Effect of BaTiO3 Filler Modification with Multiwalled Carbon Nanotubes on Electric Properties of Polymer Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
3. Results
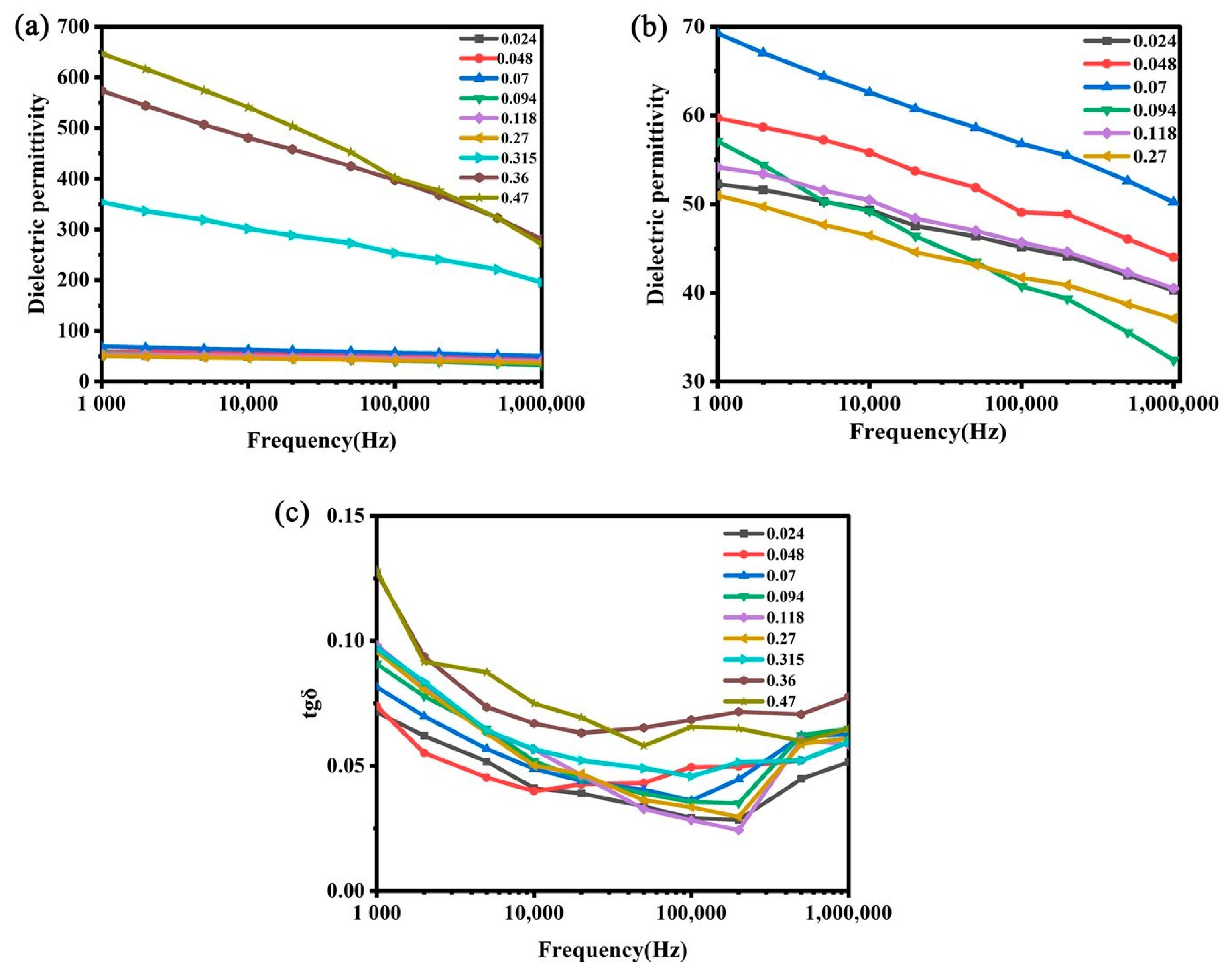
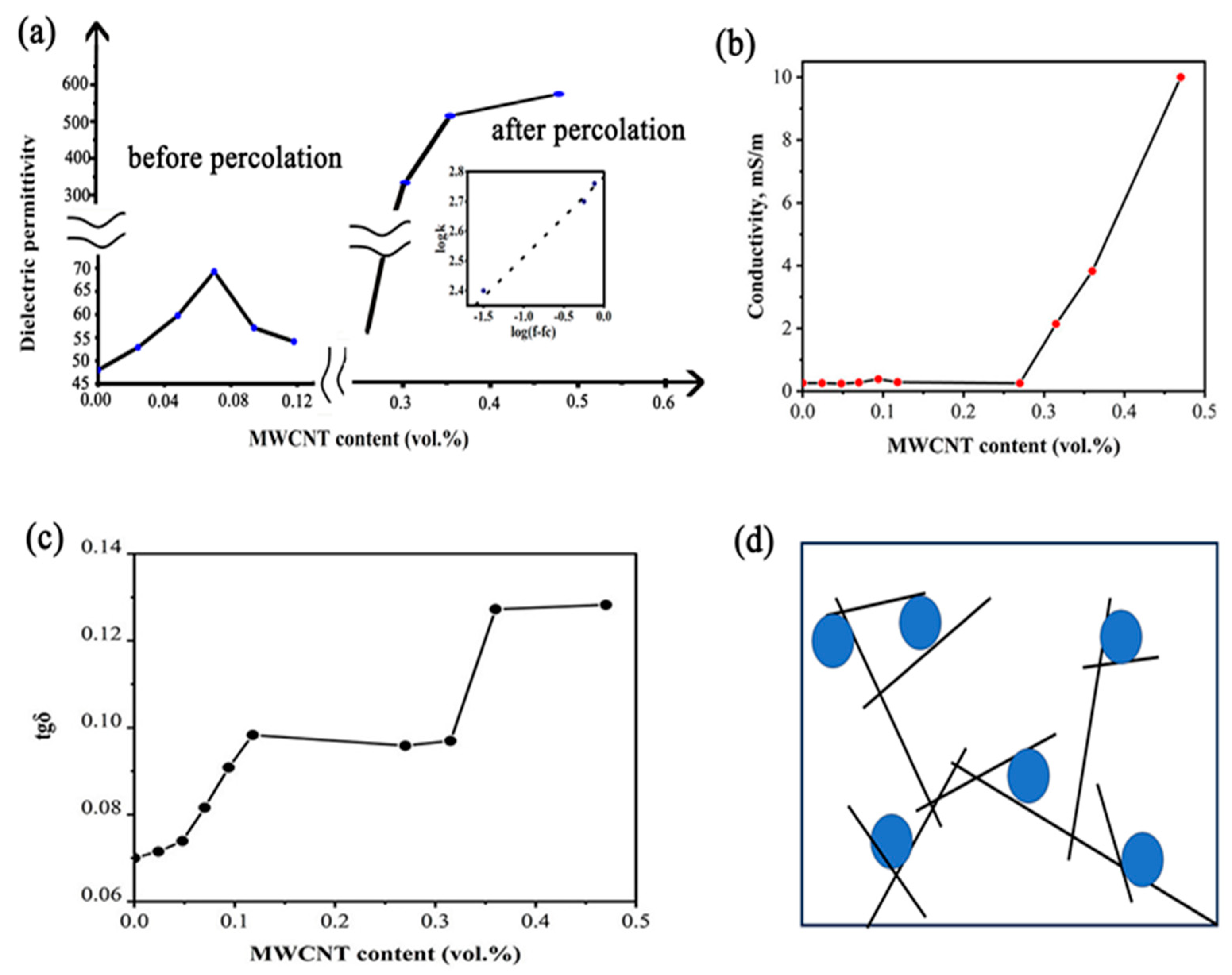
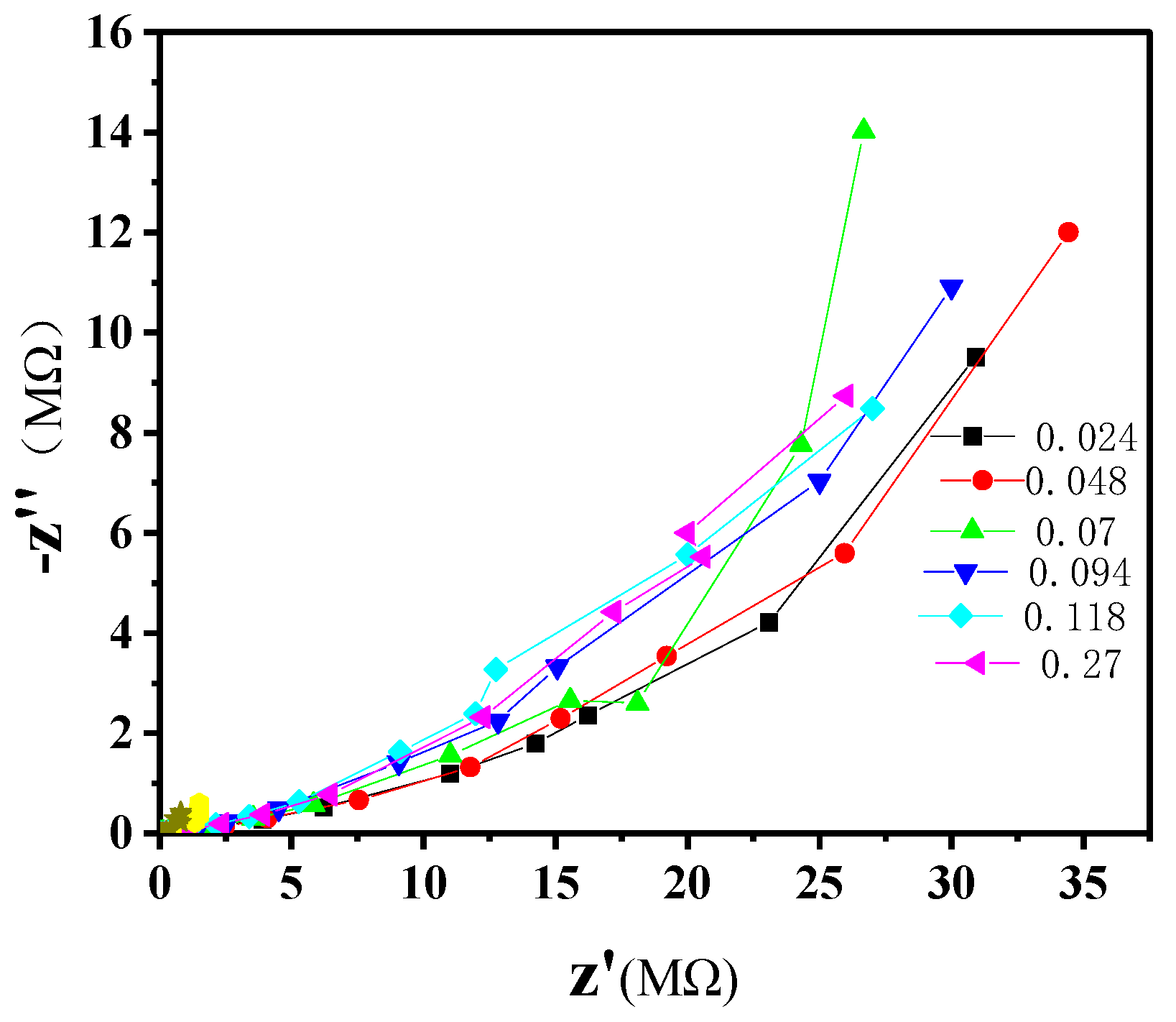
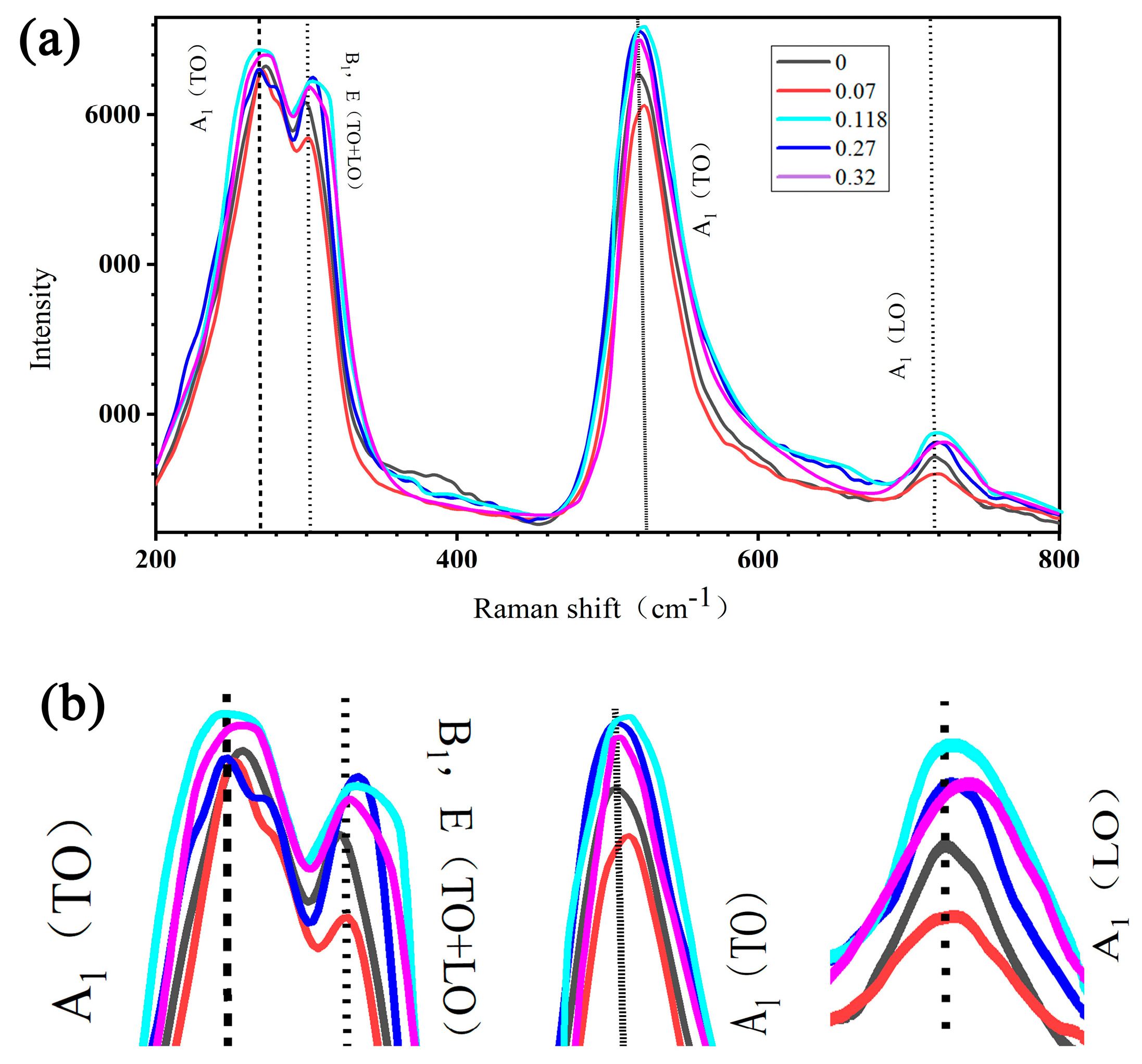
3.1. Characterization of Dielectric Properties
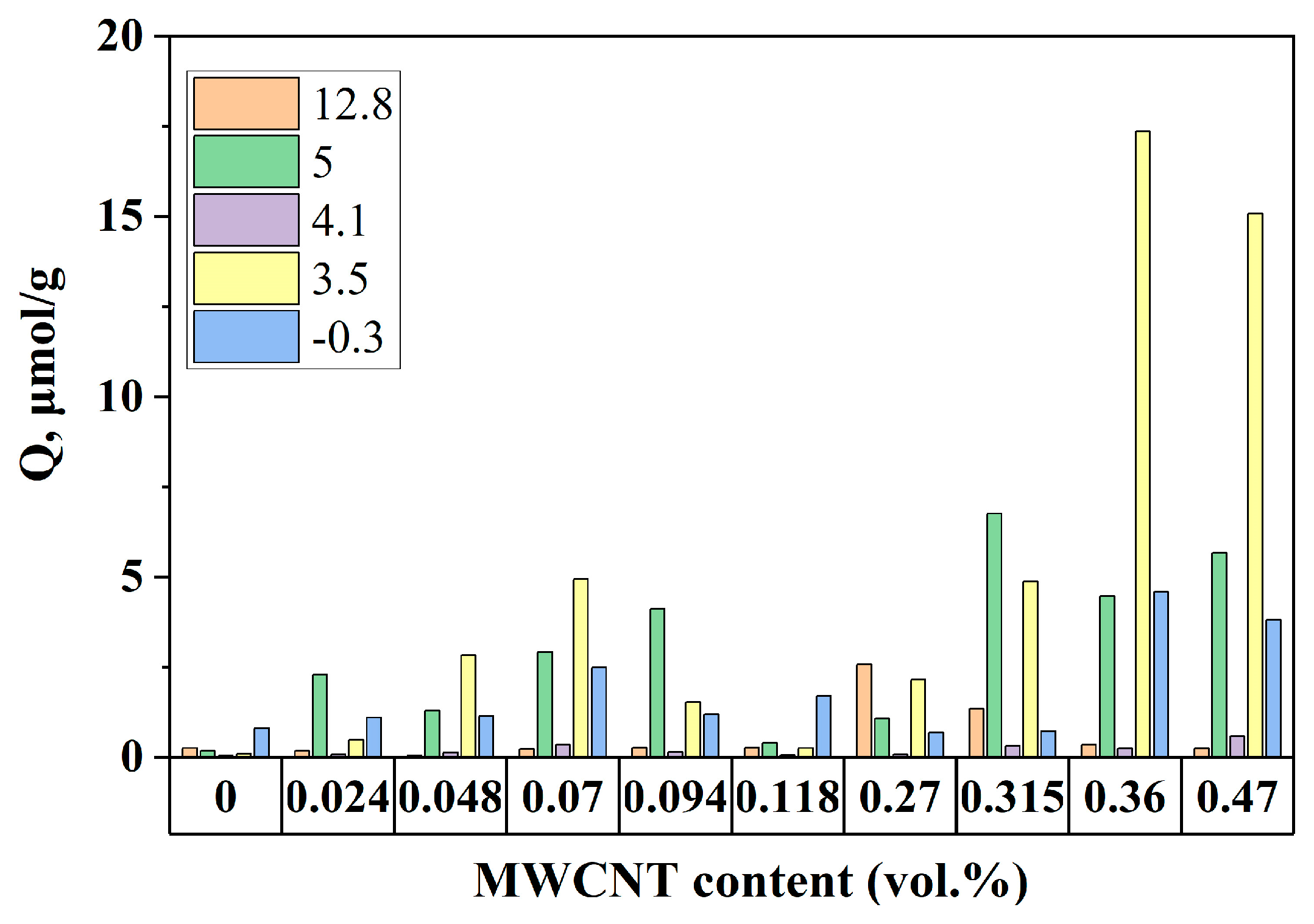
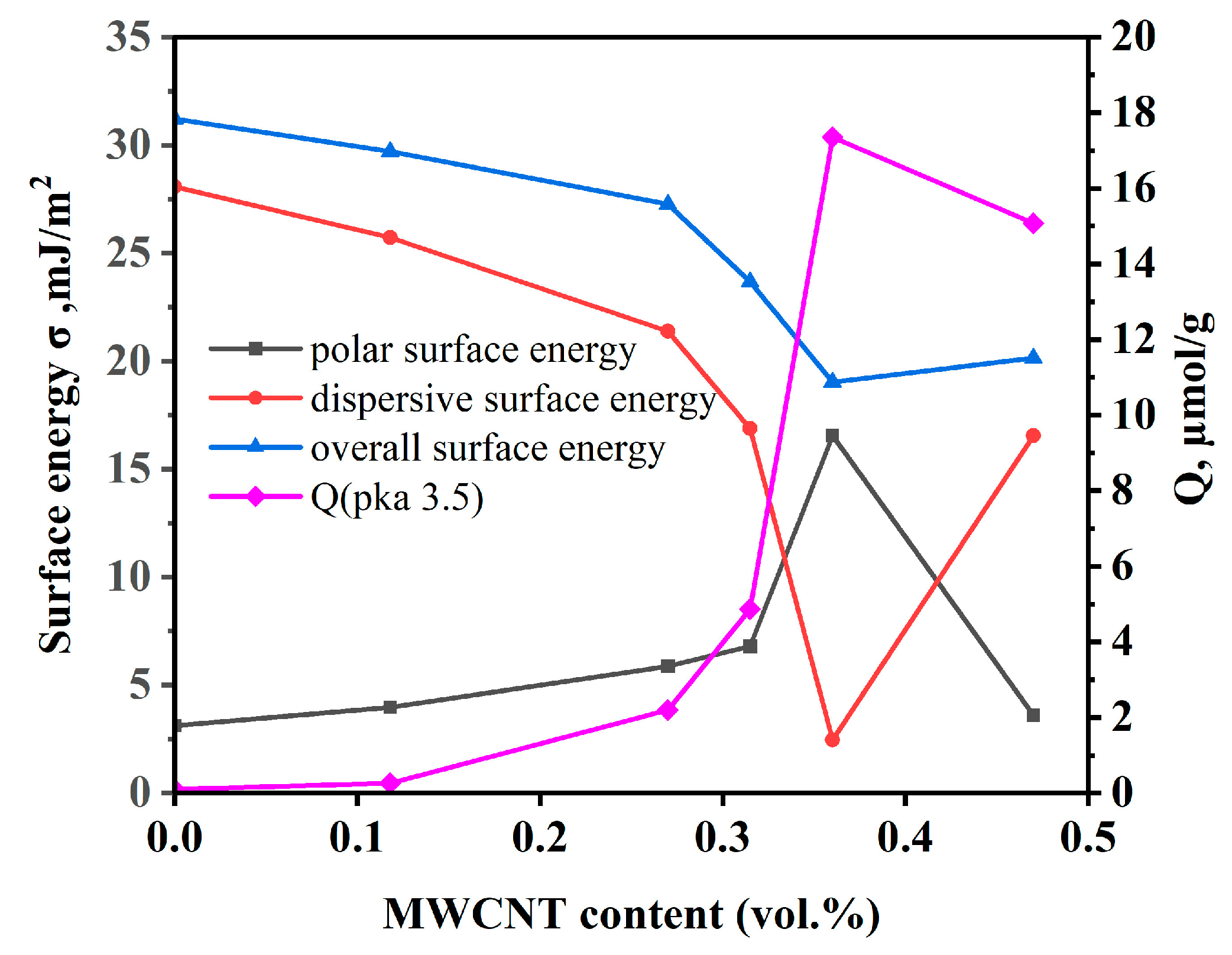
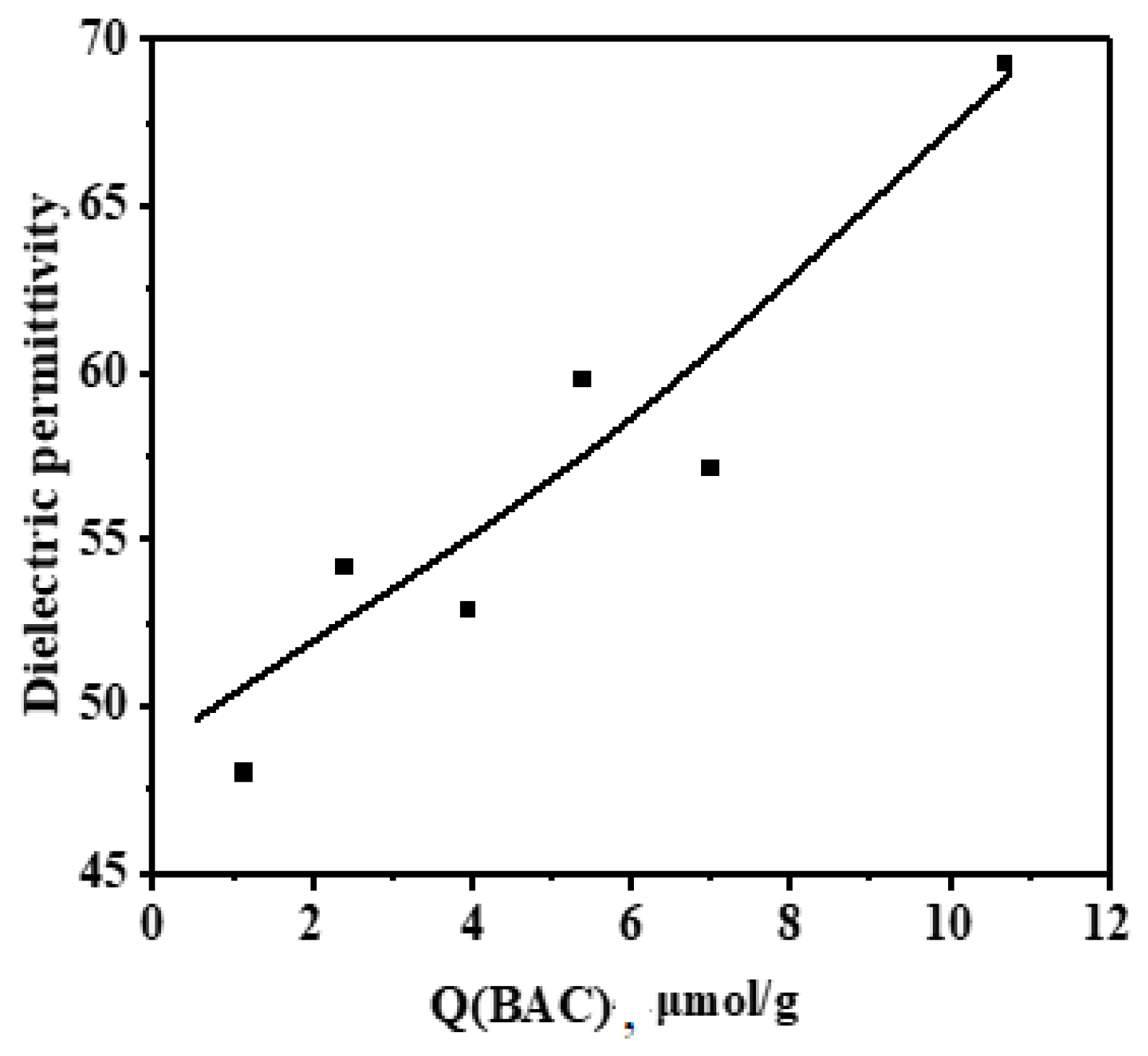
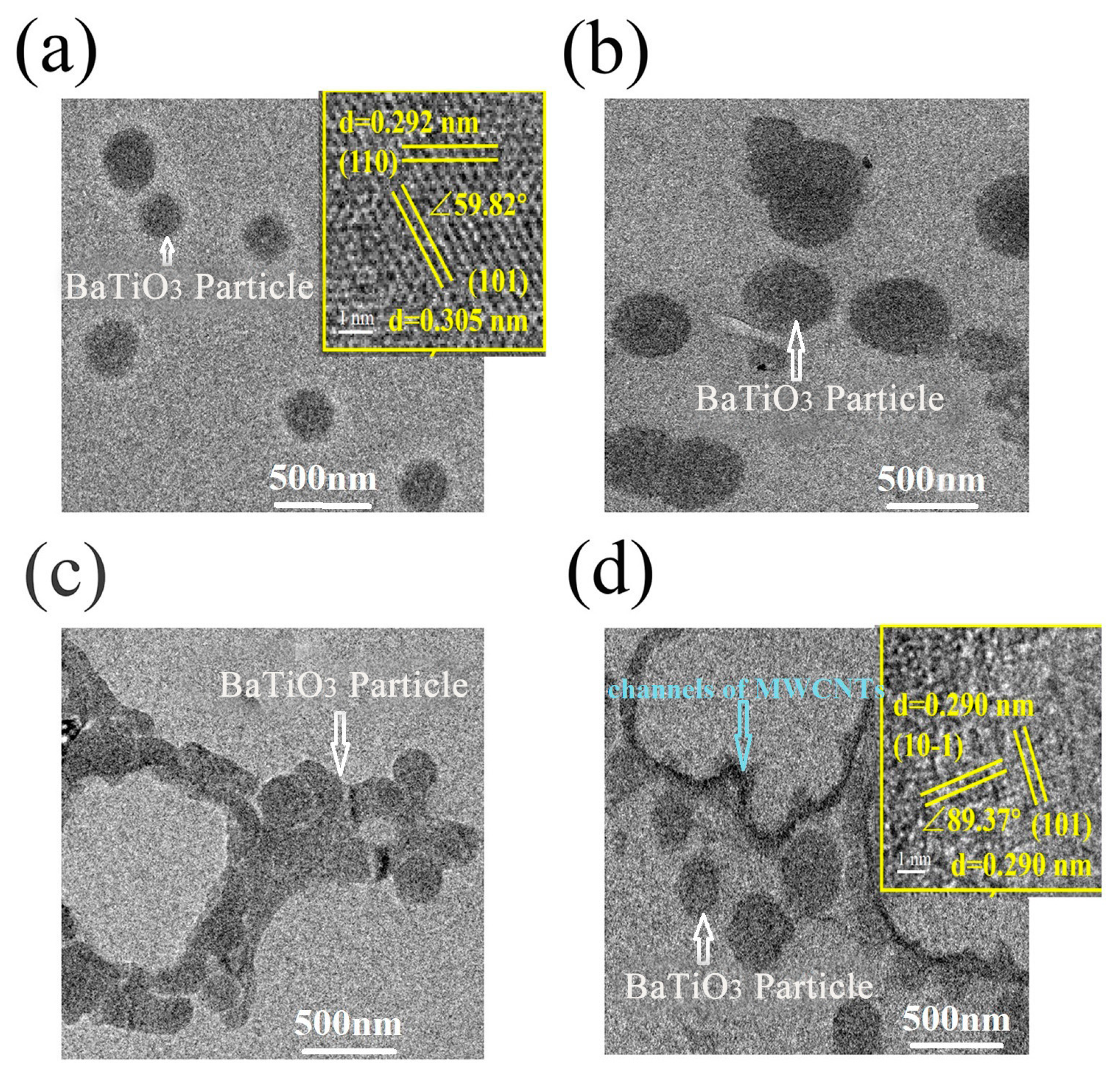
3.2. Study of the Filler and Composite Surface Characteristics Correlating with Dielectric Properties of the Composites
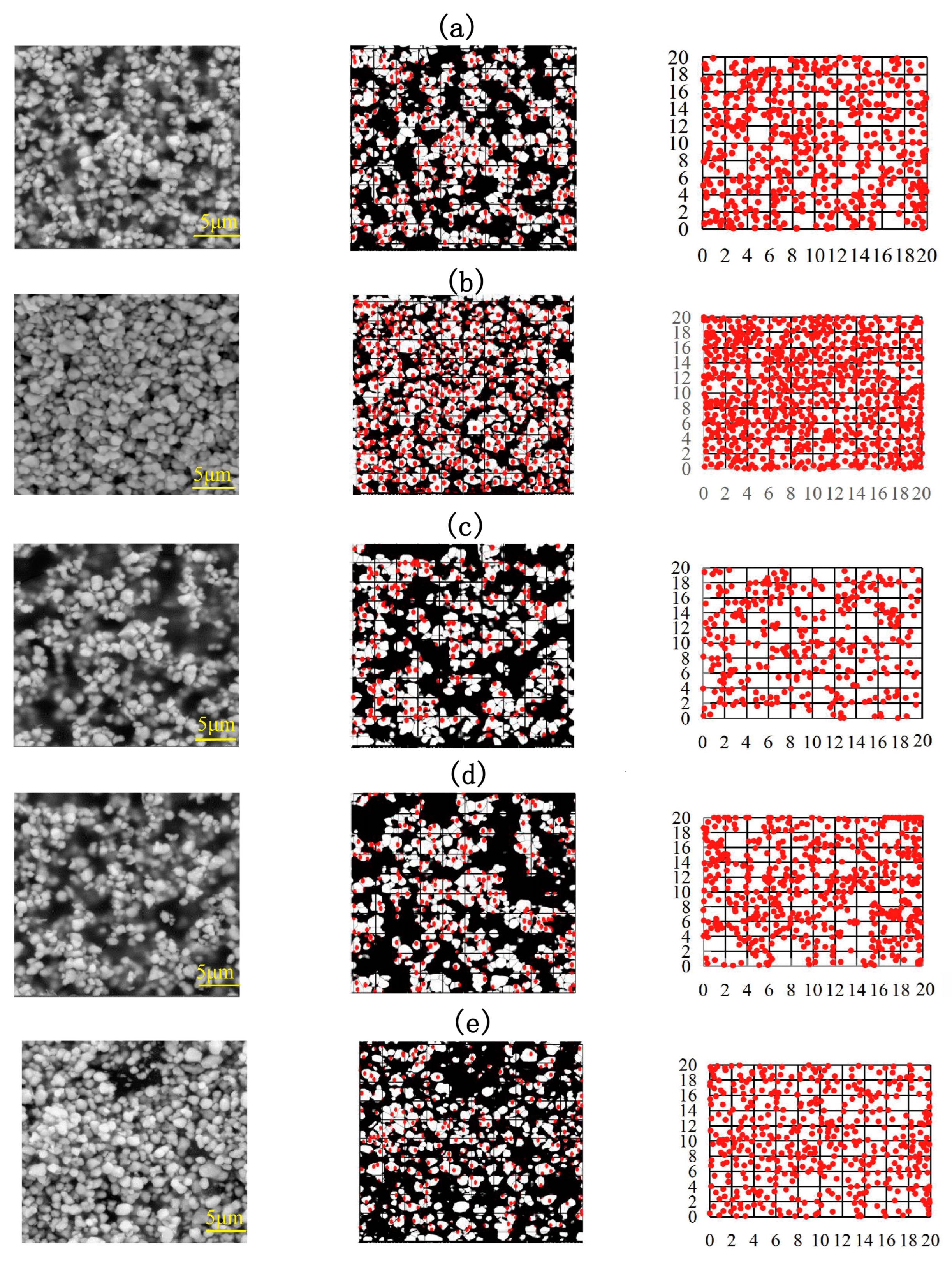
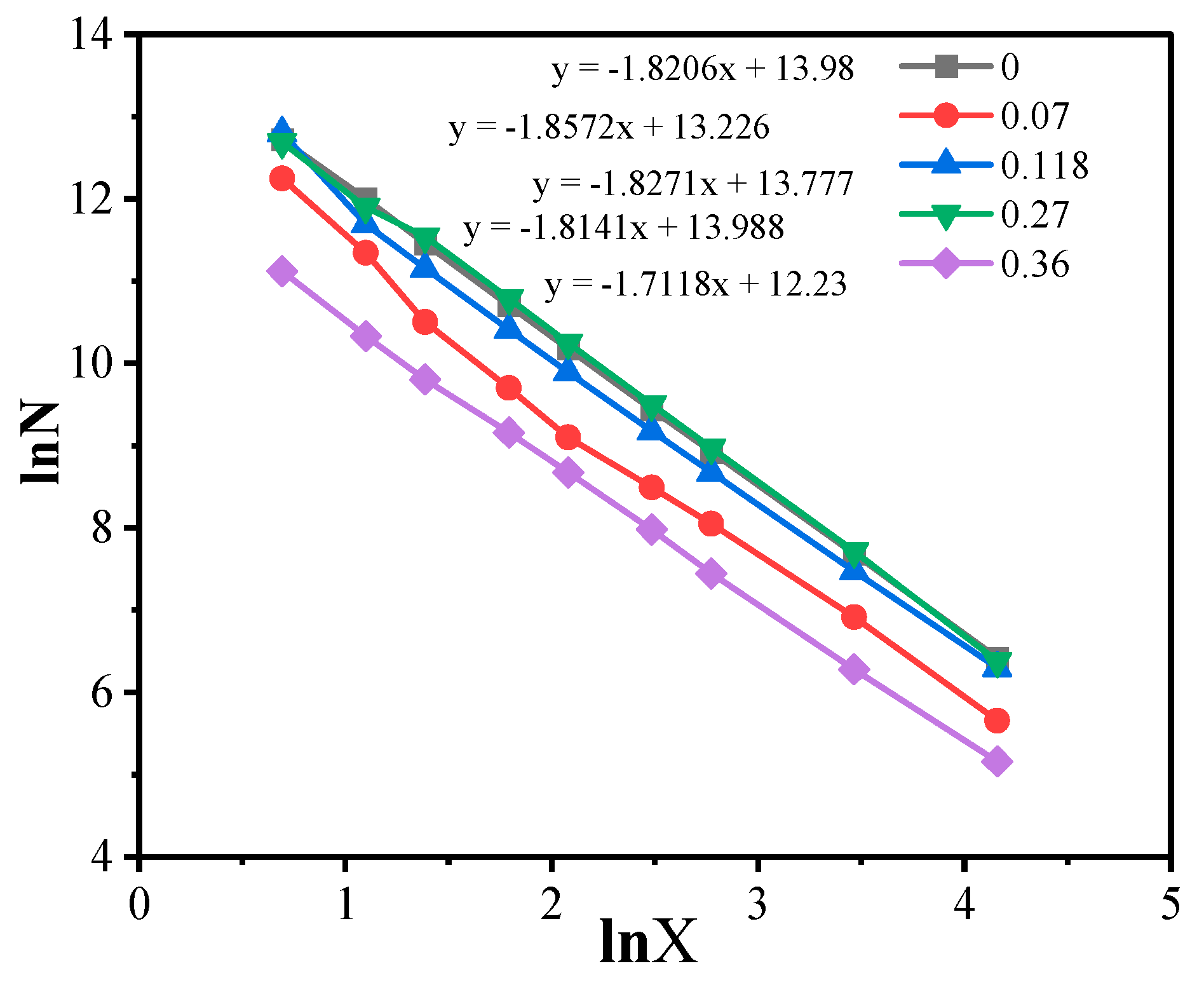
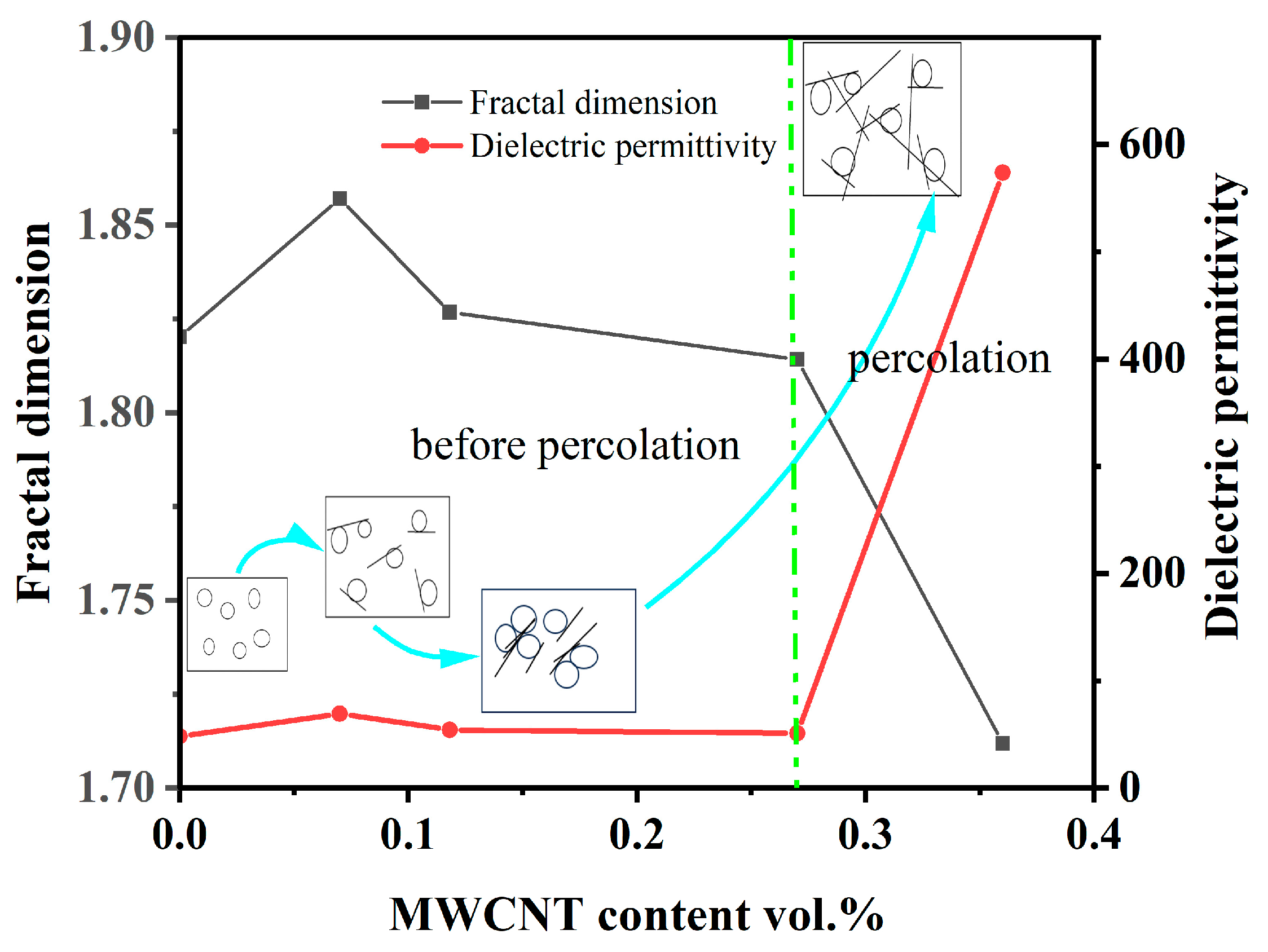
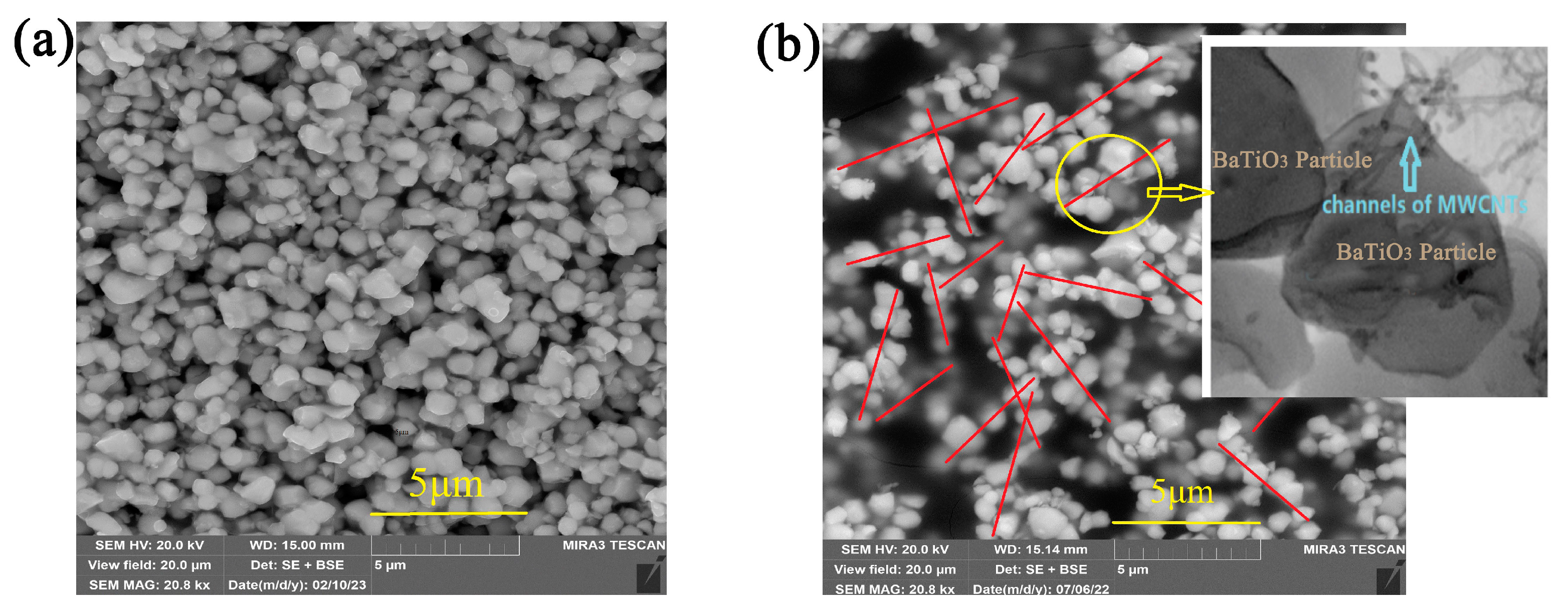
3.3. SEM Characterization of the Composites and Analysis of Fractal Characteristics
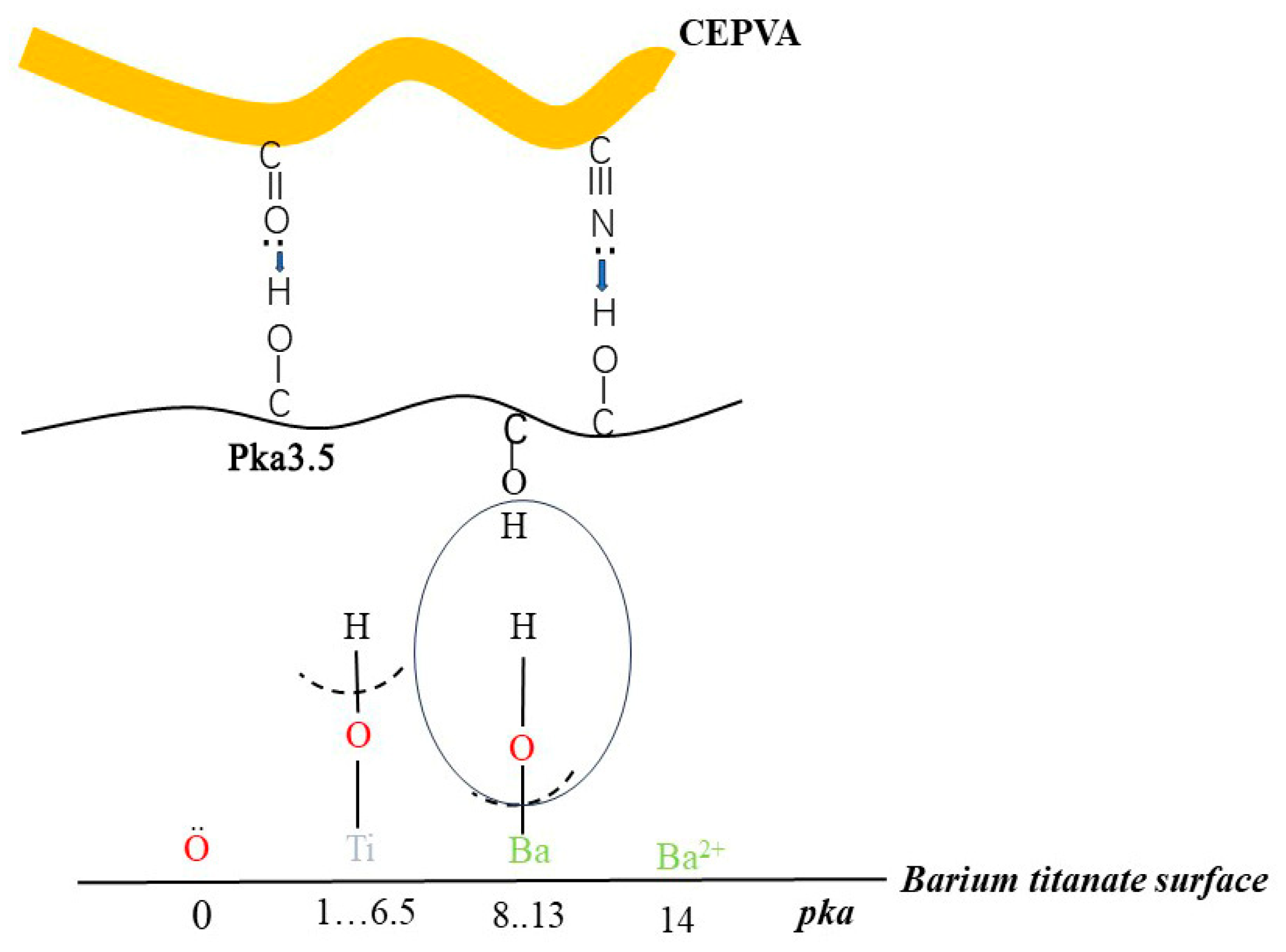
3.4. Mechanism of the Control over the Target Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Zhang, J.; Zhang, Z.; Hao, Y.; Bi, K. Enhanced dielectric properties and energy storage density of PVDF nanocomposites by co-loading of BaTiO3 and CoFe2O4 nanoparticles. Adv. Compos. Hybrid. Mater. 2020, 3, 58–65. [Google Scholar] [CrossRef]
- Costa, C.M.; Sabater i Serra, R.; Andrio Balado, A.; Gómez Ribelles, J.L.; Lanceros-Méndez, S. Dielectric relaxation dynamics in poly(vinylidene fluoride)/Pb(Zr0.53Ti0.47)O3 composites. Polymer 2020, 204, 122811. [Google Scholar] [CrossRef]
- Tomer, V.; Polizos, G.; Manias, E.; Randall, C.A. Epoxy-based nanocomposites for electrical energy storage. I: Effects of montmorillonite and barium titanate nanofillers. J. Appl. Phys. 2010, 108, 074116. [Google Scholar] [CrossRef]
