Photocatalytic CO2 Reduction Using Zinc Indium Sulfide Aggregated Nanostructures Fabricated under Four Anionic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of the Zinc Indium Sulfide (ZIS) Powders [43,44,45]
- Preparation of Precursor Solution:
- (1)
- In a 250 mL beaker, 1.25 mmol of zinc chloride (ZnCl2), 2.5 mmol of indium chloride (InCl3), 3.4 mmol of sodium citrate (Na3C6H5O7), and 5 mmol of thioacetamide (TAA) were accurately weighed and added to 100 mL of deionized water.
- (2)
- The mixture was stirred vigorously using a magnetic stirrer for 30 min at room temperature to ensure complete dissolution of the precursors.
- Hydrothermal Synthesis:
- (1)
- The precursor solution was transferred quantitatively to a 125 mL Teflon-lined stainless-steel autoclave to ensure efficient reaction conditions. The autoclave was sealed securely and placed in an oven preheated to 180 °C.
- (2)
- The reaction was allowed to proceed for 12 h under autogenous pressure to promote the nucleation and growth of ZIS nanoparticles. After the reaction time elapsed, the autoclave was allowed to cool naturally to room temperature overnight to avoid thermal shock.
- Product Purification:
- (1)
- The resulting ZIS nanoparticles were harvested by centrifugation at 11,000× g for 20 min to separate them from the reaction mixture.
- (2)
- The supernatant was carefully decanted, and the ZIS precipitate was washed twice with a mixture of deionized water and anhydrous ethanol to remove any residual precursors or by-products.
- (3)
- After washing, the ZIS sample was dried in a vacuum oven at 60 °C for 12 h to remove any remaining solvent and ensure the stability of the nanoparticles.
- Naming:
- (1)
- The final product obtained from this synthesis procedure will be designated as ZIS-Cl to denote its synthesis route using chloride salts as precursors.
2.3. Characterizations
2.4. Photocatalysis of CO2 Reduction Reaction (CO2RR)
3. Results and Discussion
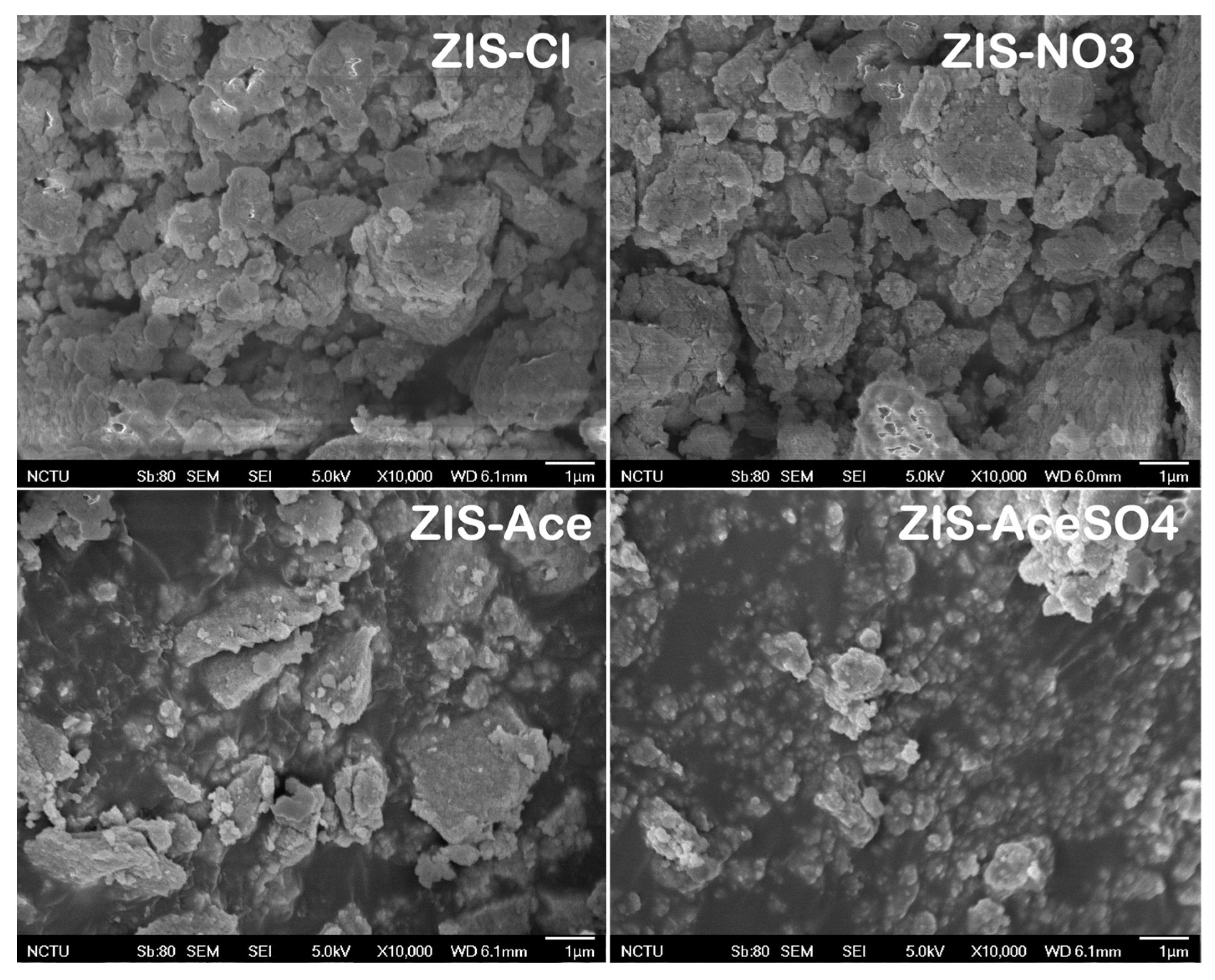
3.1. The Morphology, Crystal Structure, and Chemical State of Zinc Indium Sulfide (ZIS) Nanocrystals
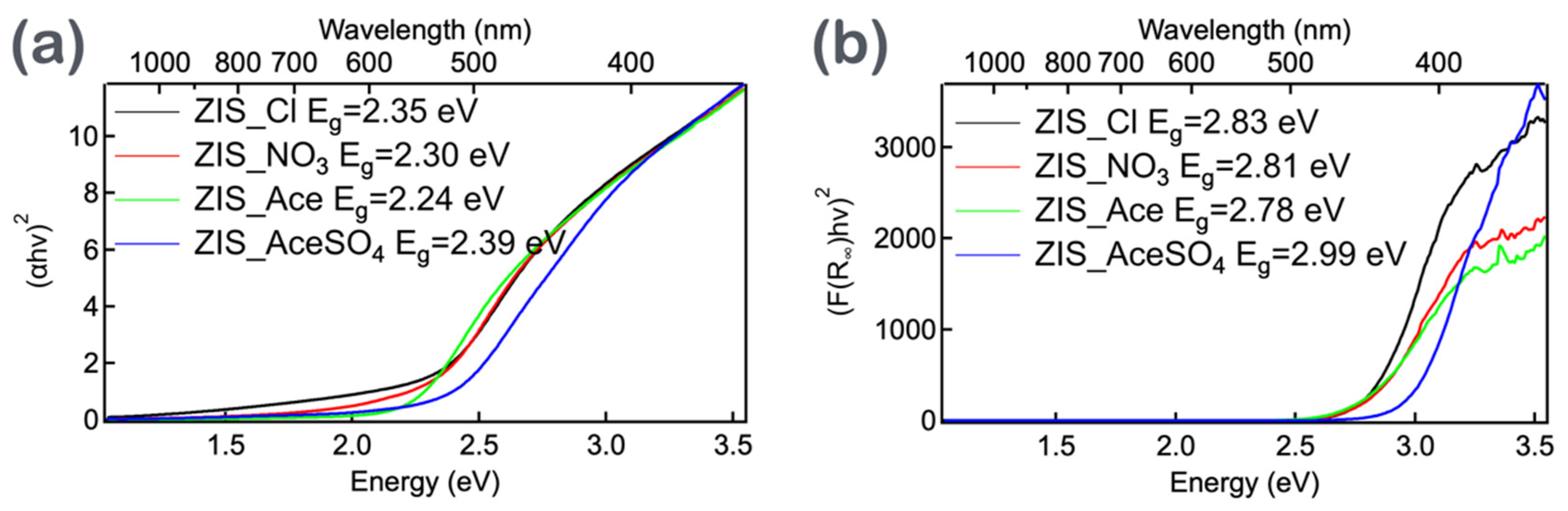
3.2. The Bandgap Estimation of Zinc Indium Sulfide (ZIS) Nanocrystals
