Combined Electrochemical Deposition and Photo-Reduction to Fabricate SERS-Active Silver Substrates: Characterization and Application for Malachite Green Detection in Aquaculture Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Characterization
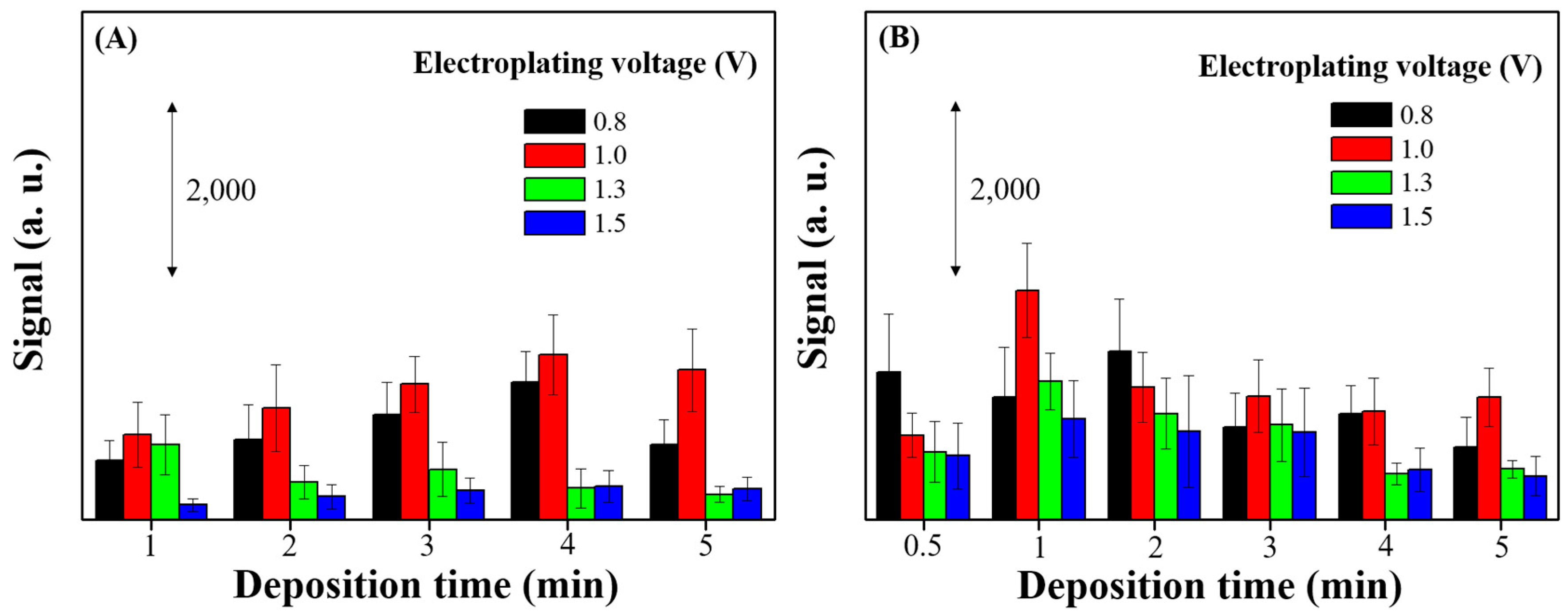
2.3. Electrochemical Deposition of AgyFTO Substrate
2.4. Photo-Reduction of X-Ag Nanoparticles
2.5. MG Detection by SERS-Active X-Ag-AgyFTO Substrate
3. Results and Discussion
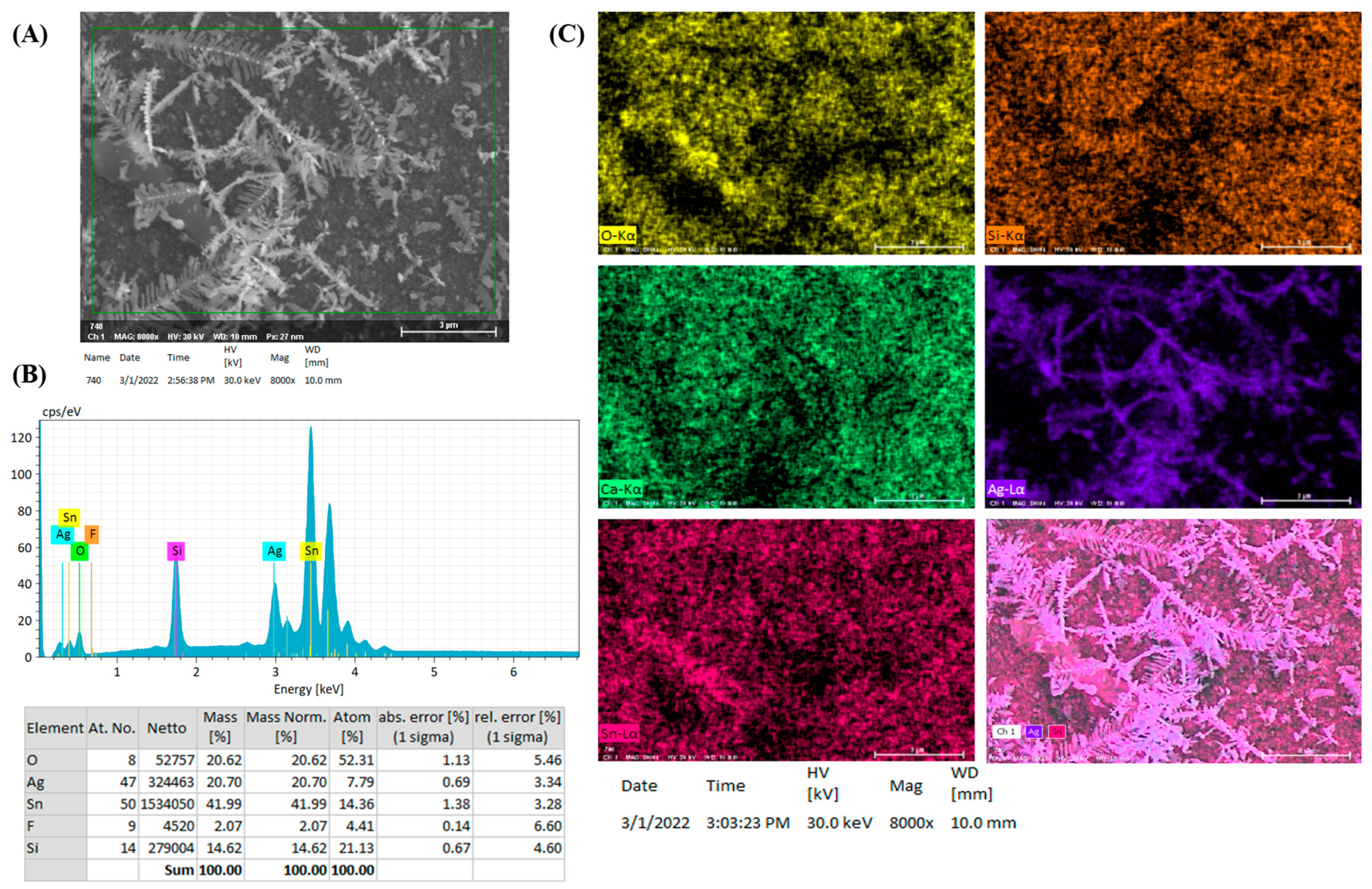
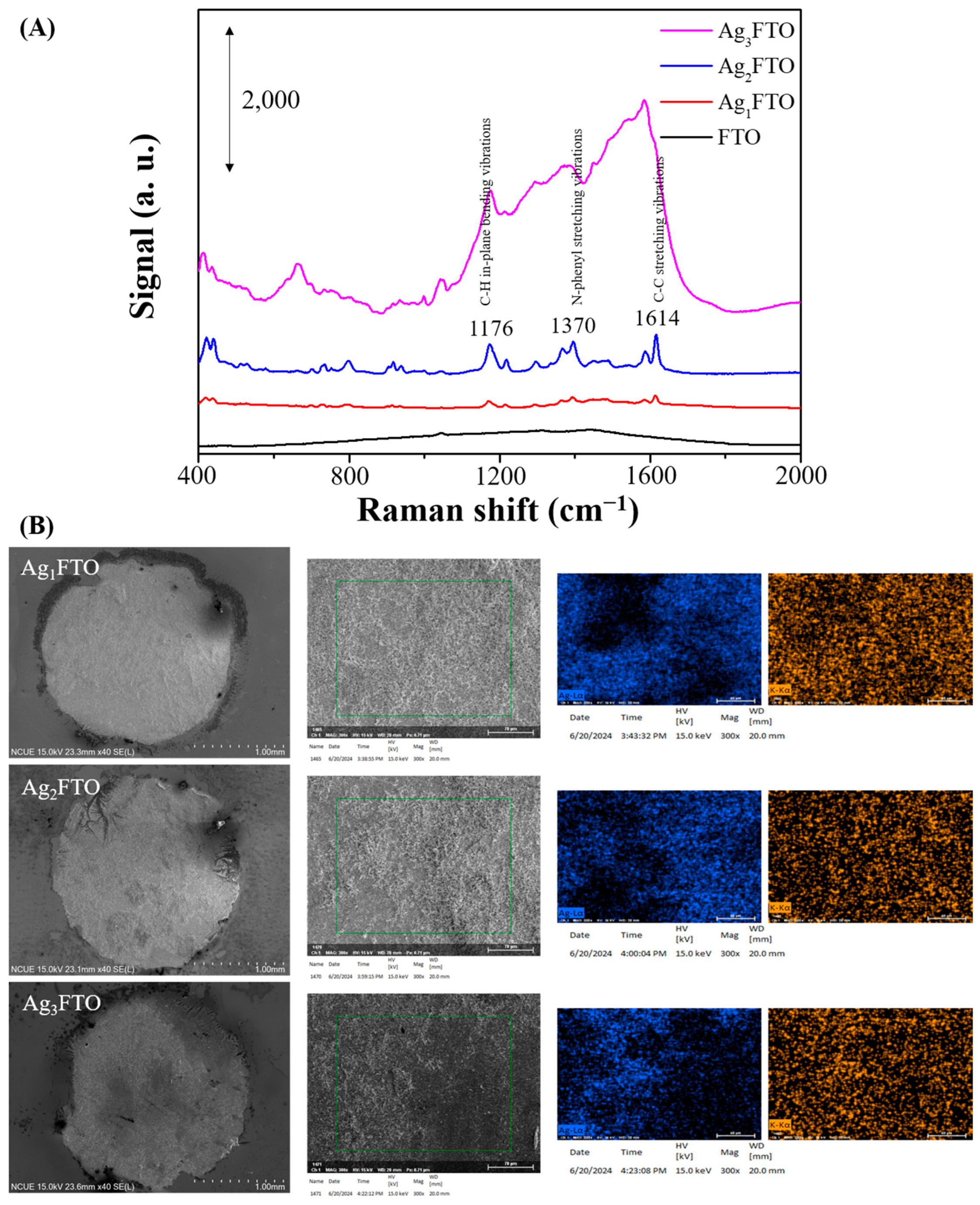
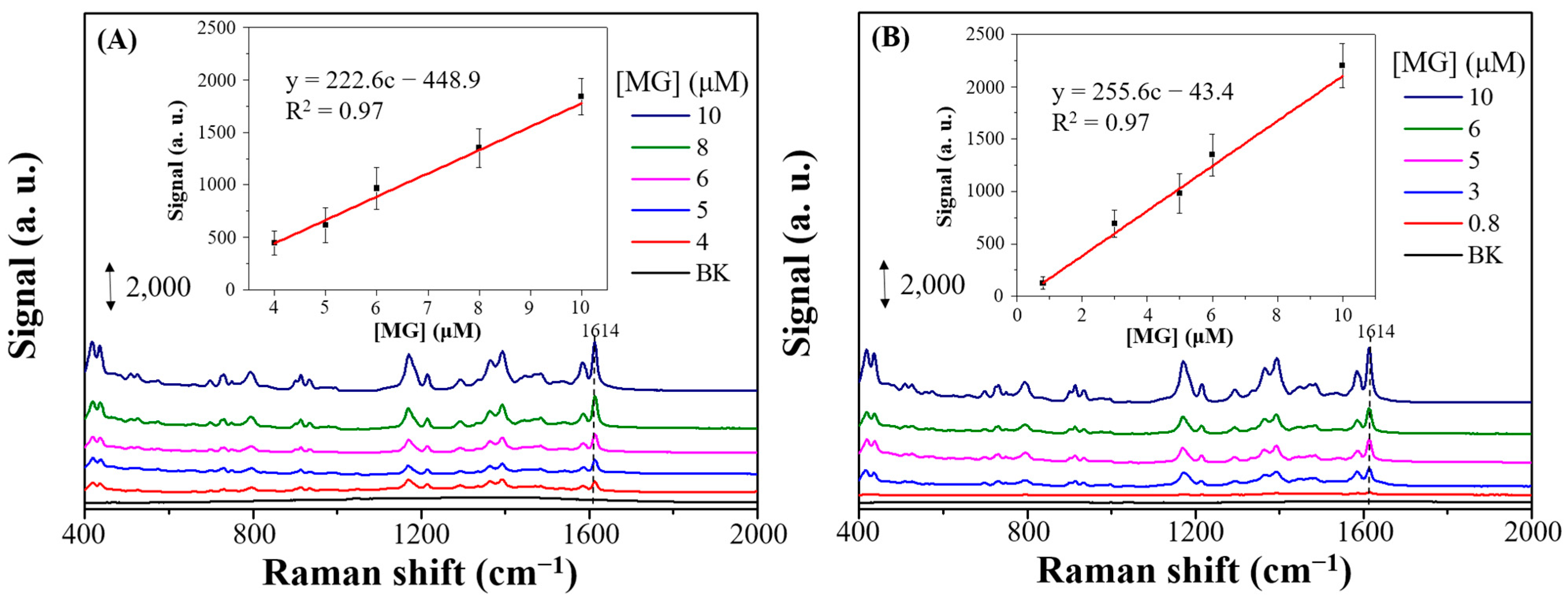
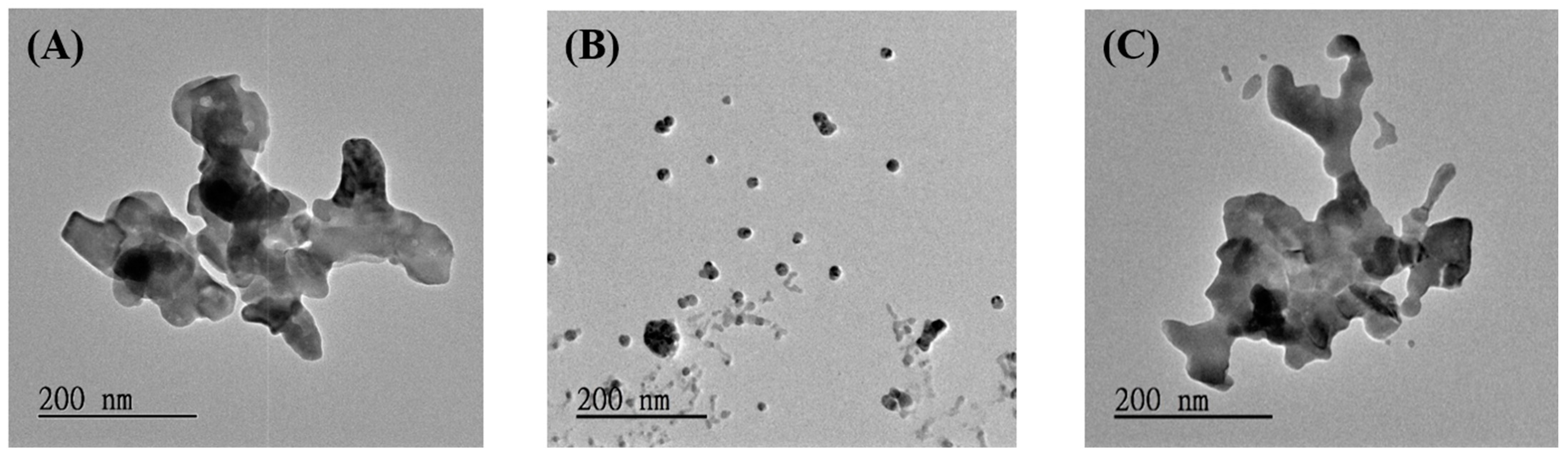
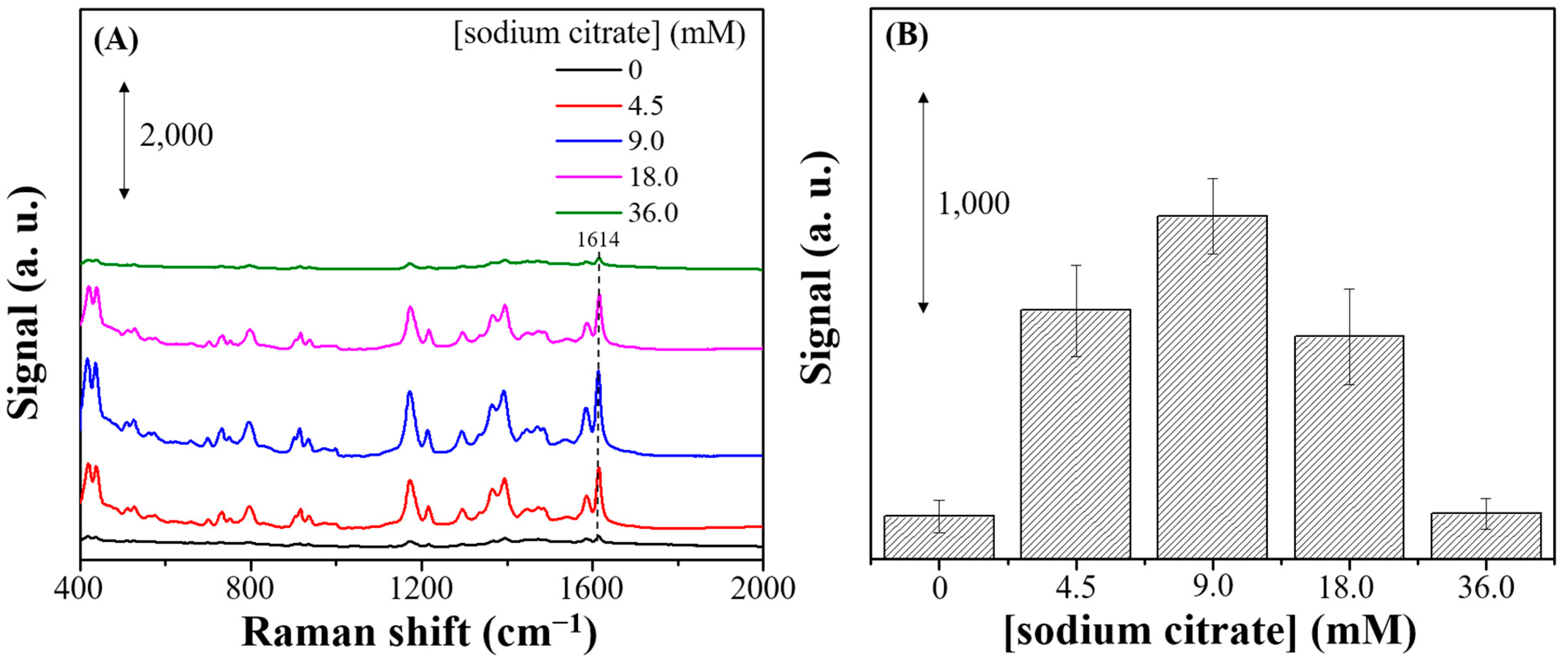
3.1. Characterization and SERS Performance of Electrochemical Deposition of AgyFTO Substrate
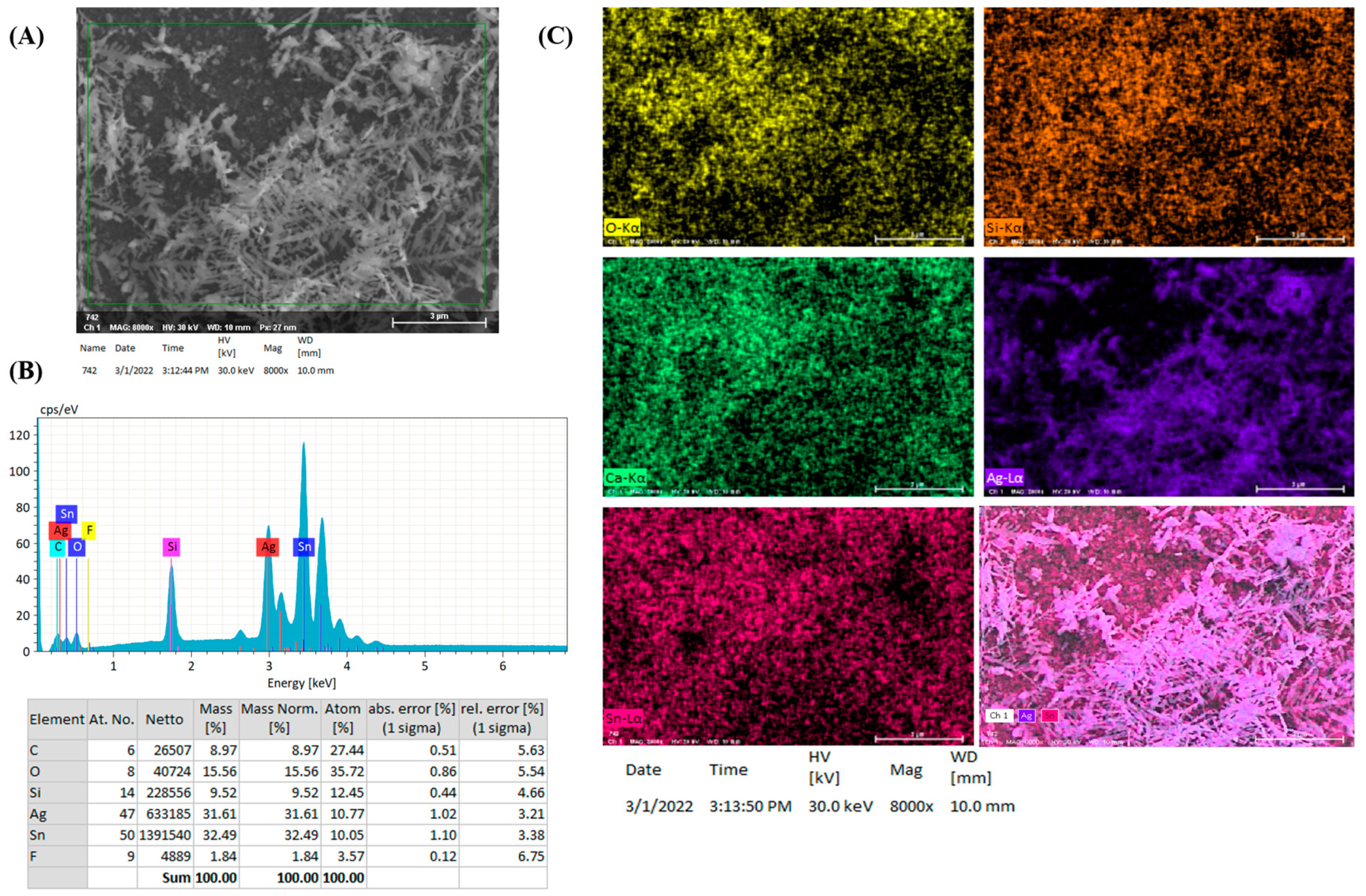
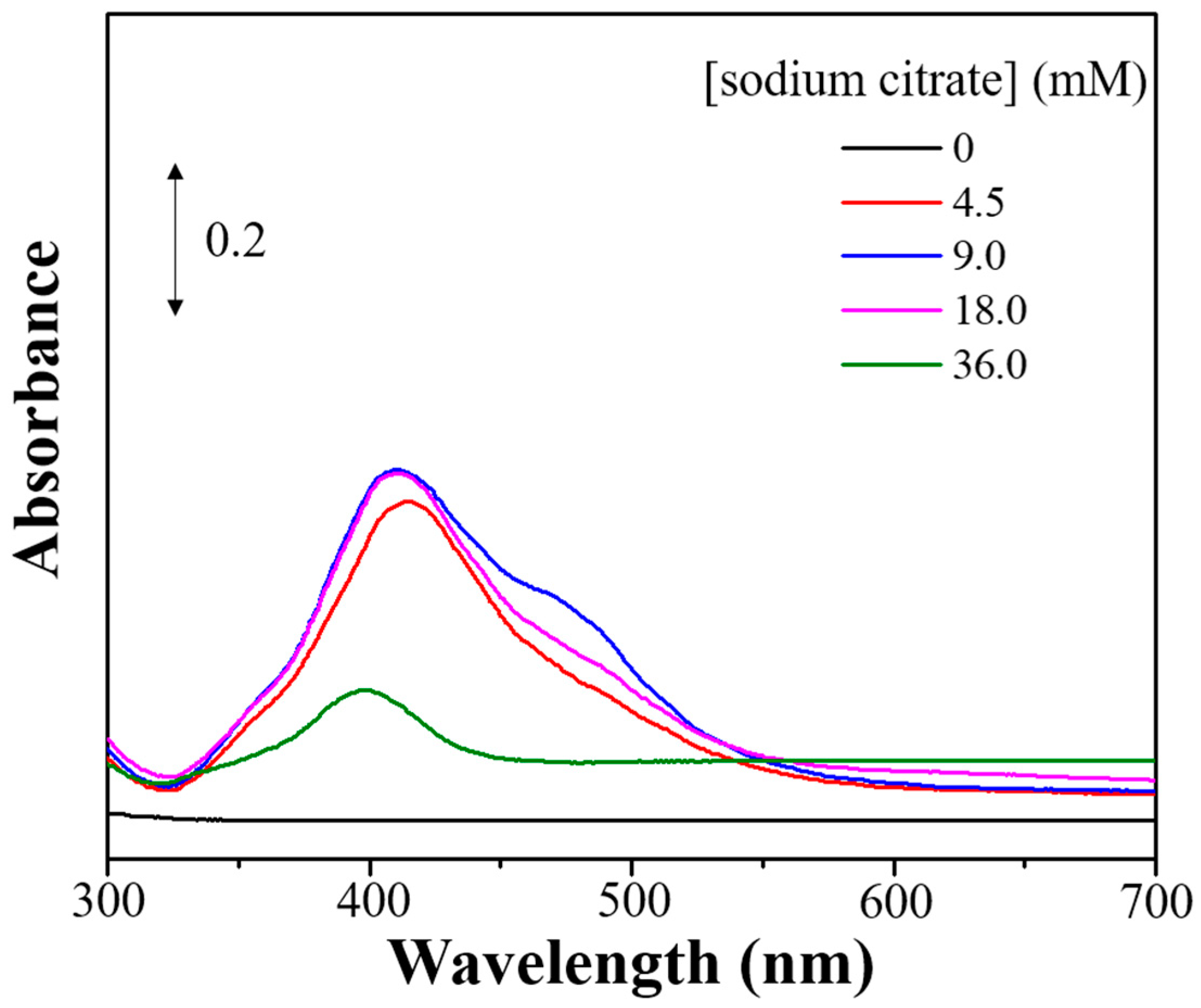
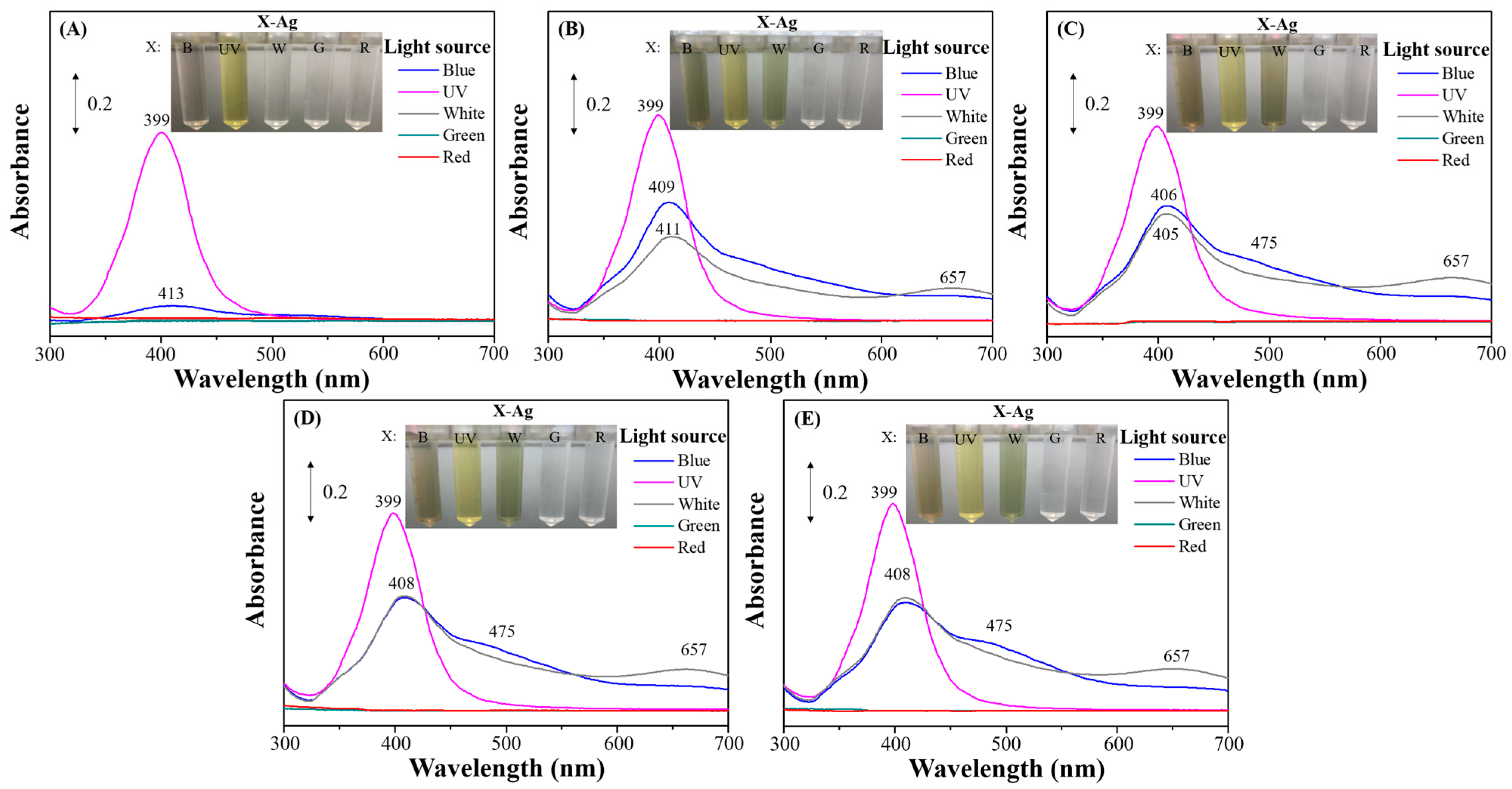
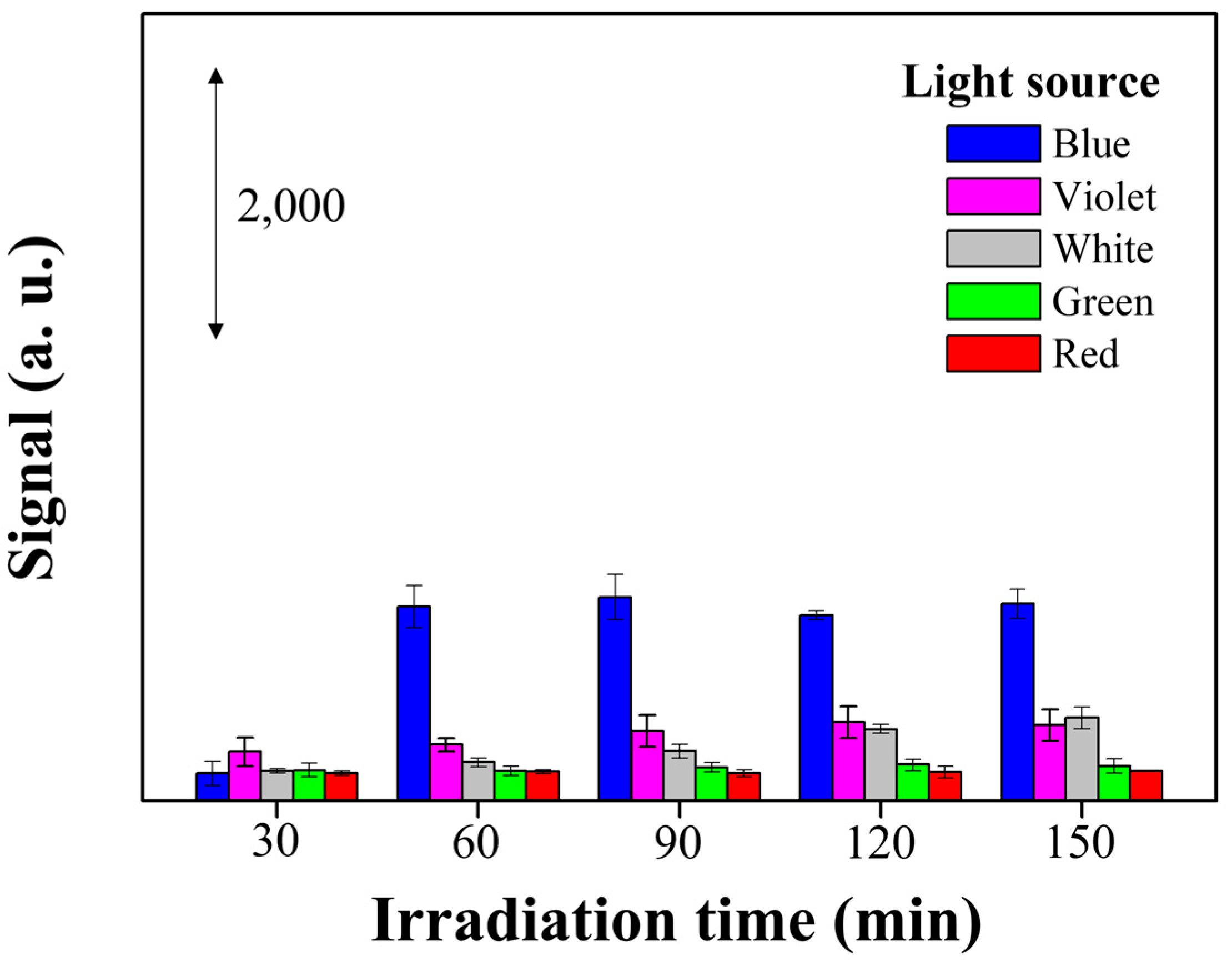
3.2. Photo-Reduction of X-Ag and Characterization
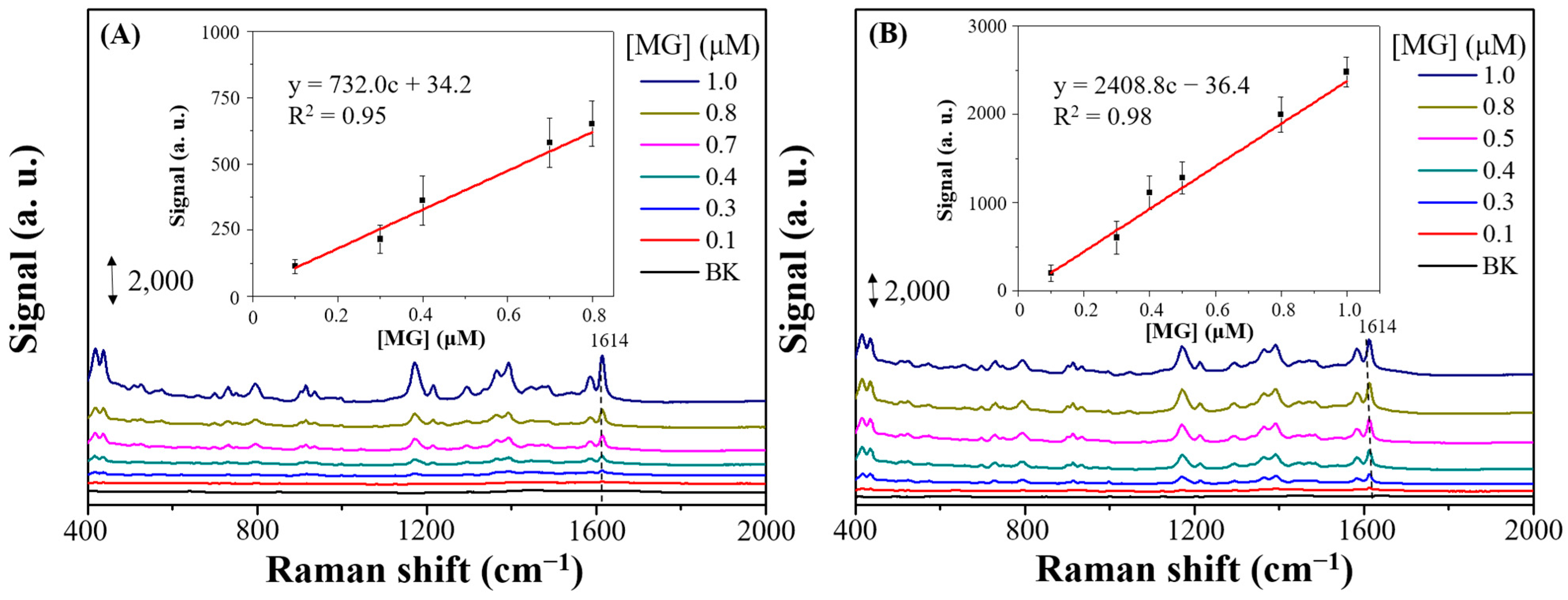
3.3. SERS Performances of X-Ag-AgyFTO Substrates and MG Detection
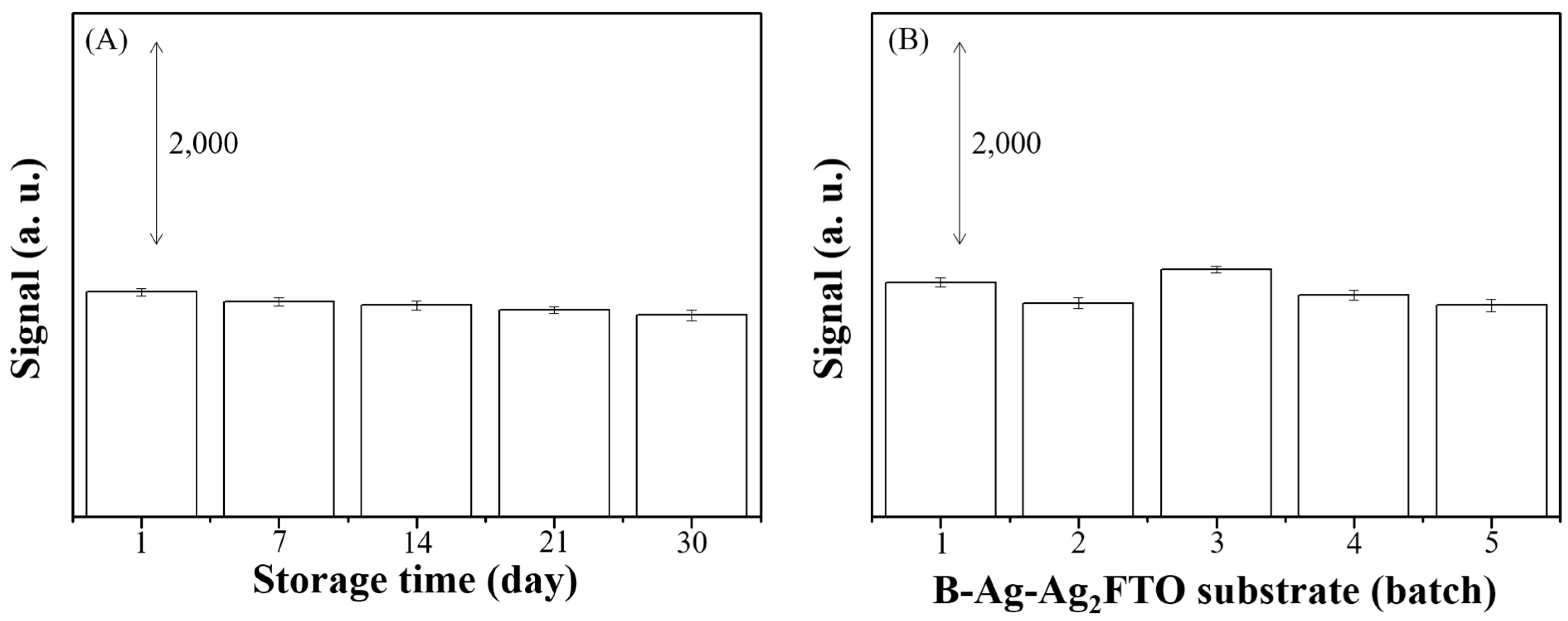
3.4. Determination of MG Concentration in Aquaculture Water Sample Using B-Ag-Ag2FTO Substrates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Umeh, C.T.; Akinyele, A.B.; Okoye, N.H.; Emmanuel, S.S.; Iwuozor, K.O.; Oyekunle, I.P.; Ocheje, J.O.; Ighalo, J.O. Recent approach in the application of nanoadsorbents for malachite green (MG) dye uptake from contaminated water: A critical review. Env. Nanotech Monit. Manag. 2023, 20, 100891. [Google Scholar] [CrossRef]
