A Systematic Microfluidic Study of the Use of Diluted Silica Sols to Enhance Oil Displacement
Abstract
1. Introduction
2. Materials and Methods
2.1. Crude Oil
2.2. Nanoparticle Suspensions
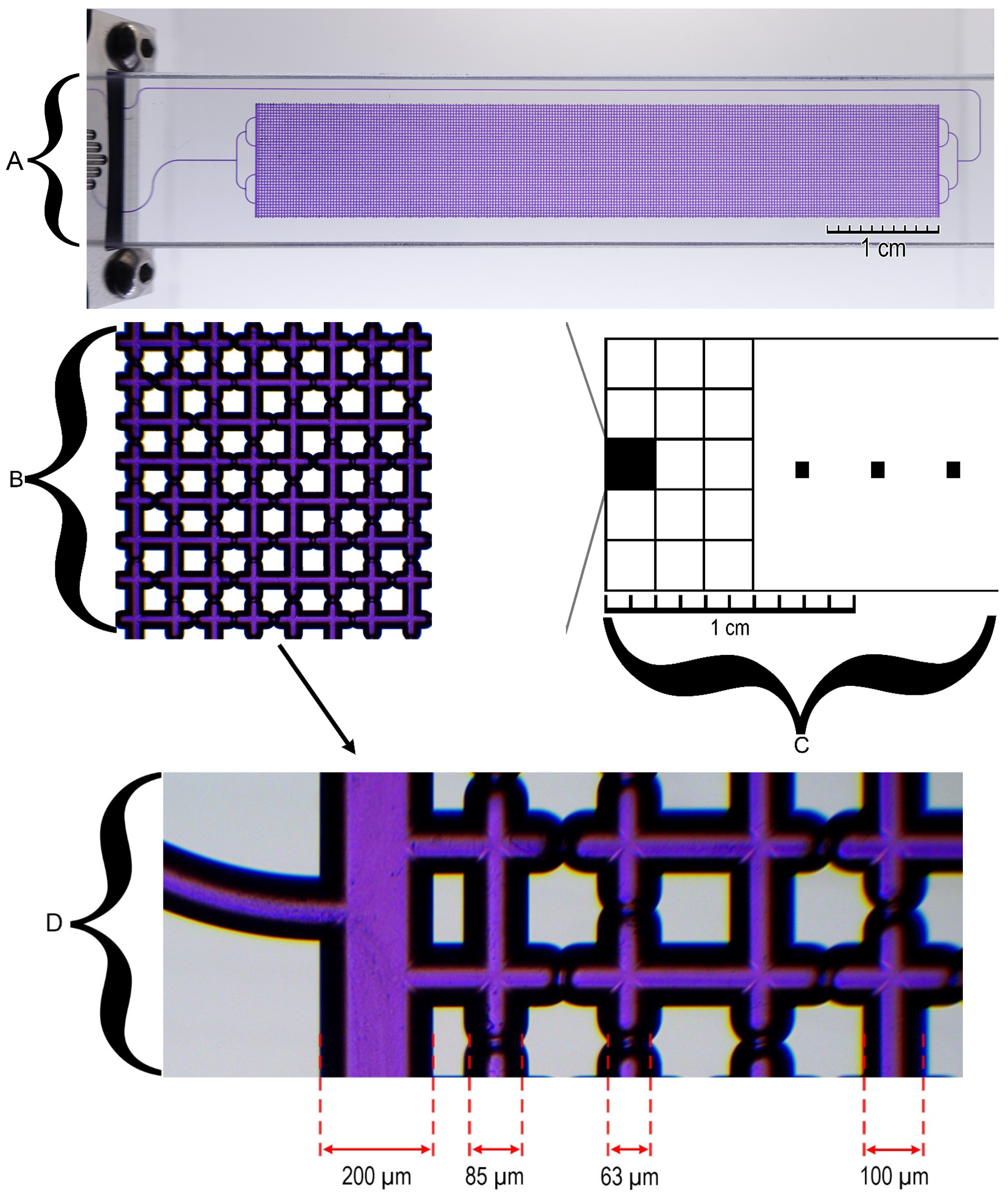
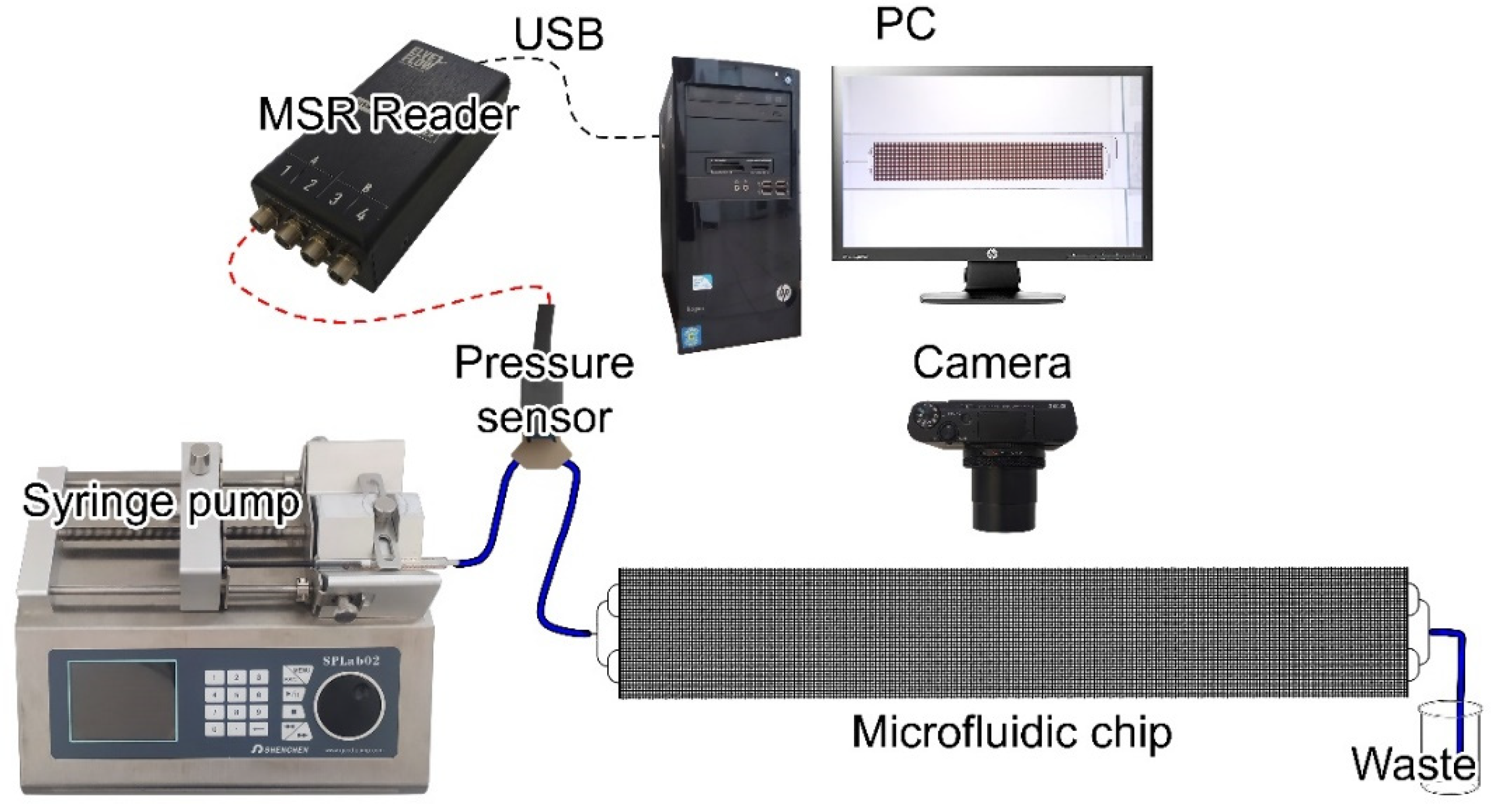
2.3. Microfluidic Chip and Experimental Technique
2.4. Experimental Technique
3. Research Results and Discussion
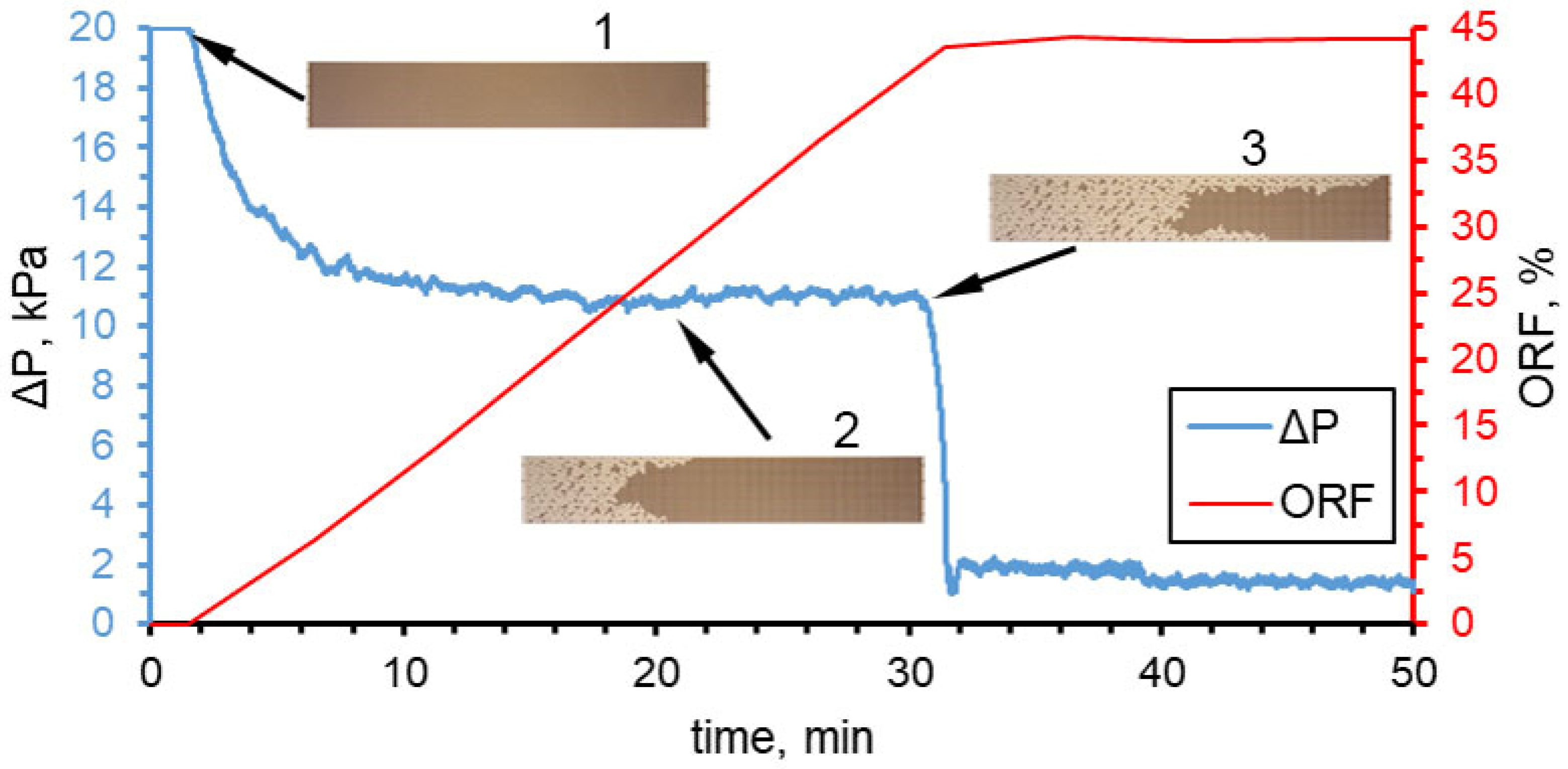
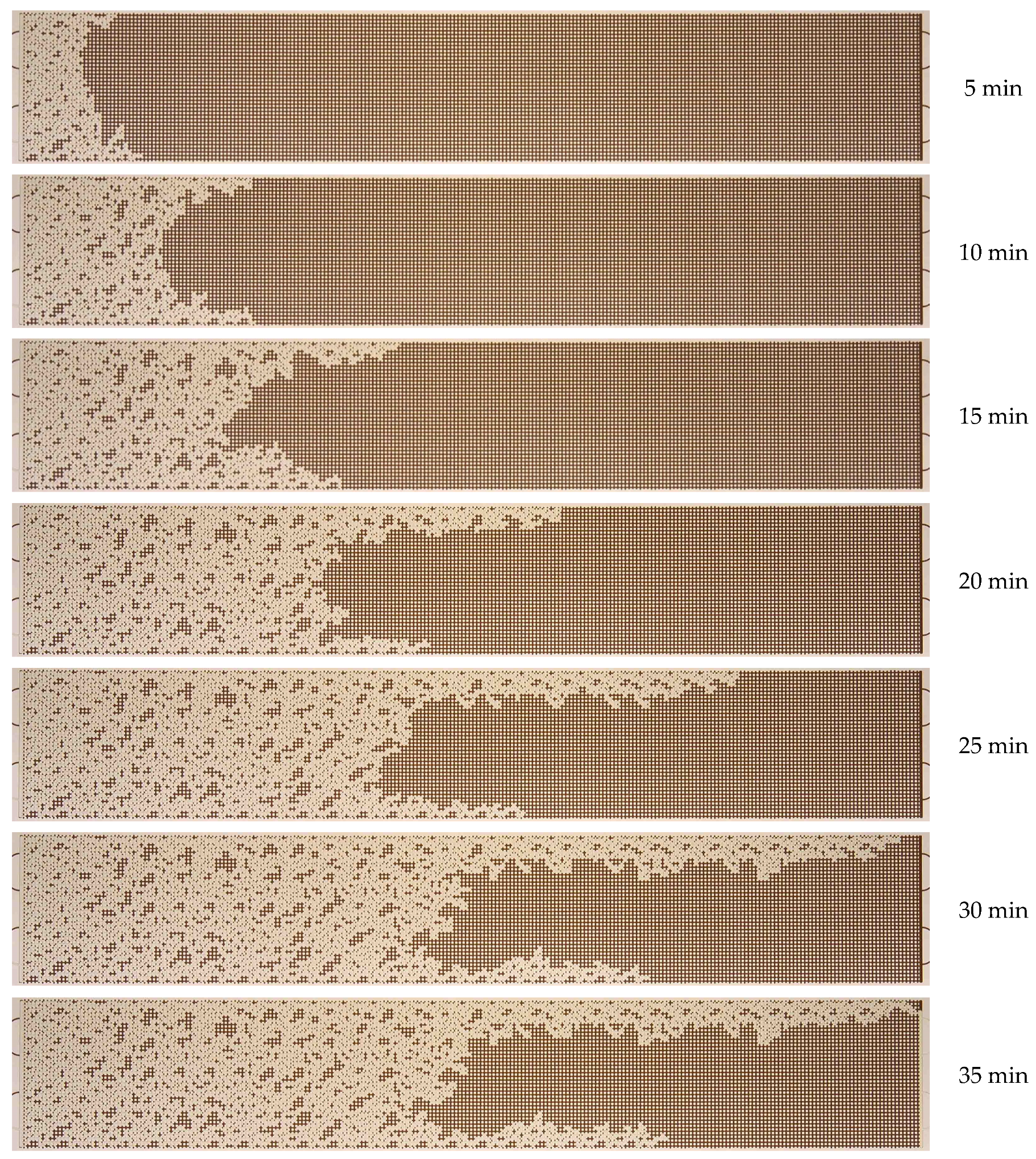
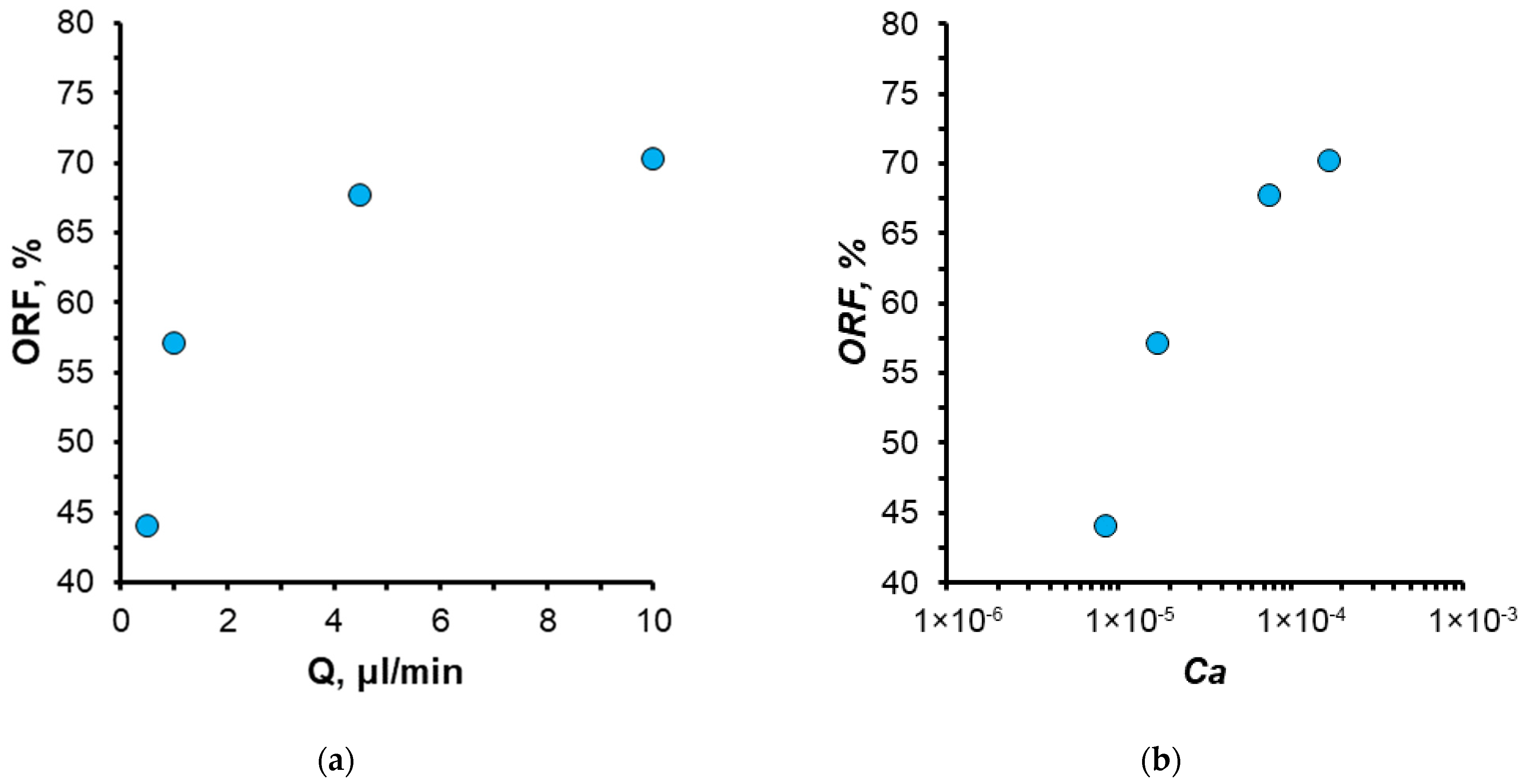
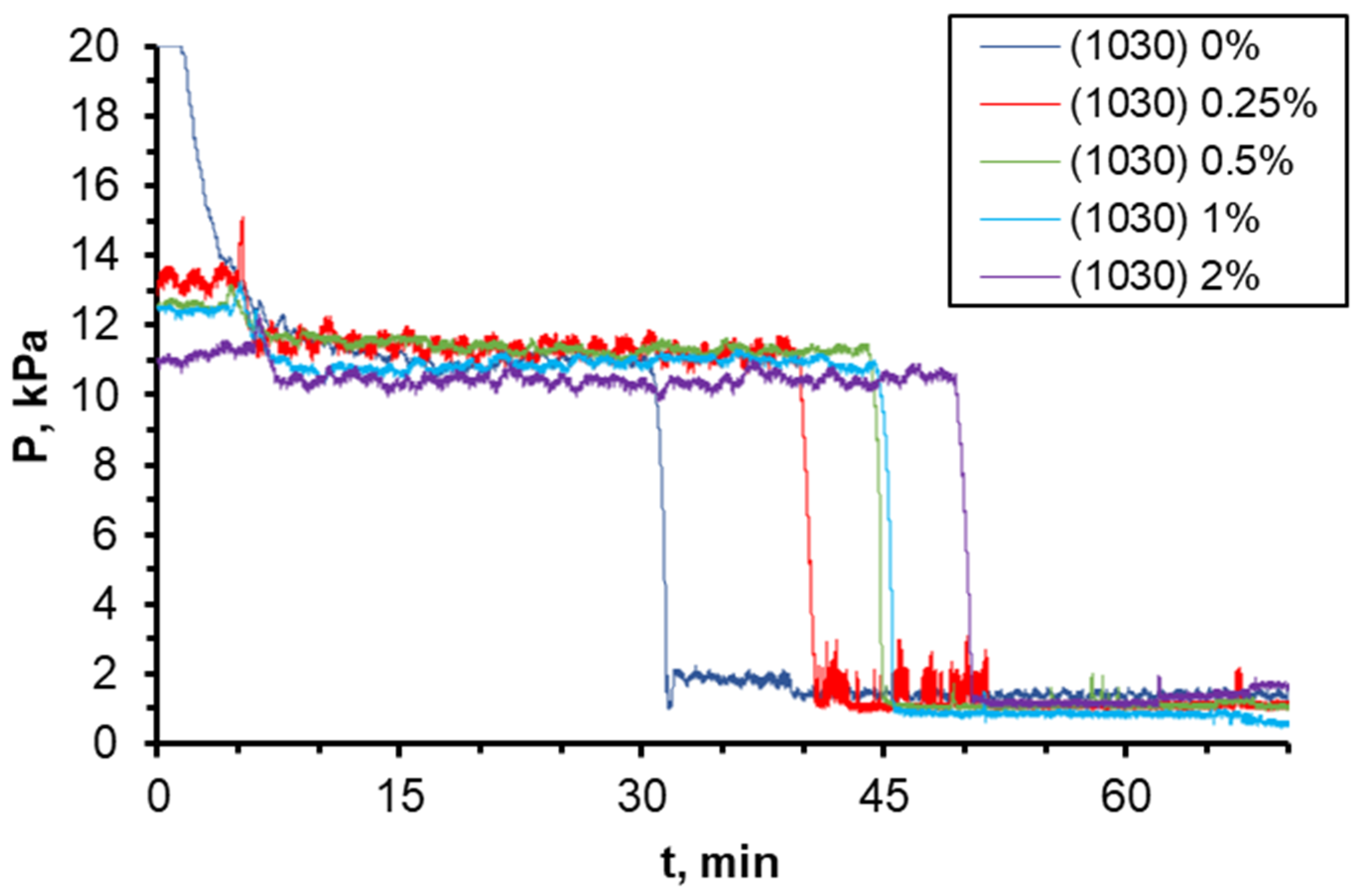
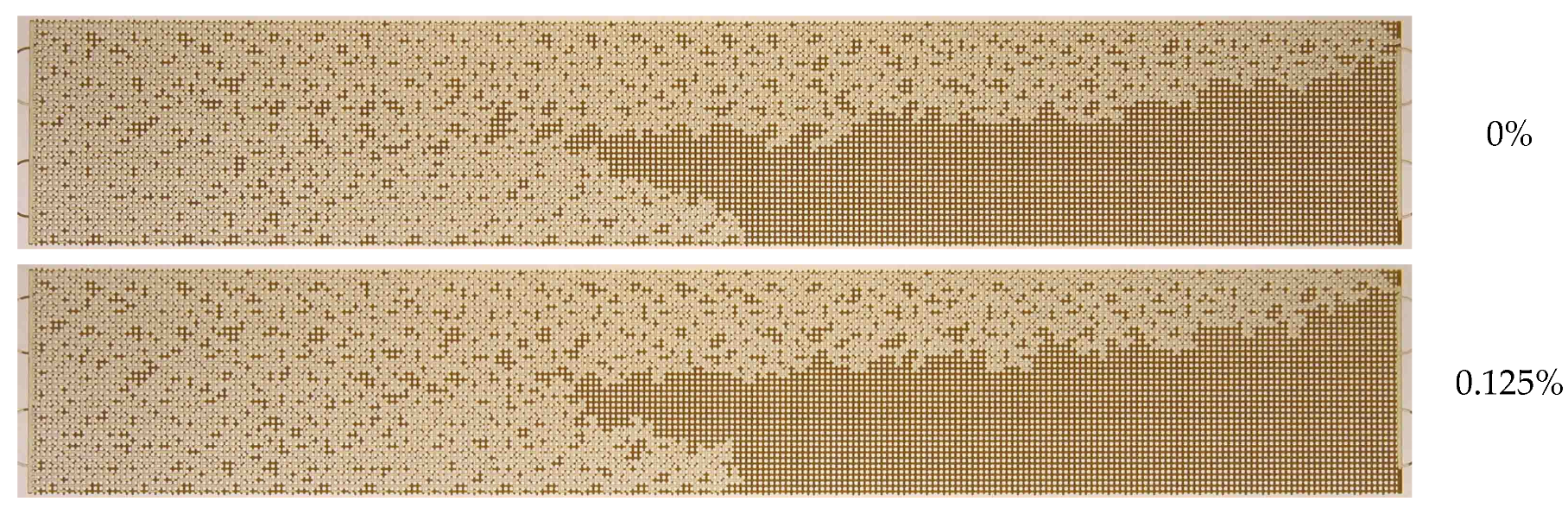
3.1. Effect of Water Flow Rate (Capillary Number)
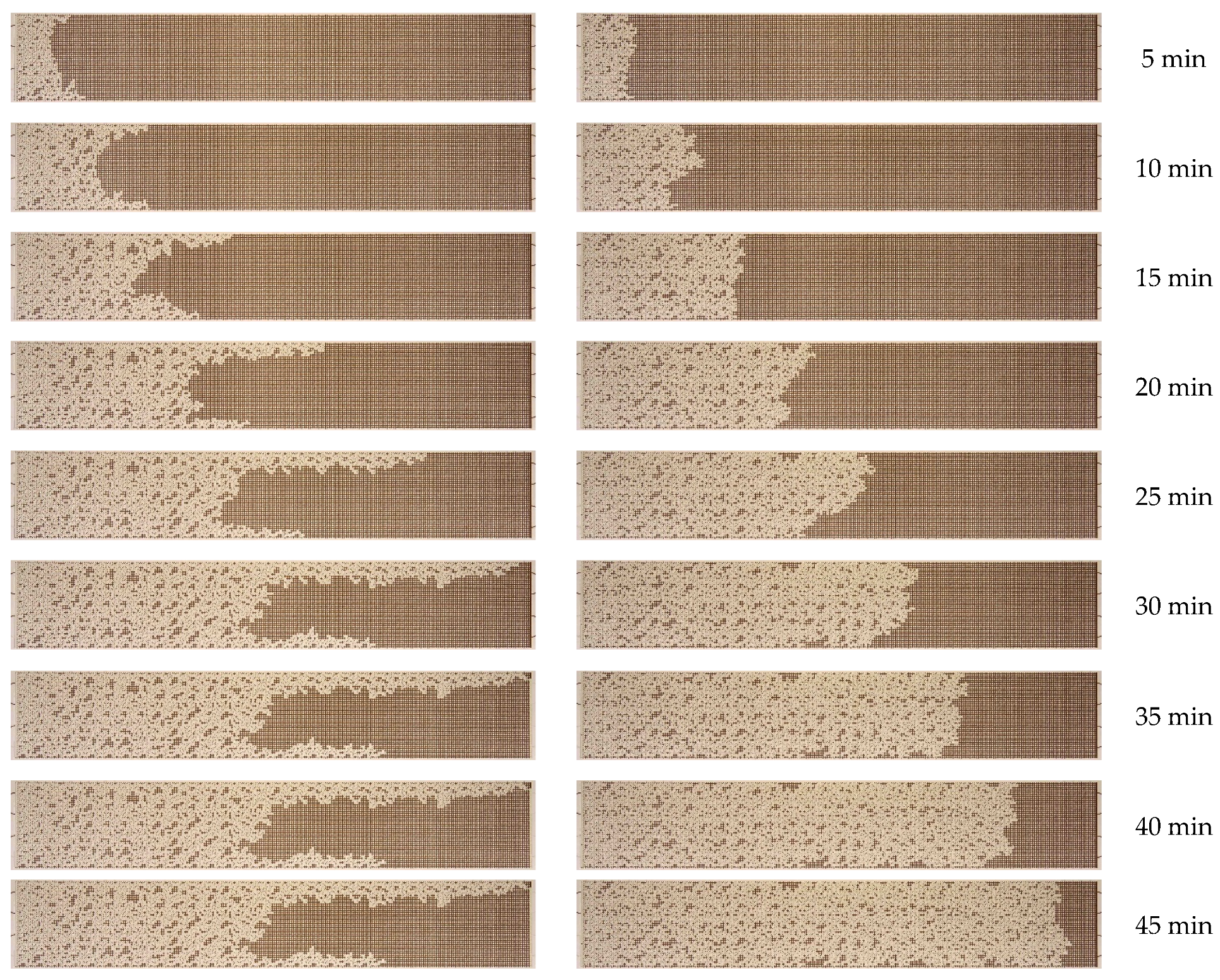
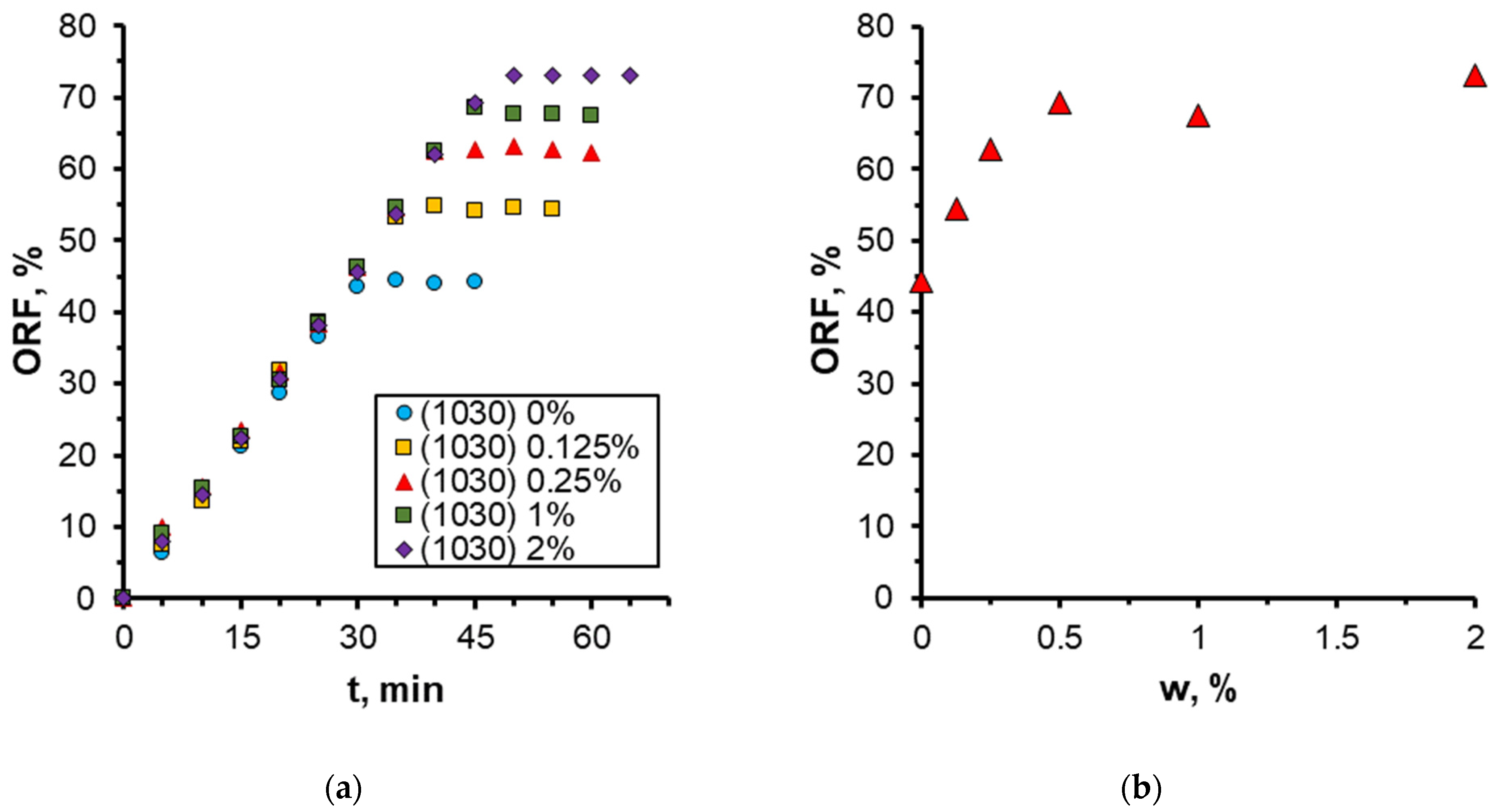
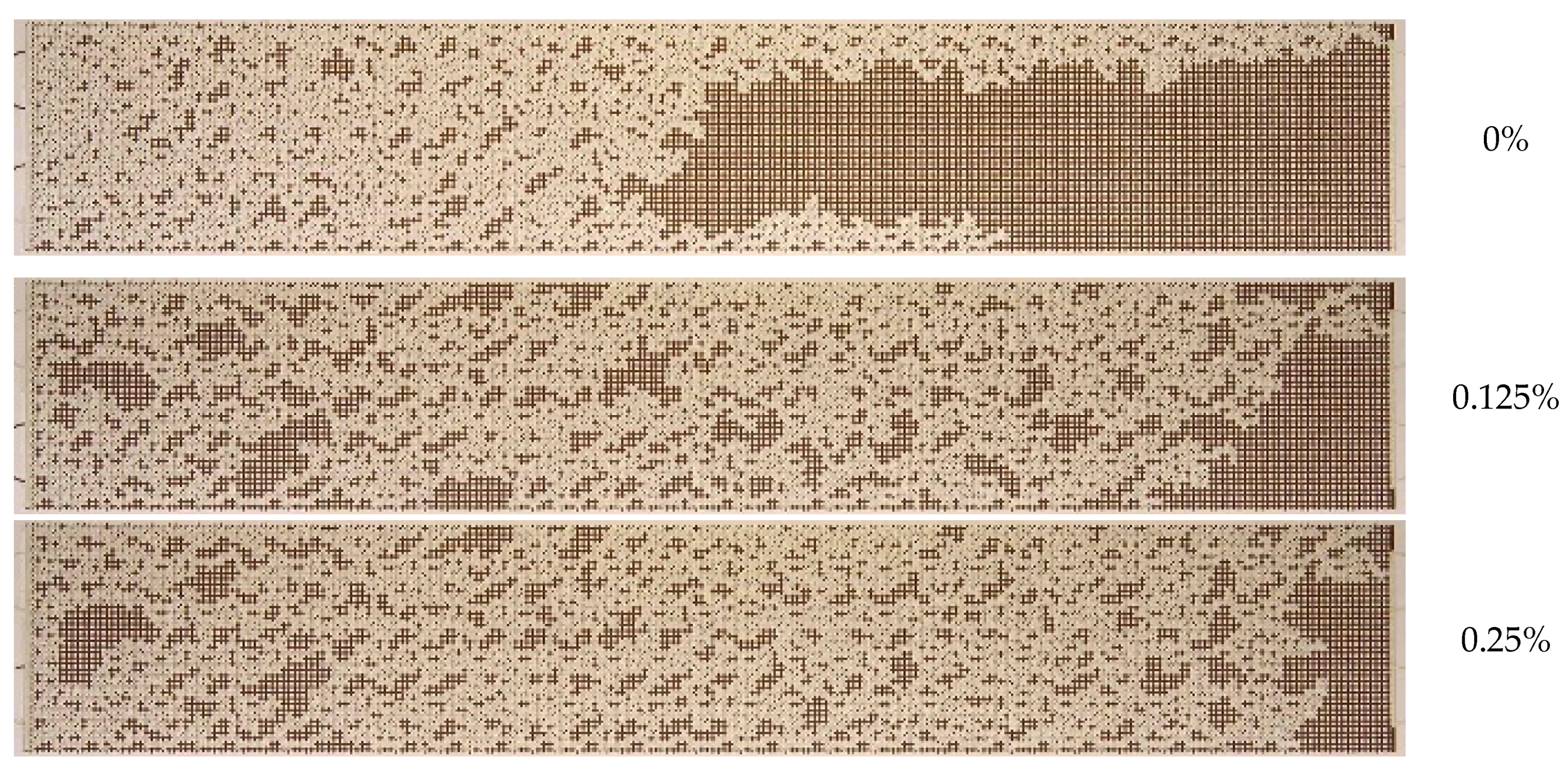
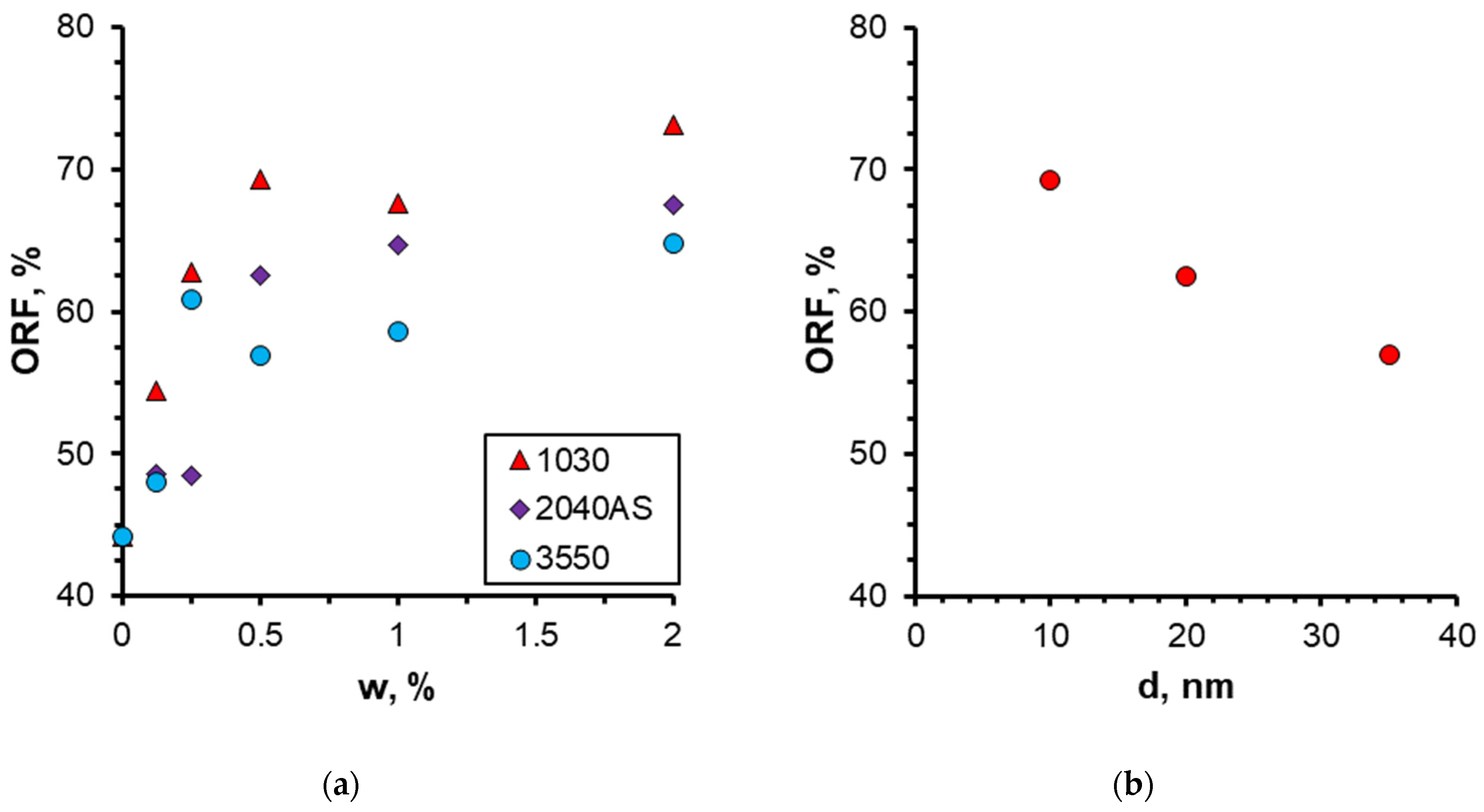
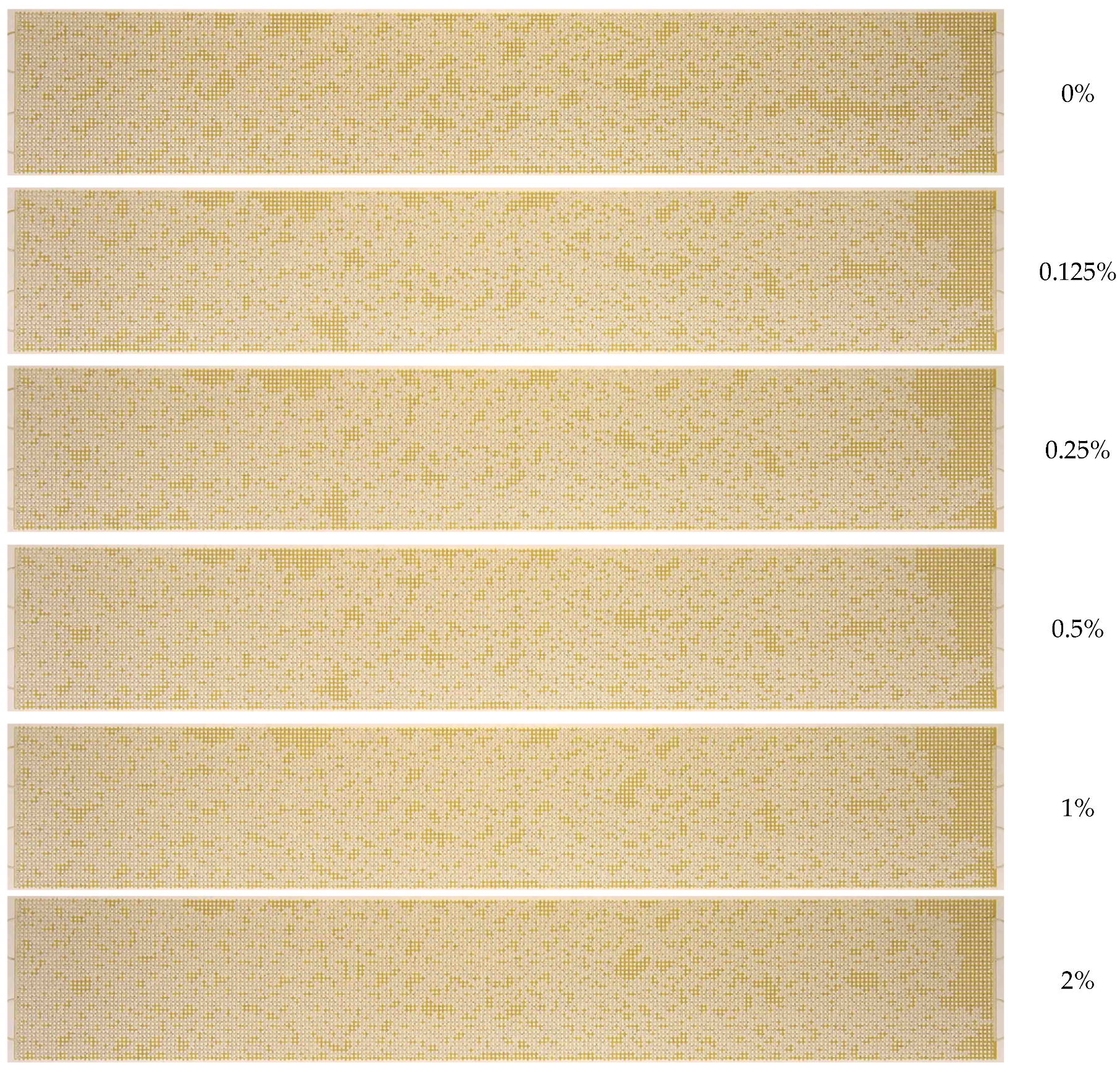
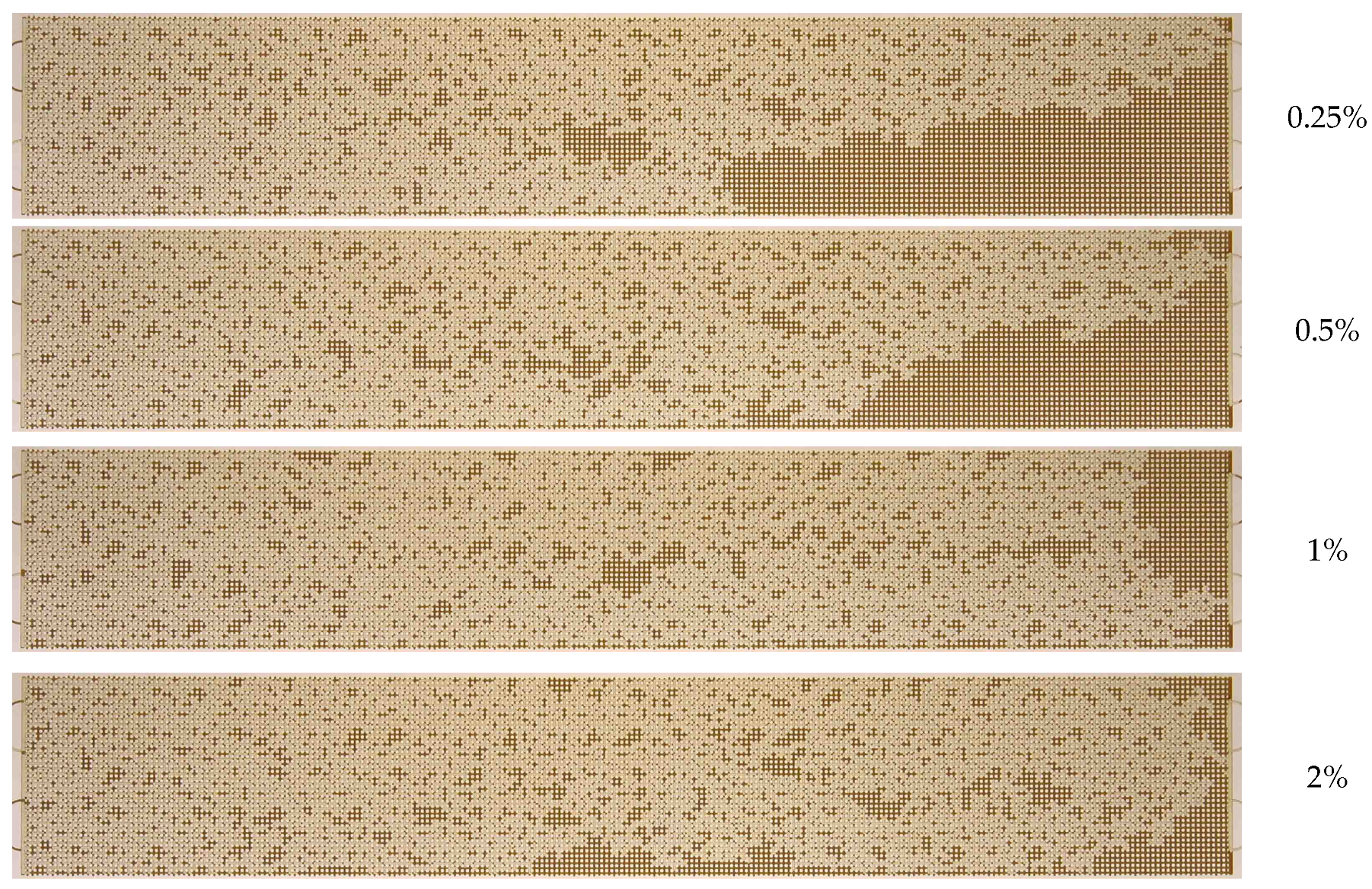
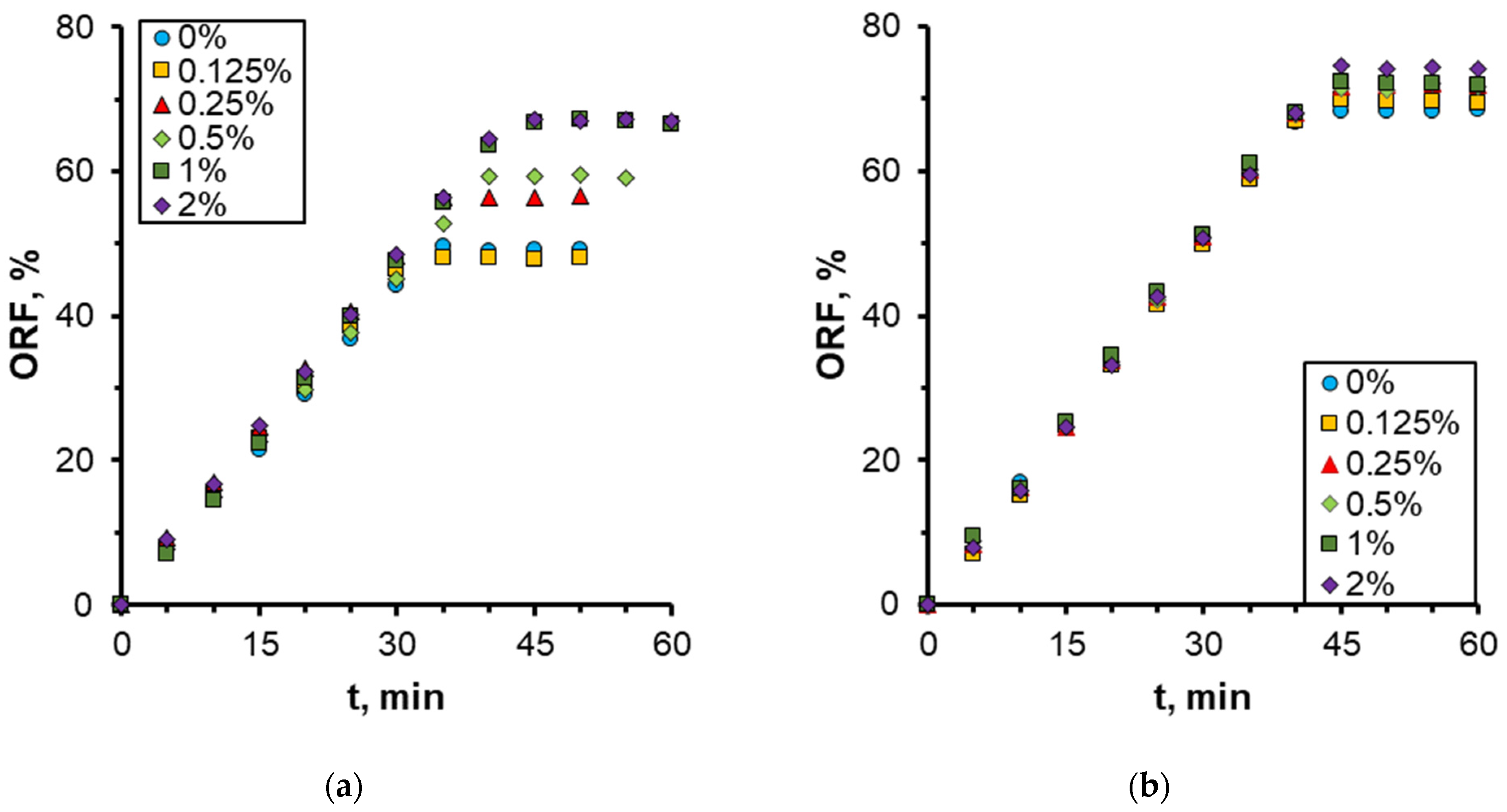
