Tunable Hydrogen-Related Defects in ZnO Nanowires Using Oxygen Plasma Treatment by Ion Energy Adjustment
Abstract
1. Introduction
2. Materials and Methods
2.1. Deposition Techniques
2.2. Plasma Treatment
2.3. Characterization Techniques
3. Results
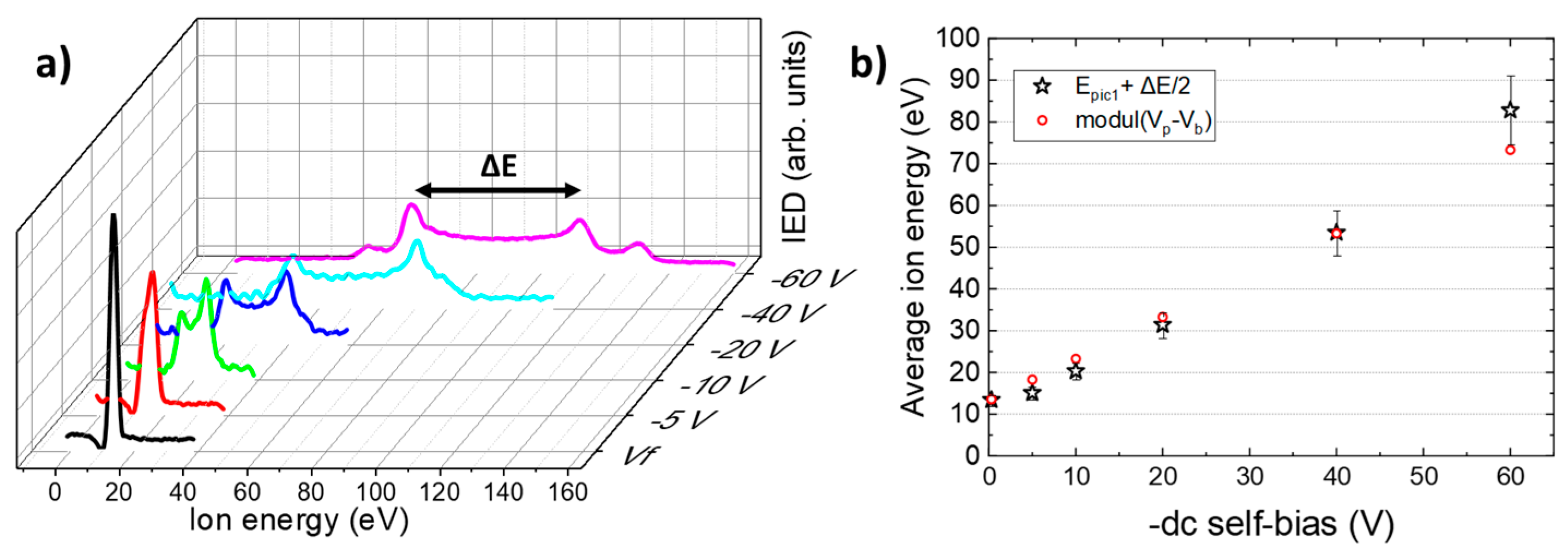
3.1. Oxygen Ion Energy
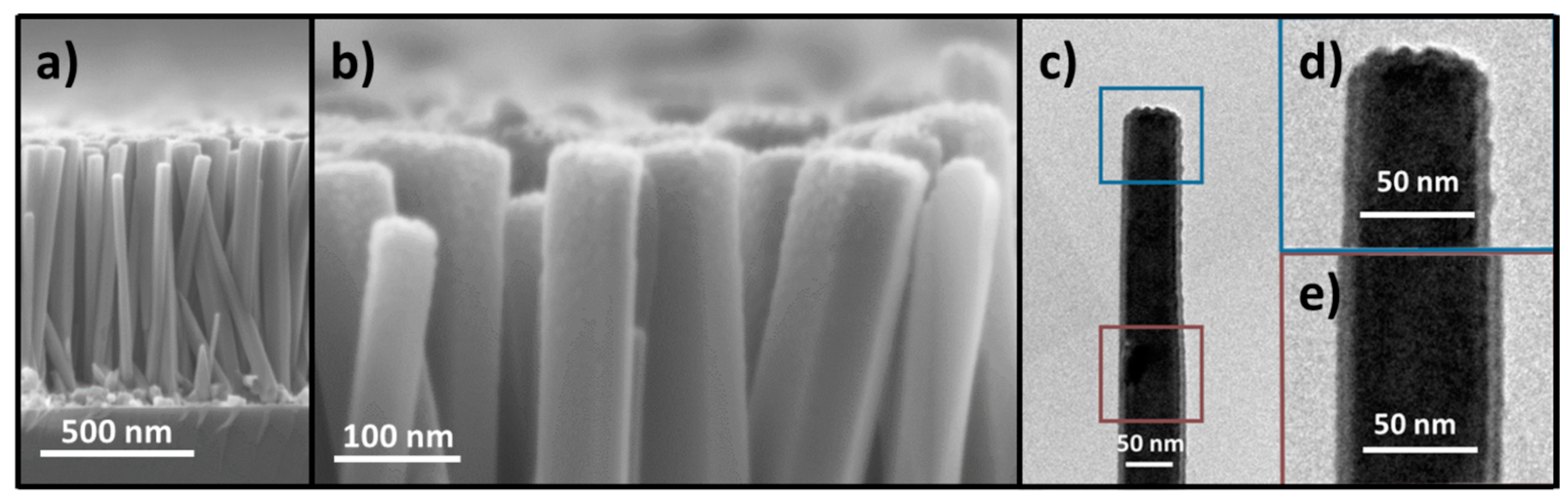
3.2. Effect of Oxygen Plasma Treatment on the Morphology of ZnO Nanowires
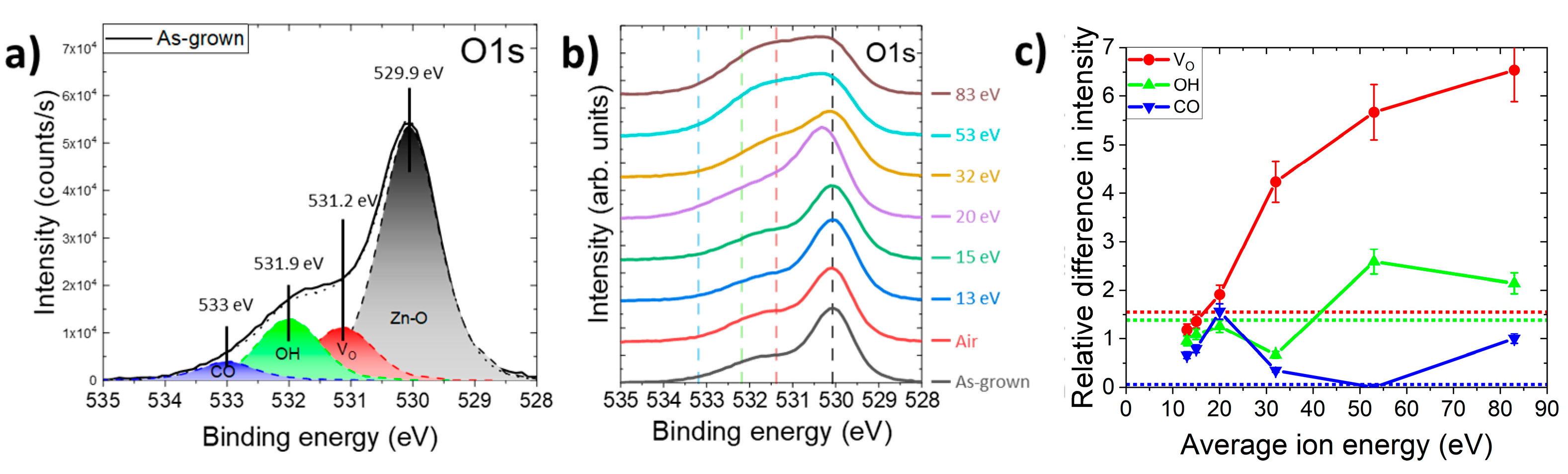
3.3. Effect of Oxygen Plasma Treatment on the Surface of ZnO Nanowires
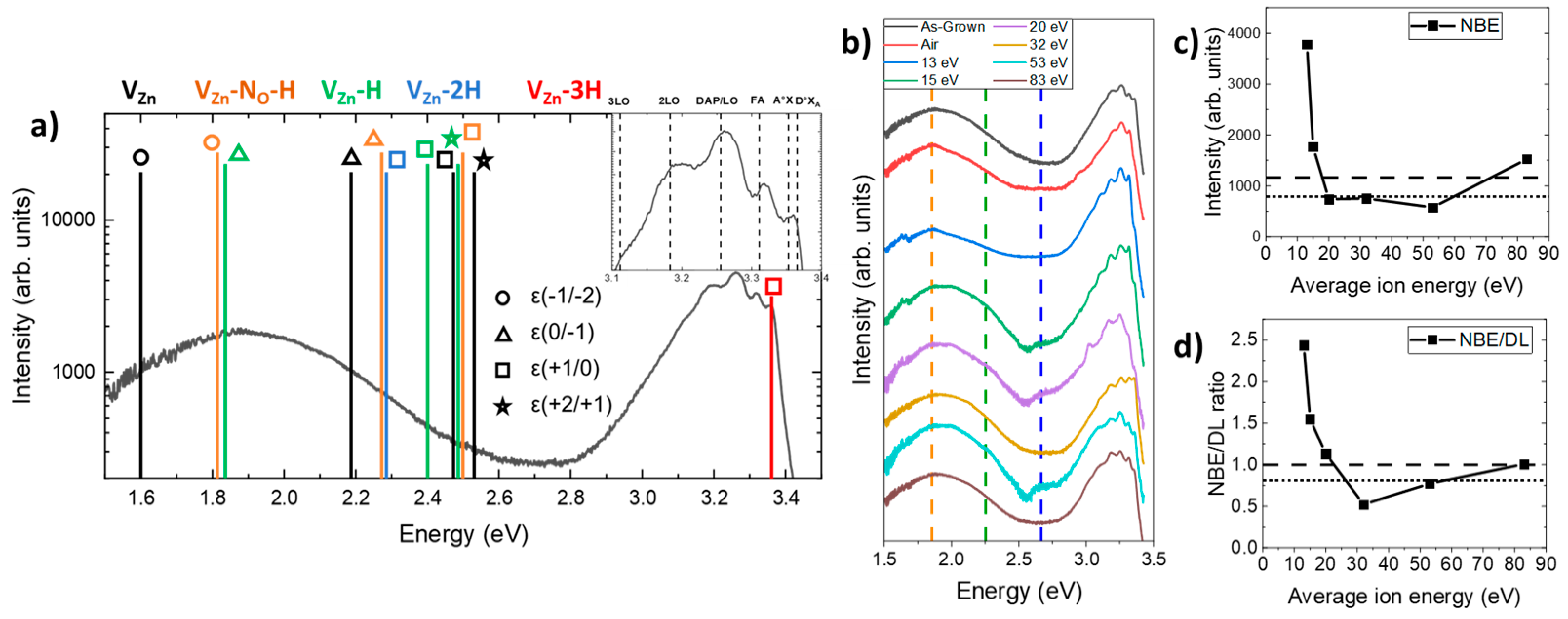
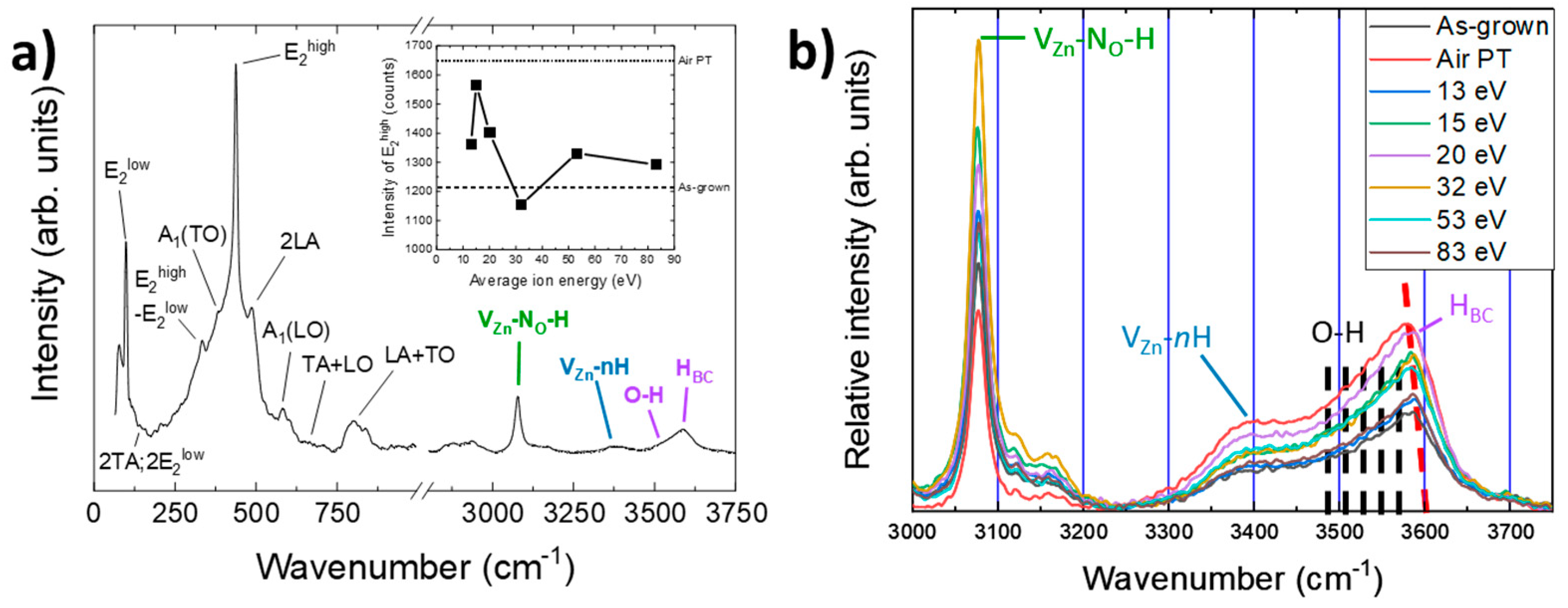
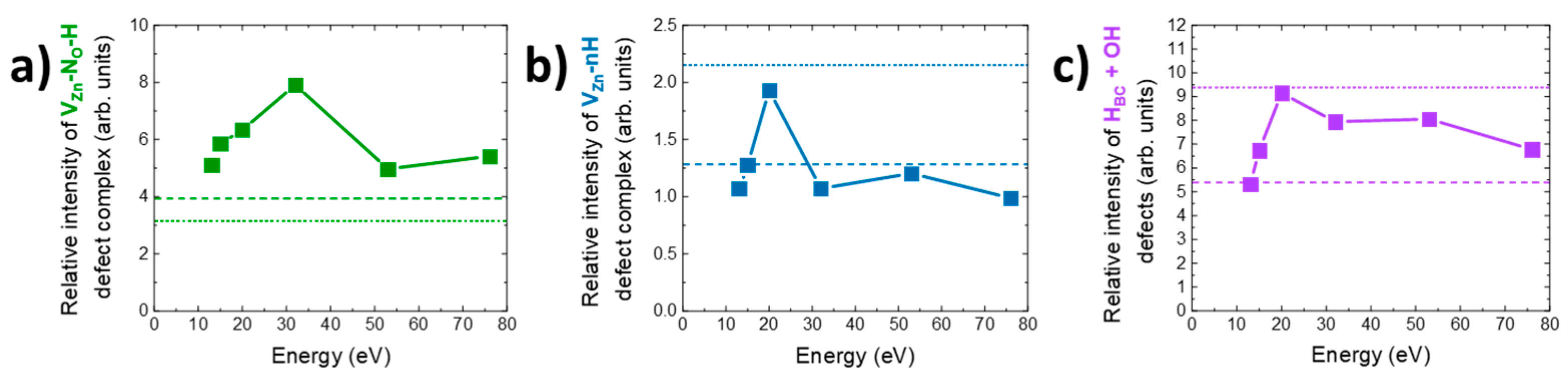
3.4. Effect of Oxygen Plasma Treatment on the Nature and Relative Concentration of Hydrogen-Related Defects
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vayssieres, L.; Keis, K.; Lindquist, S.-E.; Hagfeldt, A. Purpose-Built Anisotropic Metal Oxide Material: 3D Highly Oriented Microrod Array of ZnO. J. Phys. Chem. B 2001, 105, 3350–3352. [Google Scholar] [CrossRef]
- Wang, Z.L. Towards Self-Powered Nanosystems: From Nanogenerators to Nanopiezotronics. Adv. Funct. Mater. 2008, 18, 3553–3567. [Google Scholar] [CrossRef]
- Consonni, V.; Lord, A.M. Polarity in ZnO nanowires: A critical issue for piezotronic and piezoelectric devices. Nano Energy 2021, 83, 105789. [Google Scholar] [CrossRef]
- Pan, C.; Zhai, J.; Wang, Z.L. Piezotronics and Piezo-phototronics of Third Generation Semiconductor Nanowires. Chem. Rev. 2019, 119, 9303–9359. [Google Scholar] [CrossRef] [PubMed]
- Willander, M.; Nur, O.; Zhao, Q.X.; Yang, L.L.; Lorenz, M.; Cao, B.Q.; Zúñiga Pérez, J.; Czekalla, C.; Zimmermann, G.; Grundmann, M.; et al. Zinc oxide nanorod based photonic devices: Recent progress in growth light emitting diodes and lasers. Nanotechnology 2009, 20, 332001. [Google Scholar] [CrossRef] [PubMed]
