Carbon Quantum Dots/Cu2O Photocatalyst for Room Temperature Selective Oxidation of Benzyl Alcohol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
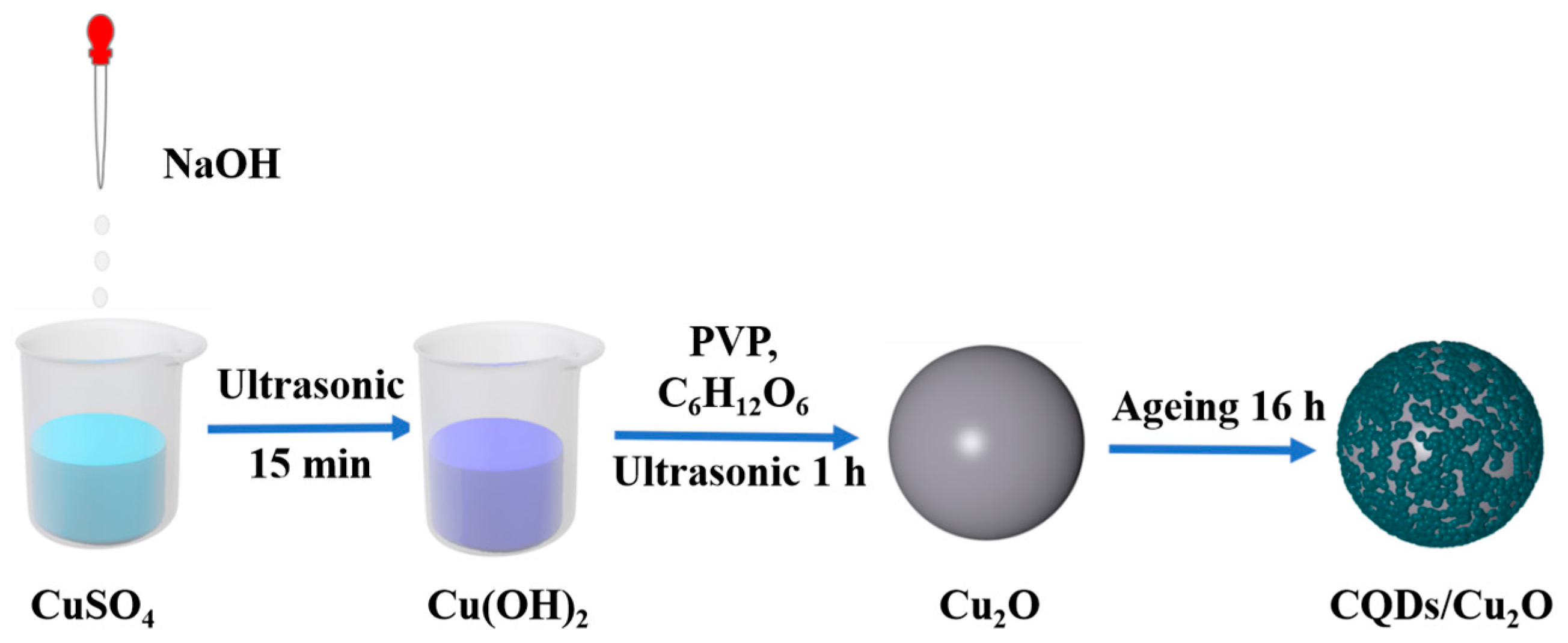
2.2. Preparation of CQDs
2.3. Preparation of CQDs/Cu2O
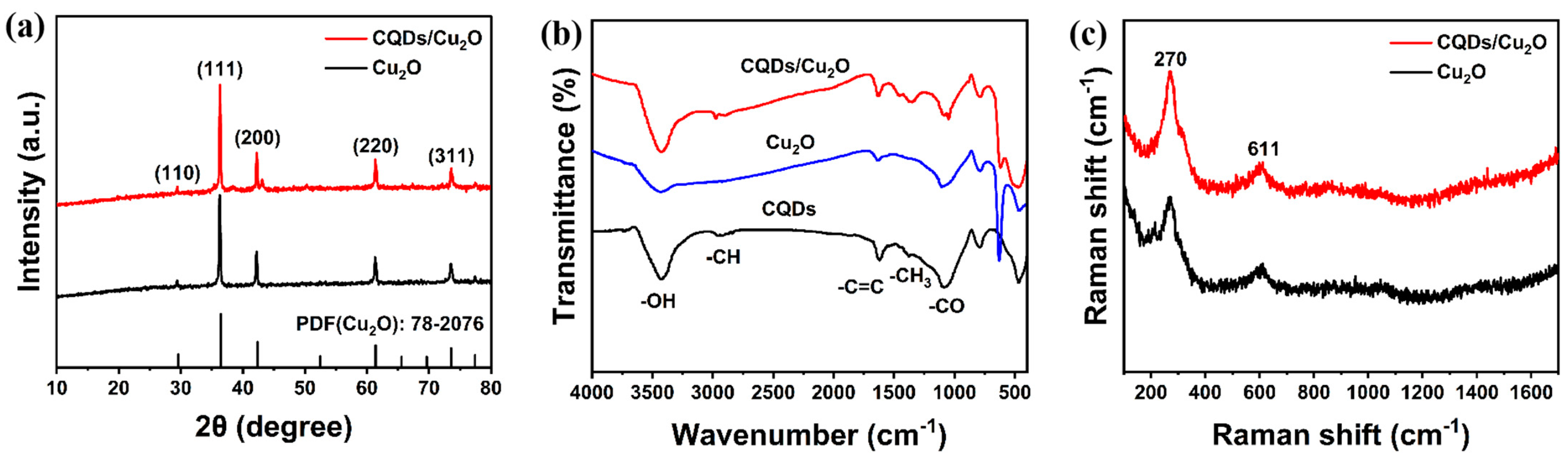
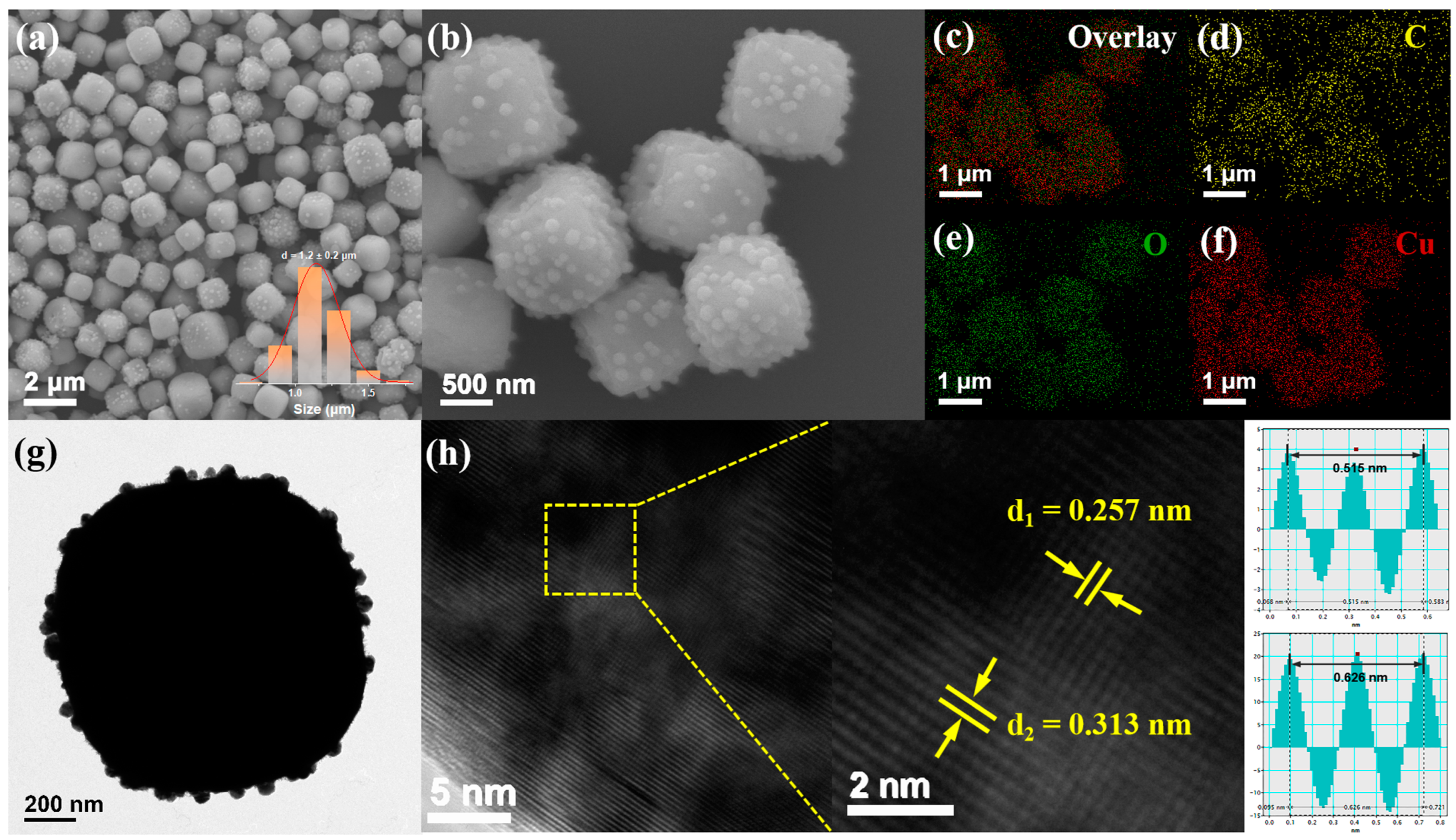
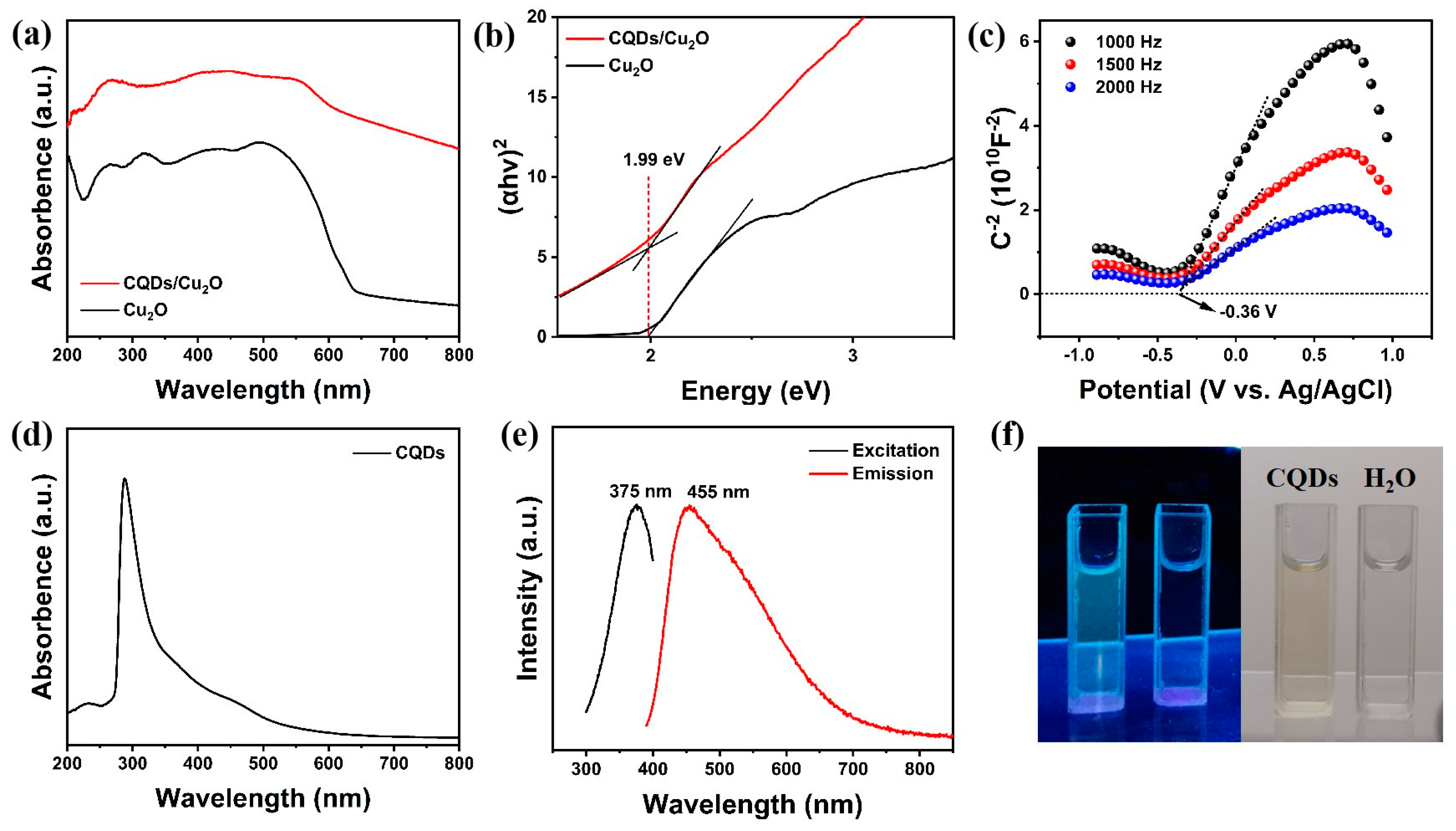
2.4. Characterization
2.5. Benzyl Alcohol Oxidation Test
2.6. Photoelectrochemical Performance Tests
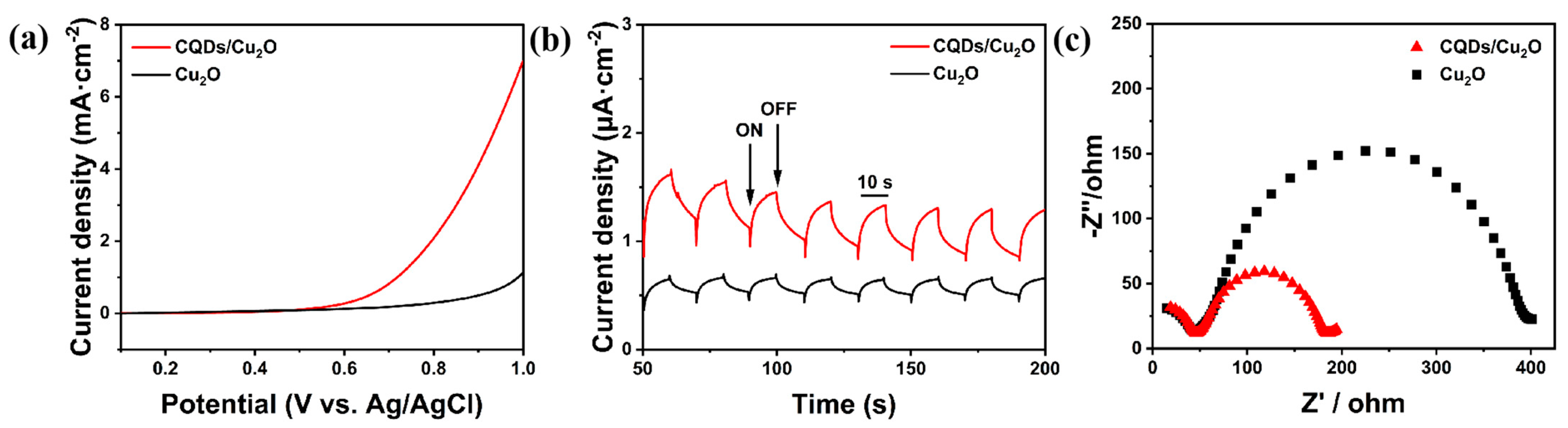
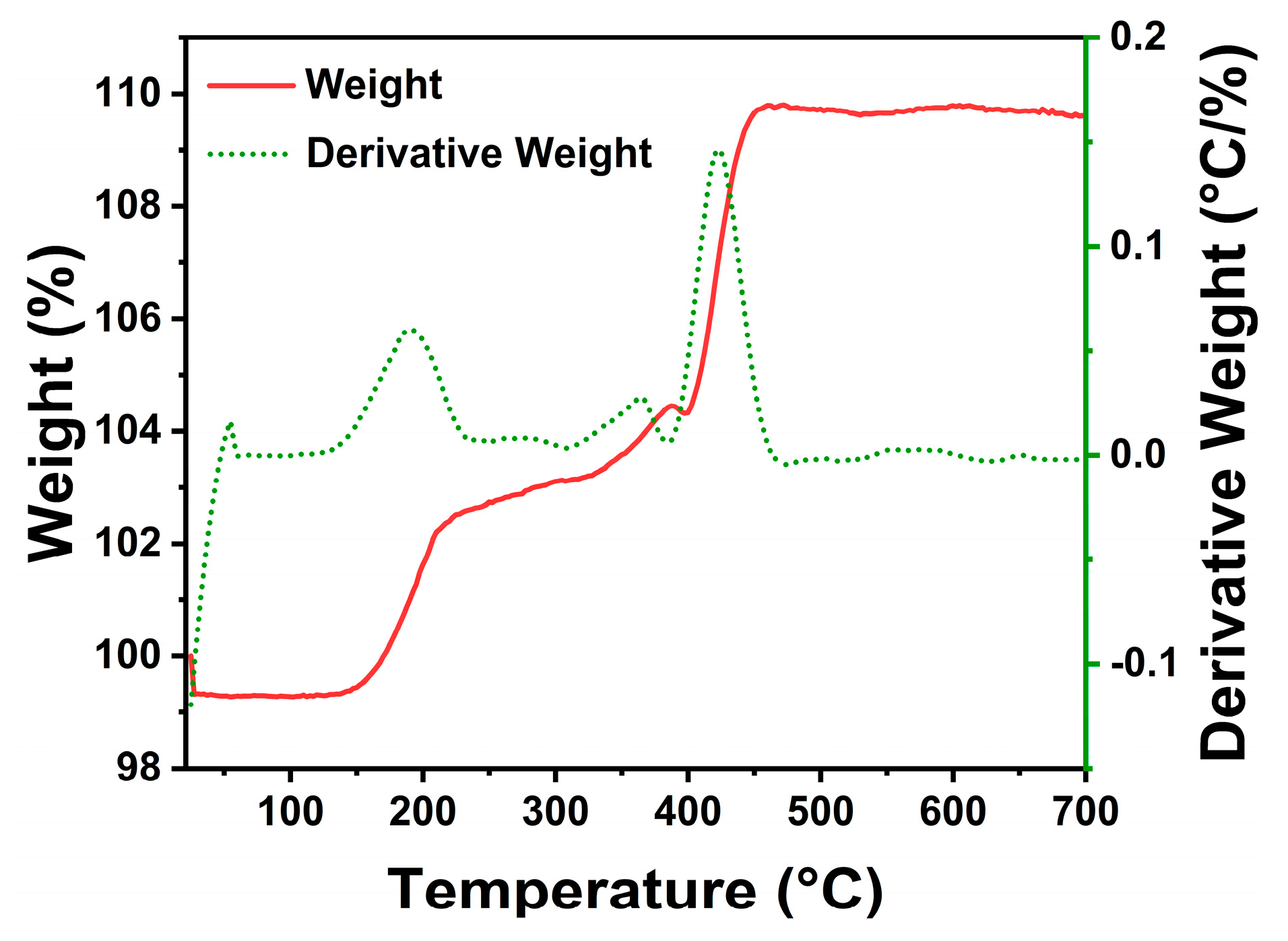
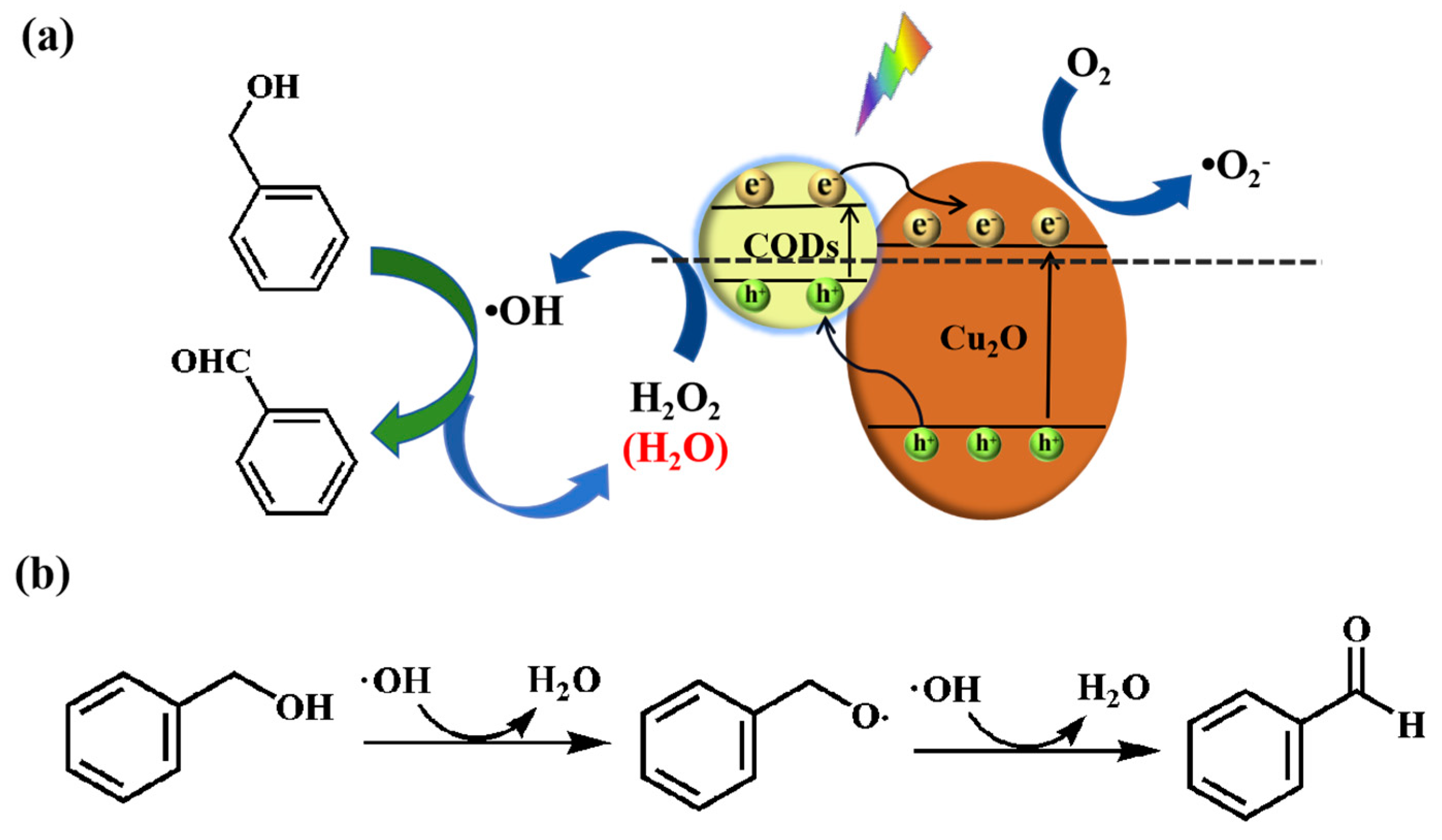
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, Y.; Feng, Y.; Li, Z.; Zhou, H.; Lv, H.; Yang, G.Y. CsPbBr3/Polyoxometalate Composites for Selective Photocatalytic Oxidation of Benzyl Alcohol. ACS Catal. 2023, 13, 14346–14355. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, Y.N.; Zhu, W.; Zhao, H.; Yang, N.; Zhao, G. Selective photoelectrocatalytic tuning of benzyl alcohol to benzaldehyde for enhanced hydrogen production. Appl. Catal. B Environ. 2021, 286, 119868. [Google Scholar]
- Lee, S.G.; Kang, M.J.; Park, M.; Kim, K.J.; Lee, H.; Kim, H.S. Selective photocatalytic conversion of benzyl alcohol to benzaldehyde or deoxybenzoin over ion-exchanged CdS. Appl. Catal. B Environ. 2022, 304, 120967. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, H.; Cui, D.; Lu, K.; Kong, X.; Cai, J.; Yu, S.; Zhang, X. Selectivity Regulation of Au/Titanate by Biochar Modification for Selective Oxidation of Benzyl Alcohol. Catalysts 2023, 13, 864. [Google Scholar] [CrossRef]
