The Recent Progresses of Electrodes and Electrolysers for Seawater Electrolysis
Abstract
1. Introduction
2. Water Splitting Reactions and Design Principles of Electrodes
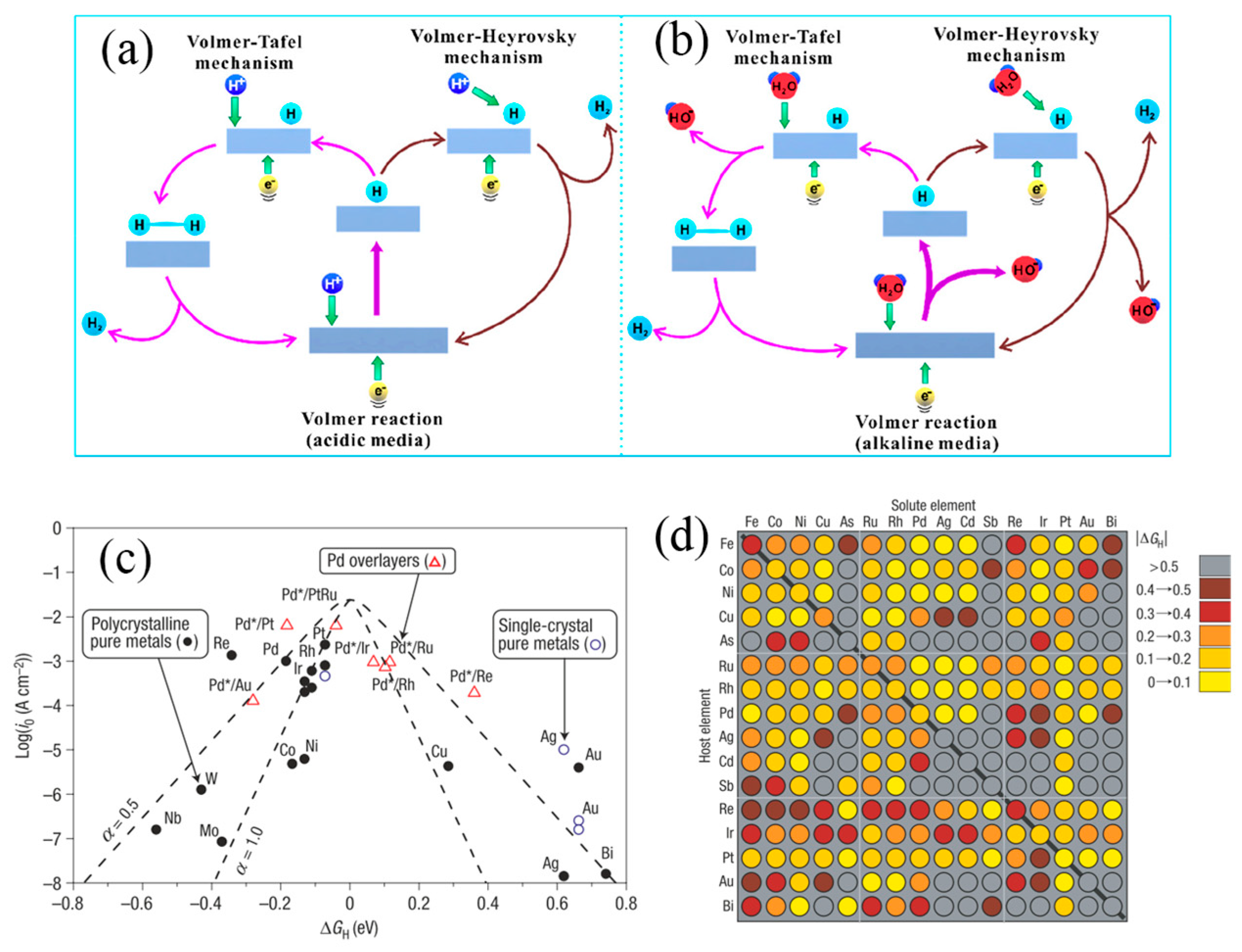
2.1. HER and OER Mechanism
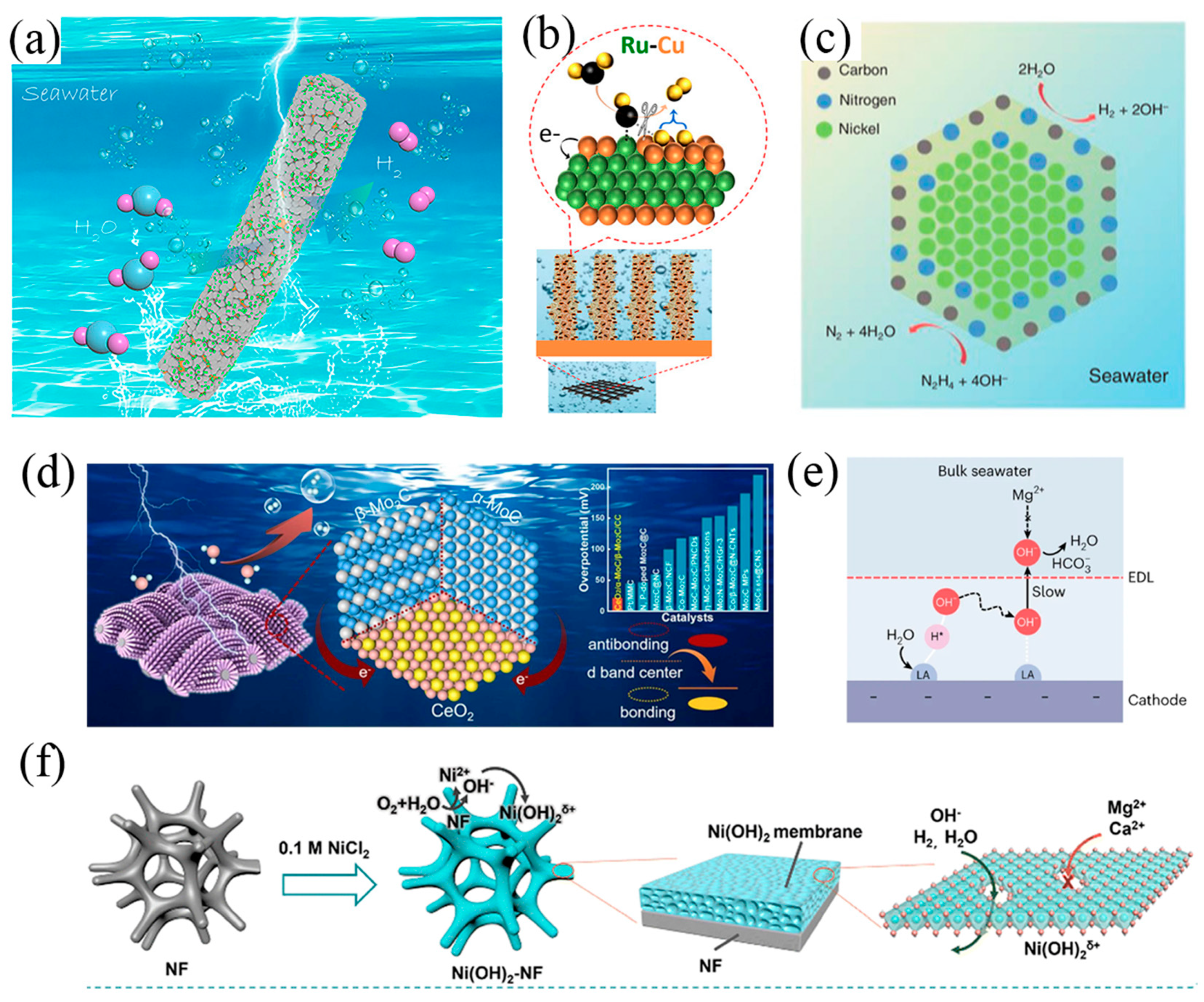
2.2. Design Principles of Cathode and Anode

3. Seawater Splitting Electrolysers
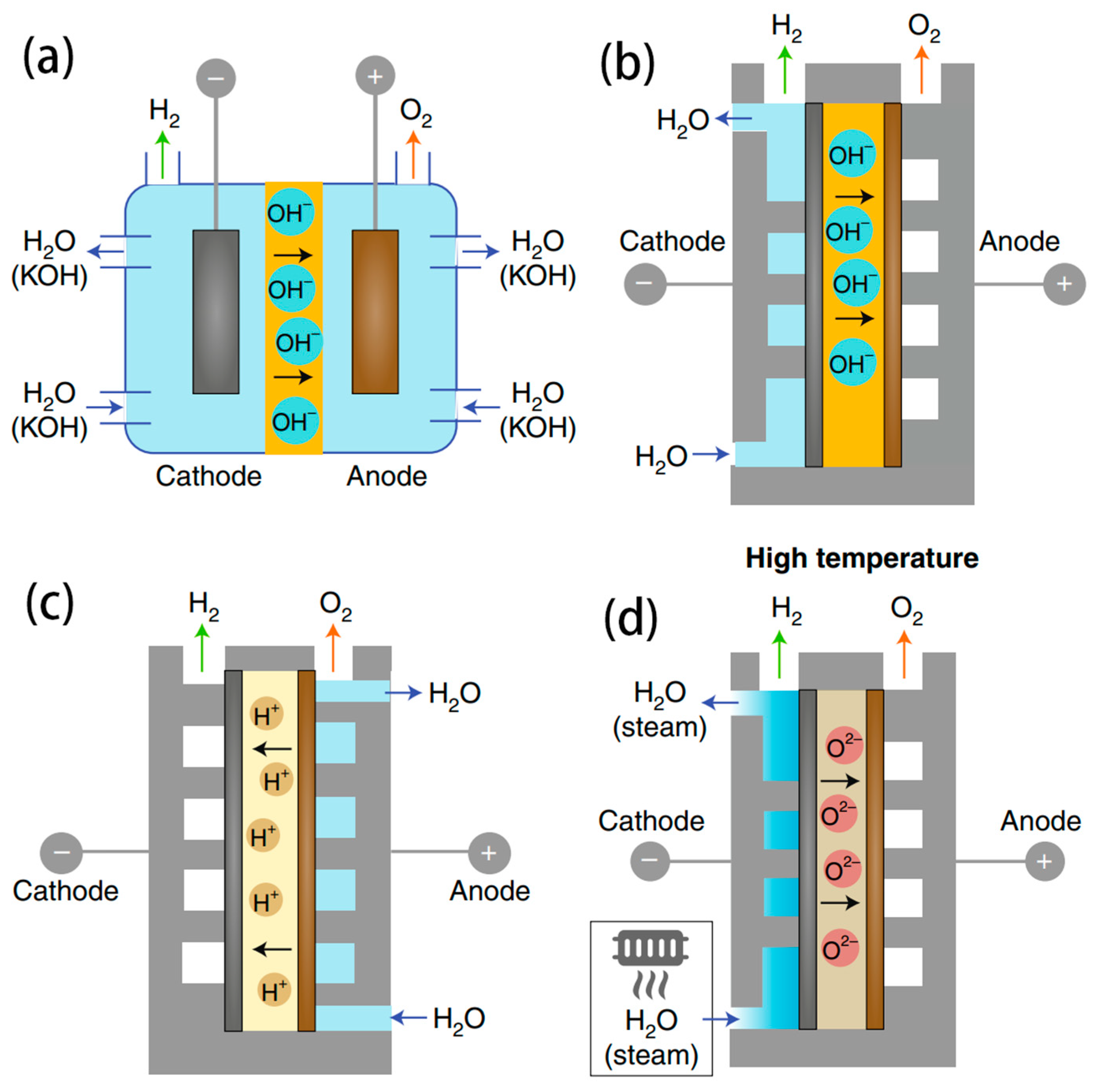
3.1. AWE Electrolyser
- (i)
- Low working current density: AWE operates at a lower current density, typically limited to 0.6 A cm−2 at the maximum, resulting in lower production and energy efficiency. Due to the high internal resistance of the electrolyte, the energy consumption for hydrogen production can be as high as 5–7 kWh/m3 H2. The produced hydrogen gas is approximately 99.7% pure but contains residual alkali, necessitating further purification.
- (ii)
- Slow response rate: Rapid shutdown or startup of AWE cells is challenging, leading to the difficulties in adjusting the H2 production rate quickly. When starting up, the cell temperature is initially insufficient for H2 production, with the consumed power used primarily to generate heat and raise the cell temperature.
- (iii)
- Reaction of Alkaline Electrolyte with CO2: Alkaline electrolytes, such as KOH, react with CO2 in the air, forming insoluble carbonates under alkaline conditions. These insoluble carbonates block the porous catalytic layer, hindering the transfer of reactants and products, and significantly reducing the performance of the electrolysis cell.
- (iv)
- Difficulty in integration with off-grid renewable energy: The slow response rate of alkaline electrolysis cells and challenges in rapid shutdown or startup, combined with the characteristics of the materials used inside the cell, means that the operational power cannot fall below a certain threshold to avoid the risk of hydrogen and oxygen crossover exceeding the explosion limit. Therefore, it is difficult to independently pair with renewable energy generation in off-grid scenarios without installing electrochemical storage or adding fuel cells to adjust the load.
- (v)
