Evaluation of the Hydrophilic/Hydrophobic Balance of 13X Zeolite by Adsorption of Water, Methanol, and Cyclohexane as Pure Vapors or as Mixtures
Abstract
1. Introduction
2. Materials and Methods
Methods
3. Results and Discussion
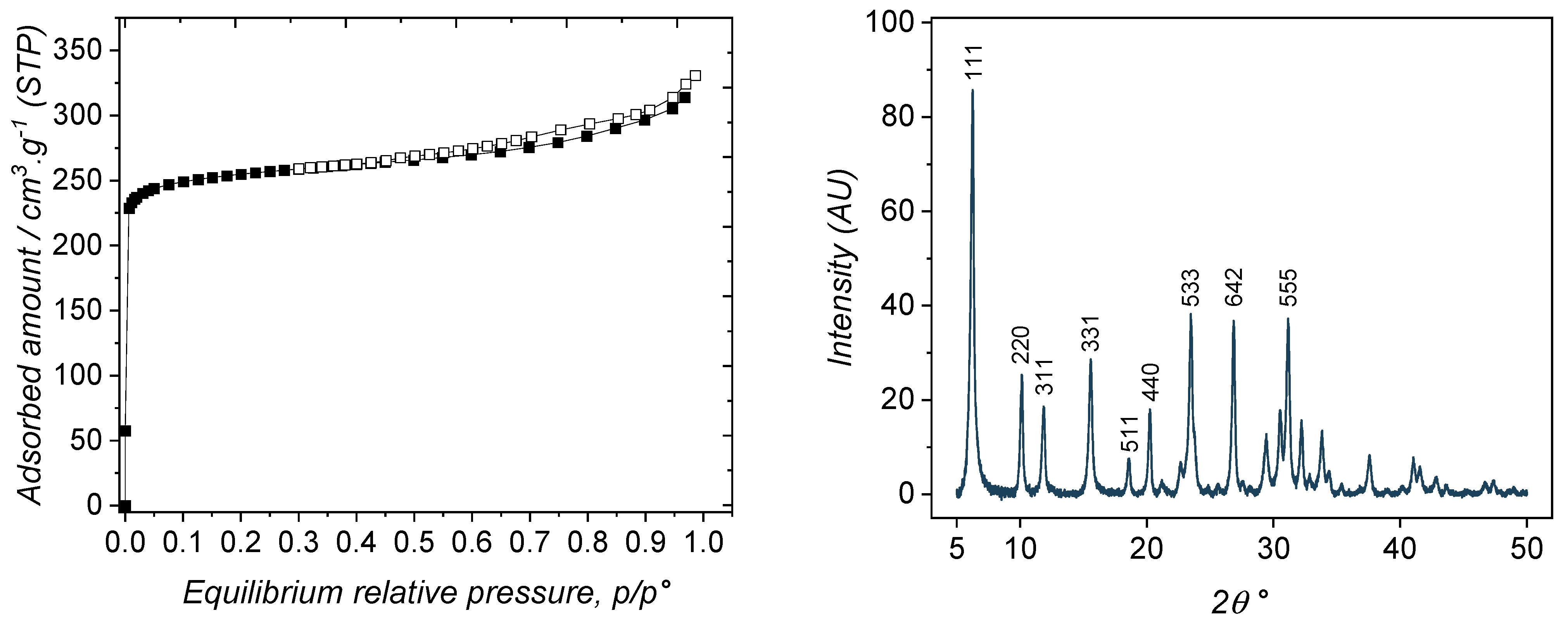
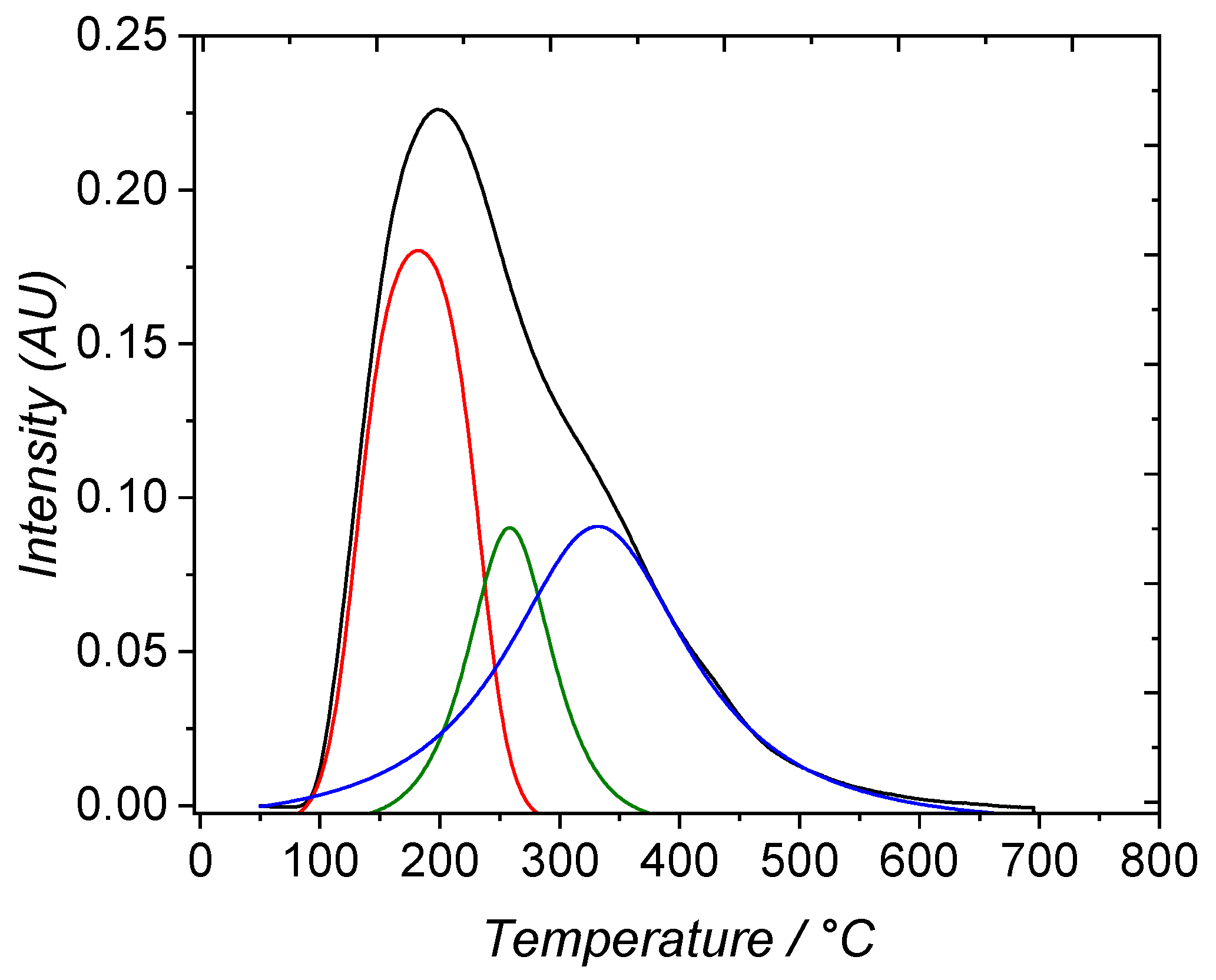
3.1. Material Characterization
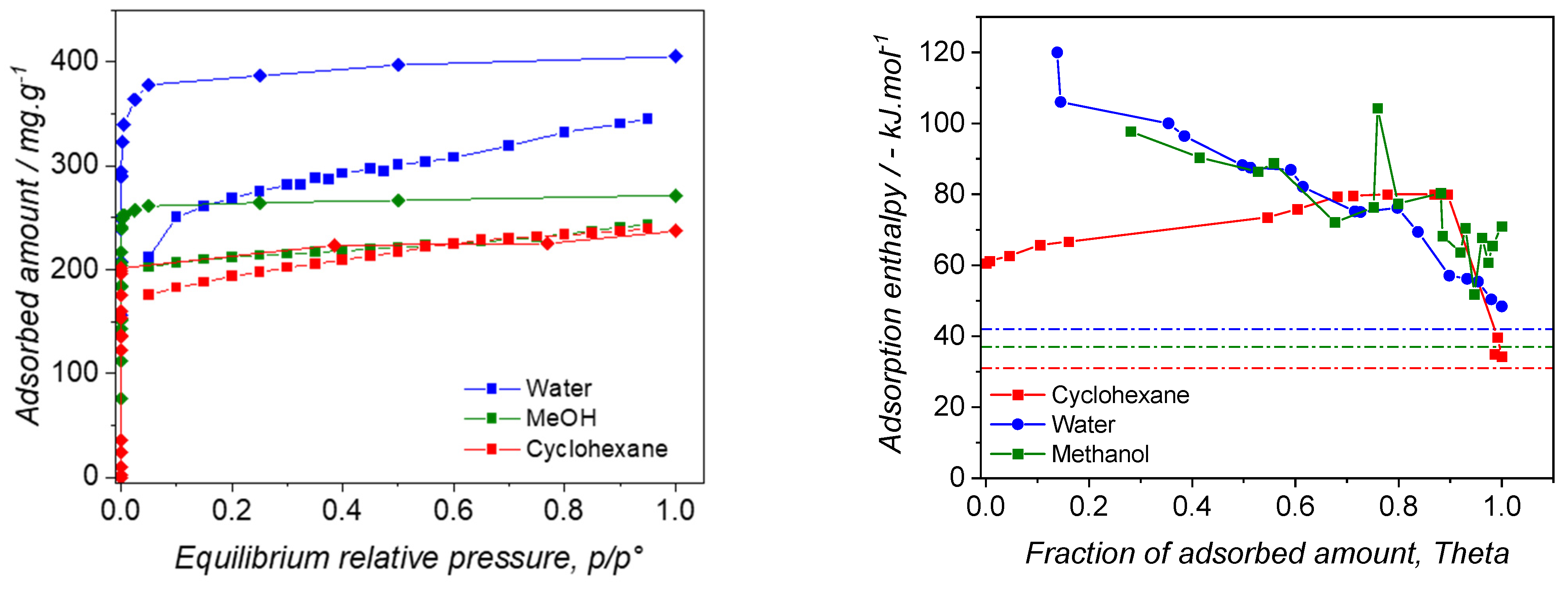
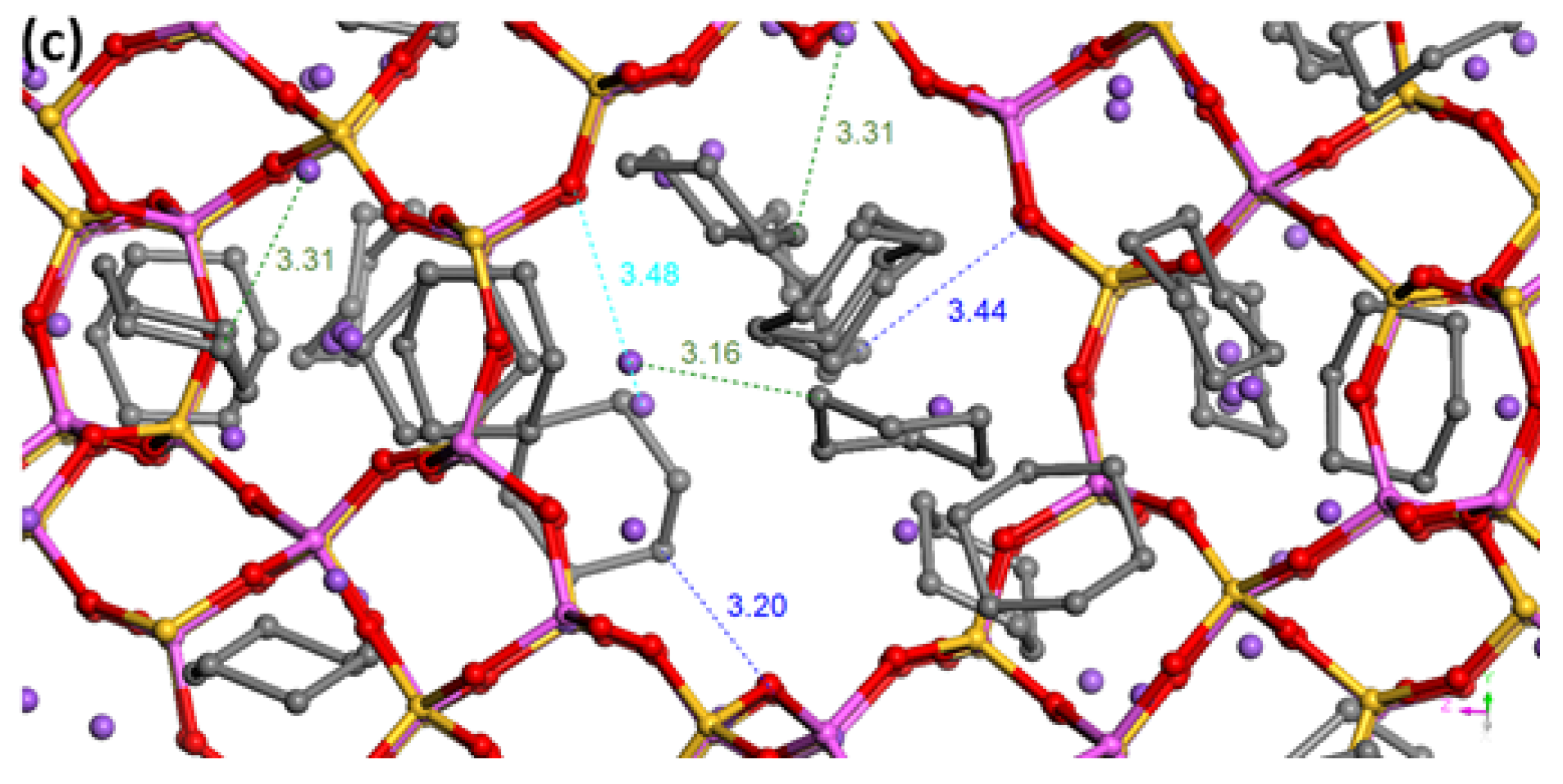
3.2. Pure Vapors Adsorption
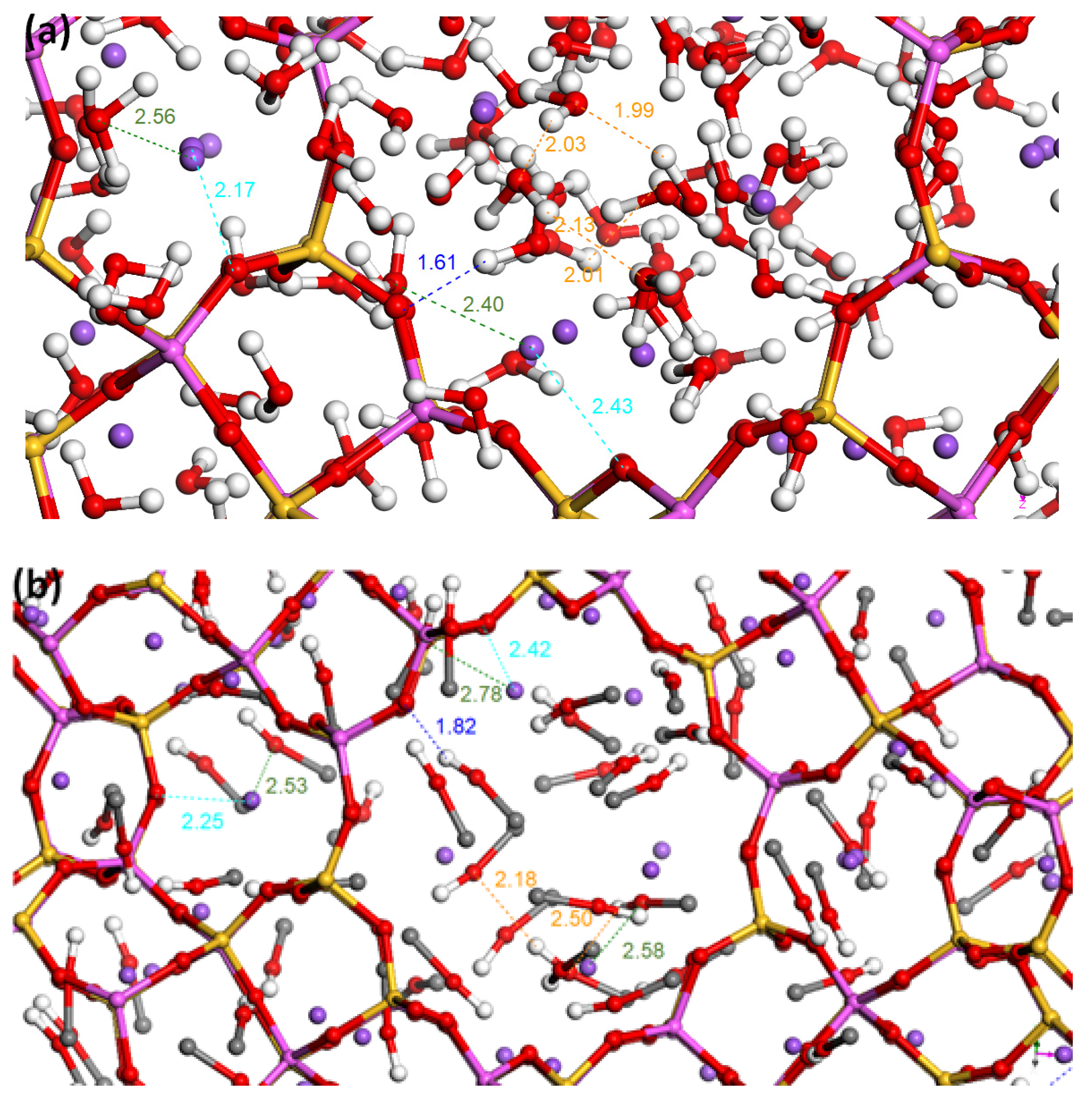
3.3. Mixtures Sorption Isotherms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Tan, S.; Horikawa, T.; Do, D.D.; Nicholson, D.; Liu, J. Water Adsorption on Carbon—A Review. Adv. Colloid Interface Sci. 2017, 250, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Peralta, R.A.; Alcántar-Vázquez, B.; Sánchez-Serratos, M.; González-Zamora, E.; Ibarra, I.A. Carbon Dioxide Capture in the Presence of Water Vapour in InOF-1. Inorg. Chem. Front. 2015, 2, 898–903. [Google Scholar] [CrossRef]
- Álvarez, J.R.; Peralta, R.A.; Balmaseda, J.; González-Zamora, E.; Ibarra, I.A. Water Adsorption Properties of a Sc(III) Porous Coordination Polymer for CO2 Capture Applications. Inorg. Chem. Front. 2015, 2, 1080–1084. [Google Scholar] [CrossRef]
- Férey, G. Hybrid Porous Solids: Past, Present, Future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial Carbon Dioxide Capture and Utilization: State of the Art and Future Challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Mantanis, B.G.I.; Young, R.A.; Rowell, R.M. Sweiling of Wood. Holzforschung 1994, 48, 480–490. [Google Scholar] [CrossRef]
