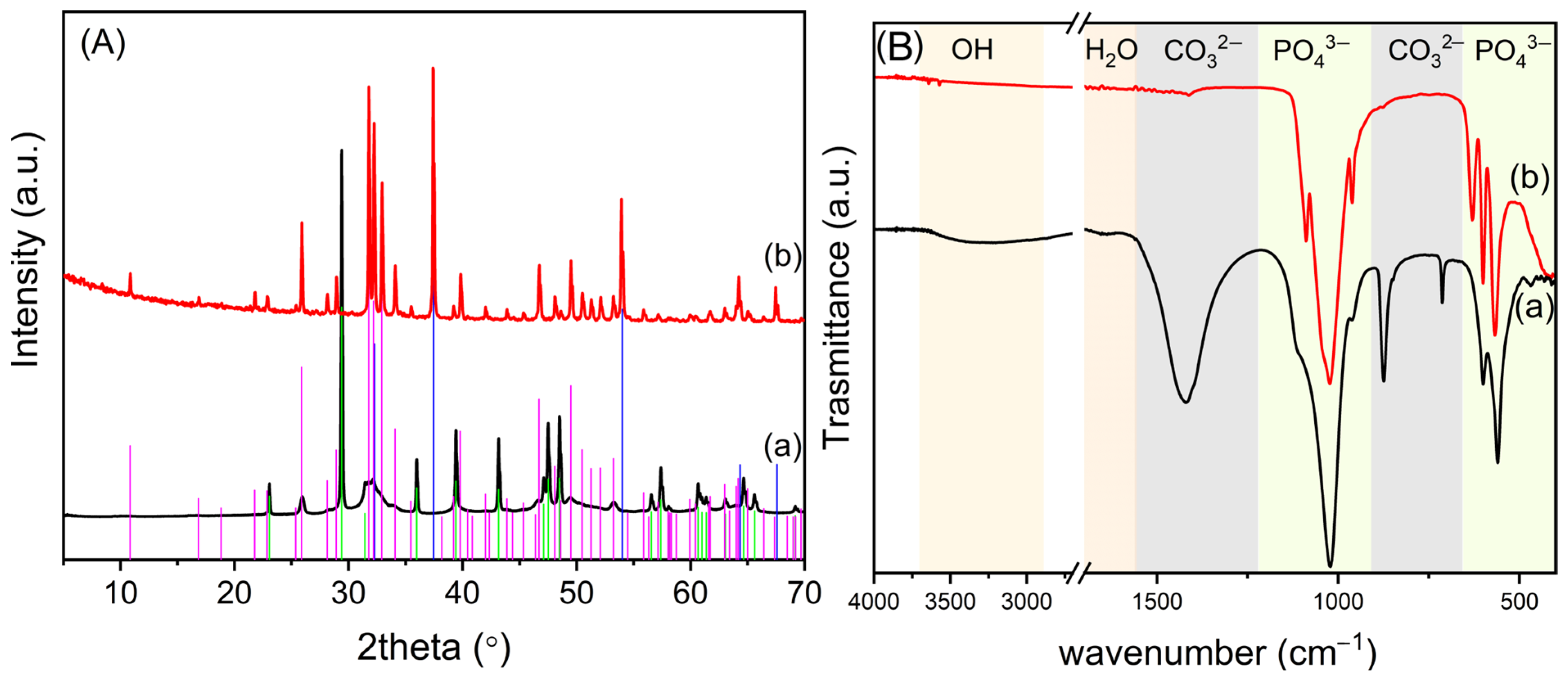
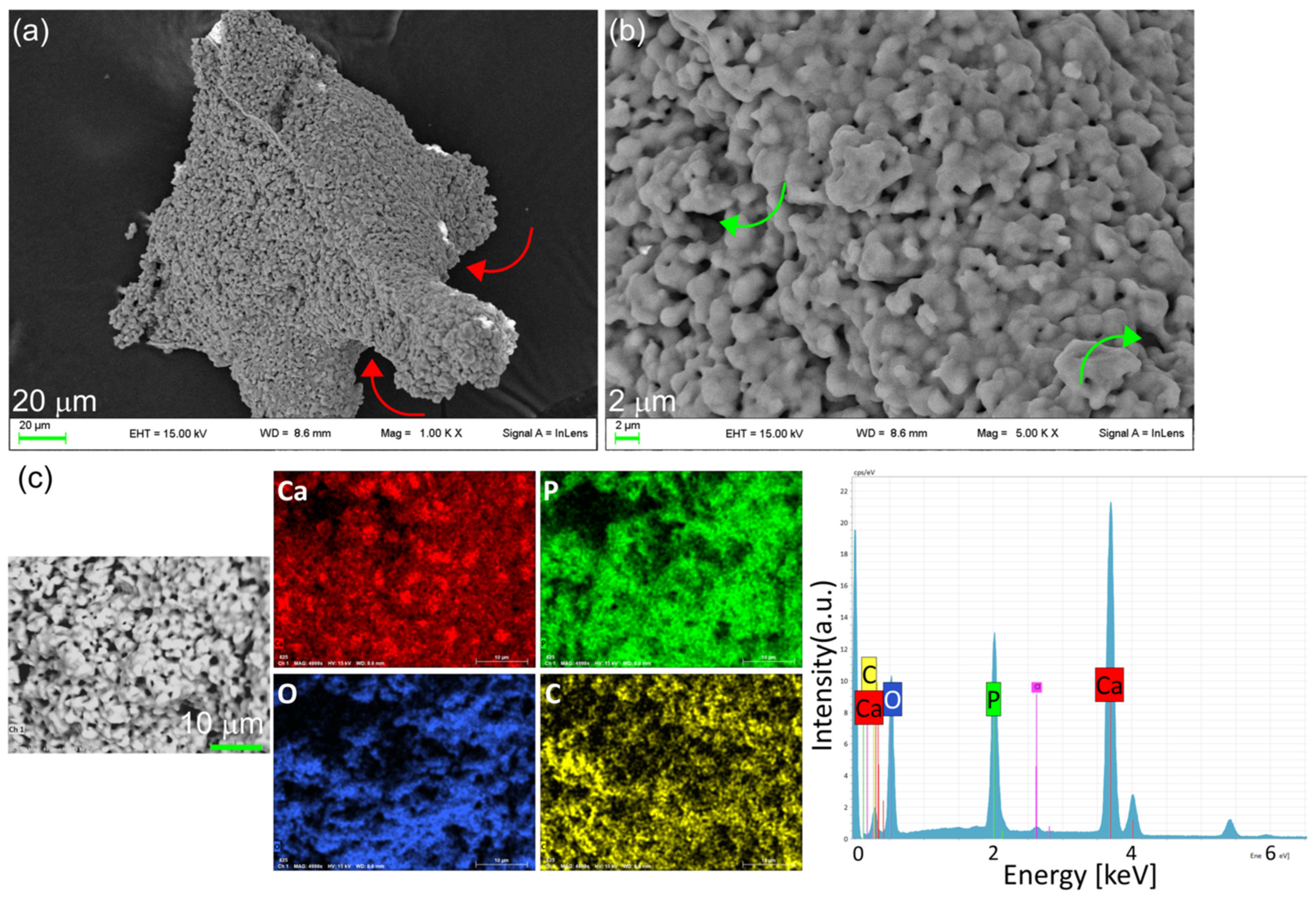
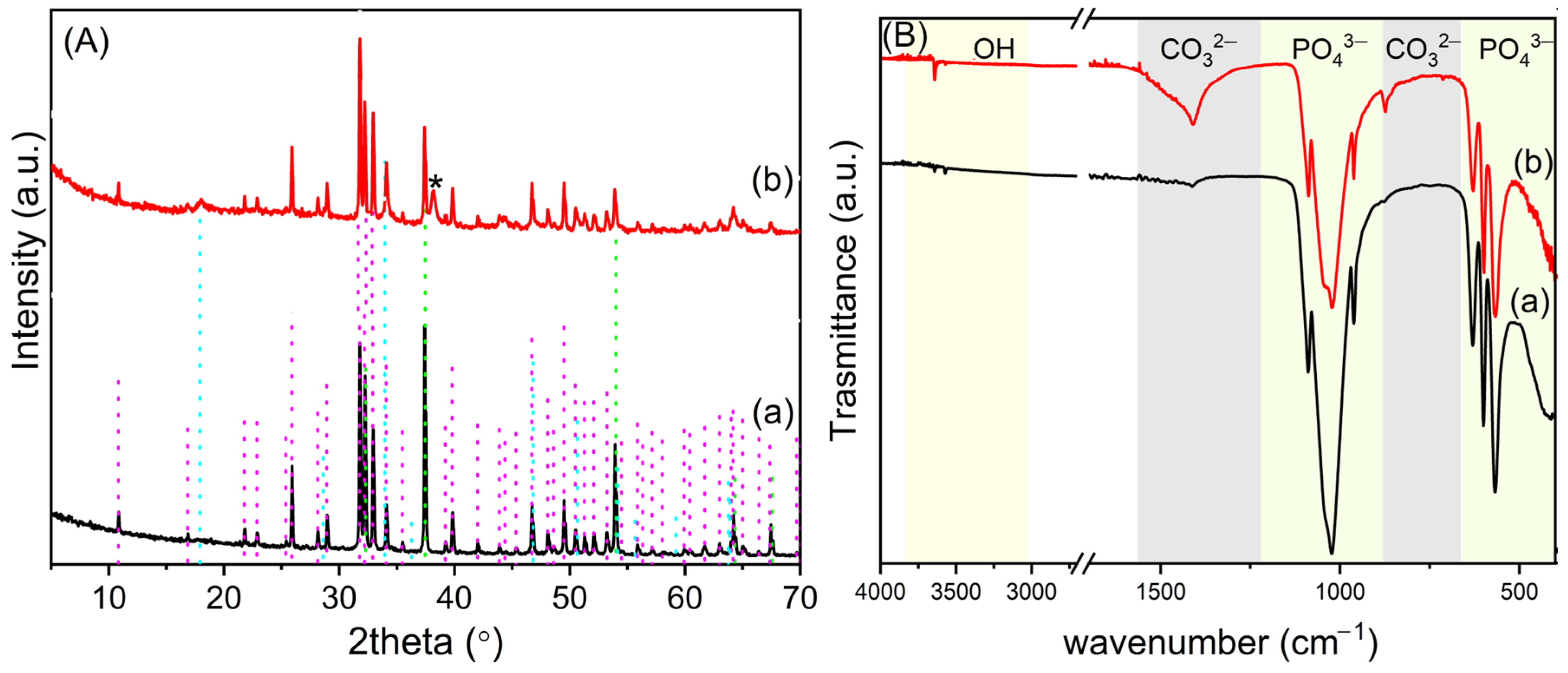
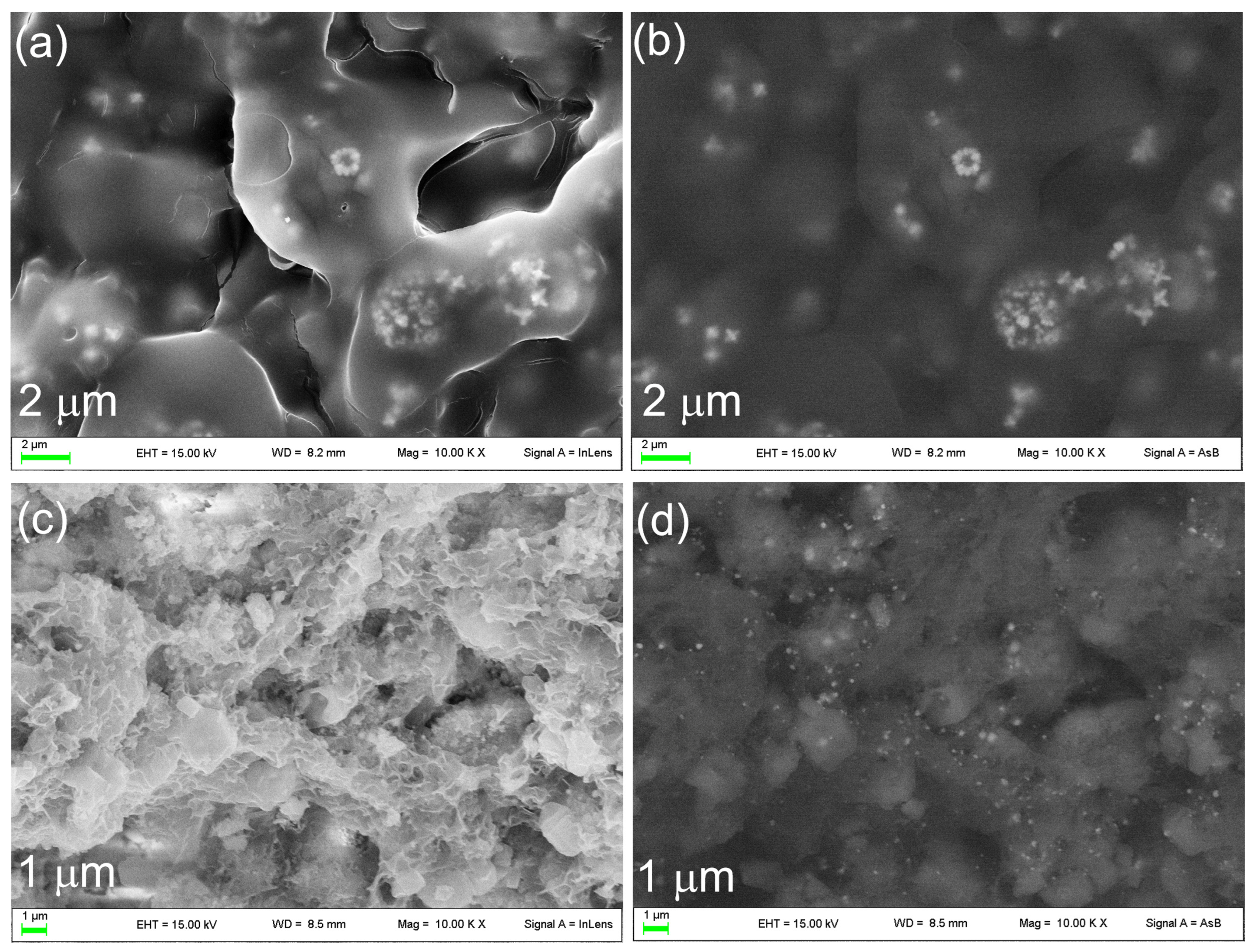
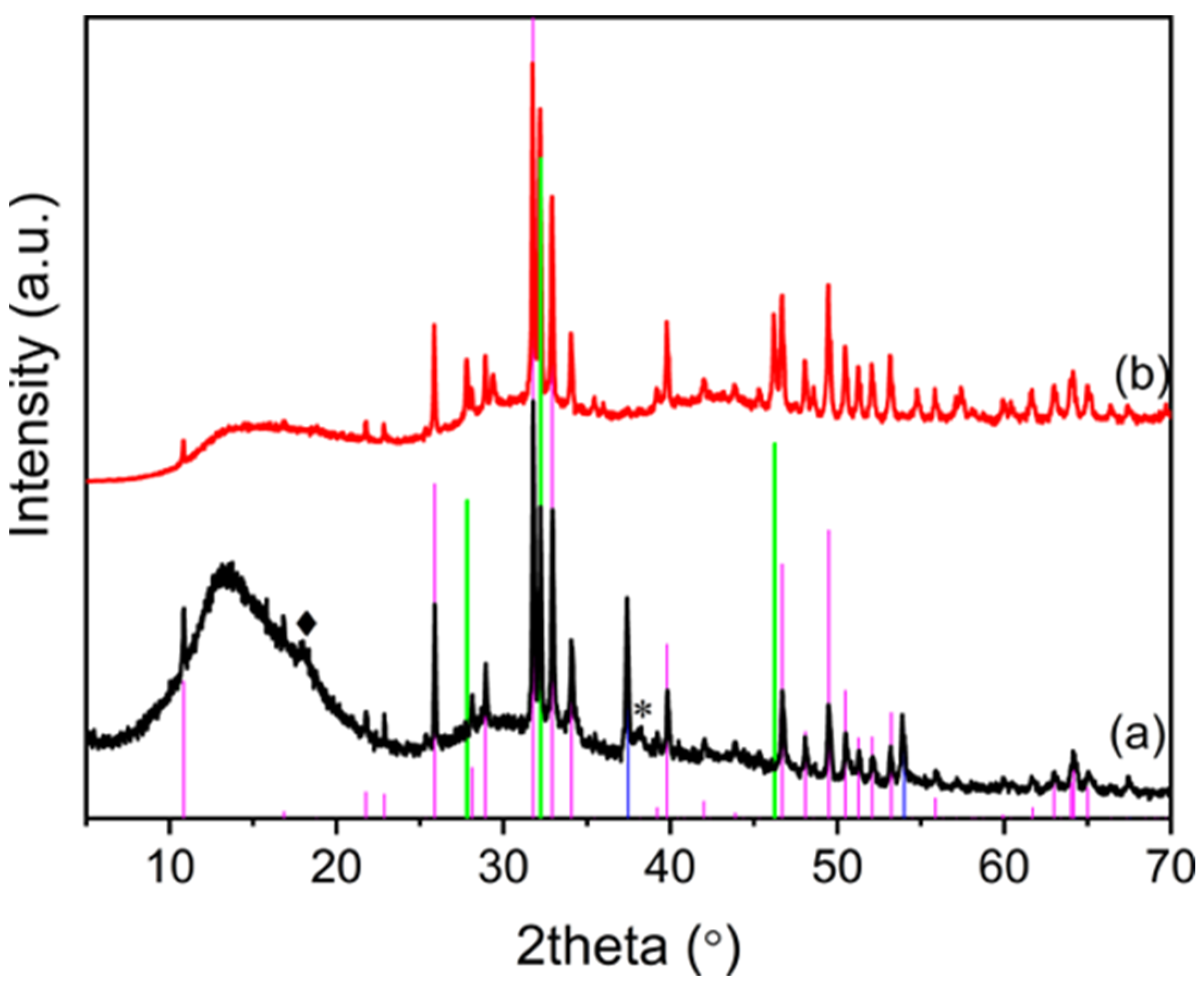
3.1. Synthesis and Characterization of OMPs
3.2. Functionalization of OMPs with Silver Nanoparticles
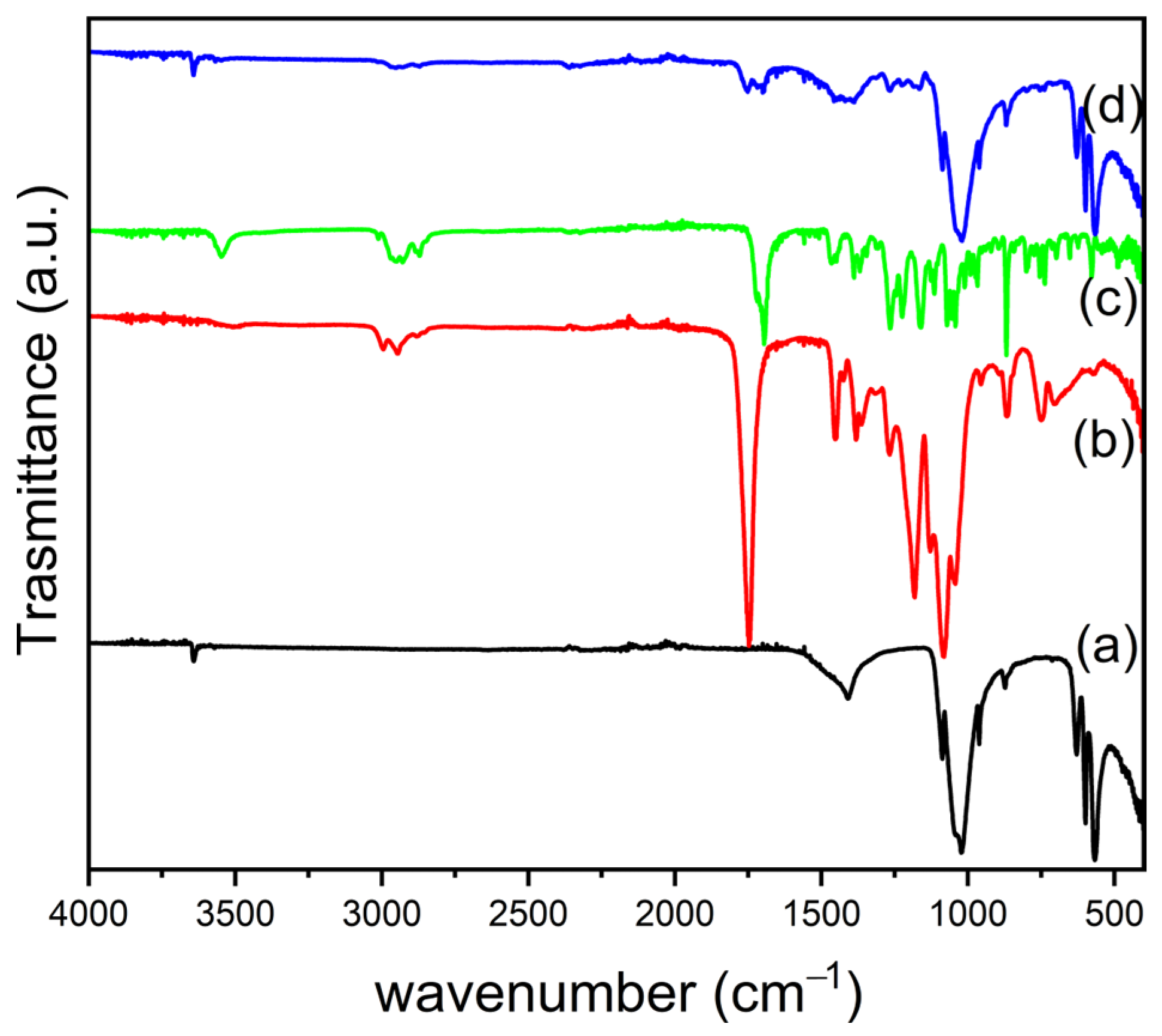
3.3. Fabrication of PLGA/OMPs and PLGA/SIMV/Ag@OMPs Composites
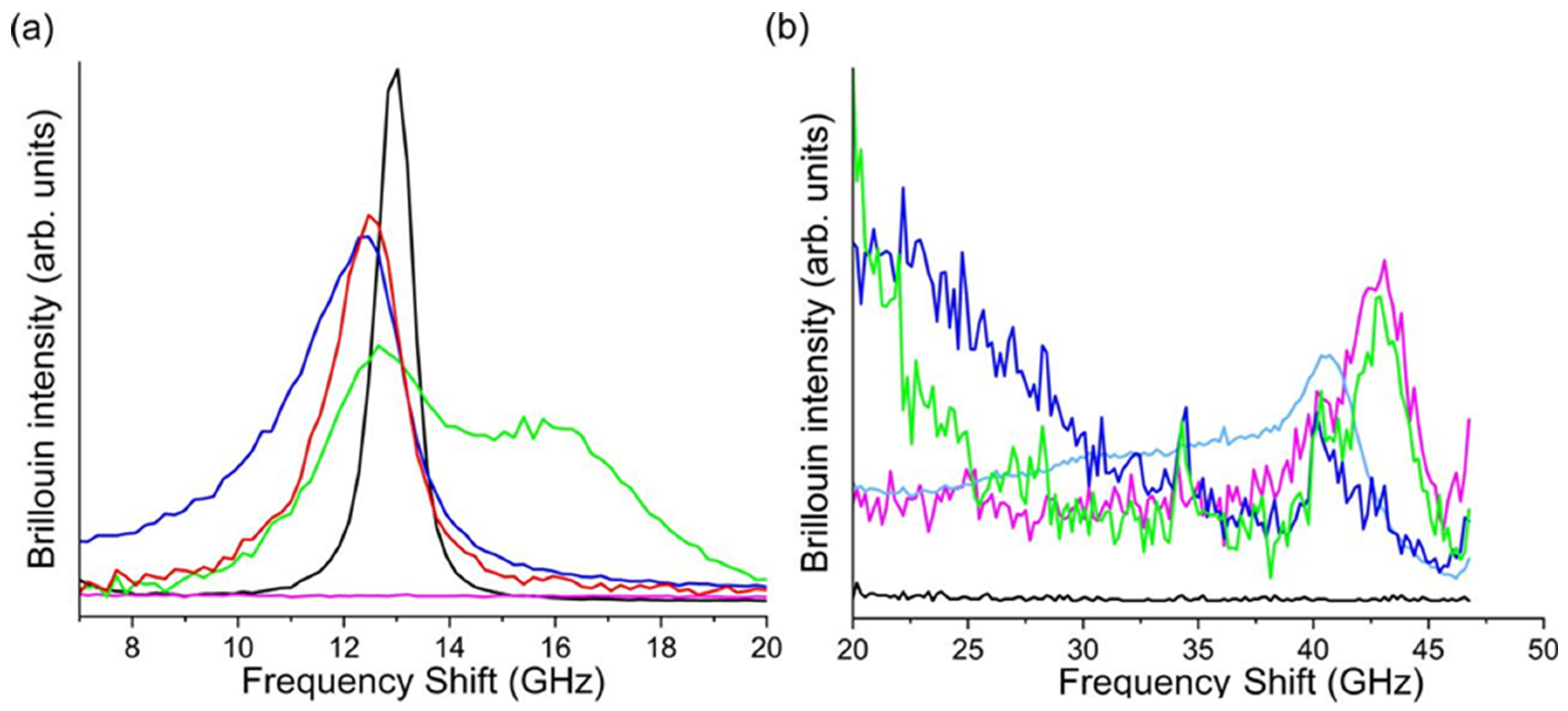
3.3.1. Mechanical Properties
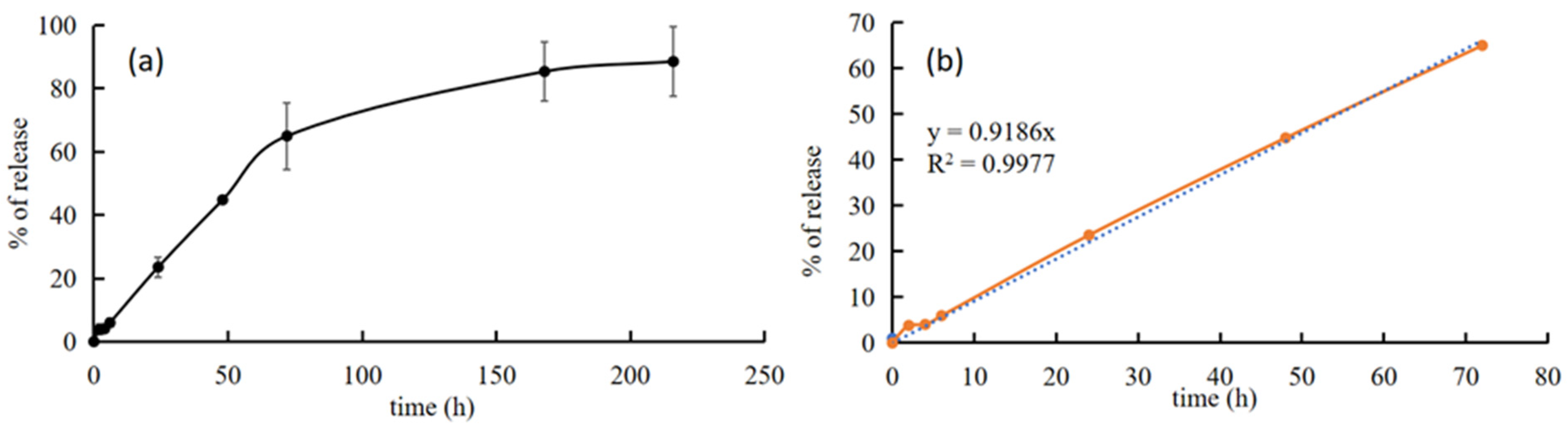
3.3.2. In Vitro Bioactivity Properties and Silver and SIMV Release of PLGA/SIMV/Ag@OMPs
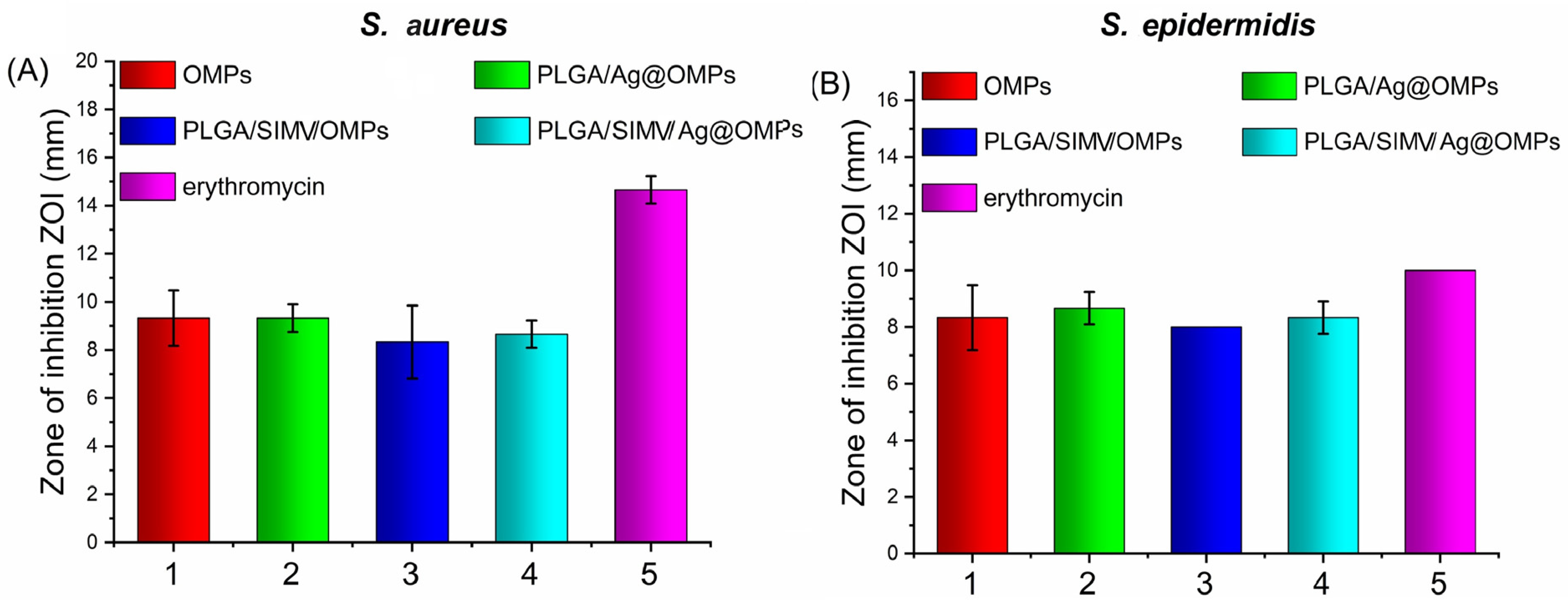
3.3.3. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5 (Suppl. S1), 125–127. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Le Gars Santoni, B.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Monchaua, F.; Lefèvre, A.; Descamps, M.; Belquin-myrdycz, A.; Laffargue, P.; Hildebrand, H.F. In vitro studies of human and rat osteoclast activity on hydroxyapatite, β-tricalcium phosphate, calcium carbonate. Biomol. Eng. 2002, 19, 143–152. [Google Scholar] [CrossRef]
- Zhong, Q.; Li, W.; Su, X.; Li, G.; Zhou, Y.; Kundu, S.C.; Yao, J.; Cai, Y. Degradation pattern of porous CaCO3 and hydroxyapatite microspheres in vitro and in vivo for potential application in bone tissue engineering. Colloids Surf. B Biointerfaces 2016, 143, 56–63. [Google Scholar] [CrossRef]
- Zhang, Q.; Schmelzer, E.; Gerlach, J.C.; Nettleship, I. A microstructural study of the degradation and calcium release from hydroxyapatite-calcium oxide ceramics made by infiltration. Mater. Sci. Eng. C 2017, 73, 684–691. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), 96–101. [Google Scholar]