- Large volume: The current density of alkaline electrolysis cells at atmospheric pressure is 0.2 A cm−2 and 1 A cm−2 under pressure, which is lower compared to other electrolysis cell designs. This necessitates a larger surface area for the same power output, resulting in larger cell volumes.
3.2. PEMWE Electrolyser
3.3. AEMWE Electrolyser
3.4. SOEC
4. Summary and Perspectives
- Integration with renewable energy: Seawater electrolysis systems are being tailored to work seamlessly with renewable energy sources like solar, wind, and wave power. This integration could lead to the creation of self-sustaining ‘green hydrogen’ production platforms that operate offshore or on remote coastlines, tapping directly into the abundance of seawater and renewable energy.
- Advanced electrolyser designs: Innovative designs that enhance the efficiency and durability of electrolysers are in development. This includes optimizing electrode materials to withstand the corrosive nature of seawater, and designing advanced membrane technologies that can handle the complex chemistry of seawater electrolysis, including the management of chloride ions.
- Catalytic efficiency: A primary focus is to enhance the efficiency of OER and HER in seawater electrolysis. Research is ongoing into non-noble metal catalysts and novel alloy compositions that could provide similar or better efficiency at a lower cost and with higher durability.
- Selective ion separation: Technological advancements in selective ion membranes or separators can prevent the formation of harmful byproducts such Cl−. These components are crucial for increasing the viability of the process, especially at the high current densities required for industrial-scale hydrogen production.
- Scale-up challenges: The scale-up of seawater electrolysis faces challenges, which causes future research directed at maintaining efficiency and stability while scaling up the operation to meet commercial and industrial demands.
- Environmental and economic viability: The development of seawater electrolysis technology is not only a technical challenge but also an environmental and economic one. It is critical for addressing the potential environmental impacts and ensuring the economic competitiveness of hydrogen production from seawater electrolysis. Life cycle assessments and cost analyses are integral to this effort.