- Bae, Y.-S.; Snurr, R.Q. Development and Evaluation of Porous Materials for Carbon Dioxide Separation and Capture. Angew. Chem. (Int. Ed. Engl.) 2011, 50, 11586–11596. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous Materials for Carbon Dioxide Separations. Nat. Mater. 2021, 20, 1060–1072. [Google Scholar]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Maharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in Solid Adsorbent Materials Development for CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Gas Process Suppliers. Association Engineering Data Book; Gas Processors Suppliers Association: Tulsa, OK, USA, 2004. [Google Scholar]
- Snurr, R.Q.; Farha, O.K.; Islamoglu, T.; Hupp, J.T.; Howarth, A.J.; Mendonca, M.L.; Bobbitt, N.S. Metal–Organic Frameworks for the Removal of Toxic Industrial Chemicals and Chemical Warfare Agents. Chem. Soc. Rev. 2017, 46, 3357–3385. [Google Scholar] [CrossRef]
- Bellat, J.P.; Moise, J.C.; Cottier, V.; Paulin, C.; Methivier, A. Effect of Water Content on the Selective Coadsorption of Gaseous P-Xylene and m-Xylene on the BaY Zeolite. Sep. Sci. Technol. 1998, 33, 2335–2348. [Google Scholar]
- Zárate, A.; Peralta, R.A.; Bayliss, P.A.; Howie, R.; Sánchez-Serratos, M.; Carmona-Monroy, P.; Solis-Ibarra, D.; González-Zamora, E.; Ibarra, I.A. CO2 Capture under Humid Conditions in NH2-MIL-53(Al): The Influence of the Amine Functional Group. RSC Adv. 2016, 6, 9978–9983. [Google Scholar] [CrossRef]
