A Review of Plant-Mediated ZnO Nanoparticles for Photodegradation and Antibacterial Applications
Abstract
1. Introduction
2. ZnO NPs Properties and Their Importance
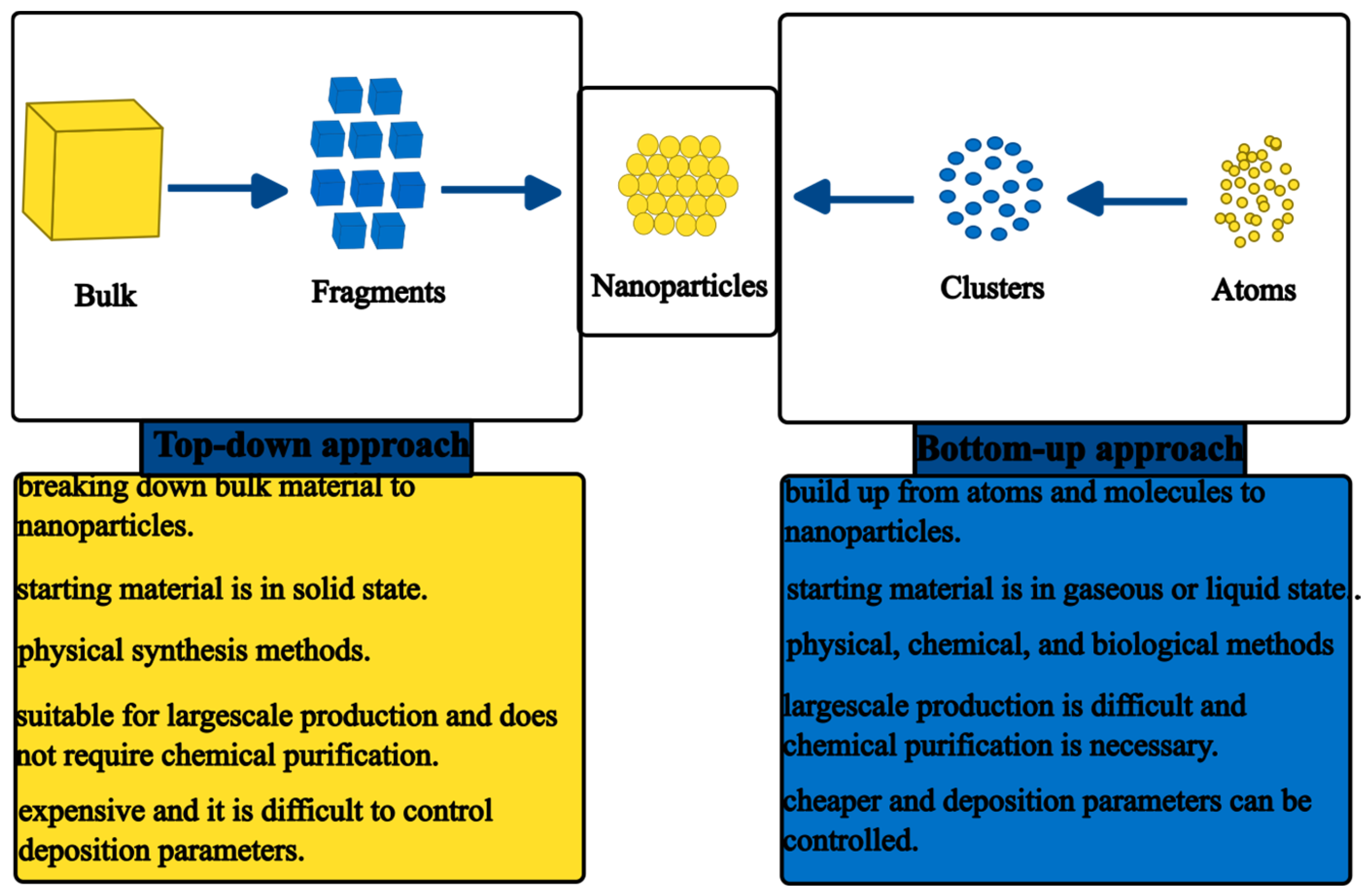
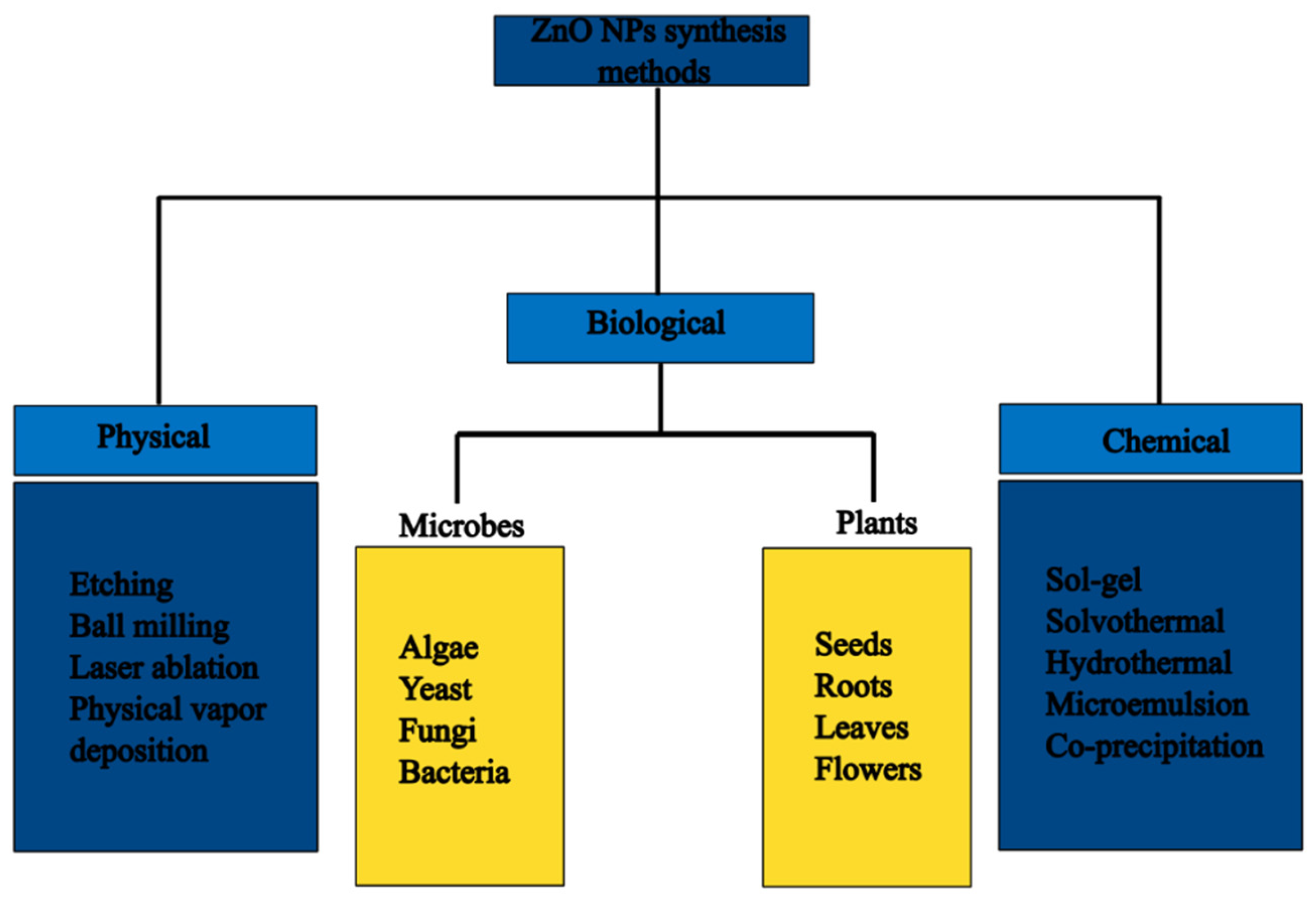
3. Synthesis of ZnO NPs
3.1. Physical Synthesis Methods of ZnO NPs
3.2. Chemical Synthesis Methods of ZnO NPs
3.3. Green Synthesis
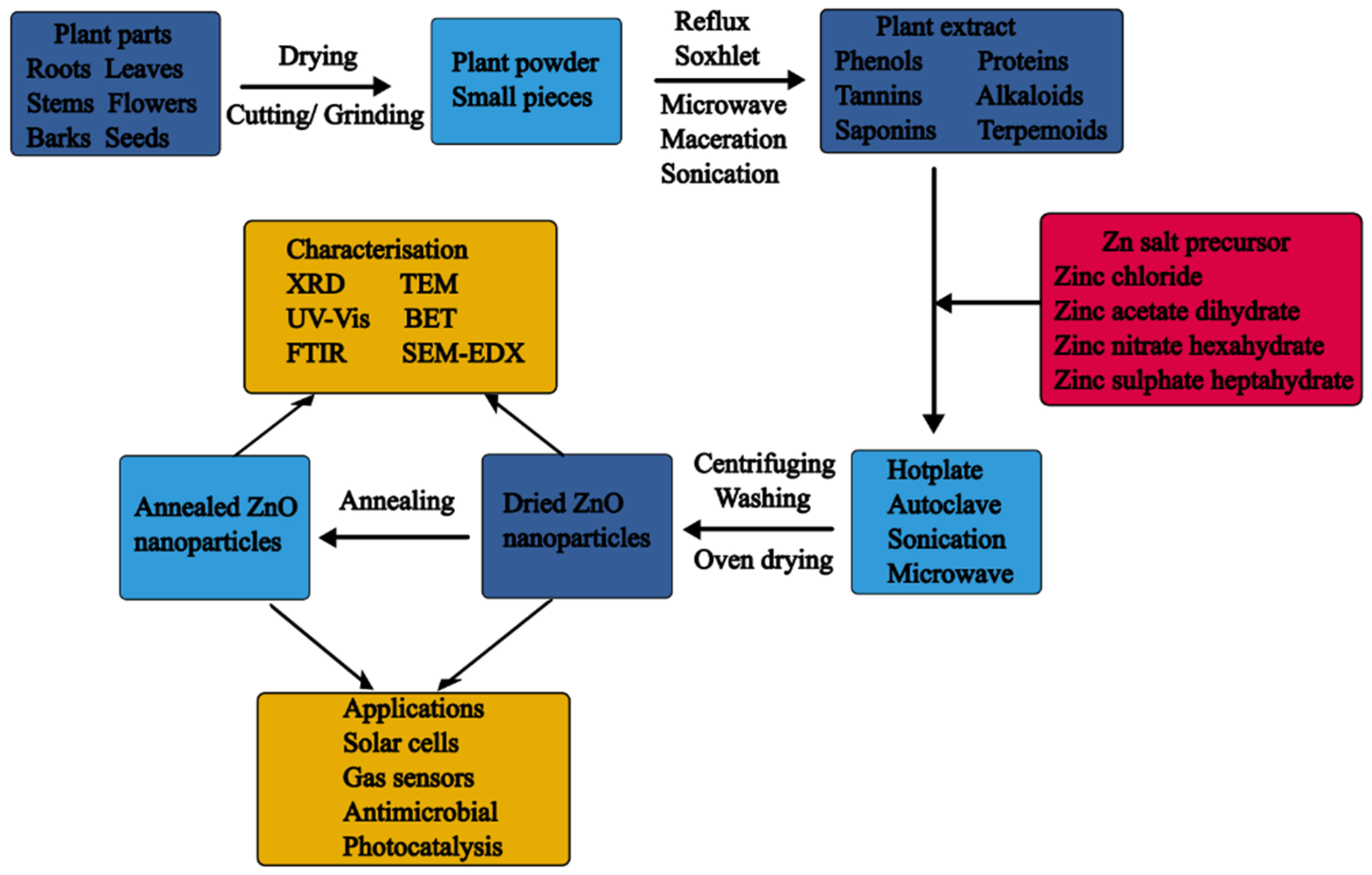
3.4. Plant-Mediated Synthesis
Plant-Mediated Synthesis of ZnO NPs
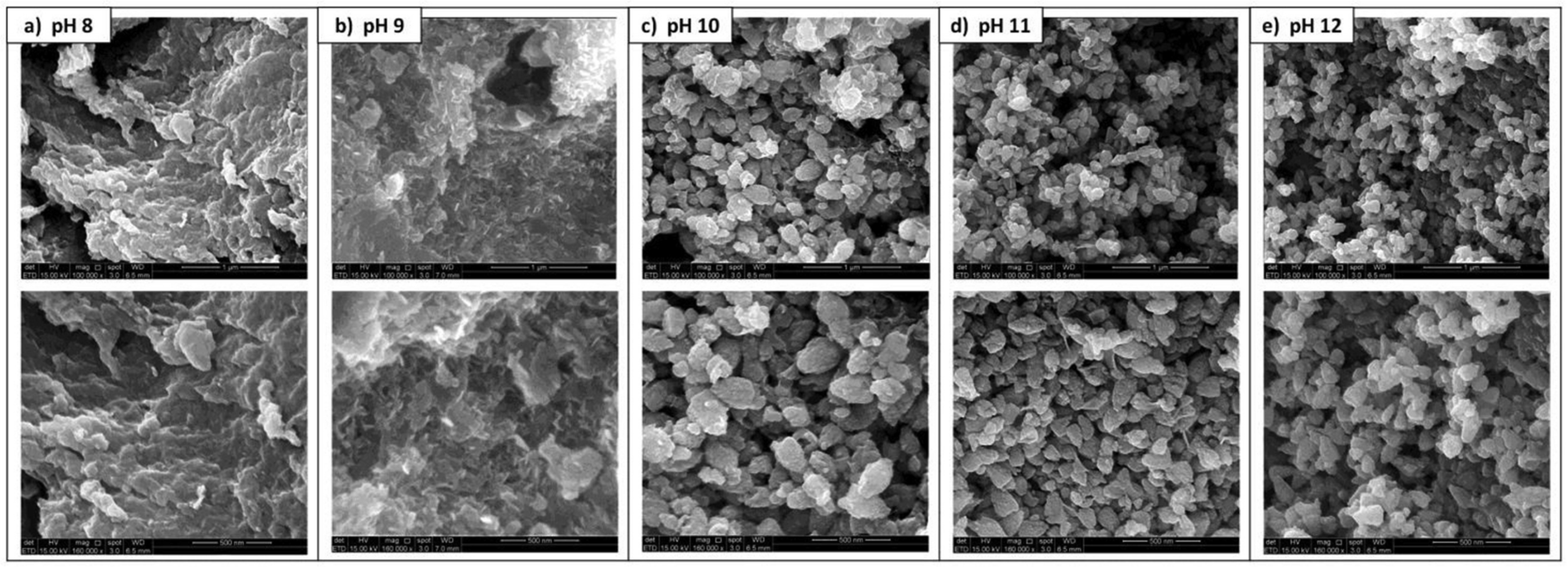
3.5. Influence of Synthesis Parameters on the Formation and the Properties of Plant-Mediated ZnO NPs
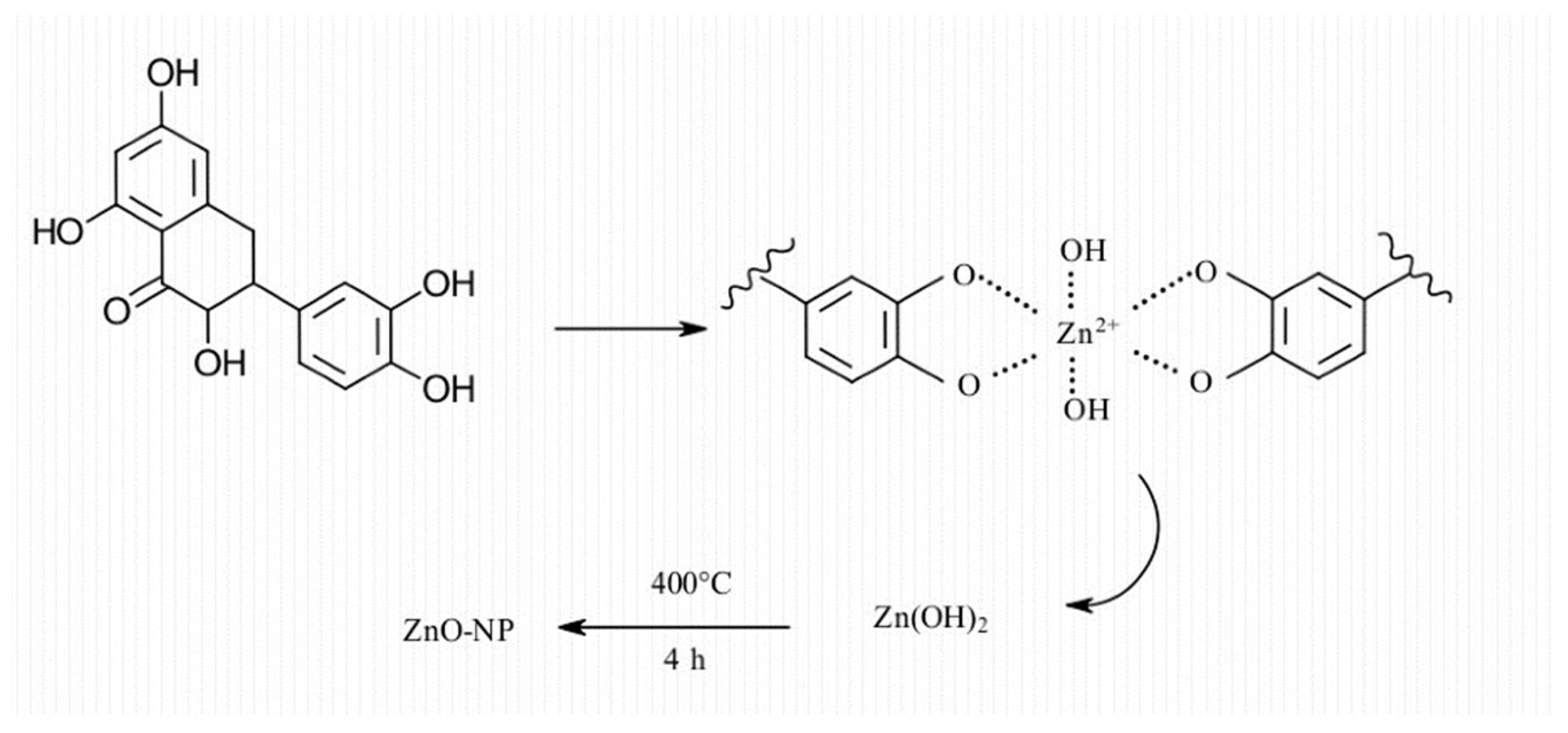
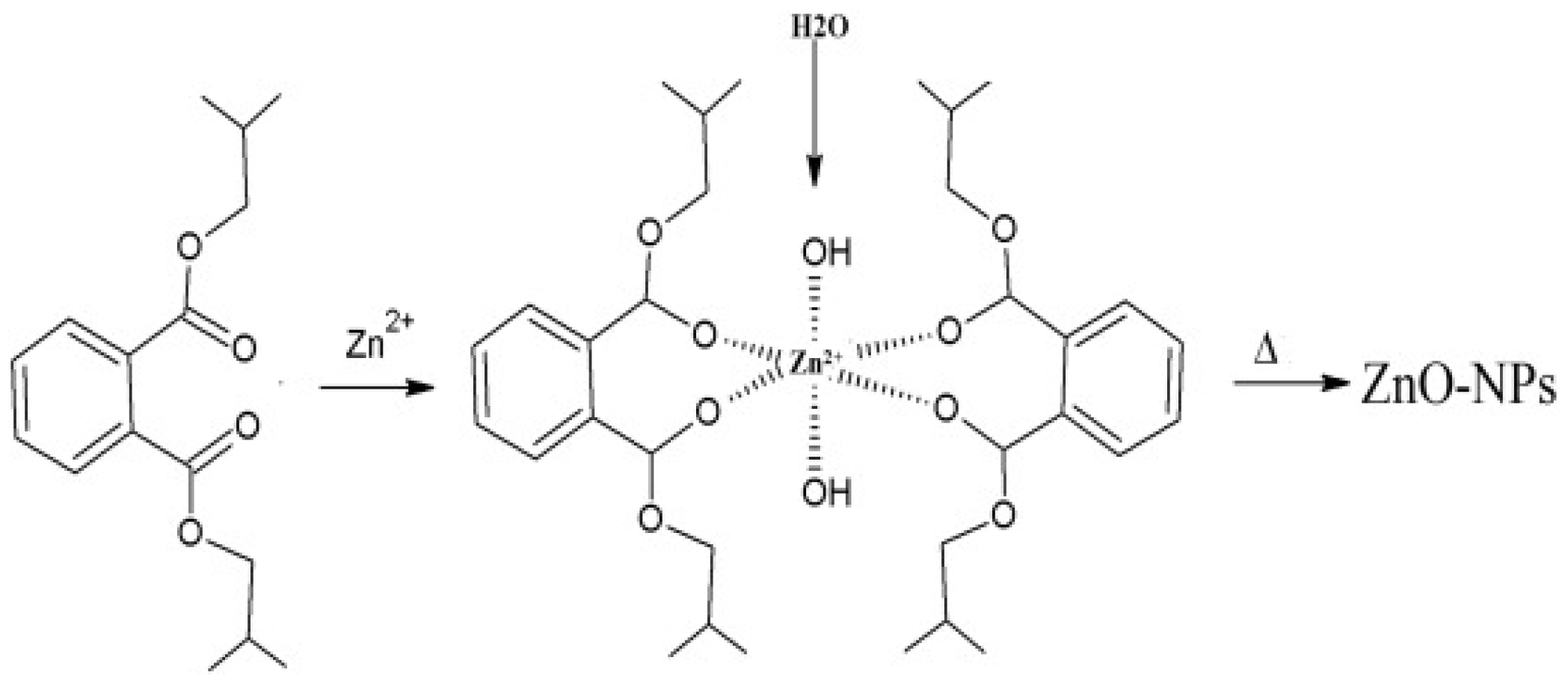
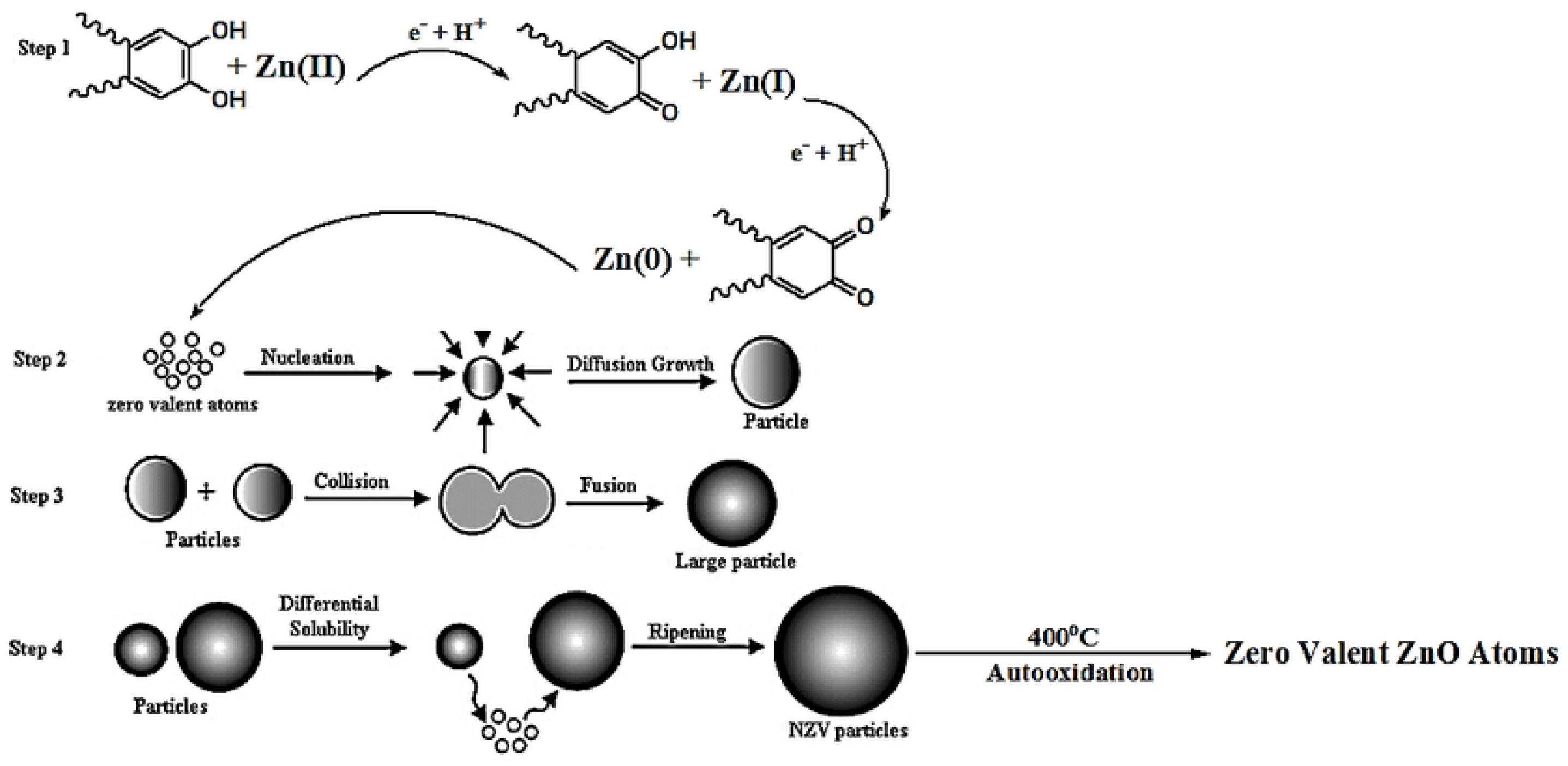
3.6. Mechanism of Formation of ZnO NPs
4. Applications
4.1. Photocatalysis
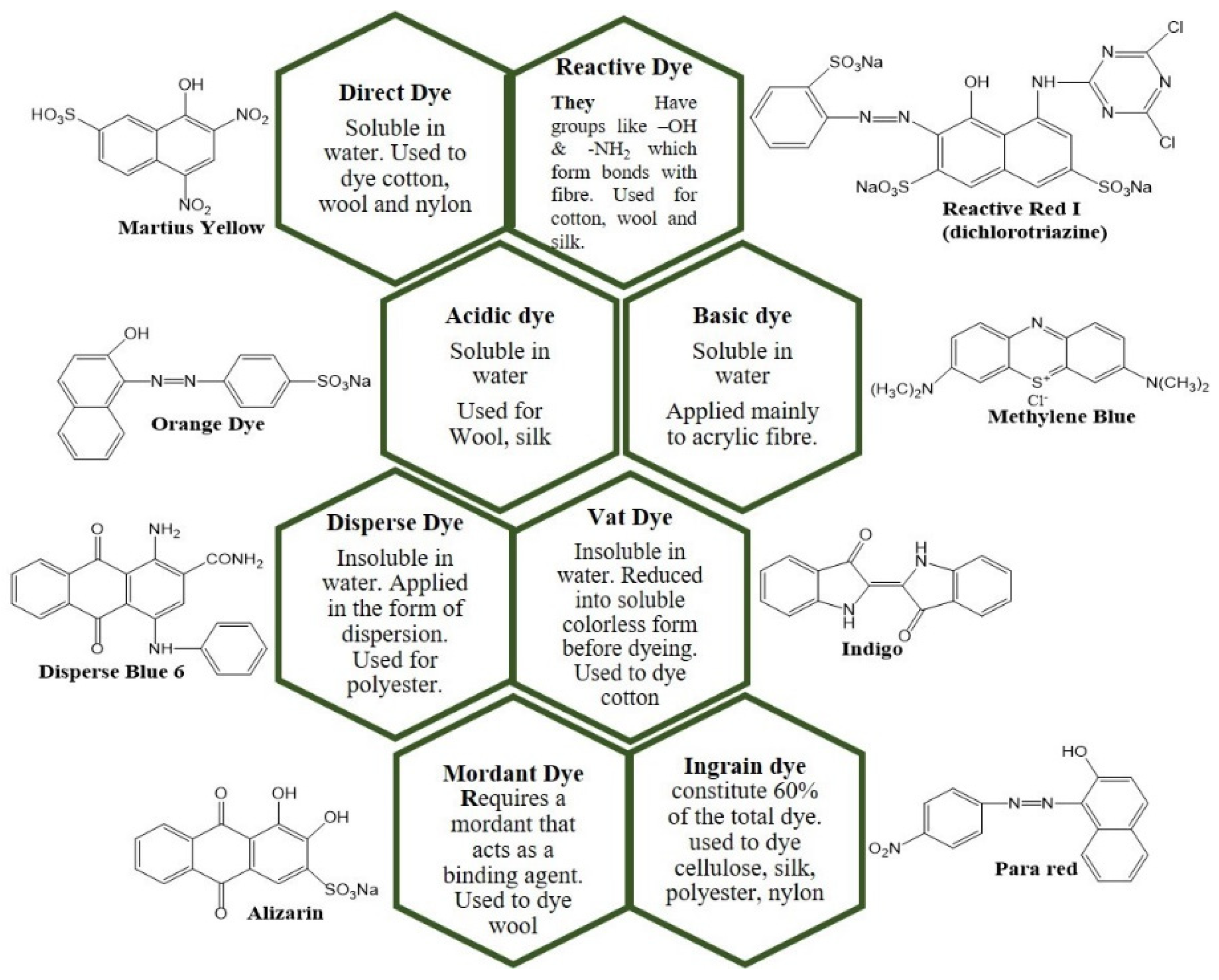
4.1.1. Dyes and Wastewater Treatment Technologies
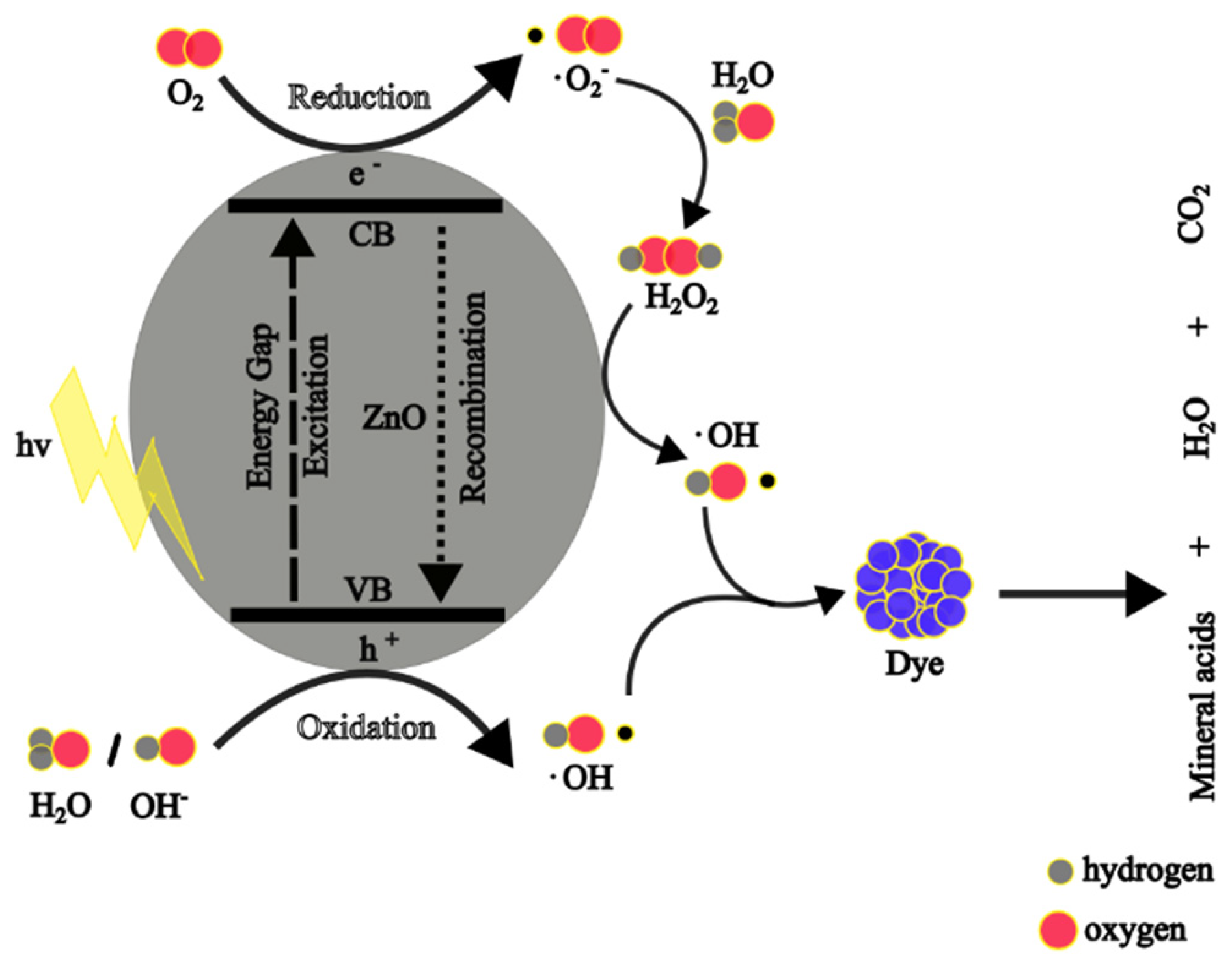
4.1.2. Photocatalytic Mechanism of Zn NPs
4.1.3. Photocatalysis Activity of Plant-Mediated ZnO NPs
4.2. Antibacterial
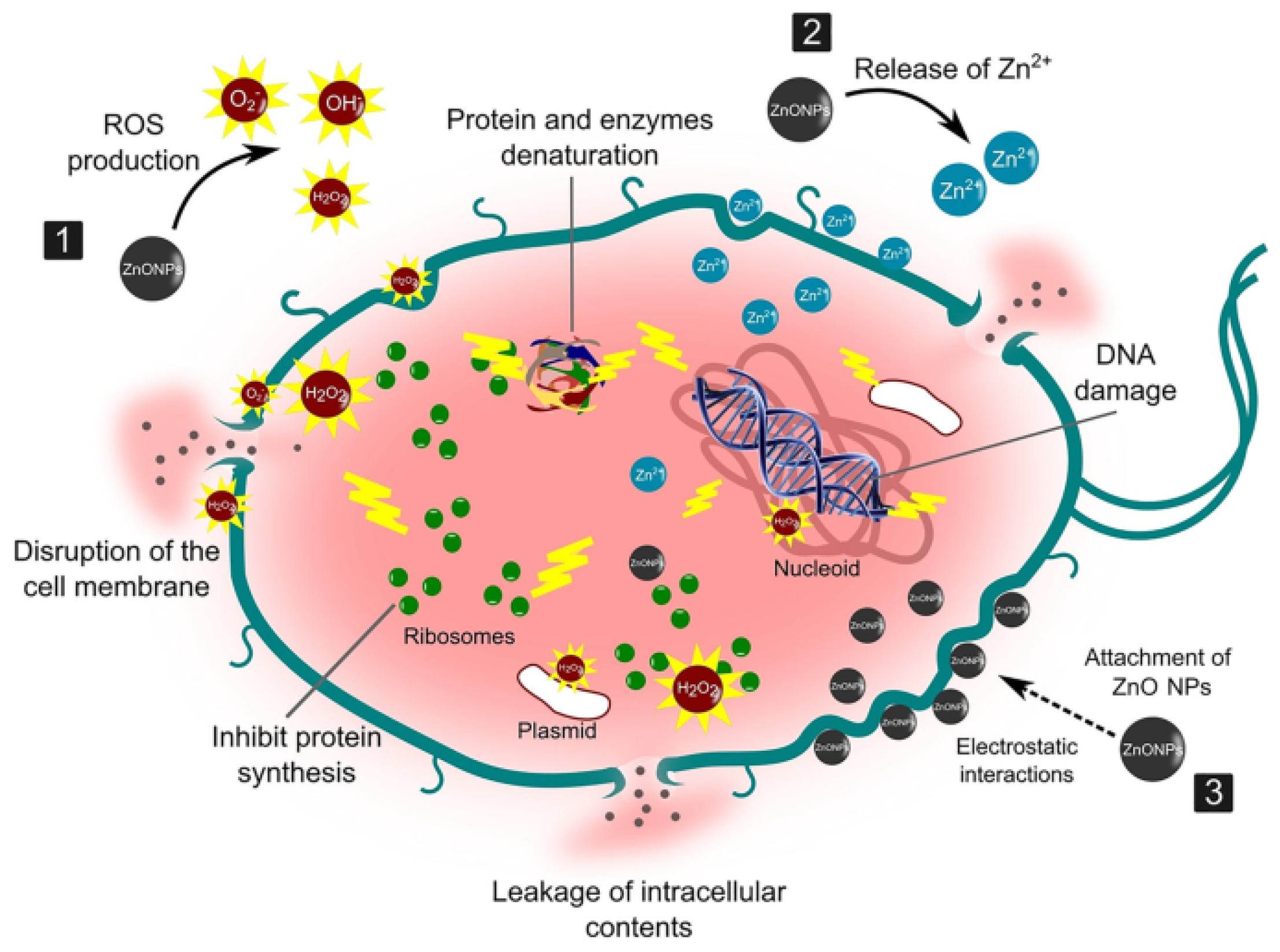
4.2.1. Antibacterial Mechanism of ZnO NPs
4.2.2. Antibacterial Activity of Plant-Mediated ZnO NPs
5. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of Green Nanoparticles for Energy, Biomedical, Environmental, Agricultural, and Food Applications: A Review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Prasad, A.R.; Williams, L.; Garvasis, J.; Shamsheera, K.O.; Basheer, S.M.; Kuruvilla, M.; Joseph, A. Applications of Phytogenic ZnO Nanoparticles: A Review on Recent Advancements. J. Mol. Liq. 2021, 331, 115805. [Google Scholar] [CrossRef]
- Hamed, R.; Obeid, R.Z.; Abu-Huwaij, R. Plant Mediated-Green Synthesis of Zinc Oxide Nanoparticles: An Insight into Biomedical Applications. Nanotechnol. Rev. 2023, 12, 20230112. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Hashem, A.H.; Elhussieny, N.I.; Saied, E. Green Biosynthesis of Zinc Oxide Nanoparticles Using Pluchea Indica Leaf Extract: Antimicrobial and Photocatalytic Activities. Molecules 2023, 28, 4679. [Google Scholar] [CrossRef] [PubMed]
- Dangana, R.S.; George, R.C.; Agboola, F.K. The Biosynthesis of Zinc Oxide Nanoparticles Using Aqueous Leaf Extracts of Cnidoscolus Aconitifolius and Their Biological Activities. Green Chem. Lett. Rev. 2023, 16, 2169591. [Google Scholar] [CrossRef]
- Gamedze, N.P.; Mthiyane, D.M.N.; Mavengahama, S.; Singh, M.; Onwudiwe, D.C. Biosynthesis of ZnO Nanoparticles Using the Aqueous Extract of Mucuna Pruriens (Utilis): Structural Characterization, and the Anticancer and Antioxidant Activities. Chem. Afr. 2024, 7, 219–228. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Krishnamoorthy, N.; Eldesoky, G.E.; Wabaidur, S.M.; Islam, M.A.; Dhanusuraman, R.; Ponnusamy, V.K. One-Pot Green Synthesis of ZnO Nanoparticles Using Scoparia Dulcis Plant Extract for Antimicrobial and Antioxidant Activities. Appl. Nanosci. 2022, 13, 6093–6103. [Google Scholar] [CrossRef]
- Shashanka, R.; Esgin, H.; Yilmaz, V.M.; Caglar, Y. Fabrication and Characterization of Green Synthesized ZnO Nanoparticle Based Dye-Sensitized Solar Cells. J. Sci. Adv. Mater. Devices 2020, 5, 185–191. [Google Scholar] [CrossRef]
- Acharya, T.R.; Lamichhane, P.; Wahab, R.; Chaudhary, D.K.; Shrestha, B.; Joshi, L.P.; Kaushik, N.K.; Choi, E.H. Study on the Synthesis of Zno Nanoparticles Using Azadirachta indica Extracts for the Fabrication of a Gas Sensor. Molecules 2021, 26, 7685. [Google Scholar] [CrossRef]
- Saini, M.; Yadav, S.; Rani, N.; Mushtaq, A.; Rawat, S.; Saini, K.; Maity, D. Biosynthesized Zinc Oxide Nanoparticles Using Seed and Bark Extract of Azadirachta indica for Antibacterial, Photocatalytic and Supercapacitor Applications. Mater. Sci. Eng. B 2022, 282, 115789. [Google Scholar] [CrossRef]
- Dönmez, S. Green Synthesis of Zinc Oxide Nanoparticles Using Zingiber Officinale Root Extract and Their Applications in Glucose Biosensor. El-Cezeri J. Sci. Eng. 2020, 7, 1191–1200. [Google Scholar] [CrossRef]
- Tanwar, N.; Dhiman, V.; Kumar, S.; Kondal, N. Plant Extract Mediated ZnO-NPs as Photocatalyst for Dye Degradation: An Overview. Mater. Today Proc. 2021, 48, 1401–1406. [Google Scholar] [CrossRef]
- Kavitha, A.; Doss, A.; Praveen Pole, R.P.; Pushpa Rani, T.P.K.; Prasad, R.; Satheesh, S. A Mini Review on Plant-Mediated Zinc Oxide Nanoparticles and Their Antibacterial Potency. Biocatal. Agric. Biotechnol. 2023, 48, 102654. [Google Scholar] [CrossRef]
- Vidya, C.; Prabha, M.N.C.; Raj, M.A.L.A. Green Mediated Synthesis of Zinc Oxide Nanoparticles for the Photocatalytic Degradation of Rose Bengal Dye. Environ. Nanotechnol. Monit. Manag. 2016, 6, 134–138. [Google Scholar] [CrossRef]
- Waseem, S.; Sittar, T.; Kayani, Z.N.; Gillani, S.S.A.; Rafique, M.; Asif Nawaz, M.; Masood Shaheen, S.; Assiri, M.A. Plant Mediated Green Synthesis of Zinc Oxide Nanoparticles Using Citrus Jambhiri Lushi Leaves Extract for Photodegradation of Methylene Blue Dye. Phys. B Condens. Matter 2023, 663, 415005. [Google Scholar] [CrossRef]
- Elango, B.; Okram, G.S.; Mathanmohun, M. Pharmacological Applications of Plant-Mediated Synthesized Nanomaterials. Curr. Pharmacol. Rep. 2023, 9, 511–522. [Google Scholar] [CrossRef]
- Al-darwesh, M.Y.; Ibrahim, S.S.; Mohammed, M.A. A Review on Plant Extract Mediated Green Synthesis of Zinc Oxide Nanoparticles and Their Biomedical Applications. Results Chem. 2024, 7, 101368. [Google Scholar] [CrossRef]
- Al-darwesh, M.Y.; Ibrahim, S.S.; Faiad Naief, M.; Mishaal Mohammed, A.; Chebbi, H. Synthesis and Characterizations of Zinc Oxide Nanoparticles and Its Ability to Detect O2 and NH3 Gases. Results Chem. 2023, 6, 101064. [Google Scholar] [CrossRef]
- Nahhas, A.M. Introductory Chapter: Overview of ZnO Based Nano Materials and Devices. In Zinc Oxide Based Nano Materials and Devices; Nahhas, A., Ed.; Intechopen: Rijeka, Croatia, 2019. [Google Scholar]