- Regulatory and safety standards: With the technology development, it is essential to establish regulatory and safety standards, which will include guidelines for the installation, operation, and maintenance of large-scale seawater electrolysis plants, particularly in off-grid environments.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef]
- Msheik, M.; Rodat, S.; Abanades, S. Methane Cracking for Hydrogen Production: A Review of Catalytic and Molten Media Pyrolysis. Energies 2021, 14, 3107. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, M.; Liang, D.; Yang, Z.; Cheng, W.; Tang, Z.; Wang, J.; Zhang, H. Recent advances during CH4 dry reforming for syngas production: A mini review. Int. J. Hydrogen Energy 2021, 46, 5852–5874. [Google Scholar] [CrossRef]
- Dehane, A.; Nemdili, L.; Merouani, S.; Ashokkumar, M. Critical Analysis of Hydrogen Production by Aqueous Methanol Sonolysis. Top. Curr. Chem. 2023, 381, 9. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Nogal, Ł.; Młotek, M.; Krawczyk, K. Enhanced production of hydrogen from methanol using spark discharge generated in a small portable reactor. Energy Rep. 2022, 8, 183–191. [Google Scholar] [CrossRef]
- Yang, Y.; Liew, R.K.; Tamothran, A.M.; Foong, S.Y.; Yek, P.N.Y.; Chia, P.W.; Van Tran, T.; Peng, W.; Lam, S.S. Gasification of refuse-derived fuel from municipal solid waste for energy production: A review. Environ. Chem. Lett. 2021, 19, 2127–2140. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen production from water electrolysis: Role of catalysts. Nano. Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- Ozturk, M.; Dincer, I. A comprehensive review on power-to-gas with hydrogen options for cleaner applications. Int. J. Hydrogen Energy 2021, 46, 31511–31522. [Google Scholar] [CrossRef]
- Chang, F.; Gao, W.; Guo, J.; Chen, P. Emerging Materials and Methods toward Ammonia-Based Energy Storage and Conversion. Adv. Mater. 2021, 33, e2005721. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sust. Energ. Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sust. Energ. Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, e2007100. [Google Scholar] [CrossRef]
- Zuo, Y.; Bellani, S.; Saleh, G.; Ferri, M.; Shinde, D.V.; Zappia, M.I.; Buha, J.; Brescia, R.; Prato, M.; Pascazio, R.; et al. Ru-Cu Nanoheterostructures for Efficient Hydrogen Evolution Reaction in Alkaline Water Electrolyzers. J. Am. Chem. Soc. 2023, 145, 21419–21431. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Liu, P.; Tang, X.; Zhang, S.; Chen, H.; Yang, Q.; Chen, X.; Tian, Z.; Dai, S.; et al. Ag Nanoparticle-Induced Surface Chloride Immobilization Strategy Enables Stable Seawater Electrolysis. Adv. Mater. 2023, 36, e2306062. [Google Scholar] [CrossRef]
- Lebrouhi, B.E.; Djoupo, J.J.; Lamrani, B.; Benabdelaziz, K.; Kousksou, T. Global hydrogen development—A technological and geopolitical overview. Int. J. Hydrogen Energy 2022, 47, 7016–7048. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, e2108133. [Google Scholar] [CrossRef]
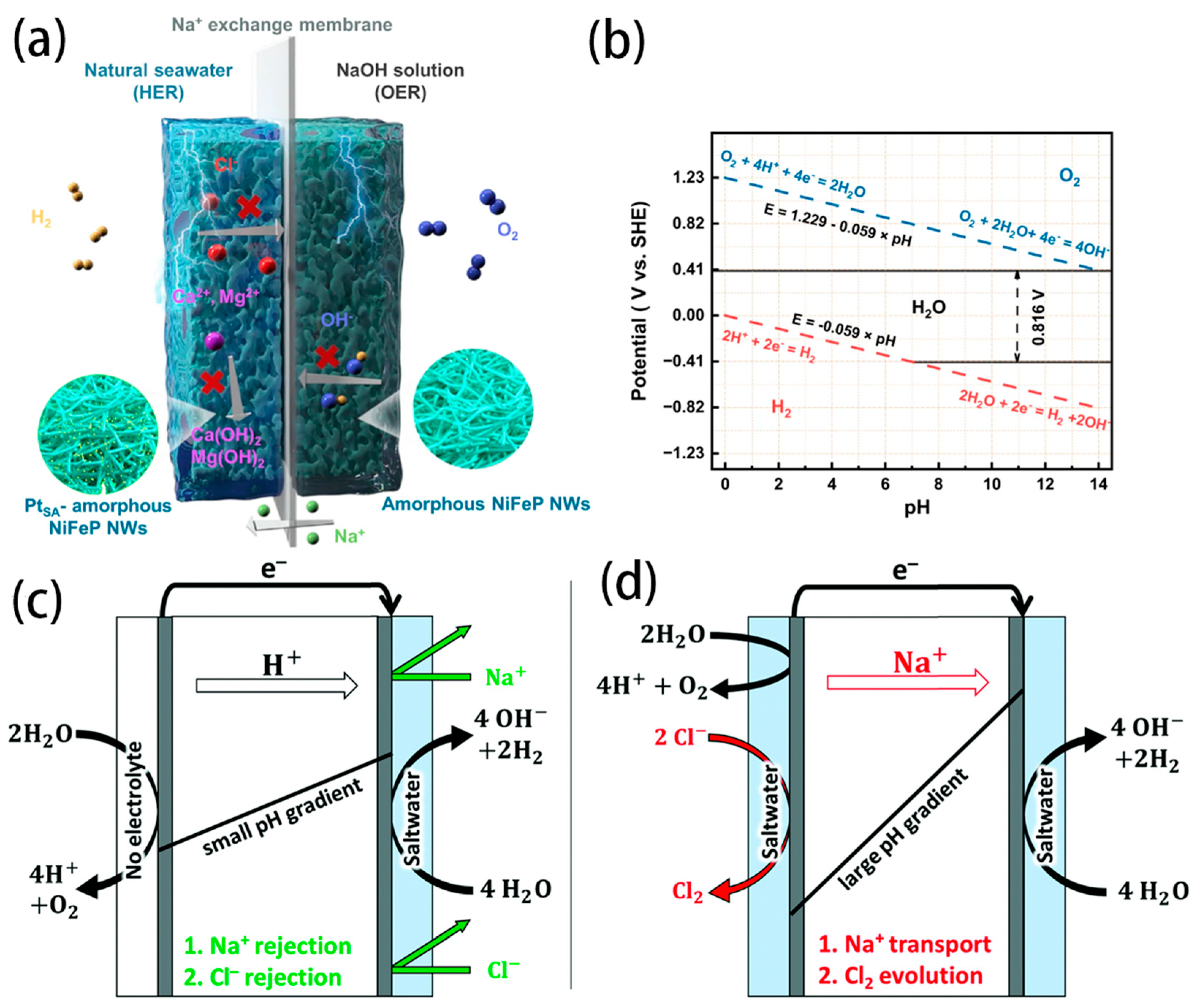
- Shi, H.; Wang, T.; Liu, J.; Chen, W.; Li, S.; Liang, J.; Liu, S.; Liu, X.; Cai, Z.; Wang, C.; et al. A sodium-ion-conducted asymmetric electrolyzer to lower the operation voltage for direct seawater electrolysis. Nat. Commun. 2023, 14, 3934. [Google Scholar] [CrossRef]
- Rossi, R.; Hall, D.M.; Shi, L.; Cross, N.R.; Gorski, C.A.; Hickner, M.A.; Logan, B.E. Using a vapor-fed anode and saline catholyte to manage ion transport in a proton exchange membrane electrolyzer. Environ. Sci. Technol. 2021, 14, 6041–6049. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, J.; Jang, M.J.; Yang, J.; Jeong, J.; Park, J.; Kim, Y.; Seo, M.H.; Chen, Z.; Choi, S.M. High-performance anion exchange membrane alkaline seawater electrolysis. J. Mater. Chem. A 2021, 9, 9586–9592. [Google Scholar] [CrossRef]
- Xing, J.; Zeng, Z.; Best, W.; Liu, Z.; Bonville, L.; Maric, R.; Bliznakov, S. Long-term durability test of highly efficient membrane electrode assemblies for anion exchange membrane seawater electrolyzers. J. Power Sources 2023, 558, 232564. [Google Scholar] [CrossRef]