3.3. The Energy Level of Zinc Indium Sulfide (ZIS) Nanocrystals
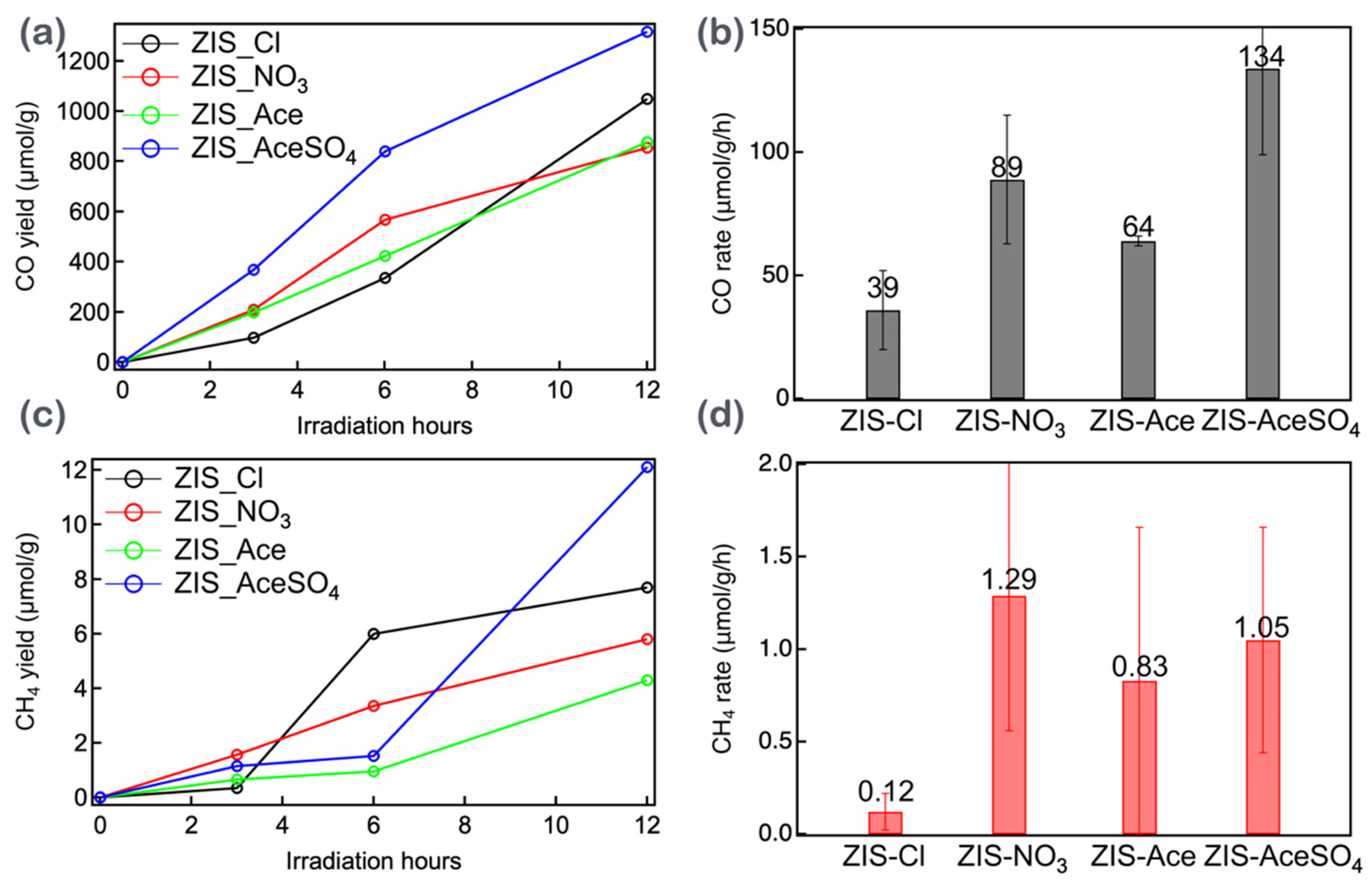
3.4. The CO2 Reduction Efficiency of Zinc Indium Sulfide (ZIS) Nanocrystals
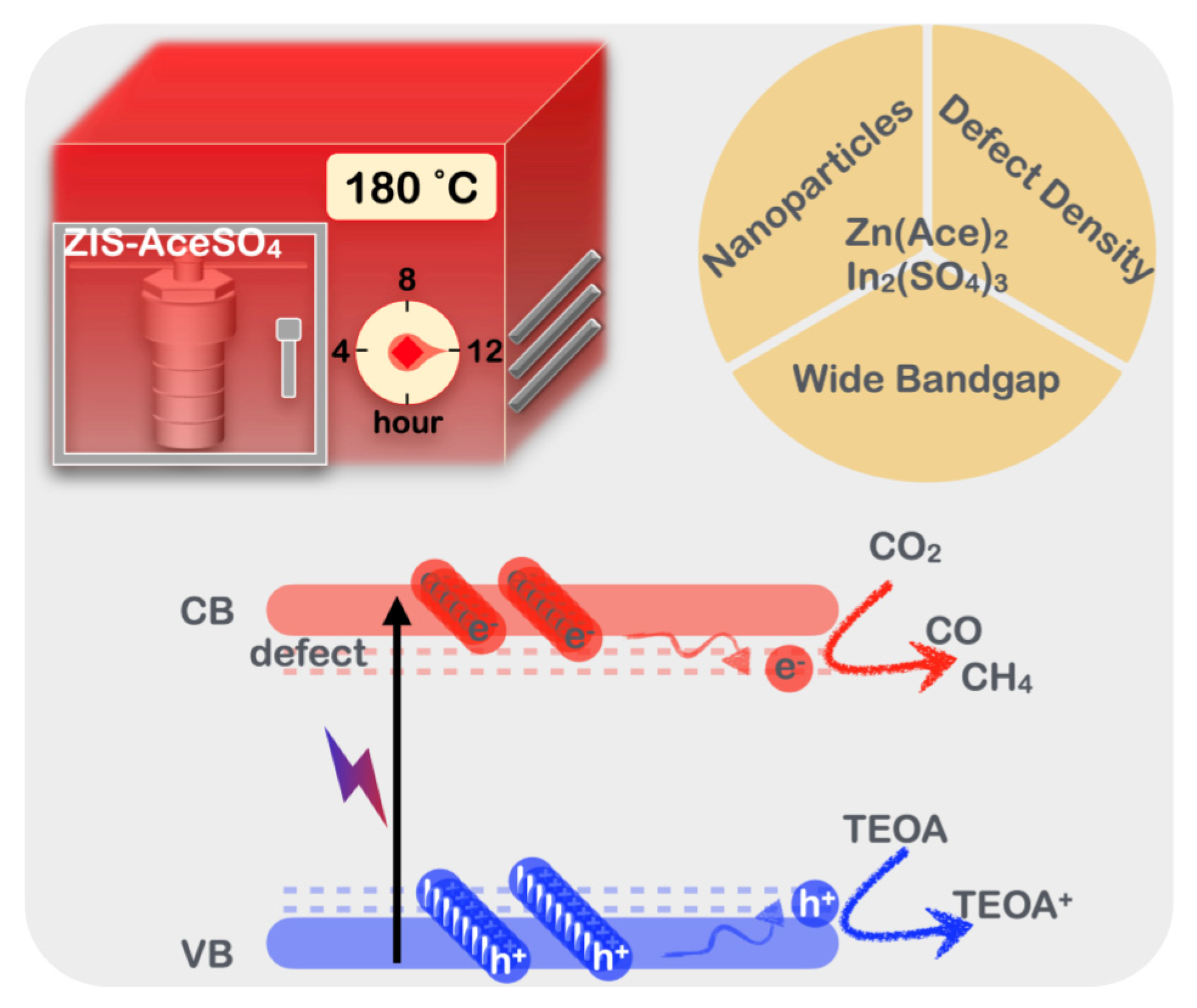
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Ahmed, S.; Irshad, M.; Yoon, W.; Karanwal, N.; Sugiarto, J.R.; Khan, M.K.; Kim, S.K.; Kim, J. Evaluation of MgO as a promoter for the hydrogenation of CO2 to long-chain hydrocarbons over Fe-based catalysts. Appl. Catal. B-Environ. 2023, 338, 123052. [Google Scholar] [CrossRef]
- Lin, R.; Guo, J.X.; Li, X.J.; Patel, P.; Seifitokaldani, A. Electrochemical Reactors for CO2 Conversion. Catalysts 2020, 10, 473. [Google Scholar] [CrossRef]
- Jung, S.C.; Chung, K.H. Enhanced hydrogen production through cracking of ammonia water using liquid plasma on titanate-based perovskite catalysts. Energy Convers. Manag. 2024, 311, 118509. [Google Scholar] [CrossRef]
- Fan, H.; Wang, D.; Liu, Z.; Xie, T.; Lin, Y. Self-assembled BiVO4/Bi2WO6 microspheres: Synthesis, photoinduced charge transfer properties and photocatalytic activities. Dalton Trans. 2015, 44, 11725–11731. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chang, W.J.; Lee, C.W.; Park, S.; Ahn, H.-Y.; Nam, K.T. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. Nat. Energy 2016, 2, 16185. [Google Scholar] [CrossRef]
- Zhang, L.; Jaroniec, M. Toward designing semiconductor-semiconductor heterojunctions for photocatalytic applications. Appl. Surf. Sci. 2018, 430, 2–17. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; García, H. Photocatalytic CO2 Reduction to C2+Products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Lu, C.; Itanze, D.S.; Aragon, A.G.; Ma, X.; Li, H.; Ucer, K.B.; Hewitt, C.; Carroll, D.L.; Williams, R.T.; Qiu, Y.; et al. Synthesis of lead-free Cs3Sb2Br9 perovskite alternative nanocrystals with enhanced photocatalytic CO2 reduction activity. Nanoscale 2020, 12, 2987–2991. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Wu, Z.H.; Qi, S.F.; Luo, J.J.; Pi, S.; Xu, H.H.; Zhang, S.M.; Xu, D.F.; Zhang, S.Y.; Yang, X.F. Construction of Inverse–Opal ZnIn2S4 with Well–Defined 3D Porous Structure for Enhancing Photocatalytic H2 Production. Nanomaterials 2024, 14, 843. [Google Scholar] [CrossRef]