- Consonni, V.; Briscoe, J.; Kärber, E.; Li, X.; Cossuet, T. ZnO nanowires for solar cells: A comprehensive review. Nanotechnology 2019, 30, 362001. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Lee, J.-H.; Majhi, M. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019, 126, 241102. [Google Scholar] [CrossRef]
- Mahapatra, S.D.; Mohapatra, P.C.; Aria, A.I.; Christie, G.; Mishra, Y.K.; Hofmann, S.; Thakur, V.K. Piezoelectric Materials for Energy Harvesting and Sensing Applications: Roadmap for Future Smart Materials. Adv. Sci. 2021, 8, 2100864. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc Oxide Nanostructures: Growth, Properties and Applications. J. Phys. Condens. Matter 2004, 16, 829–858. [Google Scholar] [CrossRef]
- Yi, G.C.; Wang, C.; Park, W.I. ZnO Nanorods: Synthesis, Characterization and Applications. Semicond. Sci. Technol. 2005, 20, 22–34. [Google Scholar] [CrossRef]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of Semiconducting Oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.C.; Zhang, Y.; Lee, C.J.; Ruh, H.; Lee, H.J. Low-Temperature Growth of ZnO Nanowire Array by a Simple Physical Vapor-Deposition Method. Chem. Mater. 2003, 15, 3294–3299. [Google Scholar] [CrossRef]
- Sun, Y.; Fuge, G.M.; Ashfold, M.N.R. Growth of Aligned ZnO Nanorod Arrays by Catalyst-Free Pulsed Laser Deposition Methods. Chem. Phys. Lett. 2004, 396, 21–26. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, S.C. Low-Temperature Growth of Well-Aligned ZnO Nanorods by Chemical Vapor Deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.W.; Yi, G.C. Metal-Organic Vapor Phase Epitaxial Growth of Vertically Well-Aligned ZnO Nanorods. Appl. Phys. Lett. 2002, 80, 4232–4234. [Google Scholar] [CrossRef]
- Zheng, M.J.; Zhang, L.D.; Li, G.H.; Shen, W.Z. Fabrication and Optical Properties of Large-Scale Uniform Zinc Oxide Nanowire Arrays by One-Step Electrochemical Deposition Technique. Chem. Phys. Lett. 2002, 363, 123–128. [Google Scholar] [CrossRef]
