Ball-Milling Enhanced UV Protection Performance of Ca2Fe-Sulisobenzone Layered Double Hydroxide Organic Clay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Intercalation Works of the Organic LDHs
2.3. Mechanochemical Treatments of the Ca2Fe-Sulisobenzone Clays
2.4. Methods of Structural Characterization
3. Results and Discussion
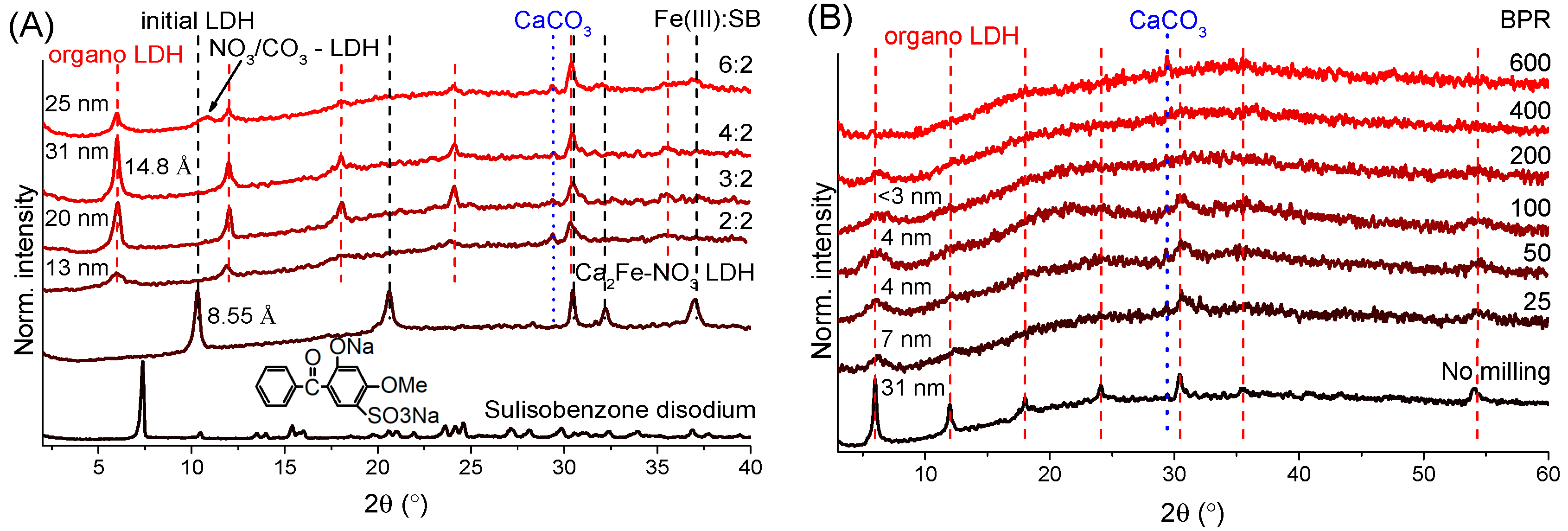
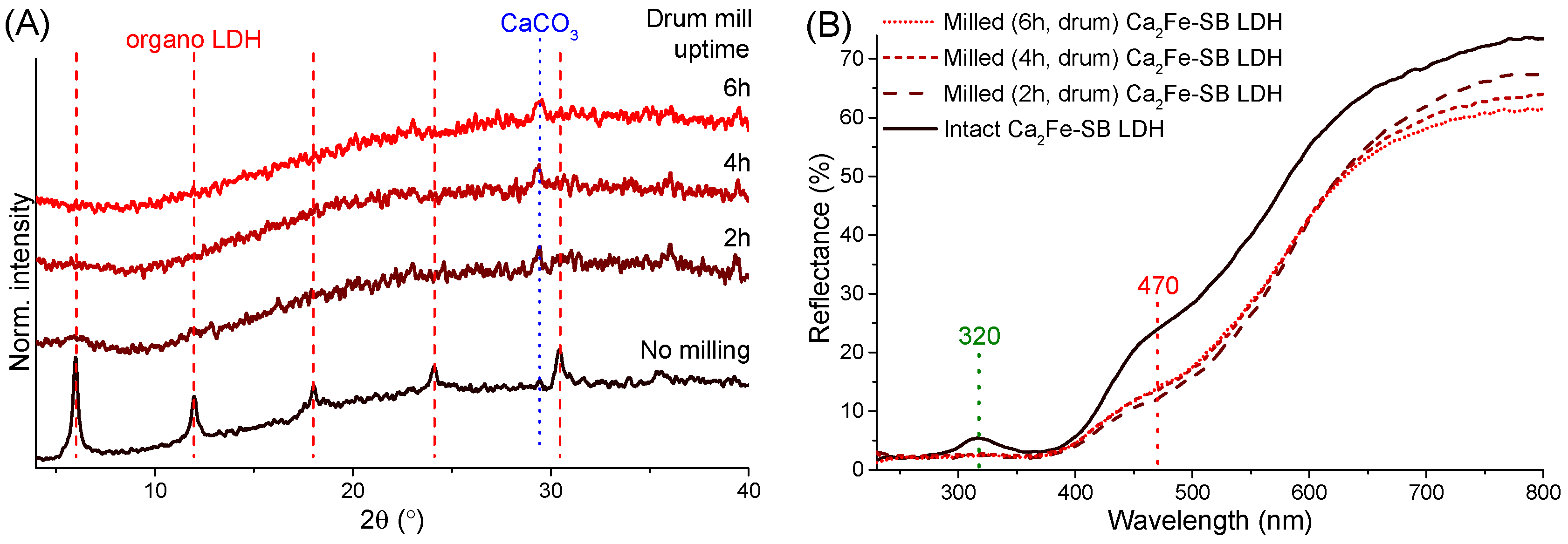
3.1. Powder X-ray Diffractometry Analysis
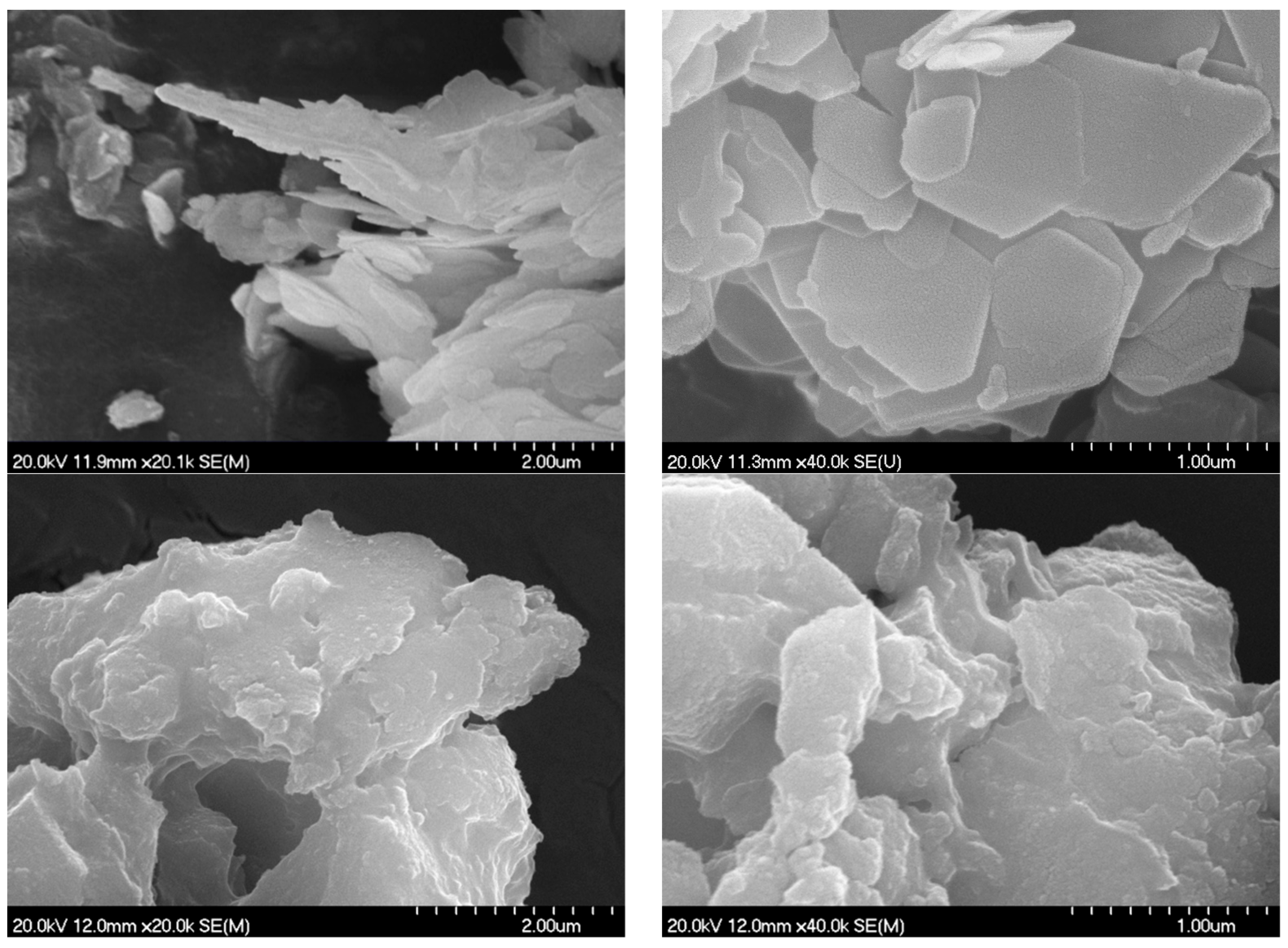
3.2. Scanning Electron Microscopy and Surface/Textural Probes
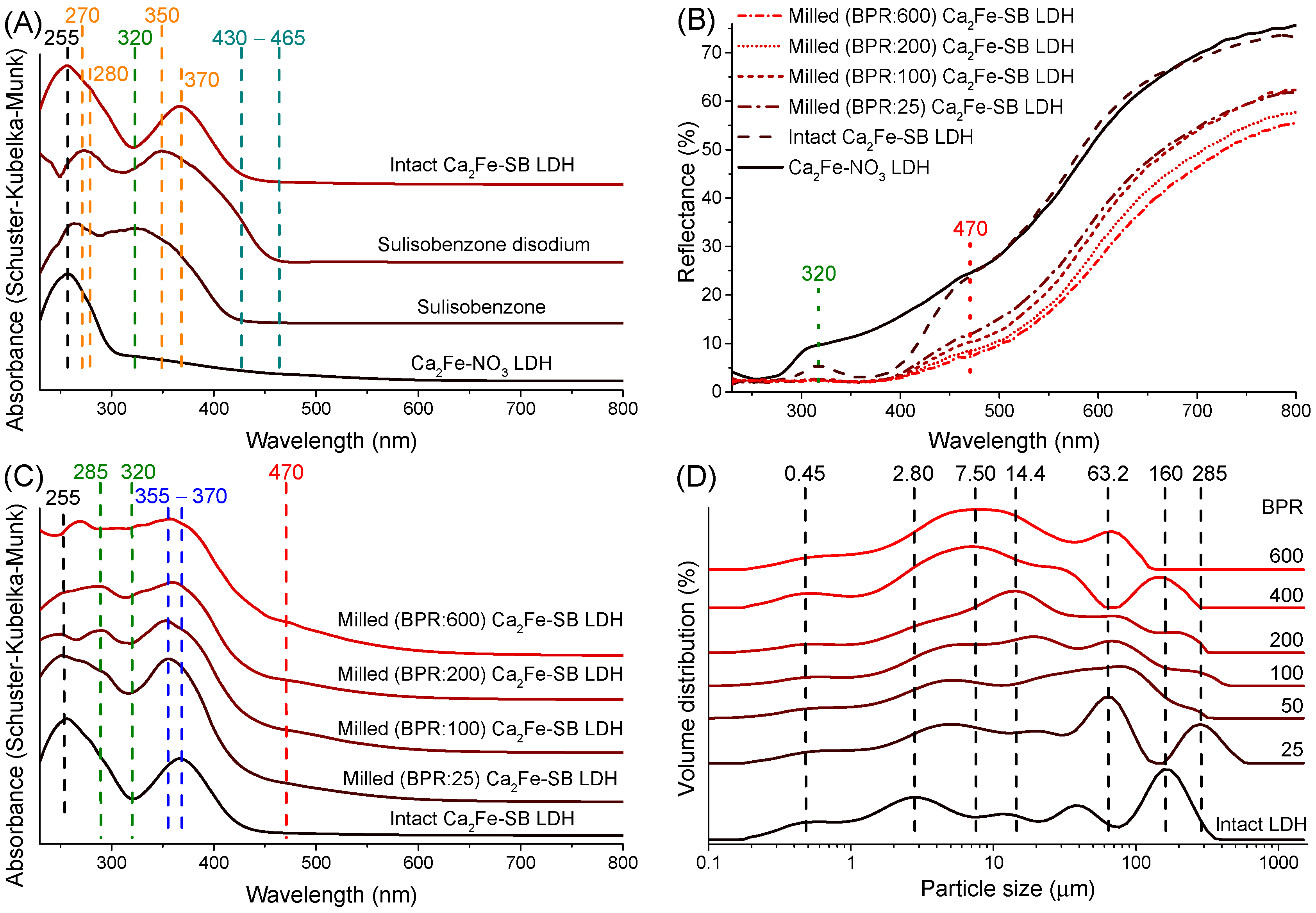
3.3. UV–Vis DRS Study
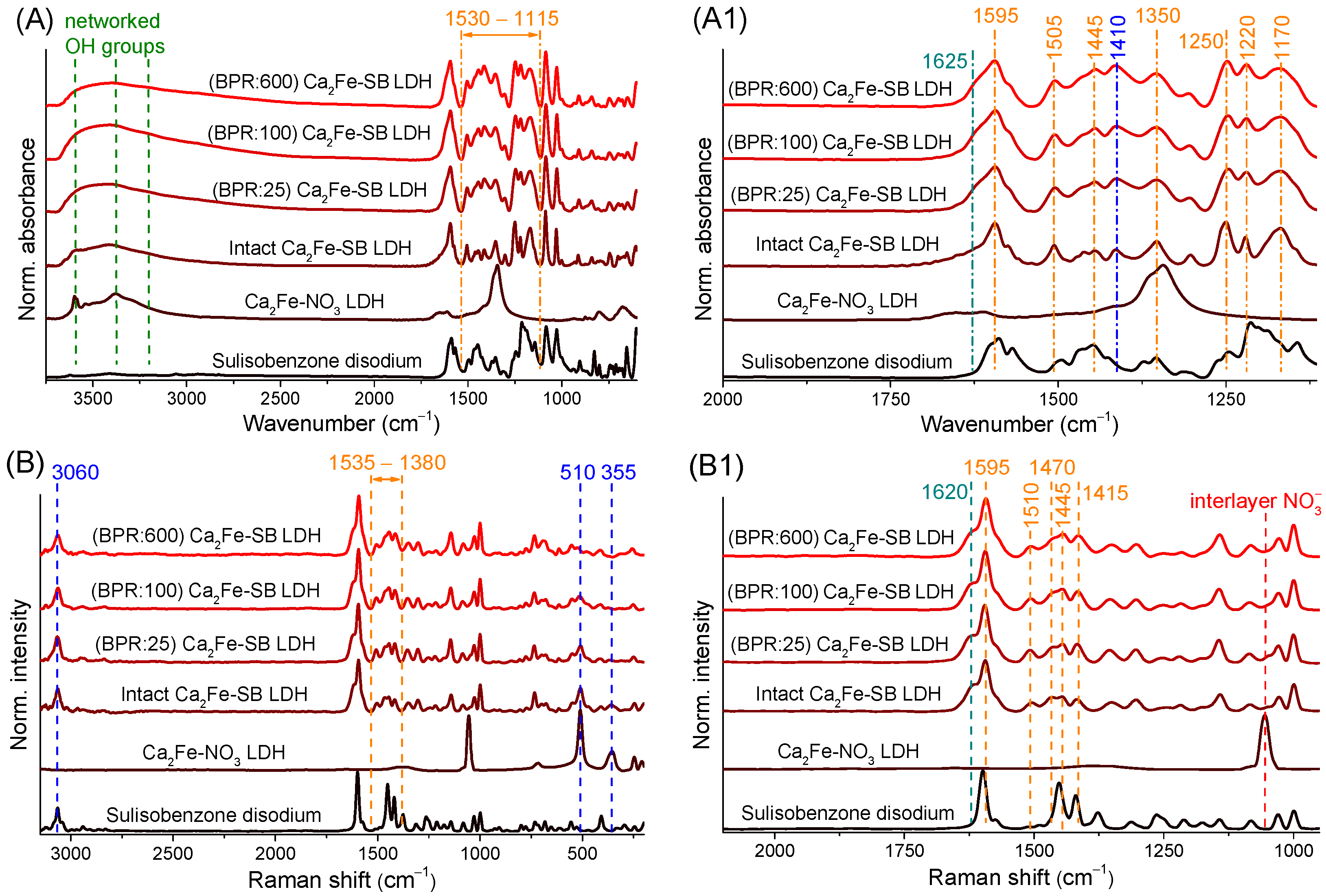
3.4. Infrared and Raman Spectroscopy Measurements
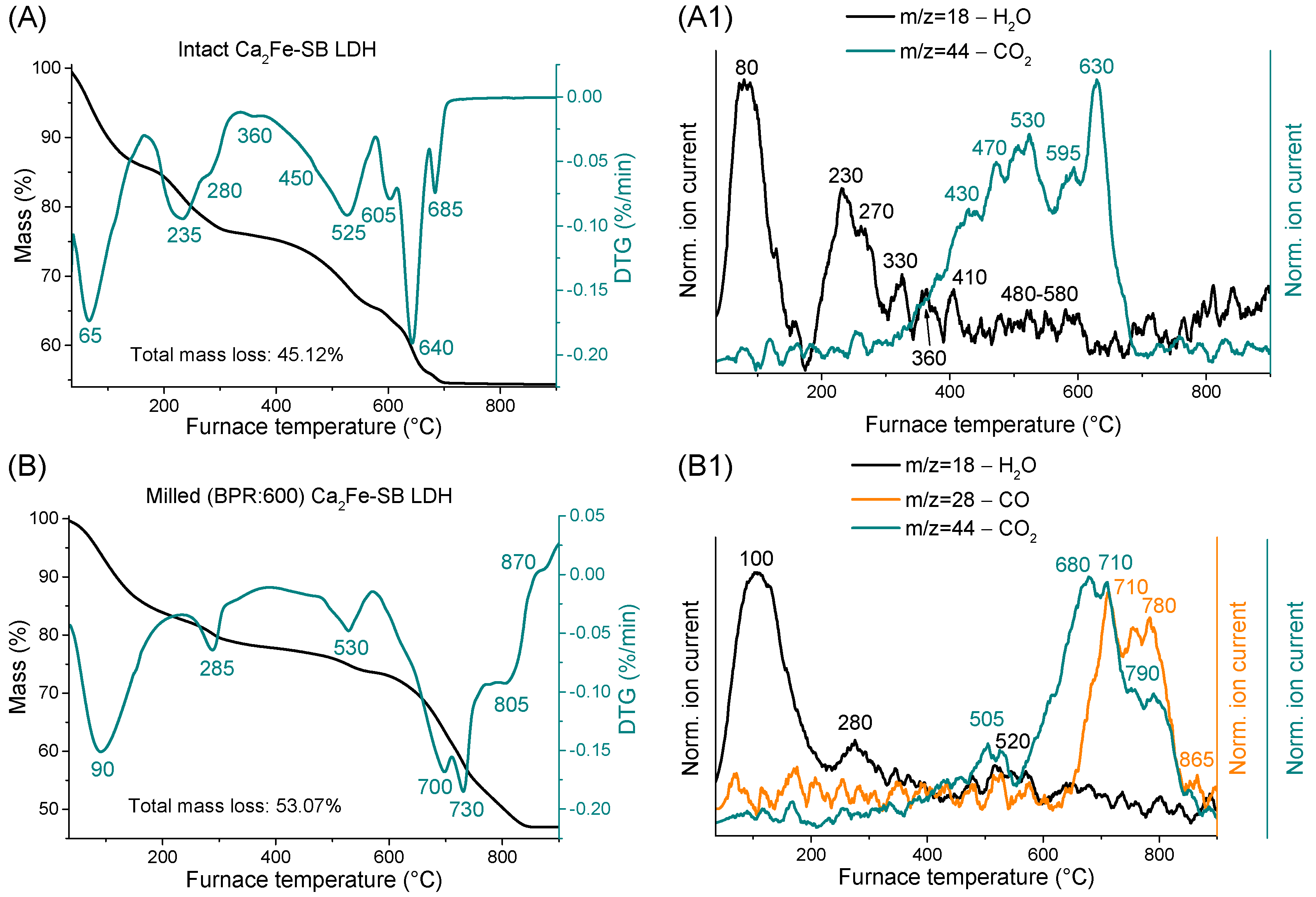
3.5. Thermogravimetric and Evolved Gas Investigations
3.6. Mechanochemical Treatment of the LDH Nanocomposites in Drum Mill
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Evans, G.D.; Slade, R.C.T. Structural aspects of layered double hydroxides. Struct. Bond. 2006, 119, 1–87. [Google Scholar] [CrossRef]
- Del Hoyo, C. Layered double hydroxides and human health: An overview. Appl. Clay Sci. 2007, 36, 103–121. [Google Scholar] [CrossRef]
- Yu, S.; Choi, G.; Choy, J.H. Multifunctional layered double hydroxides for drug delivery and imaging. Nanomaterials 2023, 13, 1102. [Google Scholar] [CrossRef]
- Da Silva Feltran, G.; Da Costa Fernandes, C.J.; Ferreira, M.R.; Kang, H.R.; De Carvalho Bovolato, A.L.; De Assis Golim, M.; Deffune, E.; Koh, I.H.J.; Constantino, V.R.L.; Zambuzzi, W.F. Sonic hedgehog drives layered double hydroxides-induced acute inflammatory landscape. Colloid Surface B 2019, 174, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Vy, N.T.T.; Khanh, D.N.N.; Khanh, P.D.; Phat, N.T.; Anh, N.T.; Nguyen, N.L.; Anh, T.N.L.; Vy, N.N.; Dan, L.T.M.; Phuong, N.T.K. Drug-intercalated Zn–Al-Layered double hydroxides as antibacterial and anti-inflammatory delivery systems for wound healing applications. J. Clust. Sci. 2023, 34, 2619–2632. [Google Scholar] [CrossRef]
- Mosconi, G.; Formica, M.L.; Palma, S.D.; Rojas, R. Carboxymethylcellulose/layered double hydroxide dispersions for topical ocular delivery of non-steroidal anti-inflammatory drugs. New J. Chem. 2024, 48, 406–415. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeung, D.-G.; Oh, J.-M. Boosting the anticancer activity of doxorubicin with a layered double hydroxide nanocarrier. Appl. Clay Sci. 2021, 203, 106000. [Google Scholar] [CrossRef]