- Peng, L.; Jurca, B.; Garcia-Baldovi, A.; Tian, L.; Sastre, G.; Primo, A.; Parvulescu, V.; Dhakshinamoorthy, A.; Garcia, H. Nanometric Cu-ZnO Particles Supported on N-Doped Graphitic Carbon as Catalysts for the Selective CO2 Hydrogenation to Methanol. Nanomaterials 2024, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Kalantari Bolaghi, Z.; Rodriguez-Seco, C.; Yurtsever, A.; Ma, D. Exploring the Remarkably High Photocatalytic Efficiency of Ultra-Thin Porous Graphitic Carbon Nitride Nanosheets. Nanomaterials 2024, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Pipelzadeh, E.; Babaluo, A.A.; Haghighi, M.; Tavakoli, A.; Derakhshan, M.V.; Behnami, A.K. Silver doping on TiO2 nanoparticles using a sacrificial acid and its photocatalytic performance under medium pressure mercury UV lamp. Chem. Eng. J. 2009, 155, 660–665. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Heng, S.; Tieng, S.; David, F.; Dine, S.; Haddad, O.; Colbeau-Justin, C.; Traore, M.; Kanaev, A. Mixed Metal Oxide W-TiO2 Nanopowder for Environmental Process: Synergy of Adsorption and Photocatalysis. Nanomaterials 2024, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Huang, H.; Li, H.; Liu, Y.; Zhong, J.; Li, Y.; Zhang, S.; Kang, Z. Metal Nanoparticle/Carbon Quantum Dot Composite as a Photocatalyst for High-Efficiency Cyclohexane Oxidation. ACS Catal. 2013, 4, 328–336. [Google Scholar] [CrossRef]
- Fernando, K.A.; Sahu, S.; Liu, Y.; Lewis, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon quantum dots and applications in photocatalytic energy conversion. ACS Appl. Mater. Interfaces 2015, 7, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Tikoo, A.; Koushik, A.K.S.; Meduri, P. Hydrogen Peroxide Production with High Solar to Chemical Conversion (0.16%) and Efficient Water Purification Using Carbon Quantum Dots-Modified Indium Sulfide. ACS Appl. Eng. Mater. 2023, 1, 1397–1407. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, Y.L.; Wu, X.; Cheng, Y.Y.; Yu, J.W.; Zhang, X.Y.; Liu, N.Y.; Liu, X.; Li, H.T. Carbon Quantum Dots/Cu2O Photocatalyst for Room Temperature Selective Oxidation of Benzyl Alcohol. Nanomaterials 2024, 14, 212. [Google Scholar] [CrossRef]