- Miccoli, I.; Spampinato, R.; Marzo, F.; Prete, P.; Lovergine, N. DC-magnetron sputtering of ZnO:Al films on (00.1)Al2O3 substrates from slip-casting sintered ceramic targets. Appl. Surf. Sci. 2014, 313, 418–423. [Google Scholar] [CrossRef]
- Lehraki, N.; Aida, M.S.; Abed, S.; Attaf, N.; Attaf, A.; Poulain, M. ZnO thin films deposition by spray pyrolysis: Influence of precursor solution properties. Curr. Appl. Phys. 2012, 12, 1283–1287. [Google Scholar] [CrossRef]
- Cossuet, T.; Donatini, F.; Lord, A.M.; Appert, E.; Pernot, J.; Consonni, V. Polarity-Dependent High Electrical Conductivity of ZnO Nanorods and Its Relation to Hydrogen. J. Phys. Chem. C 2018, 122, 22767–22775. [Google Scholar] [CrossRef]
- Huang, X.H.; Zhan, Z.Y.; Pramoda, K.P.; Zhang, C.; Zheng, L.X.; Chua, S.J. Correlating the enhancement of UV luminescence from solution-grown ZnO nanorods with hydrogen doping. CrystEngComm 2012, 14, 5163–5165. [Google Scholar] [CrossRef]
- Barbagiovanni, E.G.; Reitano, R.; Franzò, G.; Strano, V.; Terrasi, A.; Mirabella, S. Radiative mechanism and surface modification of four visible deep level defect states in ZnO nanorods. Nanoscale 2016, 8, 995–1006. [Google Scholar] [CrossRef]
- Lavrov, E.V.; Weber, J.; Bö, F.; Van De Walle, C.G.; Helbig, R. Hydrogen-related defects in ZnO studied by infrared absorption spectroscopy. Phys. Rev. B 2002, 66, 165205. [Google Scholar] [CrossRef]
- Lavrov, E.V.; Herklotz, F.; Weber, J. Identification of two hydrogen donors in ZnO. Phys. Rev. B 2009, 79, 5210. [Google Scholar] [CrossRef]
- Villafuerte, J.; Donatini, F.; Kioseoglou, J.; Sarigiannidou, E.; Chaix-Pluchery, O.; Pernot, J.; Consonni, V. Zinc Vacancy-Hydrogen Complexes as Major Defects in ZnO Nanowires Grown by Chemical Bath Deposition. J. Phys. Chem. C 2020, 124, 16652–16662. [Google Scholar] [CrossRef]