- Saini, A.; Sharma, A.; Sharma, M.; Kuanr, B.K. Yttrium iron garnet (YIG)/barium titanate (BTO) an engineered multiferroic nanocomposite. J. Alloys Compd. 2021, 879, 160422. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Kozlovskiy, A.L.; Shlimas, D.I.; Borgekov, D.B. Phase transformations in FeCo—Fe2CoO4/Co3O4-spinel nanostructures as a result of thermal annealing and their practical application. J. Mater. Sci. Mater. Electron. 2021, 32, 16694–16705. [Google Scholar] [CrossRef]
- Luo, B.; Wang, X.; Wang, Y.; Li, L. Fabrication, characterization, properties and theoretical analysis of ceramic/PVDF composite flexible films with high dielectric constant and low dielectric loss. J. Mater. Chem. A 2014, 2, 510–519. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Lee, D.; Cho, C.; Kim, B. Impact of Multi-Walled CNT Incorporation on Dielectric Properties of PVDF-BaTiO3 Nanocomposites and Their Energy Harvesting Possibilities. Coatings 2022, 12, 77. [Google Scholar] [CrossRef]
- Hoshina, T. Size effect of barium titanate: Fine particles and ceramics. J. Ceram. Soc. Jpn. 2013, 121, 156–161. [Google Scholar] [CrossRef]
- Hou, D.; Zhou, J.; Chen, W.; Zhang, P.; Shen, J.; Jian, Z. Core@double-shell structured fillers for increasing dielectric constant and suppressing dielectric loss of PVDF-based composite films. Ceram. Int. 2022, 48, 22691–22698. [Google Scholar] [CrossRef]