- Sonia, S.S.; Linda Jeeva Kumari, H.; Ruckmani, R.K.; Sivakumar, S.M. Antimicrobial and Antioxidant Potentials of Biosynthesized Colloidal Zinc Oxide Nanoparticles for a Fortified Cold Cream Formulation: A Potent Nanocosmeceutical Application. Mater. Sci. Eng. C 2017, 79, 581–589. [Google Scholar] [CrossRef]
- Dutta, G.; Sugumaran, A. Bioengineered Zinc Oxide Nanoparticles: Chemical, Green, Biological Fabrication Methods and Its Potential Biomedical Applications. J. Drug Deliv. Sci. Technol. 2021, 66, 102853. [Google Scholar] [CrossRef]
- Pradeeswari, K.; Venkatesan, A.; Pandi, P.; Karthik, K.; Hari Krishna, K.V.; Mohan Kumar, R. Study on the Electrochemical Performance of ZnO Nanoparticles Synthesized via Non-Aqueous Sol-Gel Route for Supercapacitor Applications. Mater. Res. Express 2019, 6, 105525. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphoslogies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Farahbod, F.; Farahmand, S. Empirical Investigation of Heating and Kinematic Performance of ZnO Nano Fluid in a Heat Pipe. J. Nanofluids 2017, 6, 128–135. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Bakranova, D.; Nagel, D. ZnO for Photoelectrochemical Hydrogen Generation. Clean. Technol. 2023, 5, 1248–1268. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Asiedu, T.; Agyei-Tuffour, B.; Nyankson, E.; Obada, D.; Mwabora, J.M. Photocatalytic Degradation of Rhodamine Dyes Using Zinc Oxide Nanoparticles. Mater. Today Proc. 2021, 38, 809–815. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Zhou, X.Q.; Hayat, Z.; Zhang, D.D.; Li, M.Y.; Hu, S.; Wu, Q.; Cao, Y.F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Singh, J.P.; Kumar, M.; Sharma, A.; Pandey, G.; Chae, H.; Lee, S. Bottom-Up and Top-Down Approaches for MgO. In Sonochemical Reactions; Karakuş, S., Ed.; Intechopen: Rijeka, Croatia, 2020. [Google Scholar]
- Al Jahdaly, B.A.; Elsadek, M.F.; Ahmed, B.M.; Farahat, M.F.; Taher, M.M.; Khalil, A.M. Outstanding Graphene Quantum Dots from Carbon Source for Biomedical and Corrosion Inhibition Applications: A Review. Sustainability 2021, 13, 2127. [Google Scholar] [CrossRef]
- Jin, S.E.; Jin, H.E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef]
- Gharpure, S.; Ankamwar, B. Synthesis and Antimicrobial Properties of Zinc Oxide Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 5977–5996. [Google Scholar] [CrossRef]
- Bloch, K.; Pardesi, K.; Satriano, C.; Ghosh, S. Bacteriogenic Platinum Nanoparticles for Application in Nanomedicine. Front. Chem. 2021, 9, 624344. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.K.; Thomas, S.; Thomas, S. Methods for Synthesis of Nanoparticles and Fabrication of Nanocomposites. In Synthesis of Inorganic Nanomaterials: Advances and Key Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. ISBN 9780081019757. [Google Scholar]
- Khan, F.A. Synthesis of Nanomaterials: Methods & Technology. In Applications of Nanomaterials in Human Health; Springer: Singapore, 2020; pp. 15–21. ISBN 9789811548024. [Google Scholar]
- Imran, H.J.; Hubeatir, K.A.; Aadim, K.A.; Abd, D.S. Preparation Methods and Classification Study of Nanomaterial: A Review. In Proceedings of the Journal of Physics: Conference Series; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 1818. [Google Scholar]
- Ismail, R.A.; Ali, A.K.; Ismail, M.M.; Hassoon, K.I. Preparation and Characterization of Colloidal ZnO Nanoparticles Using Nanosecond Laser Ablation in Water. Appl. Nanosci. 2011, 1, 45–49. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Jie Ying, A.N.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and Strategies for the Synthesis of Diverse Nanoparticles and Their Applications: A Comprehensive Overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Salah, N.; Habib, S.S.; Khan, Z.H.; Memic, A.; Azam, A.; Alarfaj, E.; Zahed, N.; Al-Hamedi, S. High-Energy Ball Milling Technique for ZnO Nanoparticles as Antibacterial Material. Int. J. Nanomed. 2011, 6, 863–869. [Google Scholar] [CrossRef]
- Ayyub, P.; Chandra, R.; Taneja, P.; Sharma, A.K.; Pinto, R. Synthesis of Nanocrystalline Material by Sputtering and Laser Ablation at Low Temperatures. Appl. Phys. A Mater. Sci. Process 2001, 73, 67–73. [Google Scholar] [CrossRef]
- Son, H.H.; Seo, G.H.; Jeong, U.; Shin, D.Y.; Kim, S.J. Capillary Wicking Effect of a Cr-Sputtered Superhydrophilic Surface on Enhancement of Pool Boiling Critical Heat Flux. Int. J. Heat Mass Transf. 2017, 113, 115–128. [Google Scholar] [CrossRef]
- Wender, H.; Migowski, P.; Feil, A.F.; Teixeira, S.R.; Dupont, J. Sputtering Deposition of Nanoparticles onto Liquid Substrates: Recent Advances and Future Trends. Coord. Chem. Rev. 2013, 257, 2468–2483. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, Q.; Shao, Z. Magnetron Sputtering for ZnO:Ga Scintillation Film Production and Its Application Research Status in Nuclear Detection. Crystals 2019, 9, 263. [Google Scholar] [CrossRef]
- Sun, D.; Tian, S.; Yin, C.; Chen, F.; Xie, J.; Huang, C.; Li, C. Thin Film Deposition Techniques in Surface Engineering Strategies for Advanced Lithium-Ion Batteries. Coatings 2023, 13, 505. [Google Scholar] [CrossRef]
- Gridchin, V.O.; Kotlyar, K.P.; Vershinin, A.V.; Kryzhanovskaya, N.V.; Pirogov, E.V.; Semenov, A.A.; Belyavskiy, P.Y.; Nashchekin, A.V.; Cirlin, G.E.; Soshnikov, I.P. ZnO Thin Films Synthesis by RF Magnetron Sputtering Deposition. J. Phys. Conf. Ser. 2019, 1410, 012054. [Google Scholar] [CrossRef]
- Oke, J.A.; Jen, T.C. Atomic Layer Deposition and Other Thin Film Deposition Techniques: From Principles to Film Properties. J. Mater. Res. Technol. 2022, 21, 2481–2514. [Google Scholar] [CrossRef]
- Soonmin, H. Recent Advances in the Growth and Characterizations of SILAR-Deposited Thin Films. Appl. Sci. 2022, 12, 8184. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Abd-Elrahman, N.K. Physical Methods for Preparation of Nanomaterials, Their Characterization and Applications: A Review. J. Umm Al-Qura Univ. Appl. Sci. 2024. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2022, 3, 801620. [Google Scholar] [CrossRef]