- Shi, R.; Waterhouse, G.I.N.; Zhang, T.R. Recent Progress in Photocatalytic CO2 Reduction Over Perovskite Oxides. Solar Rrl 2017, 1, 1700126. [Google Scholar] [CrossRef]
- Bhosale, S.S.; Kharade, A.K.; Jokar, E.; Fathi, A.; Chang, S.M.; Diau, E.W. Mechanism of Photocatalytic CO2 Reduction by Bismuth-Based Perovskite Nanocrystals at the Gas-Solid Interface. J. Am. Chem. Soc. 2019, 141, 20434–20442. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.S.; Kharade, A.K.; Narra, S.; Chang, S.-m.; Wei-Guang Diau, E. Self-Photocatalytic Splitting of Carbon Dioxide Using Co-cationic Perovskite Nanocrystals in the Absence of Water. ACS Energy Lett. 2022, 8, 280–288. [Google Scholar] [CrossRef]
- Awang, H.; Hezam, A.; Peppel, T.; Strunk, J. Enhancing the Photocatalytic Activity of Halide Perovskite Cesium Bismuth Bromide/Hydrogen Titanate Heterostructures for Benzyl Alcohol Oxidation. Nanomaterials 2024, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; You, W.; Liu, M.; Zhou, G.; Takata, T.; Hara, M.; Domen, K.; Li, C. Photocatalytic water reduction under visible light on a novel ZnIn2S4 catalyst synthesized by hydrothermal method. Chem. Commun. 2003, 17, 2142–2143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, S.; Liu, W.; Chen, X.; Wu, L.; Wang, X.; Liu, P.; Li, Z. Controlled syntheses of cubic and hexagonal ZnIn2S4 nanostructures with different visible-light photocatalytic performance. Dalton Trans. 2011, 40, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Shang, C.; Chen, Z.; Jin, M.; Zhang, Y.; Zhang, Z.; Wang, X.; Zhou, G. Enhanced Photocatalytic H2 Evolution over ZnIn2S4 Flower-Like Microspheres Doped with Black Phosphorus Quantum Dots. Nanomaterials 2019, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wu, H.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A mini-review on ZnIn2S4-Based photocatalysts for energy and environmental application. Green Energy Environ. 2022, 7, 176–204. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, F.; Guo, Y.; Guo, F.; Shi, W.; Yang, S. Near-Infrared Light Driven ZnIn2S4-Based Photocatalysts for Environmental and Energy Applications: Progress and Perspectives. Molecules 2023, 28, 2142. [Google Scholar] [CrossRef]