3.2. Effect of Nanoparticle Concentration
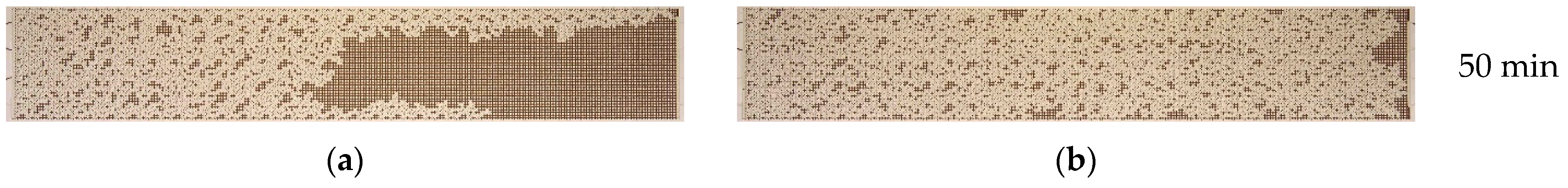
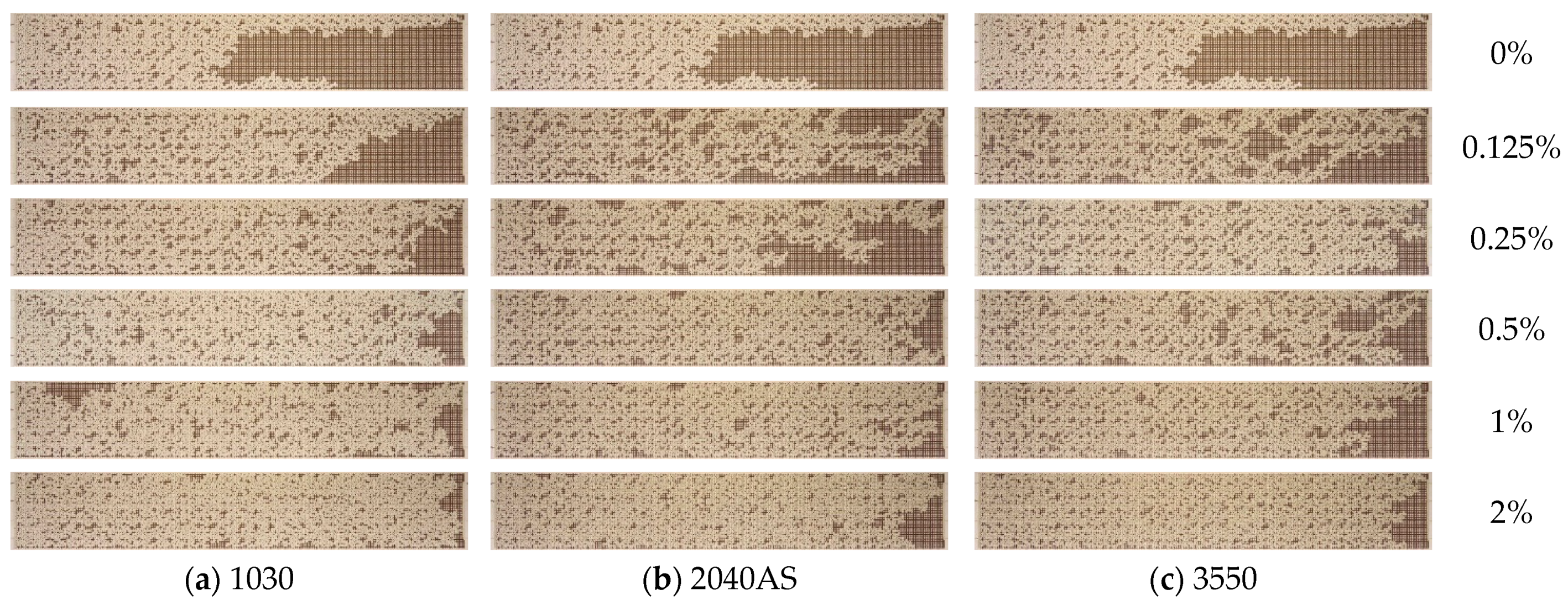
3.3. Effect of Nanoparticle Size
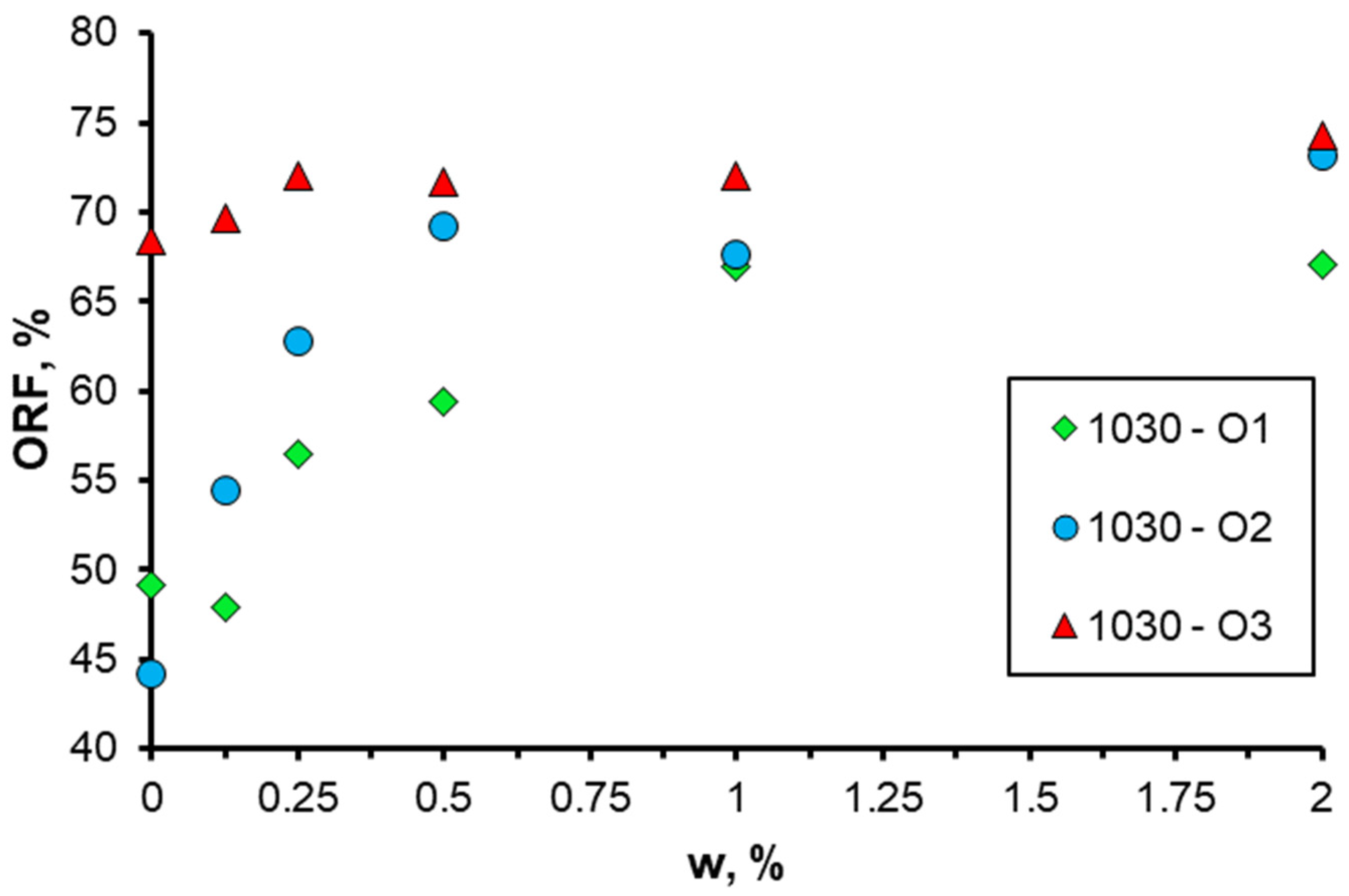
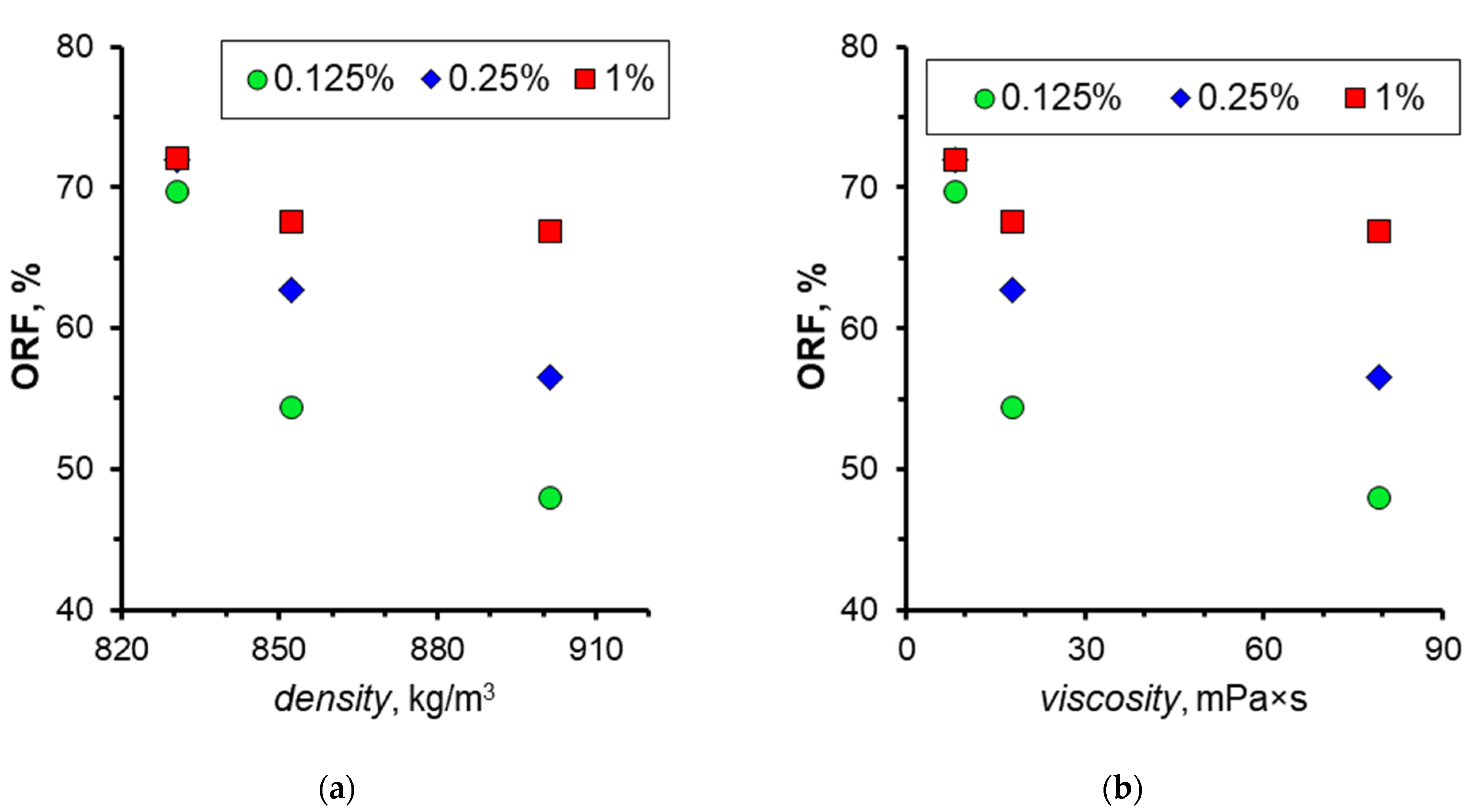
3.4. Effect of Oil Properties
4. Conclusions
- A notable enhancement in oil displacement was observed during flooding with nanosuspensions, even at low nanoparticle concentrations of 0.125–0.25%. The ORF exhibited a notable increase with rising nanoparticle concentration in nanosuspensions. The maximum increase in the ORF compared to water was approximately 28%. Increasing the nanoparticle concentration above 0.5 wt% has practically no further effect on the ORF, and thus, the concentration of 0.5 wt% should be considered optimal.
- The effect of nanoparticle size on oil recovery from microfluidic chips was investigated. The results demonstrate that the ORF increases as