Raman Study of Novel Nanostructured WO3 Thin Films Grown by Spray Deposition
Abstract
1. Introduction
2. Materials and Methods
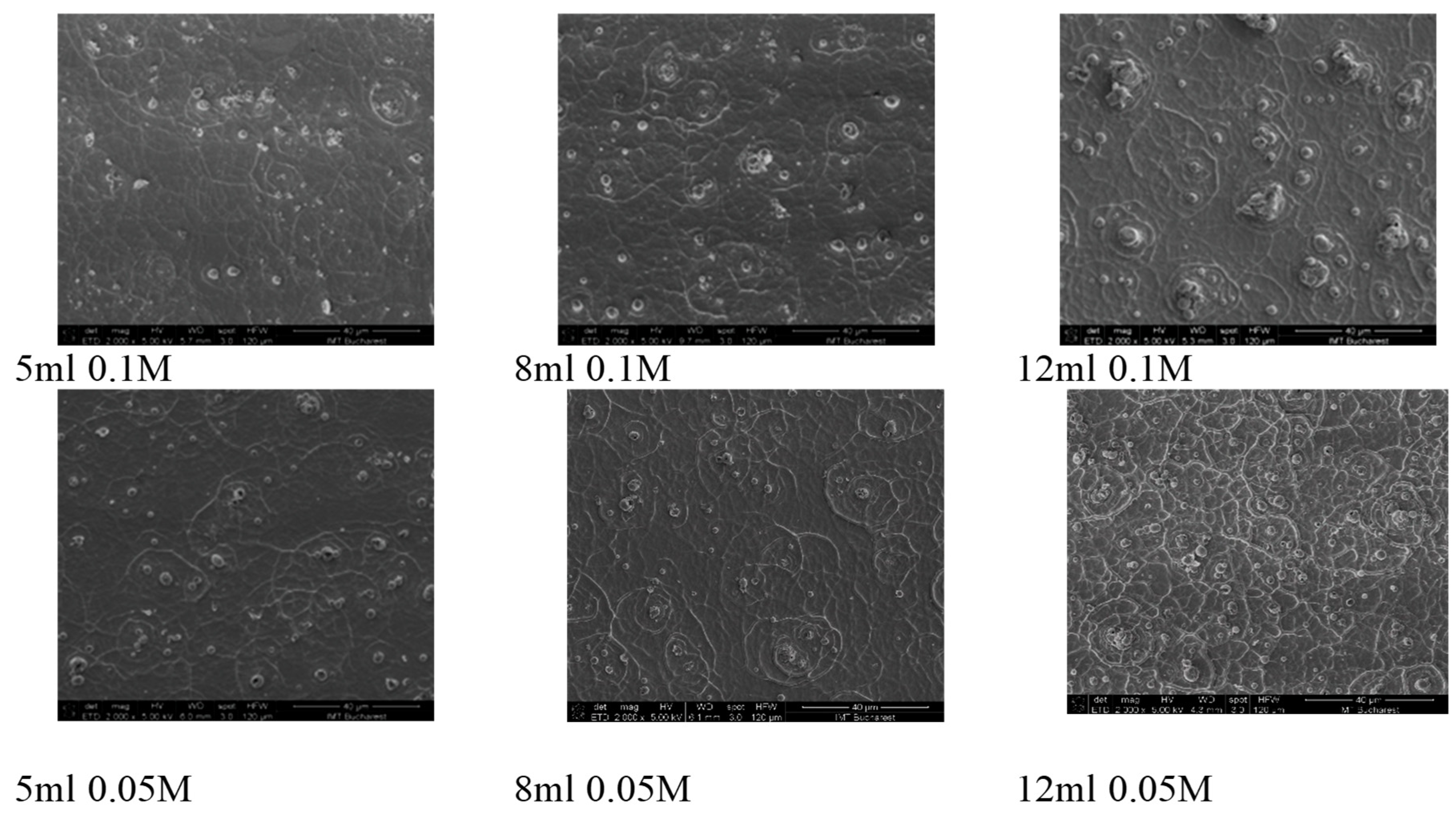
3. Results and Discussions
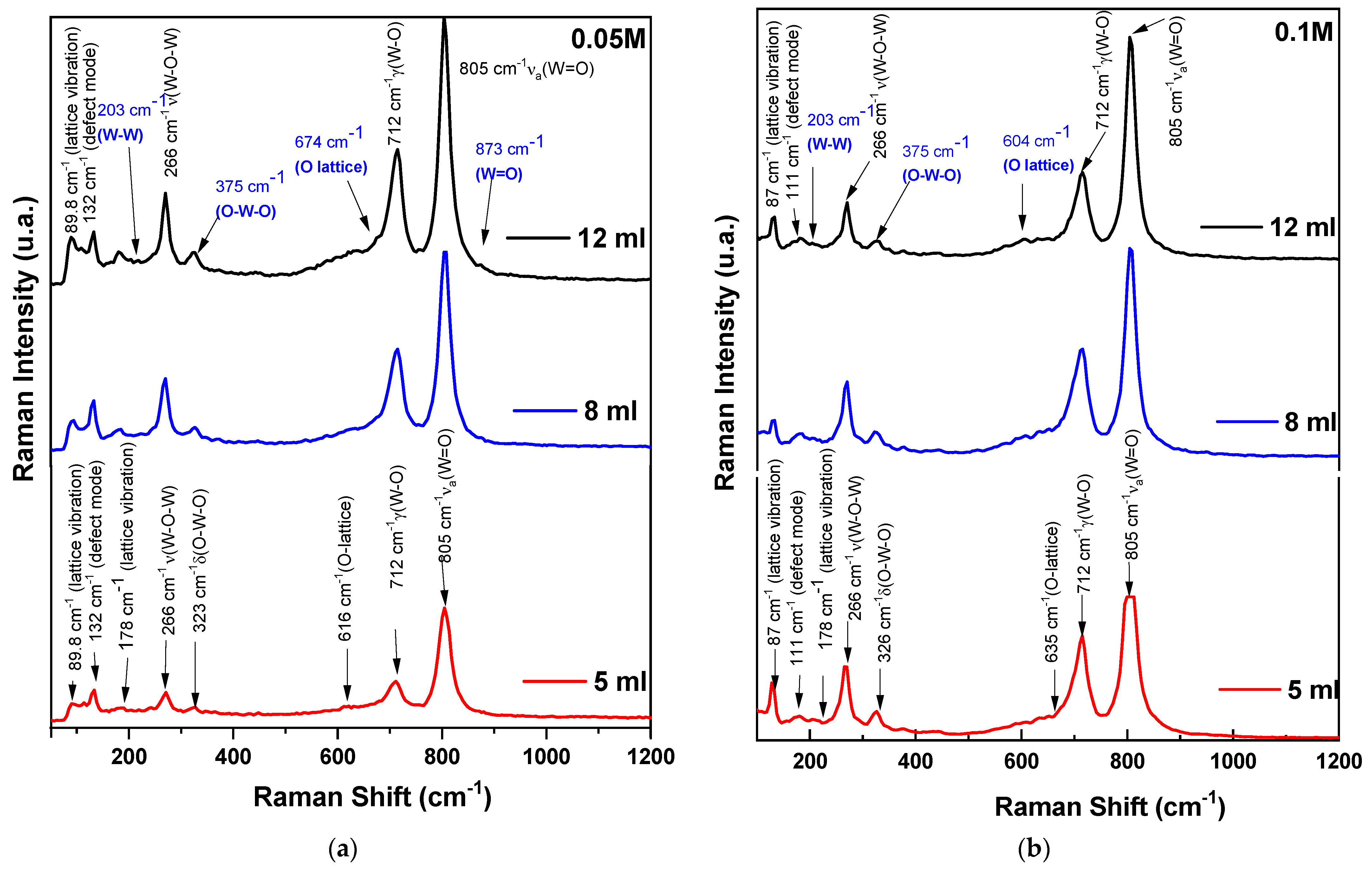
- 0.05 M Series: Thinner films (lower volume) show less intense peaks, indicating more defects and lower crystallinity. Thicker films (higher volume) exhibit sharper and more intense peaks, suggesting improved crystallinity and structural order.