- Park, Y.S.; Jeong, J.-Y.; Jang, M.J.; Kwon, C.-Y.; Kim, G.H.; Jeong, J.; Lee, J.-h.; Lee, J.; Choi, S.M. Ternary layered double hydroxide oxygen evolution reaction electrocatalyst for anion exchange membrane alkaline seawater electrolysis. J. Energy Chem. 2022, 75, 127–134. [Google Scholar] [CrossRef]
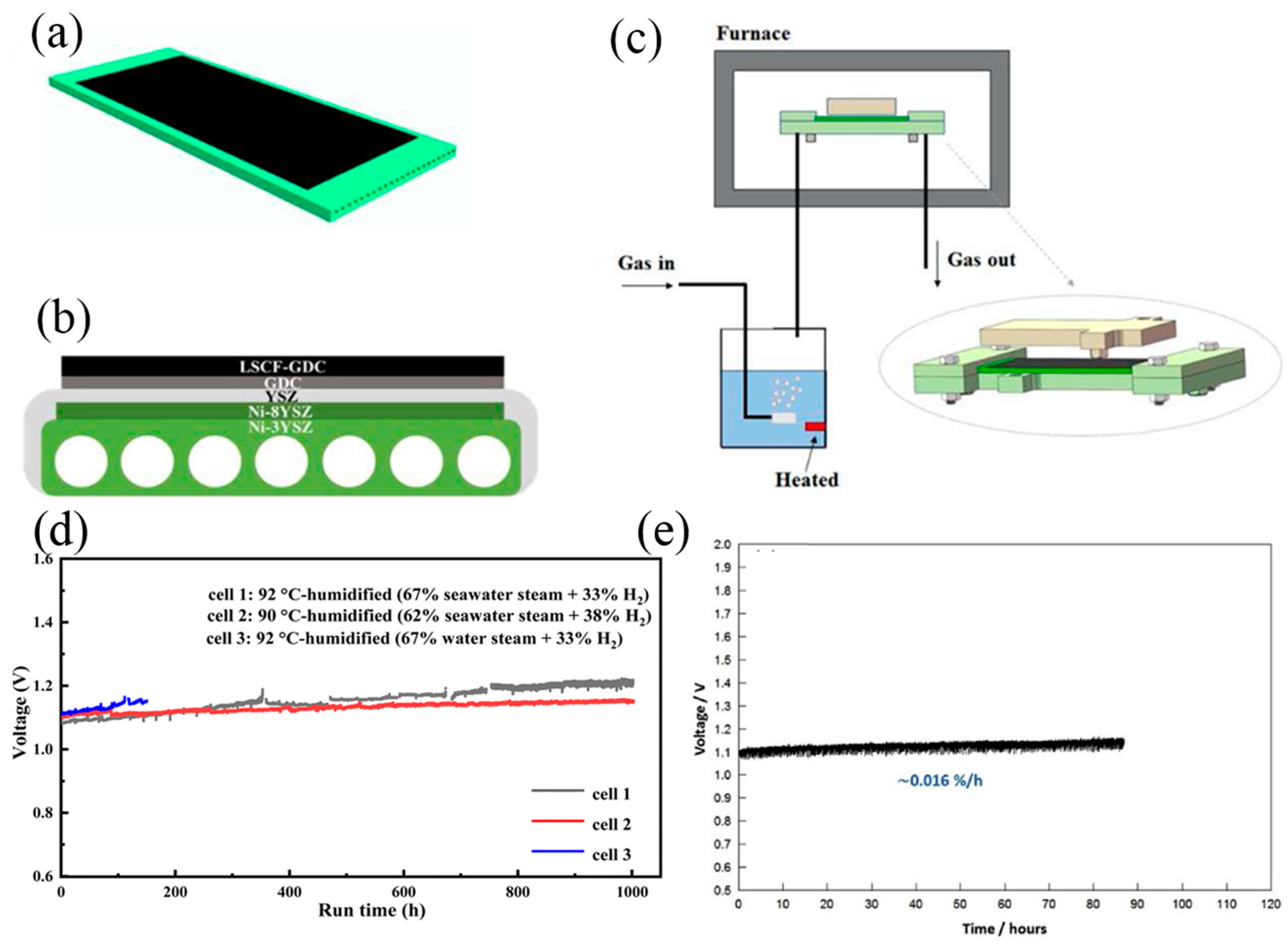
- Pan, H.; Wu, A.; Au, S.F.; Yang, Y.; Song, Z.; Liu, Z.; Gong, X.; Guan, W. Effect of the steam/hydrogen ratio on the performance of flat-tube solid oxide electrolysis cells for seawater. Sustain. Energ. Fuels 2023, 7, 3333–3341. [Google Scholar] [CrossRef]
- Song, Z.; Pan, H.; Wan, G.; Wu, A.; Chen, Q.; Guan, W.; Singhal, S.C. Enhancing durability of solid oxide cells for hydrogen production from seawater by designing nano-structured Sm0.5Sr0.5Co3-δ infiltrated air electrodes. Int. J. Hydrogen Energy 2023, 48, 27095–27104. [Google Scholar] [CrossRef]
- Hodges, A.; Hoang, A.L.; Tsekouras, G.; Wagner, K.; Lee, C.Y.; Swiegers, G.F.; Wallace, G.G. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 2022, 13, 1304. [Google Scholar] [CrossRef]
- Veroneau, S.S.; Hartnett, A.C.; Thorarinsdottir, A.E.; Nocera, D.G. Direct Seawater Splitting by Forward Osmosis Coupled to Water Electrolysis. ACS Appl. Energ. Mater. 2022, 5, 1403–1408. [Google Scholar] [CrossRef]
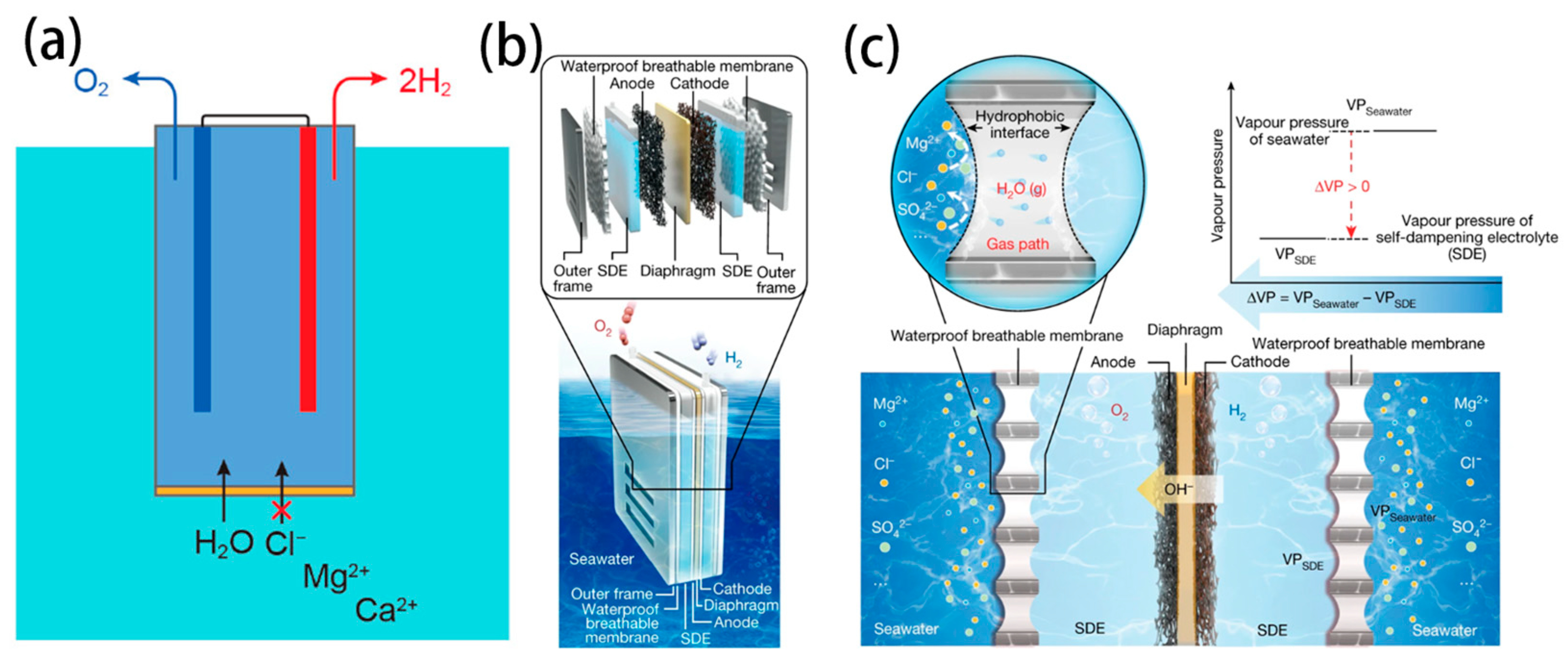
- Xie, H.; Zhao, Z.; Liu, T.; Wu, Y.; Lan, C.; Jiang, W.; Zhu, L.; Wang, Y.; Yang, D.; Shao, Z. A membrane-based seawater electrolyser for hydrogen generation. Nature 2022, 612, 673–678. [Google Scholar] [CrossRef]
- Jiang, S.; Suo, H.; Zhang, T.; Liao, C.; Wang, Y.; Zhao, Q.; Lai, W. Recent Advances in Seawater Electrolysis. Catalysts 2022, 12, 123. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Y.; Yang, T.; Jiang, L. Recent advances in electrocatalysts for seawater splitting. Nano Mater. Sci. 2023, 5, 101–116. [Google Scholar] [CrossRef]
- Feng, S.; Yu, Y.; Li, J.; Luo, J.; Deng, P.; Jia, C.; Shen, Y.; Tian, X. Recent progress in seawater electrolysis for hydrogen evolution by transition metal phosphides. Catal. Commun. 2022, 162, 106382. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.; Chorkendorff, I.B.; Norskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Hao, Y.; Hung, S.-F.; Zeng, W.-J.; Wang, Y.; Zhang, C.; Kuo, C.-H.; Wang, L.; Zhao, S.; Zhang, Y.; Chen, H.-Y.; et al. Switching the Oxygen Evolution Mechanism on Atomically Dispersed Ru for Enhanced Acidic Reaction Kinetics. J. Am. Chem. Soc. 2023, 145, 23659–23669. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design Criteria, Operating Conditions, and Nickel-Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef]
- Cho, M.K.; Park, H.-Y.; Lee, H.J.; Kim, H.-J.; Lim, A.; Henkensmeier, D.; Yoo, S.J.; Kim, J.Y.; Lee, S.Y.; Park, H.S.; et al. Alkaline anion exchange membrane water electrolysis: Effects of electrolyte feed method and electrode binder content. J. Power Sources 2018, 382, 22–29. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Z.; Zhang, Y.; Thomas, T.; Du, H.; Huang, K.; Attfield, J.P.; Yang, M. An ultra-low Pt metal nitride electrocatalyst for sustainable seawater hydrogen production. Environ. Sci. Technol. 2023, 16, 4584–4592. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, J.; Lv, E.; Li, T.; Xu, H.; Sun, L.; Nairan, A.; Zhang, Q. Integration of Alloy Segregation and Surface Co-O Hybridization in Carbon-Encapsulated CoNiPt Alloy Catalyst for Superior Alkaline Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2303833. [Google Scholar] [CrossRef]
- He, Y.; Xu, J.; Wang, F.; Mao, Q.; Huang, Y. Recent advances in Ni-Fe-based electrocatalysts for oxygen evolution reaction. Chem. Ind. Eng. Prog. 2016, 35, 2057–2062. [Google Scholar]
- Zhao, T.; Wang, S.; Jia, C.; Rong, C.; Su, Z.; Dastafkan, K.; Zhang, Q.; Zhao, C. Cooperative Boron and Vanadium Doping of Nickel Phosphides for Hydrogen Evolution in Alkaline and Anion Exchange Membrane Water/Seawater Electrolyzers. Small 2023, 19, 2208076. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Jiang, W.; Yu, T.; Tan, K.; Yin, S. Strong electronic coupling of CoNi and N-doped-carbon for efficient urea-assisted H2 production at a large current density. Carbon Energy 2023, 5, e368. [Google Scholar] [CrossRef]