- Pang, S.; Geng, C.; Fan, Z.; Hou, M.; Mao, H.; Tao, S.; Wang, J.; Wu, Y.; Wei, K.; Li, Y.; et al. Synergistic effect of layered double hydroxides nanodosage form to induce apoptosis and ferroptosis in breast cancer. Int. J. Nanomed. 2024, 19, 4199–4215. [Google Scholar] [CrossRef]
- Piao, H.; Choi, G.; Rejinold, N.S.; Yu, J.; Choi, S.-J.; Choy, J.-H. Pemetrexed-layered double hydroxide with Eudragit® S100; 2D van der Waals nanohybrid with drug delivery function. J. Ind. Eng. Chem. 2024, 130, 572–579. [Google Scholar] [CrossRef]
- Bastianini, M.; Faffa, C.; Sisani, M.; Petracci, A. Caffeic acid-layered double hydroxide hybrid: A new raw material for cosmetic applications. Cosmetics 2018, 5, 51. [Google Scholar] [CrossRef]
- Pagano, C.; Perioli, L.; Latterini, L.; Nocchetti, M.; Ceccarini, M.R.; Marani, M.; Ramella, D.; Ricci, M. Folic acid-layered double hydroxides hybrids in skin formulations: Technological, photochemical and in vitro cytotoxicity on human keratinocytes and fibroblasts. Appl. Clay Sci. 2019, 168, 382–395. [Google Scholar] [CrossRef]
- Hu, X.; Yu, J.; Sun, L.; Zhang, L.; Zhou, W.; Yan, D.; Wang, X. Synthesis of an AVB@ZnTi-LDH composite with synergistically enhance UV blocking activity and high stability for potential application in sunscreen formulations. Chin. Chem. Lett. 2024, 35, 109466. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kumar, M.; Suresh, K.; Pugazhenthi, G. Influence of organically modified NiAl layered double hydroxide (LDH) loading on the rheological properties of poly (methyl methacrylate) (PMMA)/LDH blend solution. Powder Technol. 2014, 256, 196–203. [Google Scholar] [CrossRef]
- Perioli, L.; Pagano, C.; Nocchetti, M.; Latterini, L. Development of smart semisolid formulations to enhance retinoic acid topical application. J. Pharm. Sci. 2015, 104, 3904–3912. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.A.; da Silva, T.A.; do Nascimento, T.G.; Yatsuzuka, R.E.; Grillo, L.A.M.; Dornelas, C.B. Recent advances in layered double hydroxides applied to photoprotection. Einstein 2019, 17, eRW4456. [Google Scholar] [CrossRef]
- Franco, J.G.; Ataide, J.A.; Ferreira, A.H.P.; Mazzola, P.G. Lamellar compounds intercalated with anions with solar protection function: A review. J. Drug Deliv. Sci. Technol. 2020, 59, 101869. [Google Scholar] [CrossRef]
- Ng’etich, W.K.; Martincigh, B.S. A critical review on layered double hydroxides: Their synthesis and application in sunscreen formulations. Appl. Clay Sci. 2021, 208, 106095. [Google Scholar] [CrossRef]