- Yildiz, H.; Gülşen, H.; Şahin, Ö.; Baytar, O.; Kutluay, S. Novel adsorbent for malachite green from okra stalks waste: Synthesis, kinetics and equilibrium studies. Int. J. Phytoremediat. 2024, 26, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Culp, S.J.; Beland, F.A. Malachite green: A toxicological review. J. Am. Coll. Toxicol. 1996, 15, 219–238. [Google Scholar] [CrossRef]
- Sudova, E.; Machova, J.; Svobodova, Z.; Vesely, T. Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: A review. Vet. Med. 2007, 52, 527. [Google Scholar] [CrossRef]
- Hussain Hakami, A.A.; Ahmed, M.A.; Khan, M.A.; AlOthman, Z.A.; Rafatullah, M.; Islam, M.A.; Siddiqui, M.R. Quantitative analysis of malachite green in environmental samples using liquid chromatography-mass spectrometry. Water 2021, 13, 2864. [Google Scholar] [CrossRef]
- Van de Riet, J.M.; Murphy, C.J.; Pearce, J.N.; Potter, R.A.; Burns, B.G. Determination of malachite green and leucomalachite green in a variety of aquacultured products by liquid chromatography with tandem mass spectrometry detection. J. AOAC Int. 2005, 88, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Šafařík, I.; Šafaříková, M. Detection of low concentrations of malachite green and crystal violet in water. Water Res. 2002, 36, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, J.; Pan, Z.; Li, D. Review of methods for the detection and determination of malachite green and leuco-malachite green in aquaculture. Crit. Rev. Anal. Chem. 2019, 49, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Nebot, C.; Iglesias, A.; Barreiro, R.; Miranda, J.M.; Vázquez, B.; Franco, C.M.; Cepeda, A. A simple and rapid method for the identification and quantification of malachite green and its metabolite in hake by HPLC–MS/MS. Food Control 2013, 31, 102–107. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Tian, X.-H.; Zhang, X.-Z.; Gong, X.-H.; Liu, H.-H.; Zhang, H.-J.; Huang, H.; Zhang, L.-M. Simultaneous determination of malachite green, crystal violet, methylene blue and the metabolite residues in aquatic products by ultra-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Chromatogr. Sci. 2012, 50, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.J.M.; Herrera, S.; Uclés, A.; Agüera, A.; Hernando, M.D.; Shimelis, O.; Rudolfsson, M.; Fernández-Alba, A.R. Determination of malachite green residues in fish using molecularly imprinted solid-phase extraction followed by liquid chromatography–linear ion trap mass spectrometry. Anal. Chim. Acta 2010, 665, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mitrowska, K.; Posyniak, A.; Zmudzki, J. Determination of malachite green and leucomalachite green in carp muscle by liquid chromatography with visible and fluorescence detection. J. Chromatogr. A 2005, 1089, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Lin, Q.; Li, H.; Huang, Z.; Kuang, Y.; Chen, H.; Kong, J. Rapid detection of malachite green residues in fish using a surface-enhanced Raman scattering-active glass fiber paper prepared by in situ reduction method. Talanta 2019, 200, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-X.; Guo, M.