- Saffari, N.S.; Aghabarari, B.; Javaheri, M.; Khanlarkhani, A.; Martinez-Huerta, M.V. Transforming Waste Clamshell into Highly Selective Nanostructured Catalysts for Solvent Free Liquid Phase Oxidation of Benzyl Alcohol. Catalysts 2022, 12, 155. [Google Scholar]
- Xiong, L.; Tang, J. Strategies and Challenges on Selectivity of Photocatalytic Oxidation of Organic Substances. Adv. Energy Mater. 2021, 11, 202003216. [Google Scholar]
- Tang, D.; Lu, G.; Shen, Z.; Hu, Y.; Yao, L.; Li, B.; Zhao, G.; Peng, B.; Huang, X. A review on photo-, electro- and photoelectro- catalytic strategies for selective oxidation of alcohols. J. Energy Chem. 2023, 77, 80–118. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Liu, Y.; Li, Y.; Wu, R.; Du, Y.; Askari, N.; Liu, N.; Qiao, F.; Sun, C.; et al. A core-satellite structured type II heterojunction photocatalyst with enhanced CO2 reduction under visible light. Nano Res. 2022, 15, 8880–88894. [Google Scholar]
- Dai, Y.; Ren, P.; Li, Y.; Lv, D.; Shen, Y.; Li, Y.; Niemantsverdriet, H.; Besenbacher, F.; Xiang, H.; Hao, W.; et al. Solid Base Bi24O31Br10(OH)δ with Active Lattice Oxygen for the Efficient Photo-Oxidation of Primary Alcohols to Aldehydes. Angew. Chem. Int. Ed. Engl. 2019, 58, 6265–6270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, D.; Zhou, M.; Kondamareddy, K.K.; Zhao, Z.; Wang, Z.; Wu, J.; Zhang, B.; Fan, H.; Ho, W. Boosting photocatalytic dehydrogenation and simultaneous selective oxidation of benzyl alcohol to benzaldehyde over flower-like MoS2 modified Zn0.5Cd0.5S solid solution. Mater. Today Phys. 2023, 35, 101121. [Google Scholar] [CrossRef]
- Zou, J.; Wang, Z.; Guo, W.; Guo, B.; Yu, Y.; Wu, L. Photocatalytic selective oxidation of benzyl alcohol over ZnTi-LDH: The effect of surface OH groups. Appl. Catal. B Environ. 2020, 260, 118185. [Google Scholar]