- He, Q.; Yin, S.; Sato, T. Synthesis and photochemical properties of zinc–aluminum layered double hydroxide/organic UV ray absorbing molecule/silica nanocomposites. J. Phys. Chem. Solids 2004, 65, 395–402. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.; Wang, Y.; Evans, D.G.; Duan, X. Synthesis and characterization of a UV absorbent-intercalated Zn–Al layered double hydroxide. Polym. Degrad. Stabil. 2006, 91, 789–794. [Google Scholar] [CrossRef]
- Perioli, L.; Nocchetti, M.; Ambrogi, V.; Latterini, L.; Rossi, C.; Costantino, U. Sunscreen immobilization on ZnAl-hydrotalcite for new cosmetic formulations. Micropor. Mesopor. Mater 2008, 107, 180–189. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, W.; Xue, Y.; Wanga, X.; Lin, T. Partial exfoliation of layered double hydroxides in DMSO: A route to transparent polymer nanocomposites. J. Mater. Chem. 2011, 21, 4869–4874. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, T.; Pi, H.; Guo, S. Preparation of intercalated Mg-Al layered double hydroxides and its application in PVC thermal stability. J. Appl. Polym. Sci. 2012, 124, 5180–5186. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Xiao, M.; Liu, D.; Fa, L.; Wu, K. Intercalation of 2,4-dihydroxybenzophenone-5-sulfonate anion into Zn/Al layered double hydroxides for UV absorption properties. J. Ind. Eng. Chem. 2014, 20, 1280–1284. [Google Scholar] [CrossRef]
- Mohsin, S.M.N.; Hussein, M.Z.; Sarijo, S.H.; Fakurazi, S.; Arulselvan, P.; Taufiq-Yap, Y.H. Characterisation and cytotoxicity assessment of UV absorbers-intercalated zinc/aluminium-layered double hydroxides on dermal fibroblast cells. Sci. Adv. Mater. 2014, 4, 648–658. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G.; Génin, J.-M.R.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Mineral. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Crystal structures of some double hydroxide minerals. Mineral. Mag. 1973, 39, 377–389. [Google Scholar] [CrossRef]
- Rousselot, I.; Guého, C.T.; Leroux, F.; Léone, P.; Palvadeau, P.; Besse, J.-P. Insights on the structural chemistry of hydrocalumite and hydrotalcite-like materials: Investigation of the series Ca2M3+(OH)6Cl•2H2O (M3+: Al3+, Ga3+, Fe3+, and Sc3+) by X-ray powder diffraction. J. Solid State Chem. 2002, 167, 137–144. [Google Scholar] [CrossRef]
- Renaudin, G.; Francois, M.; Evrard, O. Order and disorder in the lamellar hydrated tetracalcium monocarboaluminate compound. Cem. Concr. Res. 1999, 29, 63–69. [Google Scholar] [CrossRef]