- Huang, Z.X.; Zhao, M.L.; Zhang, G.Z.; Song, J.; Qu, J.P. Controlled localizing multi-wall carbon nanotubes in polyvinylidene fluoride/acrylonitrile butadiene styrene blends to achieve balanced dielectric constant and dielectric loss. Compos. Sci. Technol. 2021, 212, 108874. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Bhatt, U.M.; Gautam; Thote, P.S.; Joglekar, M.M.; Manhas, S.K. Fabrication and modeling of β-phase PVDF-TrFE based flexible piezoelectric energy harvester. Sens. Actuator A Phys. 2020, 304, 111879. [Google Scholar] [CrossRef]
- Dwivedi, S.; Badole, M.; Pareek, T.; Kumar, S. Multifunctional lead-free K0.5Bi0.5TiO3-based ceramic reinforced PVDF matrix composites. J. Alloys Compd. 2021, 871, 159616. [Google Scholar] [CrossRef]
- Jiao, W. Interface modification and energy storage performance of barium-based titanate/polyvinylidene fluoride composite dielectric materials. Acta Phys. Sin. 2020, 69, 217702. [Google Scholar]
- Jian, G.; Jiao, Y.; Meng, Q.; Wei, Z.; Zhang, J.; Yan, C.; Moon, K.-S.; Wong, C.-P. Enhanced dielectric constant and energy density in a BaTiO3/polymer-matrix composite sponge. Commun. Mater. 2020, 1, 91. [Google Scholar] [CrossRef]
- Tan, D. Review of polymer-based nanodielectric exploration and film scale-up for advanced capacitors. Adv. Funct. Mater. 2020, 30, 1808567. [Google Scholar] [CrossRef]
- Dang, Z.; Wang, L.; Yin, Y.; Zhang, Q.; Lei, Q. Giant dielectric permittivities in functionalized carbon-nanotube/electroactive-polymer nanocomposites. Adv. Mater. 2007, 19, 852–857. [Google Scholar] [CrossRef]
- Meisak, D.; Kinka, M.; Plyushch, A.; Macutkevič, J.; Zarkov, A.; Schaefer, S.; Selskis, A.; Samulionis, V.; Kuzhir, P.; Banys, J.; et al. Piezoelectric Nanogenerators Based on BaTiO3/PDMS Composites for High-Frequency Applications. ACS Omega 2023, 8, 13911–13919. [Google Scholar] [CrossRef]
