Reversible Tuning Electrical Properties in Ferroelectric SnS with NH3 Adsorption and Desorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Electrical Measurements
3. Results and Discussion
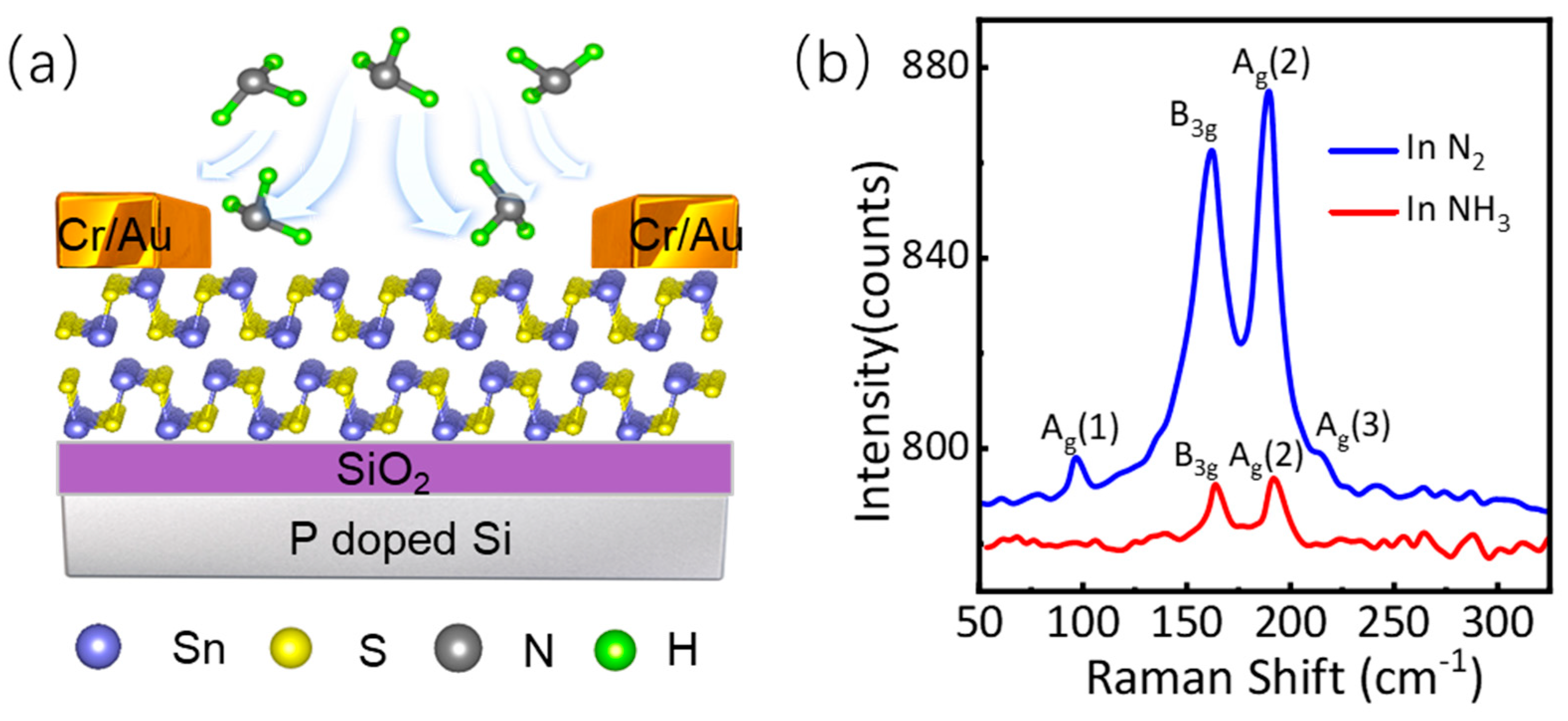
3.1. Raman Characterizations of Ferroelectric SnS
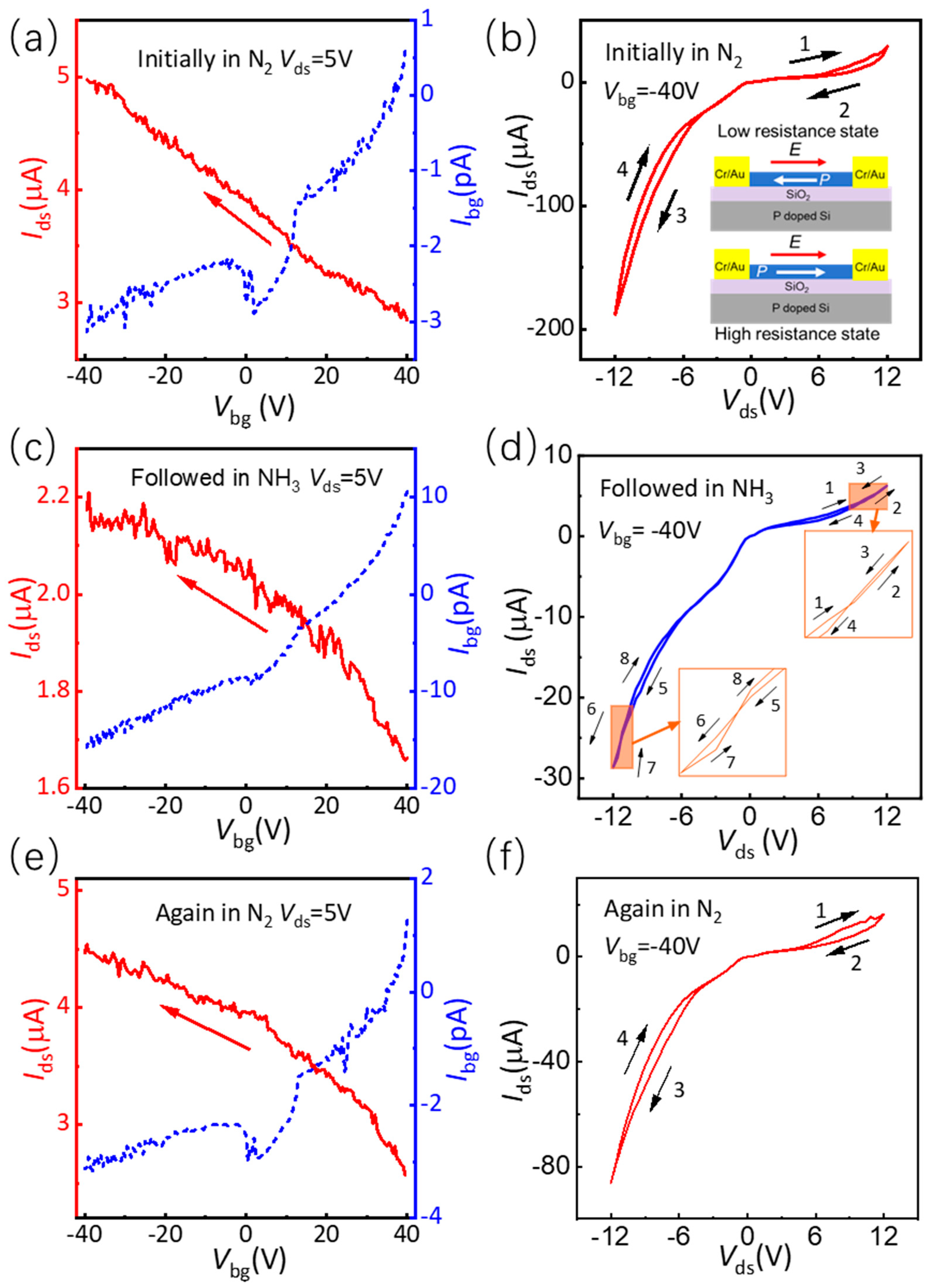
3.2. Electrical Properties of Ferroelectric SnS
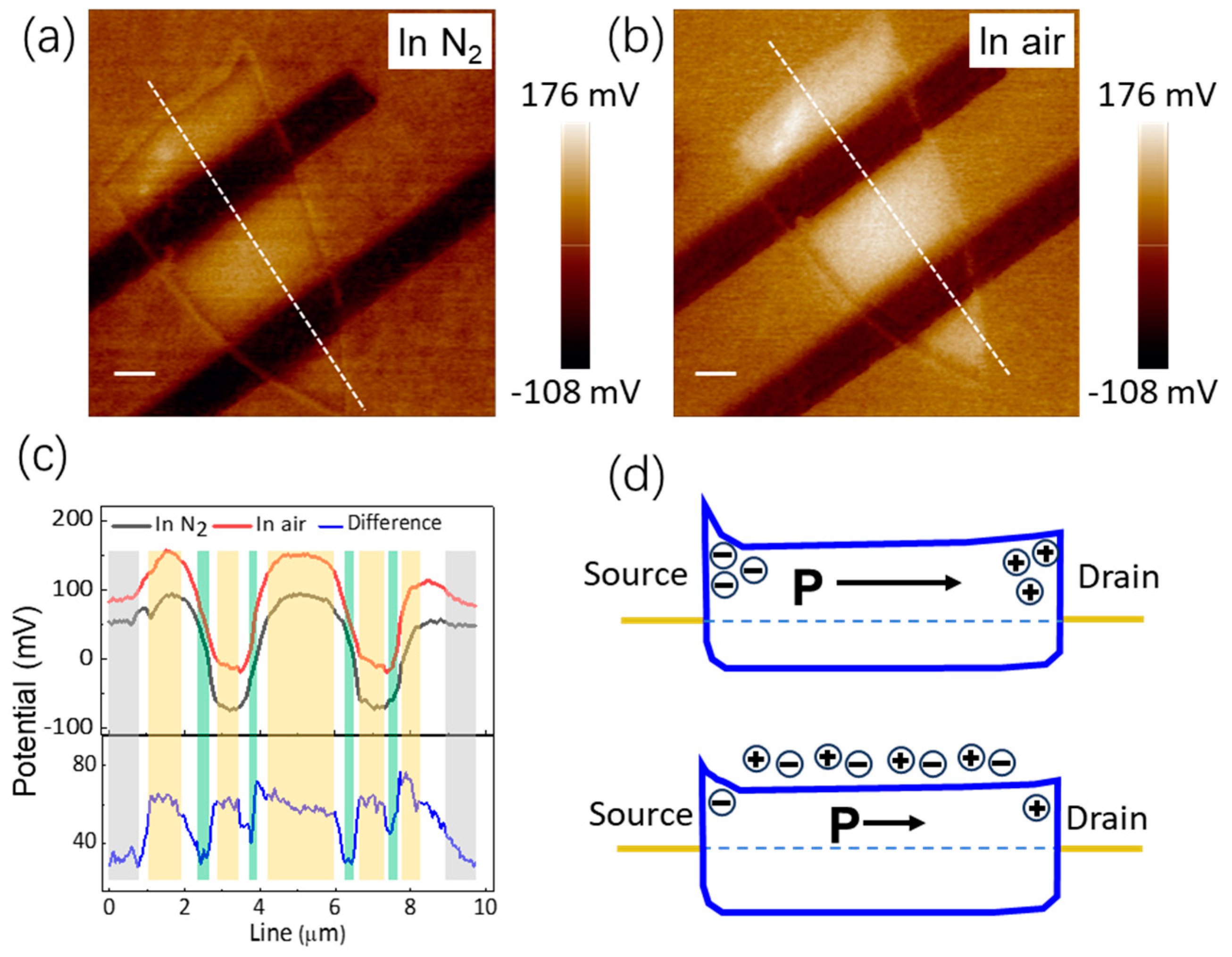
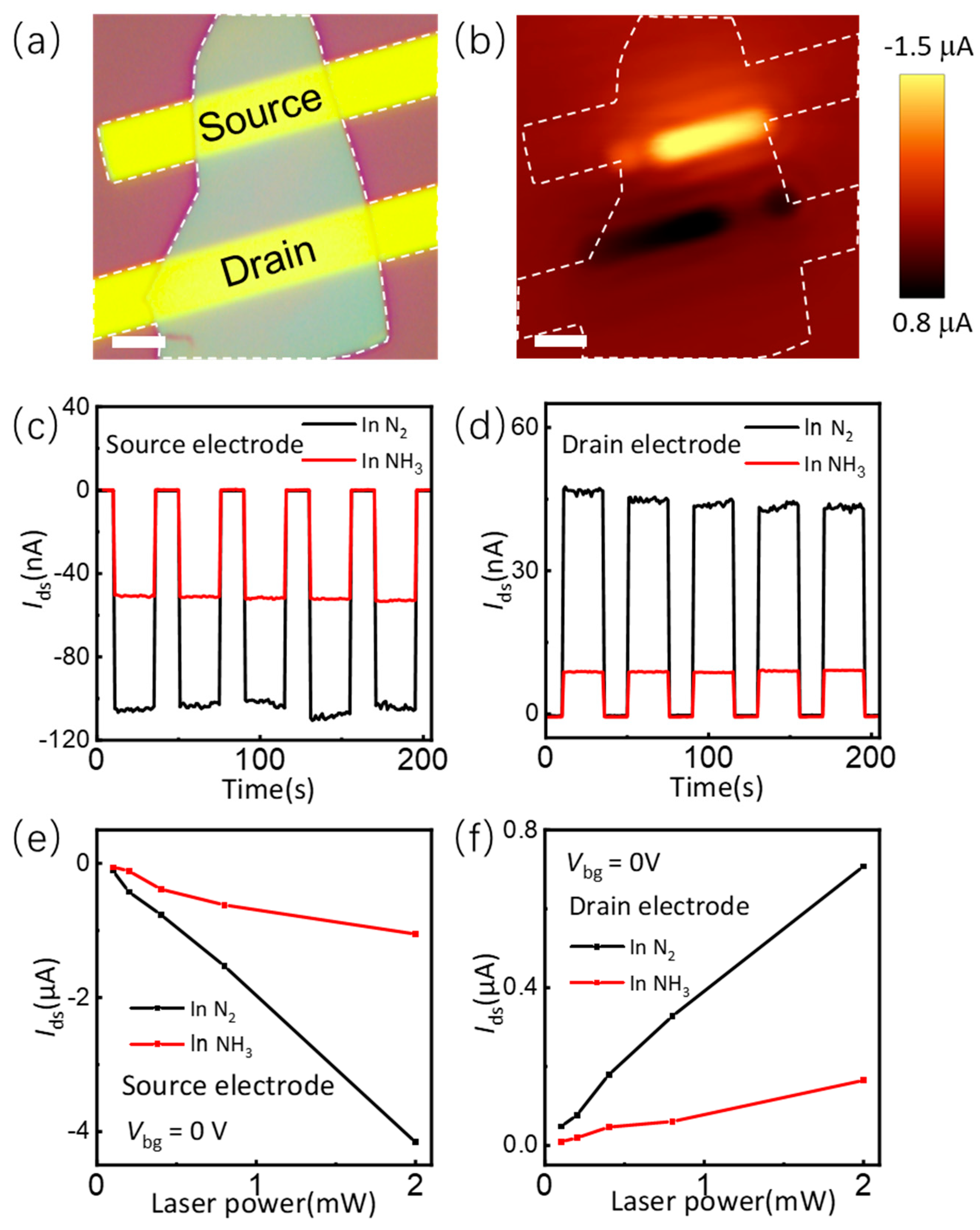
3.3. The Mechanism of NH3 Molecule Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Z.-D.; Yang, M.-M.; Liu, Y.; Alexe, M. Emerging Opportunities for 2D Semiconductor/Ferroelectric Transistor-Structure Devices. Adv. Mater. 2021, 33, 2005620. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Two-Dimensional van der Waals Ferroelectrics: Scientific and Technological Opportunities. ACS Nano 2021, 15, 9229–9237. [Google Scholar] [CrossRef]
- Jin, T.; Mao, J.; Gao, J.; Han, C.; Loh, K.P.; Wee, A.T.S.; Chen, W. Ferroelectrics-Integrated Two-Dimensional Devices toward Next-Generation Electronics. ACS Nano 2022, 16, 13595–13611. [Google Scholar] [CrossRef]
- Wang, C.; You, L.; Cobden, D.; Wang, J. Towards two-dimensional van der Waals ferroelectrics. Nat. Mater. 2023, 22, 542–552. [Google Scholar] [CrossRef]
- Xue, F.; Ma, Y.; Wang, H.; Luo, L.; Xu, Y.; Anthopoulos, T.D.; Lanza, M.; Yu, B.; Zhang, X. Two-dimensional ferroelectricity and antiferroelectricity for next-generation computing paradigms. Matter 2022, 5, 1999–2014. [Google Scholar] [CrossRef]