- Boccaccini, A.R.; Maquet, V. Bioresorbable and bioactive polymer/bioglass® composites with tailored pore structure for tissue engineering applications. Compos. Sci. Technol. 2003, 63, 2417–2429. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Ray, R.B. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J. Biomed. Mater. Res. 2001, 55, 141–150. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Yunos, D.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- Gritsch, L.; Perrin, E.; Chenal, J.M.; Fredholm, Y.; Maçon, A.L.; Chevalier, J.; Boccaccini, A.R. Combining bioresorbable polyesters and bioactive glasses: Orthopedic applications of composite implants and bone tissue engineering scaffolds. Appl. Mater. Today 2021, 22, 100923. [Google Scholar] [CrossRef]
- Stirnimann, T.; Atria, S.; Schoelkopf, J.; Gane, P.A.C.; Alles, R.; Huwyler, J.; Puchkov, M. Compaction of functionalized calcium carbonate, a porous and crystalline microparticulate material with a lamellar surface. Int. J. Pharm. 2014, 466, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Rotha, R.; Schoelkopfb, J.; Huwylera, J.; Puchkova, M. Functionalized calcium carbonate microparticles for the delivery of proteins. Eur. J. Pharm. Biopharm. 2018, 122, 96–103. [Google Scholar] [CrossRef]
- Sirkiä, S.V.; Qudsia, S.; Siekkinen, M.; Hoepfl, W.; Budde, T.; Smått, J.K.; Peltonen, J.; Hupa, L.; Heino, T.J.; Vallittu, P.K. Physicochemical and biological characterization of functionalized calcium carbonate. Materialia 2023, 28, 101742. [Google Scholar] [CrossRef]