- 0.1 M Series: Films at higher precursor concentrations show overall more intense and sharper peaks across all thicknesses, indicating higher crystallinity and fewer defects. Differences in peak intensity and presence reflect variations in film morphology and structural properties due to increased precursor concentration.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, B.; Adams, J.; Pennathur, S. Nanotechnology: Understanding Small Systems, 3rd ed.; CRC Press, Taylor & Francis Group: New York, NY, USA, 2015. [Google Scholar]
- Modak, M.; Rane, S.; Jagtap, S. WO3: A review of synthesis techniques, nanocomposite materials and their morphological effects for gas sensing application. Bull. Mater. Sci. 2023, 46, 28. [Google Scholar] [CrossRef]
- Mardare, C.C.; Hassel, A.W. Review on the Versatility of Tungsten Oxide Coatings. Phys. Status Solidi A 2019, 216, 1900047. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, P.; Gupta, G. Dimension dependency of tungsten oxide for efficient gas sensing. Environ. Sci. Nano 2022, 9, 40–60. [Google Scholar] [CrossRef]
- Dong, C.; Zhao, R.; Yao, L.; Ran, Y.; Zhang, X.; Wang, Y. A review on WO3 based gas sensors: Morphology control and enhanced sensing properties. J. Alloys Compd. 2020, 820, 153194. [Google Scholar] [CrossRef]
- Li, X.; Fu, L.; Karimi-Maleh, H.; Chen, F.; Zhao, S. Innovations in WO3 gas sensors: Nanostructure engineering, functionalization, and future perspectives. Heliyon 2024, 10, 27740. [Google Scholar] [CrossRef] [PubMed]
- Staerz, A.; Somacescu, S.; Epifani, M.; Kida, T.; Weimar, U.; Barsan, N. WO3-Based gas sensors: Identifying inherent qualities and understanding the sensing mechanism. ACS Sens. 2020, 5, 1624–1633. [Google Scholar] [CrossRef]
- Bonardo, D.; Septiani, N.L.W.; Amri, F.; Estananto; Humaidi, S.; Suyatman; Yuliarto, B. Review—Recent development of WO3 for toxic gas sensors applications. J. Electrochem. Soc. 2021, 168, 107502. [Google Scholar] [CrossRef]
- Epifani, M. Mechanistic insights into WO3 sensing and related perspectives. Sensors 2022, 22, 2247. [Google Scholar] [CrossRef]
- Wei, S.; Li, S.; Wei, R.; Liu, S.; Du, W. Different morphologies of WO3 and their exposed facets-dependent acetone sensing properties. Sens. Actuators B Chem. 2021, 329, 129188. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Yang, F. Gas sensing properties of WO3 based materials with hierarchical structural features. Ceram. Int. 2024, in press. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier Science: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Maho, A.; Nayak, S.; Gillissen, F.; Cloots, R.; Rougier, A. Film Deposition of Electrochromic Metal Oxides through Spray Coating: A Descriptive Review. Coatings 2023, 13, 1879. [Google Scholar] [CrossRef]
- Buch, V.R.; Chawla, A.K.; Rawal, S.K. Review on electrochromic property for WO3 thin films using different deposition techniques. Mater. Today Proc. 2016, 3, 1429–1437. [Google Scholar] [CrossRef]