- Sareecha, N.; Ali Shah, W.; Anis-ur-Rehman, M.; Latif Mirza, M.; Awan, M.S. Electrical Investigations of BaTiO3 Ceramics with Ba/Ti Contents under Influence of Temperature. Solid. State Ion. 2017, 303, 16–23. [Google Scholar] [CrossRef]
- Zhang, R.F.; Que, W.X. Barium Titanate Derived from Mechanochemically Activated Powders. J. Alloys Compd. 2002, 337, 226–230. [Google Scholar] [CrossRef]
- Vijatovic, M.; Vasic, M.; Bobic, J.; Zivkovic, L.; Stojanovic, B. Effect of Powder Synthesis Method on BaTiO3 Ceramics. Process. Appl. Ceram. 2008, 2, 27–31. [Google Scholar] [CrossRef]
- Luo, S.; Shen, Y.; Yu, S.; Wan, Y.; Liao, W.-H.; Sun, R.; Wong, C.-P. Construction of a 3D-BaTiO3 Network Leading to Significantly Enhanced Dielectric Permittivity and Energy Storage Density of Polymer Composites. Energy Environ. Sci. 2016, 10, 137–144. [Google Scholar] [CrossRef]
- Mallick, S.; Ahmad, Z.; Qadir, K.; Rehman, A.; Shakoor, R.; Touati, F.; Al-Muhtaseb, S. Effect of BaTiO3 on the Sensing Properties of PVDF Composite-Based Capacitive Humidity Sensors. Ceram. Int. 2020, 46, 2949–2953. [Google Scholar] [CrossRef]
- Dang, Z.M.; Yuan, J.K.; Yao, S.H.; Liao, R.J. Flexible nanodielectric materials with high permittivity for power energy storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef] [PubMed]
- Olszowy, M.; Pawlaczyk, C.; Markiewicz, E.; Kułek, J. Dielectric and pyroelectric properties of BaTiO3–PVC composites. Phys. Stat. Sol. A 2005, 202, 1848–1853. [Google Scholar] [CrossRef]
- Bouharras, F.E.; Raihane, M.; Ameduri, B. Recent progress on core-shell structured BaTiO3@polymer/fluorinated polymers nanocomposites for high energy storage: Synthesis, dielectric properties and applications. Progr. Mater. Sci. 2020, 113, 100670. [Google Scholar] [CrossRef]