- Xia, C.; Liang, H.; Zhu, J.; Schwingenschlögl, U.; Alshareef, H.N. Active Edge Sites Engineering in Nickel Cobalt Selenide Solid Solutions for Highly Efficient Hydrogen Evolution. Adv. Energy Mater. 2017, 7, 1602089. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Gao, W.; Mao, Z.; Hu, E.; Gao, X.; Zhang, J.; Chen, Z. Strategies to enhance the electrocatalytic behavior of metal selenides for hydrogen evolution reaction: A review. Int. J. Hydrogen Energy 2023, 48, 36722–36749. [Google Scholar] [CrossRef]
- Hu, M.; Chen, H.; Liu, B.; Xu, X.; Cao, B.; Jing, P.; Zhang, J.; Gao, R.; Zhang, J. Coupling ceria with dual-phased molybdenum carbides for efficient and stable hydrogen evolution electrocatalysis at large-current-density in freshwater and seawater. Appl. Catal. B 2022, 317, 121774. [Google Scholar] [CrossRef]
- Dutta, A.; Breuer, O.; Krishnappa, M.; Minnes, R.; Zak, A.; Borenstein, A. 1D transition-metal dichalcogenides/carbon core–shell composites for the hydrogen evolution reaction. J. Mater. Chem. A 2023, 11, 21806–21816. [Google Scholar] [CrossRef]
- Ritz, A.J.; Bertini, I.A.; Nguyen, E.T.; Strouse, G.F.; Lazenby, R.A. Electrocatalytic activity and surface oxide reconstruction of bimetallic iron-cobalt nanocarbide electrocatalysts for the oxygen evolution reaction. Rsc Adv. 2023, 13, 33413–33423. [Google Scholar] [CrossRef]
- Lamiel, C.; Hussain, I.; Rabiee, H.; Ogunsakin, O.R.; Zhang, K. Metal-organic framework-derived transition metal chalcogenides (S, Se, and Te): Challenges, recent progress, and future directions in electrochemical energy storage and conversion systems. Coord. Chem. Rev. 2023, 480, 215030. [Google Scholar] [CrossRef]
- Bao, X.; Wang, T.; Yang, Y. Recent progress in bimetallic carbide-based electrocatalysts for water splitting. Mater. Chem. Front. 2024; advance. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Tang, C.; Vasileff, A.; Li, L.; Slattery, A.; Qiao, S.Z. Stable and Highly Efficient Hydrogen Evolution from Seawater Enabled by an Unsaturated Nickel Surface Nitride. Adv. Mater. 2021, 33, e2007508. [Google Scholar] [CrossRef]
- Zhao, L.; Tong, Y.; Ding, Y.; Kong, W.; Wang, J.; Li, B.; Zhen, Y.; Xu, J.; Xing, W. Designing interface structures of nickel with transition metal nitrides for enhanced hydrogen electro-oxidation. Surf. Interfaces 2023, 37, 102659. [Google Scholar] [CrossRef]
- Sreehari, S.; George, N.S.; Jose, L.M.; Subramaniam, R.T.; Aravind, A. A review on 2D transition metal nitrides: Structural and morphological impacts on energy storage and photocatalytic applications. J. Alloys Compd. 2023, 950, 169888. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, C.; Liu, L.; Zhu, M. A review on the synthesis of transition metal nitride nanostructures and their energy related applications. Green Energy Environ. 2023, 8, 406–437. [Google Scholar] [CrossRef]
- Feng, C.; Chen, M.; Yang, Z.; Xie, Z.; Li, X.; Li, S.; Abudula, A.; Guan, G. Electrocatalytic seawater splitting for hydrogen production: Recent progress and future prospects. J. Mater. Sci. Technol. 2023, 162, 203–226. [Google Scholar] [CrossRef]
- Xu, S.-W.; Li, J.; Zhang, N.; Shen, W.; Zheng, Y.; Xi, P. Recent advances in direct seawater splitting for producing hydrogen. Chem. Commun. 2023, 59, 9792–9802. [Google Scholar] [CrossRef]
- Tahri, W.; Zhou, X.; Khan, R.; Sajid, M. Recent Trends in Transition Metal Phosphide (TMP)-Based Seawater Electrolysis for Hydrogen Evolution. Sustainability 2023, 15, 14389. [Google Scholar] [CrossRef]
- Dang, Y.; Wang, G.; Li, X.; Ma, X.; Yue, F.; Wang, C.; Gao, L.; Fu, F. Enhanced alkaline/seawater hydrogen evolution reaction performance of NiSe2 by ruthenium and tungsten bimetal doping. Int. J. Hydrogen Energy 2023, 48, 17035–17044. [Google Scholar] [CrossRef]
- Kim, K.; Tiwari, A.P.; Hyun, G.; Yoon, Y.; Kim, H.; Park, J.Y.; An, K.-S.; Jeon, S. Continuous 3D-nanopatterned Ni–Mo solid solution as a free-standing electrocatalyst for the hydrogen evolution reaction in alkaline medium. J. Mater. Chem. A 2021, 9, 7767–7773. [Google Scholar] [CrossRef]
- Wang, B.; Chu, S.; Zheng, L.; Li, X.; Zhang, J.; Zhang, F. Application of X-Ray Absorption Spectroscopy in Electrocatalytic Water Splitting and CO2 Reduction. Small Sci. 2021, 1, 2100023. [Google Scholar] [CrossRef]