- Espinosa, H.D.; Bernal, R.A.; Minary-Jolandan, M. A Review of Mechanical and Electromechanical Properties of Piezoelectric Nanowires. Adv. Mater. 2012, 24, 4656–4675. [Google Scholar] [CrossRef]
- Villafuerte, J.; Chaix-Pluchery, O.; Kioseoglou, J.; Donatini, F.; Sarigiannidou, E.; Pernot, J.; Consonni, V. Engineering nitrogen- and hydrogen-related defects in ZnO nanowires using thermal annealing. Phys. Rev. Mater. 2021, 5, 1–15. [Google Scholar] [CrossRef]
- Liu, Q.; Gong, M.; Cook, B.; Thapa, P.; Ewing, D.; Casper, M.; Stramel, A.; Wu, J. Oxygen Plasma Surface Activation of Electron-Depleted ZnO Nanoparticle Films for Performance-Enhanced Ultraviolet Photodetectors. Phys. Status Solidi A 2017, 214, 1700176. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, W.; Cen, J.; Stavitski, E.; Sadowski, J.T.; Vescovo, E.; Walter, A.; Attenkofer, K.; Stacchiola, D.; Liu, M. Near band edge photoluminescence of ZnO nanowires: Optimization via surface engineering. Appl. Phys. Lett. 2017, 111, 231901. [Google Scholar] [CrossRef]
- Gudmundsson, J.T.; Thorsteinsson, E.G. Oxygen discharges diluted with argon: Dissociation processes. Plasma Sources Sci. Technol. 2007, 16, 399–412. [Google Scholar] [CrossRef]
- Setsuhara, Y.; Takenaka, K.; Ebe, A.; Han, J.G. Properties of argon/oxygen mixture plasmas driven by multiple internal-antenna units. Surf. Coat. Technol. 2008, 20, 5230–5233. [Google Scholar] [CrossRef]
- Hopwood, J.; Guarnieri, C.R.; Whitehair, S.J.; Cuomo, J.J. Langmuir probe measurements of a radio frequency induction plasma. J. Vac. Sci. Technol. A 1993, 11, 152–156. [Google Scholar] [CrossRef]
- Adamovich, I.; Baalrud, S.D.; Bogaerts, A.; Bruggeman, P.J.; Cappelli, M.; Colombo, V.; Czarnetzki, U.; Ebert, U.; Eden, J.G.; Favia, P.; et al. The 2017 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2017, 50, 212001. [Google Scholar] [CrossRef]