the size of the nanoparticles decreases. The use of 1030-grade nanosuspensions with a minimum nanoparticle size (10 nm) as a displacement fluid results in the greatest enhancement of oil displacement, provided that all other parameters remain constant.
- The application of alkaline nanosuspensions to enhance oil displacement has been demonstrated to be more effective than the use of acidic nanosuspensions.
- The impact of oil viscosity on displacement efficiency when using nanosuspensions was examined. The results demonstrate that the presence of nanoparticles in the displacement fluid exerts a favorable influence on the displacement efficiency for all the examined oil samples. Concurrently, the incorporation of nanoparticles to enhance oil displacement exerts the most pronounced impact on high-viscosity oil samples.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saadat, M.; Tsai, P.; Ho, T.H.; Øye, G.; Dudek, M. Development of a Microfluidic Method to Study Enhanced Oil Recovery by Low Salinity Water Flooding. ACS Omega 2020, 5, 17521–17530. [Google Scholar] [CrossRef] [PubMed]
- Lifton, V.A. Microfluidics: An enabling screening technology for enhanced oil recovery (EOR). Lab A Chip 2016, 16, 1777–1796. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, S.; Gogoi, S.B. Review on microfluidic studies for EOR application. J. Pet. Explor. Prod. Technol. 2019, 9, 2263–2277. [Google Scholar] [CrossRef]
- Yun, W.; Chang, S.; Cogswell, D.A.; Eichmann, S.L.; Gizzatov, A.; Thomas, G.; Al-Hazza, N.; Abdel-Fattah, A.; Wang, W. Toward Reservoir-on-a-Chip: Rapid Performance Evaluation of Enhanced Oil Recovery Surfactants for Carbonate Reservoirs Using a Calcite-Coated Micromodel. Sci. Rep. 2020, 10, 782. [Google Scholar] [CrossRef] [PubMed]