- Zhu, W.; Hong, X.; Ye, P.D.; Gu, Y. 2D Piezoelectrics, pyroelectrics, and ferroelectrics. J. Appl. Phys. 2023, 133, 120402. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Lai, Q.; Fu, J.; Zhu, W.; Zeng, H. Recent Advances in Layered Two-Dimensional Ferroelectrics from Material to Device. Adv. Funct. Mater. 2023, 33, 2304139. [Google Scholar] [CrossRef]
- Tan, Y.; Zheng, J.; Niu, X.; Zhao, Y.; Zhong, N.; Tian, B.; Duan, C. Research progress on 2D ferroelectric and ferrovalley materials and their neuromorphic application. Sci. China Phys. Mech. Astron. 2023, 66, 117505. [Google Scholar] [CrossRef]
- Yuan, S.; Luo, X.; Chan, H.L.; Xiao, C.; Dai, Y.; Xie, M.; Hao, J. Room-temperature ferroelectricity in MoTe2 down to the atomic monolayer limit. Nat. Commun. 2019, 10, 1775. [Google Scholar] [CrossRef]
- Fei, Z.; Zhao, W.; Palomaki, T.A.; Sun, B.; Miller, M.K.; Zhao, Z.; Yan, J.; Xu, X.; Cobden, D.H. Ferroelectric switching of a two-dimensional metal. Nature 2018, 560, 336–339. [Google Scholar] [CrossRef]
- de la Barrera, S.C.; Cao, Q.; Gao, Y.; Gao, Y.; Bheemarasetty, V.S.; Yan, J.; Mandrus, D.G.; Zhu, W.; Xiao, D.; Hunt, B.M. Direct measurement of ferroelectric polarization in a tunable semimetal. Nat. Commun. 2021, 12, 5298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, D.; Zhu, Y.; Cho, Y.; He, Q.; Yang, X.; Herrera, K.; Chu, Z.; Han, Y.; Downer, M.C.; et al. Out-of-Plane Piezoelectricity and Ferroelectricity in Layered α-In2Se3 Nanoflakes. Nano Lett. 2017, 17, 5508–5513. [Google Scholar] [CrossRef]