- Travlou, N.A.; Rodríguez-Castellón, E.; Bandosz, T.J. Sensing of NH3on Heterogeneous Nanoporous Carbons in the Presence of Humidity. Carbon 2016, 100, 64–73. [Google Scholar] [CrossRef]
- Glaser, R.; Weitkamp, J. Surface Hydrophobicity or Hydrophilicity o f Porous Solids. Handb. Porous Solids 2002, 1, 395–431. [Google Scholar]
- Ho, L.N.; Schuurman, Y.; Farrusseng, D.; Coasne, B. Solubility of Gases in Water Confined in Nanoporous Materials: ZSM-5, MCM-41, and MIL-100. J. Phys. Chem. C 2015, 119, 21547–21554. [Google Scholar] [CrossRef]
- Boudjema, L.; Long, J.; Salles, F.; Larionova, J.; Guari, Y.; Trens, P. Switch in the Hydrophobic-Hydrophilic Character of the Lipophilic Prussian Blue Analogues: An Affinity Control for Smart Gas Sorption Processes. Chem. Eur. J. 2019, 25, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Boudjema, L.; Long, J.; Petitjean, H.; Larionova, J.; Guari, Y.; Trens, P.; Salles, F. Adsorption of Volatile Organic Compounds by ZIF-8, Cu-BTC and a Prussian Blue Analogue: A Comparative Study. Inorg. Chim. Acta 2020, 501, 119316. [Google Scholar] [CrossRef]
- Chen, L.H.; Han, W.K.; Yan, X.D.; Zhang, J.W.; Jiang, Y.Q.; Gu, Z.G. A Highly Stable Ortho-Ketoenamine Covalent Organic Framework with Balanced Hydrophilic and Hydrophobic Sites for Atmospheric Water Harvesting. ChemSusChem 2022, 15, e202201824. [Google Scholar] [CrossRef]
- Ho, P.H.; Lofty, V.; Basta, A.; Trens, P. Designing Microporous Activated Carbons from Biomass for Carbon Dioxide Adsorption at Ambient Temperature. A Comparison between Bagasse and Rice by-Products. J. Clean. Prod. 2021, 294, 126260. [Google Scholar] [CrossRef]