- Saadat, M.; Yang, J.; Dudek, M.; Tsai, P.; Øye, G.; Tsai, P.A. Microfluidic investigation of enhanced oil recovery: The effect of aqueous floods and network wettability. J. Pet. Sci. Technol. Eng. 2021, 203, 108647. [Google Scholar] [CrossRef]
- Pryazhnikov, M.I.; Minakov, A.V.; Pryazhnikov, A.I.; Denisov, I.A.; Yakimov, A.S.; Nemtsev, I.V. Application of microfluidic technologies for enhanced oil recovery. J. Phys. Conf. Ser. 2021, 2119, 012067. [Google Scholar] [CrossRef]
- Seyyedi, M.; Sohrabi, M. Oil Reservoir on a Chip: Pore-Scale Study of Multiphase Flow during Near-Miscible CO2 EOR and Storage. Transp. Porous Media 2020, 134, 331–349. [Google Scholar] [CrossRef]
- Tahir, M.; Liu, W.; Zhou, H.; Memon, A.; Ansari, U.; Akbar, I.; Zafar, A.; Shaikh, A.; Kashif, M.; Urinov, A. A review study on micro fluid chips for enhancing the oil recovery by injecting the chemical floods. Therm. Sci. 2020, 24, 423–432. [Google Scholar] [CrossRef]