- Chen, Y.; Shui, Z.; Chen, W.; Chen, G. Chloride binding of synthetic Ca–Al–NO3 LDHs in hardened cement paste. Constr. Build. Mater. 2015, 93, 1051–1058. [Google Scholar] [CrossRef]
- Silbernagel, B. Preparation and crystal growth of materials with layered structures. Physics and chemistry of materials with layered structures. Vol. 1 edited by Lieth, R.M.A. Acta Crystallogr. Sect. B 1978, 34, 3498. [Google Scholar] [CrossRef]
- Jiménez, A.; Rives, V.; Vicente, M.A. Thermal study of the hydrocalumite–katoite–calcite system. Thermochim. Acta 2022, 713, 179242. [Google Scholar] [CrossRef]
- Kim, T.-H.; Oh, J.-M. Dual nutraceutical nanohybrids of folic acid and calcium containing layered double hydroxides. J. Solid State Chem. 2016, 233, 125–132. [Google Scholar] [CrossRef]
- Fontes, D.A.F.; de Lyra, M.A.M.; de Andrade, J.K.F.; de Medeiros Schver, G.C.R.; Rolim, L.A.; da Silva, T.G.; Soares-Sobrinho, J.L.; Alves-Júnior, S.; Rolim-Neto, P.J. CaAl-layered double hydroxide as a drug delivery system: Effects on solubility and toxicity of the antiretroviral efavirenz. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 281–288. [Google Scholar] [CrossRef]
- Jensen, N.D.; Bjerring, M.; Nielsen, U.G. A solid state NMR study of layered double hydroxides intercalated with para-amino salicylate, a tuberculosis drug. Solid State Nucl. Mag. 2016, 78, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Ray, S.; Acharya, R.; Chatterjee, T.K.; Chakraborty, J. Magnesium, zinc and calcium aluminium layered double hydroxide-drug nanohybrids: A comprehensive study. Appl. Clay Sci. 2017, 135, 439–509. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mitra, M.K.; Chakraborty, J. One-pot synthesis of CaAl-layered double hydroxide–methotrexate nanohybrid for anticancer application. Bull. Mater. Sci. 2017, 40, 1203–1211. [Google Scholar] [CrossRef]
- Timóteo, T.T.R.; de Melo, C.G.; de Alencar Danda, L.J.; Silva, L.C.P.B.B.; Fontes, D.A.F.; Silva, P.C.D.; Aguilera, C.S.B.; da Paixão Siqueira, L.; Rolim, L.A.; Rolim Neto, P.J. Layered double hydroxides of CaAl: A promising drug delivery system for increased dissolution rate and thermal stability of praziquantel. Appl. Clay Sci. 2019, 180, 105197. [Google Scholar] [CrossRef]
- Pontes-Neto, J.G.; Lyra, M.A.M.; Soares, M.F.L.R.; Chaves, L.L.; Soares-Sobrinho, J.L. Intercalation of olanzapine into CaAl and NiAl Layered Double Hydroxides for dissolution rate improvement: Synthesis, characterization and in vitro toxicity. J. Drug Deliv. Sci. Technol. 2019, 52, 986–996. [Google Scholar] [CrossRef]