- Nocchetti, M.; Pietrella, D.; Antognelli, C.; Di Michele, A.; Russo, C.; Giulivi, E.; Ambrogi, V. Alginate microparticles containing silver@hydroxyapatite functionalized calcium carbonate composites. Int. J. Pharm. 2024, 661, 124393. [Google Scholar] [CrossRef]
- Ambrogi, V.; Quaglia, G.; Pietrella, D.; Nocchetti, M.; Di Michele, A.; Bolli, E.; Kaciulis, S.; Mezzi, A.; Padeletti, G.; Latterini, L. Silver@Hydroxyapatite functionalized calcium carbonate composites: Characterization, antibacterial and antibiofilm activities and cytotoxicity. Appl. Surf. Sci. 2022, 586, 152760. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Botero, L.E.; Zárate-Triviño, D.; Acevedo-Yepes, N.; Saldarriaga Escobar, J.; Pérez, V.Z.; Cruz Riano, L.J. Synthesis and characterization of a silver nanoparticle-containing polymer composite with antimicrobial abilities for application in prosthetic and orthotic devices. Biomater. Res. 2020, 24, 13. [Google Scholar] [CrossRef]
- Alsawa, G.M.; Al-Obaida, M.I.; Al-Madi, E.M. Effect of a Simvastatin-Impregnated Chitosan Scaffold onCell Growth and Osteoblastic Differentiation. Appl. Sci. 2021, 11, 5346. [Google Scholar] [CrossRef]
- Yu, W.L.; Sun, T.W.; Chao, Q.; Zhao, H.K.; Ding, Z.Y.; Zhang, Z.W.; Sun, B.B.; Shen, J.; Chen, F.; Zhu, Y.J.; et al. Enhanced osteogenesis and angiogenesis by mesoporous hydroxyapatite microspheres derived simvastatin sustained release system for superior bone regeneration. Sci. Rep. 2017, 7, 44129. [Google Scholar] [CrossRef]
- Wu, T.; Sun, J.; Tan, L.; Yan, Q.; Li, L.; Chen, L.; Liu, X.; Bin, S. Enhanced osteogenesis and therapy of osteoporosis using simvastatin loaded hybrid system. Bioact. Mater. 2020, 5, 348–357. [Google Scholar] [CrossRef]