- Goghero, D.; Goullet, A.; Borvon, G.; Turban, G. Effect of the ion bombardment energy on silicon dioxide films deposited from oxygen/tetraethoxysilane plasmas in a helicon reactor. Thin Solid. Film. 2005, 471, 123–127. [Google Scholar] [CrossRef]
- Hayden, C.; Gahan, D.; Hopkins, M.B. Ion energy distributions at a capacitively and directly coupled electrode immersed in a plasma generated by a remote source. Plasma Sources Sci. Technol. 2009, 18, 25018–25029. [Google Scholar] [CrossRef]
- Park, D.; Yang, Y.; Kim, K. Evaluation of the mechanical properties of ZnO nanorods treated with oxygen plasma using atomic force microscopy. J. Korean Inst. Met. Mater. 2021, 59, 209–216. [Google Scholar] [CrossRef]
- Ra, H.W.; Khan, R.; Kim, J.T.; Kang, B.R.; Bai, K.H.; Im, Y.H. Effects of surface modification of the individual ZnO nanowire with oxygen plasma treatment. Mater. Lett. 2009, 63, 2516–2519. [Google Scholar] [CrossRef]
- Dong, Y.; Fang, Z.Q.; Look, D.C.; Doutt, D.R.; Cantwell, G.; Zhang, J.; Song, J.J.; Brillson, L.J. Defects at oxygen plasma cleaned ZnO polar surfaces. J. Appl. Phys. 2010, 108, 103718. [Google Scholar] [CrossRef]
- Hussain, M.; Abbasi, M.A.; Ibupoto, Z.H.; Nur, O.; Willander, M. The improved piezoelectric properties of ZnO nanorods with oxygen plasma treatment on the single layer graphene coated polymer substrate. Phys. Status Solidi A 2014, 211, 455–459. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, S.; Liang, L.; Lu, W. Oxygen plasma assisted enhanced photoresponse of ZnO nanowires fabricated by catalyst-free chemical vapor deposition. RSC Adv. 2018, 8, 28928–28933. [Google Scholar] [CrossRef]
- Guillemin, S.; Appert, E.; Roussel, H.; Doisneau, B.; Parize, R.; Boudou, T.; Bremond, G.; Consonni, V. Controlling the Structural Properties of Single Step, Dip Coated ZnO Seed Layers for Growing Perfectly Aligned Nanowire Arrays. J. Phys. Chem. C 2015, 119, 21694–21703. [Google Scholar] [CrossRef]