- Zhang, X.; Shi, Q.; Liu, X.; Li, J.; Xu, H.; Ding, H.; Li, G. Facile Assembly of InVO4/TiO2 Heterojunction for Enhanced Photo-Oxidation of Benzyl. Alcohol. Nanomater. 2022, 12, 1544. [Google Scholar]
- Zeng, X.; Wang, Z.; Meng, N.; McCarthy, D.T.; Deletic, A.; Pan, J.h.; Zhang, X. Highly dispersed TiO2 nanocrystals and carbon dots on reduced graphene oxide: Ternary nanocomposites for accelerated photocatalytic water disinfection. Appl. Catal. B Environ. 2017, 202, 33–41. [Google Scholar] [CrossRef]
- Yu, Z.; Li, F.; Xiang, Q. Carbon dots-based nanocomposites for heterogeneous photocatalysis. J. Mater. Sci. Technol. 2024, 175, 244–257. [Google Scholar]
- Du, Y.; Li, Y.; Liu, Y.; Liu, N.; Cheng, Y.; Shi, Q.; Liu, X.; Tao, Z.; Guo, Y.; Zhang, J.; et al. Stalk-derived carbon dots as nanosensors for Fe3+ ions detection and biological cell imaging. Front. Bioeng. Biotechnol. 2023, 11, 1187632. [Google Scholar] [CrossRef] [PubMed]
- Manjupriya, R.; Roopan, S.M. Carbon dots-based catalyst for various organic transformations. J. Mater. Sci. 2021, 56, 17369–17410. [Google Scholar] [CrossRef]
- Li, H.; Liu, R.; Lian, S.; Liu, Y.; Huang, H.; Kang, Z. Near-infrared light controlled photocatalytic activity of carbon quantum dots for highly selective oxidation reaction. Nanoscale 2013, 5, 3289–3297. [Google Scholar]
- Mohammadi, M.; Khazaei, A.; Rezaei, A.; Zheng, H.J.; Shu, X.W. Ionic-Liquid-Modified Carbon Quantum Dots as a Support for the Immobilization of Tungstate Ions (WO42–): Heterogeneous Nanocatalysts for the Oxidation of Alcohols in Water. ACS Sustain. Chem. Eng. 2019, 7, 5283–5291. [Google Scholar]