- Liang, H.; Yan, Z.; Zeng, G. Recent Advances in In Situ/Operando Surface/Interface Characterization Techniques for the Study of Artificial Photosynthesis. Inorganics 2022, 11, 16. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, L.; Johannessen, B.; Saunders, M.; Liu, C.; Yang, S.Z.; Jiang, S.P. Designed Iron Single Atom Catalysts for Highly Efficient Oxygen Reduction Reaction in Alkaline and Acid Media. Adv. Mater. Interfaces 2020, 8, 2001788. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; Song, S.; McElhenny, B.; Zhang, F.; Chen, S.; Ren, Z. Hydrogen Generation from Seawater Electrolysis over a Sandwich-like NiCoN|NixP|NiCoN Microsheet Array Catalyst. ACS Energy Lett. 2020, 5, 2681–2689. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.-Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272. [Google Scholar] [CrossRef]
- Liu, Q.; Yan, Z.; Gao, J.; Fan, H.; Li, M.; Wang, E. Ion sieving membrane for direct seawater anti-precipitation hydrogen evolution reaction electrode. Chem. Sci. 2023, 14, 11830–11839. [Google Scholar] [CrossRef]
- Becker, H.; Murawski, J.; Shinde, D.V.; Stephens, I.E.L.; Hinds, G.; Smith, G. Impact of impurities on water electrolysis: A review. Sustain. Energ. Fuels 2023, 7, 1565–1603. [Google Scholar] [CrossRef]
- Favaro, M.; Valero-Vidal, C.; Eichhorn, J.; Toma, F.M.; Ross, P.N.; Yano, J.; Liu, Z.; Crumlin, E.J. Elucidating the alkaline oxygen evolution reaction mechanism on platinum. J. Mater. Chem. A 2017, 5, 11634–11643. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; An, P.; Tang, C.; Yu, H.; Zhang, Q.; Peng, H.-J.; Gu, L.; Zheng, Y.; Song, T.; et al. Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution. Nat. Commun. 2023, 14, 354. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.-F.; Zhu, X.-L.; Li, B.-Q.; Zhang, Q. Advances in Hybrid Electrocatalysts for Oxygen Evolution Reactions: Rational Integration of NiFe Layered Double Hydroxides and Nanocarbon. Part. Part. Syst. Charact. 2016, 33, 473–486. [Google Scholar] [CrossRef]
- Bodhankar, P.M.; Sarawade, P.B.; Singh, G.; Vinu, A.; Dhawale, D.S. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 3180–3208. [Google Scholar] [CrossRef]
- Goncalves, J.M.; Martins, P.R.; Angnes, L.; Araki, K. Recent advances in ternary layered double hydroxide electrocatalysts for the oxygen evolution reaction. New J. Chem. 2020, 44, 9981–9997. [Google Scholar] [CrossRef]
- Mondal, I.; Mahata, A.; Kim, H.; Pal, U.; De Angelis, F.; Park, J.Y. A combined experimental and theoretical approach revealing a direct mechanism for bifunctional water splitting on doped copper phosphide. Nanoscale 2020, 12, 17769–17779. [Google Scholar] [CrossRef] [PubMed]
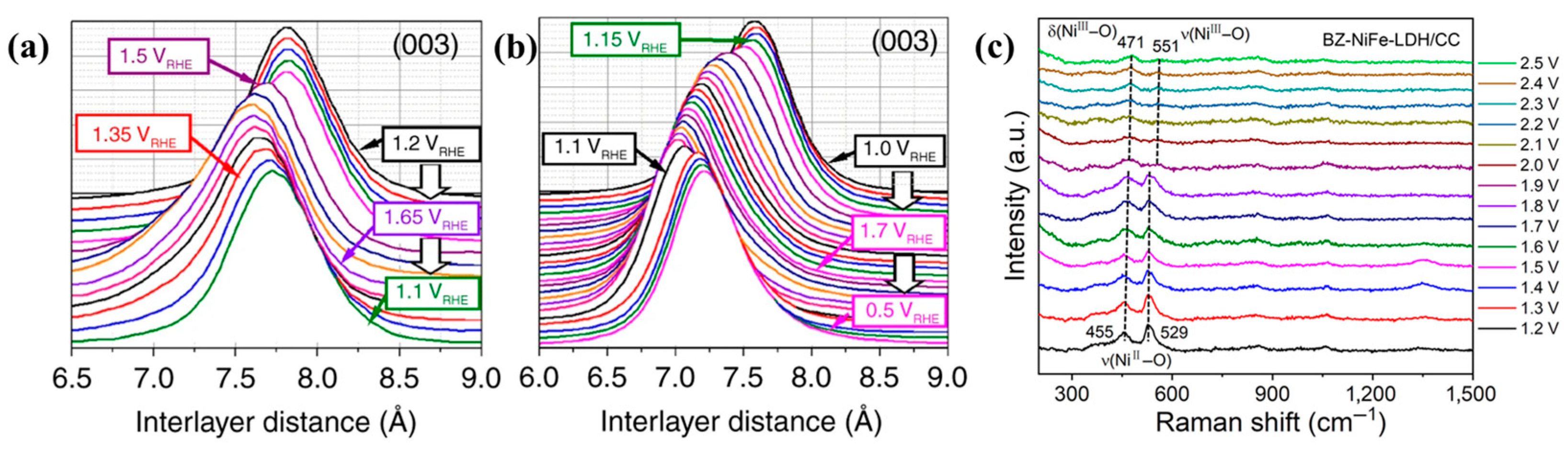
- Dionigi, F.; Zeng, Z.; Sinev, I.; Merzdorf, T.; Deshpande, S.; Lopez, M.B.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Yue, L.; Dong, K.; Li, J.; Zhao, D.; Li, Z.; Sun, S.; Luo, Y.; Liu, Q.; et al. Benzoate anions-intercalated NiFe-layered double hydroxide nanosheet array with enhanced stability for electrochemical seawater oxidation. Nano Res. Energy 2022, 1, e912002. [Google Scholar] [CrossRef]
- Scieszka, D.; Yun, J.; Bandarenka, A.S. What Do Laser-Induced Transient Techniques Reveal for Batteries? Na- and K-Intercalation from Aqueous Electrolytes as an Example. ACS Appl. Mater. Interfaces 2017, 9, 20213–20222. [Google Scholar] [CrossRef]
- Nagaiah, T.C.; Tiwari, A.; Kumar, M.; Scieszka, D.; Bandarenka, A.S. In situ Probing of Mn2O3 Activation toward Oxygen Electroreduction by the Laser-Induced Current Transient Technique. ACS Appl. Energ. Mater. 2020, 3, 9151–9157. [Google Scholar] [CrossRef]
- Hou, S.; Xu, L.; Ding, X.; Kluge, R.M.; Sarpey, T.K.; Haid, R.W.; Garlyyev, B.; Mukherjee, S.; Warnan, J.; Koch, M.; et al. Dual In Situ Laser Techniques Underpin the Role of Cations in Impacting Electrocatalysts. Angew. Chem. Int. Ed. 2022, 61, e202201610. [Google Scholar] [CrossRef]
- Bennett, J.E. Electrodes For Generation Of Hydrogen And Oxygen From Seawater. Int. J. Hydrogen Energy 1980, 5, 401–408. [Google Scholar] [CrossRef]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T.M. MnO(x)/IrO(x) as Selective Oxygen Evolution Electrocatalyst in Acidic Chloride Solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef]