-H.; Huang, Y.-P.; Li, X.-D.; Sun, J.-J. Rapid and sensitive detection of malachite green in aquaculture water by electrochemical preconcentration and surface-enhanced Raman scattering. Talanta 2018, 180, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, W.; Pei, L.; Lai, K.; Rasco, B.A.; Huang, Y. Rapid analysis of malachite green and leucomalachite green in fish muscles with surface-enhanced resonance Raman scattering. Food Chem. 2015, 169, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, J.; Chen, L.; Park, B.; Kyong, J.B.; Seong, G.H.; Choo, J.; Lee, Y.; Shin, K.-H.; Lee, E.K. Fast and sensitive trace analysis of malachite green using a surface-enhanced Raman microfluidic sensor. Anal. Chim. Acta 2007, 590, 139–144. [Google Scholar] [CrossRef]
- Lipovka, A.; Fatkullin, M.; Averkiev, A.; Pavlova, M.; Adiraju, A.; Weheabby, S.; Al-Hamry, A.; Kanoun, O.; Pašti, I.; Lazarevic-Pasti, T. Surface-enhanced Raman spectroscopy and electrochemistry: The ultimate chemical sensing and manipulation combination. Crit. Rev. Anal. Chem. 2024, 54, 110–134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, P.; Das, A.; Pathak, C.S.; Kumar, S.; Kumar, P.; Das, A.; Pathak, C. Surface-enhanced Raman scattering: Introduction and applications. In Recent Advances in Nanophotonics-Fundamentals and Applications; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.-C.; Hu, S.; Yan, S.; Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Li, Z.H.; Bai, J.H.; Zhang, X.; Lv, J.M.; Fan, C.S.; Zhao, Y.M.; Wu, Z.L.; Xu, H.J. Facile synthesis of Au nanoparticle-coated Fe3O4 magnetic composite nanospheres and their application in SERS detection of malachite green. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2020, 241, 118532. [Google Scholar] [CrossRef]