- Kong, L.B.; Ma, J.; Huang, H.; Prajapati, P.; Bhunia, R.; Siddqui, S.; Garg, A.; Gupta, R. Significantly enhanced energy density by tailoring the interface in hierarchically structured TiO2-BaTiO3-TiO2 nanofillers in PVDF-based thin-film polymer nanocomposites. ACS Appl. Mater. Interfaces 2019, 11, 14329–14339. [Google Scholar]
- Sengwa, R.J.; Kumar, N.; Saraswat, M. Morphological, structural, optical, broadband frequency range dielectric and electrical properties of PVDF/PMMA/BaTiO3 nanocomposites for futuristic microelectronic and optoelectronic technologies. Mater. Today Commun. 2023, 35, 105625. [Google Scholar] [CrossRef]
- Chekuryaev, A.G.; Sychov, M.M.; Myakin, S.V. Analysis of the structure of composite systems by means of fractal characteristics using the BaTiO3–fullerenol–CEPVA system as an example. Solid State Phys. 2021, 63, 740–746. [Google Scholar] [CrossRef]
- Sychov, M.M.; Nakanishi, Y.; Vasina, E.; Eruzin, A.; Mjakin, S.; Khamova, T.; Shilova, O.; Mimura, H. Core-shell approach to control acid-base properties of dielectric and permittivity of its composite. Chem. Lett. 2015, 44, 197–199. [Google Scholar] [CrossRef]
- Krasovskii, A.N.; Novikov, D.V.; Vasina, E.S.; Matveichikova, P.V.; Sychov, M.M.; Rozhkova, N.N. Short-range order and fractal cluster structure of aggregates of barium titanate microparticles in a composite based on cyano-ethyl ester of polyvinyl alcohol. Phys. Solid State 2015, 57, 2555–2561. [Google Scholar] [CrossRef]
- Toshikatsu, T.; Masahiro, K.; Norikazu, F. Proposal of a multi-core model for polymer nanocomposite dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 669–681. [Google Scholar]