- Szabados, M.; Gácsi, A.; Gulyás, Y.; Kónya, Z.; Kukovecz, Á.; Csányi, E.; Pálinkó, I.; Sipos, P. Conventional or mechanochemically-aided intercalation of diclofenac and naproxen anions into the interlamellar space of CaFe-layered double hydroxides and their application as dermal drug delivery systems. Appl. Clay Sci. 2021, 212, 106233. [Google Scholar] [CrossRef]
- Bohari, F.L.; Ghazali, S.A.I.S.M.; Dzulkifli, N.N.; Baharin, S.N.A.; Fatimah, I.; Poddar, S. Studies on the intercalation of calcium–aluminium layered double hydroxide-MCPA and its controlled release mechanism as a potential green herbicide. Open Chem. 2023, 21, 20220291. [Google Scholar] [CrossRef]
- Qu, J.; Sha, L.; Wu, C.; Zhang, Q. Applications of mechanochemically prepared layered double hydroxides as adsorbents and catalysts: A mini-review. Nanomaterials 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Belskaya, O.B.; Likholobov, V.A. Mechanochemical synthesis of layered double hydroxides as a promising method for the preparation of adsorbents and catalysts. Kinet. Catal. 2022, 63, 615–641. [Google Scholar] [CrossRef]
- Intasa-ard, S.G.; Imwiset, K.J.; Bureekaew, S.; Ogawa, M. Mechanochemical methods for the preparation of intercalation compounds, from intercalation to the formation of layered double hydroxides. Dalton Trans. 2018, 47, 2896–2916. [Google Scholar] [CrossRef] [PubMed]
- Ferencz, Z.; Szabados, M.; Ádok-Sipiczki, M.; Kukovecz, Á.; Kónya, Z.; Sipos, P.; Pálinkó, I. Mechanochemically assisted synthesis of pristine Ca(II)Sn(IV)-layered double hydroxides and their amino acid intercalated nanocomposites. J. Mater. Sci. 2014, 49, 8478–8486. [Google Scholar] [CrossRef]
- Qu, J.; Sha, L.; Xu, Z.; He, Z.; Wu, M.; Wu, C.; Zhang, Q. Calcium chloride addition to overcome the barriers for synthesizing new Ca-Ti layered double hydroxide by mechanochemistry. Appl. Clay Sci. 2019, 173, 29–34. [Google Scholar] [CrossRef]
- Bonifacio-Martinez, J.; Serrano-Gomez, J.; Lopez-Reyes, M.D.; Granados-Correa, F. Mechano-chemical effects on surface properties and molybdate exchange on hydrotalcite. Clay Miner. 2009, 44, 311–317. [Google Scholar] [CrossRef]
- Hongo, T.; Inoue, Y.; Yamazaki, A. Mechanochemical treatment of hydrotalcite in vibration milling and its effect on fluoride adsorption ability. Clay Sci. 2010, 14, 187–190. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Wang, Z.; Fu, Y. Structural and textural evolution of nanocrystalline Mg–Al layered double hydroxides during mechanical treatment. Appl. Clay Sci. 2013, 80–81, 334–339. [Google Scholar] [CrossRef]