- Dong, F.; Liu, H.; Qin, L.; Zhang, T.; Li, X.; Kang, S.-Z. Boosting the photocatalytic activity of ZnIn2S4-based photocatalyst for H2 evolution using porous ZnWO4 nanoflakelets as a cocatalyst. New J. Chem. 2023, 47, 13177–13185. [Google Scholar] [CrossRef]
- Shen, S.; Zhao, L.; Zhou, Z.; Guo, L. Enhanced Photocatalytic Hydrogen Evolution over Cu-Doped ZnIn2S4 under Visible Light Irradiation. J. Phys. Chem. C 2008, 112, 16148–16155. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Lin, Z.Y.; Yang, G.W. Enhanced carrier separation and increased electron density in 2D heavily N-doped ZnIn2S4 for photocatalytic hydrogen production. J. Mater. Chem. A 2020, 8, 207–217. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Si, Y.; Li, B.; Deng, F.; Yang, L.; Liu, X.; Dai, W.; Luo, S. Gradient Hydrogen Migration Modulated with Self-Adapting S Vacancy in Copper-Doped ZnIn2S4 Nanosheet for Photocatalytic Hydrogen Evolution. ACS Nano 2021, 15, 15238–15248. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, Y.; Geng, Z.; Guo, Y.; Tan, X.; Hu, Z.; Yu, T. Modulating Charge Accumulation via Electron Interaction for Photocatalytic Hydrogen Evolution: A Case of Fabricating Palladium Sites on ZnIn2S4 Nanosheets. ACS Catal. 2023, 13, 11687–11696. [Google Scholar] [CrossRef]
- Jiao, X.; Chen, Z.; Li, X.; Sun, Y.; Gao, S.; Yan, W.; Wang, C.; Zhang, Q.; Lin, Y.; Luo, Y.; et al. Defect-Mediated Electron-Hole Separation in One-Unit-Cell ZnIn2S4 Layers for Boosted Solar-Driven CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 7586–7594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Liu, C.; Luo, S.; Wang, L.; Cai, T.; Zeng, Y.; Yuan, J.; Dong, W.; Pei, Y.; et al. MoS2 Quantum Dot Growth Induced by S Vacancies in a ZnIn2S4 Monolayer: Atomic-Level Heterostructure for Photocatalytic Hydrogen Production. ACS Nano 2018, 12, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, D.; Qin, L.; Liang, J.; Huang, Y. Hydrogenated ZnIn2S4 microspheres: Boosting photocatalytic hydrogen evolution by sulfur vacancy engineering and mechanism insight. Phys. Chem. Chem. Phys. 2019, 21, 25484–25494. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.Z.; Xie, Y.; Tao, Y.; Wu, J.X.; Wang, S.J.; Zhou, W. Surface domain potential difference-mediated efficient charge separation on a defective ZnIn2S4 microsphere photocatalyst. Mater. Today Chem. 2022, 23. [Google Scholar] [CrossRef]