- Fang, Z.; Cao, X.-R.; Yu, Y.-L.; Li, M. Fabrication and Characterization of Microcapsule Encapsulating EOR Surfactants by Microfluidic Technique. Colloids Surf. A 2019, 570, 282–292. [Google Scholar] [CrossRef]
- Hou, J.; Du, J.; Sui, H.; Sun, L. A review on the application of nanofluids in enhanced oil recovery. Front. Chem. Sci. Eng. 2022, 16, 1165–1197. [Google Scholar] [CrossRef]
- Safarzadeh, S.; Bila, A.; Torsæter, O. Experimental Investigation of the Effect of Silica Nanoparticles on Interfacial Tension and Wettability during Low Salinity Water Flooding: A Micromodel Study. J. Mod. Nanotechnol. 2022, 2, 1–19. [Google Scholar] [CrossRef]
- Tuok, L.P.; Elkady, M.; Zkria, A.; Yoshitake, T.; Abdelkader, S.A.; Seyam, D.F.; El-Moneim, A.A.; Fath El-Bab, A.M.R.; Eldemerdash, U.N. Experimental investigation of copper oxide nanofluids for enhanced oil recovery in the presence of cationic surfactant using a microfluidic model. Chem. Eng. J. 2024, 488, 151011. [Google Scholar] [CrossRef]
- Pryazhnikov, A.I.; Minakov, A.V.; Pryazhnikov, M.I.; Zhigarev, V.A.; Nemtsev, I.V. Displacement of oil by SiO2-nanofluid from a microporous medium: Microfluidic experiments. J. Phys. Conf. Ser. 2022, 2150, 012019. [Google Scholar] [CrossRef]
- Rostami, P.; Sharifi, M.; Aminshahidy, B.; Fahimpour, J. The effect of nanoparticles on wettability alteration for enhanced oil recovery: Micromodel experimental studies and CFD simulation. Pet. Sci. 2019, 16, 859–873. [Google Scholar] [CrossRef]
- Pryazhnikov, M.I.; Minakov, A.V.; Pryazhnikov, A.I.; Denisov, I.A.; Yakimov, A.S. Microfluidic Study of the Effect of Nanosuspensions on Enhanced Oil Recovery. Nanomaterials 2022, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- ASTM D287-22; Standard Test Method for API Gravity of Crude Petroleum and Petroleum Products (Hydrometer/Method). ASTM: West Conshohocken, PA, USA, 2023.