- Gogova, D.; Thomas, L.-K.; Camin, B. Comparative study of gasochromic and electrochromic effect in thermally evaporated tungsten oxide thin films. Thin Solid Films 2009, 517, 3326–3331. [Google Scholar] [CrossRef]
- Gao, C.; Guo, X.; Nie, L.; Wu, X.; Peng, L.; Chen, J. A review on WO3 gasochromic film: Mechanism, preparation and properties. Int. J. Hydrog. Energy 2023, 48, 2442–2465. [Google Scholar] [CrossRef]
- Feng, W.; Zou, L.; Gao, G.; Wu, G.; Shen, J.; Li, W. Gasochromic smart window: Optical and thermal properties, energy simulation and feasibility analysis. Sol. Energy Mater. 2016, 144, 316–323. [Google Scholar] [CrossRef]
- Li, S.; Yao, Z.; Zhou, J.; Zhang, R.; Shen, H. Fabrication and characterization of WO3 thin films on silicon surface by thermal evaporation. Mater. Lett. 2017, 195, 213–216. [Google Scholar] [CrossRef]
- Cantalini, C.; Wlodarski, W.; Li, Y.; Passacantando, M.; Santucci, S.; Comini, E.; Faglia, G.; Sberveglieri, G. Investigation on the O3 sensitivity properties of WO3 thin films prepared by sol–gel, thermal evaporation and r.f. sputtering techniques. Sens. Actuators B Chem. 2000, 64, 182–188. [Google Scholar] [CrossRef]
- Kim, T.S.; Kim, Y.B.; Yoo, K.S.; Sung, G.S.; Jung, H.J. Sensing characteristics of dc reactive sputtered WO3 thin films as an NOx gas sensor. Sens. Actuators B Chem. 2000, 62, 102–108. [Google Scholar] [CrossRef]
- Johansson, M.B.; Zietz, B.; Niklasson, G.A.; Österlund, L. Optical properties of nanocrystalline WO3 and WO3-x thin films prepared by DC magnetron sputtering. Appl. Phys. 2014, 115, 213510. [Google Scholar] [CrossRef]
- Gogova, D.; Gesheva, K.; Kakanakova-Georgieva, A.; Surtchev, M. Investigation of the structure of tungsten oxide films obtained by chemical vapor deposition. Eur. Phys. J. AP 2000, 11, 167–174. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar]
- Dongale, T.D.; Mohite, S.V.; Bagade, A.A.; Gaikwad, P.; Patil, P.S.; Kamat, R.K.; Rajpure, K. Development of Ag/WO3/ITO Thin Film Memristor Using Spray Pyrolysis. Method. Electron. Mater. Lett. 2015, 11, 944–948. [Google Scholar] [CrossRef]
- Acosta, D.R.; Magaña, C.; Hernández, F.; Ortega, J. Electrical, optical and electrochromic properties of Ti:WO3 thin films deposited by the pulsed chemical spray technique. Thin Solid Films 2015, 594, 207–214. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and Raman Study of WO3 Tungsten Trioxides and W03, xHzO Tungsten Trioxide Hydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Ortega, J.M.; Martínez, A.I.; Acosta, D.R.; Magaña, C.R. Structural and electrochemical studies of WO3 films deposited by pulsed spray pyrolysis. Sol. Energy Mater. 2006, 90, 2471–2479. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Salje, E.; Viswanathan, K. Physical properties and phase transitions in WO3. Acta Cryst. 1975, A31, 356–359. [Google Scholar] [CrossRef]
- Han, W.; Qian Shi, Q.; Hu, R. Advances in Electrochemical Energy Devices Constructed with Tungsten Oxide-Based Nanomaterials. Nanomaterials 2021, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Woodward, P.M.; Sleight, A.W.; Vogt, T. Ferroelectric Tungsten Trioxide. J. Solid State Chem. 1997, 131, 9–17. [Google Scholar] [CrossRef]