- Lin, S.-C.; Zhang, X.; Zhao, W.-C.; Chen, Z.-Y.; Du, P.; Zhao, Y.-M.; Wu, Z.-L.; Xu, H.-J. Quantitative and sensitive detection of prohibited fish drugs by surface-enhanced Raman scattering. Chin. Phys. B 2018, 27, 028707. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, H.; Lin, S.; Dong, H.; Hasi, W.; Dong, B. Plasmonic nanosensor based on Ag nanocubes of high purification by extraction filtration strategy for SERS determination of malachite green in aquaculture water. Sens. Actuator B-Chem. 2022, 358, 131515. [Google Scholar] [CrossRef]
- Yang, G.; Fang, X.; Jia, Q.; Gu, H.; Li, Y.; Han, C.; Qu, L.-L. Fabrication of paper-based SERS substrates by spraying silver and gold nanoparticles for SERS determination of malachite green, methylene blue, and crystal violet in fish. Microchim. Acta 2020, 187, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, Y.; Zhang, Y.; Fan, Y.; Lai, K. Ultra sensitive detection of malachite green in fish muscle with gold nanoparticles and graphene oxide hybrid as a substrate for surface enhanced Raman scattering. J. Food Meas. Charact. 2020, 14, 658–667. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Tang, C. Electrochemical synthesis and deposition of surface-enhanced Raman scattering-active silver microstructures on a screen-printed carbon electrode. J. Phys. Chem. C 2015, 119, 24865–24874. [Google Scholar] [CrossRef]