- Pagano, C.; Marmottini, F.; Nocchetti, M.; Ramella, D.; Perioli, L. Effects of different milling techniques on the layered double hydroxides final properties. Appl. Clay Sci. 2018, 151, 124–133. [Google Scholar] [CrossRef]
- Olszówka, J.E.; Karcz, R.; Michalik-Zym, A.; Napruszewska, B.D.; Bielańska, E.; Kryściak-Czerwenka, J.; Socha, R.P.; Nattich-Rak, M.; Krzan, M.; Klimek, A.; et al. Effect of grinding on the physico-chemical properties of Mg-Al hydrotalcite and its performance as a catalyst for Baeyer-Villiger oxidation of cyclohexanone. Catal. Today 2019, 1, 147–153. [Google Scholar] [CrossRef]
- Szabados, M.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Structural reconstruction of mechanochemically disordered CaFe-layered double hydroxide. Appl. Clay Sci. 2019, 174, 138–145. [Google Scholar] [CrossRef]
- Manova, E.; Kunev, B.; Paneva, D.; Mitov, I.; Petrov, L. Mechano-synthesis, characterization, and magnetic properties of nanoparticles of cobalt ferrite, CoFe2O4. Chem. Mater. 2004, 16, 5689–5696. [Google Scholar] [CrossRef]
- Szabados, M.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Ultrasonically-assisted mechanochemical synthesis of zinc aluminate spinel from aluminium-rich layered double hydroxide. J. Solid State Chem. 2019, 272, 227–233. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Wu, T.; Feng, Y.; Wang, C.; Li, J.; Lin, Q. Mechanochemical modification of kaolin surfaces for immobilization and delivery of pesticides in alginate-chitosan composite beads. Polym. Bull. 2014, 71, 2923–2944. [Google Scholar] [CrossRef]
- Han, S.; Lee, J.; Jung, E.; Park, S.; Sagawa, A.; Shibasaki, Y.; Lee, D.; Kim, B.-S. Mechanochemical drug conjugation via pH-responsive imine linkage for polyether prodrug micelles. ACS Appl. Bio Mater. 2021, 4, 2465–2474. [Google Scholar] [CrossRef]
- Shen, H.; Cao, Y.; Lv, M.; Sheng, Q.; Zhang, Z. Polymer mechanochemistry for the release of small cargoes. Chem. Commun. 2022, 58, 4813–4824. [Google Scholar] [CrossRef]
- Balaz, P. High-Energy Milling, Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; pp. 111–114. [Google Scholar] [CrossRef]
- Franklin, K.R.; Lee, E.; Nunn, C.C. Preparation and characterisation of layered double hydroxides containing monovalent and divalent ions derived from 5-benzoyl-4-hydroxy-2-methoxybenzenesulfonic acid. J. Mater. Chem. 1995, 5, 565–569. [Google Scholar] [CrossRef]