- Wang, L.; Kalyanasundaram, K.; Stanacevic, M.; Gouma, P. Nanosensor Device for Breath Acetone Detection. Sens. Lett. 2010, 8, 709–712. [Google Scholar] [CrossRef]
- Liu, J.; Dong, X.; Li, X.W.; Shi, F. Solvothermal synthesis and characterization of tungsten oxides with controllable morphology and crystal phase. J. Alloys Compd. 2011, 509, 1482–1488. [Google Scholar] [CrossRef]
- Jain, R.; Wang, Y.; Maric, R. Tuning of WO3 Phase Transformation and Structural Modification by Reactive Spray Deposition Technology. J. Nanotechnol. Smart Mater. 2014, 1, 203. [Google Scholar]
- Adu, K.W.; Xiong, Q.; Gutierrez, H.R.; Chen, G.; Eklund, P.C. Raman scattering as a probe of phonon confinement and surface optical modes in semiconducting nanowires. Appl. Phys. A 2006, 85, 287–297. [Google Scholar] [CrossRef]
- Ashraf, S.; Blackman, C.S.; Palgrave, R.G.; Parkin, I.P. Aerosol-assisted chemical vapour deposition of WO3 thin films using polyoxometallate precursors and their gas sensing properties. J. Mater. Chem. 2007, 17, 1063–1070. [Google Scholar] [CrossRef]
- Foroushani, T.; Tavanai, H.; Ranjbar, M.; Bahrami, H. Fabrication of tungsten oxide nanofibers via electrospinning for gasochromic hydrogen detection. Sens. Actuators B Chem. 2018, 268, 319–327. [Google Scholar] [CrossRef]
- Song, W.; Zhang, R.; Bai, X.; Jia, Q.; Ji, H. Exposed crystal facets of WO3 nanosheets by phase control on NO2-sensing performance. J. Mater. Sci. Mater. Electron. 2020, 31, 610–620. [Google Scholar] [CrossRef]
- Lee, S.-H.; Cheong, H.M.; Liu, P.; Smith, D.; Tracy, C.E.; Mascarenhas, A.; Pitts, J.R.; Deb, S.K. Raman spectroscopic studies of gasochromic a-WO3 thin films. Electrochim. Acta 2001, 46, 1995–1999. [Google Scholar] [CrossRef]

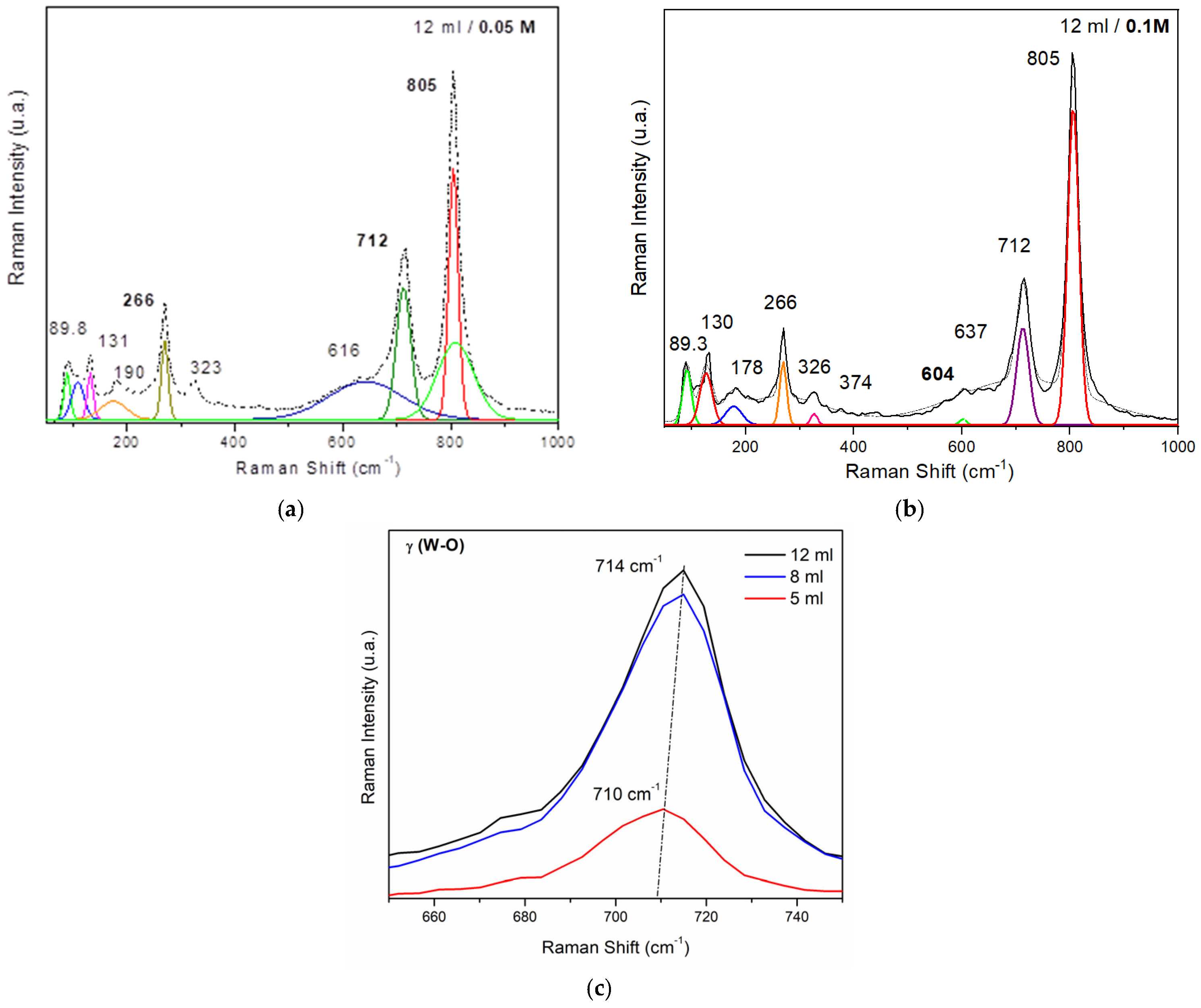
| Raman Band (cm−1) | Raman Assignment |
|---|---|
| 89.73 | Low-frequency phonon change marker |
| Low-energy lattice vibrations. Discussion: This band is typically associated with external lattice vibrations. It is prominent in the 0.05 M series, indicating structural ordering with potential lattice imperfections. Its absence or lower intensity in the 0.1 M series suggests higher crystallinity and fewer defects. | |
| 110.83 | Defect-related or localized vibrational modes. Discussion: This band may indicate specific defect states or unique structural features. It is less commonly reported and may appear as a weak peak, reflecting lattice imperfections or localized modes due to impurities. |
| 180 | Low-frequency phonon change marker |
| Lattice vibrations or phonon modes. Discussion: Present in both 0.05 M and 0.1 M series, indicating a stable characteristic of the WO3 lattice. Its consistent presence suggests that this mode is relatively unaffected by precursor concentration but varies in intensity with film thickness. | |
| 203.89 | W-W |
| Lattice vibrations. Discussion: This band is indicative of specific W-W lattice vibrational modes, observed prominently in the 0.05 M series. It reflects the differences in film morphology and thickness. | |