- Yang, Z.F.; Shao, L.H.; Wang, L.L.; Xia, X.N.; Liu, Y.T.; Cheng, S.; Yang, C.; Li, S.J. Boosted photogenerated carriers separation in Z-scheme CuP/ZnIn2S4 heterojunction photocatalyst for highly efficient H2 evolution under visible light. Int. J. Hydrogen Energy 2020, 45, 14334–14346. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Zhang, J.; Li, L.; Gu, H.; Dai, W.-L. Hierarchical fabrication of hollow Co2P nanocages coated with ZnIn2S4 thin layer: Highly efficient noble-metal-free photocatalyst for hydrogen evolution. J. Colloid Interface Sci. 2021, 590, 632–640. [Google Scholar] [CrossRef]
- Yao, H.; Jin, G.; Sui, G.; Li, J.; Guo, D.; Liang, S.; Luo, Z.; Xu, R.; Wang, C.; Tang, J. ZIF-67-derived ZnIn2S4/NiCoP Z-scheme heterojunctions for enhanced visible-light-driven photocatalytic hydrogen production. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129991. [Google Scholar] [CrossRef]
- Guo, H.; Wang, G.; Li, H.; Xia, C.; Dong, B.; Cao, L. Direct Z-scheme high-entropy metal phosphides/ZnIn2S4 heterojunction for efficient photocatalytic hydrogen evolution. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131915. [Google Scholar] [CrossRef]
- Chen, J.S.; Xin, F.; Yin, X.H.; Xiang, T.Y.; Wang, Y.W. Synthesis of hexagonal and cubic ZnIn2S4 nanosheets for the photocatalytic reduction of CO2 with methanol. RSC Adv. 2015, 5, 3833–3839. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Shawky, A.; Aljandali, M.S. Palladium/zinc indium sulfide microspheres: Enhanced photocatalysts prepare methanol under visible light conditions. J. Taiwan Inst. Chem. E 2016, 65, 498–504. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An Article on Optics of Paint Layers. Fuer Tekn. Phys. 1931, 12, 593–609. [Google Scholar]
- He, Y.Q.; Rao, H.; Song, K.P.; Li, J.X.; Yu, Y.; Lou, Y.; Li, C.G.; Han, Y.; Shi, Z.; Feng, S.H. 3D Hierarchical ZnIn2S4 Nanosheets with Rich Zn Vacancies Boosting Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2019, 29, 1905153. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, R.; Liu, D.; Zhao, X.; Huang, L.; Chen, S.; Chen, Z.; Li, M.; You, B.; Pang, Y.; et al. Exfoliated Ultrathin ZnIn2S4 Nanosheets with Abundant Zinc Vacancies for Enhanced CO2 Electroreduction to Formate. ChemSusChem 2021, 14, 852–859. [Google Scholar] [CrossRef]