- Adio, S.O.; Ganiyu, S.A.; Usman, M.; Abdulazeez, I.; Alhooshani, K. Facile and Efficient Nitrogen Modified Porous Carbon Derived from Sugarcane Bagasse for CO2 Capture: Experimental and DFT Investigation of Nitrogen Atoms on Carbon Frameworks. Chem. Eng. J. 2020, 382, 122964. [Google Scholar]
- Alabadi, A.; Razzaque, S.; Yang, Y.; Chen, S.; Tan, B. Highly Porous Activated Carbon Materials from Carbonized Biomass with High CO2 Capturing Capacity. Chem. Eng. J. 2015, 281, 600–612. [Google Scholar] [CrossRef]
- Alhassan, M.; Andrew, I.; Auta, M.; Umaru, M.; Garba, M.U.; Isah, A.G.; Alhassan, B. Comparative Studies of CO2 Capture Using Acid and Base Modified Activated Carbon from Sugarcane Bagasse. Biofuels 2018, 9, 719–728. [Google Scholar] [CrossRef]
- Phuong, N.T.T.; Tu, L.N.Q.; Hung, L.H.; Van Dung, N.; Duong, N.T.H.; Long, N.Q. Enhancement of CO2 Adsorption by Introducing Mesopores into FAU Zeolite Using Acid-Base Leaching. J. Appl. Sci. Eng. 2022, 26, 793–800. [Google Scholar]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A Review on Production of Metal Organic Frameworks (MOF) for CO2 Adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Beauvais, C.; Boutin, A.; Fuchs, A.H. Adsorption of Water in Zeolite Sodium-Faujasite. C. R. Chim. 2005, 8, 485–490. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Mintova, S. Verified Syntheses of Zeolitic Materials, XRD Patterns; Volume 3rd Revised Edition; Barrier, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-692-68539-6. [Google Scholar]