- Hennekam, R.; Sweere, T.; Tjallingii, R.; de Lange, G.J.; Reichart, G.-J. Trace metal analysis of sediment cores using a novel X-ray fluorescence core scanning method. Quat. Int. 2019, 514, 55–67. [Google Scholar] [CrossRef]
- Oprea, C.; Maslov, O.D.; Gustova, M.V.; Belov, A.G.; Szalanski, P.J.; Oprea, I.A. IGAA and XRF methods used in oil contamination research. Vacuum 2009, 83, S162–S165. [Google Scholar] [CrossRef]
- Wanatasanappan, V.V.; Kanti, P.K.; Sharma, P.; Husna, N.; Abdullah, M.Z. Viscosity and rheological behavior of Al2O3-Fe2O3/water-EG based hybrid nanofluid: A new correlation based on mixture ratio. J. Mol. Liq. 2023, 375, 121365. [Google Scholar] [CrossRef]
- Moshfeghi, R.; Toghraie, D. An analytical and statistical review of selected researches in the field of estimation of rheological behavior of nanofluids. Powder Technol. 2022, 398, 117076. [Google Scholar] [CrossRef]
- Pryazhnikov, M.I.; Zhigarev, V.A.; Minakov, A.V.; Nemtsev, I.V. Spontaneous imbibition experiments for enhanced oil recovery with silica nanosols. Capillarity 2023, 10, 73–86. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.; Minaev, K. Experimental and field applications of nanotechnology for enhanced oil recovery purposes: A review. Fuel 2022, 324, 124669. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B. The Role of Dispersed Particles in the Physicochemical Behavior of Nanofluids. Colloid J. 2023, 85, 898–907. [Google Scholar] [CrossRef]

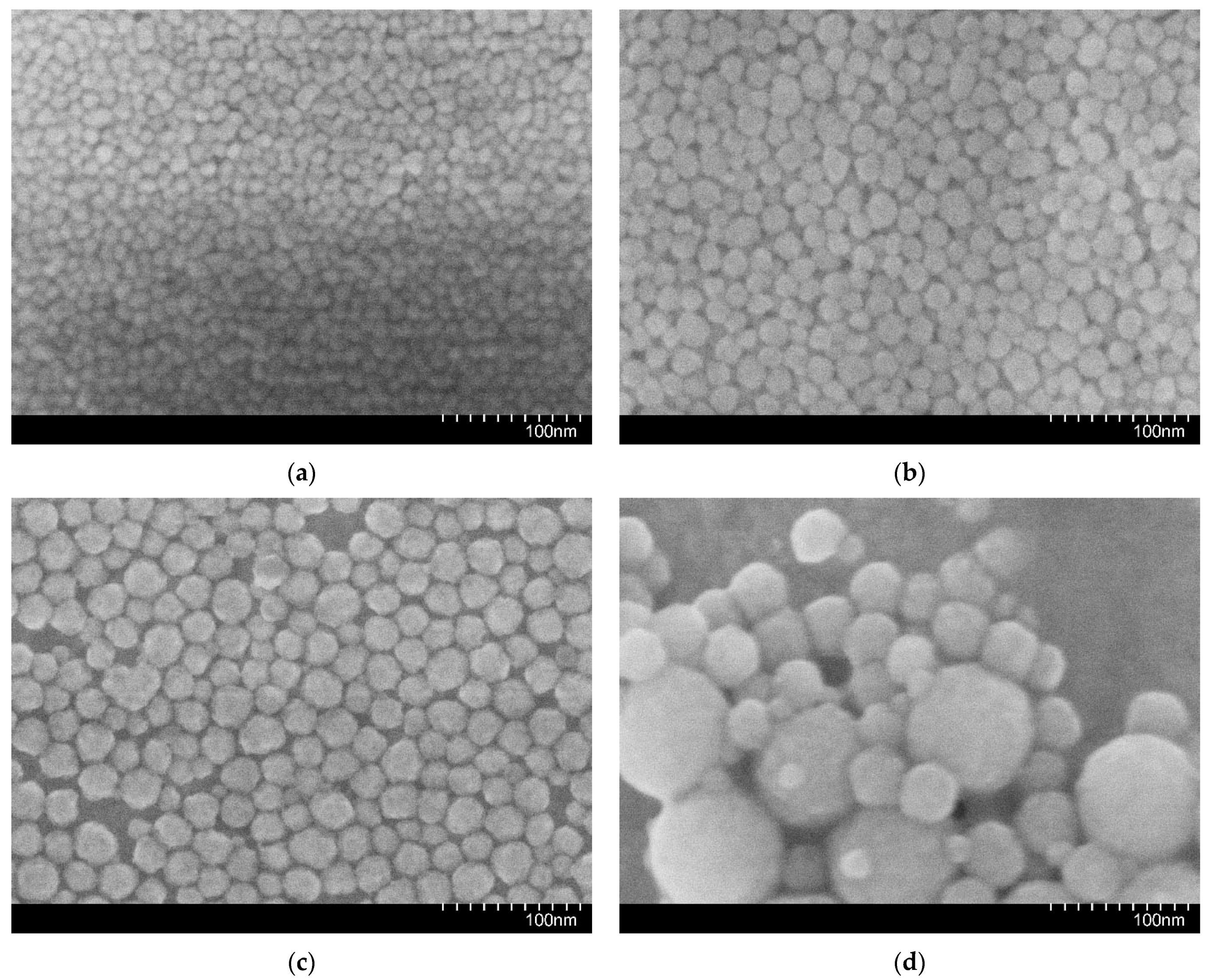





| Sample | μ, mPa × s | ρ, kg/m3 |
|---|---|---|
| O1 | 79.3 | 901.3 |
| O2 | 17.8 | 852.4 |
| O3 | 8.34 | 830.6 |
| Elements | Samples | ||
|---|---|---|---|
| O1 | O2 | O3 | |
| Ba | 19.51 | 17.58 | 24.92 |
| Ca | 30.86 | 13.10 | 122.8 |
| Cl | 617.3 | 62.89 | 457.1 |
| Mg | - | - | - |
| Mo | 11.86 | 2.774 | 11.70 |
| S | 2156 | 6328 | 2356 |
| Si | 12.02 | 6.174 | 18.40 |
| Zn | 21.28 | 20.26 | 21.64 |
| Sample | Optical Density D at the Maximum of Absorption Bands λ, cm−1 | Spectral Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1710 | 1600 | 1465 | 1380 | 1030 | 720 | C1 | C2 | C3 | C4 | C5 | |
| O1 | 0.013 | 0.062 | 0.867 | 0.452 | 0.045 | 0.096 | 0.646 | 0.015 | 0.521 | 8.839 | 0.052 |
| O2 | 0.011 | 0.050 | 0.846 | 0.445 | 0.048 | 0.119 | 0.420 | 0.013 | 0.526 | 11.280 | 0.057 |
| O3 | 0.016 | 0.033 | 0.779 | 0.383 | 0.035 | 0.130 | 0.254 | 0.021 | 0.492 | 15.545 | 0.045 |
| Sample | Manufacturer/Country | Diameter, nm | Concentration, wt% | pH | Stabilization |
|---|---|---|---|---|---|
| 1030 | RusSilica, Russia | 10 | 30 | 9.6 | Na+ |
| WA1530 | RusSilica, Russia | 15 | 30 | 2.3 | Al component |
| 2040AS | RusSilica, Russia | 20 | 40 | ||
| 3550 | RusSilica, Russia | 35 | 50 | 8.9 | NH4+ |
| Name | Concentration, wt% | Viscosity, mPa × s | Density, kg/m3 |
|---|---|---|---|
| W | 0 | 0.8909 | 997.0 |
| 0.125% 1030 | 0.125 | 0.8959 | 997.7 |
| 0.25% 1030 | 0.25 | 0.9031 | 998.4 |
| 0.5% 1030 | 0.5 | 0.9177 | 999.7 |
| 1% 1030 | 1 | 0.9467 | 1002 |
| 2% 1030 | 2 | 1.0049 | 1008 |
| 0.125% WA1530 | 0.125 | 0.8948 | 997.7 |
| 0.25% WA1530 | 0.25 | 0.9014 | 998.4 |
| 0.5% WA1530 | 0.5 | 0.9147 | 999.7 |
| 1% WA1530 | 1 | 0.9411 | 1002 |
| 2% WA1530 | 2 | 0.9940 | 1008 |
| 0.125% 2040AS | 0.125 | 0.8940 | 997.7 |
| 0.25% 2040AS | 0.25 | 0.8994 | 998.4 |
| 0.5% 2040AS | 0.5 | 0.9100 | 999.7 |
| 1% 2040AS | 1 | 0.9313 | 1002 |
| 2% 2040AS | 2 | 0.9740 | 1008 |
| 0.125% 3550 | 0.125 | 0.8932 | 997.7 |
| 0.25% 3550 | 0.25 | 0.8973 | 998.4 |
| 0.5% 3550 | 0.5 | 0.9055 | 999.7 |
| 1% 3550 | 1 | 0.9218 | 1002 |
| 2% 3550 | 2 | 0.9545 | 1008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryazhnikov, A.I.; Pryazhnikov, M.I.; Lobasov, A.S.; Minakov, A.V. A Systematic Microfluidic Study of the Use of Diluted Silica Sols to Enhance Oil Displacement. Nanomaterials 2024, 14, 1233. https://doi.org/10.3390/nano14141233
Pryazhnikov AI, Pryazhnikov MI, Lobasov AS, Minakov AV. A Systematic Microfluidic Study of the Use of Diluted Silica Sols to Enhance Oil Displacement. Nanomaterials. 2024; 14(14):1233. https://doi.org/10.3390/nano14141233
Chicago/Turabian StylePryazhnikov, Andrey I., Maxim I. Pryazhnikov, Alexander S. Lobasov, and Andrey V. Minakov. 2024. "A Systematic Microfluidic Study of the Use of Diluted Silica Sols to Enhance Oil Displacement" Nanomaterials 14, no. 14: 1233. https://doi.org/10.3390/nano14141233
APA StylePryazhnikov, A. I., Pryazhnikov, M. I., Lobasov, A. S., & Minakov, A. V. (2024). A Systematic Microfluidic Study of the Use of Diluted Silica Sols to Enhance Oil Displacement. Nanomaterials, 14(14), 1233. https://doi.org/10.3390/nano14141233