- Huang, W.; Li, Z.; Wu, C.; Zhang, H.; Sun, J.; Li, Q. Delaminating Ti3C2 MXene by blossom of ZnIn2S4 microflowers for noble-metal-free photocatalytic hydrogen production. J. Mater. Sci. Technol. 2022, 120, 89–98. [Google Scholar] [CrossRef]
- Kempken, B.; Dzhagan, V.; Zahn, D.R.T.; Alcocer, M.J.P.; Kriegel, I.; Scotognella, F.; Parisi, J.; Kolny-Olesiak, J. Synthesis, optical properties, and photochemical activity of zinc-indium-sulfide nanoplates. RSC Adv. 2015, 5, 89577–89585. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Xie, J.C.; Kuo, D.H. Transforming Zn(O,S) from UV to visible-light-driven catalyst with improved hydrogen production rate: Effect of indium and heterojunction. J. Alloys Compd. 2021, 869, 159316. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Grosse und der inneren Struktur von Kolloidteilchen mittels Rontgensrahlen. In Nachrichten von der Gesellschaft der Wissenschaften zu Gottingen, Mathematisch-Physikalische Klasse; Vandenhoeck & Ruprecht: Göttingen, Germany, 1918; Volume 2, pp. 98–100. [Google Scholar]
- Shao, Y.Q.; Wang, X.K.; Dou, Z.L.; Liang, X.Y.; Zhang, X.X.; Pang, M.; Xu, Q.; Ji, M.; Wang, M. Preparation of a ZnIn2S4–ZnAlOx nanocomposite for photoreduction of CO2 to CO. Catal. Sci. Technol. 2021, 11, 3422–3427. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D.; Ding, H.; Feng, J.; Zhang, J.Z.; Zhu, Y.; Hamid, S.; Bahnemann, D.W. Well-designed 3D ZnIn2S4 nanosheets/TiO2 nanobelts as direct Z-scheme photocatalysts for CO2 photoreduction into renewable hydrocarbon fuel with high efficiency. Appl. Catal. B: Environ. 2017, 219, 611–618. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lou, X.W.D. Construction of ZnIn2S4−In2O3 Hierarchical Tubular Heterostructures for Efficient CO2 Photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef]