- Tiantong, S.; Jie, H.; Wang, Z.; Xuanqi, Z.; Hong, W.; Jing, L.; Huijie, L.; Wanqiong, Y.; Chunli, S. Simvastatin-hydroxyapatite coatings prevent biofilm formation and improve bone formation in implant-associated infections. Bioact. Mater. 2023, 21, 44–56. [Google Scholar]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Ramay, H.R.; Zhang, M. Preparation of porous hydroxyapatite scaffolds by combination of the gel-casting and polymer sponge methods. Biomaterials 2003, 24, 3293–3302. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Santos de Oliveira, P.; Padmanabhan, S.K.; Licciulli, A. Hydroxyapatite Block Produced by Sponge Replica Method: Mechanical, Clinical and Histologic Observations. Materials 2019, 12, 3079. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Mattana, S.; Mattarelli, M.; Urbanelli, L.; Sagini, K.; Emiliani, C.; Dalla Serra, M.; Fioretto, D.; Caponi, S. Non-contact mechanical and chemical analysis of single living cells by microspectroscopic techniques. Light Sci. Appl. 2018, 7, 17139. [Google Scholar] [CrossRef]
- Passeri, A.A.; Di Michele, A.; Neri, I.; Cottone, F.; Fioretto, D.; Mattarelli, M.; Caponi, S. Size and environment: The effect of phonon localization on micro-Brillouin imaging. Biomater. Adv. 2023, 147, 213341. [Google Scholar] [CrossRef]
- Caponi, S.; Passeri, A.; Capponi, G.; Fioretto, D.; Vassalli, M.; Mattarelli, M. Non-contact elastography methods in mechanobiology: A point of view. Eur. Biophys. J. 2022, 51, 99–104. [Google Scholar] [CrossRef]
- Mattarelli, M.; Capponi, G.; Passeri, A.A.; Fioretto, D.; Caponi, S. Disentanglement of Multiple Scattering Contribution in Brillouin Microscopy. ACS Photonics 2022, 9, 2087–2091. [Google Scholar] [CrossRef]