- Parize, R.; Garnier, J.; Chaix-Pluchery, O.; Verrier, C.; Appert, E.; Consonni, V. Effects of Hexamethylenetetramine on the Nucleation and Radial Growth of ZnO Nanowires by Chemical Bath Deposition. J. Phys. Chem. C 2016, 120, 5242–5250. [Google Scholar] [CrossRef]
- Le-Quoc, H.; Lacoste, A.; Béchu, S.; Bès, A.; Bourgault, D.; Fruchart, D. Deposition of thin films of Mg2Si1−xSnx solid solution by plasma-assisted co-sputtering. J. Alloys Compd. 2012, 538, 73–78. [Google Scholar] [CrossRef]
- Le-Quoc, H.; Lacoste, A.; Hlil, E.K.; Bès, A.; Tan Vinh, T.; Fruchart, D. Thin films of thermoelectric compound Mg2Sn deposited by co-sputtering assisted by multi-dipolar microwave plasma. J. Alloys Compd. 2011, 509, 9906–9911. [Google Scholar] [CrossRef]
- Gahan, D.; Daniels, S.; Hayden, C.; O’ Sullivan, D.; Hopkins, M.B. Characterization of an asymmetric parallel plate radio-frequency discharge using a retarding field energy analyzer. Plasma Sources Sci. Technol. 2012, 21, 015002. [Google Scholar] [CrossRef]
- Moisan, M.; Pelletier, J. Microwave Excited Plasmas, 1st ed.; Plasma Technology; Hardback; Elsevier Science Publisher: Amsterdam, The Netherlands, 1992; Volume 4, ISBN 9780444888150. [Google Scholar]
- Lieberman, M.A. Plasma Discharges for Materials Processing and Display Applications. In Advanced Technologies Based on Wave and Beam Generated Plasmas; Schlüter, H., Shivarova, A., Eds.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1999; Volume 67. [Google Scholar] [CrossRef]
- Liu, J.; Huppert, G.L.; Sawin, H.H. Ion bombardment in rf plasmas. J. Appl. Phys. 1990, 68, 3916. [Google Scholar] [CrossRef]
- Janes, J.; Huth, C. Bombardment energies of O2+ in low pressure reactive ion etching. Appl. Phys. Lett. 1992, 61, 261–263. [Google Scholar] [CrossRef]
- RF Sensors. RF Voltage-Current (VI) Probes| Impedans Ltd. Available online: https://www.impedans.com/rf-voltage-current-probes/ (accessed on 16 July 2024).