- Hou, J.; Cao, S.; Wu, Y.; Gao, Z.; Liang, F.; Sun, Y.; Lin, Z.; Sun, L. Inorganic Colloidal Perovskite Quantum Dots for Robust Solar CO2 Reduction. Chemistry 2017, 23, 9481–9485. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Y.F.; Chen, B.X.; Kuang, D.B.; Su, C.Y. Synthesis and Photocatalytic Application of Stable Lead-Free Cs2AgBiBr6 Perovskite Nanocrystals. Small 2018, 14, e1703762. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S. Photocatalytic Reverse Water Gas Shift CO2 Reduction to CO over Montmorillonite Supported TiO2 Nanocomposite. Chem. Eng. Trans. 2017, 56, 319–324. [Google Scholar]
- Dong, Y.; Ghuman, K.K.; Popescu, R.; Duchesne, P.N.; Zhou, W.; Loh, J.Y.Y.; Jelle, A.A.; Jia, J.; Wang, D.; Mu, X.; et al. Tailoring Surface Frustrated Lewis Pairs of In2O3−x(OH)y for Gas-Phase Heterogeneous Photocatalytic Reduction of CO2 by Isomorphous Substitution of In3+ with Bi3+. Adv. Sci. 2018, 5, 1700732. [Google Scholar] [CrossRef]
- Yin, G.; Nishikawa, M.; Nosaka, Y.; Srinivasan, N.; Atarashi, D.; Sakai, E.; Miyauchi, M. Photocatalytic carbon dioxide reduction by copper oxide nanocluster-grafted niobate nanosheets. ACS Nano. 2015, 9, 2111–2119. [Google Scholar] [CrossRef]
- Manzi, A.; Simon, T.; Sonnleitner, C.; Doblinger, M.; Wyrwich, R.; Stern, O.; Stolarczyk, J.K.; Feldmann, J. Light-induced cation exchange for copper sulfide based CO2 reduction. J. Am. Chem. Soc. 2015, 137, 14007–14010. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, I.-H.; Diau, E.W.-G. Photocatalytic CO2 Reduction Using Zinc Indium Sulfide Aggregated Nanostructures Fabricated under Four Anionic Conditions. Nanomaterials 2024, 14, 1231. https://doi.org/10.3390/nano14141231
Tsai I-H, Diau EW-G. Photocatalytic CO2 Reduction Using Zinc Indium Sulfide Aggregated Nanostructures Fabricated under Four Anionic Conditions. Nanomaterials. 2024; 14(14):1231. https://doi.org/10.3390/nano14141231
Chicago/Turabian StyleTsai, I-Hua, and Eric Wei-Guang Diau. 2024. "Photocatalytic CO2 Reduction Using Zinc Indium Sulfide Aggregated Nanostructures Fabricated under Four Anionic Conditions" Nanomaterials 14, no. 14: 1231. https://doi.org/10.3390/nano14141231
APA StyleTsai, I.-H., & Diau, E. W.-G. (2024). Photocatalytic CO2 Reduction Using Zinc Indium Sulfide Aggregated Nanostructures Fabricated under Four Anionic Conditions. Nanomaterials, 14(14), 1231. https://doi.org/10.3390/nano14141231