- Vignesh, K.; Nair, A.S.; Udhayakeerthana, C.; Kalaivani, T. Synthesis and Characterization ZnO Nanoparticles Using Sol-Gel Method and Their Antibacterial Study. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1219, 012019. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A Review of Photocatalysts Prepared by Sol-Gel Method for VOCs Removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Zahra, S.; Bukhari, H.; Qaisar, S.; Sheikh, A.; Amin, A. Synthesis of Nanosize Zinc Oxide through Aqueous Sol–Gel Route in Polyol Medium. BMC Chem. 2022, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Sol Gel Synthesis of Zinc Oxide Nanoparticles and Their Application as Nano-Composite Electrode Material for Supercapacitor. J. Mol. Struct. 2020, 1220, 128654. [Google Scholar] [CrossRef]
- Kashyap, A.; Singh, N.K.; Soni, M.; Soni, A. Deposition of Thin Films by Chemical Solution-Assisted Techniques. In Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 79–117. ISBN 9780128197189. [Google Scholar]
- Yoshimura, M.; Byrappa, K. Hydrothermal Processing of Materials: Past, Present and Future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Droepenu, E.K.; Wee, B.S.; Chin, S.F.; Kok, K.Y.; Maligan, M.F. Zinc Oxide Nanoparticles Synthesis Methods and Its Effect on Morphology: A Review. Biointerface Res. Appl. Chem. 2022, 12, 4261–4292. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticles via Solvothermal and Hydrothermal Methods. In Handbook of Nanoparticles; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 1–28. [Google Scholar]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. A Review of Methods for Synthesis of Nanostructured Metals with Emphasis on Iron Compounds. Chem. Pap. 2007, 61, 151–170. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Liu, Y.; Fan, Y.; Dang, F.; Qiu, Y.; Zhou, H.; Wang, W.; Liu, Y. Solvothermal Synthesis of Zno Nanoparticles for Photocatalytic Degradation of Methyl Orange and P-Nitrophenol. Water 2021, 13, 3224. [Google Scholar] [CrossRef]
- Krishna, J.; Perumal, A.S.; Khan, I.; Chelliah, R.; Wei, S.; Swamidoss, C.M.A.; Oh, D.H.; Bharathiraja, B. Synthesis of Nanomaterials for Biofuel and Bioenergy Applications. In Nanomaterials: Application in Biofuels and Bioenergy Production Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 97–165. ISBN 9780128224014. [Google Scholar]
- Preeti, S.; Vijay, N. Synthesis of Nano-ZnO by Chemical Reduction Method and Their Micro Biocide Activity against Bacterial Skin Pathogens. Int. J. Life Sci. 2017, 5, 233–240. [Google Scholar]
- Zhang, Q.L.; Yang, Z.M.; Ding, B.J.; Lan, X.Z.; Guo, Y.J. Preparation of Copper Nanoparticles by Chemical Reduction Method Using Potassium Borohydride. Trans. Nonferrous Met. Soc. China Engl. Ed. 2010, 20, s240–s244. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Experimental and Computational Study of Metal Oxide Nanoparticles for the Photocatalytic Degradation of Organic Pollutants: A Review. RSC Adv. 2023, 13, 18404–18442. [Google Scholar] [CrossRef]
- Kusdianto, K.; Nugraha, D.F.; Sekarnusa, A.; Madhania, S.; Machmudah, S.; Winardi, S. ZnO-TiO2 Nanocomposite Materials: Fabrication and Its Applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012024. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Kuznetsov, N.T.; Meshalkin, V.P.; Salerno, M.; Fabiano, B. Systematical Analysis of Chemical Methods in Metal Nanoparticles Synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. [Google Scholar] [CrossRef]
- Rahimi, H.-R.; Doostmohammadi, M. Nanoparticle Synthesis, Applications, and Toxicity. In Applications in Nanotechnology; Stoytcheva, M., Zlatev, R., Eds.; Intechopen: Rijeka, Croatia, 2019. [Google Scholar]
- Nasrollahzadeh, M.; Sajjadi, M. An Introduction to Green Chemistry. In Biopolymer-Based Metal Nanoparticle Chemistry for Sustainable Applications: Volume 1: Classification, Properties and Synthesis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–22. ISBN 9780128221082. [Google Scholar]
- Annu, A.A.; Ahmed, S. Green Synthesis of Metal, Metal Oxide Nanoparticles, and Their Various Applications. In Handbook of Ecomaterials; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 4, pp. 2281–2325. ISBN 9783319682556. [Google Scholar]
- Mohd Yusof, H.; Abdul Rahman, N.; Mohamad, R.; Hasanah Zaidan, U.; Samsudin, A.A. Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An in Vitro Study. Animals 2021, 11, 2093. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, P.; Dahiya, P.; Dang, A.S.; Suneja, P. Biogenic Synthesis of Zinc Nanoparticles, Their Applications, and Toxicity Prospects. Front. Microbiol. 2022, 13, 824427. [Google Scholar] [CrossRef]
- Zikalala, N.; Matshetshe, K.; Parani, S.; Oluwafemi, O.S. Biosynthesis Protocols for Colloidal Metal Oxide Nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 288–299. [Google Scholar] [CrossRef]
- Gebre, S.H. Bio-Inspired Synthesis of Metal and Metal Oxide Nanoparticles: The Key Role of Phytochemicals. J. Clust. Sci. 2022, 34, 665–704. [Google Scholar] [CrossRef]
- Barani, M.; Masoudi, M.; Mashreghi, M.; Makhdoumi, A.; Eshghi, H. Cell-Free Extract Assisted Synthesis of ZnO Nanoparticles Using Aquatic Bacterial Strains: Biological Activities and Toxicological Evaluation. Int. J. Pharm. 2021, 606, 120878. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G. New Frontiers in the Biosynthesis of Metal Oxide Nanoparticles and Their Environmental Applications: An Overview. SN Appl. Sci. 2019, 1, 928. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Devi Rajeswari, V. Biosynthesis of Zinc Oxide Nanoparticles Using Culture Filtrates of Aspergillus Niger: Antimicrobial Textiles and Dye Degradation Studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant Extract as Reducing Agent in Synthesis of Metallic Nanoparticles: A Review. Adv. Mater. Res. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Devatha, C.P.; Thalla, A.K. Green Synthesis of Nanomaterials. In Synthesis of Inorganic Nanomaterials: Advances and Key Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–184. ISBN 9780081019757. [Google Scholar]