- Triyono, D.; Akbar, A.; Laysandra, H. Annealing-temperature dependence of the electrical properties of Ba0.8Pb0.2TiO3 as a PTC material. J. Phys. Conf. Ser. 2020, 1442, 012012. [Google Scholar] [CrossRef]
- Xie, L.; Huang, X.; Yang, K.; Li, S.; Jiang, P. “Grafting to” route to PVDF-HFPGMA/BaTiO3 nanocomposites with high dielectric constant and high thermal conductivity for energy storage and thermal management applications. J. Mater. Chem. A 2014, 2, 5244. [Google Scholar] [CrossRef]
- Jianwen, X.; Wong, C.P. Low-loss percolative dielectric composite. Appl. Phys. Lett. 2005, 87, 082907. [Google Scholar]
- Bertasiu, P.; Meisak, D.; Macutkevic, J.; Kuzhir, P.; Selskis, A.; Volnyanko, E.; Banys, J. Fine Tuning of Electrical Transport and Dielectric Properties of Epoxy/Carbon Nanotubes Composites via Magnesium Oxide Additives. Polymers 2019, 11, 2044. [Google Scholar] [CrossRef] [PubMed]
- Meisak, D.; Macutkevic, J.; Selskis, A.; Kuzhir, P.; Selskis, A.; Volnyanko, E.; Banys, J. Dielectric relaxation spectroscopy and synergy effects in epoxy/MWCNT/Ni@C composites. Nanomaterials 2021, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Bharati, A.; Wubbenhorst, M.; Moldenaers, P.; Cardinaels, R. Dielectric properties of phase-separated blends containing a microcapacitor network of carbon nanotubes: Compatibilization by a random or block copolymer. Macromolecules 2017, 50, 3856–3868. [Google Scholar] [CrossRef]
- Dang, Z.; Yao, S.; Yuan, J.; Bai, J. Tailored dielectric properties based on microstructure change in BaTiO3-carbon nanotube/polyvinylidene fluoride three-phase nanocomposites. J. Phys. Chem. C 2010, 114, 13204–13209. [Google Scholar] [CrossRef]