- Bigiani, L.; Barreca, D.; Gasparotto, A.; Andreu, T.; Verbeeck, J.; Sada, C.; Modin, E.; Lebedev, O.I.; Morante, J.R.; Maccato, C. Selective anodes for seawater splitting via functionalization of manganese oxides by a plasma-assisted process. Appl. Catal. B 2021, 284, 119684. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.; Zhang, S.; Cao, Y.; Wang, H.; Han, C.; Sun, J. High Corrosion Resistance of NiFe-Layered Double Hydroxide Catalyst for Stable Seawater Electrolysis Promoted by Phosphate Intercalation. Small 2022, 18, e2203852. [Google Scholar] [CrossRef]
- Liao, H.; Luo, T.; Tan, P.; Chen, K.; Lu, L.; Liu, Y.; Liu, M.; Pan, J. Unveiling Role of Sulfate Ion in Nickel-Iron (oxy)Hydroxide with Enhanced Oxygen-Evolving Performance. Adv. Funct. Mater. 2021, 31, 2102772. [Google Scholar] [CrossRef]
- Ma, T.; Xu, W.; Li, B.; Chen, X.; Zhao, J.; Wan, S.; Jiang, K.; Zhang, S.; Wang, Z.; Tian, Z.; et al. The Critical Role of Additive Sulfate for Stable Alkaline Seawater Oxidation on Nickel-Based Electrodes. Angew. Chem. Int. Ed. Engl. 2021, 60, 22740–22744. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, W.; Jin, H.; Xu, J.; Xi, P.; Dong, J.; Zheng, Y.; Qiao, S.Z. High-Performance Alkaline Seawater Electrolysis with Anomalous Chloride Promoted Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202311674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Li, S.; Wang, Z.; Chen, H.; Yi, L.; Chen, X.; Yang, Q.; Xu, W.; Wang, A.; et al. Concerning the stability of seawater electrolysis: A corrosion mechanism study of halide on Ni-based anode. Nat. Commun. 2023, 14, 4822. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Bocharov, D.; Isakoviča, I.; Pankratov, V.; Popov, A.A.; Popov, A.I.; Piskunov, S. Chlorine Adsorption on TiO2(110)/Water Interface: Nonadiabatic Molecular Dynamics Simulations for Photocatalytic Water Splitting. Electron. Mater. 2023, 4, 4. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S.; Jothi, V.R.; Yi, S.; Driess, M.; Menezes, P.W. Strategies and Perspectives to Catch the Missing Pieces in Energy-Efficient Hydrogen Evolution Reaction in Alkaline Media. Angew. Chem. Int. Ed. 2021, 60, 18981–19006. [Google Scholar] [CrossRef]
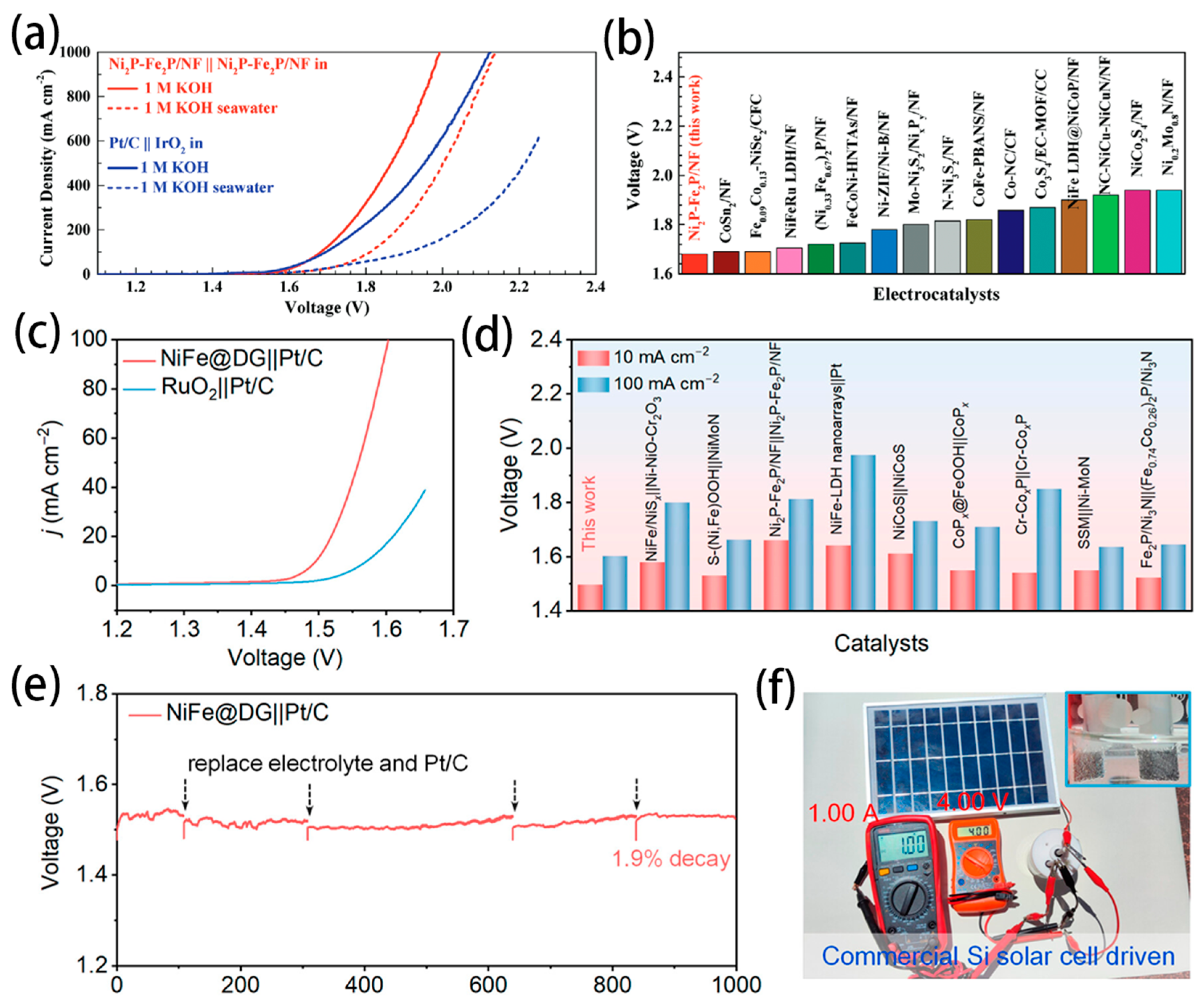
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting. Adv. Funct. Mater. 2020, 31, 2006484. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, J.; Yan, M.; Gong, H.; Ye, G.; Fei, H. Highly Durable and Efficient Seawater Electrolysis Enabled by Defective Graphene-Confined Nanoreactor. ACS Nano 2023, 17, 18372–18381. [Google Scholar] [CrossRef]
- Veroneau, S.S.; Nocera, D.G. Continuous electrochemical water splitting from natural water sources via forward osmosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2024855118. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Xu, J.; Xia, C.; Wang, P.; Xia, B.Y.; Yan, Y.; Wang, X. Key Components and Design Strategy for a Proton Exchange Membrane Water Electrolyzer. Small Struct. 2022, 4, 2200130. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, Y.-C.; Wu, Z.-P.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.-C.; et al. Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Pan, C.; Li, C.; Zhao, J. A review of membranes in proton exchange membrane fuel cells: Transport phenomena, performance and durability. Renew. Sustain. Energy Rev. 2021, 141, 110771. [Google Scholar] [CrossRef]
- Wang, X.R.; Ma, Y.; Gao, J.; Li, T.; Jiang, G.Z.; Sun, Z.Y. Review on water management methods for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 12206–12229. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef] [PubMed]
- Raja Sulaiman, R.R.; Wong, W.Y.; Loh, K.S. Recent developments on transition metal-based electrocatalysts for application in anion exchange membrane water electrolysis. Int. J. Energy Res. 2022, 46, 2241–2276. [Google Scholar] [CrossRef]
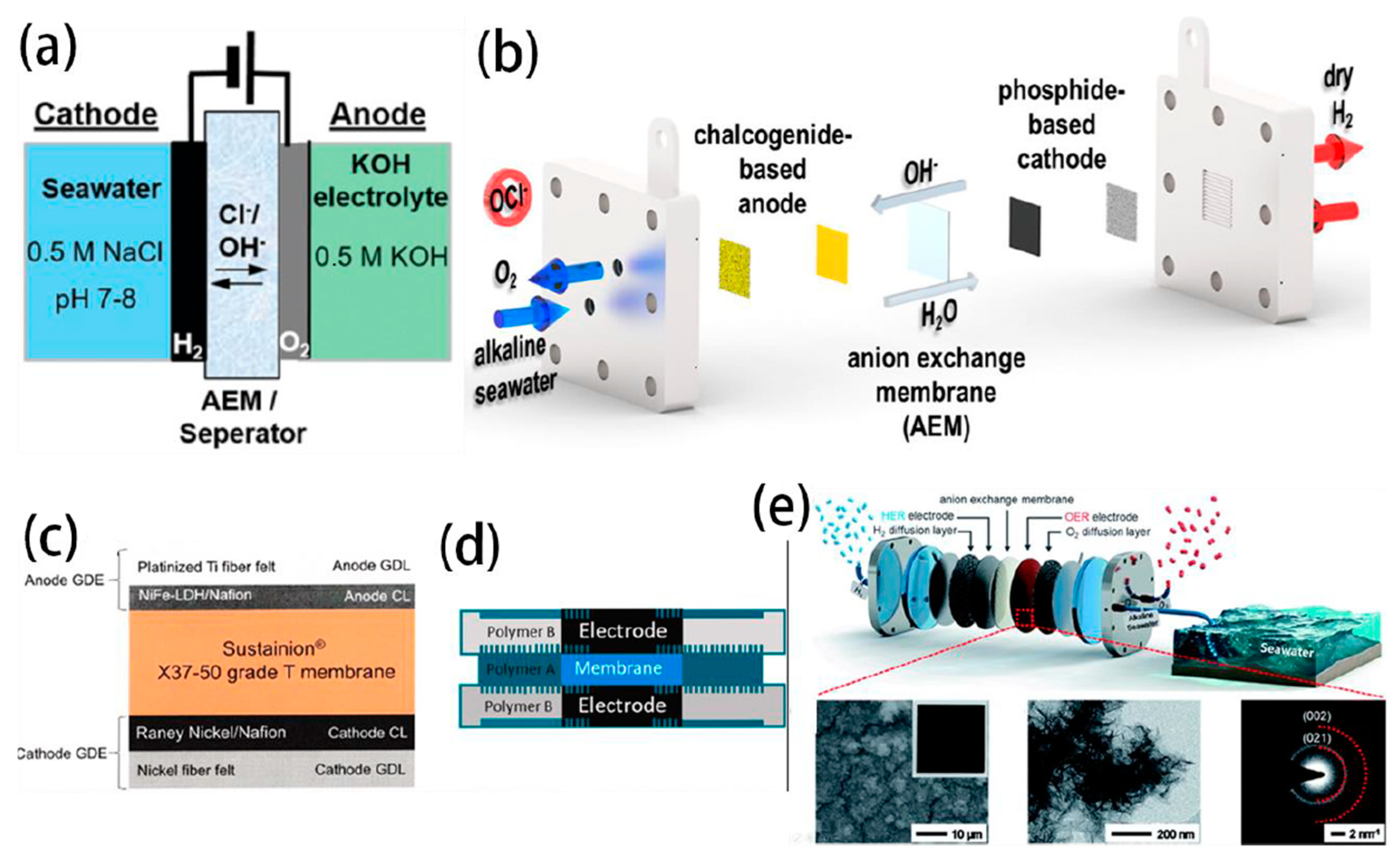
- Dresp, S.; Ngo Thanh, T.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Environ. Sci. Technol. 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Frisch, M.L.; Thanh, T.N.; Arinchtein, A.; Hager, L.; Schmidt, J.; Brückner, S.; Kerres, J.; Strasser, P. Seawater Electrolysis Using All-PGM-Free Catalysts and Cell Components in an Asymmetric Feed. ACS Energy Lett. 2023, 8, 2387–2394. [Google Scholar] [CrossRef]
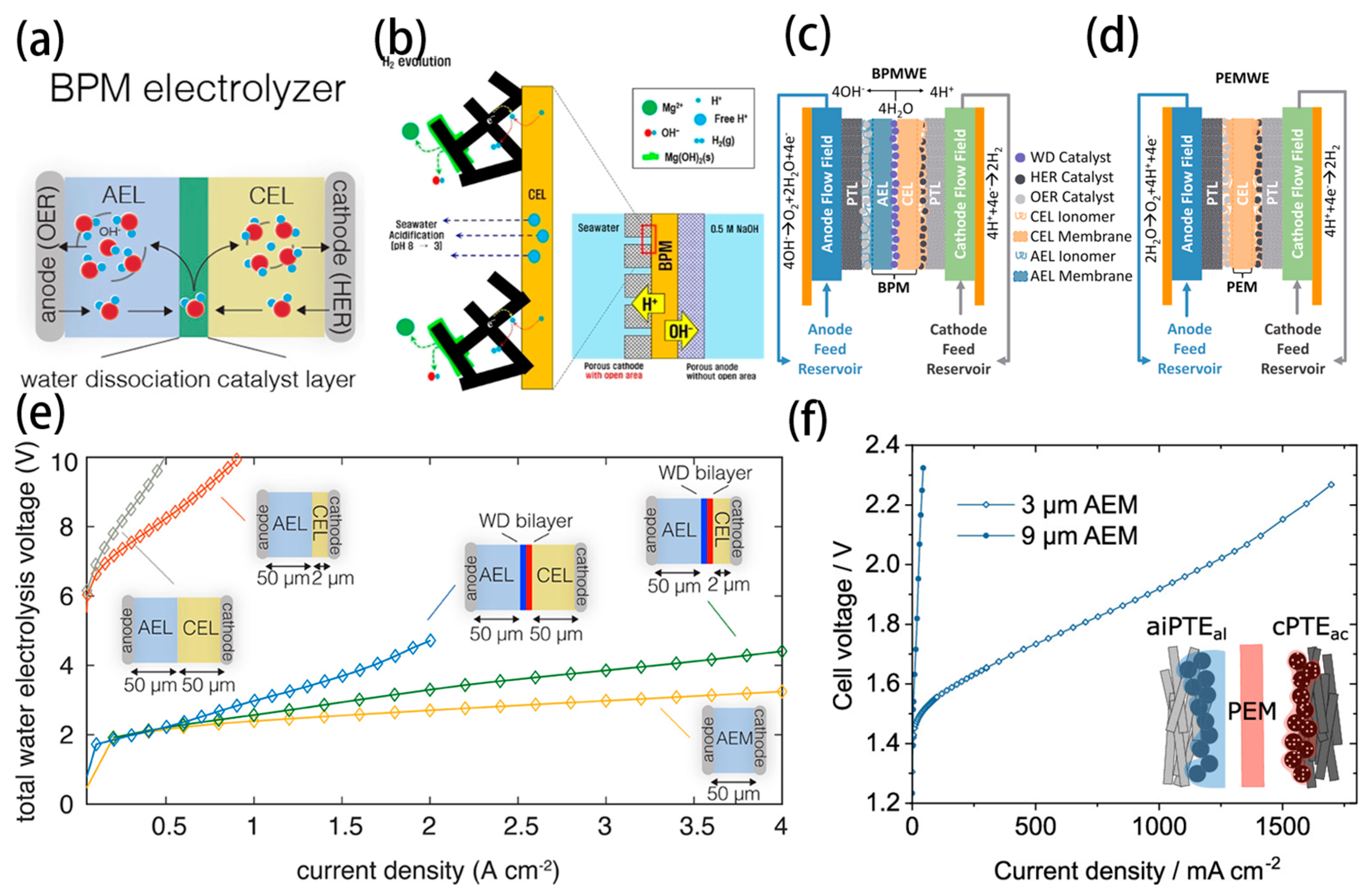