- Di Michele, A.; Nocchetti, M.; Pietrella, D.; Latterini, L.; Quaglia, G.; Mattu, I.; Padeletti, G.; Kaciulis, S.; Bolli, E.; Ambrogi, V. Ag/Ag3PO4 Nanoparticle-Decorated Hydroxyapatite Functionalized Calcium Carbonate: Ultrasound-Assisted Sustainable Synthesis, Characterization, and Antimicrobial Activity. Materials 2023, 16, 1338. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Garskaite, E.; Gross, K.A.; Yang, S.W.; Yang, T.C.K.; Yang, J.C.; Kareiva, A. Effect of processing conditions on the crystallinity and structure of carbonated calcium hydroxyapatite (CHAp). CrystEngComm 2014, 16, 3950–3959. [Google Scholar] [CrossRef]
- Poralan, G.M.; Gambe, J.E.; Alcantara, E.M.; Vequizo, R.M. X-ray diffraction and infrared spectroscopy analyses on the crystallinity of engineered biological hydroxyapatite for medical application. IOP Conf. Ser. Mater. Sci. Eng. 2015, 79, 012028. [Google Scholar] [CrossRef]
- Pleshko, N.; Boskey, A.; Mendelsohn, R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 1991, 60, 786–793. [Google Scholar] [CrossRef]
- Sayah, E.; Brouri, D.; Wu, Y.; Musi, A.; Da Costa, P.; Massiani, P.A. TEM and UV–visible study of silver reduction by ethanol in Ag–alumina catalysts. Appl. Catal. A Gen. 2011, 406, 94–101. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhu, R.; Wang, Y.; Li, B.; Ma, Y.; Yin, Y. Synthesis and characterization of serial random and block-copolymers based on lactide and glycolide. Polym. Sci. Ser. A Chem. 2016, 58, 720–729. [Google Scholar] [CrossRef]
- Mattarelli, M.; Vassalli, M.; Caponi, S. Relevant Length Scales in Brillouin Imaging of Biomaterials: The Interplay between Phonons Propagation and Light Focalization. ACS Photonics 2020, 7, 2319–2328. [Google Scholar] [CrossRef]