- Szabados, M.; Ádám, A.A.; Kónya, Z.; Kukovecz, Á.; Carlson, S.; Sipos, P.; Pálinkó, I. Effects of ultrasonic irradiation on the synthesis, crystallization, thermal and dissolution behaviour of chloride-intercalated, co-precipitated CaFe-layered double hydroxide. Ultrason. Sonochem. 2019, 55, 165–173. [Google Scholar] [CrossRef]
- Szabados, M.; Ádám, A.A.; Traj, P.; Muráth, S.; Baán, K.; Bélteky, P.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Mechanochemical and wet chemical syntheses of CaIn-layered double hydroxide and its performance in a transesterification reaction compared to those of other Ca2M(III) hydrocalumites (M: Al, Sc, V, Cr, Fe, Ga) and Mg(II)-, Ni(II)-, Co(II)- or Zn(II)-based hydrotalcites. J. Catal. 2020, 391, 282–297. [Google Scholar] [CrossRef]
- Rossi, C.; Schoubben, A.; Ricci, M.; Perioli, L.; Ambrogi, V.; Latterini, L.; Aloisi, G.G.; Rossi, A. Intercalation of the radical scavenger ferulic acid in hydrotalcite-like anionic clays. Int. J. Pharm. 2005, 5, 47–55. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Nocchetti, M.; Latterini, L.; Pagano, C.; Massetti, E.; Rossi, C. Immobilization of kojic acid in ZnAl-hydrotalcite like compounds. J. Phys. Chem. Solids 2012, 73, 94–98. [Google Scholar] [CrossRef]
- Townsend, T.K.; Sabio, E.M.; Browning, N.D.; Osterloh, F.E. Photocatalytic water oxidation with suspended alpha-Fe2O3 particles-effects of nanoscaling. Energy Environ. Sci. 2011, 4, 4270–4275. [Google Scholar] [CrossRef]
- Kása, E.; Szabados, M.; Baán, K.; Kónya, Z.; Kukovecz, Á.; Kutus, B.; Pálinkó, I.; Sipos, P. The dissolution kinetics of raw and mechanochemically treated kaolinites in industrial spent liquor—The effect of the physico-chemical properties of the solids. Appl. Clay Sci. 2021, 203, 105994. [Google Scholar] [CrossRef]
- Sangeetha, R. Raman and infrared spectra of 4-chlorobenzophenone. Sch. Natl. Sch. Leadersh. 2019, 8. [Google Scholar]
- Roy, N.; Bomzan, P.; Ghosh, B.; Roy, M.N. A combined experimental and theoretical study on p-sulfonatothiacalix[4]arene encapsulated sulisobenzone. New J. Chem. 2023, 47, 1045–1049. [Google Scholar] [CrossRef]
- Bubev, E.; Georgiev, A.; Machkova, M. ATR-FTIR spectroscopy study of the photodegradation protective properties of BP-4 and 4HBP in polyvinyl acetate thin films. J. Mol. Struc. 2016, 1118, 184–193. [Google Scholar] [CrossRef]