- Chennimalai, M.; Vijayalakshmi, V.; Senthil, T.S.; Sivakumar, N. One-Step Green Synthesis of ZnO Nanoparticles Using Opuntia Humifus Fruit Extract and Their Antibacterial Activities. Mater. Today Proc. 2021, 47, 1842–1846. [Google Scholar] [CrossRef]
- Sathappan, S.; Kirubakaran, N.; Gunasekaran, D.; Gupta, P.K.; Verma, R.S.; Sundaram, J. Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) Using Cissus Quadrangularis: Characterization, Antimicrobial and Anticancer Studies. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2021, 91, 289–296. [Google Scholar] [CrossRef]
- Ekennia, A.; Uduagwu, D.; Olowu, O.; Nwanji, O.; Oje, O.; Daniel, B.; Mgbii, S.; Emma-Uba, C. Biosynthesis of Zinc Oxide Nanoparticles Using Leaf Extracts of Alchornea Laxiflora and Its Tyrosinase Inhibition and Catalytic Studies. Micron 2021, 141, 102964. [Google Scholar] [CrossRef]
- Donga, S.; Chanda, S. Caesalpinia Crista Seeds Mediated Green Synthesis of Zinc Oxide Nanoparticles for Antibacterial, Antioxidant, and Anticancer Activities. Bionanoscience 2022, 12, 451–462. [Google Scholar] [CrossRef]
- Algarni, T.S.; Abduh, N.A.Y.; Al Kahtani, A.; Aouissi, A. Photocatalytic Degradation of Some Dyes under Solar Light Irradiation Using ZnO Nanoparticles Synthesized from Rosmarinus Officinalis Extract. Green Chem. Lett. Rev. 2022, 15, 460–473. [Google Scholar] [CrossRef]
- Nazir, A.; Raza, M.; Abbas, M.; Abbas, S.; Ali, A.; Ali, Z.; Younas, U.; Al-Mijalli, S.H.; Iqbal, M. Microwave Assisted Green Synthesis of ZnO Nanoparticles Using Rumex Dentatus Leaf Extract: Photocatalytic and Antibacterial Potential Evaluation. Z. Phys. Chem. 2022, 236, 1203–1217. [Google Scholar] [CrossRef]
- Surendra, B.S.; Swamy, M.M.; Shamala, T.; Rao, S.; Sowmy shree, A.S.; Mallikarjuna swamy, C.; Pramila, S. Development of Enhanced Electrochemical Sensor and Antimicrobial Studies of ZnO NPs Synthesized Using Green Plant Extract. Sens. Int. 2022, 3, 100176. [Google Scholar] [CrossRef]
- Fagier, M.A. Plant-Mediated Biosynthesis and Photocatalysis Activities of Zinc Oxide Nanoparticles: A Prospect towards Dyes Mineralization. J. Nanotechnol. 2021, 2021, 6629180. [Google Scholar] [CrossRef]
- Al-Kordy, H.M.H.; Sabry, S.A.; Mabrouk, M.E.M. Statistical Optimization of Experimental Parameters for Extracellular Synthesis of Zinc Oxide Nanoparticles by a Novel Haloalaliphilic Alkalibacillus Sp.W7. Sci. Rep. 2021, 11, 10924. [Google Scholar] [CrossRef]
- Abdol Aziz, R.A.; Abd Karim, S.F.; Ibrahim, U.K.; Sanuddin, N. Precursor Concentration Effect on Physicochemical Properties of Zinc Oxide Nanoparticle Synthesized with Banana Peel Extract. Key Eng. Mater. 2019, 797, 262–270. [Google Scholar] [CrossRef]
- Şimşek, T.; Ceylan, A.; Aşkin, G.Ş.; Özcan, Ş. Band Gap Engineering of ZnO Nanocrystallites Prepared via Ball-Milling. Politek. Derg. 2022, 25, 89–94. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The Effect of Shape and Size of ZnO Nanoparticles on Their Antimicrobial and Photocatalytic Activities: A Green Approach. Bull. Mater. Sci. 2020, 43, 20. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green Synthesis of Zinc Oxide Nanoparticles: A Comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Guerrero, A.; Romero, A. Efficient and Sustainable Synthesis of Zinc Salt-Dependent Polycrystal Zinc Oxide Nanoparticles: Comprehensive Assessment of Physicochemical and Functional Properties. Appl. Sci. 2024, 14, 1815. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Abu Bakar, N.H.H.; Abu Bakar, M. Low Temperature Biosynthesis of Crystalline Zinc Oxide Nanoparticles from Musa Acuminata Peel Extract for Visible-Light Degradation of Methylene Blue. Optik 2020, 206, 164279. [Google Scholar] [CrossRef]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective Antimicrobial Activity of Green ZnO Nano Particles of Catharanthus Roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Zeghoud, S.; Hemmami, H.; Ben Seghir, B.; Ben Amor, I.; Kouadri, I.; Rebiai, A.; Messaoudi, M.; Ahmed, S.; Pohl, P.; Simal-Gandara, J. A Review on Biogenic Green Synthesis of ZnO Nanoparticles by Plant Biomass and Their Applications. Mater. Today Commun. 2022, 33, 104747. [Google Scholar] [CrossRef]
- Gherbi, B.; Laouini, S.E.; Meneceur, S.; Bouafia, A.; Hemmami, H.; Tedjani, M.L.; Thiripuranathar, G.; Barhoum, A.; Menaa, F. Effect of PH Value on the Bandgap Energy and Particles Size for Biosynthesis of ZnO Nanoparticles: Efficiency for Photocatalytic Adsorption of Methyl Orange. Sustainability 2022, 14, 11300. [Google Scholar] [CrossRef]
- Mohammadi, F.M.; Ghasemi, N. Influence of Temperature and Concentration on Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Cherry Extract. J. Nanostructure Chem. 2018, 8, 93–102. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A. Green Synthesis: Proposed Mechanism and Factors Influencing the Synthesis of Platinum Nanoparticles. Green Process. Synth. 2020, 9, 386–398. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green Synthesis of Silver Nanoparticles Using Olive Leaf Extract and Its Antibacterial Activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Padalia, H.; Baluja, S.; Chanda, S. Effect of PH on Size and Antibacterial Activity of Salvadora Oleoides Leaf Extract-Mediated Synthesis of Zinc Oxide Nanoparticles. Bionanoscience 2017, 7, 40–49. [Google Scholar] [CrossRef]
- Sarillana, Z.C.; Fundador, E.O.; Fundador, N.G. Synthesis of ZnO Nanoparticles Using Theobroma Cacao L. Pod Husks, and Their Antibacterial Activities against Foodborne Pathogens. Int. Food Res. J. 2021, 28, 102–109. [Google Scholar] [CrossRef]