- Xue, F.; Hu, W.; Lee, K.-C.; Lu, L.-S.; Zhang, J.; Tang, H.-L.; Han, A.; Hsu, W.-T.; Tu, S.; Chang, W.-H.; et al. Room-Temperature Ferroelectricity in Hexagonally Layered α-In2Se3 Nanoflakes down to the Monolayer Limit. Adv. Funct. Mater. 2018, 28, 1803738. [Google Scholar] [CrossRef]
- Xue, F.; He, X.; Retamal, J.R.D.; Han, A.; Zhang, J.; Liu, Z.; Huang, J.-K.; Hu, W.; Tung, V.; He, J.-H.; et al. Gate-Tunable and Multidirection-Switchable Memristive Phenomena in a Van Der Waals Ferroelectric. Adv. Mater. 2019, 31, 1901300. [Google Scholar] [CrossRef]
- Si, M.; Zhang, Z.; Chang, S.-C.; Haratipour, N.; Zheng, D.; Li, J.; Avci, U.E.; Ye, P.D. Asymmetric Metal/α-In2Se3/Si Crossbar Ferroelectric Semiconductor Junction. ACS Nano 2021, 15, 5689–5695. [Google Scholar] [CrossRef]
- Chang, K.; Liu, J.; Lin, H.; Wang, N.; Zhao, K.; Zhang, A.; Jin, F.; Zhong, Y.; Hu, X.; Duan, W.; et al. Discovery of robust in-plane ferroelectricity in atomic-thick SnTe. Science 2016, 353, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Song, P.; Liu, Y.; Chen, Z.; Zhu, M.; Abdelwahab, I.; Su, J.; Fu, W.; Chi, X.; Yu, W.; et al. Gate-Tunable In-Plane Ferroelectricity in Few-Layer SnS. Nano Lett. 2019, 19, 5109–5117. [Google Scholar] [CrossRef]
- Yan, Y.; Deng, Q.; Li, S.; Guo, T.; Li, X.; Jiang, Y.; Song, X.; Huang, W.; Yang, J.; Xia, C. In-plane ferroelectricity in few-layered GeS and its van der Waals ferroelectric diodes. Nanoscale 2021, 13, 16122–16130. [Google Scholar] [CrossRef]