- Henson, B.F.; Hartland, G.V.; Venturo, V.A.; Felker, P.M. Raman-vibronic double-resonance spectroscopy of benzene dimer isotopomers. J. Chem. Phys. 1992, 97, 2189–2208. [Google Scholar] [CrossRef]
- Davydova, N.A.; Babkov, L.M.; Baran, J.; Kukielski, J.I.; Mel’nik, V.I.; Truchkachev, S.V. Raman spectra of benzophenone and benzopinacol crystals. J. Mol. Struc. 2002, 614, 167–172. [Google Scholar] [CrossRef]
- Tahara, T.; Hamaguchi, H.; Tasum, M. Transient resonance Raman spectra of benzophenone and its four isotopic analogues in the lowest excited triplet state. J. Phys. Chem. 1987, 91, 5875–5880. [Google Scholar] [CrossRef]
- Beloglazov, I.; Plaschinsky, V. Development MPC for the grinding process in SAG mills using DEM investigations on liner wear. Materials 2024, 17, 795. [Google Scholar] [CrossRef]
| BPR | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) | De Brouckere Mean Diameter (μm) | d(0.1) (μm) | d(0.5) (μm) | d(0.9) (μm) |
|---|---|---|---|---|---|---|---|
| 0 | 41.7 | 0.137 | 14.44 | 63.88 | 0.956 | 14.85 | 199.5 |
| 25 | 19.8 | 0.036 | 7.821 | 69.94 | 1.624 | 21.22 | 263.6 |
| 50 | 12.3 | 0.022 | 7.970 | 43.11 | 1.842 | 21.28 | 115.0 |
| 100 | 6.92 | 0.015 | 8.964 | 45.41 | 2.191 | 17.94 | 120.4 |
| 200 | 15.3 | 0.025 | 6.915 | 39.59 | 2.200 | 15.79 | 110.6 |
| 400 | 10.7 | 0.021 | 8.584 | 29.90 | 1.358 | 8.943 | 117.7 |
| 600 | 12.6 | 0.021 | 7.008 | 20.08 | 1.382 | 9.140 | 62.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabados, M.; Mészáros, R.; Dobó, D.G.; Kónya, Z.; Kukovecz, Á.; Sipos, P. Ball-Milling Enhanced UV Protection Performance of Ca2Fe-Sulisobenzone Layered Double Hydroxide Organic Clay. Nanomaterials 2024, 14, 1436. https://doi.org/10.3390/nano14171436
Szabados M, Mészáros R, Dobó DG, Kónya Z, Kukovecz Á, Sipos P. Ball-Milling Enhanced UV Protection Performance of Ca2Fe-Sulisobenzone Layered Double Hydroxide Organic Clay. Nanomaterials. 2024; 14(17):1436. https://doi.org/10.3390/nano14171436
Chicago/Turabian StyleSzabados, Márton, Rebeka Mészáros, Dorina Gabriella Dobó, Zoltán Kónya, Ákos Kukovecz, and Pál Sipos. 2024. "Ball-Milling Enhanced UV Protection Performance of Ca2Fe-Sulisobenzone Layered Double Hydroxide Organic Clay" Nanomaterials 14, no. 17: 1436. https://doi.org/10.3390/nano14171436
APA StyleSzabados, M., Mészáros, R., Dobó, D. G., Kónya, Z., Kukovecz, Á., & Sipos, P. (2024). Ball-Milling Enhanced UV Protection Performance of Ca2Fe-Sulisobenzone Layered Double Hydroxide Organic Clay. Nanomaterials, 14(17), 1436. https://doi.org/10.3390/nano14171436