- Nithya, K.; Kalyanasundharam, S. Effect of Chemically Synthesis Compared to Biosynthesized ZnO Nanoparticles Using Aqueous Extract of C. Halicacabum and Their Antibacterial Activity. OpenNano 2019, 4, 100024. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Luque, P.A.; Gómez-Gutiérrez, C.M.; Nava, O.; Vilchis-Nestor, A.R.; Lugo-Medina, E.; Ranjithkumar, R.; Castro-Beltrán, A. Study on the Effect of the Concentration of Hibiscus Sabdariffa Extract on the Green Synthesis of ZnO Nanoparticles. Results Phys. 2019, 15, 102807. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Effect of Chemically Synthesis Compared to Biosynthesized ZnO-NPs Using Solanum Nigrum Leaf Extract and Their Photocatalytic, Antibacterial and in-Vitro Antioxidant Activity. J. Environ. Chem. Eng. 2020, 8, 103705. [Google Scholar] [CrossRef]
- Mtavangu, S.G.; Machunda, R.L.; van der Bruggen, B.; Njau, K.N. In Situ Facile Green Synthesis of Ag–ZnO Nanocomposites Using Tetradenia Riperia Leaf Extract and Its Antimicrobial Efficacy on Water Disinfection. Sci. Rep. 2022, 12, 15359. [Google Scholar] [CrossRef]
- Basri, H.H.; Talib, R.A.; Sukor, R.; Othman, S.H.; Ariffin, H. Effect of Synthesis Temperature on the Size of ZnO Nanoparticles Derived from Pineapple Peel Extract and Antibacterial Activity of ZnO–Starch Nanocomposite Films. Nanomaterials 2020, 10, 1061. [Google Scholar] [CrossRef]
- Doan Thi, T.U.; Nguyen, T.T.; Thi, Y.D.; Ta Thi, K.H.; Phan, B.T.; Pham, K.N. Green Synthesis of ZnO Nanoparticles Using Orange Fruit Peel Extract for Antibacterial Activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar] [CrossRef]
- Álvarez-Chimal, R.; García-Pérez, V.I.; Álvarez-Pérez, M.A.; Tavera-Hernández, R.; Reyes-Carmona, L.; Martínez-Hernández, M.; Arenas-Alatorre, J.Á. Influence of the Particle Size on the Antibacterial Activity of Green Synthesized Zinc Oxide Nanoparticles Using Dysphania Ambrosioides Extract, Supported by Molecular Docking Analysis. Arab. J. Chem. 2022, 15, 103804. [Google Scholar] [CrossRef]
- Karthik, S.; Siva, P.; Balu, K.S.; Suriyaprabha, R.; Rajendran, V.; Maaza, M. Acalypha Indica–Mediated Green Synthesis of ZnO Nanostructures under Differential Thermal Treatment: Effect on Textile Coating, Hydrophobicity, UV Resistance, and Antibacterial Activity. Adv. Powder Technol. 2017, 28, 3184–3194. [Google Scholar] [CrossRef]
- Kem, A.; Ansari, M.R.; Prathap, P.; Jayasimhadri, M.; Peta, K.R. Eco-Friendly Green Synthesis of Stable ZnO Nanoparticles Using Citrus Limon: X-Ray Diffraction Analysis and Optical Properties. Phys. Scr. 2022, 97, 085814. [Google Scholar] [CrossRef]
- Kebede Urge, S.; Tiruneh Dibaba, S.; Belay Gemta, A. Green Synthesis Method of ZnO Nanoparticles Using Extracts of Zingiber officinale and Garlic Bulb (Allium sativum) and Their Synergetic Effect for Antibacterial Activities. J. Nanomater. 2023, 2023, 7036247. [Google Scholar] [CrossRef]
- Naseer, M.; Aslam, U.; Khalid, B.; Chen, B. Green Route to Synthesize Zinc Oxide Nanoparticles Using Leaf Extracts of Cassia Fistula and Melia Azadarach and Their Antibacterial Potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Del Buono, D.; Di Michele, A.; Costantino, F.; Trevisan, M.; Lucini, L. Biogenic Zno Nanoparticles Synthesized Using a Novel Plant Extract: Application to Enhance Physiological and Biochemical Traits in Maize. Nanomaterials 2021, 11, 1270. [Google Scholar] [CrossRef]
- Chemingui, H.; Missaoui, T.; Mzali, J.C.; Yildiz, T.; Konyar, M.; Smiri, M.; Saidi, N.; Hafiane, A.; Yatmaz, H.C. Facile Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs): Antibacterial and Photocatalytic Activities. Mater. Res. Express 2019, 6, 1050b4. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Bahadoran, A.; Bayat, S.; Rahim, R.A.; Ariff, A.; Saad, W.Z. Effect of Annealing Temperature on Antimicrobial and Structural Properties of Bio-Synthesized Zinc Oxide Nanoparticles Using Flower Extract of Anchusa Italica. J. Photochem. Photobiol. B 2016, 161, 441–449. [Google Scholar] [CrossRef]
- Mohammadi, C.; Ahmad Mahmud, S.; Mustafa Abdullah, S.; Mirzaei, Y.; Mohammadi, C.; Mahmud, S.A.; Abdulla, S.M. Green Synthesis of ZnO Nanoparticles Using the Aqueous Extract of Euphorbia Petiolata and Study of Its Stability and Antibacterial Properties. Moroc. J. Chem. 2017, 5, 476–484. [Google Scholar]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of Zinc Oxide Nanoparticles from Azadirachta indica for Antibacterial and Photocatalytic Applications. Mater. Sci. Semicond. Process 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Kathing, C.; Saini, G. A Review of Various Treatment Methods for the Removal of Dyes from Textile Effluent. Recent. Prog. Mater. 2022, 4, 21. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Damiani, S. Treatments for Color Removal from Wastewater: State of the Art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive Removal of Dyes from Wastewater Using a Metal-Organic Framework: A Review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef]
- Yeow, P.K.; Wong, S.W.; Hadibarata, T. Removal of Azo and Anthraquinone Dye by Plant Biomass as Adsorbent—A Review. Biointerface Res. Appl. Chem. 2021, 11, 8218–8232. [Google Scholar] [CrossRef]
- Roy, M.; Saha, R. Dyes and Their Removal Technologies from Wastewater: A Critical Review. In Intelligent Environmental Data Monitoring for Pollution Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 127–160. ISBN 9780128196717. [Google Scholar]
- Ismail, G.A.; Sakai, H. Review on Effect of Different Type of Dyes on Advanced Oxidation Processes (AOPs) for Textile Color Removal. Chemosphere 2022, 291, 132906. [Google Scholar] [CrossRef] [PubMed]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the Criteria Anticipated for the Fabrication of Highly Efficient ZnO-Based Visible-Light-Driven Photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, Q. Action and Mechanism of Semiconductor Photocatalysis on Degradation of Organic Pollutants in Water Treatment: A Review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100255. [Google Scholar] [CrossRef]