- Guan, Z.; Zhao, Y.; Wang, X.; Zhong, N.; Deng, X.; Zheng, Y.; Wang, J.; Xu, D.; Ma, R.; Yue, F.; et al. Electric-Field-Induced Room-Temperature Antiferroelectric–Ferroelectric Phase Transition in van der Waals Layered GeSe. ACS Nano 2022, 16, 1308–1317. [Google Scholar] [CrossRef]
- Liu, F.; You, L.; Seyler, K.L.; Li, X.; Yu, P.; Lin, J.; Wang, X.; Zhou, J.; Wang, H.; He, H.; et al. Room-temperature ferroelectricity in CuInP2S6 ultrathin flakes. Nat. Commun. 2016, 7, 12357. [Google Scholar] [CrossRef]
- Dziaugys, A.; Kelley, K.; Brehm, J.A.; Tao, L.; Puretzky, A.; Feng, T.; O’Hara, A.; Neumayer, S.; Chyasnavichyus, M.; Eliseev, E.A.; et al. Piezoelectric domain walls in van der Waals antiferroelectric CuInP2Se6. Nat. Commun. 2020, 11, 3623. [Google Scholar] [CrossRef] [PubMed]
- Io, W.F.; Pang, S.Y.; Wong, L.W.; Zhao, Y.; Ding, R.; Mao, J.; Zhao, Y.; Guo, F.; Yuan, S.; Zhao, J.; et al. Direct observation of intrinsic room-temperature ferroelectricity in 2D layered CuCrP2S6. Nat. Commun. 2023, 14, 7304. [Google Scholar] [CrossRef] [PubMed]
- Higashitarumizu, N.; Kawamoto, H.; Lee, C.-J.; Lin, B.-H.; Chu, F.-H.; Yonemori, I.; Nishimura, T.; Wakabayashi, K.; Chang, W.-H.; Nagashio, K. Purely in-plane ferroelectricity in monolayer SnS at room temperature. Nat. Commun. 2020, 11, 2428. [Google Scholar] [CrossRef]
- Zhang, D.; Schoenherr, P.; Sharma, P.; Seidel, J. Ferroelectric order in van der Waals layered materials. Nat. Rev. Mater. 2023, 8, 25–40. [Google Scholar] [CrossRef]