- Sychov, M.M.; Chekuryaev, A.G.; Bogdanov, S.P.; Kuznetsov, P.A. Digital materials science: Numerical characterization of steel microstructure. In INTER-ACADEMIA: Lecture Notes in Networks and Systems; Khakhomov, S., Semchenko, I., Demidenko, O., Kovalenko, D., Eds.; Springer: Singapore, 2021; Volume 422, pp. 159–169. [Google Scholar]
- Mjakin, S.V.; Sychov, M.M.; Vasiljeva, I.V. Electron Beam Modification of Solids: Mechanisms, Common Features and Promising Applications; Nova Science Publisher: Hauppauge, NY, USA, 2009; ISBN 978-1-60741-780-4. [Google Scholar]
- Sychov, M.M.; Zakharova, N.V.; Mjakin, S.V. Surface functional transformations in BaTiO3—CaSnO3 ceramics in the course of milling. Ceram. Int. 2013, 39, 6821–6826. [Google Scholar] [CrossRef]
- Nechiporenko, A.P. Donor-Acceptor Properties of the Surface of Solid-Phase Systems: Indicator Method; Lan Publishers: Moscow, Russia, 2017; p. 284. (in Russian) [Google Scholar]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Techol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Silakaewa, K.; Thongbai, P. Significantly improved dielectric properties of multiwall carbon nanotube-BaTiO3/PVDF polymer composites by tuning the particle size of the ceramic filler. RSC Adv. 2019, 9, 23498–23507. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Sandler, J.K.W.; Shaffer, M.S.P.; Schwarz, M.K.; Bauhofer, W.; Schulte, K.; Windle, A.H. Formation of percolating networks in multi-wall carbon-nanotube–epoxy composites. Compos. Sci. Techol. 2004, 64, 2309–2316. [Google Scholar] [CrossRef]
- Sychov, M.M.; Minakova, T.S. Acid-Base Characteristics of the Surface of Solids and Control over Properties of Materials and Composites; Khimizdat Publishers: Saint Petersburg, Russia, 2022; p. 288. (in Russian) [Google Scholar]