- Trens, P.; Ramsahye, N.; Toquer, G.; Boudjema, L.; Belarbi, H.; Shepherd, C.; Chang, J.-S. Adsorption and Separation of Hydrocarbons by the Metal Organic Framework MIL-101(Cr). Colloids Surf. Physicochem. Eng. Asp. 2017, 520, 46–52. [Google Scholar] [CrossRef]
- Vitale, G.; Mellot, C.F.; Bull, L.M.; Cheetham, A.K. Neutron Diffraction and Computational Study of Zeolite NaX: Influence of SIII‘ Cations on Its Complex with Benzene. J. Phys. Chem. B 1997, 101, 4559–4564. [Google Scholar]
- Alby, D.; Salles, F.; Fullenwarth, J.; Zajak, J. On the Use of Metal Cation-Exchanged Zeolites in Sorption Thermochemical Storage: Some Practical Aspects in Reference to the Mechanism of Water Vapor Adsorption. Sol. Energy Mater. Sol. Cells 2018, 179, 223–230. [Google Scholar]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a Full Periodic Table Force Field for Molecular Mechanics and Molecular Dynamics Simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Abascal, J.; Vega, C. A General Purpose Model for the Condensed Phases of Water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. [Google Scholar] [CrossRef]
- Chen, B.; Potoff, J.J.; Siepmann, J.I. Monte Carlo Calculations for Alcohols and Their Mixtures with Alkanes. Transferable Potentials for Phase Equilibria. 5. United-Atom Description of Primary, Secondary, and Tertiary Alcohols. J. Phys. Chem. B 2001, 105, 3093–3104. [Google Scholar] [CrossRef]