- Chen, B.B.; Liu, M.L.; Huang, C.Z. Carbon dot-based composites for catalytic applications. Green Chem. 2020, 22, 4034–4054. [Google Scholar]
- Zheng, Z.; Han, F.; Xing, B.; Han, X.; Li, B. Synthesis of Fe3O4@CdS@CQDs ternary core-shell heterostructures as a magnetically recoverable photocatalyst for selective alcohol oxidation coupled with H2O2 production. J. Colloid Interface Sci. 2022, 624, 460–470. [Google Scholar]
- Han, Y.; Wu, J.; Li, Y.; Gu, X.; He, T.; Zhao, Y.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots enhance the interface electron transfer and photoelectrochemical kinetics in TiO2 photoanode. Appl. Catal. B Environ. 2022, 304, 120983. [Google Scholar]
- Sekkat, A.; Liedke, M.O.; Nguyen, V.H.; Butterling, M.; Baiutti, F.; Sirvent Veru, J.d.D.; Weber, M.; Rapenne, L.; Bellet, D.; Chichignoud, G.; et al. Chemical deposition of Cu2O films with ultra-low resistivity: Correlation with the defect landscape. Nat. Commun. 2022, 13, 5322. [Google Scholar] [CrossRef]
- Tilley, S.D. Will Cuprous Oxide Really Make It in Water-Splitting Applications? ACS Energy Lett. 2023, 8, 2338–2344. [Google Scholar] [CrossRef]
- Rosso, C.; Filippini, G.; Prato, M. Carbon Dots as Nano-Organocatalysts for Synthetic Applications. ACS Catal. 2020, 10, 8090–8105. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; MacFarlane, D.R. Carbon Quantum Dots/Cu2O Heterostructures for Solar-Light-Driven Conversion of CO2 to Methanol. Adv. Energy Mater. 2014, 5, 201401077. [Google Scholar]
- Ge, H.; Bao, H.; Zhang, L.; Chen, G. Preparation of porous graphene using cuprous oxide microspheres as sacrificial templates for enriching proteins and peptides. Carbon 2015, 82, 579–589. [Google Scholar]