- Xu, D.-D.; Ma, R.-R.; Fu, A.-P.; Guan, Z.; Zhong, N.; Peng, H.; Xiang, P.-H.; Duan, C.-G. Ion adsorption-induced reversible polarization switching of a van der Waals layered ferroelectric. Nat. Commun. 2021, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Hu, X.; Lu, X.; Zhang, J.; Shen, X.; Huang, F. Self-organization of ferroelectric domains induced by water and reinforced via ultrasonic vibration. Commun. Mater. 2023, 4, 38. [Google Scholar] [CrossRef]
- Sutter, P.; Komsa, H.P.; Lu, H.; Gruverman, A.; Sutter, E. Few-layer tin sulfide (SnS): Controlled synthesis, thickness dependent vibrational properties, and ferroelectricity. Nano Today 2021, 37, 101082. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Luo, W.; Yang, H.; He, Y.; Liu, Y.; Zhang, X.; Peng, G. Study on adsorption and desorption of ammonia on graphene. Nanoscale Res. Lett. 2015, 10, 359. [Google Scholar] [CrossRef]
- Wang, J.; Lian, G.; Xu, Z.; Fu, C.; Lin, Z.; Li, L.; Wang, Q.; Cui, D.; Wong, C.-P. Growth of Large-Size SnS Thin Crystals Driven by Oriented Attachment and Applications to Gas Sensors and Photodetectors. ACS Appl. Mater. Interfaces 2016, 8, 9545–9551. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, L.; Zhu, S.; Guo, Y.; Yao, Y.; Yang, Y.; Gu, S.; Zhou, Z. High Sensitivity of Ammonia Sensor through 2D Black Phosphorus/Polyaniline Nanocomposite. Nanomaterials 2021, 11, 3026. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Xie, J. Humidity-enhanced NH3 sensor based on carbon quantum dots-modified SnS. Appl. Surf. Sci. 2023, 634, 157612. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Kumar, A.; Chakkar, A.G.; Betal, A.; Kumar, P.; Sahu, S.; Kumar, M. Catalytic synergy of WS2-anchored PdSe2 for highly sensitive hydrogen gas sensor. Nanoscale 2024, 16, 9593–9602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qiao, Z.; Li, C.-Y.; Sun, Y. Recent Progress in Multifunctional Gas Sensors Based on 2D Materials. Chemosensors 2023, 11, 483. [Google Scholar] [CrossRef]
- Wang, B.; Luo, H.; Wang, X.; Wang, E.; Sun, Y.; Tsai, Y.-C.; Dong, J.; Liu, P.; Li, H.; Xu, Y.; et al. Direct laser patterning of two-dimensional lateral transition metal disulfide-oxide-disulfide heterostructures for ultrasensitive sensors. Nano Res. 2020, 13, 2035–2043. [Google Scholar] [CrossRef]
- Wang, Z.; Jing, X.; Duan, S.; Liu, C.; Kang, D.; Xu, X.; Chen, J.; Xia, Y.; Chang, B.; Zhao, C.; et al. 2D PtSe2 Enabled Wireless Wearable Gas Monitoring Circuits with Distinctive Strain-Enhanced Performance. ACS Nano 2023, 17, 11557–11566. [Google Scholar] [CrossRef]
- Qin, Z.; Song, X.; Wang, J.; Li, X.; Wu, C.; Wang, X.; Yin, X.; Zeng, D. Development of flexible paper substrate sensor based on 2D WS2 with S defects for room-temperature NH3 gas sensing. Appl. Surf. Sci. 2022, 573, 151535. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Yang, J.; Zhou, J.; Zhang, Y.; Zhang, H.; Li, Y. A Fully Integrated Flexible Tunable Chemical Sensor Based on Gold-Modified Indium Selenide Nanosheets. ACS Sens. 2022, 7, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.M.; Iqbal, M.Z.; Dastgeer, G.; Nazir, G.; Mumtaz, S.; Usman, M.; Eom, J. WS2/GeSe/WS2 Bipolar Transistor-Based Chemical Sensor with Fast Response and Recovery Times. ACS Appl. Mater. Interfaces 2020, 12, 39524–39532. [Google Scholar] [CrossRef]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.-S.; Xia, J.-B.; Li, J. Photoresponsive and Gas Sensing Field-Effect Transistors based on Multilayer WS2 Nanoflakes. Sci. Rep. 2014, 4, 5209. [Google Scholar] [CrossRef]
- Das, S.; Sharma, S.; Sharma, S.K. Facile Synthesis of 2D-HfS2 Flakes for μ-IDE-Based Methanol Sensor: Fast Detection at Room Temperature. IEEE Sens. J. 2019, 19, 9090–9096. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Liu, J.; Peng, Z.; Zhou, J.; Zhang, H.; Li, Y. Optoelectronic Gas Sensor Based on Few-Layered InSe Nanosheets for NO2 Detection with Ultrahigh Antihumidity Ability. Anal. Chem. 2020, 92, 11277–11287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, J.; Wang, Q.; Zhou, J.; Ye, J.; Li, X.; Geng, Y.; Liang, Z.; Du, Y.; Tian, X. Indium oxide-black phosphorus composites for ultrasensitive nitrogen dioxide sensing at room temperature. Sens. Actuators B. Chem. 2020, 308, 127650. [Google Scholar] [CrossRef]
- Zhang, C.; Ning, J.; Wang, B.; Guo, H.; Feng, X.; Shen, X.; Jia, Y.; Dong, J.; Wang, D.; Zhang, J.; et al. Hybridized 1T/2H-MoS2/graphene fishnet tube for high-performance on-chip integrated micro-systems comprising supercapacitors and gas sensors. Nano Res. 2021, 14, 114–121. [Google Scholar] [CrossRef]