- Cardinali, M.A.; Govoni, M.; Dallari, D.; Caponi, S.; Fioretto, D.; Morresi, A. Mechano-chemistry of human femoral diaphysis revealed by correlative Brillouin–Raman microspectroscopy. Sci. Rep. 2020, 10, 17341. [Google Scholar] [CrossRef]
- Alunni Cardinali, M.; Di Michele, A.; Mattarelli, M.; Caponi, S.; Govoni, M.; Dallari, D.; Brogini, S.; Masia, F.; Borri, P.; Langbein, W.; et al. Brillouin- Raman microspectroscopy for the morpho-mechanical imaging of human lamellar bone. J. R. Soc. Interface 2022, 19, 20210642. [Google Scholar] [CrossRef]
- Ranade, V.V.; Cannon, J.B. Drug Delivery Systems; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Steele, T.W.; Huang, C.L.; Widjaja, E.; Boey, F.Y.; Loo, J.S.; Venkatraman, S.S. The effect of polyethylene glycol structure on paclitaxel drug release and mechanical properties of PLGA thin films. Acta Biomater. 2011, 7, 1973–1983. [Google Scholar] [CrossRef]
- Molleman, B.; Hiemstra, T. Time, pH, and size dependency of silver nanoparticle dissolution: The road to equilibrium. Environ. Sci. Nano 2017, 4, 1314–1327. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials 2013, 34, 8018–8029. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent evidence on bioactive glass antimicrobial and antibiofilm activity: A mini-review. Materials 2018, 11, 326. [Google Scholar] [CrossRef]
- Donnadio, A.; Bini, M.; Centracchio, C.; Mattarelli, M.; Caponi, S.; Ambrogi, V.; Pietrella, D.; Di Michele, A.; Vivani, R.; Nocchetti, M. Bioinspired reactive interfaces based on layered double hydroxides-Zn rich hydroxyapatite with antibacterial activity. ACS Biomater. Sci. Eng. 2021, 7, 1361–1373. [Google Scholar] [CrossRef]