- Li, L.; Li, Y.; Ye, Y.; Guo, R.; Wang, A.; Zou, G.; Hou, H.; Ji, X. Kilogram-Scale Synthesis and Functionalization of Carbon Dots for Superior Electrochemical Potassium Storage. ACS Nano 2021, 15, 6872–6885. [Google Scholar]
- Li, H.; Liu, R.; Liu, Y.; Huang, H.; Yu, H.; Ming, H.; Lian, S.; Lee, S.T.; Kang, Z. Carbon quantum dots/Cu2O composites with protruding nanostructures and their highly efficient (near) infrared photocatalytic behavior. J. Mater. Chem. 2012, 22, 17470–17475. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.Y.; Peng, J.F.; Wang, L.H.; Peng, D.Y.; Liu, F.Y.; Xu, S.; Yang, Z.L. Fabrication of Cu2O/ZnTi-LDH p-n heterostructure by grafting Cu2O NPs onto the LDH host layers from Cu-doped ZnTi-LDH and insight into the photocatalytic mechanism. Compos. Part B Eng. 2023, 250, 110447. [Google Scholar]
- Wang, C.; Ye, F.; Shen, J.; Xue, K.H.; Zhu, Y.; Li, C. In Situ Loading of Cu2O Active Sites on Island-like Copper for Efficient Electrochemical Reduction of Nitrate to Ammonia. ACS Appl. Mater. Interfaces 2022, 14, 6680–6688. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, X.; Mei, J.; Li, Y.; Han, W.; Xie, M.; Wang, F.; Xie, E. Improved photoelectrocatalytic hydrogen generation through BiVO4 quantum-dots loaded on nano-structured SnO2 and modified with carbon quantum-dots. Chem. Eng. J. 2018, 331, 48–53. [Google Scholar]
- Zhang, Z.; Wu, H.; Yu, Z.; Song, R.; Qian, K.; Chen, X.; Tian, J.; Zhang, W.; Huang, W. Site-Resolved Cu2O Catalysis in the Oxidation of CO. Angew. Chem. Int. Ed. Engl. 2019, 58, 4276–4280. [Google Scholar] [CrossRef]
- Wang, K.; Lv, M.; Si, T.; Tang, X.; Wang, H.; Chen, Y.; Zhou, T. Mechanism analysis of surface structure-regulated Cu2O in photocatalytic antibacterial process. J. Hazard Mater. 2024, 461, 132479. [Google Scholar] [CrossRef] [PubMed]