- Gahan, D.; Dolinaj, B.; Hopkins, M.B. Comparison of plasma parameters determined with a Langmuir probe and with a retarding field energy analyzer. Plasma Sources Sci. Technol. 2008, 17, 035026. [Google Scholar] [CrossRef]
- Andersson, J.M.; Wallin, E.; Münger, E.P.; Helmersson, U. Energy distributions of positive and negative ions during magnetron sputtering of an Al target in Ar/O2 mixtures. J. Appl. Phys. 2006, 100, 033305. [Google Scholar] [CrossRef]
- Substrate Level Measurements|Impedans Ltd. Available online: https://www.impedans.com/substrate-level-measurement/ (accessed on 16 July 2024).
- Dukes, C.A.; Baragiola, R.A. Compact plasma source for removal of hydrocarbons for surface analysis. Surf. Interface Anal. 2009, 42, 40–44. [Google Scholar] [CrossRef]
- Zhao, Q.; Cai, T.; Wang, S.; Zhu, R.; Liao, Z.; Yu, D. Enhanced near-band-edge emission and field emission properties from plasma treated ZnO nanowires. Appl. Phys. A Mater. Sci. Process. 2010, 100, 165–170. [Google Scholar] [CrossRef]
- De La Rosa, E.; Sepúlveda-Guzman, S.; Reeja-Jayan, B.; Torres, A.; Salas, P.; Elizondo, N.; Jose Yacaman, M. Controlling the Growth and Luminescence Properties of Well-Faceted ZnO Nanorods. J. Phys. Chem. C 2007, 111, 8489–8495. [Google Scholar] [CrossRef]
- Xie, F.; Yang, M.; Song, Z.Y.; Duan, W.C.; Huang, X.J.; Chen, S.H.; Li, P.H.; Xiao, X.Y.; Liu, W.Q.; Xie, P.H. Highly sensitive electrochemical detection of Hg(II) promoted by oxygen vacancies of plasma-treated ZnO: XPS and DFT calculation analysis. Electrochim. Acta 2022, 426, 140757. [Google Scholar] [CrossRef]
- Dedova, T.; Oja Acik, I.; Chen, Z.; Katerski, A.; Balmassov, K.; Gromyko, I.; Nagyné-Kovács, T.; Szilágyi, I.M.; Krunks, M. Enhanced photocatalytic activity of ZnO nanorods by surface treatment with HAuCl4: Synergic effects through an electron scavenging, plasmon resonance and surface hydroxylation. Mater. Chem. Phys. 2020, 245, 122767. [Google Scholar] [CrossRef]
- Gromyko, I.; Krunks, M.; Dedova, T.; Katerski, A.; Klauson, D.; Oja Acik, I. Surface properties of sprayed and electrodeposited ZnO rod layers. Appl. Surf. Sci. 2017, 405, 521–528. [Google Scholar] [CrossRef]
- Hu, H.; Ji, H.F.; Sun, Y. The effect of oxygen vacancies on water wettability of a ZnO surface. Phys. Chem. Chem. Phys. 2013, 15, 16557. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Electronic Structure of Defect States in Hydroxylated and Reduced Rutile TiO2 (110) Surfaces. Phys. Rev. Lett. 2006, 97, 166803. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-M.; Zhang, H.Z.; Zhou, Y.B.; Xu, J.; Zhang, J.M.; Yu, D.P. Surface effects on photoluminescence of single ZnO nanowires. Phys. Lett. A 2008, 372, 4505–4509. [Google Scholar] [CrossRef]
- Meyer, B.K.; Alves, H.; Hofmann, D.M.; Kriegseis, W.; Forster, D.; Bertram, F.; Christen, J.; Hoffmann, A.; Straßburg, M.; Dworzak, M.; et al. Bound exciton and donor-acceptor pair recombinations in ZnO. Phys. Status Solidi B 2004, 241, 231–260. [Google Scholar] [CrossRef]
- Heinhold, R.; Neiman, A.; Kennedy, J.V.; Markwitz, A.; Reeves, R.J.; Allen, M.W. Hydrogen-related excitons and their excited-state transitions in ZnO. Phys. Rev. B 2017, 95, 54120. [Google Scholar] [CrossRef]
- Rommeluère, J.F.; Svob, L.; Jomard, F.; Mimila-Arroyo, J.; Lusson, A.; Sallet, V.; Marfaing, Y. Electrical activity of nitrogen acceptors in ZnO films grown by metalorganic vapor phase epitaxy. Appl. Phys. Lett. 2003, 83, 287–289. [Google Scholar] [CrossRef]
- Pfisterer, D.; Sann, J.; Hofmann, D.M.; Plana, M.; Neumann, A.; Lerch, M.; Meyer, B.K. Incorporation of nitrogen acceptors in ZnO powder. Phys. Status Solidi B 2005, 243, 1–3. [Google Scholar] [CrossRef]