- Keasler, S.J.; Charan, S.M.; Wick, C.D.; Economou, I.G.; Siepmann, J.I. Transferable Potentials for Phase Equilibria-United Atom Description of Five- and Six-Membered Cyclic Alkanes and Ethers. J. Phys. Chem. B 2012, 116, 11234–11246. [Google Scholar] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W. Adsorption by Powders and Porous Solids; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Beaumont, R.; Barthomeuf, D. X, Y, Aluminum-Deficient and Ultrastable Faujasite-Type Zeolites: 2. Acidic strength and aluminum site reactivity. J. Catal. 1972, 27, 45–51. [Google Scholar] [CrossRef]
- Beaumont, R.; Barthomeuf, D. X, Y, Aluminum-Deficient and Ultrastable Faujasite-Typezeolites. 1. Acidic and Structural Properties. J. Catal. 1972, 26, 218–225. [Google Scholar] [CrossRef]
- Barthomeuf, D.; Beaumont, R. X, Y, Aluminum-Deficient, and Ultrastable Faujasite-Type Zeolites: 3. Catalytic Activity. J. Catal. 1973, 30, 288–297. [Google Scholar] [CrossRef]
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalysed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Kim, K.-M.; Oh, H.-T.; Lim, S.-J.; Ho, K.; Park, Y.; Lee, C.-H. Adsorption Equilibria of Water Vapor on Zeolite 3A, Zeolite 13X, and Dealuminated Y Zeolite. J. Chem. Eng. Data 2016, 61, 1547–1554. [Google Scholar] [CrossRef]
- Tang, Y.; Dubbeldam, D.; Tanase, S. Water–Ethanol and Methanol–Ethanol Separations Using In Situ Confined Polymer Chains in a Metal–Organic Framework. ACS Appl. Mater. Interfaces 2019, 11, 41383–41393. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, J.; Yang, F.; Chen, Z.; Deng, M.; Weng, L.; Ling, Y.; Zhou, Y. A Robust Etb-Type Metal–Organic Framework Showing Polarity-Exclusive Adsorption of Acetone over Methanol for Their Azeotropic Mixture. Chem. Commun. 2019, 55, 6495–6498. [Google Scholar] [CrossRef]
- Muller, J.A.; Conner, W.C. Cyclohexane in ZSM 5. 1. FTIR and x-Ray Studies. J. Phys. Chem. 1993, 97, 1451–1454. [Google Scholar] [CrossRef]
- Myers, A.L.; Prausnitz, J.M. Thermodynamics of Mixed-Gas Adsorption. AIChE J. 1965, 11, 121–127. [Google Scholar] [CrossRef]
- Benzaria, S.; Mamontova, E.; Guari, Y.; Larionova, J.; Long, J.; Trens, P.; Salles, F.; Zajac, J. Heat Release Kinetics upon Water Vapor Sorption Using Cation-Exchanged Zeolites and Prussian Blue Analogues as Adsorbents: Application to Short-Term Low-Temperature Thermochemical Storage of Energy. Energies 2021, 14, 3505. [Google Scholar] [CrossRef]
- Madero-Castro, R.M.; Luna-Triguero, A.; Sławek, A.; Vicent-Luna, J.M.; Calero, S. On the Use of Water and Methanol with Zeolites for Heat Transfer. ACS Sustain. Chem. Eng. 2023, 11, 4317–4328. [Google Scholar] [CrossRef]



| Molar Water Vapor % | Plateau mg/g | SOD mg/g | Scages mg/g | Total mg/g | Molar Adsorbed Water % |
|---|---|---|---|---|---|
| 100 | 308.0 | 24.5 | 283.5 | 308.0 | 100 |
| 66 | 281.6 | 24.5 | 256.5 | 281.0 | 75.6 |
| 50 | 274.3 | 24.5 | 249.9 | 274.5 | 64.0 |
| 33 | 261.8 | 24.5 | 237.2 | 261.7 | 49.5 |
| 0 | 224.9 | 0 | 224.9 | 224.9 | 0 |
| Molar Water Vapor % | Plateau/ mg/g | SOD/ mg/g | Scages/ mg/g | Total/ mg/g | Molar Adsorbed Water % |