- Lazarevic, Z.; Romcevic, N.; Vijatovic, M.; Paunovic, N.; Romcevic, M.; Stojanovic, B.; Dohcevic-Mitrovic, Z. Characterization of barium titanate ceramic powders by Raman spectroscopy. Acta Phys. Pol. A 2009, 115, 808–810. [Google Scholar]
- Asiaie, R.; Zhu, W.; Akbar, S.A.; Dutta, P.K. Characterization of submicron particles of tetragonal BaTiO3. Chem. Mater. 1996, 8, 226–234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sychov, M.; Guan, X.; Mjakin, S.; Boridko, L.; Khristyuk, N.; Gravit, M.; Diachenko, S. Effect of BaTiO3 Filler Modification with Multiwalled Carbon Nanotubes on Electric Properties of Polymer Nanocomposites. Nanomaterials 2024, 14, 1232. https://doi.org/10.3390/nano14141232
Sychov M, Guan X, Mjakin S, Boridko L, Khristyuk N, Gravit M, Diachenko S. Effect of BaTiO3 Filler Modification with Multiwalled Carbon Nanotubes on Electric Properties of Polymer Nanocomposites. Nanomaterials. 2024; 14(14):1232. https://doi.org/10.3390/nano14141232
Chicago/Turabian StyleSychov, Maxim, Xingyu Guan, Sergey Mjakin, Lyubov Boridko, Nikolay Khristyuk, Marina Gravit, and Semen Diachenko. 2024. "Effect of BaTiO3 Filler Modification with Multiwalled Carbon Nanotubes on Electric Properties of Polymer Nanocomposites" Nanomaterials 14, no. 14: 1232. https://doi.org/10.3390/nano14141232
APA StyleSychov, M., Guan, X., Mjakin, S., Boridko, L., Khristyuk, N., Gravit, M., & Diachenko, S. (2024). Effect of BaTiO3 Filler Modification with Multiwalled Carbon Nanotubes on Electric Properties of Polymer Nanocomposites. Nanomaterials, 14(14), 1232. https://doi.org/10.3390/nano14141232