- Caponi, S.; Liguori, L.; Giugliarelli, A.; Mattarelli, M.; Morresi, A.; Sassi, P.; Urbanelli, L.; Musio, C. Raman micro-spectroscopy: A powerful tool for the monitoring of dynamic supramolecular changes in living cells. Biophys. Chem. 2013, 12, 58–63. [Google Scholar] [CrossRef]
- Cazzolli, G.; Caponi, S.; Defant, A.; Gambi, C.M.C.; Marchetti, S.; Mattarelli, M.; Montagna, M.; Rossi, B.; Rossi, F.; Viliani, G. Aggregation processes in micellar solutions: A Raman study. J. Raman Spectrosc. 2012, 43, 1877–1883. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Cusco, R.; Guitian, F.; de Aza, S.; Artus, L. Differentiation between Hydroxyapatite and β-Tricalcium Phosphate by Means of μ-Raman Spectroscopy. J. Eur. Ceram. Soc. 1998, 18, 1301–1305. [Google Scholar] [CrossRef]
- Khan, A.F.; Awais, M.; Khan, A.S.; Tabassum, S.; Chaudhry, A.A.; Rehman, I.U. Raman Spectroscopy of Natural Bone and Synthetic Apatites. Appl. Spectrosc. Rev. 2013, 48, 329–355. [Google Scholar] [CrossRef]
- Biswal, A.K.; Hariprasad, P.; Saha, S. Efficient and prolonged antibacterial activity from porous PLGA microparticles and their application in food preservation. Mater. Sci. Eng. 2020, 108, 110496. [Google Scholar] [CrossRef] [PubMed]
- van Apeldoorn, A.A.; Aksenov, Y.; Stigter, M.; Hofland, I.; de Bruijn, J.D.; Koerten, H.K.; Otto, C.; Greve, J.; van Blitterswijk, C.A. Parallel high-resolution confocal Raman SEM analysis of inorganic and organic bone matrix constituents. J. R. Soc. Interface 2015, 2, 39–45. [Google Scholar] [CrossRef]
- Heilmann, M.T.; Simoes, R.G.; Bernardes, C.E.; Ramisch, Y.; Bienert, R.; Röllig, M.; Emmerling, F.; Minas da Piedade, M.E. Real-Time In situ XRD Study of Simvastatin Crystallization in Levitated Droplets. Cryst. Growth Des. 2021, 21, 4665–4673. [Google Scholar] [CrossRef]