|---|---|---|---|---|---|
| 100 | 308.0 | 24.5 | 283.5 | 308.0 | 100 |
| 66 | 270.1 | 24.5 | 245.5 | 270.0 | 71.5 |
| 50 | 253.2 | 24.5 | 228.5 | 253.0 | 23.0 |
| 33 | 233.0 | 24.5 | 225.0 | 249.5 | Undefined |
| 0 | 225.0 | 0 | 224.9 | 224.9 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saidi, M.; Bihl, F.; Gimello, O.; Louis, B.; Roger, A.-C.; Trens, P.; Salles, F. Evaluation of the Hydrophilic/Hydrophobic Balance of 13X Zeolite by Adsorption of Water, Methanol, and Cyclohexane as Pure Vapors or as Mixtures. Nanomaterials 2024, 14, 213. https://doi.org/10.3390/nano14020213
Saidi M, Bihl F, Gimello O, Louis B, Roger A-C, Trens P, Salles F. Evaluation of the Hydrophilic/Hydrophobic Balance of 13X Zeolite by Adsorption of Water, Methanol, and Cyclohexane as Pure Vapors or as Mixtures. Nanomaterials. 2024; 14(2):213. https://doi.org/10.3390/nano14020213
Chicago/Turabian StyleSaidi, Meryem, François Bihl, Olinda Gimello, Benoit Louis, Anne-Cécile Roger, Philippe Trens, and Fabrice Salles. 2024. "Evaluation of the Hydrophilic/Hydrophobic Balance of 13X Zeolite by Adsorption of Water, Methanol, and Cyclohexane as Pure Vapors or as Mixtures" Nanomaterials 14, no. 2: 213. https://doi.org/10.3390/nano14020213
APA StyleSaidi, M., Bihl, F., Gimello, O., Louis, B., Roger, A.-C., Trens, P., & Salles, F. (2024). Evaluation of the Hydrophilic/Hydrophobic Balance of 13X Zeolite by Adsorption of Water, Methanol, and Cyclohexane as Pure Vapors or as Mixtures. Nanomaterials, 14(2), 213. https://doi.org/10.3390/nano14020213