- Dandia, A.; Saini, P.; Sethi, M.; Kumar, K.; Saini, S.; Meena, S.; Meena, S.; Parewa, V. Nanocarbons in quantum regime: An emerging sustainable catalytic platform for organic synthesis. Catal. Rev. 2023, 65, 874–928. [Google Scholar] [CrossRef]
- Luo, J.; Liu, X.; Gu, J.; Zhao, W.; Gu, M.; Xie, Y. Construction of novel g-C3N4/β-FeOOH Z-Scheme heterostructure photocatalyst modified with carbon quantum dots for efficient degradation of RhB. J. Mater. Sci. Technol. 2023, 181, 11–19. [Google Scholar] [CrossRef]
- Miao, R.; Luo, Z.; Zhong, W.; Chen, S.Y.; Jiang, T.; Dutta, B.; Nasr, Y.; Zhang, Y.; Suib, S.L. Mesoporous TiO2 modified with carbon quantum dots as a high-performance visible light photocatalyst. Appl. Catal. B Environ. 2016, 189, 26–38. [Google Scholar]
- Shi, H.; Niu, Z.; Wang, H.; Ye, W.; Xi, K.; Huang, X.; Wang, H.; Liu, Y.; Lin, H.; Shi, H.; et al. Endowing matrix-free carbon dots with color-tunable ultralong phosphorescence by self-doping. Chem. Sci. 2022, 13, 4406–4412. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Rezaei, A.; Khazaei, A.; Shu, X.W.; Zheng, H.J. Targeted Development of Sustainable Green Catalysts for Oxidation of Alcohols via Tungstate-Decorated Multifunctional Amphiphilic Carbon Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 33194–33206. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar]
- Xie, R.; Zhang, L.; Xu, H.; Zhong, Y.; Sui, X.; Mao, Z. Construction of up-converting fluorescent carbon quantum dots/Bi20TiO32 composites with enhanced photocatalytic properties under visible light. Chem. Eng. J. 2017, 310, 79–90. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.; Yang, X.; Lee, S.T. Water-soluble fluorescent carbon quantum dots and photocatalyst design. Angew. Chem. Int. Ed. Engl. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Xu, W.; Li, N.; Xue, C.; Wang, Y.; Li, Y.; Wang, H.; Yang, J.; Hu, S. Dynamic restructuring of carbon dots/copper oxide supported on mesoporous hydroxyapatite brings exceptional catalytic activity in the reduction of 4-nitrophenol. Appl. Catal. B Environ. 2020, 263, 118299. [Google Scholar]
- Zhang, Y.; Wu, L.; Zhao, X.; Zhao, Y.; Tan, H.; Zhao, X.; Ma, Y.; Zhao, Z.; Song, S.; Wang, Y.; et al. Leaf-Mosaic-Inspired Vine-Like Graphitic Carbon Nitride Showing High Light Absorption and Efficient Photocatalytic Hydrogen Evolution. Adv. Energy Mater. 2018, 8, 1801139. [Google Scholar]
- Zayed, M.F.; Eisa, W.H.; Anis, B. Gallic acid-assisted growth of cuprous oxide within polyvinyl alcohol; a separable catalyst for oxidative and reductive degradation of water pollutants. J. Clean. Prod. 2021, 279, 123826. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Wu, Y.; Chen, J.; Zhao, J.; Jin, F.; Na, P. Carbon dots-TiO2 nanosheets composites for photoreduction of Cr(VI) under sunlight illumination: Favorable role of carbon dots. Appl. Catal. B Environ. 2018, 224, 508–517. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Li, Y.; Ma, C.; Huang, Z.; Wang, C.; Li, J.; Chen, Z.; Liu, S. One-step hydrothermal synthesis of fluorescent nanocrystalline cellulose/carbon dot hydrogels. Carbohydr. Polym. 2017, 175, 7–17. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Biesold-McGee, G.V.; Huang, J.; Ng, Y.H.; Sun, H.; Wang, J.; Lai, Y.; Lin, Z. Silk fibroin-derived nitrogen-doped carbon quantum dots anchored on TiO2 nanotube arrays for heterogeneous photocatalytic degradation and water splitting. Nano Energy 2020, 78, 105313. [Google Scholar]