- Oener, S.Z.; Twight, L.P.; Lindquist, G.A.; Boettcher, S.W. Thin Cation-Exchange Layers Enable High-Current-Density Bipolar Membrane Electrolyzers via Improved Water Transport. ACS Energy Lett. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Han, J.H. Exploring the Interface of Porous Cathode/Bipolar Membrane for Mitigation of Inorganic Precipitates in Direct Seawater Electrolysis. ChemSusChem 2022, 15, e202200372. [Google Scholar] [CrossRef]
- Marin, D.H.; Perryman, J.T.; Hubert, M.A.; Lindquist, G.A.; Chen, L.; Aleman, A.M.; Kamat, G.A.; Niemann, V.A.; Stevens, M.B.; Regmi, Y.N.; et al. Hydrogen production with seawater-resilient bipolar membrane electrolyzers. Joule 2023, 7, 765–781. [Google Scholar] [CrossRef]
- Mayerhöfer, B.; McLaughlin, D.; Böhm, T.; Hegelheimer, M.; Seeberger, D.; Thiele, S. Bipolar Membrane Electrode Assemblies for Water Electrolysis. ACS Appl. Energ. Mater. 2020, 3, 9635–9644. [Google Scholar] [CrossRef]
- Lim, C.K.; Liu, Q.; Zhou, J.; Sun, Q.; Chan, S.H. High-temperature electrolysis of synthetic seawater using solid oxide electrolyzer cells. J. Power Sources 2017, 342, 79–87. [Google Scholar] [CrossRef]

| Gray Hydrogen | Blue Hydrogen | Green Hydrogen | |
|---|---|---|---|
| Process | Reforming or gasification | Reforming or gasification with carbon capture | Electrolysis |
| Energy sources | Fossil fuels | Fossil fuel | Renewable electricity |
| Estimates of emission from the production process | Reforming: 9–11 Gasification: 8–20 | 0.4–4.5 | 0 |
| AWE | PEM | AEM | SOEC | |
|---|---|---|---|---|
| Electrolyte | KOH 5–7 mol L−1 | PFSA membranes | DVB polymer support with KOH or NaHCO3 1 mol L−1 | Yttria-stabilized Zirconia (YSZ) |
| Separator | ZrO2 stabilized with PPS mesh | Solid electrolyte | Solid electrolyte | Solid electrolyte |
| Electrode/Catalyst (Anode) | Nickel coated stainless steel | Iridium oxide | Nickel or NiFeCo alloys | Perovskite-type |
| Electrode/Catalyst (Cathode) | Nickel coated stainless steel | Platinum nanoparticles | High surface area nickel | Ni/YSZ |
| Bipolar plate | Nickel coated stainless steel | Platinum-coated titanium | Nickel-coated stainless steel | none |
| Sealing | PSU, PTFE | PTFE, PSU, ETFE | PTFE, Silicon | Ceramic glass |
| Current density | <0.6 A cm−2 | 0–4 A cm−2 | 0.2–2 A cm−2 | 0.2–0.4 A cm−2 |
| Power consumption | 4.3–5.7 kWh/Nm3 | 5.8–7.3 kWh/Nm3 | 5.2–4.8 kWh/Nm3 | - |
| H2 purity | >99.8% | >99.99% | >99.99% | - |
| Work temperature | 65–100 °C | 50–95 °C | 50–85 °C | 600–800 °C |
| Cell Pressure | 25–30 bar | <30 bar | <35 bar | - |
| Advantages | Well established; Low cost | Immediate response; High current densities; High H2 purity | PGM-free electrocatalysts; Low corrosion; | High electricity efficiency; Security; No pollution; |
| Disadvantages | Low current densities; Alkaline corrosion; Expensive maintain cost; Low H2 purity; High gas crossover; | Acid corrosion; Noble metal catalysts; Highly cost components; | Low OH− conductivity; Short lifetime; Membrane degradation; High catalyst loading; | High temperature; Laboratory stage |
| Development status | Small scale application | R&D | R&D | R&D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Zhou, J.; Chen, X.; Zhao, S.; Zhao, Y.; Tang, Y.; Tian, Z.; Yang, Q.; Slavcheva, E.; Lin, Y.; et al. The Recent Progresses of Electrodes and Electrolysers for Seawater Electrolysis. Nanomaterials 2024, 14, 239. https://doi.org/10.3390/nano14030239
Zhang F, Zhou J, Chen X, Zhao S, Zhao Y, Tang Y, Tian Z, Yang Q, Slavcheva E, Lin Y, et al. The Recent Progresses of Electrodes and Electrolysers for Seawater Electrolysis. Nanomaterials. 2024; 14(3):239. https://doi.org/10.3390/nano14030239
Chicago/Turabian StyleZhang, Fan, Junjie Zhou, Xiaofeng Chen, Shengxiao Zhao, Yayun Zhao, Yulong Tang, Ziqi Tian, Qihao Yang, Evelina Slavcheva, Yichao Lin, and et al. 2024. "The Recent Progresses of Electrodes and Electrolysers for Seawater Electrolysis" Nanomaterials 14, no. 3: 239. https://doi.org/10.3390/nano14030239
APA StyleZhang, F., Zhou, J., Chen, X., Zhao, S., Zhao, Y., Tang, Y., Tian, Z., Yang, Q., Slavcheva, E., Lin, Y., & Zhang, Q. (2024). The Recent Progresses of Electrodes and Electrolysers for Seawater Electrolysis. Nanomaterials, 14(3), 239. https://doi.org/10.3390/nano14030239