- Zhang, B.P.; Binh, N.T.; Segawa, Y.; Wakatsuki, K.; Usami, N. Optical properties of ZnO rods formed by metalorganic chemical vapor deposition. Appl. Phys. Lett. 2003, 83, 1635–1637. [Google Scholar] [CrossRef]
- Yang, X.D.; Xu, Z.Y.; Sun, Z.; Sun, B.Q.; Ding, L.; Wang, F.Z.; Ye, Z.Z. Recombination property of nitrogen-acceptor-bound states in ZnO. J. Appl. Phys. 2006, 99, 046101. [Google Scholar] [CrossRef]
- Look, D.C.; Reynolds, D.C.; Litton, C.W.; Jones, R.L.; Eason, D.B.; Cantwell, G. Characterization of homoepitaxial p-type ZnO grown by molecular beam epitaxy. Appl. Phys. Lett. 2002, 81, 1830. [Google Scholar] [CrossRef]
- Lautenschlaeger, S.; Eisermann, S.; Haas, G.; Zolnowski, E.A.; Hofmann, M.N.; Laufer, A.; Pinnisch, M.; Meyer, B.K.; Wagner, M.R.; Reparaz, J.S.; et al. Optical signatures of nitrogen acceptors in ZnO. Phys. Rev. B 2012, 85, 235204. [Google Scholar] [CrossRef]
- Wang, Y.G.; Lau, S.P.; Zhang, X.H.; Hng, H.H.; Lee, H.W.; Yu, S.F.; Tay, B.K. Enhancement of near-band-edge photoluminescence from ZnO films by face-to-face annealing. J. Cryst. Growth 2003, 259, 335–342. [Google Scholar] [CrossRef]
- Cuscó, R.; Alarcón-Lladó, E.; Ibáñez, J.; Artús, L.; Jiménez, J.; Wang, B.; Callahan, M.J. Temperature dependence of Raman scattering in ZnO. Phys. Rev. B 2007, 75, 165202. [Google Scholar] [CrossRef]
- Villafuerte, J.; Sarigiannidou, E.; Donatini, F.; Kioseoglou, J.; Chaix-Pluchery, O.; Pernot, J.; Consonni, V. Modulating the growth of chemically deposited ZnO nanowires and the formation of nitrogen- and hydrogen-related defects using pH adjustment. Nanoscale Adv. 2022, 4, 1793–1807. [Google Scholar] [CrossRef]
- Koch, S.G.; Lavrov, E.V.; Weber, J. Interplay between interstitial and substitutional hydrogen donors in ZnO. Phys. Rev. B 2014, 89, 235203. [Google Scholar] [CrossRef]
- Gao, J.; Qin, R.; Luo, G.; Lu, J.; Leprince-Wang, Y.; Ye, H.; Liao, Z.; Zhao, Q.; Yu, D. First-principles study of the formation mechanisms of nitrogen molecule in annealed ZnO. Phys. Lett. A 2010, 374, 3546–3550. [Google Scholar] [CrossRef]



| Reaction Number | Reaction |
|---|---|
| R1 | VZn + n HBC → VZn-nH |
| R2 | VZn + 2 VZn-3H → 3 VZn-2H |
| R2 bis | VZn + VZn-3H → VZn-2H + VZn-H |
| R3 | HBC + HBC → H2 |
| R4 | Adsorption of hydroxyl groups |
| R5 | VZn + NO-H → VZn-NO-H |
| R5 bis | VZn-nH + NO-H → VZn-NO-H + nHBC |
| R6 | Exo-diffusion of H2 |
| R7 | Exo-diffusion of nitrogen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dieulesaint, A.; Chaix-Pluchery, O.; Weber, M.; Donatini, F.; Lacoste, A.; Consonni, V.; Sarigiannidou, E. Tunable Hydrogen-Related Defects in ZnO Nanowires Using Oxygen Plasma Treatment by Ion Energy Adjustment. Nanomaterials 2024, 14, 1225. https://doi.org/10.3390/nano14141225
Dieulesaint A, Chaix-Pluchery O, Weber M, Donatini F, Lacoste A, Consonni V, Sarigiannidou E. Tunable Hydrogen-Related Defects in ZnO Nanowires Using Oxygen Plasma Treatment by Ion Energy Adjustment. Nanomaterials. 2024; 14(14):1225. https://doi.org/10.3390/nano14141225
Chicago/Turabian StyleDieulesaint, Alexandre, Odette Chaix-Pluchery, Matthieu Weber, Fabrice Donatini, Ana Lacoste, Vincent Consonni, and Eirini Sarigiannidou. 2024. "Tunable Hydrogen-Related Defects in ZnO Nanowires Using Oxygen Plasma Treatment by Ion Energy Adjustment" Nanomaterials 14, no. 14: 1225. https://doi.org/10.3390/nano14141225
APA StyleDieulesaint, A., Chaix-Pluchery, O., Weber, M., Donatini, F., Lacoste, A., Consonni, V., & Sarigiannidou, E. (2024). Tunable Hydrogen-Related Defects in ZnO Nanowires Using Oxygen Plasma Treatment by Ion Energy Adjustment. Nanomaterials, 14(14), 1225. https://doi.org/10.3390/nano14141225