- Abbas, A.N.; Liu, B.; Chen, L.; Ma, Y.; Cong, S.; Aroonyadet, N.; Köpf, M.; Nilges, T.; Zhou, C. Black Phosphorus Gas Sensors. ACS Nano 2015, 9, 5618–5624. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High-Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Monolayer MoS2 Transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Luo, W.; Zhang, S.; Zeng, C.; Xie, F.; Deng, C.; Wang, G.; Peng, G. Reversible Tuning Electrical Properties in Ferroelectric SnS with NH3 Adsorption and Desorption. Nanomaterials 2024, 14, 1638. https://doi.org/10.3390/nano14201638
Wang W, Luo W, Zhang S, Zeng C, Xie F, Deng C, Wang G, Peng G. Reversible Tuning Electrical Properties in Ferroelectric SnS with NH3 Adsorption and Desorption. Nanomaterials. 2024; 14(20):1638. https://doi.org/10.3390/nano14201638
Chicago/Turabian StyleWang, Wanqian, Wei Luo, Sen Zhang, Chayuan Zeng, Fei Xie, Chuyun Deng, Guang Wang, and Gang Peng. 2024. "Reversible Tuning Electrical Properties in Ferroelectric SnS with NH3 Adsorption and Desorption" Nanomaterials 14, no. 20: 1638. https://doi.org/10.3390/nano14201638
APA StyleWang, W., Luo, W., Zhang, S., Zeng, C., Xie, F., Deng, C., Wang, G., & Peng, G. (2024). Reversible Tuning Electrical Properties in Ferroelectric SnS with NH3 Adsorption and Desorption. Nanomaterials, 14(20), 1638. https://doi.org/10.3390/nano14201638