- Lanjwani, M.F.; Tuzen, M.; Khuhawar, M.Y.; Saleh, T.A. Trends in Photocatalytic Degradation of Organic Dye Pollutants Using Nanoparticles: A Review. Inorg. Chem. Commun. 2024, 159, 111613. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of Photocatalytic Degradation of Organic Compounds: A Mini-Review and New Approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, Y.; Al-Gheethi, A.; Mohamed, R.; Arifin, N.H.; Salleh, N.A. Green ZnO Nanoparticles Photocatalyst for Efficient BR51 Degradation: Kinetics and Mechanism Study. Environ. Prog. Sustain. Energy 2021, 40, e13559. [Google Scholar] [CrossRef]
- Gangwar, J.; Sebastian, J.K. Unlocking the Potential of Biosynthesized Zinc Oxide Nanoparticles for Degradation of Synthetic Organic Dyes as Wastewater Pollutants. Water Sci. Technol. 2021, 84, 3286–3310. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Som, I.; Saha, R.; Mondal, S. A Critical Review on Novel Eco-Friendly Green Approach to Synthesize Zinc Oxide Nanoparticles for Photocatalytic Degradation of Water Pollutants. Int. J. Environ. Anal. Chem. 2022, 104, 489–516. [Google Scholar] [CrossRef]
- Maro, C.A.G.; Gálvez, H.E.G.; de Olivas, O.J.N.; Morales, M.L.; Hernández, D.V.; Flores, H.G.; Carmona, V.M.O.; de Chinchillas, M.J.C. Peumus Boldus Used in the Synthesis of ZnO Semiconductor Nanoparticles and Their Evaluation in Organic Contaminants. Materials 2023, 16, 4344. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.S.; Zafar, A.; Waqas, M.; Hassan, S.G.; ul Haq, A.; Tariq, T.; Batool, S.; Dilshad, M.; Hasan, M.; Shu, X. Phyto-Reflexive Zinc Oxide Nano-Flowers Synthesis: An Advanced Photocatalytic Degradation and Infectious Therapy. J. Mater. Res. Technol. 2021, 13, 2375–2391. [Google Scholar] [CrossRef]
- Bekele, E.T.; Sintayehu, Y.D.; Murthy, H.C.A.; Shume, M.S.; Ayanie, G.T.; Turunesh, D.J.; Balachandran, R.; Tan, K.B.; Chan, K.Y.; Ghotekar, S.; et al. Synthesis of ZnO Nanoparticles Mediated by Natural Products of Acanthus Sennii Leaf Extract for Electrochemical Sensing and Photocatalytic Applications: A Comparative Study of Volume Ratios. Chem. Pap. 2022, 76, 5967–5983. [Google Scholar] [CrossRef]
- Rafique, M.; Sohaib, M.; Tahir, R.; Tahir, M.B.; Khalid, N.R.; Shakil, M.; Gillani, S.S.A.; Khan, M.I.; Alrobei, H.; Shahzad, K.; et al. Novel, Facile and First Time Synthesis of Zinc Oxide Nanoparticles Using Leaves Extract of Citrus Reticulata for Photocatalytic and Antibacterial Activity. Optik 2021, 243, 167495. [Google Scholar] [CrossRef]
- Shanavas, S.; Duraimurugan, J.; Kumar, G.S.; Ramesh, R.; Acevedo, R.; Anbarasan, P.M.; Maadeswaran, P. Ecofriendly Green Synthesis of ZnO Nanostructures Using Artabotrys Hexapetalu and Bambusa Vulgaris Plant Extract and Investigation on Their Photocatalytic and Antibacterial Activity. Mater. Res. Express 2019, 6, 105098. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Alghamdi, A.A.; Alothman, A.A.; Almarhoon, Z.M.; Alsulaiman, M.F.; Al-Zaqri, N. Green Synthesis of Zno Nanostructures Using Salvadora Persica Leaf Extract: Applications for Photocatalytic Degradation of Methylene Blue Dye. Crystals 2020, 10, 441. [Google Scholar] [CrossRef]
- Wijesinghe, U.; Thiripuranathar, G.; Menaa, F.; Iqbal, H.; Razzaq, A.; Almukhlifi, H. Green Synthesis, Structural Characterization and Photocatalytic Applications of ZnO Nanoconjugates Using Heliotropium Indicum. Catalysts 2021, 11, 831. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Synthesis Method, Antibacterial and Photocatalytic Activity of ZnO Nanoparticles for Azo Dyes in Wastewater Treatment: A Review. Inorg. Chem. Commun. 2020, 120, 108140. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An Overview of Photocatalytic Degradation: Photocatalysts, Mechanisms, and Development of Photocatalytic Membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef]
- Supin, K.K.; Parvathy Namboothiri, P.N.; Vasundhara, M. Enhanced Photocatalytic Activity in ZnO Nanoparticles Developed Using Novel Lepidagathis Ananthapuramensis Leaf Extract. RSC Adv. 2023, 13, 1497–1515. [Google Scholar] [CrossRef]
- Kadhim, M.J.; Mahdi, M.A.; Selman, A.M.; Al-Ani, S.K.J.; Hassan, J.J.; Ahmed, N.M. The Most Important Parameters That Affect the Photocatalytic Activity of ZnO Nanostructures against Organic Dyes: A Review. Iran. J. Catal. 2023, 13, 1–21. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Degradation of Wastewaters Containing Organic Dyes Photocatalysed by Zinc Oxide: A Review. Desalination Water Treat. 2012, 41, 131–169. [Google Scholar] [CrossRef]
- Nethravathi, P.C.; Shruthi, G.S.; Suresh, D.; Udayabhanu; Nagabhushana, H.; Sharma, S.C. Garcinia Xanthochymus Mediated Green Synthesis of ZnO Nanoparticles: Photoluminescence, Photocatalytic and Antioxidant Activity Studies. Ceram. Int. 2015, 41, 8680–8687. [Google Scholar] [CrossRef]
- Ragavendran, C.; Kamaraj, C.; Jothimani, K.; Priyadharsan, A.; Anand Kumar, D.; Natarajan, D.; Malafaia, G. Eco-Friendly Approach for ZnO Nanoparticles Synthesis and Evaluation of Its Possible Antimicrobial, Larvicidal and Photocatalytic Applications. Sustain. Mater. Technol. 2023, 36, e00597. [Google Scholar] [CrossRef]
- Sreelekshmi, P.B.; Pillai, R.R.; Unnimaya, S.; Anju, A.L.; Meera, A.P. Biofabrication of Novel ZnO Nanoparticles for Efficient Photodegradation of Industrial Dyes. Clean. Technol. Environ. Policy 2023, 1–4. [Google Scholar] [CrossRef]
- Ramesh, A.M.; Purushotham, D.; Kodandaram, A.; Shilpa, N.; Singh, S.B.; Aiyaz, M.; Gowtham, H.G.; Rahdar, A.; Kaviyarasu, K.; Murali, M. Visible Light Driven Photocatalytic and Competent Antioxidant Properties of Phyto-Fabricated Zinc Oxide Nanoparticles (ZnO-NPs) from Borreria Hispida. J. Mol. Struct. 2023, 1293, 136152. [Google Scholar] [CrossRef]