- You, Y.-H.; Lin, Y.-W.; Chen, C.-Y. Surface-enhanced Raman scattering-active desert-rose-like Ag mesoparticles prepared using cyclic voltammetric methods. RSC Adv. 2015, 5, 93293–93300. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zou, S.; Zhang, H. Electrodeposition of Ag nanodendrites SERS substrates for detection of malachite green. Microchem J. 2019, 150, 104127. [Google Scholar] [CrossRef]
- Wicaksono, W.P.; Dang, H.; Lee, S.; Choo, J. Electrochemical surface-enhanced Raman spectroscopy analysis of malachite green on gold substrates. Appl. Surf. Sci. 2024, 649, 159163. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, L.; Wu, Y.; Chen, Y.; Yan, J.; Zhu, W.; Tan, X.; Wang, Q. Fabrication of sea urchin-like Au@SiO2 nanoparticles SERS substrate for the determination of malachite green in tilapia. Vib. Spectrosc. 2022, 118, 103319. [Google Scholar] [CrossRef]
- Huang, H.J.; Shiao, M.-H.; Lin, Y.-W.; Lin, B.-J.; Su, J.; Lin, Y.-S.; Chang, H.-W. Au@Ag dendritic nanoforests for surface-enhanced Raman scattering sensing. Nanomaterials 2021, 11, 1736. [Google Scholar] [CrossRef] [PubMed]
- Shiao, M.-H.; Wu, T.; Huang, H.J.; Peng, C.-Y.; Lin, Y.-S.; Lai, T.-Y.; Lin, Y.-W. Dendritic forest-like Ag nanostructures prepared using fluoride-assisted galvanic replacement reaction for SERS applications. Nanomaterials 2021, 11, 1359. [Google Scholar] [CrossRef] [PubMed]