| 270.02 | ν (O-W-O) in monoclinic phase |
| Bending modes. Discussion: Associated with W-O-W bending vibrations. Its presence is related to monoclinic phase structural characteristics specific to the WO3 thin films. | |
| 326.31 | δ (O-W-O) |
| Out-of-plane wagging modes. Discussion: Indicates out-of-plane bending or wagging of W-O bonds. Prominent in both concentration series, reflecting the structural integrity of the WO3 lattice. | |
| 374 | δ (O-W-O) |
| Lattice vibrations. Discussion: This band, observed in the 0.1 M series, indicates complex lattice dynamics and interactions. Its presence suggests differences in structural properties influenced by higher precursor concentration. | |
| 604 | O-lattice |
| Bending modes. Discussion: This band is related to O-W-O bending vibrations. More prominent in the 0.1 M series, indicating a more ordered structure due to higher precursor concentration. | |
| 674 | O-lattice |
| W=O stretching vibrations. Discussion: Indicates strong W=O stretching, present in the 0.05 M series. It suggests moderate crystallinity and possible defects. | |
| 714.96 | γ (W-O) |
| W=O stretching vibrations. Discussion: Common in both concentration series, indicating strong W=O stretching vibrations. Higher intensity in the 0.1 M series reflects better crystallinity. | |
| 803.91 | νa (anti-symmetric O-W-O) monoclinic phase |
| W=O stretching vibrations. Discussion: Present in both series, indicating strong W=O stretching. The higher intensity in the 0.1 M series suggests improved structural order. | |
| 873 | νs (symmetric W=O terminal) |
| High-frequency stretching modes. Discussion: This band is indicative of high-frequency W=O stretching vibrations, observed prominently in both series. Its intensity reflects the crystallinity and thickness of the films. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, A.G.M.; Tudose, I.V.; Romanitan, C.; Popescu, M.; Manica, M.; Schiopu, P.; Vladescu, M.; Suchea, M.P.; Pachiu, C. Raman Study of Novel Nanostructured WO3 Thin Films Grown by Spray Deposition. Nanomaterials 2024, 14, 1227. https://doi.org/10.3390/nano14141227
Popescu AGM, Tudose IV, Romanitan C, Popescu M, Manica M, Schiopu P, Vladescu M, Suchea MP, Pachiu C. Raman Study of Novel Nanostructured WO3 Thin Films Grown by Spray Deposition. Nanomaterials. 2024; 14(14):1227. https://doi.org/10.3390/nano14141227
Chicago/Turabian StylePopescu, Andreea Gabriela Marina, Ioan Valentin Tudose, Cosmin Romanitan, Marian Popescu, Marina Manica, Paul Schiopu, Marian Vladescu, Mirela Petruta Suchea, and Cristina Pachiu. 2024. "Raman Study of Novel Nanostructured WO3 Thin Films Grown by Spray Deposition" Nanomaterials 14, no. 14: 1227. https://doi.org/10.3390/nano14141227
APA StylePopescu, A. G. M., Tudose, I. V., Romanitan, C., Popescu, M., Manica, M., Schiopu, P., Vladescu, M., Suchea, M. P., & Pachiu, C. (2024). Raman Study of Novel Nanostructured WO3 Thin Films Grown by Spray Deposition. Nanomaterials, 14(14), 1227. https://doi.org/10.3390/nano14141227