- Bopape, D.A.; Motaung, D.E.; Hintsho-Mbita, N.C. Green Synthesis of ZnO: Effect of Plant Concentration on the Morphology, Optical Properties and Photodegradation of Dyes and Antibiotics in Wastewater. Optik 2022, 251, 168459. [Google Scholar] [CrossRef]
- Mashentseva, A.A.; Aimanova, N.A.; Parmanbek, N.; Temirgaziyev, B.S.; Barsbay, M.; Zdorovets, M.V. Serratula coronata L. Mediated Synthesis of ZnO Nanoparticles and Their Application for the Removal of Alizarin Yellow R by Photocatalytic Degradation and Adsorption. Nanomaterials 2022, 12, 3293. [Google Scholar] [CrossRef]
- Goutham, R.; Badri Narayan, R.; Srikanth, B.; Gopinath, K.P. Supporting Materials for Immobilisation of Nano-Photocatalysts. In Nanophotocatalysis and Environmental Applications. Environmental Chemistry for a Sustainable World; Inamuddin, Sharma, G., Kumar, A., Lighfouse, E., Asiri, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 29, pp. 49–82. [Google Scholar]
- Ramadhani, S.; Helmiyati, H. Alginate/CMC/ZnO Nanocomposite for Photocatalytic Degradation of Congo Red Dye. AIP Conf. Proc. 2022, 2242, 1–8. [Google Scholar]
- Zyoud, A.H.; Zorba, T.; Helal, M.; Zyoud, S.; Qamhiya, N.; Hajamohideen, A.R.; Zyoud, S.; Hilal, H.S. Direct Sunlight-Driven Degradation of 2-Chlorophenol Catalyzed by Kaolinite-Supported ZnO. Int. J. Environ. Sci. Technol. 2019, 16, 6267–6276. [Google Scholar] [CrossRef]
- Bel Hadjltaief, H.; Ben Ameur, S.; Da Costa, P.; Ben Zina, M.; Elena Galvez, M. Photocatalytic Decolorization of Cationic and Anionic Dyes over ZnO Nanoparticle Immobilized on Natural Tunisian Clay. Appl. Clay Sci. 2018, 152, 148–157. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Correa, M.G.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial Metal-Based Nanoparticles: A Review on Their Synthesis, Types and Antimicrobial Action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Ogly, G.-Z.N.G.; et al. Modern Physical Methods and Technologies in Agriculture. Physics-Uspekhi 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.S.; Mendoza, R.M.O.; Lu, M.C. Zinc Oxide Nanoparticles for Water Disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of Physical and Chemical Interactions in the Antibacterial Behavior of ZnO Nanoparticles against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef]
- Ijaz, M.; Zafar, M.; Islam, A.; Afsheen, S.; Iqbal, T. A Review on Antibacterial Properties of Biologically Synthesized Zinc Oxide Nanostructures. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2815–2826. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Mohamad, R.; Zaidan, U.H.; Abdul Rahman, N.A. Microbial Synthesis of Zinc Oxide Nanoparticles and Their Potential Application as an Antimicrobial Agent and a Feed Supplement in Animal Industry: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Akbar, S.; Tauseef, I.; Subhan, F.; Sultana, N.; Khan, I.; Ahmed, U.; Haleem, K.S. An Overview of the Plant-Mediated Synthesis of Zinc Oxide Nanoparticles and Their Antimicrobial Potential. Inorg. Nano-Met. Chem. 2020, 50, 257–271. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting Antibiotic-Resistant Bacteria Using Nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.E.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review. Hygiene 2023, 3, 269–290. [Google Scholar] [CrossRef]
- Serov, D.A.; Burmistrov, D.E.; Simakin, A.V.; Astashev, M.E.; Uvarov, O.V.; Tolordava, E.R.; Semenova, A.A.; Lisitsyn, A.B.; Gudkov, S.V. Composite Coating for the Food Industry Based on Fluoroplast and ZnO-NPs: Physical and Chemical Properties, Antibacterial and Antibiofilm Activity, Cytotoxicity. Nanomaterials 2022, 12, 4158. [Google Scholar] [CrossRef] [PubMed]
- Ravbar, M.; Kunčič, A.; Matoh, L.; Smole Možina, S.; Šala, M.; Šuligoj, A. Controlled Growth of ZnO Nanoparticles Using Ethanolic Root Extract of Japanese Knotweed: Photocatalytic and Antimicrobial Properties. RSC Adv. 2022, 12, 31235–31245. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, S.; Hema, N.; Vijaya, P.P. In Vitro Biocompatibility and Antimicrobial Activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route—A Comparative Study. Bionanoscience 2020, 10, 112–121. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S. Green Synthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from the Leaf Extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef] [PubMed]
- Hosseindokht, Z.; Kolahdouz, M.; Hajikhani, B.; Sasanpour, P. Photoacoustic Based Evaluation of Viscoelastic Properties of Gram-Negative and Gram-Positive Bacterial Colonies. Sci. Rep. 2023, 13, 14656. [Google Scholar] [CrossRef]
- Imade, E.E.; Ajiboye, T.O.; Fadiji, A.E.; Onwudiwe, D.C.; Babalola, O.O. Green Synthesis of Zinc Oxide Nanoparticles Using Plantain Peel Extracts and the Evaluation of Their Antibacterial Activity. Sci. Afr. 2022, 16, e01152. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green Synthesis of Zinc Oxide Nanoparticles Using Phoenix Dactylifera Waste as Bioreductant for Effective Dye Degradation and Antibacterial Performance in Wastewater Treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef] [PubMed]
- Archana, P.; Janarthanan, B.; Bhuvana, S.; Rajiv, P.; Sharmila, S. Concert of Zinc Oxide Nanoparticles Synthesized Using Cucumis Melo by Green Synthesis and the Antibacterial Activity on Pathogenic Bacteria. Inorg. Chem. Commun. 2022, 137, 109255. [Google Scholar] [CrossRef]
- Alnehia, A.; Al-Odayni, A.B.; Al-Sharabi, A.; Al-Hammadi, A.H.; Saeed, W.S. Pomegranate Peel Extract-Mediated Green Synthesis of ZnO-NPs: Extract Concentration-Dependent Structure, Optical, and Antibacterial Activity. J. Chem. 2022, 2022, 9647793. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; Kalpana, V.N.; Rajalakshmi, G.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A. Enhanced Photocatalytic Degradation of Water Pollutants Using Bio-Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs). J. Environ. Chem. Eng. 2021, 9, 105772. [Google Scholar] [CrossRef]