- Kitahama, Y.; Itoh, T.; Aoyama, J.-i.; Nishikata, K.; Ozaki, Y. SERRS fiber probe: Fabrication of silver nanoparticles at the aperture of an optical fiber used for SNOM. Chem. Commun. 2009, 43, 6563–6565. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-C.; Lai, Y.-S.; Tsai, C.-M.; Kong, Y.-T.; Lee, C.-I.; Huang, C.-L. One-pot synthesis of monodispersed silver nanodecahedra with optimal SERS activities using seedless photo-assisted citrate reduction method. J. Phys. Chem. C 2012, 116, 24292–24300. [Google Scholar] [CrossRef]
- Ciou, S.-H.; Cao, Y.-W.; Huang, H.-C.; Su, D.-Y.; Huang, C.-L. SERS enhancement factors studies of silver nanoprism and spherical nanoparticle colloids in the presence of bromide ions. J. Phys. Chem. C 2009, 113, 9520–9525. [Google Scholar] [CrossRef]

| Substrate | Linear Range (μM) | R2 | LOD (μM) | EF Value |
|---|---|---|---|---|
| Ag1FTO | 4.0–10.0 | 0.97 | 0.52 | 4.52 × 104 |
| Ag2FTO | 0.8–10.0 | 0.97 | 0.21 | 6.15 × 104 |
| B-Ag-Ag1FTO | 0.1–0.8 | 0.92 | 0.07 | 2.56 × 105 |
| B-Ag-Ag2FTO | 0.1–1.0 | 0.98 | 0.02 | 2.79 × 105 |
| Spiked (μM) | Detected (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|
| 0.20 | 0.18 | 90.0 | 4.3 |
| 0.40 | 0.43 | 107.5 | 6.3 |
| 0.60 | 0.63 | 105.0 | 5.3 |
| 0.80 | 0.78 | 97.5 | 3.9 |
| 1.00 | 1.10 | 110.0 | 5.5 |
| Substrate | Linear Range (M) | LOD (M) | Ref. |
|---|---|---|---|
| Fe3O4@Au MCS | 1.0 × 10−7–1.0 × 10−3 | 1.0 × 10−7 | [21] |
| Au/cicada wing | 1.0 × 10−7–1.0 × 10−3 | 1.0 × 10−7 | [22] |
| AgNCs | 5.0 × 10−7–5.0 × 10−4 | 2.6 × 10−7 | [23] |
| Paper-based Au/AgNPs | 3.9 × 10−8–1.0 × 10−5 | 4.3 × 10−9 | [24] |
| AuNPs–GO | 2.7 × 10−11–2.7 × 10−8 | 2.7 × 10−11 | [25] |