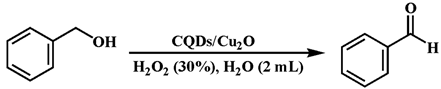 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Time (h) | T (°C) | Conversion (%) | Selectivity (%) |
| 1 | CQDs/Cu2O (hv) | 6 | r.t. | 35 | 90 |
| 2 | CQDs/Cu2O | 6 | r.t. | 2 | 100 |
| 3 | Cu2O (hv) | 6 | r.t. | 10 | 100 |
| 4 | Cu2O | 6 | r.t. | - | - |
| 5 | None | 6 | r.t. | - | - |
| 6 | TiO2 (hv) | 6 | r.t. | 13 | 100 |
| 7 | g-C3H4 (hv) | 6 | r.t. | 12 | 100 |
| 8 | CQDs (hv) | 6 | r.t. | 18 | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Z.; Liu, Y.; Wu, X.; Cheng, Y.; Yu, J.; Zhang, X.; Liu, N.; Liu, X.; Li, H. Carbon Quantum Dots/Cu2O Photocatalyst for Room Temperature Selective Oxidation of Benzyl Alcohol. Nanomaterials 2024, 14, 212. https://doi.org/10.3390/nano14020212
Tong Z, Liu Y, Wu X, Cheng Y, Yu J, Zhang X, Liu N, Liu X, Li H. Carbon Quantum Dots/Cu2O Photocatalyst for Room Temperature Selective Oxidation of Benzyl Alcohol. Nanomaterials. 2024; 14(2):212. https://doi.org/10.3390/nano14020212
Chicago/Turabian StyleTong, Zhuang, Yunliang Liu, Xin Wu, Yuanyuan Cheng, Jingwen Yu, Xinyue Zhang, Naiyun Liu, Xiang Liu, and Haitao Li. 2024. "Carbon Quantum Dots/Cu2O Photocatalyst for Room Temperature Selective Oxidation of Benzyl Alcohol" Nanomaterials 14, no. 2: 212. https://doi.org/10.3390/nano14020212
APA StyleTong, Z., Liu, Y., Wu, X., Cheng, Y., Yu, J., Zhang, X., Liu, N., Liu, X., & Li, H. (2024). Carbon Quantum Dots/Cu2O Photocatalyst for Room Temperature Selective Oxidation of Benzyl Alcohol. Nanomaterials, 14(2), 212. https://doi.org/10.3390/nano14020212