| Sample a | PLGA (wt%) | Ag (wt%) | SIMV (wt%) |
|---|---|---|---|
| 1PLGA/OMPs | 9.3 | - | |
| 2PLGA/OMPs | 15.0 | - | |
| 3PLGA/OMPs | 18.0 | - | |
| 4PLGA/OMPs | 24.1 | - | |
| PLGA/Ag@OMPs | 7.4 | 4.3 | - |
| PLGA/SIMV/OMPs | 12.9 | - | 6.4 |
| PLGA/SIMV/Ag@OMPs | 12.6 | 4.1 | 6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocchetti, M.; Piccotti, C.; Piccinini, M.; Caponi, S.; Mattarelli, M.; Pietrella, D.; Di Michele, A.; Ambrogi, V. Silver Nanoparticles and Simvastatin-Loaded PLGA-Coated Hydroxyapatite/Calcium Carbonate Scaffolds. Nanomaterials 2024, 14, 1637. https://doi.org/10.3390/nano14201637
Nocchetti M, Piccotti C, Piccinini M, Caponi S, Mattarelli M, Pietrella D, Di Michele A, Ambrogi V. Silver Nanoparticles and Simvastatin-Loaded PLGA-Coated Hydroxyapatite/Calcium Carbonate Scaffolds. Nanomaterials. 2024; 14(20):1637. https://doi.org/10.3390/nano14201637
Chicago/Turabian StyleNocchetti, Morena, Chiara Piccotti, Michela Piccinini, Silvia Caponi, Maurizio Mattarelli, Donatella Pietrella, Alessandro Di Michele, and Valeria Ambrogi. 2024. "Silver Nanoparticles and Simvastatin-Loaded PLGA-Coated Hydroxyapatite/Calcium Carbonate Scaffolds" Nanomaterials 14, no. 20: 1637. https://doi.org/10.3390/nano14201637
APA StyleNocchetti, M., Piccotti, C., Piccinini, M., Caponi, S., Mattarelli, M., Pietrella, D., Di Michele, A., & Ambrogi, V. (2024). Silver Nanoparticles and Simvastatin-Loaded PLGA-Coated Hydroxyapatite/Calcium Carbonate Scaffolds. Nanomaterials, 14(20), 1637. https://doi.org/10.3390/nano14201637