| B-Ag-Ag2FTO | 1.0 × 10−7–1.0 × 10−6 | 2.0 × 10−8 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Chen, Y.-T.; Chang, C.-T.; Ting, C.Y.; Arta, Y.; Wu, M.-Y.; Wu, T.; Lin, Y.-S.; Lin, Y.-W. Combined Electrochemical Deposition and Photo-Reduction to Fabricate SERS-Active Silver Substrates: Characterization and Application for Malachite Green Detection in Aquaculture Water. Nanomaterials 2024, 14, 1226. https://doi.org/10.3390/nano14141226
Li Y-X, Chen Y-T, Chang C-T, Ting CY, Arta Y, Wu M-Y, Wu T, Lin Y-S, Lin Y-W. Combined Electrochemical Deposition and Photo-Reduction to Fabricate SERS-Active Silver Substrates: Characterization and Application for Malachite Green Detection in Aquaculture Water. Nanomaterials. 2024; 14(14):1226. https://doi.org/10.3390/nano14141226
Chicago/Turabian StyleLi, Yu-Xuan, Yi-Ting Chen, Cheng-Tse Chang, Chao Yi (Anso) Ting, Yaumalika Arta, Mei-Yao Wu, Tsunghsueh Wu, Yu-Shen Lin, and Yang-Wei Lin. 2024. "Combined Electrochemical Deposition and Photo-Reduction to Fabricate SERS-Active Silver Substrates: Characterization and Application for Malachite Green Detection in Aquaculture Water" Nanomaterials 14, no. 14: 1226. https://doi.org/10.3390/nano14141226
APA StyleLi, Y.-X., Chen, Y.-T., Chang, C.-T., Ting, C. Y., Arta, Y., Wu, M.-Y., Wu, T., Lin, Y.-S., & Lin, Y.-W. (2024). Combined Electrochemical Deposition and Photo-Reduction to Fabricate SERS-Active Silver Substrates: Characterization and Application for Malachite Green Detection in Aquaculture Water. Nanomaterials, 14(14), 1226. https://doi.org/10.3390/nano14141226