- Darvishi, E.; Kahrizi, D.; Arkan, E. Comparison of Different Properties of Zinc Oxide Nanoparticles Synthesized by the Green (Using Juglans Regia L. Leaf Extract) and Chemical Methods. J. Mol. Liq. 2019, 286, 110831. [Google Scholar] [CrossRef]

| Advantages | Disadvantages |
|---|---|
| Cost-effective | Reproducibility issues |
| Biocompatible | Relatively less stable NPs |
| Low energy consumption | Difficult to control morphology |
| Avoids hazardous chemicals | - |
| Does not require expensive equipment | - |
| Property | Value |
|---|---|
| Appearance | White solid |
| Odour | Odourless |
| Molecular weight | 81.38 g/mol |
| Density | 5.6 g/cm3 |
| Coordination geometry | Tetrahedral |
| Crystal structure | Hexagonal wurtzite |
| Melting point | (decomposes) |
| Flash point | |
| Toxicity hazards | None |
| Refractive index | 2.0041 |
| Bandgap | Direct and 3.37 eV at RT |
| Electrical conductivity | Semiconductor |
| Hole effective mass | 0.59 |
| Electron effective mass | 0.24 |
| Exciton binding energy | 60 meV |
| Bohr radius | 2.34 nm |
| Luminescence | Luminescent in UV and visible light |
| Synthesis Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Ball milling | Efficient and ideal for large-scale production. | Lengthy processing times. | [39] |
| Thermal evaporation | High deposition rates. | Poor coverage due to low vacuum. | [48] |
| Physical vapour deposition | Simple and relatively low-temperature method. | High production costs and low productivity. | [49] |
| Molecular beam epitaxy | Produces high-purity materials. | High operational cost and expensive equipment. | [48] |
| Sputtering | The nature of sputtering gas may affect the properties of the thin films. | High cost of target and low purity. | [47,48] |
| Synthesis Method | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Sol–gel | Easy and low-temperature synthesis. | Utilises chemical agents and metal alkoxides are expensive | [65] |
| Hydrothermal | A simple method that produces high-yield and uniform NPs. | Requires an autoclave, and high temperatures. Risk of explosion from the autoclave. | [66] |
| Chemical reduction | It is cost-effective and has a good production rate. | Utilises hazardous agents and the formation of toxic by-products. | [67] |
| Chemical vapour deposition | Produces high-purity NPs. | Operates at high temperatures and utilises chemical agents. | [68] |
| Microemulsion | No energy requirements. | Difficult to scale up and requires large amounts of surfactants. | [65] |
| Type of Zn Salt Precursor | Morphology |
|---|---|
| Zinc chloride anhydrous | Mixture of random, conglomerated, packed, clustered, and rod-shaped NPs with face-centred rhombohedral, spherical, hexagonal, and cubic-like structures |
| Zinc acetate dihydrate | Needle-shaped nanorods, thinner nanoplates, and flower-like nanolayers |
| Zinc nitrate hexahydrate | Nanorods, flower-like, nanolayered, and some cubic structures |
| Zinc sulphate monohydrate | Nanorods, nanoplate flower-like nanolayered, and hollow spherical |
| Plant | XRD | Particle Size | Shape | Application | Ref. |
|---|---|---|---|---|---|
| M. acuminata | 30 nm 33.3 nm 40.0 nm | - | Leaf-like, triangular-like, triangular with tapered tips | Photocatalysis | [97] |
| C. roseus | Average 36.83 nm | 62–94 nm SEM 50–92 nm TEM | Spherical | Antimicrobial | [98] |
| Cherry | 20.18 nm | 87.5–116 nm | Spherical | - | [101] |
| H. sabdariffa | 8.71 nm 9.05 nm 38.63 nm | 5–12 nm 20–40 nm TEM | Spherical | Photocatalysis | [107] |
| S. nigrum | Average 2.07 nm | Average 49 nm DLS | Spherical | Antibacterial Antioxidant Photocatalysis | [108] |
| M. acuminata | Average 14.93 nm | Average 74.19 nm BET | - | - | [92] |
| P. oleracea | 25.39 nm 26.04 nm | Average size distribution 70 nm | Spherical and oval | Photocatalysis | [100] |
| T. cacao | - | Average 81 nm TEM | Irregular | Antibacterial Photocatalysis | [105] |
| C. halicacabum | Average 48 nm | Average 48 nm DLS | Hexagonal quartzite | Antibacterial | [107] |
| P. dactylifera | 9.3–22.6 nm | 3.7–10.2 nm TEM | nanorods, nanoplate flower-like, and hollow spherical; needle-shaped nanorods, thinner nanoplate, and flower-like; nanorods, flower-like, and some cubic; clustered and rod-shaped NPs with face-centred rhombohedral, spherical, hexagonal, and cubic-like | Antioxidant | [96] |
| A. indica | Average 39.34 nm 43.63 nm | 53.39 nm TEM 65.13 nm | Spherical and irregular | UV protection Antibacterial Water repellent | [113] |
| D. ambrosioides | - | Average 7, 14, 22, 70 nm FESEM | Quasi-spherical and hexagonal prisms | Antibacterial | [112] |
| S. oleoides | - | 26.62 and 38.62 nm TEM | Irregular, spherical and round | Antibacterial | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutukwa, D.; Taziwa, R.T.; Khotseng, L. A Review of Plant-Mediated ZnO Nanoparticles for Photodegradation and Antibacterial Applications. Nanomaterials 2024, 14, 1182. https://doi.org/10.3390/nano14141182
Mutukwa D, Taziwa RT, Khotseng L. A Review of Plant-Mediated ZnO Nanoparticles for Photodegradation and Antibacterial Applications. Nanomaterials. 2024; 14(14):1182. https://doi.org/10.3390/nano14141182
Chicago/Turabian StyleMutukwa, Dorcas, Raymond Tichaona Taziwa, and Lindiwe Khotseng. 2024. "A Review of Plant-Mediated ZnO Nanoparticles for Photodegradation and Antibacterial Applications" Nanomaterials 14, no. 14: 1182. https://doi.org/10.3390/nano14141182
APA StyleMutukwa, D., Taziwa, R. T., & Khotseng, L. (2024). A Review of Plant-Mediated ZnO Nanoparticles for Photodegradation and Antibacterial Applications. Nanomaterials, 14(14), 1182. https://doi.org/10.3390/nano14141182









